Abstract
Neuroplastin 65 (Np65) is an immunoglobulin superfamily cell adhesion molecule involved in synaptic formation and plasticity. Our recent study showed that Np65-knockout (KO) mice exhibit abnormal cognition and emotional disorders. However, the underlying mechanisms remain unclear. In this study, we found 588 differentially-expressed genes in Np65-KO mice by microarray analysis. RT-PCR analysis also revealed the altered expression of genes associated with development and synaptic structure, such as Cdh1, Htr3a, and Kcnj9. In addition, the expression of Wnt-3, a Wnt protein involved in development, was decreased in Np65-KO mice as evidenced by western blotting. Surprisingly, MRI and DAPI staining showed a significant reduction in the lateral ventricular volume of Np65-KO mice. Together, these findings suggest that ablation of Np65 influences gene expression, which may contribute to abnormal brain development. These results provide clues to the mechanisms underlying the altered brain functions of Np65-deficient mice.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0251-5) contains supplementary material, which is available to authorized users.
Keywords: Neuroplastin 65, Microarray analysis, Gene expression profile, Htr3a, Wnt
Introduction
Neuroplastin (Np) is a member of the immunoglobulin (Ig) superfamily of cell adhesion molecules and exists in two isoforms, Np65 and Np55 [1]. Np65 contains extracellular Ig1-2-3 modules, while Np55 only contains extracellular Ig2-3. Thus, Np65 can be differentiated from Np55 by its extracellular Ig1. Np55 is expressed in various organs and cell types, whereas the expression of Np65 is brain-specific and restricted to neurons.
Np65 undergoes trans- and cis-homophilic bindings as well as several heterophilic bindings with fibroblast growth factor receptors, the α1 or α2 subunit of GABAA receptors, and the basigin-monocarboxylate transporter [2–4]. Np65 has been implicated in the regulation of synaptic plasticity and the maintenance of excitatory/inhibitory balance. Antibodies specific for Np65 or recombinant Np65 block long-term potentiation (LTP) in the hippocampal CA1. The induction of LTP also increases the expression of Np65 in postsynaptic densities [5]. In addition, Nptn-deficient neurons exhibit impaired inhibitory transmission [6].
Previous studies have suggested that Np65 is associated with cognition and emotional states. Polymorphisms in the human NPTN gene have been shown to correlate with cortical thickness and intellectual abilities in adolescents as well as in patients with schizophrenia [7, 8]. Nptn-deficient mice exhibit retrograde amnesia, depressive-like behaviors, and decreased social interactions [9]. In addition, mutation of the Nptn gene results in deafness in mice, suggesting that NPTN is a novel deafness gene [10, 11]. We have previously demonstrated that Np65 knock-out (KO) mice exhibit enhanced hippocampal-dependent spatial memory in the Morris water maze and step-through passive avoidance tests [12], but the underlying mechanisms were unclear. In this study, we used custom-designed microarray analysis to profile differentially-expressed genes in Np65-KO mice, in order to explain the altered brain functions in Np65-deficient mice.
Materials and Methods
Animals
The homozygous Np65-KO mice were obtained from engineered mouse models; this caused Np65-Ig1 deficiency in single chromosome as previously described [12]. Wild-type (WT) littermates served as controls. Animals were housed in a temperature-controlled environment under a 12 h light/dark cycle (08:00–20:00) with food and water ad libitum. All protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Ethics Committee of Tongji University School of Medicine, and conformed to Directive 2010/63/EU and NIH guidelines.
Microarray Experiments
Microarray analysis was performed as previously described [13]. Briefly, animals were sacrificed after deep anesthesia with intraperitoneal (i.p.) injection of 1% pentobarbital sodium (30 mg/kg). Hippocampi from adult Np65-KO mice (3 months old) and age-matched WT mice (n = 3/genotype) were dissected and immediately frozen in liquid nitrogen. The samples were stored at −80°C until use.
Total RNA was extracted from the hippocampal tissue using TRIzol (15596026, Thermo Fisher Scientific, Waltham, MA) and further purified with an RNeasy Mini Kit (74104, Qiagen, Hilden, Germany). RNA concentration and quality were evaluated by spectrophotometry (NanoDrop ND-1000, Thermo Fisher Scientific, Waltham, MA). One microgram of total RNA was amplified and labeled with a One-Color Quick Amp Labeling Kit (5190-0442, Agilent Technologies, Santa Clara, CA). The fluorescence-labeled cRNA was hybridized onto the Whole Mouse Genome Oligo Microarray (4 × 44K, Agilent Technologies, Santa Clara, CA) using the Agilent Gene Expression Hybridization Kit (5188-5242, Agilent Technologies, Santa Clara, CA). Chips were washed and scanned by a microarray scanner (G2565BA, Agilent Technologies, Santa Clara, CA). Raw data were then normalized and analyzed using the GeneSpring GX Software Package (v11.5, Agilent Technologies). The microarray experiment was performed with 3 biological and experimental repeats. Normalized values were used to screen for differentially-expressed genes from biological and experimental repeats before all replicates were combined. Genes with a fold-change of > 2.0 and a P value < 0.05 were selected for Gene Ontology (GO) and pathway analysis.
Gene Ontology and Pathway Analysis
The fold-changes of differential expression were determined by the abundance ratio of Np65-KO and WT mice. Hierarchical clustering was used to analyze the differentially-expressed genes. GO analysis was applied to analyze the cellular components, biological functions, and biological processes of the differentially-expressed genes (www.geneontology.org). Pathway analysis was used to reveal significant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of the differentially-expressed genes.
Quantitative Real-Time Reverse-Transcription PCR
Quantitative real-time reverse-transcription PCR (RT-PCR) was performed as previously described [14]. Adult Np65-KO and WT mice (n = 4/genotype) were sacrificed after anesthesia with 1% pentobarbital sodium (30 mg/kg, i.p.) and the forebrain was harvested to extract total RNA using TRIzol (15596026, Thermo Fisher Scientific, Waltham, MA). RNA concentration and quality were determined by NanoDrop (ND-1000, Thermo Fisher Scientific, Waltham, MA). cDNA was generated using reverse transcriptase (PrimeScript™ RT reagent Kit, RR0747Q, Takara Bio, Tokyo, Japan). The first-strand cDNA was used as a template for RT-PCR analysis. The primers for RT-PCR analysis (Table 1) were designed by the NCBI primer designing tool [15] and synthesized by Sangon (Shanghai, China). Each RT-PCR reaction was carried out in a 20 μL volume using SYBR Green Master Mix (RR820Q, Takara Bio, Tokyo, Japan), started at 30 s at 95°C for initial denaturation, followed by 40 cycles of 5 s at 95°C and 34 s at 60°C in the ABI 7500 Real-Time PCR System. A total of 3 independent samples per subject were run in duplicate for RT-PCR. β-actin was used as the reference gene. The 2− ΔΔCt method was used to determine the relative expression levels of genes.
Table 1.
Primers used in RT-PCR.
| Gene | NCBI Accession | Forward Primer | Reverse Primer |
|---|---|---|---|
| Cdh1 | NM_009864 | CAGCCGGTCTTTGAGGGATT | TGACGATGGTGTAGGCGATG |
| Cdh4 | NM_009867 | ACAACCGTCCCGAGTTCATC | TCATCTGCATCGTTGGCTGT |
| Htr3a | NM_013561 | CAGACCACCTCCTGGCTAAC | GATGCTGTCTGTGGGGATGG |
| Htr4 | NM_008313 | ACGTCCTCATGCCCATTTCC | ACCACTGCAAGGAACGTGAG |
| Kcnj9 | NM_008429 | TCTTCTTCGTGCTCGCCTAC | CGAAGCCGTTGAGGTTGTTG |
| Pla2g4e | NM_177845 | CTCCAACTGCCTACACCCAG | CCTCTGGGTTGAGTGGGAAC |
| Xaf1 | NM_001037713 | AGAGCCCATCCCAGAGTCAA | CAGATTGCTAAGCTGCACGG |
| Lactb | NM_030717 | GGCTATGCAGACGTGGAGAA | CAGTTTAGCCAGAGCCACCA |
| Actb | NM_007393 | GCTGTATTCCCCTCCATCGTG | AGTCCTTCTGACCCATTCCCA |
Western Blotting
Adult Np65-KO and WT mice (4 months old, n = 3/genotype) were used for western blotting. Briefly, animals were decapitated after deep anesthesia with 1% pentobarbital sodium (30 mg/kg i.p.). Forebrains were collected and frozen in nitrogen and then stored at −80°C until use. The total proteins were extracted using RIPA lysis buffer (P0013B, Beyotime) with 1 mmol/L PMSF (ST506, Beyotime). Protein concentrations were measured using the BCA Protein Assay Kit (P0010, Beyotime, Jiangsu, China), then 10 ng of total protein was separated by SDS-PAGE and transferred to the PVDF membrane. After blocking with 5% bovine serum albumin (BSA), the membranes were incubated overnight at 4°C with primary antibody against Wnt-3 (1:1,000, Santa Cruz Biotechnology, Dallas, TX) and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1,000, Santa Cruz Biotechnology, Dallas, TX). Subsequently, the membranes were incubated with HRP-conjugated goat anti-mouse secondary antibody (1:1,000, Beyotime) for 2 h at room temperature. The labeled proteins were detected by using the ImageQuant LAS 4000mini system (GE Healthcare Life Sciences, Chicago, IL). The protein levels were normalized to that of GAPDH from three independent experiments.
Magnetic Resonance Imaging (MRI)
A separate cohort of mice (4 months old, n = 5/genotype) was used in MRI analysis. MRI data were acquired with a 7.0 T animal MRI scanner (PharmaScan, Bruker Biospin GmbH, Germany) with 4-channel phased array coil. T2-weighed (T2-wt) MRI was performed using a rapid acquisition with relaxation enhancement (RARE) sequence with TR/TE = 4200/36 ms, RARE factor = 8, and averaging number = 3. The geometric parameters for the scan were: slice number = 18, slice thickness = 0.5 mm, matrix = 256 × 256, and FOV = 21 × 21 mm2.
4′,6-Diamidino-2-Phenylindole (DAPI) Staining
Adult Np65-KO and WT mice (n = 4, 2 months old) were anesthetized with 1% pentobarbital sodium intraperitoneally and perfused with 4% paraformaldehyde. The brain was removed, postfixed for 10 h–16 h, and cryoprotected in 20% sucrose. Coronal sections (10 μm, at the level of the lateral ventricle, 0 mm–2 mm from bregma) were prepared for DAPI staining. In brief, the sections were blocked in 5% BSA (B2064-100G, Sigma-Aldrich, St. Louis, MO) with 0.3% Triton X-100 (ST795, Beyotime), then incubated with DAPI (1:300, Beyotime) diluted in 1% BSA with 0.3% Triton X-100 for 10 min at room temperature. The sections were then rinsed with PBS and covered with Permount for fluorescent microscopy (Eclipse 80i, Nikon Corp., Tokyo, Japan).
Statistics
Statistics were calculated using SPSS Statistics software (v22.0, IBM). All data are presented as mean ± SEM. Independent samples were tested by the unpaired Student’s t-test (two-tailed). The Mann-Whitney U test was used to determine the significance of data with an abnormal distribution or unequal variance. Statistical significance was set at P < 0.05.
Results
Microarray Analysis of Differentially-Expressed Genes in the Hippocampus of Np65-KO Mice
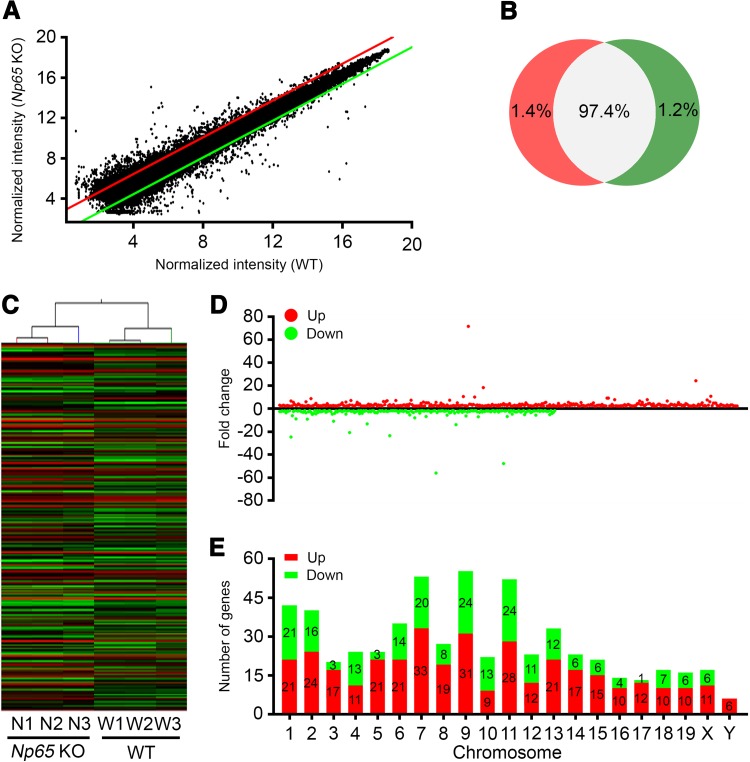
All genes are shown in a scatter plot with normalized intensity in Fig. 1A (details in Table S1). Of the 34397 targeted genes by the Mouse 4 × 44K Gene Chip, 481 (1.4%) were up-regulated and 418 (1.2%) were down-regulated by 2-fold in the Np65-KO mice (Fig. 1B). Using P < 0.05 as the criterion, 367 genes were significantly higher and 221 genes were significantly lower in Np65-KO mice as compared to age-matched WT mice (Fig. 1C and D, Table S2). These differentially-expressed genes were primarily located on chromosomes 7, 9 and 11. Notably, the NPTN gene resides on chromosome 9 (Fig. 1E).
Fig. 1.
Differentially-expressed genes in the hippocampus of Np65-KO mice. A Scatter plot of normalized intensity derived from microarray chips with WT and Np65-KO mice. Dots above the red line denote upregulated genes, and dots below the green line denote downregulated genes. B Venn diagram showing the percentages of differentially-expressed genes categorized by fold-change. C Hierarchical clustering of differentially-expressed genes. N1–N3, Np65-KO mice; W1–W3, WT mice. D Fold changes of differentially-expressed genes in Np65-KO mice. E Chromosome distributions of differentially-expressed genes in Np65-KO mice. Red bars, numbers of upregulated genes; green bars, numbers of downregulated genes.
Gene Ontology and Pathway Analysis of Differentially-Expressed Genes
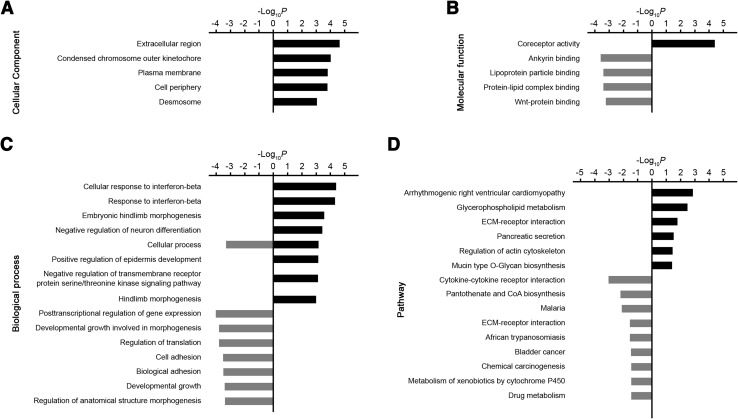
Using the criterion of P < 0.001, GO analysis showed that the upregulated genes were involved in several cellular components, including extracellular region, plasma membrane, and desmosome (Fig. 2A). The molecular functions of the upregulated genes were mainly associated with co-receptor activity. The down-regulated genes were involved in various binding actions, such as ankyrin binding, lipoprotein binding, and Wnt-protein binding (Fig. 2B). The main biological processes were the cellular response to interferon-beta, embryonic hindlimb morphogenesis, negative regulation of neuron differentiation, cellular process, positive regulation of epidermis development, and negative regulation of transmembrane receptor protein serine/threonine kinase signaling pathway. The downregulated genes were associated with several biological processes, including post-transcriptional regulation of gene expression, developmental growth involved in morphogenesis, regulation of translation, cell adhesion, developmental growth, and response to stimuli (Fig. 2C).
Fig. 2.
GO and pathway analysis of differentially-expressed genes in Np65-KO mice. A–C Cellular components (A), molecular functions (B), and biological processes (C) of differentially-expressed genes. D Significantly changed pathways in Np65-KO mice.
According to the pathway analysis, 8 pathways were significantly up-regulated in Np65-KO mice, including glycerophospholipid metabolism, pancreatic secretion, and regulation of actin cytoskeleton. Among the 9 down-regulated pathways, the most prominent was cytokine-cytokine receptor interaction. The other downregulated pathways were involved in bladder cancer, chemical carcinogenesis, and drug metabolism (Fig. 2D).
Functional Analysis of Differentially-Expressed Genes in Np65-KO Mice
The differentially-expressed genes were divided into four categories: cell adhesion, development, neurotransmission and ion channel, and signal transduction. Previous studies have suggested that Np65 may interact with other cell adhesion molecules like fibroblast growth factor receptor (FGFR) to activate intracellular signaling [2]. Interestingly, the expression of several cell adhesion molecules was altered in Np65-KO mice. Fgfr4, immunoglobulin superfamily member 1 (Igsf1), interleukin 7 receptor (Il7r), and members of the integrin superfamily such as integrin alpha 1 (Itga1) and integrin alpha 9 (Itga9) were upregulated, while interleukin 1 receptor, type II (Il1r2) and protein tyrosine phosphatase receptor type D (Ptprd) were down-regulated in Np65-KO mice. In Np65-KO mice, the expression levels of genes associated with Ca2+ binding, including Ca2+-binding protein 5 (Cabp5), calbindin 1 (Calb1), and calmodulin-like 4 (Calml4) were significantly increased. However, several cadherins, including cadherin 1 (Cdh1), cadherin 4 (Cdh4), cadherin 6 (Cdh6), protocadherin 7 (Pcdh7), protocadherin 12 (Pcdh12), and protocadherin 17 (Pchd17) were down-regulated in Np65-KO mice (Table 2).
Table 2.
Selected differentially-expressed genes in the hippocampus of Np65-KO mice.
| Category | NCBI accession | Full name | Fold change | P value |
|---|---|---|---|---|
| Cell adhesion | ||||
| Fgfr4 | NM_008011 | Fibroblast growth factor receptor 4 | 2.0 | 0.004 |
| Igsf1 | NM_177591 | Immunoglobulin superfamily, member 1 | 2.3 | 0.002 |
| Il7r | NM_008372 | Interleukin 7 receptor | 2.1 | 0.006 |
| Il1r2 | NM_010555 | Interleukin 1 receptor, type II | −2.1 | 0.002 |
| Ptprd | XR_107615 | Protein tyrosine phosphatase, receptor type D | −2.3 | 0.015 |
| Cdh1 | NM_009864 | Cadherin 1 | −2.4 | 0.010 |
| Cdh4 | NM_009867 | Cadherin 4 | −2.1 | 0.004 |
| Cdh6 | NM_007666 | Cadherin 6 | −2.2 | 0.043 |
| Pcdh7 | NM_001122758 | Protocadherin 7 | −5.8 | 0.020 |
| Pcdh12 | NM_017378 | Protocadherin 12 | −2.3 | 0.045 |
| Pcdh17 | NM_001013753 | Protocadherin 17 | −2.2 | 0.000 |
| Itga1 | NM_001033228 | Integrin alpha 1 | 3.0 | 0.047 |
| Itga9 | NM_133721 | Integrin alpha 9 | 10.4 | 0.000 |
| Development | ||||
| Ccr5 | NM_009917 | Chemokine (C-C motif) receptor 5 | −2.1 | 0.009 |
| Foxo3 | AK143198 | Forkhead box O3 | −2.0 | 0.004 |
| Mbp | NM_010777 | Myelin basic protein | −2.2 | 0.014 |
| Mef2c | NM_025282 | Myocyte enhancer factor 2C | −2.1 | 0.034 |
| Wif1 | NM_011915 | Wnt inhibitory factor 1 | −2.1 | 0.001 |
| Ntf3 | NM_001164034 | Neurotrophin 3 | 3.2 | 0.044 |
| Gcm1 | NM_008103 | Glial cells missing homolog 1 | 2.9 | 0.019 |
| Trp73 | NM_011642 | Transformation related protein 73 | 2.3 | 0.040 |
| Aldh1a3 | NM_053080 | Aldehyde dehydrogenase family 1, subfamily A3 | −2.1 | 0.008 |
| Rpgrip1 | NM_023879 | Retinitis pigmentosa GTPase regulator interacting protein 1 | −2.5 | 0.020 |
| Crb1 | NM_133239 | Crumbs homolog 1 | −2.5 | 0.039 |
| Vsx1 | NM_054068 | Visual system homeobox 1 homolog | −3.4 | 0.015 |
| Krt12 | NM_010661 | Keratin 12 | −2.9 | 0.000 |
| Sfrp5 | NM_018780 | Secreted frizzled-related sequence protein 5 | −2.0 | 0.006 |
| Myo7a | NM_008663 | Myosin VIIA | −2.8 | 0.040 |
| Cthrc1 | NM_026778 | Collagen triple helix repeat containing 1 | −2.1 | 0.000 |
| Neurotransmission and ion channel | ||||
| Cabp5 | NM_013877 | Ca2+ binding protein 5 | 3.5 | 0.031 |
| Calb1 | AK038856 | Calbindin 1 | 2.1 | 0.004 |
| Calml4 | NM_138304 | Calmodulin-like 4 | 3.0 | 0.007 |
| Cplx2 | NM_009946 | Complexin 2 | 2.1 | 0.033 |
| Htr4 | NM_008313 | 5-hydroxytryptamine (serotonin) receptor 4 | 2.4 | 0.043 |
| Htr3a | NM_013561 | 5-hydroxytryptamine (serotonin) receptor 3A | −2.5 | 0.001 |
| Clca5 | NM_178697 | Cl– channel Ca2+ activated 5 | 2.9 | 0.000 |
| Kcnj9 | NM_008429 | K+ inwardly-rectifying channel, subfamily J, member 9 | 4.2 | 0.000 |
| Signal transduction | ||||
| Map2k7 | NM_001042557 | Mitogen-activated protein kinase kinase 7 | −2.67 | 0.000 |
| Pla2g4e | NM_177845 | Phospholipase A2, group IVE | 4.64 | 0.000 |
| Stk38l | NM_172734 | Serine/threonine kinase 38 like | 4.80 | 0.013 |
| Ppp6r1 | NM_172894 | Protein phosphatase 6, regulatory subunit 1 | 2.95 | 0.033 |
| Xaf1 | NM_001037713 | XIAP associated factor 1 | 5.99 | 0.000 |
| Lactb | NM_030717 | Lactamase, beta | 24.30 | 0.000 |
In addition, a subset of genes associated with neuronal development, such as chemokine (C-C motif) receptor 5 (Ccr5), forkhead box O3 (Foxo3), myelin basic protein (Mbp), myocyte enhance factor 2C (Mef2c), and Wnt inhibitory factor 1 (Wif1), were significantly downregulated in Np65-KO mice, while several genes involved in glial cell development, including neurotrophin 3 (Ntf3), glial cell missing homolog 1 (Gcm1), and transformation related protein 73 (Trp73), were upregulated. More interestingly, the expression of eye development-related genes was also altered in Np65-KO mice. The expression of aldehyde dehydrogenase family 1 subfamily A3 (Aldh1a3), retinitis pigmentosa GTPase regulator interacting protein 1 (Rpgrip1), crumbs homolog 1 (Crb1), visual system homeobox 1 homolog (Vsx1), keratin 12 (Krt12), and secreted frizzled-related sequence protein 5 (Sfrp5) were down-regulated. Myosin VIIA (Myo7a) and collagen triple helix repeat containing 1 (Cthrc1), which are associated with inner ear receptor cell development, were also decreased in Np65-KO mice (Table 2).
The expressions of genes related to the structure and function of synapses were also altered in Np65-KO mice. Notably, expression of the serotonin receptor 4 (Htr4) gene was significantly increased, whereas the expression of serotonin receptor 3A (Htr3a) was significantly decreased in Np65-KO mice. Moreover, two ion channel-related genes displayed significant downregulations in Np65-KO mice: Cl– channel Ca2+ activated 5 (Clca5) and K+ inwardly-rectifying channel subfamily J member 9 (Kcnj9) (Table 2).
MAPK signaling is essential for various physiological and pathological processes, such as neural plasticity and memory. We found that the expression of mitogen-activated protein kinase kinase 7 (Map2k7) was significantly decreased, while phospholipase A2, group IVE (Pla2g4e), serine/threonine kinase 38 like (Stk38l), and protein phosphatase 6, regulatory subunit 1 (Ppp6r1) were significantly increased in Np65-KO mice. In addition, the expression level of X-linked inhibitor of apoptosis protein associated factor 1 (Xaf1), an apoptosis-promoting factor, was decreased. Most notably, β-lactamase (Lactb), which is involved in mitochondrial metabolism, was also significantly down-regulated in Np65-KO mice (24.30-fold, P < 0.001) (Table 2).
RT-PCR Analysis of Differentially-Expressed Genes
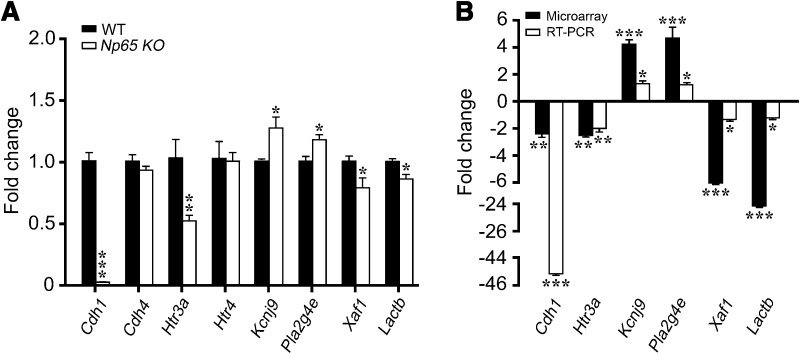
Np65 is highly expressed in the hippocampus and other brain regions, such as cortex and striatum [1]. Therefore, 8 genes related to the functions of Np65 were further selected for RT-PCR analysis. The results showed that 6 of these were also significantly changed in the forebrain of Np65-KO mice (Fig. 3A), including the downregulated Cdh1 (fold change, 45.34, P < 0.001), Htr3a (fold change, 1.94, P < 0.01), Xaf1 and Lactb, and the increased Kcnj9 (fold change, 1.26, P < 0.05) and Pla2g4e (fold change, 1.17, P < 0.05) (Fig. 3B).
Fig. 3.
Relative expression levels of selected genes in Np65-KO and WT mice. A Results of quantitative real-time PCR (RT-PCR) (n = 4 mice). B RT-PCR and microarray experimental results for relative gene expression in Np65-KO and WT mice. The relative expression levels were calculated as the ratio of the target gene expression level to the β-actin expression level in the same sample. Fold changes are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Decreased Expression of Wnt-3 in Np65-KO Mice
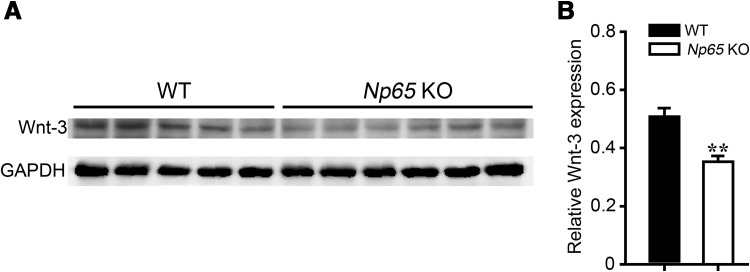
Microarray and RT-PCR analysis showed that the expression levels of several genes associated with development were altered in Np65-KO mice. Some differentially-expressed genes, such as Wif1 and Cdh1, are involved in Wnt signaling. Wnt signaling is a crucial regulator of many developmental processes, such as cell proliferation, maintenance of stem cells, and cell fate determination [16]. Therefore, we examined the protein level of Wnt-3 in Np65-KO mice by western blotting. The results showed that the protein level of Wnt-3 was significantly lower in the forebrain of Np65-KO mice than in WT mice (Fig. 4).
Fig. 4.
Reduced expression of Wnt-3 in Np65-KO mice. A Representative Wnt-3 bands from the forebrain of WT (n = 5) and Np65-KO mice (n = 6). B Quantitative results of western blotting analysis showed that the expression level of Wnt-3 was significantly decreased in the forebrain of Np65-KO mice. All data are presented as mean ± SEM. **P < 0.01.
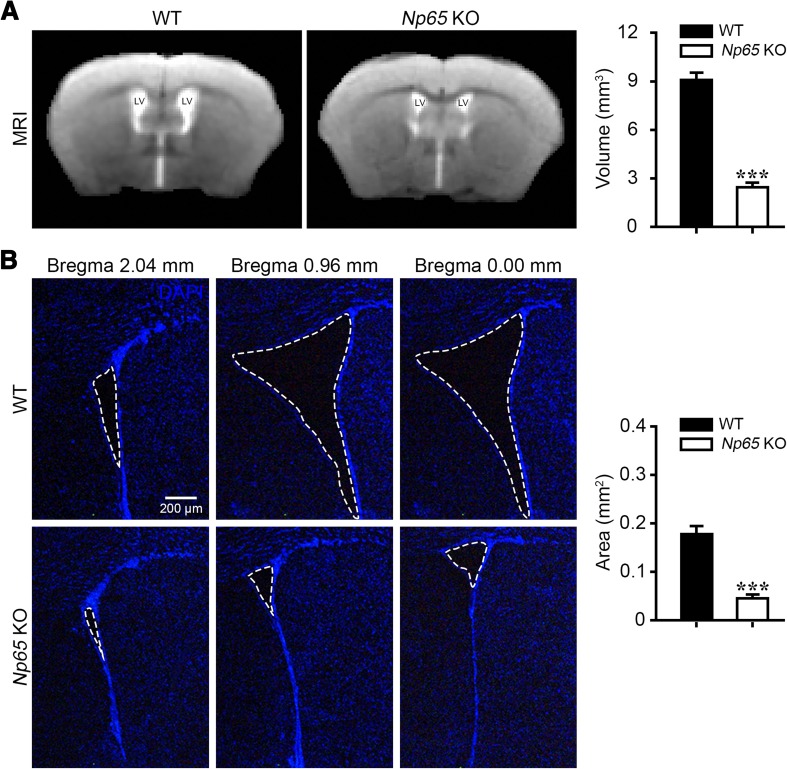
Reduced Lateral Ventricles in Np65-KO Mice
Given that the dysfunction of Wnt signaling may influence brain development, we then assessed whether ablation of Np65 affects the brain morphology of mice. T2-wt images were used to assess region-specific volume changes. The gross brain architecture was not affected in Np65-KO mice. MRI morphometry also showed normal anatomy of the cerebral cortex, hippocampus, thalamus, hypothalamus, basal ganglia, and caudatoputamen of Np65-KO mice. However, the lateral ventricular volume was significantly reduced compared to WT mice (Fig. 5A), and this was further confirmed by DAPI staining (Fig. 5B). Thus, these results suggested that the absence of Np65 leads to altered architecture of the mouse brain.
Fig. 5.
Reduction in lateral ventricles in Np65-KO mice. A T2-wt MRI showing a significant reduction in the lateral ventricles (LV) compared to WT mice. B DAPI staining showing a significant reduction in the lateral ventricles in coronal sections from adult Np65-KO mice compared to WT mice. Scale bar, 200 µm. ***P < 0.001.
Discussion
Np65 is specifically expressed in the brain and has been reported to mediate several cellular processes including cell-cell adhesion, neurite outgrowth, and synaptic plasticity [2, 5, 17, 18]. Our previous studies have shown that Np65-KO mice exhibit abnormal cognitive and emotional behaviors [12]. To investigate the underlying mechanisms, we further analyzed the gene expression profiles in Np65-KO mice in this study. Our microarray analysis demonstrated a large number of differentially-expressed genes in Np65-KO mice; these genes are crucially involved in development, ion channels, neurotransmission, and signal transduction.
Our study identified many differentially-expressed genes involved in neuronal development, such as the decreased expressions of Cdh1, Ccr5, Foxo3, Mbp, Wif1, and Mef2c, as well as upregulation of Ntf3, Gcm1, and Trp73, implying that Np65 deletion affects the configuration of the brain. Coincidently, T2-wt MRI morphometry and brain slices stained with DAPI showed a significant reduction in lateral ventricular volume in Np65-KO mice compared to WT mice. The expression of Wnt-3 was significantly decreased in Np65-KO mice. Wnt signaling is a crucial regulator of developmental processes like cell proliferation and cell fate determination [16]. Dysregulation of Wnt signaling may contribute to neuropsychiatric disorders, such as depression and schizophrenia [19]. In this study, our findings suggested that Np65 deletion affects the Wnt signaling pathway by decreasing Wnt expression. Together, these differentially-expressed genes associated with development may contribute, at least in part, to changes in the ventricles and abnormal behaviors in Np65-KO mice.
Recent studies have reported that mutation of the NPTN gene results in deafness in mice [10]. It has been reported that Np65 may regulate the properties of synapses connecting the inner hair cells with spiral ganglion neurons [10]. Intriguingly, Zeng et al. reported that Np55 is expressed in stereocilia of outer but not inner hair cells and affects interactions of stereocilia with the tectorial membrane and cochlear amplification in mice with NPTN mutation [11]. Together, these recent findings clearly confirm NPTN as a novel deafness gene. Consistent with their reports, our microarray analysis showed that the genes associated with inner ear receptor cell development, myosin VIIA (Myo7a) and collagen triple helix repeat containing 1 (Cthrc1) were significantly decreased in Np65-KO mice, supporting the hypothesis that Np65 is involved in hearing.
It has been shown that Np65 is linked with ribbon synapse formation in the plexiform layers of the rat retina [20]. Retinal function, as assessed using the electroretinogram, is unaffected by the absence of NPTN [10]. Surprisingly, the involvement of Np65 in vision was demonstrated using the pupillary light reflex and flash visual evoked potentials (our unpublished data). In agreement with our finding, our microarray analysis showed that the expression of eye development-related genes, including Aldh1a3, Rpgrip1, Crb1, Vsx1, Krt12, and Sfrp5, was significantly downregulated in Np65-KO mice. Although these alterations in eye-development genes need to be confirmed, the reduced amplitude of the pupil in the pupillary light reflex and reduced first negative and positive amplitude of flash visual evoked potentials (our unpublished data) suggest that Np65 plays roles in vision.
Our previous studies have shown that Np65-KO mice appear to show enhanced memory in the Morris water maze and increased anxiety [12]. Central 5-hydroxytryptamine (5-HT) activity is involved in emotional and cognitive activities [21, 22]. Generally, stimulation of central 5-HT activity impairs cognition, while its inhibition enhances cognition in rodent models. Tropisetron, a selective 5-HT3 receptor antagonist, has been confirmed to reverse the cognitive deficit in rats injected with Aβ (1–42) [23]. In addition, central 5-HT activity is closely associated with anxiety [24–27]. Among the 5-HT receptors, 5-HT3 is the only ligand-gated ion channel that increases intracellular cations such as Ca2+, Na+, and K+. Stimulation of 5-HT3 receptors induces the rapid and transient depolarization of neurons. 5-HT receptor 3A-null mice exhibit anxiolytic behaviors, indicating that this receptor influences anxiety-like behavior [28]. More surprisingly, microarray and RT-PCR analysis demonstrated that Htr3a mRNA was significantly reduced in Np65-KO mice. How deletion of Np65 affects the expression of Htr3a remains to be determined. To date, the decreased expression of Htr3a may explain, at least in part, the changed cognitive and anxiety behaviors in Np65-KO mice.
In conclusion, the present study demonstrates that a large number of genes are differentially expressed in Np65-KO mice. Notably, microarray analysis in Np65-KO mice revealed altered expression of Htr3a and genes associated with development, hearing, and vision, which may provide important insights for understanding the role of Np65 in brain development as well as brain functions like cognition and emotion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (XLSX 11345 kb)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81371213, 81070987, and 30971531) and the grants from the Ministry of Science and Technology of China (2010CB945600 and 2010CB945601).
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
References
- 1.Langnaese K, Beesley PW, Gundelfinger ED. Synaptic membrane glycoproteins gp65 and gp55 are new members of the immunoglobulin superfamily. J Biol Chem. 1997;272:821–827. doi: 10.1074/jbc.272.2.821. [DOI] [PubMed] [Google Scholar]
- 2.Owczarek S, Soroka V, Kiryushko D, Larsen MH, Yuan Q, Sandi C, et al. Neuroplastin-65 and a mimetic peptide derived from its homophilic binding site modulate neuritogenesis and neuronal plasticity. J Neurochem. 2011;117:984–994. doi: 10.1111/j.1471-4159.2011.07269.x. [DOI] [PubMed] [Google Scholar]
- 3.Sarto-Jackson I, Milenkovic I, Smalla KH, Gundelfinger ED, Kaehne T, Herrera-Molina R, et al. The cell adhesion molecule neuroplastin-65 is a novel interaction partner of gamma-aminobutyric acid type A receptors. J Biol Chem. 2012;287:14201–14214. doi: 10.1074/jbc.M111.293175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson MC, Kraus M, Marzban H, Sarna JR, Wang Y, Hawkes R, et al. The neuroplastin adhesion molecules are accessory proteins that chaperone the monocarboxylate transporter MCT2 to the neuronal cell surface. PLoS One. 2013;8:e78654. doi: 10.1371/journal.pone.0078654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smalla KH, Matthies H, Langnase K, Shabir S, Bockers TM, Wyneken U, et al. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc Natl Acad Sci U S A. 2000;97:4327–4332. doi: 10.1073/pnas.080389297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera-Molina R, Sarto-Jackson I, Montenegro-Venegas C, Heine M, Smalla KH, Seidenbecher CI, et al. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J Biol Chem. 2014;289:8973–8988. doi: 10.1074/jbc.M113.514992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desrivieres S, Lourdusamy A, Tao C, Toro R, Jia T, Loth E, et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol Psychiatry. 2015;20:263–274. doi: 10.1038/mp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito A, Fujikura-Ouchi Y, Kuramasu A, Shimoda K, Akiyama K, Matsuoka H, et al. Association study of putative promoter polymorphisms in the neuroplastin gene and schizophrenia. Neurosci Lett. 2007;411:168–173. doi: 10.1016/j.neulet.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Herrera-Molina R, Sabanov V, Ahmed T, Iscru E, Stober F, et al. Genetically induced retrograde amnesia of associative memories after neuroplastin ablation. Biol Psychiatry. 2017;81:124–135. doi: 10.1016/j.biopsych.2016.03.2107. [DOI] [PubMed] [Google Scholar]
- 10.Carrott L, Bowl MR, Aguilar C, Johnson SL, Chessum L, West M, et al. Absence of neuroplastin-65 affects synaptogenesis in mouse inner hair cells and causes profound hearing loss. J Neurosci. 2016;36:222–234. doi: 10.1523/JNEUROSCI.1808-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng WZ, Grillet N, Dewey JB, Trouillet A, Krey JF, Barr-Gillespie PG, et al. Neuroplastin isoform Np55 is expressed in the stereocilia of outer hair cells and required for normal outer hair cell function. J Neurosci. 2016;36:9201–9216. doi: 10.1523/JNEUROSCI.0093-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amuti S, Tang Y, Wu S, Liu L, Huang L, Zhang H, et al. Neuroplastin 65 mediates cognitive functions via excitatory/inhibitory synapse imbalance and ERK signal pathway. Neurobiol Learn Mem. 2016;127:72–83. doi: 10.1016/j.nlm.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Dong S, Li C, Wu P, Tsien JZ, Hu Y. Environment enrichment rescues the neurodegenerative phenotypes in presenilins-deficient mice. Eur J Neurosci. 2007;26:101–112. doi: 10.1111/j.1460-9568.2007.05641.x. [DOI] [PubMed] [Google Scholar]
- 14.Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- 15.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 17.Owczarek S, Berezin V. Neuroplastin: cell adhesion molecule and signaling receptor. Int J Biochem Cell Biol. 2012;44:1–5. doi: 10.1016/j.biocel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Empson RM, Buckby LE, Kraus M, Bates KJ, Crompton MR, Gundelfinger ED, et al. The cell adhesion molecule neuroplastin-65 inhibits hippocampal long-term potentiation via a mitogen-activated protein kinase p38-dependent reduction in surface expression of GluR1-containing glutamate receptors. J Neurochem. 2006;99:850–860. doi: 10.1111/j.1471-4159.2006.04123.x. [DOI] [PubMed] [Google Scholar]
- 19.Hussaini SM, Choi CI, Cho CH, Kim HJ, Jun H, Jang MH. Wnt signaling in neuropsychiatric disorders: ties with adult hippocampal neurogenesis and behavior. Neurosci Biobehav Rev. 2014;47:369–383. doi: 10.1016/j.neubiorev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreutz MR, Langnaese K, Dieterich DC, Seidenbecher CI, Zuschratter W, Beesley PW, et al. Distribution of transcript and protein isoforms of the synaptic glycoprotein neuroplastin in rat retina. Invest Ophthalmol Vis Sci. 2001;42:1907–1914. [PubMed] [Google Scholar]
- 21.McEntee WJ, Crook TH. Serotonin, memory, and the aging brain. Psychopharmacology (Berl) 1991;103:143–149. doi: 10.1007/BF02244194. [DOI] [PubMed] [Google Scholar]
- 22.Ma G, Fan H, Shen C, Wang W. Genetic and neuroimaging features of personality disorders: State of the art. Neurosci Bull. 2016;32:286–306. doi: 10.1007/s12264-016-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahimian R, Fakhfouri G, Ejtemaei Mehr S, Ghia JE, Genazzani AA, Payandemehr B, et al. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur J Clin Invest. 2013;43:1039–1051. doi: 10.1111/eci.12141. [DOI] [PubMed] [Google Scholar]
- 24.Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- 25.Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, et al. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinhababu AK, Borchardt RT. Molecular mechanism of biological action of the serotonergic neurotoxin 5,7-dihydroxytryptamine. Neurochem Int. 1988;12:273–284. doi: 10.1016/0197-0186(88)90165-9. [DOI] [PubMed] [Google Scholar]
- 27.Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, et al. Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci U S A. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/S0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 2 (XLSX 11345 kb)