Abstract
Background
Induced premature menopause and cardio-toxic therapy increase cardiovascular disease risk in female cancer survivors.
Objective
To compare the effects of a 12 month aerobic-resistance fitness center intervention to home based physical activity on cardiovascular function and metabolic risk factors.
Methods
Subjects (N = 154) who had completed primary and/or adjuvant chemotherapy (past 3 years) were randomized to a fitness center intervention or a home based group. The fitness center intervention was a structured thrice weekly aerobic (30 min brisk walking treadmill in target heart range) combined with resistance (30 min of lower body strength training) exercise program, supervised for the first 6 months. The home based group received national guidelines for 30 min moderate intensity exercise most days of the week. Fasting serum samples were collected at baseline, 6 and 12 months for insulin, glucose, lipids and hemoglobin A-1C. A graded exercise stress test was also performed at baseline and 6 months.
Results
The majority of subjects were white (85.7%), had breast cancer (83.1%) and the average age was 51.9 years. Subjects in the fitness center intervention had significantly improved time on treadmill (p = .039), improved heart rate recovery at 1 min (p = .028), greater MET minutes/week (p ≤ .0001), a trend for improved insulin resistance (p = .067) and stable insulin levels (p = .045) compared to the home based physical activity group.
Conclusions
Exercise represents a potential cardiac risk reduction intervention for cancer survivors.
Clinical Trials.gov
Keywords: Breast cancer, Cardiovascular fitness, Heart rate recovery, Exercise, Insulin resistance
Introduction
Cancer and cardiovascular disease are integrally related and the growing specialty of cardio-oncology has identified the need for interdisciplinary collaboration in clinical care and research.1,2 The menopausal transition increases cardiovascular disease risk.3 For women diagnosed with breast cancer, the known presence of cardiovascular risk factors (e.g. overweight, obesity, sedentary behavior), premature treatment induced menopause, cardiotoxicity associated with chemotherapy and targeted agents, deconditioning during treatment and aromatase inhibitor endocrine therapy have resulted in lower cardiovascular reserve and a predicted increased risk for cardiovascular disease.4–8 There are more than 3 million breast cancer survivors and due to advances in breast cancer treatment, all-cause mortality has now surpassed breast cancer specific mortality.9 Assessment of risk factors and risk reduction interventions are urgently needed. As one example, weight gain during and after breast cancer treatment is common,10–12 which is associated with an increased risk of recurrence and lower survival13–16 and represents a modifiable risk factor for tertiary prevention.17,18
Healthy lifestyle behaviors are recognized as essential risk reduction interventions for cardiovascular health in women.1,19,20 Exercise has been shown to stabilize or prevent weight gain in healthy women21 improves insulin resistance,22 decreases the risk of diabetes23 improves lipid profile24,25 and reduces overall cardiovascular disease risk.26 For cancer survivors, physical activity has been shown to improve aerobic capacity, quality of life, strength, fatigue and depression, although there are wide variations in the exercise protocols in terms of duration, intensity, and types of delivery.27–31 The increased risk of cardiovascular disease among breast cancer survivors due to aging, presence of cardiovascular risk factors and/or cardiotoxicity from cancer treatment32–34 in the context of prolonged cancer survival rates supports an ongoing need to evaluate lifestyle behaviors as a cardiovascular risk reduction strategy. The purpose of the Yale Fitness Intervention Trial (Yale FIT), a randomized controlled trial (RCT), was to determine the effect of a 12-month aerobic-resistance exercise intervention compared to a home based physical activity group on cardiovascular function and metabolic risk factors in female cancer survivors. The hypothesis of the trial was that the aerobic-resistance exercise intervention would have significantly improved cardiovascular fitness outcomes and a better metabolic profile compared to the home based physical activity group at 6 and 12 months.
Methods
Subject recruitment
Subject recruitment, enrollment, attrition and intervention design has been previously described in detail.35 Briefly, eligible subjects (breast, colorectal, gynecologic cancer or lymphoma diagnosis; ≤3 years since completion of non-endocrine cancer therapy; menopausal [peri or early postmenopausal, the latter defined as ≤5 years since last menstrual period], no resistance exercise ≥3 times/week; physically able to participate [physician permission]) were recruited predominantly from two hospital tumor registries supplemented by physician or self referrals from clinical sites. After baseline data were collected, we used a stratified randomization of blocks of 6 to help control the wave enrollment. We also stratified based on cancer site, as most subjects were expected to have a diagnosis of breast cancer. The study was approved by the Yale University Human Subjects Review Committee.
Exercise intervention protocol
The exercise intervention was an aerobic-resistance intervention delivered by trained research interventionists at community fitness centers (matched to where women lived or worked) 3 times per week, supervised for the first 6 months and unsupervised the last 6 months. The first 4 weeks were designed as progressive training to achieve the intervention target of 30 min of aerobic activity in the subject’s target heart rate goal (65%–75%) through brisk walking on a treadmill and approximately 30 min of lower body resistance exercises including lunges, squats and 8 repetitions, specifically with leg press, toe press, leg extension, leg curl up and bent knee sit ups. The BORG Rating of Perceived Exertion Scale36 was reviewed with subjects on beta blockers with the goal by the end of the four week training to have subjects exercising at 13–16 perceived exertion rating. Warm up and cool down stretching exercises were performed before and at the end of each exercise session. Subjects were encouraged to perform aerobic activity on other days of the week. For the subjects randomized to the home based group, national guidelines for physical activity for adults (30 min moderate level activity most days of the week) were provided and reviewed, supported by the American Cancer Society’s “Smart Steps” booklet which describes examples of moderate intensity activities. Both the intervention and home-based groups received the American Cancer Society Nutrition Guidelines for cancer survivors.37
Metabolic data acquisition
Data were collected at the Yale Center for Clinical Investigation’s Hospital Research Unit (HRU) at baseline, 6 and 12 months in the morning after a 12 h fast. Serum was collected for insulin, glucose, Hemoglobin A1-C, total cholesterol, triglycerides, and high density and low density lipoprotein. Insulin resistance was determined by the homeostatic model of assessment (HOMA) index (fasting plasma glucose [in millimoles per liter] X fasting plasma insulin [in microunits per millimeter] divided by 22.5).38 Weight was recorded on a balance beam scale with light clothing and no shoes to the nearest pound and height measured to the nearest inch.
Hemodynamic monitoring and exercise testing
The study research nurse took blood pressure after a 10 min period of complete rest (supine) with a mercury sphygmomanometer. The mean of three resting BP readings taken 1 min apart was used as the resting blood pressure reading. A graded exercise treadmill test was performed at the Yale New Haven Hospital, Cardiovascular Nuclear Imaging and Exercise Laboratory at baseline and 6 months using a standard modified Bruce protocol with progressive 3 min stages of exercise.39 A modified twelve lead electrocardiogram was obtained prior to exercise and recorded continuously throughout the stress protocol and monitored continuously for a minimum of 5 min post exercise. Heart rate and blood pressure were recorded in each stage of exercise.40 Patients were evaluated for the change in total exercise treadmill time at 6 months and change in heart rate from peak exercise to one and 5 min post exercise was assessed as an index of autonomic function and predictor of cardiovascular outcome as previously applied in the general population.41,42
Monitoring physical activity
The International Physical Activity Questionnaire (IPAQ)43,44 was administered by a research assistant to document leisure, moderate and vigorous activity which summarizes all the types of physical activity to yield a score, reported as MET minutes per week. MET minutes per week were calculated in subscales of walking, moderate intensity, and vigorous intensity as the product of MET level, minutes per episode, and time spent per week. The total MET minutes per week was the sum of the three subscales, which represented to total amount of physical activity.
Statistical analysis
Data analysis was performed with SAS version 9.3. Chi-square test, Cochran-Armitage trend test and t-tests were used to examine baseline equivalence in demographic and clinical variables between two groups. The change of cardiac outcomes physical activity were estimated using a general linear model (GLM) and least square means (LS-mean) were estimated after controlling for age and baseline outcome. To examine the improvements in metabolic risk factors, the changed scores at 6 and 12 months from baseline between two groups were estimated using mixed effect model with a within-subject correlation structure. LS-means of change score were estimated from group-time interaction term after controlling for age, baseline BMI, and baseline score.
Results
From 1264 potential subjects identified from the tumor registries and 81 referrals, 672 were screened, and of those, 308 were determined eligible, and 154 (76 in the fitness center and 78 in the home-based group) women were enrolled.35 There were four women who never started the exercise program, and over the first six months, four who were lost to follow-up and seven withdrew for non-study injuries or illness conditions leaving 139 women (67 in gym exercise vs. 72 in home-based activity) evaluable (Fig. 1).
Fig. 1.
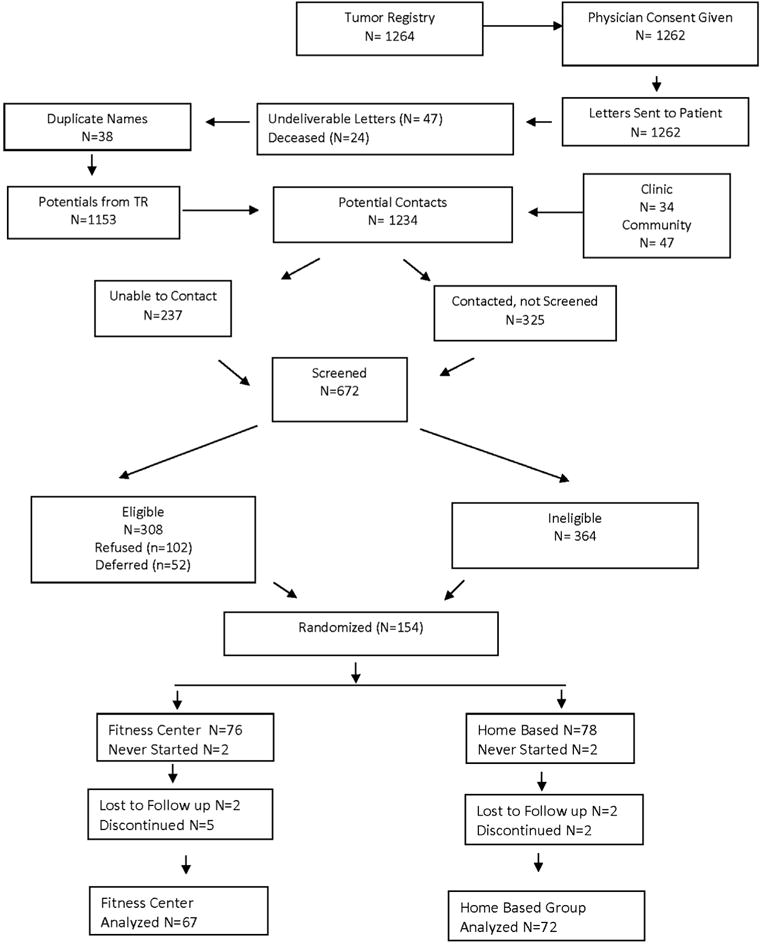
Enrollment.
The sample consisted of primarily White women (85.7%) who were married (70%), employed either full or part time (82.8%), had a breast cancer diagnosis (83.1%) and an average age of 51.9 years (SD = 6.1). Half of the subjects had received adjuvant chemotherapy (52.7%) and 69.4% were on adjuvant endocrine therapy with either tamoxifen or an aromatase inhibitor.35 Comparison of baseline characteristics for cardiac, body composition, physical activity level and metabolic risk factors between the fitness center intervention and home based group are shown in Table 1. Lower triglycerides (p = .0129) and higher peak heart rate (p = .0187) in exercise intervention subjects compared to home-based physical activity subjects were observed at baseline. None of the other baseline characteristics were significantly different between the two groups.
Table 1.
Baseline comparisons between fitness center and home based groups.
| Fitness center N = 76 |
Home-based N = 78 |
Baseline difference (P-value) |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Body composition | |||
| Weight (lb) | 173.6 (42.8) | 166.7 (41.0) | (ǂ).2654 |
| BMI | 29.5 (6.8) | 28.5 (6.8) | (ǂ).2984 |
| Waist circumference (cm) | 87.1 (15.0) | 85.5 (15.6) | (ǂ).4611 |
| Total fat (kg) | 31.5 (11.2) | 30.3 (11.9) | (ǂ).3815 |
| Total lean (kg) | 44.1 (7.6) | 42.8 (7.4) | (ǂ).2832 |
| Total mass (kg) | 77.8 (17.4) | 75.3 (18.3) | (ǂ).3039 |
| % Fat | 39.6 (6.3) | 39.1 (6.6) | .6154 |
| Metabolic risk factors | |||
| Insulin | 13.9 (8.4) | 14.1 (9.3) | (ǂ).7403 |
| Glucose | 5.44 (0.98) | 5.78 (1.79) | (ǂ).1564 |
| Hemoglobin A1c | 0.056 (0.004) | 0.058 (0.012) | (ǂ).2247 |
| Total lipid | 187.6 (27.2) | 185.5 (28.0) | .6411 |
| Triglycerides | 96.0 (41.3) | 119.1 (66.3) | (ǂ).0129 |
| HDL-Cholesterol | 53.6 (14.6) | 50.6 (14.3) | .1978 |
| LDL-Cholesterol | 107.3 (22.6) | 105.2 (23.0) | .5716 |
| Cardiac variables | |||
| Resting heart rate | 75.6 (9.5) | 76.3 (11.8) | .7072 |
| Peak heart rate | 166.6 (14.9) | 160.6 (16.2) | .0187 |
| Recovery heart rate | 100.5 (15.0) | 99.1 (16.8) | .6077 |
| (*) Change heart rate in 1 min (%) | 15.7 (5.5) | 14.7 (6.0) | .3466 |
| Resting Systolic Blood Pressure | 116.7 (14.4) | 119.9 (16.3) | .2182 |
| Resting diastolic blood pressure | 73.2 (8.4) | 74.8 (9.5) | .2803 |
| Level of physical activity | |||
| MET minutes per week | 1327 (1494) | 1446 (1843) | (ǂ).4218 |
Note. (*) % Change Heart Rate in 5 min = 100* (Peak HR − Recovery HR)/Peak HR (%). P-values were obtained from two-side t-test with log-transformed score when variables are skewed.
Cardiovascular fitness
Cardiovascular fitness outcomes improved in the fitness center exercise intervention group compared to the home based physical activity group. Both groups improved time on treadmill at 6 months (<.001) (Fig. 2) but the fitness center group had significantly greater improvement (P = .039).
Fig. 2.
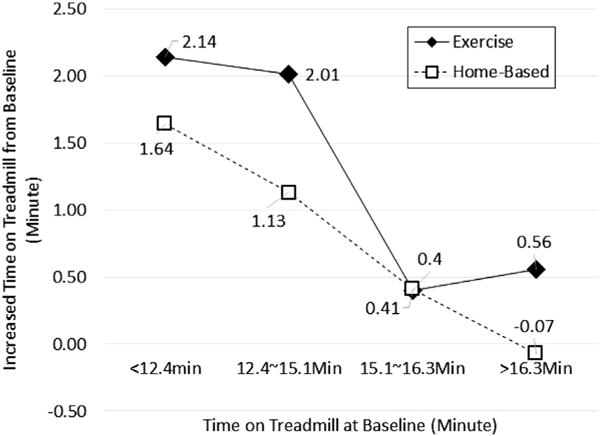
Increased time on treadmill at 6 months from baseline. Note. Mean of increased time on treadmill was calculated by the quartile of time at baseline.
We evaluated change from peak heart rate to 1 min post exercise an established index of autonomic function and cardiovascular fitness. After controlling for peak heart rate and age at baseline, the fitness center group had significantly improved heart rate recovery (HRR) at 1 min compared to the home based group (p = .028) (Table 2).
Table 2.
Changes in heart rate reduction (HRR) over 6 months, blood pressure, and physical activity (MET minutes per week) between the fitness center intervention and home-based physical activity groups. (N = 139).
| Baseline mean (SD) | 6 Month mean (SD) | Delta LS-mean (95% CI) | Comparison of delta (p-value) | |
|---|---|---|---|---|
| Heart rate reduction at 1 min | ||||
| Fitness Center | 15.7 (5.5) | 16.9 (5.9) | 1.30 (−0.45, 3.05) | .0286 |
| Home-Based | 14.7 (6.1) | 13.3 (8.6) | −1.55 (−3.38, 0.29) | |
| Systolic blood pressure (mmHg) | ||||
| Fitness Center | 116.7 (14.3) | 115.0 (14.7) | −2.44 (−5.40, 0.52) | .5646 |
| Home-Based | 119.5 (16.7) | 118.1 (14.4) | −1.22 (−4.14, 1.69) | |
| Diastolic blood pressure (mmHg) | ||||
| Fitness Center | 73.3 (8.7) | 71.3 (9.2) | −2.77 (−5.03, −0.52) | .2326 |
| Home-Based | 74.7 (9.8) | 73.7 (9.3) | −0.85 (−3.06, 1.36) | |
| Total MET minutes per week | ||||
| Fitness Center | 1309.7 (1512.9) | 2816.8 (2538.6) | 1482.4 (1038.9, 19025.9) | <.0001 |
| Home-Based | 1433.8 (1741.3) | 1223.0 (870.3) | −173.6 (−591.5, 244.2) |
Note. Delta is the changed score during 6 months from baseline and least-square means (LS-Mean) were estimated from general linear regression model after controlling for baseline score and age.
HRR during a cool-down period of 5 min after peak exercise was positively associated with time on treadmill (r = .225, p = .006). There were no significant differences in blood pressure between groups. Significantly greater improvement in total MET minutes per week was observed in the fitness center group compared to the home based group (p < .0001).
Metabolic risk factors
After controlling for age, baseline BMI, and baseline values, Table 3 shows the least square mean and 95% confidence interval of changed metabolic outcomes from baseline. There was a trend for improved insulin resistance in the fitness center intervention group at 6 months (p = .067) and insulin was significantly increased in the home-based group compared to no change in the fitness center group at 12 months (p = .0450).
Table 3.
Least square mean changed metabolic outcomes at 6 and 12 months between fitness center and home based groups.
| Cardiac Variables | Intervention | 6 months
|
12 months
|
||
|---|---|---|---|---|---|
| LS-mean difference (95% CI) | p-value | LS-mean difference (95% CI) | p-value | ||
| Insulin | Fitness Center Home-based | 1.30 (−0.33, 2.94) 2.61 (0.97, 4.24) | .2702 | 1.29 (−0.41, 2.99) 3.72 (2.08, 5.35) | .0450 |
| HOMA | Fitness Center Home-based | 0.09 (−0.45, 0.64) 0.82 (0.27, 1.36) | .0673 | 0.02 (−0.55, 0.58) 0.71 (0.17, 1.25) | .0843 |
| HbA1c | Fitness Center Home-based | −0.01 (−0.09, 0.07) −0.03 (−0.10, 0.05) | .7459 | −0.09 (−0.02, −0.01) −0.07 (−0.14, 0.01) | .7144 |
| Total Cholesterol | Fitness Center Home-based | 1.51 (−4.44, 7.46) 1.85 (−3.82, 7.53) | .9347 | 6.71 (0.61, 12.80) 8.27 (2.57, 13.98) | .7132 |
| Triglyceride(ǂ) | Fitness Center Home-based | −0.05 (−0.13, 0.03) −0.00 (−0.08, 0.07) | .4092 | −0.02 (−0.11, 0.06) 0.03 (−0.04, 0.11) | .3187 |
| HDL Cholesterol | Fitness Center Home-based | 1.76 (−0.41, 3.93) 2.11 (0.04, 4.17) | .8221 | 4.76 (2.53, 6.98) 3.79 (1.72, 5.87) | .5381 |
| LDL Cholesterol | Fitness Center Home-based | 3.82 (−1.01, 8.66) 1.27 (−3.34, 5.88) | .4551 | 7.90 (2.96, 12.84) 8.00 (3.37, 12.62) | .9775 |
Note. Least square mean (LS-mean) and 95% confidence intervals were estimated after controlling for age, baseline BMI, and baseline score. (ǂ) triglyceride was log-transformed to hold a normal assumption of residuals.
When cholesterol levels were analyzed by type of adjuvant endocrine therapy (30% aromatase inhibitor [AI], 31% tamoxifen, 39% no endocrine therapy) (Table 4), women on AI therapy had increased cholesterol levels over time. Those in the home-based group had a statistically significant greater increase compared to the exercise group (p = .0378). Among women on tamoxifen, the home-based group had significantly higher insulin resistance (p = .0486) compared to the fitness center group (Table 4).
Table 4.
Least square mean of changed metabolic outcomes at 6 and 12 months between the fitness center and home-based groups by adjuvant endocrine therapy type.
| Variables | Intervention | 6 months
|
12 months
|
||
|---|---|---|---|---|---|
| LS-mean (95% CI) | Difference p-value | LS-mean (95% CI) | Difference p-value | ||
| Aromatase inhibitor (N = 41) | |||||
| Insulin | Fitness center Home-based | 0.11 (−2.34,2.56) 1.35 (−1.04,3.74) | .4910 | 0.70 (−1.75,3.16) 3.46 (1.02,5.90) | .1338 |
| HOMA | Fitness Center Home-based | 0.12 (−0.59,0.82) 0.55 (−0.14,1.23) | .4098 | 0.07 (−0.63,0.77) 0.89 (0.19,1.59) | .1236 |
| HbA1c | Fitness Center Home-based | 0.12 (−0.15,0.19) −0.02 (−0.17,0.13) | .7359 | −0.04 (−0.21,0.12) −0.10 (−0.25,0.06) | .6623 |
| Total Lipid | Fitness Center Home-based | 2.77 (−6.66,12.19) 5.87 (−2.83,14.58) | .6442 | 5.87 (−2.83,14.58) 17.78 (8.90,26.67) | .0378 |
| Triglyceride(ǂ) | Fitness Center Home-based | −0.03 (−0.19, 0.13) −0.01 (−0.16, 0.14) | .8767 | −0.15 (−0.32, 0.01) 0.07 (−0.08, 0.23) | .0589 |
| HDL Cholesterol | Fitness Center Home-based | −0.95 (−5.94,4.03) 2.95 (−1.20,8.77) | .2794 | 3.78 (−1.20,8.77) 5.22 (0.54,9.90) | .6905 |
| LDLCholesterol | Fitness Center Home-based | 7.78 (0.49,15.08) 4.00 (−2.75,10.75) | .4649 | 9.20 (1.91,16.50) 12.94 (6.05,19.83) | .4741 |
| Tamoxifen (N = 42) | |||||
| Insulin | Fitness Center Home-based | 0.61 (−3.34,4.56) 5.08 (1.34,8.82) | .1107 | 0.31 (−3.63,4.27) 5.43 (1.78,9.08) | .0655 |
| HOMA | Fitness Center Home-based | −0.08 (−1.19,1.03) 1.16 (0.11,2.21) | .1153 | −0.33 (−1.44,0.78) 1.21 (0.18,2.24) | .0486 |
| HbA1c | Fitness Center Home-based | 0.03 (−0.10,0.17) 0.03 (−0.15,0.10) | .5106 | −0.02 (−0.16,0.11) −0.08 (−0.21,0.04) | .5238 |
| Total lipid | Fitness Center Home-based | −3.08 (−14.21,8.05) −4.37 (−14.64,5.90) | .8666 | 2.13 (−9.00,13.26) 1.83 (−8.22,11.88) | .9684 |
| Triglyceride(ǂ) | Fitness Center Home-based | −0.01 (−0.16, 0.14) 0.05 (−0.09, 0.19) | .5880 | 0.03 (−0.13, 0.18) 0.07 (−0.07, 0.20) | .7058 |
| HDL Cholesterol | Fitness Center Home-based | 2.23 (−2.02,6.48) 0.33 (−3.58,4.25) | .5238 | 2.18 (−2.07,6.43) 1.92 (−1.91,5.75) | .9286 |
| LDLCholesterol | Fitness Center Home-based | 0.00 (−9.40,9.40) −1.86 (−10.47,6.75) | .7738 | 5.63 (−3.77,15.03) 2.55 (−5.93,11.03) | .6310 |
| No hormone therapy (N = 52) | |||||
| Insulin | Fitness Center Home-based | 3.30 (0.94,5.65) 1.36 (−1.03,3.76) | .2514 | 1.94 (−0.69,4.58) 2.34 (−0.05,4.74) | .8209 |
| HOMA | Fitness Center Home-based | 0.45 (−0.56,1.45) 0.56 (−0.47,1.58) | .8749 | 0.19 (−0.93,1.32) −0.04 (−1.06,0.99) | .7608 |
| HbA1c | Fitness Center Home-based | −0.03 (−0.16,0.10) −0.07 (−0.20,0.06) | .6444 | −0.15 (−0.29, −0.01) −0.05 (−0.18,0.07) | .3023 |
| Total lipid | Fitness Center Home-based | 2.77 (−8.23,13.77) 8.75 (−1.94,19.45) | .4354 | 12.25 (0.66,23.84) 9.00 (−1.81,19.82) | .6816 |
| Triglyceride(ǂ) | Fitness Center Home-based | −0.09 (−0.21, 0.04) −0.02 (−0.14, 0.10) | .4337 | 0.04 (−0.09, 0.18) −0.03 (−0.16, 0.09) | .4214 |
| HDL Cholesterol | Fitness Center Home-based | 3.80 (0.79,6.80) 3.25 (0.37,6.13) | .7936 | 7.73 (4.50,10.95) 4.05 (1.12,6.98) | .0973 |
| LDL Cholesterol | Fitness Center Home-based | 2.30 (−6.75,11.34) 5.05 (−3.74,13.84) | .6622 | 8.83 (−0.68,18.33) 10.94 (2.05,19.82) | .7459 |
Note. Least square mean (LS-mean) and 95% confidence intervals were estimated after controlling for age, baseline BMI, and baseline score.
Baseline BMI was an important predictor of increased insulin (p = .0020), insulin resistance (p = .0188), and LDL cholesterol (p = .0469). As weight is also a known factor in metabolic outcomes, weight was categorized as a loss (≥5 lbs), stable (within ± 5 lbs) or gain (≥5lbs). Of 130 evaluable subjects with weight data at baseline and 12 months, 20% lost weight, 30% gained and 50% were weight stable. There was no change in weight by fitness center or home based group (data not shown) but women who gained weight had significantly higher insulin (p = .0434), and insulin resistance (p = .0194), with no significant changes in other metabolic variables after controlling for age, baseline BMI, and baseline score (Table 5).
Table 5.
Least square mean of changed metabolic outcomes at 12 months by weight gain over 12 months.
| Weight lose (< −5lb) N = 26 LS-Mean (95% CI) |
Stable weight (within ±5lb) N = 65 LS-Mean (95% CI) |
Weight gain (>5lb) N = 39 LS-Mean (95% CI) |
P-value | |
|---|---|---|---|---|
| Metabolic outcomes | ||||
| Insulin | 1.60 (−0.99, 4.19) | 1.52 (−0.13, 3.18) | 4.79 (2.71, 6.88) | .0434 |
| HOMA | −0.07 (−0.79, 0.65) | 0.12 (−0.34, 0.58) | 1.07 (0.49, 1.65) | .0194 |
| HbA1c | −0.20 (−0.32, −0.08) | −0.03 (−0.11, 0.04) | −0.06 (−0.16, 0.04) | .0717 |
| Total lipid | 6.02 (−3.14, 15.17) | 11.14 (5.29, 16.98) | 3.22 (−4.26, 10.70) | .2576 |
| Triglyceride(ǂ) | 0.04 (−0.08, 0.16) | −0.02 (−0.10, 0.05) | 0.41 (−0.05, 0.14) | .5057 |
| HDL Cholesterol | 6.70 (2.83, 10.57) | 4.98 (2.51, 7.44) | 1.82 (−1.32, 4.97) | .1228 |
| LDL Cholesterol | 4.04 (−3.10, 11.19) | 11.83 (7.26, 16.40) | 3.35 (−1049, 9.20) | .0535 |
Note. Least square mean (LS-mean) and 95% confidence intervals were estimated after controlling for age, baseline BMI, and baseline score.
In summary, cardiac autonomic function as reflected by post exercise HRR was significantly improved for subjects in the fitness center intervention compared to the home based group. The aerobic-resistance fitness center intervention also stabilized insulin and insulin resistance for women on AI and Tamoxifen endocrine therapies compared to the observed increases for subjects in the home based group. Baseline BMI was a predictor of increased insulin and insulin resistance and subjects who gained weight during the year of the study also had significant increases in insulin and insulin resistance.
Discussion
The findings of the Yale FIT study confirm the importance of exercise in a high risk cancer survivor population related to cardiovascular outcomes. Both time on treadmill and HRR were significantly improved for subjects in the Yale FIT aerobic-resistance intervention group. Improvement in HRR is clinically important as it is a prognostic predictor,41,45 an indicator of cardiac autonomic function46 and overall cardiovascular fitness has been reported to reduce cancer mortality, independent of adiposity.47 As two-thirds of the subjects in Yale FIT were overweight or obese, improvement in fitness supports the cardiovascular health benefits of exercise in an at risk population of cancer survivors.
Being overweight, obese and weight gain after treatment is common among breast cancer survivors,10–12,48 weight gain in non-obese healthy postmenopausal women is associated with insulin resistance,49 and higher fasting insulin levels and insulin resistance are associated with poorer outcomes for cancer survivors.50–52 Thus, investigators have evaluated the effect of exercise on insulin levels and insulin resistance.42,53–56 While two studies showed a decrease in insulin levels in the exercise intervention group,53,56 it was not significantly different compared to the control group in either study (p =.08 and p = .07 respectively). The findings of Yale FIT demonstrated stabilization of fasting insulin levels in the exercise group compared to statistically increased insulin levels in the home based group and a trend for improvement in insulin resistance for the exercise group, but only at 12 months. Two of the previous cited studies were only of 4 months56 or 6 months duration53 and perhaps longer sustained exercise is needed to alter these metabolic outcomes. Weight did influence insulin and insulin resistance with women with stable weight or who lost weight having significantly lower levels, which is similar to a recently published weight loss trial in breast cancer survivors.57 There was no significant change in triglycerides by group or type of adjuvant therapy. The effect of AI and Tamoxifen endocrine therapy on lipids is mixed58 and it is unknown even if there are favorable changes in lipids, if that translates into a clinical benefit.59 However, endocrine therapy is now recommended for up to 10 years and the cardiovascular effects are yet to be fully identified.
BMI has been reported to be a predictor of increased cardiovascular disease8 and increased recurrence rate and decreased survival.16,48,51 In Yale FIT, long term cancer outcomes could not be evaluated but baseline BMI was a predictor of increased insulin and insulin resistance. The relationship of BMI and insulin on breast cancer progression is complex60,61 but a comprehensive risk reduction approach related to cardiovascular disease, diabetes, cancer specific and all-cause mortality is needed.61 The effect of weight reduction for women who are overweight or obese at diagnosis on disease free and overall survival is unknown as we await results of ongoing trials.60 However, what is known are the health benefits of regular sustained exercise as a non-pharmacologic intervention for cardiovascular and overall health of women who are cancer survivors.14,17,18 The combination of aerobic plus resistance exercise is recommended for all women >50 years of age and resistance exercise has the potential to decrease chronic illness risks associated with central adiposity, increased fat mass, and elevated waist circumference in normal, overweight and obese breast cancer survivors.62,63
Limitations of the study are the sample size, especially for the sub-analyses by type of adjuvant endocrine therapy, the lack of a cardiovascular assessment at 12 months and the downward drift in level of physical activity in the intervention group during the last nonsupervised 6 months. Also, the average age of the subjects was relatively young across the age spectrum of women with cancer and perhaps the intervention would have greater impact on older age survivors with known cardiovascular risk factors.7,8
In summary, we demonstrated that a 12-month aerobic-resistance exercise intervention delivered at a fitness center resulted in significantly better cardiovascular fitness and metabolic risk factors compared to a national guideline home-based physical activity group. We had a 77.4% adherence rate to the supervised thrice weekly exercise intervention at the fitness centers across the first 6 months.35 However, based on the MET minutes/week, there was a decline in physical activity in the last six months when women were not being supervised and there was little change in physical activity among the home-based group. A major challenge is how to motivate, engage and sustain cancer survivors in physical activity to achieve a risk reduction benefit. Text messaging, feedback with accelerometers and peer coaching offer potential strategies to promote adherence over time. Innovative and cost effective interventions are needed to increase physical activity and prevent weight gain in order to decrease co-morbid health risks.
Acknowledgments
This study was made possible by NIH/NCI R01CA122658, NIH/NHLBI 5T32HL098069-07, and the Yale CTSA grant UL1TR000142 from the National Center for Advancing Translational Science (NCATS), NIH.
References
- 1.Barac A, Murtagh G, Carver JR, et al. Cardiovascular health of patients with cancer and cancer survivors. J Am Coll Cardiol. 2015;65:2739. doi: 10.1016/j.jacc.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khakoo AY, Yeh ET. Therapy insight: management of cardiovascular disease in patients with cancer and cardiac complications of cancer therapy. Nat Clin Pract Oncol. 2008;5(11):655. doi: 10.1038/ncponc1225. [DOI] [PubMed] [Google Scholar]
- 3.Crawford SL, Casey VA, Avis NE, McKinlay SM. A longitudinal study of weight and menopause transition: results from the Massachusetts women’s health study. Menopause. 2000;7:96. doi: 10.1097/00042192-200007020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Jones LW, Haykowsky M, Peddle CJ, et al. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomark Prev. 2007;16:1026. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 5.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013 doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed]
- 8.Obi N, Gornyk D, Heinz J, et al. Determinants of newly diagnosed comorbidities among breast cancer survivors. J Cancer Surviv. 2014;8:384. doi: 10.1007/s11764-013-0338-y. [DOI] [PubMed] [Google Scholar]
- 9.Chapman JW, Meng, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin M, McTiernan A, Baumgarten RN, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic and lifestyle factors. J Clin Oncol. 2005;23:774. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McInnes J, Knobf MT. Weight gain and quality of life in women treated with adjuvant therapy for breast cancer. Oncol Nurs Forum. 2001;28:675. [PubMed] [Google Scholar]
- 12.Makari-Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13:258. doi: 10.1111/j.1524-4741.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 14.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copson ER, Cutress RI, Maishman T, et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26:101. doi: 10.1093/annonc/mdu509. [DOI] [PubMed] [Google Scholar]
- 16.Ewertz M, Jenson M, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2010;29:25. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 17.Denlinger CS, Ligibel JA, Baker KS, et al. Survivorship: nutrition and weight management version 2-2014. Clinical practice guidelines in oncology. J NCCN. 2014;12:1396. doi: 10.6004/jnccn.2014.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demark-Wahnefried W, Rogers LQ, Alfano C, et al. Practical clinical interventions for diet, physical activity and weight control in cancer survivors. CA Cancer J Clin. 2015;65:167. doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
- 19.Knobf MT, Coviello J. Lifestyle interventions for cardiovascular risk reduction in women with breast cancer. Curr Cardiol Rev. 2011;7:250. doi: 10.2174/157340311799960627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eijsvogels MH, Molossi S, Lee D, Emery MS, Thompson PD. Exercise at the extremes. J Am Coll Cardiol. 2016;67:316. doi: 10.1016/j.jacc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JM, Jacobsen D, Heelan KS, et al. The effects of 18 months of intermittent vs. continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previous sedentary moderately obese females. J Obes Rel Metabol Disord. 2000;24:560. doi: 10.1038/sj.ijo.0801198. [DOI] [PubMed] [Google Scholar]
- 22.Franks LL, Sorensen BE, Yasui Y, et al. Effects of exercise on metabolic risk variable in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13:615. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 23.Hagey AR, Warren MP. Exercise and Menopause: positive health effects. Menopause Manag. 2006;15:19–25. [Google Scholar]
- 24.Kelly GA, Kelly KS, Tran ZU. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Women’s Health. 2004;13:1148. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly GA, Kelly KS, Tran ZU. Exercise, lipids and lipoproteins in older adults: a metaanalysis. Prev Cardiol. 2005;8:206. doi: 10.1111/j.0197-3118.2005.03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk reduction in adults: a scientific statement from the American heart association. Circulation. 2010;122:406. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speck RM, Courneya KS, Masse L, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim C, Kang D, Park J. A meta-analysis of aerobic exercise interventions for women with breast cancer. West J Nurs Res. 2009;31:437. doi: 10.1177/0193945908328473. [DOI] [PubMed] [Google Scholar]
- 29.Mares M, Brockow T. Exercise for Women Receiving Adjuvant Therapy for Breast Cancer (Review) Cochrane Collection. John Wiley & Sons; 2009. [DOI] [PubMed] [Google Scholar]
- 30.Fong DYT, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomized controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive-theory based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surv. 2015;9:305. doi: 10.1007/s11764-014-0413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh E, Bickford C. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 33.Russell R. Anthracyclines and cardiotoxicity. Curr Cardiol Rev. 2011;7(4):214. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chien KR. Herceptin and the heart: a molecular modifier of cardiac failure. N Engl J Med. 2006;358(8):789. doi: 10.1056/NEJMp058315. [DOI] [PubMed] [Google Scholar]
- 35.Knobf MT, Jeon S, Smith B, et al. Effect of a randomized controlled exercise trial on bone outcomes: influence of adjuvant endocrine therapy. Breast Cancer Res Treat. 2016 doi: 10.1007/s10549-016-3693-3. [DOI] [PubMed]
- 36.Borg GV. Rating of perceived exertion scale. Med Sci Sports Exerc. 1982;14:377–387. [PubMed] [Google Scholar]
- 37.Brown JK, Byers T, Doyle C, et al. Nutrition and physical activity during and after cancer treatment: an American cancer society guide for informed choices. CA Cancer J Clin. 2003;53:268. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.McInnes KJ, Balady GJ, Weiner DA, Ryan TJ. Comparison of ischemic and physiologic responses during exercise testing in men using the standard and modified bruce protocols. Am J Cardiol. 1992;69:84. doi: 10.1016/0002-9149(92)90680-w. [DOI] [PubMed] [Google Scholar]
- 40.Wackers F. Nuclear Cardiology: The Basics. Humana Press; 2004. [Google Scholar]
- 41.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 42.Giallauria F, Maresca L, Vitelli A, et al. Exercise training improves heart rate recovery in women with breast cancer. Springer Plus. 2015;4:388. doi: 10.1186/s40064-015-1179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Physical Activity Questionnaire. www.ipaq.ki.se.
- 44.Booth ML. Assessment of physical activity: an international perspective. Res Quart Exer Sports. 2000;71:s114. [PubMed] [Google Scholar]
- 45.Kohli P, Gulati M. Exercise stress testing in women. Circulation. 2010;122:2570. doi: 10.1161/CIRCULATIONAHA.109.914754. [DOI] [PubMed] [Google Scholar]
- 46.Lakowski SG, Jones LW, Krone RJ, et al. Autonomic dysfunction in early stage breast cancer: incidence, clinical importance and underlying mechanisms. Am Heart J. 2015;170:231. doi: 10.1016/j.ahj.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26:272. doi: 10.1093/annonc/mdu250. [DOI] [PubMed] [Google Scholar]
- 48.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012 doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed]
- 49.LeMay A, Turcot L, Dechene F, Dodin S, Forest J. Hyperinsulinemia in nonobese women reporting moderate weight gain at the beginning of menopause: a useful early measure of susceptibility to insulin resistance. Menopause. 2010;17:321. doi: 10.1097/gme.0b013e3181b7c521. [DOI] [PubMed] [Google Scholar]
- 50.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer:results of a prospective cohort study. J Clin Oncol. 2002;20:42. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 51.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin-and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2011;30:164. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 52.Duggan C, Irwin ML, Xiao L, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. 2010 doi: 10.1200/jco2009.26.4473. [DOI] [PMC free article] [PubMed]
- 53.Irwin ML, Vanna K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale exercise and survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:EPI-08-0531. doi: 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fairey AS, Courneya KS, Field CJ, et al. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721. [PubMed] [Google Scholar]
- 55.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14:1672. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 56.Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 57.Harrigan M, Cartmel B, Loftfiled E, et al. Randomized trial comparing telephone versus in person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise and nutrition (LEAN) study. J Clin Oncol. 2016;34:669. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ewer MS, Gluck S. A woman’s heart. The impact of adjuvant endocrine therapy on cardiovascular health. Cancer. 2009;115:1813. doi: 10.1002/cncr.24219. [DOI] [PubMed] [Google Scholar]
- 59.Esteva FJ, Hortobagyi GN. Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women. Breast. 2006;15:301. doi: 10.1016/j.breast.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 60.Chlebowski RT. Obesity and breast cancer outcome: adding to the evidence. J Clin Oncol. 2011;30:126. doi: 10.1200/JCO.2011.39.7877. [DOI] [PubMed] [Google Scholar]
- 61.DeCensi A, Gennari A. Insulin breast cancer connection: confirmatory data set the stage for better care. J Clin Oncol. 2011;29:7. doi: 10.1200/JCO.2010.32.3022. [DOI] [PubMed] [Google Scholar]
- 62.Sturgeon KM, Dean LT, Heroux M, et al. Commercially available lifestyle modification program: randomized controlled trial addressing heart and bone health in BRCA1/2+ breast cancer survivors after risk-reducing salpingo-oophorectomy. J Cancer Surviv. 2017;11:246–255. doi: 10.1007/s11764-016-0582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cespedo Feliciano EM, Kwan ML, Kushi LH, Weltzien EK, Castillo AL, Caan BJ. Adiposity, post-diagnosis weight change and risk of cardiovascular events among early-stage breast cancer survivors. Breast Cancer Res Treat. 2017;162:549–557. doi: 10.1007/s10549-017-4133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


