Abstract
Purpose
To evaluate microstructural retinal abnormalities on spectral domain-optical coherence tomography (SD-OCT) imaging of eyes with Coats’ disease.
Methods
This is a multicenter, retrospective study in which SD-OCT images of patients with treatment-naive Coats’ disease were correlated to clinical examination and visual acuity (VA) and, when available, followed longitudinally over time.
Results
Macular SD-OCT of 27 eyes with Coats’ disease revealed intraretinal edema (59%), intraretinal exudates (67%), subretinal fluid (SRF, 37%), subretinal exudate (48%), ellipsoid zone (EZ) disruption (52%), external limiting membrane (ELM) disruption (41%), and subfoveal nodule (26%). All of these microstructural abnormalities correlated with worse baseline and final VA (p<0.05) on univariate analysis, except for intraretinal edema which exhibited a non-statistically significant trend towards worse baseline VA (p=0.16). Within stage 2b eyes, ELM disruption and subretinal nodule on SD-OCT were associated with worse baseline VA (p=0.02 for both), and there was a trend toward worse final VA with ELM disruption and subretinal nodule (p=0.17 for both) and worse baseline (p=0.08) and final (p=0.13) VA with EZ disruption. No microstructural abnormalities were noted on OCT of fellow eyes.
Conclusion
SD-OCT can identify microstructural abnormalities in Coats’ disease that are associated on univariate analysis with worse baseline VA and visual prognosis. Further larger studies are necessary.
Keywords: Coats’ disease, ellipsoid zone, external limiting membrane, subfoveal nodule, optical coherence tomography, spectral domain
INTRODUCTION
Coats’ disease is an idiopathic congenital retinal vasculopathy in which retinal vascular telangiectasias can result in exudation and exudative retinal detachment. The diagnosis of Coats’ disease primarily involves clinical examination along with characteristic findings on fluorescein angiography (FA).1 Although there have been several case reports and small case series describing optical coherence tomography (OCT) findings during the course of Coats’ disease and its management, the microstructural abnormalities noted on OCT imaging in Coats’ disease have not been well characterized or systematically evaluated.2–5 A report by Shields et al reported OCT findings in cases of tumors and simulating lesions, including 4 cases of Coats’ disease; however, specific OCT features of the Coats’ disease cases were not described.6 More recently, there was a retrospective report of OCT and OCT-angiography (OCT-A) findings in 8 cases of Coats’ disease, with OCT findings reported only in a few cases in case report fashion.7 In the present multicenter retrospective cohort study, we systematically evaluated microstructural abnormalities noted on spectral domain (SD)-OCT of eyes with Coats’ disease and evaluated their associations with Coats’ disease stage and visual acuity (VA).
METHODS
This is a multicenter, retrospective, observational cohort study of all patients with Coats’ disease who presented at Weill Cornell Medical College or Mass Eye & Ear Infirmary/Harvard Medical School between January 2007 – September 2016 and had SD-OCT imaging performed. The study protocol was approved by the Institutional Review Boards of both institutions, and the study was performed in a manner compliant with the Healthcare Insurance Portability and Accountability Act and the tenets put forth in the Declaration of Helsinki.
The electronic medical record database was queried for all patients with a billing diagnosis of “Coats’ disease” (ICD9 362.12; ICD10 H35.021, H35.022, H35.023, H35.029). The charts of these patients were reviewed to identify those who met the following inclusion criteria: (1) diagnosis of Coats’ disease based on characteristic clinical and FA findings and (2) SD-OCT imaging available for retrospective review. Exclusion criteria were: (1) atypical Coats’ cases such as bilateral Coats’ or syndromic cases (Coats’ plus or Coats’ syndrome (fascioscapulohumeral muscular dystrophy)); (2) any prior treatment of Coats’ disease (intravitreal anti-vascular endothelial growth factor (VEGF) injection, laser photocoagulation, cryotherapy, and/or surgery) and/or (3) presence of media opacity limiting interpretability of SD-OCT imaging.
For subjects who met the inclusion criteria, the electronic medical record was evaluated to record demographic information and clinical examination findings including: baseline VA, presence of vitreoretinal interface abnormality, macular edema, macular exudate, macular SRF (retinal detachment), and final VA. Coats’ disease stage1 (Table 1) was determined based on the review of evaluating clinician’s staging diagnosis (when available in the assessment section), clinical examination findings, and, whenever available, fundus photographs and FA. SD-OCT volume scans were obtained using the Heidelberg Spectralis HRA (Heidelberg Retinal Angiograph) + OCT system (Heidelberg Engineering, Inc, Heidelberg, Germany) (25 eyes) or the Cirrus HD-OCT (2 eyes, Carl Zeiss Meditec, Dublin, CA, USA). For the Heidelberg Spectralis images, volume scans ranged from 20º × 15º [5.9 × 4.4 mm] with 37 B-scans and 123 μm between B-scan images to 20º × 25º [6.3 × 7.8mm] with 61 B-scans and 131 μm between B-scan images). For the Cirrus HD-OCT images, volume scans using the 512×128 protocol with 56 to 68 B-scans, 125 μm scan separation, and 9mm scan length were used. Two independent readers evaluated all images (MPG and KX), with any discrepancies in interpretations reconciled by a third independent reviewer (YY). SD-OCT volume scans were evaluated for presence of vitreoretinal interface abnormality, intraretinal edema, intraretinal exudate, subretinal exudate, subretinal fluid (SRF), ellipsoid zone (EZ) disruption, external limiting membrane (ELM) disruption, or subfoveal nodule (defined as a sharply demarcated hyperreflective subfoveal lesion with posterior shadowing effect). Central retinal thickness was measured at the foveal center using the calipers on the OCT machine’s imaging review software, with boundaries defined as internal limiting membrane and retinal pigment epithelium. When available, subfoveal choroidal thickness, measured from the outer portion of the RPE to the inner surface of the sclera, was assessed using the calipers available on the OCT machine’s imaging review software.
Table 1.
Coats’ disease classification schema1
| Stage | Clinical |
|---|---|
| 1 | Retinal telangiectasia only |
| 2a | Retinal telangiectasia with exudation, not involving the fovea |
| 2b | Retinal telangiectasia with exudation involving the fovea |
| 3a1 | Subtotal exudative retinal detachment, not involving the fovea |
| 3a2 | Subtotal exudative retinal detachment, involving the fovea |
| 3b | Total retinal detachment |
| 4 | Total retinal detachment and glaucoma |
| 5 | Advanced end-stage disease |
Snellen VA were converted to logarithm of the minimal angle of resolution (logMAR) units for analyses IBM Statistical Package for the Social Sciences (SPSS) software was utilized for statistical analyses. Statistical analyses for the non-parametric variable of logMAR VA were performed using Mann Whitney U test for two groups (baseline versus final VA; correlations of OCT variables (presence or absence) with baseline or final VA) and the Kruskal Wallis H test for two or more groups (for correlation of logMAR VA relative to Coats’ disease stage). Student’s t-test with paired comparisons was performed for evaluation of subfoveal choroidal thickness between the two eyes of the same subject. Fisher’s exact test or Chi-squared test was performed for frequency comparisons of categorical variables. Logistic regression analyses were performed for multivariable analyses of baseline logMAR VA, Coats’ disease stage, and SD-OCT features, with the outcome measure being final logMAR VA. All analyses were two-tailed with p-values < 0.05 considered significant.
RESULTS
Twenty-seven eyes of 27 subjects met inclusion criteria. The average age of subjects was 10.5 years (median 9 years; range 4–44 years). Twenty-two were male (82%) and 5 were female. Average VA at presentation was 0.80 LogMAR (Snellen equivalent 20/125; range 20/15 to hand motions). Coats’ disease stage at the time of diagnosis was: stage 1 in 2 eyes (7%), stage 2a in 6 eyes (22%), stage 2b in 12 eyes (44%), stage 3a1 in 3 eyes (11%), and stage 3a2 in 4 eyes (15%). There were no cases of more advanced Coats’ disease stages (stage 3b, 4, 5) that had SD-OCT imaging available. The average length of follow-up was 27.7 (range 1–67) months. Two subjects were lost to follow-up after initial presentation. Of the remaining 25 subjects, initial therapy was: laser (10 eyes), combination laser and injection of intravitreal bevacizumab (13 eyes), and surgery (2 eyes, 1 with external drainage of SRF, laser, and intravitreal injection of bevacizumab and another with external drainage of SRF, pars plana vitrectomy, endolaser, and fluid-air exchange and intravitreal injection of bevacizumab). The average VA at final follow-up was 0.63 LogMAR (Snellen equivalent 20/85; range 20/20 to hand motions).
Baseline SD-OCT abnormalities and correlations to baseline and final VA
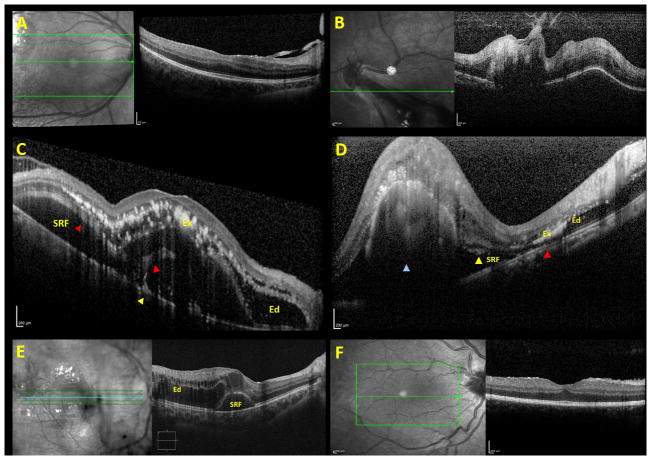
At presentation, only 2 of 27 eyes (7.4%) had vitreoretinal interface abnormalities, with two stage 3 eyes (7.4%) exhibiting hyaloid organization with traction (Figure 1A–B). There were no epiretinal membranes (ERMs) noted in any of the eyes (0/27, 0%). Table 2 outlines intra and subretinal microstructural abnormalities identified on OCT and their correlations to VA. Intraretinal macular edema (Figure 1C and 1E) was noted by SD-OCT in 16/27 eyes (59.3%) and by clinical examination in 10/27 eyes (37.0%) (p = 0.10). Presence of intraretinal edema on SD-OCT correlated with worse final VA (0.86 (~20/145) vs. 0.33 (~20/43), p = 0.04), and there was a trend toward a similar association with baseline VA (0.98 (~20/190) vs. 0.53 (~20/67), p = 0.16). Intraretinal exudates (Figure 1C–1D) were noted in 17/27 eyes (63%) on SD-OCT and in18/27 (67%) by clinical exam (p = 1.0). Presence of intraretinal exudate on SD-OCT was associated with worse initial VA (1.07 (~20/235) vs. 0.38 (~20/48), p = 0.02) and final VA (1.01 (~20/205) vs. 0.13 (~20/27), p = 0.001) There was a predilection for involvement of the outer plexiform layer for both intraretinal edema and exudates (Figure 1C–1E), although other inner and outer retinal layers were variably involved. Macular SRF (Figure 1C and 1E) was noted on SD-OCT in 10/27 eyes (37%) versus 4/27 eyes (15%) by clinical exam (p=0.06), and presence of SRF on SD-OCT was associated with worse baseline (1.34 (~20/438) vs. 0.47 (~20/59), p = 0.005) and final (1.05 (~20/224) vs. 0.38 (~20/48), p = 0.02) VA. Subretinal exudates (Figure 1C–1D) were noted in 13/27 eyes (48%) and were associated with worse baseline (1.23 (~20/340) vs. 0.44 (~20/55), p = 0.005) and final (1.08 (~20/240) vs. 0.3 (~20/40), p=0.003) VA. Disruption of the EZ (Figure 1C–1D) was noted in 14/27 eyes (52%) and was associated with worse baseline (1.26 (~20/264) vs. 0.35 (~20/45), p = <0.001) and final (1.12 (~20/264) vs 0.19 (~20/31), p < 0.001) VA. Disruption of the ELM (Figure 1C–1D) was noted in 11/17 eyes (41%), all of which also had EZ abnormalities, and was associated with worse baseline (1.51 (~20/647) vs. 0.36 (~20/45), p < 0.001) and final (1.21 (~20/324) vs 0.28 (~20/38), p = 0.001) VA. Subfoveal nodule (Figure 1D) was noted in 7/27 (26%) eyes and was associated with worse baseline (1.12 (~20/264) vs 0.71 (~20/102), p=0.03) and final (1.15 (~20/282) vs. 0.43 (~20/54), p=0.04) VA (Table 2). Baseline logMAR VA did not correlate statistically with final VA (p=0.46). A Kruskal-Wallis H test showed that there was a strong trend towards a statistically significant difference in baseline VA based on Coats’ disease staging (p = 0.06), with mean rank logMAR baseline VA of 5.75 for Stage 1, 9.83 for Stage 2a, 14.45 for Stage 2b, 10.83 for Stage 3a, and 22.25 for Stage 3b. There was a trend towards statistical significant difference in final VA based on Coats’ disease staging (p = 0.09), with mean rank logMAR baseline VA of 3.00 for Stage 1, 7.10 for Stage 2a, 13.80 for Stage 2b, 11.50 for Stage 3a, and 14.25 for Stage 3b. Multivariable logistic regression analyses showed a trend toward a significant prediction of final VA based on Coats’ disease stage, baseline VA, and SD-OCT findings of intraretinal edema, intraretinal exudates, subretinal fluid, subretinal exudate, EZ disruption, ELM disruption, or subfoveal nodule (F(8,12) = 1.96, p = 0.14 with an R2 of 0.57). However, none of the individual variables contributed significantly to the prediction (p = 0.29, 0.31, 0.81, 8.98, 0.43, 0.35, 0.33, 0.62, and 0.30, respectively).
Figure 1. Microstructural abnormalities identified on baseline SD-OCT imaging of eyes with Coats’ disease.
Few eyes exhibited vitreoretinal interface changes such as posterior hyaloidal organization with traction (A–B). Retinal microstructural abnormalities (C–E) noted on SD-OCT included intraretinal edema (C–E, labelled “Ed”), intraretinal exudates (C–D, labelled “Ex”), subretinal exudates (C–D, yellow arrowhead), subretinal fluid (C–E, labelled “SRF”), disruption of both the ellipsoid zone and external limiting membrane (C–D, red arrowhead), and subfoveal nodule (D, blue arrowhead). Subjects with Stage 2b Coats’ disease frequently exhibited intraretinal edema (labelled “Ed”) with a secondary SRF (labelled “SRF”) pocket (E). Persistent fetal foveal architecture with retention of inner retinal layers in the foveal center was infrequently noted (F).
Table 2.
Retinal microstructural abnormalities identified by optical coherence tomography and correlations to logMAR visual acuity
| Present | Absent | p-value | ||
|---|---|---|---|---|
| Intraretinal edema | Number (%) | 16 (59) | 11 (41) | |
| Initial logMAR visual acuity (Snellen) | 0.98 (20/190) | 0.53 (20/67) | 0.16 | |
| Final logMAR visual acuity (Snellen) | 0.86 (20/145) | 0.33 (20/43) | 0.04 | |
| Intraretinal exudate | Number (%) | 17 (63) | 9 (33) | |
| Initial logMAR visual acuity (Snellen) | 1.07 (20/235) | 0.38(20/48) | 0.02 | |
| Final logMAR visual acuity (Snellen) | 1.01 (20/205) | 0.13 (20/27) | 0.001 | |
| Subretinal fluid | Number (%) | 10 (37) | 17 (63) | |
| Initial logMAR visual acuity (Snellen) | 1.34 (20/438) | 0.47 (20/59) | 0.005 | |
| Final logMAR visual acuity (Snellen) | 1.05 (20/224) | 0.38 (20/48) | 0.02 | |
| Subretinal exudate | Number (%) | 13 (48) | 14 (52) | |
| Initial logMAR visual acuity (Snellen) | 1.23 (20/340) | 0.44 (20/55) | 0.005 | |
| Final logMAR visual acuity (Snellen) | 1.08 (20/240) | 0.3 (20/40) | 0.003 | |
| Disruption of the ellipsoid zone | Number (%) | 14 (52) | 13 (48) | |
| Initial logMAR visual acuity (Snellen) | 1.26 (20/264) | 0.35 (20/45) | <0.001 | |
| Final logMAR visual acuity (Snellen) | 1.12 (20/264) | 0.19 (20/31) | <0.001 | |
| Disruption of the external limiting membrane | Number (%) | 11 (41) | 16 (59) | |
| Initial logMAR visual acuity (Snellen) | 1.51 (20/647) | 0.36 (20/45) | <0.001 | |
| Final logMAR visual acuity (Snellen) | 1.21 (20/324) | 0.28 (20/38) | 0.001 | |
| Subfoveal nodule | Number (%) | 7 (26) | 20 (74) | |
| Initial logMAR visual acuity (Snellen) | 1.12 (20/264) | 0.71 (20/102) | 0.03 | |
| Final logMAR visual acuity (Snellen) | 1.15 (20/282) | 0.43 (20/54) | 0.04 | |
One eye (4%) exhibited persistent fetal foveal architecture (retention of the inner retinal layers in the central fovea) (Figure 1F). The mean subfoveal choroidal thickness was 233.8 microns in the involved eyes at baseline and 225.3 microns in the fellow eyes (p = 0.54). No vitreoretinal interface nor intraretinal microstructural abnormalities were noted on SD-OCT of any of the fellow eyes.
Baseline SD-OCT findings and correlations to Coats’ disease stage
The distribution of microstructural intraretinal abnormalities in relation to Coats’ disease staging are noted in Table 3. As expected, no microstructural abnormalities were noted in OCT in stage 1 Coats’ disease (telangiectasia without exudation). Most stage 2a eyes (telangiectasia and extrafoveal exudation) (5/6 eyes, 83%) exhibited no macular abnormalities on SD-OCT. One of 6 patients (17%) did demonstrate a cystic change in the fovea on SD-OCT imaging, however there was no macular leakage on FA. As expected, all stage 2b Coats’ disease eyes (telangiectasia and foveal exudation but no retinal detachment) exhibited either intraretinal edema (10 eyes, 88%) and/or intraretinal exudation (11 eyes, 91%) on SD-OCT. Of the stage 2b eyes, 50% (6/12 eyes) exhibited SRF (Figure 1E), though no detachment nor SRF was noted clinically; 67% (8/12 eyes) exhibited EZ disruption; 50% (6/12 eyes) exhibited disruption of the ELM; and 50% (6/12 eyes) exhibited subfoveal nodule. In these stage 2b eyes, SRF was seen in discrete pockets associated with marked intraretinal edema. Of 3 subjects with stage 3a1 Coats’ disease (subtotal exudative retinal detachment not involving the fovea), 1 (33%) had intraretinal edema involving the fovea, 2 of 3 (67%) had intraretinal exudates involving the fovea, and 2 (67%) had EZ and/or ELM disruption. None (0%) had subfoveal nodule. All stage 3a2 eyes (subtotal exudative retinal detachment involving the fovea) had intraretinal foveal edema, intraretinal foveal exudates, SRF, subretinal exudates, and disruption of both the EZ and ELM (4/4 eyes, 100%), although only 1 of the 4 eyes (25%) exhibited subfoveal nodule.
Table 3.
Microstructural abnormalities identified on spectral domain optical coherence tomography imaging, according to Coats’ disease stage
| Stage | Intraretinal edema (No. (%)) | Intraretinal exudate (No. (%)) | Subretinal fluid (No. (%)) | Subretinal exudate (No. (%)) | Disruption of the ellipsoid zone (No. (%)) | Disruption of the external limiting membrane (No. (%)) | Subfoveal nodule (No. (%)) |
|---|---|---|---|---|---|---|---|
| 1 (n=2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2a (n=6) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2b (n=12) | 10 (83) | 11 (91) | 6 (50) | 8 (67) | 8 (67) | 6 (50) | 6 (50) |
| 3a1 (n=3) | 1 (33) | 2 (67) | 0 (0) | 1 (33) | 2 (67) | 1 (33) | 0 (0) |
| 3a2 (n=4) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 1 (25) |
Abbreviations: No.: number
Within stage 2b eyes, all of which had intraretinal edema and/or intreretinal exudates, disruption of the ELM and presence of subfoveal nodule were associated with worse baseline VA (1.08 (~20/240) vs. 0.47 (~20/59), p = 0.02 for both analyses). Small sample size limited the analyses, although there were trends towards association of ELM disruption and subfoveal nodule with final VA (1.32 (~20/418) vs 0.54 (~20/69), p = 0.17 for both analyses) and of EZ disruption with baseline VA (0.94 (~20/174) vs 0.4 (~20/50), p = 0.08) and final VA (1.16 (~20/289) vs. 0.4 (~20/50), p = 0.13). There was no statistically significant difference in eyes with or without SRF in terms of baseline VA (0.88 (20/152) vs. 0.59 (20/78), p = 0.51) or final VA (1.13 (20/270) vs. 0.63 (20/85), p = 0.45). Subgroup analyses within other stages could not be performed due to small subgroup sample sizes.
Longitudinal follow-up of SD-OCT abnormalities
Of the 27 eyes, 20 had subsequent SD-OCT available, at an average of 28.6 (range 1–67) months after the initial SD-OCT. At final follow-up, SD-OCT showed no additional eyes with vitreomacular traction. Three of 20 eyes (15%) exhibited ERM (p=0.07 vs. baseline). Table 4 outlines longitudinal changes in intraretinal microstructural abnormalities on SD-OCT. At final follow-up, intraretinal edema was noted in 8/20 eyes (40%; p = 0.24 vs. baseline), intraretinal exudates in 13/20 of eyes (65%, p = 1.00 vs. baseline), and subretinal exudates in 7/20 eyes (35%, p = 0.37 vs. baseline). Intraretinal edema, intraretinal exudates, and subretinal exudates stabilized or improved in 19/20 eyes (95%) and worsened in 1/20 eyes (5%). Subretinal fluid was noted on final follow-up SD-OCT in 3/20 eyes (15%, p = 0.09 vs. baseline), and subretinal fluid was improved in 7/20 eyes (35%), stable in 13/20 eyes (65%), and worse in 0/20 eyes (0%), as compared to baseline SD-OCT. At final follow-up, SD-OCT revealed EZ disruption in 11/20 eyes (55%, p = 1.00 vs. baseline) and ELM disruption in 9/20 eyes (45%, p = 1.00 vs. baseline). ELM and EZ integrity were stable in 19/20 eyes (95%), while 1/20 eyes (5%) exhibited interval disruption of an initially intact EZ and ELM. There were no cases in whom baseline disrupted EZ or ELM normalized after therapy. Eight of 20 eyes (40%) exhibited a macular scar on SD-OCT at final follow-up, and final VA correlated with presence of macular scar (1.1 (~20/252) vs. 0.37 (~20/47), p=0.01). Presence of subfoveal nodule at initial SD-OCT was associated with later development of macular scar (p=0.002). All eyes (6/6 eyes, 100%) with subfoveal nodule at baseline who had subsequent OCT imaging developed macular scar by final follow-up, while 2/12 eyes (17%) without subfoveal nodule at baseline developed macular scar by final follow-up. Average central retinal thickness on SD-OCT was 474.8 microns at baseline and 383.6 microns at final follow-up (p = 0.04).
Table 4.
Longitudinal evolution of retinal microstructural abnormalities identified in Coats’ disease by optical coherence tomography
| Intraretinal edema | Baseline SD-OCT | 16/27 (59%) |
| Improved | 10/20 (50%) | |
| Stable | 9/20 (45%) | |
| Worse | 1/20 (5%) | |
| Final SD-OCT | 8/20 (40%) | |
| Intraretinal exudates | Baseline SD-OCT | 17/27 (63%) |
| Improved | 11/20 (55%) | |
| Stable | 8/20 (40%) | |
| Worse | 1/20 (5%) | |
| Final SD-OCT | 13/20 (65%) | |
| Subretinal fluid | Baseline SD-OCT | 10/27 (37%) |
| Improved | 7/20 (35%) | |
| Stable | 13/20 (65%) | |
| Worse | 0/20 (0%) | |
| Final SD-OCT | 3/20 (15%) | |
| Subretinal exudates | Baseline SD-OCT | 13/27 (48%) |
| Improved | 10/20 (50%) | |
| Stable | 9/20 (45%) | |
| Worse | 1/20 (5%) | |
| Final SD-OCT | 7/20 (35%) | |
| Ellipsoid zone disruption | Baseline SD-OCT | 14/27 (52%) |
| Improved | 0/20 (0%) | |
| Stable | 19/20 (95%) | |
| Worse | 1/20 (5%) | |
| Final SD-OCT | 11/20 (55%) | |
| External limiting membrane disruption | Baseline SD-OCT | 11/27 (41%) |
| Improved | 0/20 (0%) | |
| Stable | 19/20 (95%) | |
| Worse | 1/20 (5%) | |
| Final SD-OCT | 9/20 (45%) |
Abbreviations: SD-OCT: spectral domain optical coherence tomography
DISCUSSION
Coats’ disease is a sporadic, idiopathic, congenital retinal vasculopathy involving retinal telangiectasias that result in exudation, exudative retinal detachment, and in end stage eyes, glaucoma and phthisis bulbi. The diagnosis of Coats’ disease involves characteristic clinical findings, along with FA demonstrating leaking retinal telangiectasias.1 To our knowledge, the SD-OCT findings in this condition have not been systematically evaluated, although several prior manuscripts have described findings qualitatively or in case report fashion.2–7 In this study, we retrospectively reviewed microstructural abnormalities noted on SD-OCT imaging of eyes with Coats’ disease and correlated these to Coats’ disease stage and baseline VA and final VA, as well as determined how the SD-OCT findings evolve over time. The key findings of this study were: (1) SD-OCT can identify microstructural retinal abnormalities in Coats’ disease, and some microstructural abnormalities such as intraretinal edema and subretinal fluid may be identified more frequently with SD-OCT than with clinical examination; (2) SD-OCT findings in Coats’ disease generally correlate well with clinical Coats’ disease staging; and (3) microstructural abnormalities on SD-OCT of Coats’ disease may correlate with VA and visual prognosis and may refine visual prognosis within a given Coats’ disease stage
Our SD-OCT data identified microstructural abnormalities in Coats’ disease eyes which have the potential to affect vision. Vitreoretinal interface abnormalities such as posterior hyaloidal organization with secondary traction were noted on baseline SD-OCT in two eyes with stage 3a disease. SD-OCT did not identify any ERMs at baseline, perhaps reflecting the young age of the Coats’ disease patient population and the pathophysiology of Coats’ disease, which is considered a retinopathy, rather than a vitreoretinopathy. In contrast, ERM was noted on final SD-OCT imaging in several eyes, likely related to treatment effect from laser. In this study, SD-OCT imaging was able to identify intraretinal edema, intraretinal exudate, SRF, subretinal exudate, disruption of the outer retinal and photoreceptors (ELM, EZ), and presence of subfoveal nodule in eyes with Coats’ disease. Prior descriptive case reports and series have similarly described presence of ERM,7 intraretinal edema,7 intraretinal exudates,2–5,7 subretinal exudates,4,5 and SRF2,3,7 in Coats’ disease. A prior study described subfoveal nodule in patient’s with stage 2b and 3a Coats’ disease. 8 To our knowledge, EZ and ELM integrity has not previously been evaluated. These findings are of particular interest since neither are evident clinically and because both correlated strongly with VA and potential. Recently, abnormalities in choroidal thickness has been implicated in a number of retinal diseases, including the related condition of macular telangiectasia type 2 (in which choroidal thickening was noted).9 In this study, choroidal thickness appeared normal for age, and was no difference in baseline subfoveal choroidal thickness between the involved and uninvolved eyes. However, the evaluation of the choroid was limited by lack of enhanced-depth imaging. Additionally, it is possible that there are choroidal abnormalities localized to the area of retinal telangiectasia and leakage that were not appreciable by the macular SD-OCT imaging utilized in this study.
Compared to clinical examination, SD-OCT appeared to have a similar rate of identification of intraretinal exudate, perhaps reflecting the readily apparent nature of yellow exudates on clinical examination. In contrast, SD-OCT exhibited a trend towards increased identification of intraretinal edema (60% vs. 37%, p=0.10) and subretinal fluid (37% vs 14%, p=0.06), as compared to clinical examination. Larger studies are necessary to fully establish these findings. As noted below, several OCT features that are clinically imperceptible (EZ and ELM disruption) correlated strongly with baseline and final VA.
Overall, SD-OCT findings correlated well with Coats’ disease staging based on examination, FA, and fundus photographs. As expected, none of the stage 1 eyes (telangiectasia without exudation) exhibited microstructural abnormalities such as edema or exudate in the macula on SD-OCT imaging, whereas all of the stage 3a2 eyes (subtotal exudative detachment involving the fovea) exhibited all of these abnormalities. Consistent with the definition of stage 2a Coats’ disease (exudation without foveal involvement), none of these eyes exhibited intraretinal exudate or SRF on SD-OCT. One of 6 eyes (17%) with stage 2a Coats’ disease exhibited a foveal cystic change on SD-OCT. Although both ischemia and/or inflammation-driven VEGF load10 could result in parafoveal capillary leakage with cystoid edema in Coats’ disease, the FA in this patient exhibited no leakage. Non-leaking cystoid macular edema may occur in the setting of nicotinic acid, taxanes, and other medications, X-linked retinoschisis (XLRS), myopic foveal schisis, vitreoretinal traction, pseudohole from epiretinal membrane, and retinal degenerations such as Goldmann-Favre Syndrome. In this case, the patient was emmetropic, with no relevant medications, and no retinal findings suggestive of XLRS or retinal degeneration. The contralateral eye shows no abnormalities. Thus, the non-leaking cystoid change may be degenerative secondary to Coat’s disease, although the pathophysiology is unclear. Variable abnormalities were noted in all of the stage 3a1 eyes (subtotal exudative detachment not involving the fovea), although consistent with their staging, none had macular SRF on SD-OCT. In stage 2b eyes (telangiectasia with exudation involving the fovea but no exudative detachment), although no retinal detachment (nor SRF) was noted clinically (by definition), 50% exhibited SRF on SD-OCT imaging. These cases all involved relatively shallow subretinal fluid that secondary to marked intraretinal edema. Whether the potential increased sensitivity of SD-OCT in determining SRF has any implications for staging or prognosis of Coats’ disease is unclear from this small study. Previous studies correlating visual outcome to Coats’ staging were based upon clinical diagnosis and it is quite likely that a clinically apparent detachment used to define Coats’ stage has different implications for retinal injury than small pockets of SRF such as those identified by OCT alone. Additionally, it should be noted that Coats’ disease staging has important implications beyond visual prognostication – namely determining the optimal treatment strategy. Whether additional insights from SD-OCT imaging can inform treatment is unknown and beyond the scope of this study. Finally, since the study was retrospective in nature, it is possible that SD-OCT findings were incorporated by the examining clinician when assigning stage. If so, the correlation between SD-OCT findings and staging may be over-estimated in this study and the potential impact of SD-OCT on Coats’ disease diagnosis and management may be under-estimated. Future prospective studies are necessary to determine how SD-OCT findings correlate with and influence disease staging. Several microstructural abnormalities identified on SD-OCT were associated in univariate analysis with worse baseline and/or final VA in this study. Overall, presence of intraretinal exudate, macular SRF, subretinal exudates, EZ disruption, ELM disruption, and presence of subfoveal nodule on baseline SD-OCT all correlated to worse baseline and final VA. Intraretinal edema correlated to worse final VA and exhibited a trend towards worse baseline VA (p=0.16). While Coats’ disease stage overall correlates well to VA due to the overall higher disease activity in higher stage eyes, macular findings and/or SD-OCT findings may further refine visual prognosis within or across stages. In this study, several SD-OCT findings that are not clinically perceivable correlated to worse vision in stage 2b eyes. Disruption of the ELM and presence of subfoveal nodule were associated with worse final VA, and there were trends towards worse baseline vision with ELM disruption and subfoveal nodule (p=0.17 for both) and worse baseline (p=0.08) and final (p=0.13) vision with EZ disruption. Our study lends further support to a prior study in which presence of subfoveal nodule (by exam, FA, or OCT) correlated to worse visual outcomes overall and in particular in stage 2b eyes. This prior study had suggested therefore that stage 2b eyes be further dividing into stage 2b1 and 2b2 eyes depending on the presence or absence of subfoveal nodule, respectively. It is also possible that a stage 3a eye may have a peripheral exudative detachment and yet less vision-affecting macular changes than a stage 2b eye with massive macular exudation and edema. In these cases, the additional component of macular SD-OCT findings may help further clarify visual prognosis. However, as expected, SD-OCT features were noted at higher frequencies in higher Coats’ disease stages and the associations noted for SD-OCT and VA on univariate analysis may indeed reflect Coats’ disease stage. There was a strong trend towards significant association between advanced Coats’ disease stage and worse baseline and final VA. Samples sizes were too small to allow analyses within subgroups other than Stage 2b. Multivariate analysis did not achieve statistical significance for any variables, likely reflecting the small sample size relative to number of variables included in the regression model. In the future, larger studies may provide further insight.
Over time, the majority of treated eyes with subsequent SD-OCT imaging exhibited stabilization or improvement in retinal microstructural abnormalities such as intraretinal edema, intraretinal exudates, subretinal exudates, and SRF, consistent with prior reports showing improvement in these parameters after therapy.2,3,5 Rates of improvement of edema, intraretinal exudate, and SRF were noted to be higher in this study than in a prior study, which likely reflect the less advanced Coats’ stages in this population or the longer length of followup in our study.3 In addition, although several reports noted that intraretinal exudates on SD-OCT improved but did not resolve after treatment,3,5 we noted resolution in 1 of 13 eyes in this study. There were no cases of improvement in EZ or ELM abnormalities. Given their strong correlations to final VA and static nature over time in this study, these may be useful markers for visual prognosis.
This study had several limitations inherent to its retrospective nature and small sample size. In addition to the inherent limitations of a retrospective study, this study was limited by small sample size, resulting in several associations which exhibited trends towards statistical significance without falling within our designated p-value. Additionally, subgroup analyses such as effect of SD-OCT findings on VA within each stage of Coats’ disease (other than Stage 2b) may be instructive to determine whether SD-OCT findings can provide any additional insight beyond the already well-established association of Coats’ disease staging to VA. Similarly, larger sample sizes would enable multivariate analyses to determine whether the univariate associations between SD-OCT findings and VA are maintained when accounting for additional variables such as Coats’ disease stage and baseline VA. Although such analysis was conducted in this study, none of the variables achieved a statistical significance, likely due to the small sample size. As noted above, analyses relating SD-OCT findings to Coats’ disease staging are also limited by the possibility that the examining physician incorporated OCT imaging when determining the clinical stage. Another limitation is the lack of correlation of SD-OCT findings to other imaging modalities. For example, further prospective studies comparing clinical examination, fundus photography, and FA imaging to SD-OCT findings would be instructive. Such analysis was not incorporated in this study due to a significant proportion of patients who did not have all such imaging available for retrospective review. The findings of the present demonstrating clinically meaningful features on SD-OCT imaging provide support for such studies in the future. Similarly, fundus autofluorescence has increasingly been applied in the diagnosis and evaluation of retinal diseases, but its features have not yet been well characterized in Coats’ disease. Studies are necessary to clarify the autofluorescence findings in various stages of Coats’ disease and their response to treatment. Subsequently, studies correlating these findings to SD-OCT features may be worthwhile. Recent studies have demonstrated the ability of wide-field OCT imaging to demonstrate peripheral retinal pathology and of OCT-angiography (OCT-A) to non-invasively image vascular and microvascular abnormalities in a variety of retinal diseases. Prior reports have demonstrated nonperfusion of the superficial capillary plexus with arborizing, rarefied, looped, and/or dilated capillary networks on OCT-A of Coats’ disease eyes.7,11,12 These imaging modalities may provide further insights into the pathophysiology of Coats’ disease, and evaluation of their relationship to vision and macular SD-OCT findings could be useful. However, wide-field OCT and OCT-A were not performed in any of the patients in this study.
In summary, we report microstructural abnormalities on macular SD-OCT of treatment-naïve Coats’ disease patients. SD-OCT was able to provide some additional insights beyond those evident from stage or clinical exam alone. SD-OCT features correlated with visual prognosis within stage 2b eyes. These findings, in addition to the strong univariate associations of SD-OCT microstructural abnormalities with VA and visual prognosis, suggest a role for SD-OCT in Coats’ disease diagnosis and management. However, additional, larger studies with multivariate analyses are necessary to determine whether these associations are maintained, to determine how SD-OCT findings compare to clinical examination or staging for VA prognostication, and to further determine whether SD-OCT findings can influence management.
SUMMARY STATEMENT.
In this retrospective, multicenter study, we identified microstructural retinal abnormalities of eyes with Coats’ disease using SD-OCT imaging and correlated the SD-OCT findings with Coats’ disease staging, visual acuity, and visual outcome.
Acknowledgments
Funding/Grants: Unrestricted departmental grant from Research to Prevent Blindness (MPG, RVPC); gifts to the Mukai Fund at the Mass Eye and Ear Infirmary (SM); National Institute of General Medical Sciences through the Medical Scientist Training Program GM07739 (ED); National Institutes of Health R01 EY019474, Bethesda, Maryland (RVPC); National Science Foundation SCH-1622679, Arlington, Virginia (RVPC); National Institutes of Health P30 EY001792 Core Grant for Vision Research (RVPC)
Footnotes
Financial Disclosures/Conflicts of Interest: none
Presentations at prior meetings: none
References
- 1.Shields JA, Shields CL. Review: Coats disease – The 2001 LuEsther T. Mertz Lecture. RETINA. 2002;22:80–91. doi: 10.1097/00006982-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Li S, et al. The effects of a treatment combination of anti-VEGF injections, laser coagulation, and cryotherapy on patients with type 3 Coat’s disease. BMC Ophthalmology. 2017;17:76–82. doi: 10.1186/s12886-017-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, et al. Successful use of intravitreal ranibizumab injection and combined treatment in the management of Coats’ disease. Acta Ophthalmologica. 2016;94:401–406. doi: 10.1111/aos.13067. [DOI] [PubMed] [Google Scholar]
- 4.Henry CR, et al. Intraoperative spectral-domain optical coherence tomography in Coats’ disease. OSLI Retina. 2012:e80–e84. doi: 10.3928/15428877-20120719-01. [DOI] [PubMed] [Google Scholar]
- 5.Kessner R, et al. Intraretinal exudates in Coats’ disease as demonstrated by spectral-domain OCT. Case Reports in Ophthalmology. 2012;3:11–15. doi: 10.1159/000335897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields CL, et al. Optical coherence tomography in children: analysis of 44 eyes with ocular tumors and simulating conditions. J Pediatr Ophthalmol Strabismus. 2004;41:338–344. doi: 10.3928/01913913-20041101-04. [DOI] [PubMed] [Google Scholar]
- 7.Hautz W, et al. Optical coherence tomography and optical coherence angiography in monitoring Coats’ disease. J Ophthalmol. 2017 doi: 10.1155/2017/7849243. e-print ahead of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daruich AL, et al. Subfoveal nodule in Coats’ disease: toward an updated classification predicting visual prognosis. RETINA. 2017;37:1591–1598. doi: 10.1097/IAE.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes RP, et al. Spectral-domain optical coherence tomography measurements of choroidal thickness and outer retinal disruption in macular telangiectasia type 2. OSLI Retina. 2015;46:162–170. doi: 10.3928/23258160-20150213-17. [DOI] [PubMed] [Google Scholar]
- 10.Kase S, et al. Expression of vascular endothelial growth factor in eyes with Coats’ disease. IOVS. 2013;54:57–62. doi: 10.1167/iovs.12-10613. [DOI] [PubMed] [Google Scholar]
- 11.Yonekawa Y, et al. Optical coherence tomography angiography findings in Coats’ disease. Ophthalmology. 2016;123:1964. doi: 10.1016/j.ophtha.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Muakkassa NW, et al. Optical coherence tomography findings in Coats’ disease. OSLI Retina. 2016;47:632–635. doi: 10.3928/23258160-20160707-04. [DOI] [PubMed] [Google Scholar]