Abstract
Although osteoporosis affects 10 million people in the United States, screening and treatment rates remain low. We performed a systematic review and meta-analysis of the efficacy of quality improvement strategies to improve osteoporosis screening (bone mineral density (BMD)/dual-energy x-ray absorptiometry (DXA) testing) and/or treatment (pharmacotherapy) initiation rates. We developed broad literature search strategies for PubMed, Embase, and Cochrane Library databases, and applied inclusion/exclusion criteria to select relevant studies. Random-effects meta-analyses were performed for outcomes of BMD/DXA testing and/or osteoporosis treatment. Forty-three randomized clinical studies met inclusion criteria. For increasing BMD/DXA testing in patients with recent or prior fracture, meta-analyses demonstrated several efficacious strategies including orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management (risk difference 44%, 95%CI 26%–63%), fracture liaison service/case management (risk difference 43%, 95%CI 23%–64%), multifaceted interventions targeting providers and patients (risk difference 24%, 95%CI 15%–32%), and patient education and/or activation (risk difference 16%, 95%CI 6%–26%). For increasing osteoporosis treatment in patients with recent or prior fracture, meta-analyses demonstrated significant efficacy for interventions of fracture liaison service/case management (risk difference 20%, 95%CI 1%–40%) and multifaceted interventions targeting providers and patients (risk difference 12%, 95%CI 6%–17%). The only quality improvement strategy for which meta-analysis findings demonstrated significant improvement of osteoporosis care for patient populations including individuals without prior fracture was patient self-scheduling of DXA plus education, for increasing the outcome of BMD testing (risk difference 13%, 95% CI 7%–18%). The meta-analyses findings were limited by small number of studies in each analysis; high between-study heterogeneity; sensitivity to removal of individual studies; and unclear risk of bias of included studies. Despite the limitations of the current body of evidence, our findings indicate there are several strategies that appear worthwhile to enact to try to improve osteoporosis screening and/or treatment rates.
Keywords: osteoporosis, health services research, fracture prevention, screening, antiresorptives
Introduction
Osteoporosis affects approximately 10 million people in the United States (8 million women and 2 million men,(1) and approximately 50% of postmenopausal women and 20% of white men will sustain an osteoporotic fracture in their lifetimes.(2) The morbidity, mortality, and costs associated with osteoporotic fractures in the U.S. are significant,(1–6) and the prevalence of osteoporosis and fractures are projected to increase with the aging of the U.S. population in upcoming years.(2) Despite the high prevalence and health impact of osteoporosis and ample evidence and guidelines supporting osteoporosis screening and treatment for older women and men,(7–12) screening and treatment rates remain low in the U.S. For example, studies have shown that fewer than 30% of women over the age of 65 with a known diagnosis of osteoporosis receive treatment,(13,14) and only 23% of women age 50 and older who sustain an osteoporotic fracture receive treatment within the first year after fracture.(15,16) A recent large study that analyzed insurance claims data of more than 1.5 million U.S. women age 50 and older found that fewer than 1 in 4 had osteoporosis screening within the most recent 2-year continuous enrollment period.(17) Osteoporosis screening and treatment rates are even lower for older men in the U.S..(18–20) One study found that fewer than 20% of men age 70–75 received screening with dual-energy x-ray absorptiometry (DXA).(18) A large cohort study found that only 15% of men age 50 and older underwent diagnostic testing and/or initiated pharmacotherapy within one year following a fragility fracture.(19)
Given the large gaps between recommended screening and treatment practices for osteoporosis and current clinical practice, a number of studies have evaluated different types of quality improvement strategies to improve osteoporosis screening and/or treatment rates in clinical practice. We performed a systematic review and meta-analysis of randomized clinical trials that have evaluated the efficacy of osteoporosis quality improvement strategies to improve screening and/or treatment initiation rates.
Materials and Methods
Data sources and search strategies
We developed literature search strategies for PubMed, Embase, and Cochrane Library databases to locate randomized clinical trials evaluating the efficacy of osteoporosis quality improvement strategies. The search strategies were designed to be broad to have high sensitivity for identifying relevant literature, and included search terms for osteoporosis, randomized clinical trials,(21) and different quality improvement strategies. We performed the literature searches on 2/24/17 for PubMed and Cochrane Library databases, and 2/27/17 for Embase. The PubMed search strategy is shown in Supplemental Table 1; the Embase and Cochrane Library search strategies are available upon request. We searched for additional relevant studies by reviewing the reference lists of studies identified with the database search strategies that met our inclusion criteria.
Study selection
We selected relevant studies by applying inclusion and exclusion criteria to the literature retrieved with the search strategies. We included studies in women and men that evaluated an osteoporosis quality improvement strategy; were randomized clinical trials; and reported quantitative measures of the efficacy of the quality improvement strategy compared to a comparator or control strategy on outcomes of osteoporosis screening (bone mineral density (BMD)/DXA testing) rates, treatment (pharmacotherapy) initiation rates, and/or fracture rates. We excluded studies in which the focus was on strategies to improve adherence to osteoporosis pharmacotherapy, as strategies to improve adherence to pharmacotherapy were not the focus of this review. We also excluded studies in which the treatment outcome was calcium and/or vitamin D initiation only, as our treatment focus was on initiation of pharmacotherapy. We did not apply any language, country, or patient sociodemographic exclusion criteria. When multiple publications reported data from the same study, we included the publication with the most complete data and excluded publications reporting duplicate data. Studies were reviewed for inclusion or exclusion in two stages – first, titles and abstracts were assessed, and then studies identified as possibly relevant by title/abstract screen received full-text review.
Data extraction
We extracted relevant information from eligible studies, including number of participants; participant sociodemographic characteristics; study location/setting; year of publication; funding sources; quality improvement strategy or strategies evaluated; comparator or control strategy evaluated; relevant outcome(s) evaluated; outcome results for intervention and comparator groups; and information about potential sources of bias.
Data analysis
We performed random effects meta-analysis using the DerSimonian and Laird method(22) to calculate summary estimates of effect size (relative risk and risk difference) for each type of quality improvement strategy for which two or more studies reported the same outcome measure(s), and reported the number of study participants who did and did not experience the outcome(s) of interest in the intervention and comparator groups (2 × 2 table values); or provided enough information to calculate these numbers. We performed separate meta-analyses for studies that based inclusion on a recent or prior fracture history, and studies that included patients who did not have a prior fracture. We preferentially used intention-to-treat analysis data from studies when available. We assessed between-study heterogeneity in each performed meta-analysis with I2 values. When three or more studies were included in a meta-analysis, we additionally performed influence (sensitivity) analysis to assess whether meta-analysis summary estimates were sensitive to removal of individual studies. We used Stata version 11.0 (StataCorp, College Station, TX) to perform all meta-analyses.
In addition to performing meta-analyses, we qualitatively described findings and characteristics of included studies and study quality. To assess study quality we applied measures recommended by the Cochrane Collaboration to assess risk of bias in domains of performance bias, selection bias, detection bias, reporting bias, and attrition bias.(23)
Results
Literature search and study selection
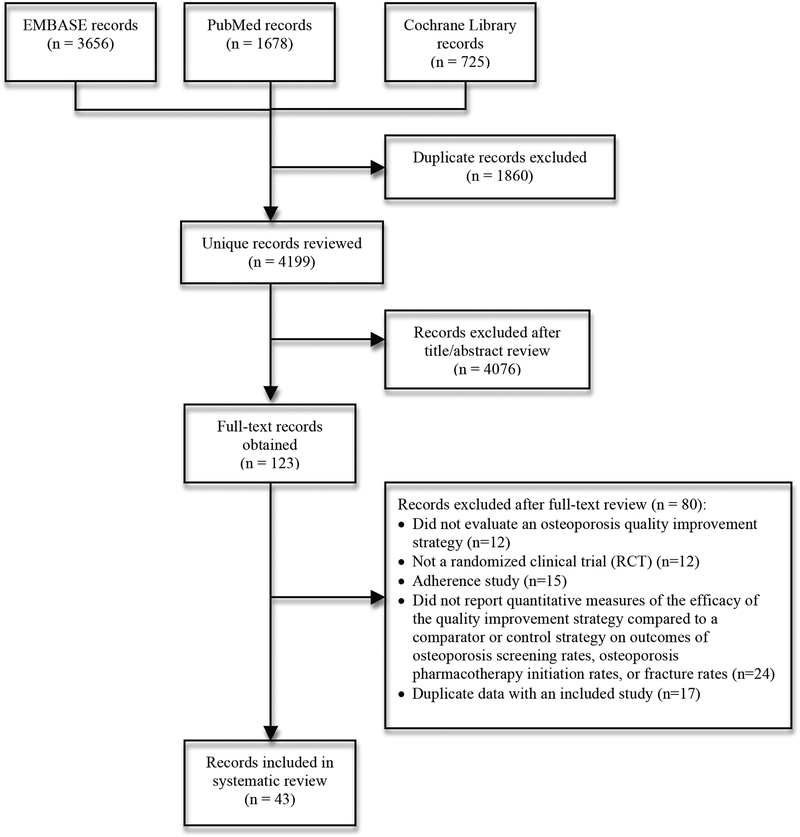
A total of 4199 unique records (citations) were identified with the literature search strategies, of which 43 met inclusion criteria.(24–66) A flow diagram of the literature search and inclusion/exclusion process is shown in Figure 1.
Figure 1.
Flow diagram of literature search and study selection.
Study characteristics
The characteristics of included studies are shown in Supplemental Table 2. The studies were published between 2004 and 2017, a large majority (84%) were performed in North America (24 (56%) in the United States and 12 (28%) in Canada), and number of patients in included studies ranged from 46 to 13,455. A variety of different types of quality improvement strategies were evaluated, including patient education, patient activation, provider education, provider reminders, provider notification, fracture liaison services/case management, orthopedic surgeon or fracture clinic initiation of osteoporosis workup, pharmacist interventions, patient self-referral for screening, and multifaceted interventions, among others. In a majority of studies, patients rather than providers were the unit of randomization. Approximately half of the studies evaluated quality improvement strategies for patient populations composed entirely of individuals with recent or prior fracture. Most studies reported outcomes of osteoporosis treatment and/or screening (BMD/DXA testing); only four reported fracture outcomes.(28,29,40,61) Almost all included studies compared quality improvement strategies to control or usual care rather than an active comparator. Approximately two-thirds of studies reported a significant positive impact of an intervention on at least one outcome measure of interest. About one-third of studies reported pharmaceutical company funding.(25,27–29,35–38,43,50,56–58,60,64)
Study quality and potential sources of bias
Study quality assessment findings are shown in Supplemental Table 3. In every bias domain category evaluated, most studies were assessed as having unclear risk of bias. The bias domain category in which the greatest number of studies (14 or 33% of all studies) were assessed as having low risk of bias was attrition bias due to incomplete outcome data; for other bias domains, even fewer studies were assessed as having low risk of bias. Almost all included studies received a summary assessment of unclear risk of bias, due to an unclear risk of bias in at least one bias domain category. No study received a summary assessment of low risk of bias.
Meta-analyses
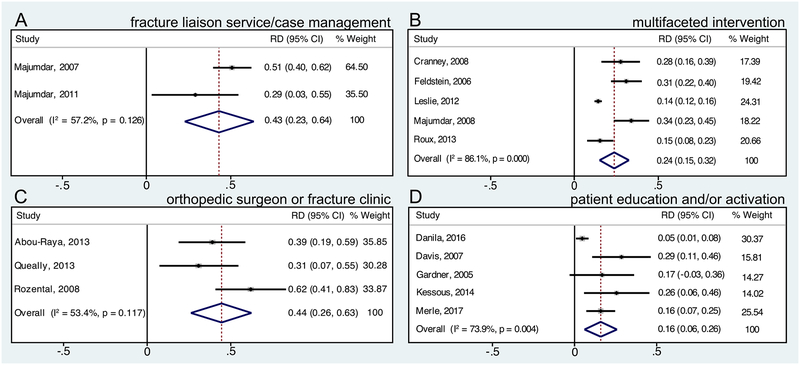
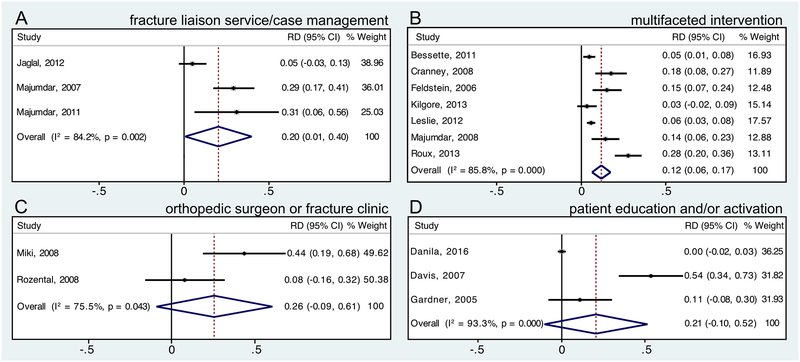
We performed meta-analyses for several osteoporosis quality improvement strategy categories for outcomes of osteoporosis screening (BMD/DXA testing), osteoporosis treatment (pharmacotherapy), and/or screening or treatment. Table 1 shows meta-analysis results for the outcome of BMD/DXA testing, and Figure 2 shows meta-analysis forest plots for the outcome of BMD/DXA testing for studies with patient populations with recent or prior fracture. Table 2 shows meta-analysis results for the outcome of osteoporosis treatment (pharmacotherapy), and Figure 3 shows meta-analysis forest plots for the outcome of osteoporosis treatment for studies with patient populations with recent or prior fracture. Table 3 shows meta-analysis results for the outcome of BMD testing and/or osteoporosis treatment.
Table 1.
Meta-Analysis Results, Bone Mineral Density (BMD)/Dual-Energy X-ray Absorptiometry (DXA) Testing Outcome
| Type of quality improvement strategy | Included studies | Summary estimate of relative risk (RR)a (95%CI; I2 valueb) | Summary estimate of risk difference (RD)a (95%CI; I2 valueb) |
|---|---|---|---|
| Meta-analysis findings for studies in which all patients had recent or prior fracture | |||
| Fracture liaison service/case management | Majumdar 2007,(46) Majumdar 2011(48) | 2.11 (1.19–3.74; I2=78.9%) | 0.43 (0.23–0.64; I2=57.2%) |
| Multifaceted intervention targeting providers and patients | Cranney 2008,(32) Feldstein 2006,(36) Leslie 2012,(44) Majumdar 2008,(47) Roux 2013c(56) | 3.05 (1.69–5.50; I2=92.9%)d | 0.24 (0.15–0.32; I2=86.1%)d |
| Orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management | Abou-Raya 2013,(24) Queally 2013,(55) Rozental 2008(57) | 2.26 (1.65–3.08; I2=0.0%)d | 0.44 (0.26–0.63; I2=53.4%)d |
| Patient education and/or activation | Danila 2016,(34) Davis 2007,(35) Gardner 2005,(37) Kessous 2014,(41) Merle 2017(51) | 1.61 (1.07–2.43; I2=76.8%)e | 0.16 (0.06–0.26; I2=73.9%)d |
| Meta-analysis findings for studies that included patients without prior fracture | |||
| Multifaceted intervention targeting providers and patientsf | Solomon 2007,(60) Solomon 2007(61) |
1.17 (0.80–1.71; I2=82.7%) | 0.02 (−0.02–0.06; I2=86.0%) |
| Patient education and/or activationg | Heyworth 2014,(38) Solomon 2007(61) | 1.11 (0.98–1.25; I2=0.0%) | 0.01 (0.00–0.02; I2=0.0%) |
| Patient self-scheduling of DXA plus education | Warriner 2012,(64) Warriner 2014(65) | 3.36 (2.74–4.12; I2=61.6%) | 0.13 (0.07–0.18; I2=95.1%) |
| Pharmacist initiation of screening | McConaha 2014,(49) Yuksel 2010(66) | 1.36 (0.52–3.58; I2=81.0%) | 0.06 (−0.12–0.23; I2=57.8%) |
Random-effects meta-analysis using DerSimonian and Laird method; results for intervention compared to comparator/control
Percentage of variation across studies attributable to heterogeneity
For Roux et al. study(56), results from both intervention groups combined were used for meta-analysis
Significance of meta-analysis findings robust to removal of individual studies in influence/sensitivity analysis
Significance of meta-analysis findings sensitive to removal of Gardner et al.(37), Kessous et al.(41), or Merle et al.(51) studies in influence/sensitivity analysis
Lafata et al. study(43) could not be included due to lack of clarity on 2×2 table values from reporting in paper. Contacted author but numbers were still unclear. However, if we assumed all randomized patients in Lafata et al. study(43) were included in osteoporosis screening analysis, meta-analysis results for RR and RD would still not be significant.
Lafata et al. study(43) could not be included due to lack of clarity on 2×2 table values from reporting in paper. Contacted author but numbers were still unclear. However, if we assumed all randomized patients in Lafata et al. study(43) were included in osteoporosis screening analysis, meta-analysis results for RR and RD would still not be significant.
Figure 2.
Forest plots of risk difference (RD) of bone mineral density (BMD)/dual-energy X-ray absorptiometry (DXA) testing for studies in which all patients had recent or prior fracture. Forest plots for quality improvement strategies of (A) fracture liaison service/case management, (B) multifaceted intervention targeting providers and patients, (C) orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management, and (D) patient education and/or activation.
Table 2.
Meta-Analysis Results, Osteoporosis Treatment (Pharmacotherapy) Outcome
| Type of quality improvement strategy | Included studies | Summary estimate of relative risk (RR)a (95%CI; I2 valueb) | Summary estimate of risk difference (RD)a (95%CI; I2 valueb) |
|---|---|---|---|
| Meta-analysis findings for studies in which all patients had recent or prior fracture | |||
| Fracture liaison service/case management | Jaglal 2012,(39) Majumdar 2007,(46) Majumdar 2011(48) | 1.94 (1.15–3.27; I2=62.1%)c | 0.20 (0.01–0.40; I2=84.2%)c |
| Multifaceted intervention targeting providers and patients | Bessette 2011,(25) Cranney 2008,(32) Feldstein 2006,(36) Kilgore 2013,(42) Leslie 2012,(44) Majumdar 2008,(47) Roux 2013d(56) | 1.95 (1.50–2.55; I2=67.0%)e | 0.12 (0.06–0.17; I2=85.8%)e |
| Orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management | Miki 2008,(52) Rozental 2008(57) | 1.99 (1.19–3.32; I2=0.0%) | 0.26 (−0.09–0.61; I2=75.5%) |
| Patient education and/or activation | Danila 2016,(34) Davis 2007,(35) Gardner 2005(37) | 1.64 (0.65–4.15; I2=66.8%)e | 0.21 (−0.10–0.52; I2=93.3%)e |
| Meta-analysis findings for studies that included patients without prior fracture | |||
| Multifaceted intervention targeting providers and patientsf | Ciaschini 2010,(27) Solomon 2007,(60) Solomon 2007(61) | 1.44 (0.90–2.30; I2=81.7%)g | 0.02 (−0.02–0.06; I2=85.6%)e |
| Patient education and/or activationh | Cram 2006,(30) Heyworth 2014,(38) Solomon 2007(61) | 0.93 (0.82–1.06; I2=0.0%)e | −0.01 (−0.02–0.00; I2=0.0%)e |
| Pharmacist initiation of screening | McConaha 2014,(49) Yuksel 2010(66) | 2.62 (0.76–9.00; I2=0.0%) | 0.05 (−0.02–0.12; I2=46.3%) |
Random-effects meta-analysis using DerSimonian and Laird method; results for intervention compared to comparator/control
Percentage of variation across studies attributable to heterogeneity
Significance of meta-analysis findings sensitive to removal of Majumdar et al. 2007(46) or Majumdar et al. 2011(48) studies in influence/sensitivity analysis
For Roux et al. study(56), results from both intervention groups combined were used for meta-analysis
Significance of meta-analysis findings robust to removal of individual studies in influence/sensitivity analysis
Lafata et al. study(43) could not be included due to lack of clarity on 2×2 table values from reporting in paper. Contacted author but numbers were still unclear. However, if we assume all randomized patients in Lafata et al. study(43) included in osteoporosis treatment analysis, meta-analysis results for RR would be significant, but RD meta-analysis results would remain not significant.
Significance of meta-analysis findings sensitive to removal of Solomon et al. 2007 study(61) in influence/sensitivity analysis
Lafata et al. study(43) could not be included due to lack of clarity on 2×2 table values from reporting in paper. Contacted author but numbers were still unclear. However, if we assume all randomized patients in Lafata et al. study(43) included in osteoporosis treatment analysis, meta-analysis results for RR and RD would still not be significant.
Figure 3.
Forest plots of risk difference (RD) of osteoporosis treatment (pharmacotherapy) for studies in which all patients had recent or prior fracture. Forest plots for quality improvement strategies of (A) fracture liaison service/case management, (B) multifaceted intervention targeting providers and patients, (C) orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management, and (D) patient education and/or activation.
Table 3.
Meta-Analysis Results, Bone Mineral Density (BMD) Testing and/or Osteoporosis Treatment Outcome
| Type of quality improvement strategy | Included studies | Summary estimate of relative risk (RR)a (95%CI; I2 valueb) | Summary estimate of risk difference (RD)a (95%CI; I2 valueb) |
|---|---|---|---|
| Meta-analysis findings for studies in which all patients had recent or prior fracture | |||
| Fracture liaison service/case managementc | Jaglal 2012,(39) Majumdar 2007,(46) Majumdar 2011(48) | 2.05 (1.50–2.81; I2=36.3%)d | 0.27 (0.06–0.48; I2=85.6%)e |
| Multifaceted intervention targeting providers and patients | Feldstein 2006,(36) Leslie 2012,(44) Olegario 2012(53) | 2.44 (1.63–3.65; I2=84.3%)f | 0.19 (0.09–0.29; I2=93.4%)f |
| Meta-analysis findings for studies that included patients without prior fracture | |||
| Multifaceted intervention targeting providers and patients | Solomon 2007,(60) Solomon 2007(61) | 1.19 (0.83–1.71; I2=85.5%) | 0.02 (−0.02–0.06; I2=86.4%) |
Random-effects meta-analysis using DerSimonian and Laird method; results for intervention compared to comparator/control
Percentage of variation across studies attributable to heterogeneity
Meta-analysis results reported in this row for fracture liaison service/case management studies are for outcome of “appropriate management”, defined in included studies as BMD testing and treatment if bone mass low
Significance of meta-analysis findings robust to removal of individual studies in influence/sensitivity analysis
Significance of meta-analysis findings sensitive to removal of Majumdar et al. 2011 study(48) in influence/sensitivity analysis
Significance of meta-analysis findings sensitive to removal of Leslie et al. study(44) in influence/sensitivity analysis
Meta-analyses findings for osteoporosis quality improvement strategies in studies with patient populations composed of individuals with recent or prior fracture
Meta-analysis findings demonstrated that fracture liaison service/case management interventions and multifaceted interventions targeting providers and patients significantly increased BMD testing and osteoporosis treatment outcomes in patient populations with recent or prior fracture. The multifaceted interventions in most of the studies included in these analyses were composed of patient education plus provider education, notification, and/or reminders. Meta-analysis findings for orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management demonstrated significant efficacy for increasing the outcome of BMD testing (both relative risk and risk difference) and the relative risk (but not risk difference) of osteoporosis treatment compared to control/comparator. Meta-analysis findings for patient education and/or activation in studies with patients with recent or prior fracture demonstrated significant efficacy for increasing BMD testing, but not osteoporosis treatment. The summary estimates of relative risk and risk difference (absolute difference) for different quality improvement strategies compared to control/comparator were generally larger for the outcome of BMD testing than osteoporosis treatment; and summary estimates of risk difference for quality improvement strategies on these outcomes were sizable, although confidence intervals were wide. For example, summary estimates of risk difference were as large as 44% (95% CI 26%–63%) for orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management for the outcome of BMD testing, 27% (95% CI 6%–48%) for fracture liaison service/case management for the outcome of “appropriate management” (BMD testing and treatment if low BMD), and 20% (95% CI 1%–40%) for fracture liaison service/case management for the outcome of osteoporosis treatment.
The meta-analysis findings for quality improvement strategies in studies composed of patients with recent or prior fracture were limited by the small number of studies, ranging from two to seven, included in each analysis. Furthermore, a number of studies included in these meta-analyses had relatively small sample sizes, including all studies in the analyses for orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management. Additionally, influence analysis showed that the significance of the findings of several of the meta-analyses were sensitive to removal of individual studies. Furthermore, there was significant between-study heterogeneity in most of the meta-analyses performed, with I2 values indicating moderate or high heterogeneity.
Meta-analyses findings for osteoporosis quality improvement strategies in studies with patient populations including individuals without prior fracture
We performed meta-analyses for several types of quality improvement strategies for studies that included participants without a prior fracture. These meta-analyses were limited by the small number of studies (two or three) included in each analysis. The only quality improvement strategy for which meta-analysis findings demonstrated significant improvement of osteoporosis care for these populations was patient self-scheduling of DXA plus education, for improving the BMD testing outcome, with a summary estimate of risk difference compared to control of 13% (95% CI 7%–18%). However, I2 values for this analysis demonstrated that the two included studies were highly heterogeneous.
Meta-analysis findings for quality improvement strategies of multifaceted interventions targeting providers and patients, patient education and/or activation, and pharmacist initiation of screening in studies including patients without prior fractures did not demonstrative significant efficacy for increasing BMD testing or osteoporosis treatment. However, influence (sensitivity) and additional analyses showed that removal of the Solomon et al. 2007 study(61), or inclusion of the Lafata et al. study(43) with the assumption that all randomized patients were included in their analysis, resulted in significant meta-analysis findings for efficacy of multifaceted interventions targeting providers and patients on the outcome of relative risk (but not risk difference) of osteoporosis treatment. I2 values indicated high between-study heterogeneity in the meta-analyses of studies that evaluated multifaceted interventions targeting providers and patients; between-study heterogeneity ranging from low to high in the meta-analyses of studies that evaluated pharmacist initiation of screening, and low between-study heterogeneity in the meta-analyses for patient education and/or activation interventions.
Discussion
Our systematic review identified 43 randomized clinical trials that have evaluated the efficacy of a variety of different types of quality improvement strategies to improve osteoporosis care. Our meta-analysis findings indicate that a number of strategies appear to be efficacious for increasing BMD/DXA testing and/or osteoporosis treatment rates for patients with recent or prior fracture, including fracture liaison service/case management, multifaceted interventions targeting providers and patients, orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management, and patient education and/or activation. For populations including individuals without prior fracture, the only intervention for which meta-analysis results showed significant improvement in osteoporosis care was patient self-scheduling of DXA plus education, which increased BMD testing rates. For efficacious quality improvement strategies, summary estimates of risk difference (absolute difference) for outcomes of BMD/DXA testing or osteoporosis treatment were relatively large compared to control/comparator; however, confidence intervals were wide. Furthermore, summary estimates of risk difference were generally larger for the outcome of BMD/DXA testing than treatment.
With respect to strategies to improve care for patients with recent or prior fracture, orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management and fracture liaison service/case management were the interventions with the largest meta-analysis summary estimates of risk difference (absolute difference) for the outcome of BMD/DXA testing compared to comparator/control, with summary estimates of risk difference of 44% (95% CI 26%–63%) and 43% (95% CI 23%–64%), respectively. Meta-analysis results for fracture liaison service/case management also demonstrated a significant increase in the outcome of osteoporosis treatment, with a summary estimate of risk difference compared to comparator/control of 20% (95% CI 1%–40%). Meta-analysis of studies evaluating orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or treatment demonstrated statistically significant increased relative risk but not risk difference of osteoporosis treatment compared to comparator/control; although the summary estimate of risk difference was sizable (26%), the 95% confidence interval was very wide and crossed zero (−9%–61%). Meta-analysis of studies evaluating multifaceted interventions targeting providers and patients for populations with recent or prior fracture demonstrated sizable and significant increases in BMD testing (summary estimate of risk difference 24%, 95% CI 15%–32%) and osteoporosis treatment (summary estimate of risk difference 12%, 95% CI 6%–17%) compared to comparator/control; the studies included in these meta-analyses that showed significant findings tended to have interventions composed of a provider reminder or notification, plus patient education.(32,36,44,47,53,56) Meta-analysis of studies that evaluated patient education and/or activation strategies in populations with recent or prior fracture demonstrated significant efficacy for improving BMD/DXA testing (summary estimate of risk difference 16%, 95%CI 6%–26%) but not osteoporosis treatment compared to comparator/control; although the summary estimate of risk difference for treatment was sizable (21%), the 95% confidence interval was wide and crossed zero (−10%–52%).
With respect to quality improvement strategies in patient populations including individuals without prior fracture, the only intervention for which meta-analysis results demonstrated significant improvement was patient self-scheduling of DXA plus education, which increased BMD testing rates compared to control (summary estimate of risk difference 13%, 95% CI 7%–18%). Meta-analysis findings for strategies of multifaceted interventions targeting providers and patients, patient education and/or activation, and pharmacist initiation of screening in populations that included individuals without prior fracture did not show statistically significant improvement in outcomes of BMD/DXA testing or osteoporosis treatment. Furthermore, although we did not have enough similar studies that evaluated provider education and/or audit and feedback to perform meta-analyses for these strategies, qualitative review of studies with interventions composed primarily of these strategies revealed that almost all of the studies did not find significant improvement in BMD testing and/or osteoporosis treatment outcomes.(28,29,33,40,58,61)
Our meta-analysis findings were limited by the small number of studies included in each analysis; relatively small sample sizes of several studies; generally high between-study heterogeneity in most of the analyses performed; sensitivity of several of the meta-analyses findings to individual studies; and unclear risk of bias of the studies. Possible sources of between-study heterogeneity include different patient populations and settings; for example, studies performed in different countries (e.g., U.S. and Canada) and within different types of healthcare systems (e.g., systems in which incentives to screen and treat patients for osteoporosis are aligned, such as single payer systems, and systems in which incentives are not aligned). Given the challenges of performing meta-analysis with a wide variety of types of quality improvement strategies, we performed separate analyses for groups of studies with similar intervention characteristics, and did not feel it was appropriate to perform meta-analyses with larger groups of studies including more disparate intervention types. Another limitation of our review is that publication bias may have led to positive studies being more likely to be published, thus biasing our findings in favor of the efficacy of the interventions.
Despite the limitations of the current body of evidence and our systematic review, our findings suggest that several types of interventions may have a sizable impact on improving BMD testing and/or treatment rates for patient populations with recent or prior fracture, including fracture liaison services/case management, multifaceted interventions that include provider reminders and/or notification plus patient education, orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management, and patient education and/or activation. Furthermore, patient self-scheduling of DXA appears to be an efficacious strategy to increase DXA testing rates in patient populations that include individuals without prior fracture. Additional high-quality RCTs evaluating the osteoporosis quality improvement strategies that our analyses suggested would be beneficial would help further clarify the expected impact of each of these strategies on osteoporosis screening rates, treatment rates, and fracture rates; the specific features of efficacious strategies; and how the impact of these strategies may vary in different patient populations and settings. Moreover, additional RCTs to evaluate other types of quality improvement strategies not included in our meta-analyses to improve osteoporosis care for patients without prior fracture would be welcome, given that our meta-analysis findings only demonstrated the effectiveness of one of the evaluated quality improvement strategies, patient self-scheduling of DXA, to improve screening rates in this population. One relatively large non-randomized controlled trial by Loo et al. suggested that panel management may be an effective strategy to improve osteoporosis screening rates;(67) it may be worthwhile to evaluate this type of strategy in a randomized controlled trial.
Our study is the most comprehensive systematic review and meta-analysis of randomized clinical trials of osteoporosis quality improvement strategies to date. A systematic review and meta-analysis of randomized controlled trials of interventions to improve post-fracture care for patients at risk for osteoporosis by Little et al. published in 2010 included nine RCTs evaluating a variety of interventions.(68) Little et al. performed meta-analyses that included all studies regardless of the specific type of intervention they evaluated, and found that interventions collectively significantly improved BMD testing and osteoporosis treatment rates.(68) Our systematic review included 43 studies, and we performed separate meta-analyses for different types of quality improvement interventions. Furthermore, we also included studies evaluating osteoporosis quality improvement strategies for populations that included individuals without prior fracture.
In conclusion, a number of strategies appear to be efficacious for improving BMD/DXA testing and/or osteoporosis treatment rates in patient populations with recent or prior fracture, including fracture liaison service/case management, multifaceted interventions including provider reminders and/or notification plus patient education, orthopedic surgeon or fracture clinic initiation of osteoporosis evaluation or management, and patient education and/or activation, with potentially sizable impact. For populations that include individuals without prior fracture, patient self-scheduling of DXA appears to be an efficacious strategy to increase DXA testing rates. Given the current body of evidence, these would be worthwhile strategies to enact to try to improve osteoporosis screening and/or treatment rates for relevant patient populations. Additional high-quality RCTs evaluating the osteoporosis quality improvement strategies that our analyses suggested would be beneficial would be helpful to further clarify the expected impact of each of these strategies on osteoporosis screening rates, treatment rates, and fracture rates; the specific features of efficacious strategies; and how the impact of these strategies may vary in different patient populations and settings. Furthermore, additional RCTs to evaluate other types of quality improvement strategies not included in our meta-analyses to improve osteoporosis care for patients without prior osteoporotic fracture would be useful to identify additional beneficial strategies for this population.
Supplementary Material
Acknowledgements
Sources of funding:
Drs. Nayak and Greenspan were supported by grant 1R21AR072930–01 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Greenspan was also supported by NIH grants P30AG024827 and K07AG052668 from the National Institute on Aging.
Footnotes
Supplemental data:
Supplemental data has been included with the submission.
Disclosures:
Drs. Nayak and Greenspan have no conflicts of interests.
References
- 1.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General, 2004. [Google Scholar]
- 3.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17(1):1–45. [PubMed] [Google Scholar]
- 4.Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res. 2004;(425):126–34. [PubMed] [Google Scholar]
- 5.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22(6):781–8. [DOI] [PubMed] [Google Scholar]
- 6.Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22(6):1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–64. [DOI] [PubMed] [Google Scholar]
- 8.Qaseem A, Forciea MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med. 2017;166(11):818–39. [DOI] [PubMed] [Google Scholar]
- 9.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis: executive summary of recommendations. Endocr Pract. 2010;16(6):1016–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–22. [DOI] [PubMed] [Google Scholar]
- 11.Lim LS, Hoeksema LJ, Sherin K. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med. 2009;36(4):366–75. [DOI] [PubMed] [Google Scholar]
- 12.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siris ES, Yu J, Bognar K, DeKoven M, Shrestha A, Romley JA, et al. Undertreatment of osteoporosis and the role of gastrointestinal events among elderly osteoporotic women with Medicare Part D drug coverage. Clin Interv Aging. 2015;10:1813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Sajjan S, Modi A. High rate of non-treatment among osteoporotic women enrolled in a US Medicare plan. Curr Med Res Opin. 2016;32(11):1849–56. [DOI] [PubMed] [Google Scholar]
- 15.Wilk A, Sajjan S, Modi A, Fan CP, Mavros P. Post-fracture pharmacotherapy for women with osteoporotic fracture: analysis of a managed care population in the USA. Osteoporos Int. 2014;25(12):2777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillespie CW, Morin PE. Osteoporosis-Related Health Services Utilization Following First Hip Fracture Among a Cohort of Privately-Insured Women in the United States, 2008–2014: An Observational Study. J Bone Miner Res. 2017;32(5):1052–61. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie CW, Morin PE. Trends and Disparities in Osteoporosis Screening Among Women in the United States, 2008–2014. Am J Med. 2017;130(3):306–16. [DOI] [PubMed] [Google Scholar]
- 18.Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311–3. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian A, Tosi LL, Lane JM, Dirschl DR, Ho PR, O’Malley CD. Declining rates of osteoporosis management following fragility fractures in the U.S., 2000 through 2009. J Bone Joint Surg Am. 2014;96(7):e52. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd AJ, Cass AR, Ray LA, Tan A, Wilkinson GS. Treatment for older men with fractures. Osteoporos Int. 2012;23(3):1041–51. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Box 6.4.b: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity- and precision-maximizing version (2008 revision); PubMed format. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou-Raya S, Abou-Raya A, Khadrawi T. A randomized controlled trial of early initiation of osteoporosis assessment and management in the acute setting of the fracture clinic. Annals of the rheumatic diseases [serial on the Internet]. 2013. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/507/CN-01011507/frame.html. [Google Scholar]
- 25.Bessette L, Davison KS, Jean S, Roy S, Ste-Marie LG, Brown JP. The impact of two educational interventions on osteoporosis diagnosis and treatment after fragility fracture: a population-based randomized controlled trial. Osteoporos Int. 2011;22(12):2963–72. [DOI] [PubMed] [Google Scholar]
- 26.Bliuc D, Eisman JA, Center JR. A randomized study of two different information-based interventions on the management of osteoporosis in minimal and moderate trauma fractures. Osteoporos Int. 2006;17(9):1309–17. [DOI] [PubMed] [Google Scholar]
- 27.Ciaschini PM, Straus SE, Dolovich LR, Goeree RA, Leung KM, Woods CR, et al. Community based intervention to optimize osteoporosis management: randomized controlled trial. BMC Geriatr. 2010;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colón-Emeric CS, Lyles KW, House P, Levine DA, Schenck AP, Allison J, et al. Randomized trial to improve fracture prevention in nursing home residents. Am J Med. 2007;120(10):886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox H, Puffer S, Morton V, Cooper C, Hodson J, Masud T, et al. Educating nursing home staff on fracture prevention: a cluster randomised trial. Age Ageing. 2008;37(2):167–72. [DOI] [PubMed] [Google Scholar]
- 30.Cram P, Schlechte J, Christensen A. A randomized trial to assess the impact of direct reporting of DXA scan results to patients on quality of osteoporosis care. J Clin Densitom. 2006;9(4):393–8. [DOI] [PubMed] [Google Scholar]
- 31.Cram P, Wolinsky FD, Lou Y, Edmonds SW, Hall SF, Roblin DW, et al. Patient-activation and guideline-concordant pharmacological treatment after bone density testing: the PAADRN randomized controlled trial. Osteoporos Int. 2016;27(12):3513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cranney A, Lam M, Ruhland L, Brison R, Godwin M, Harrison MM, et al. A multifaceted intervention to improve treatment of osteoporosis in postmenopausal women with wrist fractures: a cluster randomized trial. Osteoporos Int. 2008;19(12):1733–40. [DOI] [PubMed] [Google Scholar]
- 33.Curtis JR, Westfall AO, Allison J, Becker A, Melton ME, Freeman A, et al. Challenges in improving the quality of osteoporosis care for long-term glucocorticoid users: a prospective randomized trial. Arch Intern Med. 2007;167(6):591–6. [DOI] [PubMed] [Google Scholar]
- 34.Danila MI, Outman RC, Rahn EJ, Mudano AS, Redden DT, Li P, et al. The Activating Patients at Risk for Osteoporosis Study: A Randomized Trial within the Global Longitudinal Study of Osteoporosis in Women Cohort. Arthritis Rheumatol. 2016; 68 (supp 10). http://acrabstracts.org/abstract/the-activating-patients-at-risk-for-osteoporosis-study-a-randomized-trial-within-the-global-longitudinal-study-of-osteoporosis-in-women-cohort/. [Google Scholar]
- 35.Davis JC, Guy P, Ashe MC, Liu-Ambrose T, Khan K. HipWatch: osteoporosis investigation and treatment after a hip fracture: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(8):888–91. [DOI] [PubMed] [Google Scholar]
- 36.Feldstein A, Elmer PJ, Smith DH, Herson M, Orwoll E, Chen C, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450–7. [DOI] [PubMed] [Google Scholar]
- 37.Gardner MJ, Brophy RH, Demetrakopoulos D, Koob J, Hong R, Rana A, et al. Interventions to improve osteoporosis treatment following hip fracture. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(1):3–7. [DOI] [PubMed] [Google Scholar]
- 38.Heyworth L, Kleinman K, Oddleifson S, Bernstein L, Frampton J, Lehrer M, et al. Comparison of interactive voice response, patient mailing, and mailed registry to encourage screening for osteoporosis: a randomized controlled trial. Osteoporos Int. 2014. May;25(5):1519–26. [DOI] [PubMed] [Google Scholar]
- 39.Jaglal SB, Donescu OS, Bansod V, Laprade J, Thorpe K, Hawker G, et al. Impact of a centralized osteoporosis coordinator on post-fracture osteoporosis management: a cluster randomized trial. Osteoporos Int. 2012;23(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy CC, Ioannidis G, Thabane L, Adachi JD, Marr S, Giangregorio LM, et al. Successful knowledge translation intervention in long-term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials. 2015;16:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kessous R, Weintraub AY, Mattan Y, Dresner-Pollak R, Brezis M, Liebergall M, et al. Improving compliance to osteoporosis workup and treatment in postmenopausal patients after a distal radius fracture. Taiwan J Obstet Gynecol. 2014;53(2):206–9. [DOI] [PubMed] [Google Scholar]
- 42.Kilgore ML, Outman R, Locher JL, Allison JJ, Mudano A, Kitchin B, et al. Multimodal intervention to improve osteoporosis care in home health settings: results from a cluster randomized trial. Osteoporos Int. 2013;24(10): 2555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lafata JE, Kolk D, Peterson EL, McCarthy BD, Weiss TW, Chen YT, et al. Improving osteoporosis screening: results from a randomized cluster trial. J Gen Intern Med. 2007;22(3):346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leslie WD, LaBine L, Klassen P, Dreilich D, Caetano PA. Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184(3):290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy BT, Hartz A, Woodworth G, Xu Y, Sinift S. Interventions to improving osteoporosis screening: an Iowa Research Network (IRENE) study. J Am Board Fam Med. 2009;22(4):360–7. [DOI] [PubMed] [Google Scholar]
- 46.Majumdar SR, Beaupre LA, Harley CH, Hanley DA, Lier DA, Juby AG, et al. Use of a case manager to improve osteoporosis treatment after hip fracture: results of a randomized controlled trial. Arch Intern Med. 2007;167(19):2110–5. [DOI] [PubMed] [Google Scholar]
- 47.Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178(5):569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majumdar SR, Johnson JA, Bellerose D, McAlister FA, Russell AS, Hanley DA, et al. Nurse case-manager vs multifaceted intervention to improve quality of osteoporosis care after wrist fracture: randomized controlled pilot study. Osteoporosis Int. 2011;22(1):223–30. [DOI] [PubMed] [Google Scholar]
- 49.McConaha JL, Berdine HJ, Skomo ML, Laux RV, Higginbotham SK, O’Neil CK. Impact of the fracture risk assessment on patient and physician behavior in osteoporosis prevention. J Pharm Pract. 2014;27(1):25–30. [DOI] [PubMed] [Google Scholar]
- 50.McDonough RP, Doucette WR, Kumbera P, Klepser DG. An evaluation of managing and educating patients on the risk of glucocorticoid-induced osteoporosis. Value Health. 2005;8(1):24–31. [DOI] [PubMed] [Google Scholar]
- 51.Merle B, Chapurlat R, Vignot E, Thomas T, Haesebaert J, Schott AM. Post-fracture care: do we need to educate patients rather than doctors? The PREVOST randomized controlled trial. Osteoporos Int. 2017;28(5):1549–58. [DOI] [PubMed] [Google Scholar]
- 52.Miki RA, Oetgen ME, Kirk J, Insogna KL, Lindskog DM. Orthopaedic management improves the rate of early osteoporosis treatment after hip fracture. A randomized clinical trial. J Bone Joint Surg Am. 2008;90(11):2346–53. [DOI] [PubMed] [Google Scholar]
- 53.Olegario RC, Kjell J, Teng D, Nessim S, Isobe C. Improving osteoporosis outreach effort: A randomized, controlled study of program effectiveness. Journal of managed care pharmacy [serial on the Internet]. 2012. (2):184 Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/239/CN-01004239/frame.html. [Google Scholar]
- 54.Prihar BJ, Katz S. Patient education as a tool to increase screening for osteoporosis. J Am Geriatr Soc. 2008;56(5):961–2. [DOI] [PubMed] [Google Scholar]
- 55.Queally JM, Kiernan C, Shaikh M, Rowan F, Bennett D. Initiation of osteoporosis assessment in the fracture clinic results in improved osteoporosis management: a randomised controlled trial. Osteoporos Int. 2013;24(3):1089–94. [DOI] [PubMed] [Google Scholar]
- 56.Roux S, Beaulieu M, Beaulieu MC, Cabana F, Boire G. Priming primary care physicians to treat osteoporosis after a fragility fracture: an integrated multidisciplinary approach. J Rheumatol. 2013;40(5):703–11. [DOI] [PubMed] [Google Scholar]
- 57.Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953–61. [DOI] [PubMed] [Google Scholar]
- 58.Solomon DH, Katz JN, Tourette AM, Coblyn JS. Multifaceted intervention to improve rheumatologists’ management of glucocorticoid-induced osteoporosis: a randomized controlled trial. Arthritis Rheum. 2004;51(3):383–7. [DOI] [PubMed] [Google Scholar]
- 59.Solomon DH, Finkelstein JS, Polinski JM, Arnold M, Licari A, Cabral D, et al. A randomized controlled trial of mailed osteoporosis education to older adults. Osteoporosis Int. 2006;17(5):760–7. [DOI] [PubMed] [Google Scholar]
- 60.Solomon DH, Polinski JM, Stedman M, Truppo C, Breiner L, Egan C, et al. Improving care of patients at-risk for osteoporosis: a randomized controlled trial. J Gen Intern Med. 2007;22(3):362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon DH, Katz JN, Finkelstein JS, Polinski JM, Stedman M, Brookhart MA, et al. Osteoporosis improvement: a large-scale randomized controlled trial of patient and primary care physician education. J Bone Miner Res. 2007;22(11):1808–15. [DOI] [PubMed] [Google Scholar]
- 62.Stephens MH, Grey A, Fernandez J, Kalluru R, Faasse K, Horne A, et al. 3-D bone models to improve treatment initiation among patients with osteoporosis: A randomised controlled pilot trial. Psychol Health. 2016;31(4):487–97. [DOI] [PubMed] [Google Scholar]
- 63.Tso LS, Loi D, Mosley DG, Yi D, Stockl KM, Lew HC, et al. Evaluation of a Nationwide Pharmacist-Led Phone Outreach Program to Improve Osteoporosis Management in Older Women with Recently Sustained Fractures. J Manag Care Spec Pharm. 2015;21(9):803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warriner AH, Outman RC, Kitchin E, Chen L, Morgan S, Saag KG, et al. A randomized trial of a mailed intervention and self-scheduling to improve osteoporosis screening in postmenopausal women. J Bone Miner Res. 2012;27(12):2603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warriner AH, Outman RC, Feldstein AC, Roblin DW, Allison JJ, Curtis JR, et al. Effect of self-referral on bone mineral density testing and osteoporosis treatment. Med Care. 2014;52(8):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Community pharmacist-initiated screening program for osteoporosis: randomized controlled trial. Osteoporos Int. 2010;21(3):391–8. [DOI] [PubMed] [Google Scholar]
- 67.Loo TS, Davis RB, Lipsitz LA, Irish J, Bates CK, Agarwal K, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552–8. [DOI] [PubMed] [Google Scholar]
- 68.Little EA, Eccles MP. A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci. 2010;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.