Abstract
In colorectal cancers (CRCs) with tumour mismatch repair (MMR) deficiency, genes involved in the host immune response that contain microsatellites in their coding regions, including beta-2-microglobulin (B2M), can acquire mutations that may alter the immune response, tumour progression and prognosis. We screened the coding microsatellites within B2M for somatic mutations in MMR-deficient CRCs and adenomas to determine associations with tumour subtypes, clinicopathological features and survival. Incident MMR-deficient CRCs from Australasian Colorectal Cancer Family Registry (ACCFR) and the Melbourne Collaborative Cohort Study participants (n = 144) and 63 adenomas from 41 MMR gene mutation carriers from the ACCFR were screened for somatic mutations within five coding microsatellites of B2M. Hazard ratios (HR) and 95% confidence intervals (CI) for overall survival by B2M mutation status were estimated using Cox regression, adjusting for age at CRC diagnosis, sex, AJCC stage and grade. B2M mutations occurred in 30 (20.8%) of the 144 MMR-deficient CRCs (29% of the MLH1-methylated, 17% of the Lynch syndrome and 9% of the suspected Lynch CRCs). No B2M mutations were identified in the 63 adenomas tested. B2M mutations differed by site, stage, grade and lymphocytic infiltration although none reached statistical significance (p > 0.05). The HR for overall survival for B2M mutated CRC was 0.65 (95% CI 0.29–1.48) compared with B2M wild-type. We observed differences in B2M mutation status in MMR-deficient CRC by tumour subtypes, site, stage, grade, immune infiltrate and for overall survival that warrant further investigation in larger studies before B2M mutation status can be considered to have clinical utility.
Keywords: B2M, Colorectal cancer, Mismatch repair deficiency, Microsatellite instability, Lynch syndrome, MLH1 methylation
Introduction
Colorectal cancer (CRC) is a leading cause of global morbidity and mortality [1, 2]. Loss of DNA mismatch repair function (MMR-deficiency), identified by high levels of microsatellite instability (MSI) or loss of MMR protein expression, predicts higher survival from CRC [3, 4]. Lynch syndrome is an inherited form of MMR-deficiency that increases risk of CRC, and is caused by a germline mutation in one of the DNA MMR genes (MLH1, MSH2, MSH6 or PMS2). Additionally, mutations in EPCAM, a gene upstream of MSH2, have been shown to disrupt MSH2 expression and predispose to MSH2-deficient cancers [5]. Non-inherited explanations of MMR-deficiency in CRC include somatic hypermethylation of the MLH1 gene promoter (MLH1-methylated) [6] and double somatic MMR gene mutations [7–9]. The suspected Lynch syndrome group comprises those CRCs with an unidentified cause for their tumour MMR-deficiency [10]. Together, these clinically relevant subgroups of MMR-deficient CRC comprise 10–15% of all CRCs [11–13].
MMR-deficient CRCs accumulate high numbers of insertion/deletion somatic mutations (replication errors) at mono- or di-nucleotide repetitive DNA sequences (micro-satellites), and are therefore said to be MSI-High. Micro-satellite sequences are more prevalent in intronic and intergenic regions, although relatively short microsatellite sequences do occur within the coding sequence of some genes. When somatic mutations occur within these coding microsatellites (cMS) during tumourigenesis, altered or truncated protein products can be generated that are recognised by the immune system (neoantigens), thus MMR-deficient tumours can be highly immunogenic [14], and vulnerable to the natural protection afforded by the immune system. On the other hand, cMS mutations can impair the immune system when the gene containing a mutated cMS is part of an immune response pathway [14].
Beta-2-microglobulin (B2M) functions as a component of the human leukocyte antigen (HLA) class I complex, which presents peptides to cytotoxic CD8 + T-cells for cell lysis. B2M contains five short coding microsatellite sequences which have been previously found to acquire somatic mutations in approximately 25% of MMR-deficient CRCs [15–17], but rarely in MMR-proficient tumours [18]. It is hypothesised that when B2M is somatically mutated, tumours have a diminished ability to present antigens through the HLA class I complex, which allows tumours to evade cytotoxic T-cells [18]. Despite this, tumours with B2M somatic mutations have been associated with reduced metastases and disease relapse [16, 17]. Previous studies investigating B2M and prognosis in MMR-deficient CRCs have reported conflicting findings [15, 17, 19] (see Supplementary Table 1), which may have been due to small sample sizes.
In this study, we screened a cohort of MMR-deficient CRCs (n = 144) and adenomas (n = 63) (MMR-deficient adenomas (n = 42)/MMR-proficient adenomas (n = 21)), obtained from the Australian Colorectal Cancer Family Registry (ACCFR) [20] and the Melbourne Collaborative Cohort Study (MCCS) [21], for somatic mutations within the cMS of B2M. We aimed to determine: (i) the prevalence of B2M somatic mutations across three different clinically-relevant subgroups of MMR-deficient CRCs (Lynch syndrome, MLH1-methylated, and suspected Lynch syndrome); (ii) the association of B2M somatic mutations with tumour histopathological features and (iii) whether B2M somatic mutation status is associated with overall survival.
Methods
Study sample
Participants recruited to the MCCS [20] and the population-based recruitment arm of the ACCFR [21] with an incident CRC were assessed for tumour MMR-deficiency by immunohistochemistry for loss of MMR protein expression and MSI analysis as previously described [13, 22]. One hundred and forty four primary tumours from 144 participants had DNA available for somatic mutation screening across both studies. Subsequent tumour and germline characterisation of these MMR-deficient CRC cases was performed to identify tumours caused by: (1) MLH1 methylation; (2) a germline MMR gene mutation (Lynch syndrome) and (3) those with suspected Lynch syndrome; as previously reported [13]. In addition, 63 adenomas (42 MMR-deficient adenomatous polyps and 21 MMR-proficient adenomatous polyps), a subset of which have been previously reported [23], obtained from 41 ACCFR participants recruited from family cancer clinics across Australia [21] and who were proven MMR gene mutation carriers, were also screened for B2M mutations.
MMR-deficient CRCs underwent a standardised histopathology review, by a specialist in gastrointestinal pathology, for tumour site, histological type (adenocarcinoma, mucinous carcinoma, signet ring cell carcinoma), histological grade (for adenocarcinoma only), tumour margin, peritumoural lymphocytes, Crohn’s-like lymphocytic reaction and tumour-infiltrating lymphocytes (TILs); as previously described [13, 24]. Tumours from the ileo-caecal junction through the caecum, ascending colon, hepatic flexure, and transverse colon were grouped as right-sided (proximal). Tumours from the splenic flexure, descending, sigmoid colon and recto-sigmoid junction and rectum were classified as left-sided (distal). The American Joint Committee on Cancer (AJCC) staging system was used to categorise stage: stage I (T1–2, N0, M0), stage II (T3 –4, N0, M0), stage III (Tany, N1–2, M0) and stage IV (Tany, Nany, M1).
B2M somatic mutation detection
Cancerous and adenomatous regions were marked by pathologist (CR) on each H&E slide and regions macro-dissected from unstained sections of formalin-fixed paraffin-embedded tissue to enrich for >70% tumor cellularity. Genomic DNA was extracted from the macrodissected CRC and adenoma tissue using QIAamp DNA Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA was quantified using Qubit HS kit (ThermoFisher Scientific). Five coding microsatellites within B2M (NM_004048: c.37–44, c.188–195, c.200–204, c.272–276 and c.281–285) were assessed for frameshift mutations using standard Sanger sequencing protocols. Briefly, tumour DNA was PCR amplified across exons 1 and 2; the resultant products were then purified and sequenced to capture all five coding microsatellites (primers available on request). A threshold of 15% mutant allele fraction was used based on the chromatogram traces from CRCs and level of background noise. A subset CRCs and adenomas for each amplicon (n = 30; 14.5%) were repeated to confirm B2M mutation status with 100% concordance.
Statistical analysis
Frequency distributions of B2M status by demographic and tumour-related factors were calculated. For the survival analysis, observation time started from the date of CRC diagnosis and ended at the date of death from any cause or censored at last known data alive for ACCFR and 31st August 2015 for MCCS. We derived Kaplan–Meier survival curves and mortality rates (per 1000 person-years). We used Cox regression [25] with the time axis defined as ‘time since CRC diagnosis’ to estimate hazard ratios (HR) and 95% confidence intervals (CI) for overall survival by B2M mutation status. Multivariable models were adjusted for age at CRC diagnosis (categorical: <40, 40–49, 50–59, 60–69, ≥70 years), sex, AJCC stage (categorical: stage I, stage II, stage III, stage IV) and grade (categorical: low-well/moderately differentiated, high-poorly differentiated). We examined each model for outliers and influential points and used Schoenfeld residuals to assess the proportional hazard assumptions; there was no evidence that they were violated. All statistical analyses were performed using Stata 14.1 (StataCorp, College Station, TX).
Results
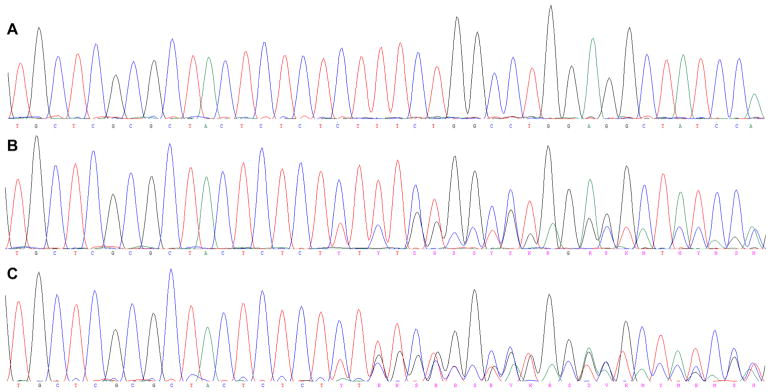
Assessment of B2M mutation status was performed for 144 MMR-deficient CRCs from the ACCFR and MCCS resources and for 63 adenomas from MMR gene mutation carriers from the ACCFR. The characteristics of the participants, CRCs and adenomas are shown in Table 1. Of the five coding microsatellites tested in the B2M gene, at least one somatic mutation was observed in 30 out of 144 (20.8%) of the CRCs. No mutation was identified in any of the 63 adenomas tested. The frequency of somatic mutations across each of the five coding microsatellites is shown in Table 2. The most common somatic mutation was the c.43_44delCT mutation in exon 1 (16/35; 45.7%; Fig. 1). Five CRCs (5/144; 3.5%) showed two distinct mutations within B2M. None of the samples containing a frameshift mutation had sequencing profiles suggestive of loss of heterozygosity of the wildtype allele.
Table 1.
Characteristics of study participants with MMR-deficient CRCs and adenomas
| Characteristic | Total 144 (%) |
|---|---|
| Study | |
| ACCFR | 63 (43.8) |
| MCCS | 81 (56.2) |
| Age at diagnosis (years) | |
| <50 | 51 (35.4) |
| ≥50 | 93 (64.6) |
| Median (range) | 62 (18–83) |
| Sex | |
| Male | 68 (47.2) |
| Female | 76 (52.8) |
| B2M mutation status | |
| B2M mutant | 30 (20.8) |
| B2M wildtype | 114 (79.2) |
| AJCC stage | |
| Stage I + II | 111 (77.1) |
| Stage III + IV | 30 (20.8) |
| Unknown | 3 (2.1) |
| Histological grade | |
| Moderate/well differentiated | 89 (61.8) |
| Poorly differentiated | 49 (34.0) |
| Unknown | 6 (4.2) |
| MMR IHC pattern of loss | |
| MLH1/PMS2 + MLH1/PMS2&MSH6 | 101 (70.1) |
| MSH2/MSH6 | 18 (12.5) |
| Othera | 25 (17.4) |
| MMR-deficient subtype | |
| MMR gene mutation carrier/Lynch syndrome | 41 (28.5) |
| Somatic MLH1 methylation | 69 (47.9) |
| Suspected Lynch syndromeb | 34 (23.6) |
| CRC site | |
| Right | 108 (75.0) |
| Left/rectum | 31 (21.5) |
| Unknown | 5 (3.5) |
| Histological type | |
| Mucinous | 37 (25.7) |
| Adenocarcinoma + signet + undifferentiated | 103 (71.5) |
| Unknown | 4 (2.8) |
| Tumour infiltrating lymphocytes | |
| No | 36 (25.0) |
| Yes | 101 (70.1) |
| Unknown | 7 (4.9) |
| Peritumoural lymphocytes | |
| No | 60 (41.7) |
| Yes | 74 (51.4) |
| Unknown | 10 (6.9) |
| Crohn’s like lymphocytes | |
| No | 63 (43.8) |
| Yes | 69 (47.9) |
| Unknown | 12 (8.3) |
| Adenomatous polyps | 63 (%) |
| Age at diagnosis median in years (range) | 43 [18–72] |
| Sex | |
| Male | 33 (52.4) |
| Female | 30 (47.6) |
| MMR IHC pattern of loss | |
| MLH1/PMS2 loss | 24 (38) |
| MSH2/MSH6 loss | 16 (25) |
| PMS2 only loss | 1 (2) |
| Normal expression | 22 (34) |
AJCC American Joint Committee on Cancer, MMR mismatch repair, CRC colorectal cancer
Other MMR patterns of loss include solitary MSH6 (n = 4) and solitary PMS2 (n = 1),
Suspected Lynch syndrome = no germline mutation identified and no evidence of somatic hypermethylation of the MLH1 promoter
Table 2.
The frequency of B2M somatic mutations across the five cMS tested in the 144 MMR-deficient CRCs
| B2M mutations total | 35a | |
|---|---|---|
| c.43_44delCT | p.(Leu15Phefs*41) | 16 (46%) |
| c.276delC | p.(Thr93Leufs*10) | 6 (17%) |
| c.276dupC | p.(Thr93Hisfs*2) | 4 (11%) |
| c.204delA | p.(Val69Trpfs*34) | 4 (11%) |
| c.45_48delTTCT | p.(Ser16Alafs*27) | 2 (6%) |
| c.285delA | p.(Asp97Metfs*7) | 2 (6%) |
| c.222_226delGTCTT | p.(Trp80Cysfs*8) | 1 (3%) |
Five tumours contained two separate mutations
Fig. 1.
Example sequencing profiles from B2M exon 1. A Wild type sequence chromatogram spanning the 8 bp dinucleotide cMS in exon 1. B Example of the most common mutation identified in our cohort c.43_44delCT. C Two samples gave sequencing traces suggestive of multiple mutations (i.e. triplicate reads), which could most simply be explained by a compound mutation of c.43_44delCT and c.45_48delTTCT
Tumours demonstrating loss of MLH1/PMS2 protein expression were enriched for B2M somatic mutations compared with CRCs showing loss of MSH2/MSH6 expression (p = 0.08). B2M mutations occurred in 29.0% of the MLH1 methylated CRCs, 17.1% of the Lynch syndrome CRCs and 8.8% of the suspected Lynch CRCs, respectively (p = 0.1) (Table 3). B2M mutations occurred predominately in AJCC stage II and stage III tumours, 26.4 and 17.2%, respectively (Table 3). B2M mutations were higher in poorly differentiated CRCs and right-sided CRCs compared with B2M wild-type CRCs (Table 3). TILs and Crohn’s-like lymphocytes were also increased in B2M mutated CRCs while peritumoural lymphocytes were decreased, however, none of these differences in tumour features or immune infiltrate reached statistical significance (Table 3).
Table 3.
Analysis of B2M somatic mutations by tumour histopathological characteristics
| Characteristic |
B2M status
|
||
|---|---|---|---|
| B2M mutants 30 (20.8%) | B2M wildtype 114 (79.2%) | p value | |
| AJCC stage | |||
| Stage I | 1 (4.2) | 23 (95.8) | 0.6a |
| Stage II | 23 (26.4) | 64 (73.6) | |
| Stage III | 5 (17.2) | 24 (82.8) | |
| Stage IV | 0 | 1 (100) | |
| Unknown | 1 (33.3) | 2 (66.7) | |
| Histological grade | |||
| Moderate/well differentiated | 16 (18.0) | 73 (82.0) | 0.4 |
| Poorly differentiated | 12 (24.5) | 37 (75.5) | |
| Unknown | 2 (33.3) | 4 (66.7) | |
| MMR IHC pattern of loss | |||
| MLH1/PMS2 + MLH1/PMS2&MSH6 | 24 (23.8) | 77 (76.2) | 0.08 |
| MSH2/MSH6 | 1 (5.6) | 17 (94.4) | |
| Other | 5 (20.0) | 20 (80.0) | |
| MMR-deficient subtype | |||
| MMR gene mutation carrier/Lynch syndrome | 7 (17.1) | 34 (82.9) | 0.1 |
| Somatic MLH1 methylation | 20 (29.0) | 49 (71.0) | |
| Suspected Lynch | 3 (8.8) | 31 (91.2) | |
| CRC site | |||
| Right | 23 (21.3) | 85 (78.7) | 0.5 |
| Left/rectum | 5 (16.1) | 26 (83.9) | |
| Unknown | 2 (40.0) | 3 (60.0) | |
| Histological type | |||
| Mucinous | 7 (18.9) | 30 (81.1) | 0.8 |
| Adenocarcinoma + signet + undifferentiated | 21 (20.4) | 82 (79.6) | |
| Unknown | 2 (50.0) | 2 (50.0) | |
| Tumour infiltrating lymphocytes | |||
| No | 6 (16.7) | 30 (83.3) | 0.6 |
| Yes | 21 (20.8) | 80 (79.2) | |
| Unknown | 3 (42.9) | 4 (57.1) | |
| Peritumoural lymphocytes | |||
| No | 15 (25.0) | 45 (75.0) | 0.1 |
| Yes | 11 (14.9) | 63 (85.1) | |
| Unknown | 4 (40.0) | 6 (60.0) | |
| Crohn’s like lymphocytes | |||
| No | 9 (14.3) | 54 (85.7) | 0.1 |
| Yes | 17 (24.6) | 52 (75.4) | |
| Unknown | 4 (33.3) | 8 (66.7) | |
AJCC American Joint Committee on Cancer, MMR mismatch repair, CRC colorectal cancer; Missing information for that characteristic was not available, NA not applicable
Given stage IV tumours have insufficient numbers (no B2M mutations and only 1 B2M wild-type), we have combined stage I/II and stage III/IV for analysis
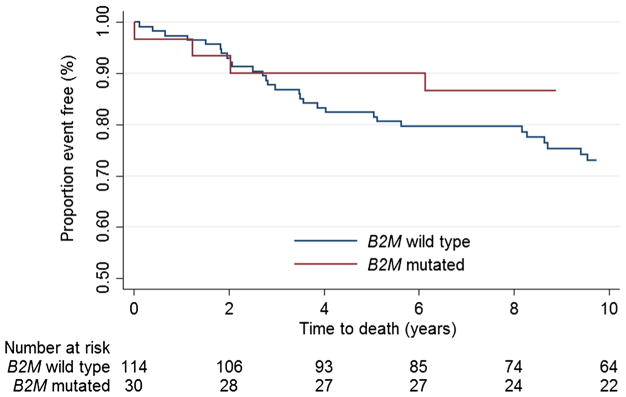
The adjusted HR for overall survival for individuals with B2M mutated CRC compared with those with B2M wildtype CRC (5-year survival: 90% [95% CI 72–97%] versus 82% [95% CI 74–88%], respectively) was 0.65 for B2M mutated CRC ([95% CI 0.29–1.48]; p = 0.3) (Table 4; Fig. 2). The HR did not change when we repeated the analysis excluding those with unknown AJCC stage or grade (HRadjusted, 0.59 [95% CI 0.25–1.40]; Supplementary Table 2).
Table 4.
Overall survival for B2M mutation status
| Cases | Deaths | Mortality rate/1000 person-years | 5-year survival rate (95% CI) (%) | 10-year survival rate (95% CI) (%) | Unadjusted HR (95% CI) | p valuea | Adjusted HR (95% CI)b, c | p valuea | |
|---|---|---|---|---|---|---|---|---|---|
| B2M mutation status | |||||||||
| B2M wild type | 114 | 38 | 32.5 | 82 (74–88) | 73 (63–80) | Reference | Reference | ||
| B2M mutated | 30 | 8 | 23.7 | 90 (72–97) | 87 (68–95) | 0.75 (0.35–1.60) | 0.5 | 0.65 (0.29–1.48) | 0.3 |
| All | 144 | 46 | 30.5 | 84 (77–89) | 76 (68–82) | – | – | – | – |
HR hazard ratio, CI confidence interval
Wald test from cox regression models
Adjusted for age at diagnosis, sex, AJCC (American Joint Committee on Cancer) stage and grade
Those with unknown AJCC stage or grade included as separate categories under each variable
Fig. 2.
Kaplan–Meier curve for overall survival for B2M mutation status
Discussion
The immune system plays a key role in tumour initiation, evolution and progression, including in CRC, where the presence of a strong T-cell response is associated with higher patient survival [26]. MMR-deficiency leads to genome-wide accumulation of frameshift mutations in microsatellite repeats, and when they occur in coding regions, can result in frameshift mutation-derived peptides (FSP) which act as highly immunogenic neoantigens eliciting T-cell responses [27, 28]. This has been shown specifically for FSPs derived from TGFBR2, OGT and CASP5 [27, 29, 30]. These FSP-specific T-cell responses are detectable in peripheral blood from Lynch syndrome mutation carriers, even if they have no previous history of any clinically apparent neoplastic lesion [31]. As such, the potential to vaccinate with FSP antigens as a treatment of Lynch syndrome-associated cancers is being explored [32]. Furthermore, this FSP-driven immune response in MMR-deficient CRCs has been successfully exploited to develop immune checkpoint blockade therapy with dramatic effects [33, 34]. B2M encodes a component of the HLA Class I antigen presentation complex, and as such somatic mutations within B2M may lead to impaired antigen presentation, immune evasion and immunotherapy resistance [35]; conversely, high somatic mutation rates in B2M, due to MMR-deficiency, can result in the production of immunogenic neoantigens which may be potential targets for immunotherapy [14]. Thus, we investigated somatic mutations in B2M and relationship with clinicopathological features and survival from MMR-deficient CRC.
We identified somatic cMS mutations in 20.8% of the MMR-deficient CRCs tested, whereas none were identified in the 63 adenomas from the MMR gene mutation carriers tested (including 42 MMR-deficient adenomas). Previous studies of somatic B2M mutations in MMR-deficient CRCs have reported frequencies of 42.7% [15], 29.4% [17], 27.8% [16] and 19.4% [19]. Using similar mutation detection techniques, both our study and that of Kloor et al. [16] identified c.43_44delCT as being the most frequently occurring B2M mutation in MMR-deficient CRCs and that a small subset of MMR-deficient tumours had multiple B2M somatic mutations. In our study, the frequency of B2M mutations was highest in MLH1 methylated CRCs (29.0%) compared with Lynch syndrome CRCs (17.1%) and suspected Lynch CRCs (8.8%). In contrast, Kloor et al. [16] reported B2M mutations to be more frequent in MLH1 and MSH2 (36%) germline mutation carriers compared with those without germline mutations (15.4%), although it is unknown if this group of non-carriers was comprised solely of MLH1 methylated MMR-deficient CRCs or included suspected Lynch syndrome MMR-deficient CRCs. These inconsistent results suggest that acquired B2M mutations may be independent of the underlying mechanism causing MMR-deficiency.
The absence of B2M somatic mutations in the 63 adenomas from the MMR gene mutation carriers suggests that B2M mutations do not occur early in tumourigenesis even if MMR-deficiency is present in these lesions. This is further supported by our finding that only 4.2% of stage I tumours had B2M mutations (a higher proportion was seen for stage-II CRCs), suggesting that these cMS mutations may have been acquired later during tumour development. The lack of stage IV CRCs in our study is consistent with the existing literature, which indicates that the MMR-deficient subgroup of CRC metastasises less frequently than MMR-proficient CRCs [36]. As such, we were limited in our ability to draw conclusions regarding B2M mutations and metastatic MMR-deficient CRC. We could not replicate the findings of Kloor et al. [16], who found 11.8% of the 17 Lynch syndrome adenomas they tested had cMS B2M somatic mutations and that the frequency of B2M mutations increased with increasing localised stage. Koelzer et al. [19], described a higher B2M mutation frequency in lower staged tumours (p = 0.035). Further understanding of the timing of these key somatic mutations may allow opportunities for prevention or early treatment of cancers, especially for MMR-deficient CRCs.
Analogous to our study, the studies by Yamamoto et al. [15], Kloor et al. [16], Tikidzhieva et al. [17] or Koelzer et al. [19], did not find differences in distribution of B2M mutations by age and gender. For the analysis of tumour features, we observed that B2M mutations occurred in 1 in 4 tumours with a higher histological grade (i.e. poorer differentiation) and approximately 1 in 6 tumours of lower histological grade. Although this difference was not statistically significant (p = 0.4) it was consistent with the findings by Koelzer et al. [19] which did report this finding as a statistically significant association. Yamamoto et al. [15] found no association between histological grade and B2M mutation status. B2M mutations are thought to alter the immune response to tumours [14], with a previous study reporting fewer TILs in B2M mutated CRCs [15]. We observed an increase in Crohn’s like lymphocytes and a decrease in peritumoural lymphocytes in B2M mutated CRCs, however, neither of these differences reached statistical significance (p = 0.1).
Previous studies of B2M mutations and survival from cancer have reported conflicting results (Supplementary Table 1). B2M mutations have been associated with poorer survival in patients with MMR-deficient CRC (p < 0.01) [15], however, more recent studies have suggested B2M somatic mutations [17] or loss of B2M protein expression [19] result in more favourable patient outcomes (absence of disease relapse, distant metastasis or tumour-related deaths). In our study, the confidence intervals from this analysis were wide and overlapping and we would not conclude that survival differed by B2M mutation status.
To the best of our knowledge, the present body of work represents the largest study profiling B2M cMS somatic mutations in MMR-deficient CRC and adenomas to date. Despite this, many of our estimates of associations had wide confidence intervals and a larger sample size would have improved the statistical power to identify any existing associations between B2M mutations and clinicopathological features or survival from CRC. An additional limitation is that we did not confirm whether B2M somatic mutations translated to aberrant expression of B2M protein and, therefore, comparisons to findings of Koelzer et al. [19] may not be appropriate. Furthermore, we assessed lymphocytic infiltration on a crude and binary level, and did not differentiate between various T-cell markers, such as CD8+, CD3+, or FOXP3+ which are known to differ even within MMR-deficient CRC [37, 38]. Recently, FOXP3+ has been shown to differ markedly in expression levels within the normal colonic mucosa adjacent to the CRC based on B2M mutation status [39]. The inclusion of additional genes with cMS that are associated with MHC class I and class II function including TAP1, TAP2, RFX5 and CIITA, may improve the prediction of survival for this subgroup of CRCs [14].
Conclusion
In summary, we observed differences in the distribution of B2M cMS somatic mutations by pattern of MMR protein loss of expression by IHC, MMR-deficiency subtype, site, grade, stage and the presence of peritumoural lymphocytes or Crohn’s like lymphocytes, however these differences did not reach statistical significance. Also, while people with B2M mutated CRC had better overall survival, our analysis was underpowered to conclude that survival differed significantly by B2M mutation status. Larger studies are needed to resolve some of the current inconsistencies with the findings from previous studies before B2M mutation status can be considered to have clinical utility.
Supplementary Material
Acknowledgments
This work was supported by Grant UM1 CA167551 from the National Cancer Institute and through cooperative agreements with Australasian Colorectal Cancer Family Registry (U01 CA074778 and U01/U24 CA097735) and was conducted under Colon-CFR approval C-AU-1014-01. The Melbourne Collaborative Cohort Study for colorectal cancer was funded by NHMRC project Grant 509348 (PI-Dallas English) “Risk Factors for Molecular Subtypes of Colorectal Cancer”. Aung K. Win is an Australian National Health and Medical Council (NHMRC) Early Career Fellow. Melissa C. Southey is a NHMRC Senior Research Fellow. Mark A. Jenkins is a NHMRC Senior Research Fellow. John L. Hopper is a NHMRC Senior Principal Research Fellow and Distinguished Visiting Professor at Seoul National University, Korea. Christophe Rosty is the Jass Pathology Fellow. Daniel D. Buchanan is a University of Melbourne Research at Melbourne Accelerator Program (R@MAP) Senior Research Fellow and NHMRC R.D. Wright Career Development Fellow.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10689-017-0013-y) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare they hold no conflict of interest with respect to this work.
Disclaimer The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Cancer Family Registries, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the Cancer Family Registry. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
References
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 4.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–2798. doi: 10.1016/j.ejca.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet. 2009;41(1):112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 6.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95(12):6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–16e1. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–6e8. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Sourrouille I, Coulet F, Lefevre JH, et al. Somatic mosaicism and double somatic hits can lead to MSI colorectal tumors. Fam Cancer. 2013;12(1):27–33. doi: 10.1007/s10689-012-9568-9. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan DD, Rosty C, Clendenning M, Spurdle AB, Win AK. Clinical problems of colorectal cancer and endometrial cancer cases with unknown cause of tumor mismatch repair deficiency (suspected Lynch syndrome) Appl Clin Genet. 2014;7:183–193. doi: 10.2147/TACG.S48625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–87e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan DD, Clendenning M, Rosty C, et al. Tumor testing to identify lynch syndrome in two Australian colorectal cancer cohorts. J Gastroenterol Hepatol. 2017;32(2):427–438. doi: 10.1111/jgh.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloor M, von Knebel Doeberitz M. The immune biology of microsatellite-unstable cancer. Trends Cancer. 2016;2(3):121–133. doi: 10.1016/j.trecan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto H, Yamashita K, Perucho M. Somatic mutation of the beta2-microglobulin gene associates with unfavorable prognosis in gastrointestinal cancer of the microsatellite mutator phenotype. Gastroenterology. 2001;120(6):1565–1567. doi: 10.1053/gast.2001.24497. [DOI] [PubMed] [Google Scholar]
- 16.Kloor M, Michel S, Buckowitz B, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121(2):454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 17.Tikidzhieva A, Benner A, Michel S, et al. Microsatellite instability and Beta2-Microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer. 2012;106(6):1239–1245. doi: 10.1038/bjc.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6(12):1695–1697. doi: 10.1016/s0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 19.Koelzer VH, Baker K, Kassahn D, Baumhoer D, Zlobec I. Prognostic impact of beta-2-microglobulin expression in colorectal cancers stratified by mismatch repair status. J Clin Pathol. 2012;65(11):996–1002. doi: 10.1136/jclinpath-2012-200742. [DOI] [PubMed] [Google Scholar]
- 20.Giles GG, English DR. The Melbourne collaborative cohort study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 21.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomark Prev. 2007;16(11):2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 22.Cicek MS, Lindor NM, Gallinger S, et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn. 2011;13(3):271–281. doi: 10.1016/j.jmoldx.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh MD, Buchanan DD, Pearson SA, et al. Immunohistochemical testing of conventional adenomas for loss of expression of mismatch repair proteins in Lynch syndrome mutation carriers: a case series from the Australasian site of the colon cancer family registry. Mod Pathol. 2012;25(5):722–730. doi: 10.1038/modpathol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosty C, Young JP, Walsh MD, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013;26(6):825–834. doi: 10.1038/modpathol.2012.240. [DOI] [PubMed] [Google Scholar]
- 25.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 26.Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linnebacher M, Gebert J, Rudy W, et al. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93(1):6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 28.Saeterdal I, Bjorheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98(23):13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripberger E, Linnebacher M, Schwitalle Y, Gebert J, von Knebel Doeberitz M. Identification of an HLA-A0201-restricted CTL epitope generated by a tumor-specific frameshift mutation in a coding microsatellite of the OGT gene. J Clin Immunol. 2003;23(5):415–423. doi: 10.1023/a:1025329819121. [DOI] [PubMed] [Google Scholar]
- 30.Schwitalle Y, Linnebacher M, Ripberger E, Gebert J, von Knebel Doeberitz M. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004;4:14. [PubMed] [Google Scholar]
- 31.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134(4):988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 32.von Knebel Doeberitz M, Kloor M. Towards a vaccine to prevent cancer in Lynch syndrome patients. Fam Cancer. 2013;12(2):307–312. doi: 10.1007/s10689-013-9662-7. [DOI] [PubMed] [Google Scholar]
- 33.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5(1):16–18. doi: 10.1158/2159-8290.CD-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13(13):3831–3839. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 37.Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44(3):698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Masugi Y, Nishihara R, Yang J, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2016 doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echterdiek F, Janikovits J, Staffa L, et al. Low density of FOXP3-positive T cells in normal colonic mucosa is related to the presence of beta2-microglobulin mutations in Lynch syndrome-associated colorectal cancer. Oncoimmunology. 2016;5(2):e1075692. doi: 10.1080/2162402X.2015.1075692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.