Abstract
Background:
Posttraumatic stress is associated with elevated risk for cardiovascular disease (CVD). Relatively little research, particularly among women, has documented mechanisms by which PTSD might confer CVD risk during early adulthood. The purpose of the present study was to examine whether the number and relative levels of CVD risk factors are associated with posttraumatic stress symptom severity among young, trauma-exposed women.
Methods:
Participants were premenopausal women ages 19–49 with varying levels of posttraumatic stress and no history of chronic medical illness (n=54), and were recruited from mental health clinics and the general community. Posttraumatic stress severity was assessed with a structured clinical interview (Clinician-Administered PTSD Scale). The CVD risk factors assessed were lipids (total cholesterol, triglycerides, high- and low-density lipoproteins), resting blood pressure (BP), body mass index (BMI), no exercise in typical week, and cigarette smoking.
Results:
Posttraumatic stress severity was associated with lower high-density lipoprotein levels and higher triglycerides, greater systolic and diastolic BP, greater BMI, and a greater number of total CVD risk factors.
Limitations:
The main limitation is the limited number of participants who displayed clinical levels on some of the CVD risk factors (e.g., BP). Nonetheless, most participants exhibited more than one CVD risk factor, indicating the potential for many of the women in this relatively young sample to progress toward greater risk later in life.
Conclusions:
The present results support the contention that, in the absence of medical illness, posttraumatic stress symptom severity among young women is associated with several CVD risk factors early in life.
Keywords: cardiovascular, posttraumatic stress, women
Introduction
Research in the area of psychological trauma has revealed a relationship between posttraumatic stress disorder (PTSD) and poor cardiovascular health (Bedi & Arora, 2007; Boscarino & Chang, 1999; Jordan et al., 2011; Kubzansky et al., 2009; Ouimette et al., 2004; Sumner et al., 2015; Xue et al., 2012). Much of this evidence has involved retrospective examination of PTSD status among combat veterans who exhibit clinical symptoms of cardiovascular disease (CVD). Many civilians also suffer from PTSD resulting from the occurrence of threatening incidents including sexual abuse/rape, physical assault, and automobile accidents (e.g., McGruder-Johnson et al., 2000; Norris et al., 2002). Studies have revealed high rates of lifetime exposure to potentially traumatic events among young women (e.g., McGruder-Johnson et al., 2000; Norris et al., 2002), and women are more likely than men to develop chronic PTSD after a trauma exposure (Davidson, 2000; Galea et al., 2002; Norris et al., 2002).
The available evidence concerning PTSD-related CVD risk among women suggests that women may display similarly high levels of cardiovascular risk relative to males (e.g., Kubzansky et al., 2009; Ouimette et al., 2004; Sumner et al., 2015). The relationship between heart disease and PTSD symptoms was examined in 1,059 community-dwelling women ages 19–93 (mean age = 44 years at the start of 4-year follow-up) from the Baltimore cohort of the Epidemiologic Area Catchment study (Kubzansky et al., 2009). Results indicated that with each additional PTSD symptom the risk of developing incident CHD increased 17%. Sumner et al. (2015) subsequently demonstrated, among 49,978 female nurses followed longitudinally in the Nurses’ Health Study II (ages 25–42 in year 1 of the 20-year study period), that women with four or more PTSD symptoms had the highest likelihood of incident CVD events (myocardial infarction or stroke) compared to those with fewer symptoms. Additional analyses from this large-scale study indicated that elevated levels of posttraumatic stress were also associated with the occurrence of a thromboembolism (Sumner, Kubzansky, Kabrhel et al., 2016). In a study of 134 men and women in VA medical clinics (mean ± SD age = 52 ± 15), PTSD was associated with an odds ratio of 3.7 for greater ICD-9 coded circulatory problems (Ouimette et al., 2004); gender did not moderate this relationship. Civilian women suffering from PTSD have been understudied with regard to CVD risk factors. Thus, there is a need to investigate the potential cardiovascular manifestations of trauma among young women, prior to the development of CVD, in order to improve understanding of the mechanisms that contribute to this relationship.
There are several hypotheses about specific behavioral, psychological, and physiological risk factors and mechanisms that may contribute to CVD in PTSD (e.g., Dedert et al., 2010; Gustafson & Sarwer, 2004; Harrington et al., 2010; Kubzansky et al., 2014; McShane & Zirkel, 2008; Robinson, 2000; Wiederman et al., 1999; Weiner & Stephens, 1999; Wonderlich et al., 2001). Although it is beyond the scope of the present paper to review all potential explanatory mechanisms with regard to PTSD/CVD risk associations, we review a number of the most prevalent behavioral/psychological and physiological theories/concepts. From a behavioral perspective, the approaches utilized by individuals with PTSD to cope with trauma and other stressors are sometimes physically unhealthy; in particular, weight management may be disrupted among individuals with PTSD (e.g., Dedert et al., 2010; Kubzansky et al., 2014). In a sample of civilian women, Dedert et al. (2010) found support for PTSD as a potential mediating variable in the relationship between childhood traumatic stress (childhood sexual and physical trauma) and weight outcomes as adults [body mass index (BMI) and hip-to-waist ratios]. Subsequently, Kubzansky et al. (2014) demonstrated a positive relationship between PTSD symptoms and the prospective risk of becoming overweight or obese in the large-scale Nurse’s Health Study II.
Given the relationship of physical activity to weight and CVD risk, assessing exercise patterns for women with PTSD could yield additional information about risk. Although, there is little evidence regarding physical activity levels in PTSD, some studies have indicated that PTSD has a negative impact on physical activity levels (e.g., de Assis et al., 2008; Winning et al., 2017). Further study of exercise levels in PTSD may provide greater insight into physical activity as a mechanism that may be associated with CVD. Smoking/tobacco use is another widely recognized health risk behavior associated with PTSD (Breslau et al., 2003; Fu et al., 2007). This research has outlined a causal effect of PTSD on smoking.
There is some evidence that unhealthy lipid levels and elevated blood pressure (BP) are CVD risks that occur with increased prevalence among individuals with PTSD (e.g., Dedert et al., 2013; Dennis et al., 2014; Filakovic et al., 1997; Kagan et al., 1999; Kibler et al., 2009; Maia et al., 2007; Sumner, Kubzansky, Roberts et al., 2016). Most of the research associating PTSD with unhealthy lipid profiles has been conducted with male combat veterans (e.g., Filakovic et al., 1997; Kagan et al., 1999; Maia et al., 2007). Among Croatian soldiers, elevated total cholesterol and triglycerides were observed in mental health patients with PTSD relative to patients without PTSD (Filakovic et al., 1997). Brazilian police officers with PTSD exhibited significantly higher total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides than those without PTSD (Maia et al., 2007). The limited research examining lipids in relation to PTSD with female samples has yielded mixed results. Dedert et al. (2013) did not observe differences between women with and without PTSD for triglycerides, LDL levels or high-density lipoprotein (HDL) levels. In a sample of combined men and women, Dennis et al. (2014) observed significantly lower HDL levels between a PTSD group and non-PTSD controls, but no significant differences in triglycerides or LDL levels; when PTSD and depressive symptoms were placed on a continuum and combined as a latent variable in this study, the PTSD/depressive symptoms were negatively associated with HDL and positively associated with triglycerides. Given the scarcity of research related to PTSD and lipids with female samples, and the mixed findings, further examination is needed to evaluate whether lipid alterations are observed among women with PTSD. With regard to BP, data from the National Comorbidity Survey (Kibler et al., 2009) indicated greater rates of hypertension among women and men with PTSD compared with a no mental illness group and a group with MDD only (no PTSD). These differences in hypertension rates were significant when controlling for the relationship between age and hypertension rate. Subsequently, results from 47,514 civilian women in the Nurses’ Health Study II indicated that elevated levels of posttraumatic stress were associated with the occurrence of hypertension (Sumner, Kubzansky, Roberts et al., 2016).
Taken together, the evidence suggests a convergence of behavioral and physiological risks that may help to explain the higher rates of CVD in women with PTSD. The physiological pathways involved in maladaptive stress responses involving autonomic, metabolic, and/or immune dysregulation (e.g., Brudey et al., 2015; Sumner et al., 2017; Thayer et al, 2017) may contribute to hypertension and cardiovascular events in PTSD. However, the underrepresentation of civilian women in most preliminary studies of PTSD-related CVD risk factors underscores the need for additional research with this population. Research focused on cardiovascular risk factors in young women with PTSD and no current CVD is critical in understanding pre-clinical progression of PTSD toward CVD. Studies of young, relatively healthy individuals with posttraumatic stress, permits detection of health behaviors or physiological risks prior to complication by most age-related or other physiological (e.g., menopausal factors in women) or illness-related confounds that occur later in life.
The present study was conducted to examine whether the number and relative levels of CVD risk factors are associated with posttraumatic stress symptom severity among young, trauma-exposed women. Most studies that have examined CVD risk factors in PTSD have not examined more than 1 or 2 risk variables, such as BMI or lipids. The proposed research permits assessment of a total CVD risk factor cluster by assessing several indices of CVD risk. Another major strength of the present study is the clinical assessment of PTSD symptoms and other psychiatric disorders using structured clinical interviews. Most studies of CVD risk in PTSD have either relied on PTSD screening instruments or short participant-rated or researcher-rated checklists. The use of structured interviews represents the only true method of establishing clinical PTSD severity.
Methods
Participants
A total of 54 young women (M ± SD age = 30 ± 8) completed the study. Participants were recruited via posting of flyers, advertisement in scientific and public websites, and referrals from a community mental health clinic. Inclusion criteria were 1) no history of major chronic illness, 2) pre-menopausal, and 3) not currently taking any medications that could significantly influence physiological measures (e.g., beta-blockers, lipid-lowering agents). Chronic illness was an exclusion criterion, because it complicates our focus on factors that may increase risk for developing CVD. Exclusion for most axis I disorders was implemented in an effort to focus our findings on phenomena associated with PTSD severity. The study was open to participants both with and without trauma histories. However, all of the participants reported exposure to at least one potentially traumatic event (see the Results for descriptive data on trauma exposure). This study was approved by the appropriate University IRB. All participants completed an informed consent procedure prior to inclusion.
Measures
Trauma Life Event Questionnaire (TLEQ).
Prior to the structured clinical interviews, participants completed an assessment of prior exposure to potentially traumatic events using the TLEQ (Kubany et al., 2000). The TLEQ is a brief survey used to assess a broad range of potentially traumatic events such as motor vehicle accidents, combat, physical assault, and sexual abuse. Events are described in behaviorally descriptive terms consistent with the language for the Diagnostic and Statistical Manual of Mental Disorders 4th edition - revised (DSM-IV) PTSD criterion A traumatic event (Kubany et al., 2000). The TLEQ has yielded good reliability, and good concurrent and predictive validity (Kubany et al., 2000).
Clinician-Administered PTSD Scale (CAPS).
The clinical interview to assess PTSD severity followed the format of the CAPS - an international standard in diagnostic assessments for PTSD (Blake et al., 1995; Blanchard et al., 1995; Hovens et al., 1994; King et al., 1998; Weathers et al., 1999). The CAPS is a semi-structured interview designed to assess current and lifetime PTSD, and has been shown to be reliable based on analyses of inter-rater reliability (.92-.99), test-retest reliability (.91-.95), and internal consistency (.73-.95) (Blake et al., 1995; Blanchard et al., 1995; Hovens et al., 1994; Weathers et al., 1999). Studies of the CAPS have also shown high inter-rater agreement and good convergent validity (Blake et al., 1995; Hovens et al., 1994; King et al., 1998; Weathers et al., 1999).
Structured Clinical Interview for DSM-IV Axis I Disorders (SCID).
The clinical interview for other Axis I disorders followed the format of the SCID, research version (Basco et al., 2000). Studies have demonstrated superior validity of the SCID relative to standard unstructured interviews (e.g., Basco et al., 2000; Kranzler et al., 1996).
Fasting Lipid Profile.
The Cholestech LDX System (Cholestech Corp., Hayward, CA) was utilized to assess lipid profile using finger stick capillary whole blood (Cobbaert et al., 1993; Drimmer & Girgenti, 1995). The Cholestech LDX Analyzer is designed for point of service lipid analysis using finger stick capillary whole blood. The system uses reflectance photometry to obtain lipid results that are available within 4 minutes. Lipid values derived from the Cholestech system are highly correlated with serum-derived reference values (r values = .96–.98) with approximate percent bias of 0–3% for total cholesterol, HDL, and LDL and 0–8% for triglycerides (Cobbaert et al., 1993; Drimmer & Girgenti, 1995; Polito et al., 2000). The following lipid levels were determined for the present study: 1) triglycerides, 2) total cholesterol, 3) HDL, and 4) LDL.
Body Mass Index.
Body weight relative to height was utilized for calculation of BMI and determination of obesity status.
Resting BP.
The participant sat quietly for 5 min after which 3 seated mercury sphygmomanometer-determined systolic and diastolic BP readings were taken at 2 min intervals from the dominant arm. The average of the last 2 measures provided an index of the participant’s resting systolic and diastolic BP.
Secondary Self-Report Measures.
Basic demographic information was assessed by self-report including age, race/ethnicity, and family income. Physical activity was assessed by self-report using a 7-day recall originally developed for use in the Stanford Heart Prevention Program (Richardson, Ainsworth, Jacobs, & Leon, 2001; Sallis et al., 1985). Smoking status (current use) was also assessed by self-report.
Procedures
Data collection took place during two separate assessment sessions - a structured interview session and a laboratory assessment session. The interval between the interview and laboratory assessment was minimized (no more than 2 weeks) due to the possibility that symptom presentation could change over time. To control for menstrual cycle effects on the cardiovascular measures, participants completed the laboratory session during the follicular stage of the menstrual cycle (approximately days 5–9, depending on the participant’s estimate of cycle length).
The structured interviews (i.e., CAPS, SCID) were conducted in a University-based psychological services center by advanced clinical psychology doctoral students under supervision of the first author. Interviewers received extensive, focused training consistent with the recommendations given by the designers of the CAPS and SCID from the first author prior to conducting the interviews. Each interview was audiotaped, and an interviewer who did not conduct the original interview provided ratings of the participants’ symptoms offline for the purpose of calculating inter-rater reliability of the diagnostic assessments. The overall inter-rater reliability correlations were .87 for the CAPS and 0.69 for the SCID.
Participants presented to a psychophysiological assessment laboratory for the cardiovascular risk assessment. Separate written informed consent was required for this session. Prior to arriving at the laboratory, participants were asked to refrain from eating for 12 hours, caffeine and strenuous exercise for 3 hours, and smoking for 30 minutes prior to this session. When the participant arrived, compliance with restrictions was assessed. Noncompliance would result in rescheduling. However, there was only one instance of rescheduling due to noncompliance (coffee consumed in the morning before the scheduled session). In general, reminder calls conducted the day before each session were effective in promoting compliance with the restrictions. This session began early in the morning (approx. 8:00 am) due to the requirement of fasting for the lipid assessment. A standardized light breakfast snack (e.g., granola bar and water or juice) was provided after the lipid assessment. For the BP assessment, the participant sat quietly for 5 min after which 3 seated mercury sphygmomanometer-determined systolic and diastolic BP readings were taken at 2 min intervals from the dominant arm. After the physical risk factor measures (i.e., height, weight, lipids, BP) were completed, the brief self-report surveys were administered. The research assistants were present and available to answer any questions of the participants throughout the laboratory session.
Data Reduction and Analyses
Preliminary analyses were conducted to check for outliers and examine distributions. Univariate correlation coefficients (Pearson) were calculated for the purpose of evaluating the associations of PTSD severity with the CVD risk variables. The qualitative cutoffs for determining the total number of CVD risk factors were consistent with standard guidelines (Flegal et al., 2013; Whelton et al., 2017) established for traditional risk factors (i.e., BMI > 25, total cholesterol > 200, HDL < 40, LDL > 130, triglycerides > 150, systolic BP ≥ 130, diastolic BP ≥ 80), no exercise in a typical week, and current use of cigarettes. The dependent variable for the analysis involving total number of CVD risks consisted of non-normally distributed count data (Poisson distribution). To approximate a normal distribution and stabilize the variances, we followed the standard recommendation to use a log(y+1) transformation for Poisson distributions with a mean of less than 3.0 with zero-counts included (Hinkelmann & Kempthorne, 2005; Kuehl, 1999). Follow-up analyses were conducted for significant univariate associations, adjusting for the potential confounding effects of age and oral contraceptive use on the CVD risks (nine of the participants were using oral contraceptives).
Results
Descriptive Data
Participants ranged in age from 19–49 (M ± SD = 30 ± 8). The ethnic breakdown of the sample was as follows: 56% Caucasian, 21% African American, 19% Hispanic, and 4% other. One third of participants reported family income less than $20,000, another 37% reported $20,000–50,000, 21% reported $50,000–100,000, and 9% reported over $100,000. With regard to education, 26% reported high school or equivalent as the terminal degree, 36% reported a Bachelor’s degree, 19% reported Associate’s, 15% reported graduate or professional degrees, and 3% reported “other”. Only 7% of the sample were sedentary (as defined by no exercise in a typical week), and 21% reported current cigarette use. Overall, 69% of participants had at least one CVD risk factor and 40% exhibited more than one CVD risk factor. Descriptive data for the continuous cardiovascular risk variables and PTSD symptoms are depicted in Table 1.
Table 1.
Descriptive data for primary study variables.
| Risk Variable | Mean | SD | Range | % clinically elevated |
|---|---|---|---|---|
| PTSD Total CAPS Score | 25.9 | 30.1 | 0 – 92 | |
| Number of CVD Risks | 1.8 | 2.0 | 0 – 8 | |
| Body Mass Index | 33.1 | 8.4 | 17.7 – 51.2 | 50% |
| Triglycerides | 95.4 | 72.8 | 40 – 384 | 12% |
| Total Cholesterol | 174.0 | 36.9 | 114 – 311 | 17% |
| HDL Cholesterol | 52.1 | 15.6 | 28 – 98 | 24% |
| LDL Cholesterol | 102.0 | 36.2 | 43 – 212 | 14% |
| SBP | 107.6 | 10.3 | 87 – 130 | 2% |
| DBP | 69.1 | 8.3 | 48.5 – 92.5 | 10% |
Abbreviations: Cardiovascular disease (CVD); Clinician-Administered PTSD Scale (CAPS); High-density lipoproteins (HDL); Low-density lipoproteins (LDL); Systolic blood pressure (SBP); Diastolic blood pressure (DBP)
With regard to trauma histories, the most distressing “index” events consisted of sexual assault/abuse (24%), physical assault/abuse/victim of domestic violence (18%), witnessing violence (12%), motor vehicle accident (4%), natural disaster (4%), sudden unexpected death of a loved one (20%), stalking (4%), abortion (4%), sexual harassment (2%), and other traumas (e.g., jailed/lost in foreign country) or life-threatening accidents requiring hospitalization (8%). This was a sample with high levels of trauma exposure protracted over an extended period - in terms of frequency, all participants reported exposure to more than one potentially traumatic event/incident (M ± SD = 13 ± 11). The PTSD symptom severity varied across the full range, and not all participants endorsed PTSD symptoms. Of the participants with the highest PTSD severity, 14 evidenced full PTSD criteria, and 5 women had significant subthreshold PTSD symptoms but did not meet full criteria (≥ 4 symptoms with ≥ 1 re-experiencing).
Primary Analyses
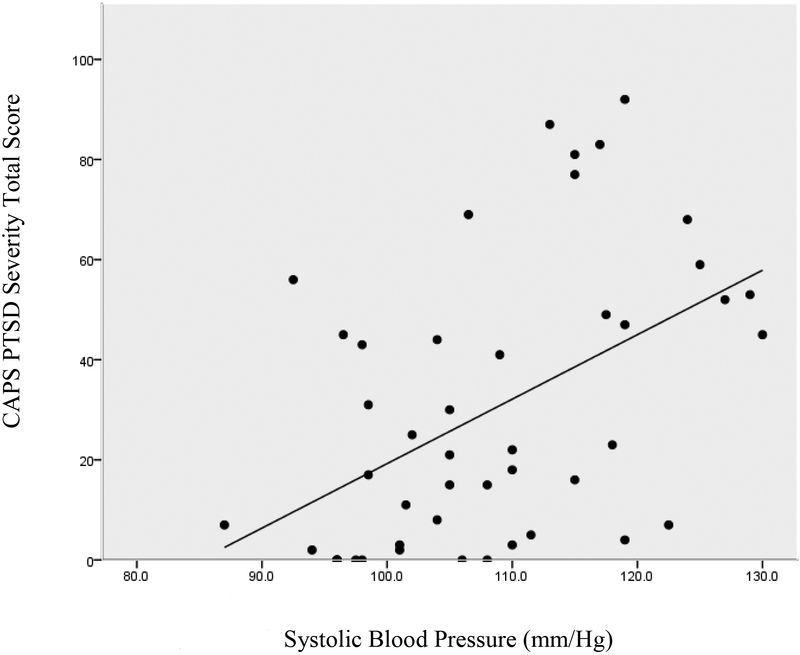
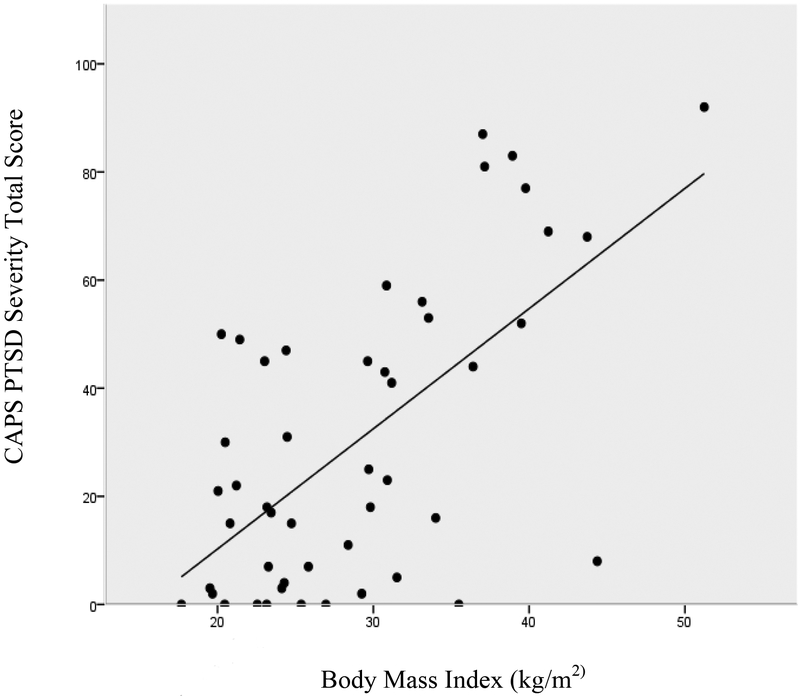
Posttraumatic stress severity was associated with lower HDL levels (r = −.40, p < .01) and higher triglycerides (r = .36, p < .05), but was not significantly associated with total cholesterol (r = .12) or LDL levels (r = .15). The posttraumatic stress severity scores were also significantly associated with greater systolic BP (r = .48, p < .01) and diastolic BP (r = .30, p < .05), greater BMI (r = .62, p < .001), and a greater number of total CVD risk factors (r = .34, p < .05). The associations of PTSD severity with systolic BP and BMI are illustrated in Figures 1 and 2, respectively.
Figure 1.
The correlation between PTSD severity and systolic blood pressure.
Figure 2.
The correlation between PTSD severity and body mass index.
Adjusting for the potential confounding effects of age, the associations of posttraumatic stress severity with HDL levels (r = −.40, p < .01), triglycerides (r = .36, p < .05), systolic BP (r = .45, p < .01) and BMI (r = .61, p < .001) remained significant. After adjusting for age, the association of PTSD severity with total CVD risk factors only approached significance (r = .30; p = .05), and the association of PTSD severity with diastolic BP was not significant (p = .397). The analyses adjusting for oral contraceptive use as a potential confound did not impact the significance of CVD risk factor/PTSD severity associations, with the exception of diastolic BP - this correlation was no longer significant after controlling for oral contraceptive use (p = .468).
Post-hoc exploratory analyses were conducted to determine whether the type of trauma (assaultive/interpersonal vs. non-assaultive) were systematically related to the CVD risk outcomes. Although participants identifying assaultive trauma had significantly higher PTSD severity, and tended to have greater levels of CVD risk across the outcome variables (aside from diastolic BP), none of these differences in CVD risk factors were statistically significant (see Table 2).
Table 2.
Descriptive data for primary study variables by trauma type (assaultive/interpersonal vs. non-assaultive).
| Assaultive | Non-assaultive | ||||
|---|---|---|---|---|---|
| Risk Variable | Mean | SD | Mean | SD | p value |
| PTSD Total CAPS Score | 36.8 | 29.0 | 14.7 | 27.4 | p = .009 |
| Number of CVD Risks | 2.0 | 2.0 | 1.4 | 1.7 | p = .256 |
| Body Mass Index | 29.4 | 8.6 | 27.9 | 7.5 | p = .482 |
| Triglycerides | 100.3 | 72.4 | 95.3 | 72.1 | p = .813 |
| Total Cholesterol | 176.3 | 35.6 | 172.2 | 40.3 | p = .709 |
| HDL Cholesterol | 48.7 | 16.0 | 55.7 | 15.2 | p = .118 |
| LDL Cholesterol | 107.5 | 34.6 | 97.5 | 37.4 | p = .328 |
| SBP | 108.7 | 11.4 | 106.6 | 9.5 | p = .473 |
| DBP | 68.5 | 9.0 | 69.6 | 7.9 | p = .623 |
Abbreviations: Cardiovascular disease (CVD); Clinician-Administered PTSD Scale (CAPS); High-density lipoproteins (HDL); Low-density lipoproteins (LDL); Systolic blood pressure (SBP); Diastolic blood pressure (DBP)
Discussion
Among women with no diagnosed medical illnesses, PTSD severity was associated with a greater number of CVD risks than controls. The comprehensive assessment of CVD risk factors in the present study is a relative strength that permitted quantification of this risk factor total index. The PTSD-related CVD risks observed in prior studies of predominantly male veterans appear to be salient health risks for women. The present findings are consistent with recent studies of posttraumatic stress and CVD risks among women (Kubzansky et al., 2014; Sumner, Kubzansky, Roberts et al., 2016). With a mean age of 30 in the present study, our sample is younger than other studies of premorbid CVD risk factors; this factor is significant in emphasizing the early nature of CVD risks that may in part derive from unhealthy behaviors, and may also begin to develop as early as childhood for those who experience early traumatic events.
For some of the specific risk variables (i.e., BMI, BP, triglycerides), there were significant associations between levels of CVD risk and PTSD symptom severity. These findings generally reflected effects in the moderate or small-to-moderate range. The finding that greater PTSD severity was associated higher BMI is consistent with prior research (e.g., Kubzansky et al., 2014; Scott et al., 2008). It is notable that the largest effect was for BMI and PTSD severity in the present study; this finding underscores the extent of the weight management problem associated with PTSD. Therefore, further examination is needed with regard to theories concerning overweight and obesity among women with abuse histories and PTSD. With regard to behaviors/factors that may influence body weight, several trauma types have been associated with binge eating to cope with daily stressors (Harrington et al., 2010; Wonderlich et al., 2001). Binging may represent a means of inducing dissociation or focusing attention away from stressful thoughts and negative emotions (McShane & Zirkel, 2008). Another salient construct for women with PTSD is the effect of negative self-image on body weight. Women who have been abused commonly have a disrupted sense of self-worth and often experience disgust regarding their own bodies; these perceptions can be manifested in diminished self-care, including overeating and physical inactivity (Robinson, 2000). Few studies have explored physical activity levels among women with PTSD. However, Winning et al. (2017) tracked physical activity of women over more than 20 years using data from Nurse’s Health Study, and found that women with high levels of PTSD symptoms exhibited steep declines in physical activity over the study period (compared to women with no PTSD symptoms who had steady levels on average). Another construct related to weight-management in PTSD is the desire to be overweight, which serves as a protective mechanism for some sexual trauma victims who may consider themselves less attractive to other potential perpetrators - this phenomenon has been referred to as sexual barrier weight (Gustafson & Sarwer, 2004; Wiederman et al., 1999; Weiner & Stephens, 1999). Given the literature on eating and other factors that may influence body weight for women with PTSD, further examination of obesity/BMI as a preventable CVD risk in this population is needed.
Our findings concerning BP and lipids in relation to PTSD severity are consistent with prior studies (Filakovic et al., 1997; Kagan et al., 1999; Maia et al., 2007), and further expand these findings to women. In large samples from the US National Comorbidity Study (Kibler et al., 2009) and the Nurses’ Health Study II (Sumner, Kubzansky, Roberts et al., 2016), elevated levels of posttraumatic stress were associated with the occurrence of hypertension. The finding in the present study that young women with greater PTSD severity had higher BP levels (within the normal range) supports the possibility that early BP elevations may eventually manifest in hypertension. Although lipid levels are determined by physiological factors, in addition to diet, research of this issue generally indicates behavioral factors such as diet and exercise confer at least an interactive effect with genes (e.g., Cooper et al., 1992; Vinson et al., 2008). The findings that levels of triglycerides and HDL were significantly associated with PTSD severity in the present study, have the most apparent implications for dietary behaviors. Although it is not yet clear from the PTSD literature, it is generally accepted that disruption in these lipid measures results from the digestion of fats from food. Therefore, there is a need for further research of the behavioral aspects (i.e., dietary factors) that impact lipid levels among young women with PTSD.
Primary limitations of the present study include the cross-sectional design and the limited number of participants who displayed clinical levels on some of the CVD risk factors (e.g., systolic BP). Nonetheless, most of the participants had at least one CVD risk factor and nearly half exhibited more than one CVD risk factor, indicating the potential for many of the women in this relatively young sample to progress toward greater risk later in life. Identifying risk factors (or even elevations in the normal range) among young adults, adolescents, and children, has implications for early identification of these problems and timely intervention studies and prevention programs. Thus, despite its limitations, the present study provides data with practical implications. We utilized a comprehensive assessment of CVD risk variables, and the findings may assist researchers in further understanding the CVD risks and enhancing prevention and health-promotion for young women with PTSD.
The cardiovascular health risks that are associated with PTSD are not directly addressed by traditional PTSD treatments - clinicians are generally trained to utilize evidence-based treatments that focus on the mental health symptoms. Therefore, this area of research has implications for the multidisciplinary training of clinicians. Ideally, clinical trainees could benefit from increased focus on behavioral medicine approaches to managing health risks among individuals with PTSD.
Highlights.
Little research, particularly among women, has documented mechanisms by which PTSD might confer cardiovascular disease (CVD) risk during early adulthood.
This study was designed to examine whether the number and relative levels of CVD risk factors are associated with posttraumatic stress symptom severity among young, trauma-exposed women.
Posttraumatic stress severity was associated with lower high-density lipoprotein levels and higher triglycerides, greater systolic and diastolic blood pressure, greater body mass index, and a greater number of total CVD risk factors.
These findings support the contention that, in the absence of medical illness, posttraumatic stress symptom severity among young women is associated with several CVD risk factors early in life.
Acknowledgements:
The authors thank the following individuals for their assistance in data collection and other related supportive services to this study: Karen Findon, Kathryn Greene, Kavita Joshi, Rachel Lerner, Morgan Levy, Reeva Ramcharan, and Annmarie Wacha-Montes
Funding: This work was supported by NIH/NHLBI (grant #1R15HL085121-01). The funding source had no involvement in the study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: Each of the authors: Kibler, Ma, Tursich, Malcolm, Llabre, Gold, Greenbarg, and Beckham declares that they have no conflict of interest.
Contributor Information
Mischa Tursich, Mankato Clinic
Lydia Malcolm, Nova Southeastern University
Maria M. Llabre, University of Miami
Jean C. Beckham, Durham Veterans Affairs Medical Center and Duke University Medical Center
References
- Basco MR, Bostic JQ, Davies D, Rush AJ, Witte B, Hendrickse W, Barnett V (2000). Methods to improve diagnostic accuracy in a community mental health setting. American Journal of Psychiatry, 157, 1599–1605. [DOI] [PubMed] [Google Scholar]
- Bedi US, Arora R (2007). Cardiovascular manifestations of posttraumatic stress disorder. Journal of the National Medical Association, 99(6), 642–649. [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Forneris CA, Loos W, Jaccard J (1995). Effects of varying scoring rules of the Clinician-Administered PTSD Scale (CAPS) for the diagnosis of posttraumatic stress disorder in motor vehicle accident victims. Behaviour Research and Therapy, 33, 471–475. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Chang J (1999). Electrocardiogram abnormalities among men with stressrelated psychiatric disorders: Implications for coronary heart disease and clinical research. Annals of Behavioral Medicine, 21, 227–234. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR (2003). Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry, 60, 289–294. [DOI] [PubMed] [Google Scholar]
- Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ (2015).Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol, 309, R315–R321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbaert C, Boerma GJM, Lindemans J (1993). Evaluation of the Cholestech L.D.X. desktop analyzer for cholesterol, HDL-cholesterol, and triacylglycerols in heparinized venous blood. European Journal of Clinical Chemical and Clinical Biochemical, 32, 391–394. [PubMed] [Google Scholar]
- Cooper GR, Myers GL, Smith SJ, Schlant RC (1992). Blood lipid measurements: Variations and practical utility. JAMA, 267, 1652–60. [PubMed] [Google Scholar]
- Davidson JRT (2000). Trauma: The impact of post-traumatic stress disorder. Journal of Psychopharmacology, 14, S5–S12. [DOI] [PubMed] [Google Scholar]
- de Assis MA, Mello MF, Scorza FA, Cadrobbi MP, Schooedl AF, da Silva SG,…Arida RM (2008). Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics, 63, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedert EA, Becker ME, Fuemmeler BF, Braxton LE, Calhoun PS, Beckham JC (2010). Childhood traumatic stress and obesity in women: The intervening effects of PTSD and MDD. Journal of Traumatic Stress, 23, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedert EA, Harper LA, Calhoun PS, Dennis MF, Beckham JC (2013). The impact of race on metabolic disease risk factors in women with and without posttraumatic stress disorder. Journal of Clinical Psychology in Medical Settings, 20, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Ulmer CS, Calhoun PS, Sherwood A, Watkins LL, Dennis MF, Beckham JC (2014). Behavioral health mediators of the link between posttraumatic stress disorder and dyslipidemia. Journal of Psychosomatic Research, 77, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drimmer A, Girgenti AJ (1995). Cholestech LDX System cholesterol and HDL-C accuracy and precision. College of American Pathologists. [Abstract]. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (2001). Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA, 285(19), 2486–2497. [DOI] [PubMed] [Google Scholar]
- Filakovic P, Barkic J, Kadoic D, Crncevic-Orlic Z, Grguric-Radanovic L, Karner I,…Mandic N (1997). Biological parameters of posttraumatic stress disorder. Psychiatria Danubina, 9, 207–211. [Google Scholar]
- Flegal KM, Kit BK, Orpana H, Graubard BI (2013). Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA, 309, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, & Joseph AM (2007). Post-traumatic stress disorder and smoking: A systematic review. Nicotine & Tobacco Research, 9 (11), 1071–1084. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, & Vlahov D (2002). Psychological sequelae of the September 11 terrorist attacks in New York City. New England Journal of Medicine, 13, 982–987. [DOI] [PubMed] [Google Scholar]
- Gustafson TB, Sarwer DB (2004). Childhood sexual abuse and obesity. Obesity Review, 5, 129–135. [DOI] [PubMed] [Google Scholar]
- Harrington EF, Crowther JH, Shipherd JC (2010). Trauma, binge eating, and the “strong Black woman”. Journal of Consulting and Clinical Psychology, 78(4), 469–479. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Kempthorne O (2005). Design and analysis of experiments, volume 2 New York: Wiley and Sons. [Google Scholar]
- Hovens JE, Van Der Ploeg HM, Klaarenbeek MTA, Bramsen I, Schreuder JN, Rivero VV (1994). The assessment of posttraumatic stress disorder with the Clinician Administered PTSD Scale: Dutch results. Journal of Clinical Psychology, 50, 325–340. [DOI] [PubMed] [Google Scholar]
- Jordan HT, Miller-Archie S, Cone JE, Morabia A, Stellman SD (2011). Heart disease among adults exposed to the September 11, 2001 World Trade Center disaster: Results from the World Trade Center Health Registry. Preventive Medicine, 53, 370–376. [DOI] [PubMed] [Google Scholar]
- Kagan BL, Leskin G, Haas B, Wilkins J, Foy D (1999). Elevated lipid levels in Vietnam veterans with chronic posttraumatic stress disorder. Biological Psychiatry, 45, 374–377. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Joshi K, Ma M (2009). Hypertension in relation to posttraumatic stress disorder and depression in the U.S. National Comorbidity Survey. Behavioral Medicine, 34, 125–131. [DOI] [PubMed] [Google Scholar]
- King DW, Leskin GA, King LA, Weathers FW (1998). Confirmatory factor analysis of the clinician-administered PTSD Scale: Evidence for the dimensionality of posttraumatic stress disorder. Psychological Assessment, 10, 90–96. [Google Scholar]
- Kranzler HR, Kadden R, Barbor TF, Tennen H, Rounsaville BJ (1996). Validity of the SCID in substance abuse patients. Addiction, 91, 859–868. [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K (2000). Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychological Assessment, 12, 210–224. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC (2014). The weight of traumatic stress: A prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry, 71(1), 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Jones C, Eaton WW (2009). A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychology, 28(1), 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl RO (1999). Design of experiments: Statistical principles of research design and analysis. Belmont, CA: Duxbury Press. [Google Scholar]
- Maia DB, Marmar CR, Mendlowicz MV, Metzler T, Nóbrega A, Peres MC, Coutinho ES,…Figueira I (2007). Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. Journal of Affective Disorders, 107, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGruder-Johnson AK, Davidson ES, Gleaves DH, Stock W, Finch JF (2000). Interpersonal violence and posttraumatic symptomatology: The effects of ethnicity, gender, and exposure to violent events. Journal of Interpersonal Violence, 15, 205–221. [Google Scholar]
- McShane JM., Zirkel S (2008). Dissociation in the binge-purge cycle of bulimia nervosa. Journal of Trauma & Dissociation, 9(4), 463–479. [DOI] [PubMed] [Google Scholar]
- Norris FH, Foster JD, Weishaar DL (2002). The epidemiology of sex differences in PTSD across developmental, societal, and research contexts In Kimerling R, Ouimette P & Wofle J (Eds.), Gender and PTSD (pp. 3–42). New York: The Guilford Press. [Google Scholar]
- Ouimette P, Cronkite R, Henson BR, Prins A, Gima K, Moos RH (2004). Posttraumatic stress disorder and health status among female and male medical patients. Journal of Traumatic Stress, 17(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Polito FA, Malenka DJ, Robb JF, Burke WC, Yeo KT (2000). Clinical performance of the Cholestech L.D.X. analyzer for the determination of lipid profiles in patients undergoing cardiac catheterization. AACC 52nd Annual Meeting. (Abstract). [Google Scholar]
- Richardson M, Ainsworth B, Jacobs D, & Leon A (2001). Validation of the Stanford 7-day recall to assess habitual physical activity. Annals of Epidemiology, 11, 145–153. [DOI] [PubMed] [Google Scholar]
- Robinson S (2000). Body image and body recovery In Shalev AY & Yehuda R (Eds.), International handbook of human response to trauma. The Plenum Series on Stress and Coping (pp. 163–177). Dordrecht, Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Sallis J, Haskell W, Wood P, Fortmann SP, Rogers T, Blair SN, & Paffenbarger RS (1985). Physical activity assessment methodology in the Five-City Project. American Journal of Epidemiology, 121, 91–106. [DOI] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells E, Oakley-Browne MA (2008). Obesity and mental disorders in the adult general population. Journal of Psychosomatic Research, 64, 97–105. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P,…Kubzansky LD (2017). Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biological Psychiatry, 82, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q,…Koenen KC (2015). Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation, 132, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Kabrhel C, Roberts AL, Chen Q, Winning A,…Koenen KC (2016). Associations of trauma exposure and posttraumatic stress symptoms with venous thromboembolism over 22 years in women. Journal of the American Heart Association, 5, e003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Roberts AL, Gilsanz P, Chen Q, Winning A,…Koenen KC (2016). Posttraumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychological Medicine, 46(15), 3105–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer Z, Barbosa-Leiker C, McDonell M, Nelson L, Buchwald D, Manson S. (2017). Early life trauma, post-traumatic stress disorder, and allostatic load in a sample of American Indian adults. Am J Hum Biol, 29, e22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson A, Mahaney MC, Diego VP, Cox LA, Rogers J, VandeBerg JL (2008). Genotype-by-diet effects on co-variation in Lp-PLA2 activity and LDL-cholesterol concentration in baboons fed an atherogenic diet. Journal of Lipid Research, 49, 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM (1999). Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment, 11, 124–133. [Google Scholar]
- Weiner EJ, Stephens L (1996). Sexual barrier weight: A new approach In Schwartz MF, & Cohn L (Eds.), Sexual abuse and eating disorders (pp. 68–77). New York: Brunner/Mazel. [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison HC,… Wright JT (2017). ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, HYP.0000000000000065. [Google Scholar]
- Wiederman MW, Sansone RA, Sansone LA (1999). Obesity among sexually abused women: An adaptive function for some? Women’s Health, 29, 89–100. [DOI] [PubMed] [Google Scholar]
- Winning A, Gilsanz P, Koenen KC, Roberts AL, Chen Q, Sumner JA,…Kubzansky LD (2017). Post-traumatic stress disorder and 20-year physical activity trends among women. American Journal of Preventive Medicine, 52(6), 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich SA, Crosby RD, Mitchell JE, Thompson KM, Redlin J, Demuth G,…Haseline B (2001). Eating disturbance and sexual trauma in childhood and adulthood. International Journal of Eating Disorders, 30(4), 401–412. [DOI] [PubMed] [Google Scholar]
- Xue Y, Taub PR, Iqbal N, Fard A, Wentworth B, Redwine L,…Maisel A (2012). Cardiac biomarkers, mortality, and posttraumatic stress disorder in military Veterans. The American Journal of Cardiology, 109, 1215–1218. [DOI] [PubMed] [Google Scholar]