Abstract
Rationale
Occupational exposures at the WTC site after September 11, 2001 have been associated with several presumably inflammatory lower airway diseases. In this study, we describe the trajectories of expiratory air flow decline, identify subgroups with adverse progression, and investigate the association of a quantitative computed tomography (QCT) imaging measurement of airway wall thickness, and other risk factors for adverse progression.
Methods
We examined the trajectories of expiratory air flow decline in a group of 799 former WTC workers and volunteers with QCT-measured (with two independent systems) wall area percent (WAP) and at least 3 periodic spirometries. We calculated individual regression lines for first–second forced expiratory volume (FEV1), identified subjects with rapidly declining and increasing (“gainers”), and compared them to subjects with normal and “stable” FEV1 decline. We used multivariate logistic regression to model decliner vs. stable trajectories.
Results
The mean longitudinal FEV1slopes for the entire study population, and its stable, decliner, and gainer subgroups were, respectively, −35.8, −8, −157.6, and + 173.62 ml/year. WAP was associated with “decliner” status (ORadj 1.08, 95% CI 1.02, 1.14, per 5% increment) compared to stable. Age, weight gain, baseline FEV1 percent predicted, bronchodilator response, and pre-WTC occupational exposures were also significantly associated with accelerated FEV1 decline. Analyses of gainers vs. stable subgroup showed WAP as a significant predictor in unadjusted but not consistently in adjusted analyses.
Conclusions
The apparent normal age-related rate of FEV1 decline results from averaging widely divergent trajectories. WAP is significantly associated with accelerated air flow decline in WTC workers.
Keywords: Multidetector computed tomography, Smoke inhalation injury, Spirometry, World Trade Center Attack, 2001, Chronic bronchitis, Occupational disease
Introduction
Occupational exposures at the World Trade Center (WTC) disaster site in 2001–2002 have been associated with a variety of adverse health effects [1], including chronic lower airway diseases [1, 2]. Despite their heterogeneity, we have postulated that the latter have airway wall inflammation as their common denominator. Quantitative CT measurements have emerged as powerful research tools in the non-invasive evaluation of the airway, pulmonary parenchymal, and vascular and other thoracic structures, allowing further phenotypical characterization of a variety of lung diseases [3]. Although longitudinal spirometric follow-up of the WTC occupational cohorts suggests a normal age-related expiratory flow decline [4, 5], this study sought to demonstrate and characterize subgroups with widely divergent, and more adverse respiratory health trajectories, and hypothesized that wall area percent (WAP), a quantitative CT marker of airway inflammation, is associated with those adverse outcomes.
Methods
Subjects and Clinical Data Acquisition
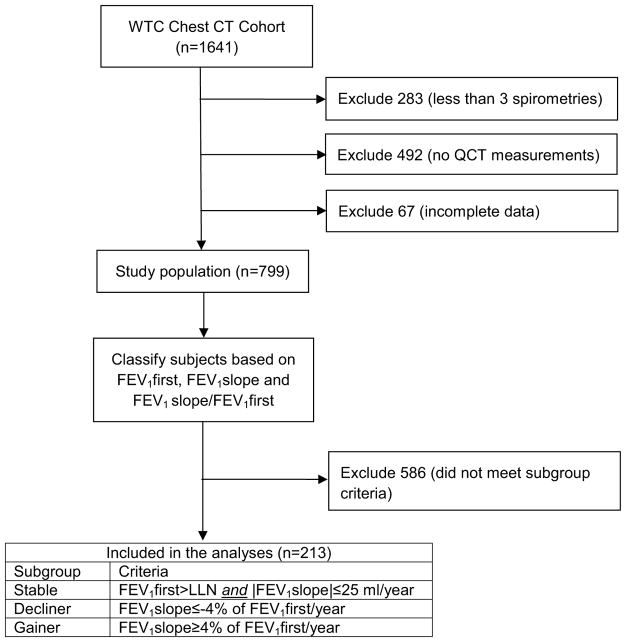
All subjects participated in the screening, surveillance, and clinical programs of the World Trade Center (WTC) Clinical Center of Excellence at Mount Sinai Medical Center, in New York City, and were part of the subcohort (n= 1641) evaluated by the WTC Pulmonary Evaluation Unit (WTC PEU), who underwent chest computed tomography (CT) scanning between 2003 and 2012, as part of their diagnostic evaluation. The study was approved by the Mount Sinai Program for the Protection of Human Subjects (HS12-00925). Details on subject recruitment, eligibility criteria, and screening and surveillance protocols have been previously reported [6]. In brief, participants were all workers and volunteers who performed rescue, recovery, and service restoration duties at the WTC disaster site from September 11, 2001 to June 2002 (Fig. 1). This cohort includes all occupational groups, except firefighters [7]. Beginning in July 2002, all subjects underwent a baseline screening evaluation, which included questionnaires on respiratory symptoms, pre-WTC- and WTC-related occupational exposures, laboratory testing, and spirometry. Subsequent (“monitoring”) health surveillance visits included a similar evaluation at 12- to 18-month intervals, and clinical services were offered for individualized diagnostic and treatment services [1, 2].
Fig. 1.
Study flow chart
CT Imaging Procedures
All CT studies were obtained at Mount Sinai in General Electric® or Siemens® multidetector row chest CT scanners. Chest CT studies were performed using a protocol [8] with a radiation dose at 120 kVp, and a mean of 146 (SD 69) mAs, with subjects in the supine position. CT scans were obtained from the lung apices to the bases in a single breath hold at maximum inspiration, with section thickness not exceeding 1.5 mm. All deidentified and coded chest CT images were stored and cataloged during the past 5 years in the WTC PEU Chest CT Image Archive (ClinicalTrials.gov identifier NCT03295279) [9].
Inclusion Criteria and QCT Systems
Inclusion into this study required that the WTC workers had (1) adequate quality study for quantitative chest CT scan (QCT) measurements of their airways performed with the Simba system (http://www.via.cornell.edu/simba/simba) [10], (2) at least three screening and surveillance spirometries, and (3) complete data for all covariates of interest (this criterion alone excluded 90 subjects). A total of 799 subjects met those three criteria, and were thus included in this study. In order to confirm the consistency of our findings, we conducted the same analyses described herein using a second QCT system, the Chest Imaging Platform (CIP, formerly Airway Inspector, http://www.chestimagingplatform.org/), an open source and well-validated system that has been used in large studies [11]. We had CIP WAP measurements available on 455 subjects (described in Table S2) meeting the same three listed criteria. Both QCT measurements were performed independently, and blinded to each other, any identifier, and all clinical information. None of the included subjects had interstitial lung disease, infectious or neoplastic processes, and other disorders.
Spirometry
Spirometry was performed using the EasyOne® portable flow device (ndd, Zurich, Switzerland), selected for its accuracy, and quality feedback [12, 13]. Bronchodilator response (BDR) was assessed at least once (and most often at the baseline visit) by repeating spirometry 15 min after administration of 180 mcg of albuterol via metered dose inhaler. Predicted values for spirometric measurements were calculated for all subjects’ acceptable tests, based on reference equations from the third National Health and Nutrition Examination Survey (NHANES III) [14], and all testing, quality assurance, ventilatory impairment pattern definitions, and interpretative approaches followed American Thoracic Society recommendations [15–17]. Spirometries in this study were selected if deemed acceptable, and more than 95% also had a good quality grade (computer quality grade A or B, or C if at least 5 trials had been obtained). Although airway obstructive impairment was defined by FEV1/FVC below the lower limit of normal (LLN), post-bronchodilator FEV1/FVC ratio < 0.70 on at least two occasions [18, 19], defined chronic obstructive pulmonary disease (COPD).
Measurements
Our outcome of interest was the risk of having experienced accelerated air flow decline (“decliner” status), compared to normal baseline FEV1 and age-related decline (“stable” status). The expiratory air flow indicator was the first–second forced expiratory volume (FEV1). We used linear regression to calculate the slope of every patient’s FEV1 (FEV1slope) over a minimum of 3 periodic measurements [20]. We first identified subjects (“stable” status) with FEV1 above the lower limit of their predicted normal at their baseline visit, and a FEV1slope, calculated from at least 3 subsequent visits, not exceeding a presumably and a priori determined “normal” age-related decline (or gain) of 25 ml/year. Our primary comparison was with those whose FEV1 declined over the next two follow-up visits (“decliners”), defined by FEV1 slope decrements of at least 4%/year from the baseline FEV1 (regardless of whether the latter was above the subject’s lower limit of normal at baseline). In a secondary analysis, we also compared the “stable” subgroup to those subjects who experienced accelerated FEV1 gain (“gainers”), defined as a positive FEV1slope from the first 3 spirometries exceeding 4%/year of their baseline FEV1. We estimated the root mean squared error (RMSE) as an indicator of group FEV1 fluctuation or variability.
Our main predictor of interest was airway wall area percent (WAP), measured by QCT in the 3rd bronchial generation of the right upper lobe [3], using the Simba system [10, 21]. The automated process starts with identification of the airways and their branch points on inspiratory scans. Airways can be followed out up to five generations, depending on the resolution of the images. Based primarily on density differences between the luminal air, airway wall, and surrounding parenchyma, the airway lumen area (Ai), total airway area (Ao), and airway wall area (Aaw) are measured. These cross-sectional area measurements are averaged along the length of the bronchus. Wall area percentage (WAP) is calculated as (Ao − Ai)/Ao × 100%, and was averaged over all measurable airways. An increase in WAP suggests airway wall thickening, in relation to the lumen, which is in turn suggestive of airway inflammatory changes.
Covariates of interest included age on September 11, 2001, gender, height, race/ethnicity (grouped as Latino, non-Latino white, and non-Latino of other races), body mass index (BMI, expressed in kg/m2) at first evaluation, weight change on follow-up (BMIslope, in kg/m2/year), baseline percent predicted FEV1, evidence of bronchodilator response (BDR) at one or more visits, baseline smoking status (never, former and current smokers), and pre-WTC and WTC occupational exposure categories. BMIslope (kg/m2/year) was calculated as the slope of the linear regression of each subject’s BMI, over all visits available, and used as an indicator of longitudinal weight gain or decline.
Smoking status was assessed at the baseline examination. A subject was considered a lifetime non-smoker if (s) he had smoked less than 20 packs of cigarettes (or 12 oz. of tobacco) in their lifetime, or less than 1 cigarette/day (or 1 cigar/week) for 1 year. A minimum of 12 months without tobacco use was required to deem a subject a former smoker.
WTC occupational exposure relied on two self-reported variables: arrival at the WTC site within 48 h of the terrorist attack (dichotomous) and cumulative exposure duration (in days) [1]. Pre-WTC occupational exposures were assessed dichotomously as self-reported daily exposure, in the course of usual occupation before September 11, 2001, to any of the following list of 11 vapors, dust, gases, and fumes: asbestos, Cadmium, diesel and non-diesel exhaust, general, mineral, and silica/sand dust, wood dust, fiberglass, industrial cleaning solutions, and welding fumes. For descriptive purposes, an occupational physician (RED) recoded, grouped, and labeled occupations into the following 6 categories: (1) management, business, science, arts, service, sales, or office occupations (“management/services”); (2) construction trades, maintenance, and natural resources (“construction trades”); (3) construction and demolition laborers, asbestos handlers, and building cleaners (“laborers/cleaners”) [22]; (4) production, transportation, and material moving (“transportation”); (5) law enforcement specific and military (“law enforcement”); and (6) unemployed, retired, or unknown (“unemployed/retired”).
Statistical Analyses
Descriptive statistics included mean and standard deviation and median and interquartile ranges (IQR) for normally, and non-normally distributed continuous variables, respectively, and counts and proportions for categorical variables. Unadjusted bivariate analyses included t test, χ2 test, or Pearson correlation test, as appropriate. As mentioned before, linear regression was used to estimate the rate of change of FEV1 and BMI, and to define subgroups for comparisons. We calculated the root mean squared error (RMSE) of the group linear regressions, as indicators of FEV1 fluctuation or variability over time. Using the “stable” as the comparison group, we then used multivariate logistic regression to model decliner status vs. WAP, adjusting for the above-listed and described covariates. Logistic regression models were fitted to model the odds of being a decliner using a modified backward selection procedure, with a significance level of 0.2 to remain in the model. All unselected variables were subsequently added to the resulting model one at a time and retained in the model if they altered the β estimate for WAP by greater than 10%. The model was adjusted for baseline smoking status, even if non-significant. Although some of the predictors were correlated, multicollinearity was excluded by the variance inflation factor method. Model goodness of fit was assessed by means of the c statistic. The odds ratios for WAP and BMIslope were calculated per each 5%, and 0.2 kg/m2/year units, respectively. Due to the small number of subjects meeting criteria to be in the gainer subgroup, and the number of variables under consideration, only unadjusted bivariate analyses were performed. A two-sided p value less than 0.05 defined statistical significance. The SAS program, version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
The study group consisted of 799 subjects. Subjects were predominantly male (81.7%), with mean age on September-11-2001 of 42.6 years (SD 8.8 years; Table 1). The most frequent occupations were laborers/building cleaners and law enforcement. Subjects had their baseline spirometry a median of 3.39 years (IQR 1.77–5.39 years) after 11-September-2001, and the interval between the first and third available spirometry for the entire group was a median of 4.65 years (IQR 3.76, 5.72). The prevalence of baseline overweight and obesity were 47.9 and 34%, respectively. Table S1 presents the comparison of the included and excluded subjects.
Table 1.
Characteristics of the entire study group, and the stable, decliner, and gainer subgroups
| Entire group (n = 799) | Stable (n = 103) | Decliner (n = 81) | Gainer (n = 29) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | p* | Mean | SD | p* | |
| FEV1slope, ml/year | −35.7 | 0.15a | −7.8 | 0.15a | −157.6 | 0.2a | – | 173.6 | 0.26a | – |
| Wall area percent | 62.1 | 7.76 | 60.7 | 7.46 | 65.1 | 7.31 | < 0.0001 | 63.5 | 7.8 | 0.0824 |
| Age, years | 42.6 | 8.81 | 41.6 | 7.8 | 45.1 | 8.8 | 0.0046 | 41.0 | 8.3 | 0.7100 |
| Height, cm | 171.5 | 10.0 | 169.8 | 10.3 | 171.2 | 9.6 | 0.3392 | 175.1 | 8.5 | 0.0119 |
| Baseline BMI, kg/m2 | 28.96 | 4.76 | 28.85 | 4.35 | 29.74 | 4.79 | 0.1428 | 29.61 | 5.4 | 0.3738 |
| BMIslope, kg/m2/year | 0.11 | 0.36 | 0.01 | 0.30 | 0.16 | 0.48 | 0.0128 | −0.04 | 0.6 | 0.7020 |
| Baseline FEV1%predicted | 87.1 | 16.9 | 95.6 | 11.7 | 82.3 | 17.1 | < 0.0001 | 56.6 | 15.6 | < 0.0001 |
| WTC exposure duration, days | 93.9 | 74.1 | 85.1 | 73.2 | 83.8 | 79.8 | 0.9059 | 88.5 | 78.3 | 0.8303 |
|
| ||||||||||
| n | % | n | % | n | % | p | n | % | p | |
|
| ||||||||||
| Spirometry result | ||||||||||
| Normal | 483 | 60.5 | 89 | 86.4 | 44 | 54.3 | – | 2 | 6.9 | – |
| Reduced FVC | 233 | 29.2 | 11 | 10.7 | 25 | 30.9 | 12 | 41.4 | ||
| Obstruction | 83 | 10.4 | 0 | 0 | 12 | 14.8 | 15 | 51.7 | ||
| COPD | 44 | 5.5 | 0 | 0 | 9 | 11.1 | 6 | 20.7 | ||
| Occupation | ||||||||||
| Management/services | 141 | 17.6 | 15 | 14.6 | 22 | 27.2 | – | 8 | 27.6 | – |
| Construction trades | 150 | 18.8 | 16 | 16.0 | 17 | 21.0 | 7 | 24.1 | ||
| Laborers/cleaners | 269 | 33.7 | 39 | 37.9 | 17 | 21.0 | 4 | 13.8 | ||
| Transportation | 41 | 5.1 | 4 | 3.9 | 4 | 4.9 | 1 | 3.4 | ||
| Law enforcement | 171 | 21.4 | 23 | 22.3 | 19 | 23.5 | 8 | 27.6 | ||
| Unemployed/retired | 27 | 3.4 | 6 | 5.8 | 2 | 2.5 | 1 | 3.4 | ||
| Baseline smoking status | ||||||||||
| Never | 407 | 50.9 | 64 | 62.1 | 38 | 46.9 | 15 | 51.7 | ||
| Former | 235 | 29.4 | 31 | 30.1 | 27 | 33.3 | 0.0296 | 6 | 20.7 | 0.0146 |
| Current | 157 | 19.7 | 8 | 7.8 | 16 | 19.8 | 8 | 27.6 | ||
| Pre-WTC exposure | ||||||||||
| No | 370 | 46.3 | 48 | 46.6 | 47 | 58.0 | 0.1238 | 8 | 27.6 | 0.0672 |
| Yes | 429 | 53.7 | 55 | 53.4 | 34 | 42.0 | 21 | 72.4 | ||
| Gender | ||||||||||
| Male | 653 | 81.7 | 85 | 82.5 | 61 | 75.3 | 0.2300 | 26 | 89.7 | 0.5654 |
| Female | 146 | 18.3 | 18 | 17.5 | 20 | 24.7 | 3 | 10.3 | ||
| WTC arrival < 48 h | ||||||||||
| No | 413 | 51.7 | 56 | 54.4 | 25 | 30.9 | 0.0014 | 8 | 27.6 | 0.0108 |
| Yes | 386 | 48.3 | 47 | 45.6 | 56 | 69.1 | 21 | 72.4 | ||
| Race/ethnicity | ||||||||||
| Latino | 276 | 34.5 | 35 | 34.0 | 20 | 24.7 | 6 | 20.7 | ||
| Non-Latino white | 425 | 53.2 | 52 | 50.5 | 47 | 58.0 | 0.3920 | 14 | 48.3 | 0.1237 |
| Non-Latino other | 98 | 12.3 | 16 | 15.5 | 14 | 17.3 | 9 | 31.0 | ||
| Any BDR | ||||||||||
| No | 618 | 77.4 | 89 | 86.4 | 47 | 58.0 | < 0.0001 | 13 | 44.8 | < 0.0001 |
| Yes | 181 | 22.7 | 14 | 13.6 | 34 | 42.0 | 16 | 55.2 | ||
WAP measured with the Simba system. The p values relate to the unadjusted comparisons of the decliner and gainer subgroups, with the stable subgroup
p values for continuous measures from t test, p value for categorical measures from χ2 or Fisher’s exact test as appropriate, for comparisons between the stable vs. the decliner or the gainer subgroups. Statistically significant differences are bolded
Root mean squared error, instead of SD
The mean longitudinal FEV1slopes (with RMSE) for the entire study population, and its stable (n= 103), decliner (n = 81), and gainer (n = 29) subgroups were, respectively, −35.8 (0.15), −8 (0.13), −157.6 (0.20), and + 173.6 (0.26) ml/year. For the entire group, we observed that WAP was correlated with baseline BMI (r = 0.243) and FEV1%predicted (r=−0.208), and associated with evidence of BDR (all p < 0.001).
Table 1 shows and contrasts the characteristics of the decliner (n = 81) vs stable (n= 103) subgroups. Unadjusted analyses showed statistically significant associations of accelerated FEV1 decline with WAP, age on 9/11/2001, BMIslope, baseline FEV1%predicted, baseline smoking status, arrival at the WTC within 48 h of the terrorist attack, and BDR. The adjusted logistic regression model (Table 2) confirmed the association for WAP, age on 9/11/2001, BMIslope, baseline FEV1% predicted, and BDR, but also for pre-WTC occupational exposures. The c statistic for the final model was 0.85. Except for age on 9/11/2001, and pre-WTC occupational exposures, we obtained remarkably similar results with the CIP platform QCT measurements, with significant adjusted associations for WAP, BMIslope, baseline FEV1%predicted, and BDR (Table S3).
Table 2.
Logistic regression model for the comparison of decliner (n = 81) versus stable (n = 103) subgroups
| Adjusted comparison | ||||
|---|---|---|---|---|
|
| ||||
| OR | 95% CI | p | ||
| Wall area percent*, 5% unit | 1.47 | 1.12 | 1.95 | 0.0063 |
| Age, years | 1.07 | 1.01 | 1.12 | 0.0134 |
| BMI slope, 0.2 kg/m2/year unit | 1.48 | 1.18 | 1.87 | 0.0009 |
| Baseline FEV1%predicted | 0.95 | 0.92 | 0.98 | 0.0013 |
| Pre-911 exposure | ||||
| No | 1 | – | – | 0.0090 |
| Yes | 0.32 | 0.14 | 0.75 | |
| Any BDR | ||||
| No | 1 | – | – | 0.0185 |
| Yes | 2.88 | 1.20 | 6.96 | |
WAP measured with the Simba system. The model was also adjusted for baseline smoking status, height, gender, and WTC arrival within 48 h. Statistically significant estimates are bolded
Table 1 also shows and contrasts the characteristics of the gainer (n= 29) vs stable (n= 103) subgroups. In unadjusted comparisons, accelerated FEV1 gain was associated with baseline FEV1%predicted, baseline smoking status, BDR, height, and early arrival at the WTC disaster site (Table 1). WAP was not associated with this trajectory in unadjusted or adjusted analyses with the Simba measurements. In contrast, with the CIP system there were both unadjusted and adjusted associations, but estimates were unstable due to a substantially smaller sample size (Table S4).
Discussion
We demonstrated a fairly normal average overall longitudinal FEV1 decline in this group of exposed WTC workers (−35 ml/year). That finding belies widely divergent trajectories with subgroups with excessive air flow decline, as well as gain. We found that accelerated longitudinal FEV1 decline in this WTC occupational cohort is predicted by quantitative CT measurement of bronchial wall area percent, after adjusting for significant predictors like bronchodilator response, age on September 11, 2001, weight gain/loss (as indicated by BMIslope), initial FEV1%predicted, and pre-WTC occupational exposures.
We chose WAP as an indicator of proximal airway wall thickening, presumably as a result of inflammatory changes. This has been demonstrated in other studies of chronic airway diseases, including tobacco-[23, 24] and wood smoke-related COPD [25, 26]. In our classification of WTC-related airway disorders [1], a substantial proportion of cases does not meet established criteria for the diagnoses of asthma or COPD, but are instead diagnosed with non-specific chronic bronchitis or bronchiolitis, based on the presence of lower respiratory symptoms, non-specific lung function abnormalities, and/or chest CT imaging evidence of end-expiratory air trapping [1, 2, 8]. Our findings suggest that WAP, possibly due to proximal bronchial inflammation and/or remodeling, is significantly associated with accelerated FEV1 decline in this cohort.
The additional predictors of accelerated expiratory flow decline are not surprising. To the extent that significant BDR reflects bronchial inflammation, it is expected to be associated with accelerated expiratory flow decline, and BDR has been associated with increased susceptibility to tobacco-smoke pulmonary toxicity [27]. Indeed, WAP was associated with the presence of BDR in this study population (p < 0.001). On the other hand, data from the subgroup with accelerated expiratory flow gain suggest that BDR may be associated with improved function, presumably as a result of mitigation or resolution of toxicant-induced inflammation, pharmacologic treatment, both, or some other unrecognized factor. Further investigation is warranted of this heretofore underrecognized subgroup.
The negative impact of weight gain on longitudinal expiratory flow decline has been identified in community [28], and occupational cohort studies [29], with the effect ostensibly stronger among men [28] (the vastly predominant gender in the WTC occupational cohorts), and surpassing that of obesity at baseline [28]. Our finding confirms an earlier report on a similar cohort [4], but does not appear to have been examined in the firefighters’ cohort [30], where obesity and overweight are more prevalent than in ours [31].
Several studies have identified the association of early arrival at the WTC disaster site [1, 32, 33] with adverse respiratory health outcomes, with the larger ones also describing a significant association for occupational WTC exposure duration [33]. As in our previous study [1], we found significant unadjusted association for early arrival, but not for prolonged exposure at the disaster site. In the adjusted model, however, early arrival fell short of statistical significance. We cannot exclude that a sample size limitation explained this finding. Similarly, we found an unadjusted association of baseline smoking status with abnormal expiratory flow decline or gain, but this variable was correlated with multiple covariates in our models, and was added as an adjusting but non-significant predictor to the final model.
We confirmed that overall longitudinal expiratory flow decline in this WTC occupational cohort is similar to what has been reported in other occupational cohorts [34]. It was noticeable, however, that our cohort had a larger fluctuation in longitudinal expiratory air flow trajectory [35] than has been reported in other occupational cohorts that, like ours, included exposed and symptomatic workers [36]. That finding motivated the investigation of the extremes of those trajectories in both directions. The secondary analyses of excessive expiratory flow increase (gainer subgroup) demonstrated unadjusted significant associations with FEV1%predicted, baseline smoking status, bronchodilator response, height, and early arrival at the WTC, but the significantly reduced FEV1%predicted at baseline of this subgroup appeared to be by far the strongest predictor. Gainers had a higher prevalence of current smoking (as of September-11-2001), and all ventilatory impairments, and also demonstrated the largest variability or fluctuation of their FEV1 over time (Table 1).
The strengths of this study relate to the richness and diversity of the patient population, the amount of data available for covariates of interest, the availability of imaging data from the largest established WTC chest CT archive to date, and the consistency of the findings using two different, independent, and blinded QCT measurement systems. This study also has some limitations. We lacked comparison QCT imaging data from a well-defined control group of occupationally and WTC unexposed, totally asymptomatic subjects, with normal spirometry and chest radiograph. However, our subgroup case definitions served well the intended goal of identifying workers with adverse longitudinal lung function trajectory, as well as unexpected expiratory air flow gain. We used retrospective chest CT imaging data, which were subject to variations in protocols over time. However, most studies were performed in a very small number of scanners with an intended technical consistency, and quality control was exerted to exclude a priori studies that did not meet technical standards for QCT. We recently published the findings on systematic readings of the CT scans [9], noted the paucity of interstitial lung disease features, and the group included in the present study did not include any subject with that type of disease. Despite the richness of our data, we lacked information on other factors that can relate to airway disease outcomes, like atopy, smoking status after baseline, and smoking intensity. Preliminary studies, however, have not suggested, an association between atopy and WTC lower airway disease [37], and occupational airway disease [38, 39], and periodic cross-sectional assessments of smoking status in this cohort do not suggest increasing group smoking rates (data not presented).
In summary, our study demonstrates the different expiratory flow trajectories followed by workers at the WTC disaster site, characterizes subgroups in need of further study, and supports a role for quantitative CT measurements in the investigation of the lower airway diseases observed in this cohort, and in occupational bronchitis [40–43] in general. The findings also provide supportive evidence for the bronchial inflammation that seems to underlie the different WTC-related lower airway diseases [1, 44], as has been recently emphasized for other dust-related occupational diseases [45], and underscores the often neglected importance of considering occupational and environmental exposures in any study of chronic inflammatory airway disorders [43].
Supplementary Material
Acknowledgments
This work was supported by Grant U01-OH010401 from the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health (CDCP/NIOSH). The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the CDCP/NIOSH. ClinicalTrials.gov identifier NCT03295279. The authors would like to thank all participants in this study, and the staff of the Mount Sinai WTC Health Program Clinical Center of Excellence, and Data Center. We also acknowledge the able support of Lilliam Tirado and Raymond Mathews as research coordinators.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00408-018-0125-7) contains supplementary material, which is available to authorized users.
References
- 1.de la Hoz RE, Shohet MR, Chasan R, Bienenfeld LA, Afilaka AA, Levin SM, Herbert R. Occupational toxicant inhalation injury: the World Trade Center (WTC) experience. Int Arch Occup Environ Health. 2008;81(4):479–485. doi: 10.1007/s00420-007-0240-x. [DOI] [PubMed] [Google Scholar]
- 2.de la Hoz RE. Occupational asthma and lower airway disease in former World Trade Center workers and volunteers. Curr Allergy Asthma Rep. 2010;10(4):287–294. doi: 10.1007/s11882-010-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San José Estépar R, Reilly JJ, Silverman EK, Washko GR. Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc. 2008;5(9):905–909. doi: 10.1513/pats.200809-104QC. [DOI] [PubMed] [Google Scholar]
- 4.Skloot GS, Schechter CB, Herbert R, Moline JM, Levin SM, Crowley LE, Luft BJ, Udasin IG, Enright PL. Longitudinal assessment of spirometry in the World Trade Center Medical Monitoring Program. Chest. 2009;135(2):492–498. doi: 10.1378/chest.08-1391. [DOI] [PubMed] [Google Scholar]
- 5.Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, Cosenza K, Christodoulou V, Glass L, Al Othman F, Weiden MD, Kelly KJ, Prezant DJ. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362(14):1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbert R, Moline J, Skloot G, Metzger K, Barron S, Luft B, Markowitz S, Udasin I, Harrison D, Stein D, Todd AC, Enright P, Stellman JM, Landrigan PJ, Levin SM. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect. 2006;114(12):1853–1858. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woskie SR, Kim H, Freund A, Stevenson L, Park BY, Baron S, Herbert R, Siegel de Hernandez M, Teitelbaum S, de la Hoz RE, Wisnivesky JP, Landrigan P. World Trade Center disaster: assessment of responder occupations, work locations, and job tasks. Am J Ind Med. 2011;54(9):681–695. doi: 10.1002/ajim.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendelson DS, Roggeveen M, Levin SM, Herbert R, de la Hoz RE. Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic World Trade Center rescue and recovery workers. J Occup Environ Med. 2007;49(8):840–845. doi: 10.1097/JOM.0b013e3180d09e87. [DOI] [PubMed] [Google Scholar]
- 9.de la Hoz RE, Weber J, Xu D, Doucette JT, Liu X, Carson DA, Celedón JC. Chest CT scan findings in World Trade Center workers. Arch Environ Occup Health. doi: 10.1080/19338244.2018.1452712. in press. [DOI] [PMC free article] [PubMed]
- 10.Lee J, Reeves AP, Fotin SV, Apananosovich TV, Yankelevitz DF. Human airway measurement from CT Images. SPIE International Symposium on Medical Imaging, SPIE; Bellingham, WA. 2008 Feb; 2008. p. 691518. [Google Scholar]
- 11.San José Estépar R, Washko GG, Silverman EK, Reilly JJ, Kikinis R, Westin C-F. [Accessed 7 July 2017];Airway inspector: an open source application for lung morphometry. 2008 http://lmi.bwh.harvard.edu/papers/pdfs/2008/sanjoseMICCAI08.pdf.
- 12.Walters JA, Wood-Baker R, Walls J, Johns DP. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11(3):306–310. doi: 10.1111/j.1440-1843.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Padilla R, Vázquez-García JC, Márquez MN, Jardim JR, Pertuze J, Lisboa C, Muiño A, López MV, Tálamo C, de Oca MM, Valdivia G, Menezes AM. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006;51(10):1167–1171. [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencratz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Enright PL, Skloot GS, Cox-Ganser JM, Udasin IG, Herbert R. Quality of spirometry performed by 13,599 participants in the World Trade Center Worker and Volunteer Medical Screening Program. Respir Care. 2010;55(3):303–309. [PubMed] [Google Scholar]
- 18.Pérez-Padilla R, Wehrmeister FC, Montes de Oca M, López MV, Jardim JR, Muiño A, Valdivia G, Pertuze J, Menezes AM. Instability in the COPD diagnosis upon repeat testing vary with the definition of COPD. PLoS One. 2015;10(3):e0121832. doi: 10.1371/journal.pone.0121832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaron SD, Tan WC, Bourbeau J, Sin DD, Loves RH, MacNeil J, Whitmore GA. Diagnostic instability and reversals of chronic obstructive pulmonary disease diagnosis in individuals with mild to moderate airflow obstruction. Am J Respir Crit Care Med. 2017;196(3):306–314. doi: 10.1164/rccm.201612-2531OC. [DOI] [PubMed] [Google Scholar]
- 20.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Reeves AP, Yankelevitz DF, Henschke CI. SPIE Medical Imaging. SPIE; Bellingham: 2009. Bronchial segment matching in low-dose lung CT scan pairs. [Google Scholar]
- 22.de la Hoz RE, Hill S, Chasan R, Bienenfeld LA, Afilaka AA, Wilk-Rivard E, Herbert R. Health care and social issues of immigrant rescue and recovery workers at the World Trade Center site. J Occup Environ Med. 2008;50(12):1329–1334. doi: 10.1097/JOM.0b013e31818ff6fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutten EP, Grydeland TB, Pillai SG, Wagers S, Dirksen A, Coxson HO, Gulsvik A, Wouters EF, Bakke PS. Quantitative CT: associations between emphysema, airway wall thickness and body composition in COPD. Pulm Med. 2011;2011:419328. doi: 10.1155/2011/419328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchetti N, Garshick E, Kinney GL, McKenzie A, Stinson D, Lutz SM, Lynch DA, Criner GJ, Silverman EK, Crapo JD, Investigators C. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med. 2014;190(7):756–762. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreira MAC, Barbosa MA, de Queiroz MC, Teixeira KISS, Torres PPTS, de Santana PJ, Júnior, Montadon ME, Júnior, Jardim JR. Pulmonary changes on HRCT scans in nonsmoking females with COPD due to wood smoke exposure. J Bras Pneumol. 2013;39(2):155–163. doi: 10.1590/S1806-37132013000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-García M, Maldonado Gómez D, Torres-Duque CA, Barrero M, Jaramillo Villegas C, Pérez JM, Varón H. Tomographic and functional findings in severe COPD: comparison between the wood smoke-related and smoking-related disease. J Bras Pneumol. 2013;39(2):147–154. doi: 10.1590/S1806-3713201300020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celedón JC, Speizer FE, Drazen JM, Weiss ST, Campbell EJ, Carey VJ, Reilly JJ, Ginns L, Silverman EK. Bronchodilator responsiveness and serum total IgE levels in families of probands with severe early-onset COPD. Eur Respir J. 2000;14:1009–1014. doi: 10.1183/09031936.99.14510099. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993;48(4):375–380. doi: 10.1136/thx.48.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ML, McCabe L, Petsonk EL, Hankinson JL, Banks DE. Weight gain and longitudinal changes in lung function in steel workers. Chest. 1997;111(6):1526–1532. doi: 10.1378/chest.111.6.1526. [DOI] [PubMed] [Google Scholar]
- 30.Aldrich TK, Vossbrinck M, Zeig-Owens R, Hall CB, Schwartz TM, Moir W, Webber MP, Cohen HW, Nolan A, Weiden MD, Christodoulou V, Kelly KJ, Prezant DJ. Lung function trajectories in World Trade Center-exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149(6):1419–1427. doi: 10.1016/j.chest.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webber MP, Lee R, Soo J, Gustave J, Hall CB, Kelly K, Prezant D. Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath. 2011;15(3):283–294. doi: 10.1007/s11325-010-0379-7. [DOI] [PubMed] [Google Scholar]
- 32.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, Kelly KJ. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347(11):806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler K, McKelvey W, Thorpe L, Perrin M, Cone J, Kass D, Farfel M, Thomas P, Brackbill R. Asthma diagnosed after September 11, 2001 among rescue and recovery workers: findings from the World Trade Center Health Registry. Environ Health Perspect. 2007;115(11):1584–1590. doi: 10.1289/ehp.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang ML, Avashia BH, Petsonk EL. Interpreting periodic lung function tests in individuals: the relationship between 1- to 5-year and long-term FEV 1 changes. Chest. 2006;130:493–499. doi: 10.1378/chest.130.2.493. [DOI] [PubMed] [Google Scholar]
- 35.Kaminsky DA, Wang LL, Bates JH, Thamrin C, Shade DM, Dixon AE, Wise RA, Peters S, Irvin CG. Fluctuation analysis of peak expiratory flow and its association with treatment failure in asthma. Am J Respir Crit Care Med. 2017;195(8):993–999. doi: 10.1164/rccm.201601-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ML, Avashia BH, Wood J, Petsonk EL. Excessive longitudinal FEV1 decline and risks to future health: a case-control study. Am J Ind Med. 2009;52(12):909–915. doi: 10.1002/ajim.20764. [DOI] [PubMed] [Google Scholar]
- 37.de la Hoz RE, Shohet MR, Wisnivesky JP, Bienenfeld LA, Afilaka AA, Herbert R. Atopy and upper and lower airway disease among former World Trade Center workers and volunteers. J Occup Environ Med. 2009;51(9):992–995. doi: 10.1097/JOM.0b013e3181b32093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarlo SM, Broder I. Irritant-induced occupational asthma. Chest. 1989;96:297–300. doi: 10.1378/chest.96.2.297. [DOI] [PubMed] [Google Scholar]
- 39.Demir A, Joseph L, Becklake MR. Work-related asthma in Montreal, Quebec: population attributable risk in a community-based study. Can Respir J. 2008;15(8):406–412. doi: 10.1155/2008/391269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilson JC. Occupational bronchitis? Proc R Soc Med. 1970;63(9):857–864. doi: 10.1177/003591577006300901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan WKC. Industrial bronchitis. Br J Ind Med. 1978;35(4):285–291. doi: 10.1136/oem.35.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140(Suppl):S85–S91. doi: 10.1164/ajrccm/140.3_Pt_2.S85. [DOI] [PubMed] [Google Scholar]
- 43.Martinez CH, Kim V, Chen Y, Kazerooni EA, Murray S, Criner GJ, Curtis JL, Regan EA, Wan E, Hersh CP, Silverman EK, Crapo JD, Martinez FJ, Han MK. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108(3):491–499. doi: 10.1016/j.rmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de la Hoz RE. Occupational lower airway disease in relation to World Trade Center inhalation exposure. Curr Opin Allergy Clin Immunol. 2011;11(2):97–102. doi: 10.1097/ACI.0b013e3283449063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petsonk EL, Rose C, Cohen R. Coal mine dust lung disease–New lessons from old exposure. Am J Respir Crit Care Med. 2013;187(11):1178–1185. doi: 10.1164/rccm.201301-0042CI. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.