Abstract
Stress is a contributing factor in the etiology of several mood and anxiety disorders, and social defeat models are used to investigate the biological basis of stress-related psychopathologies. Male Syrian hamsters are highly aggressive and territorial, but after social defeat they exhibit a conditioned defeat (CD) response which is characterized by increased submissive behavior and a failure to defend their home territory against a smaller, non-aggressive intruder. Hamsters with dominant social status show increased c-Fos expression in the infralimbic (IL) cortex following social defeat and display a reduced CD response at testing compared to subordinates and controls. In this study, we tested the prediction that dominants would show increased defeat-induced neural activity in IL, but not prelimbic (PL) or ventral hippocampus (vHPC), neurons that send efferent projections to the basolateral amygdala (BLA) compared to subordinates. We performed dual immunohistochemistry for c-Fos and cholera toxin B (CTB) and found that dominants display a significantly greater proportion of double-labeled c-Fos + CTB cells in both the IL and PL. Furthermore, dominants display more c-Fos-positive cells in both the IL and PL, but not vHPC, compared to subordinates. These findings suggest that dominant hamsters selectively activate IL and PL, but not vHPC, projections to the amygdala during social defeat, which may be responsible for their reduced CD response. This project extends our understanding of the neural circuits underlying resistance to social stress, which is an important step towards delineating a circuit-based approach for the prevention and treatment of stress-related psychopathologies.
Keywords: Social Dominance, Amygdala, Medial Prefrontal Cortex, Social Defeat, Cholera Toxin B, Resilience
Introduction
Stress is a contributing factor in the etiology of several mood and anxiety disorders (Abelson et al., 2007; Heim et al., 2008; Meewisse et al., 2007). However, many individuals who experience stressful events do not develop a stress-related psychopathology. Resilience refers to the ability to maintain normal levels of psychological, biological, and social functioning following a trauma or stressful event. Because some people cope better with stress than others, resilience to the negative effects of aversive and traumatic events varies within the human population. Post-traumatic stress disorder (PTSD) is one example of how symptoms such as hyper-vigilance, helplessness, avoidance, and increased arousal may develop in some, but not all, people who experience trauma.
Rodent models of social defeat stress have strong face, construct, and predictive validity for stress-induced psychopathologies (Huhman, 2006; Nestler and Hyman, 2010). One example of striking behavioral changes that result from social defeat is observed in Syrian hamsters. Male Syrian hamsters are highly aggressive and territorial animals; but after a single defeat experience, they no longer defend their home territory in a subsequent social interaction test, even when confronted with a smaller, non-aggressive intruder. Instead, a defeated hamster will show defensive postures and display submissive behaviors such as flees and tail raises. This defeat-induced change in agonistic behavior is called conditioned defeat (CD) (Huhman et al., 2003). We have previously shown that dominant hamsters exhibit less submissive and defensive behavior at CD testing compared to subordinates and controls and thereby show a reduced CD response (Morrison et al., 2011). Importantly, dominant hamsters acquire resistance to CD during the long-term maintenance of their social status, because hamsters that win encounters for 14 days show CD resistance, but hamsters that win encounters for one or seven days do not (Morrison et al., 2014). In sum, these findings suggest that differential CD responses of dominant and subordinate hamsters are experience-dependent.
The medial prefrontal cortex (mPFC), which includes the infralimbic (IL) and prelimbic (PL) cortices, controls emotion regulation, working memory, and executive function (Dalley et al., 2004; Davidson, 2002; Wang et al., 2006). Importantly, the mPFC also regulates many aspects of the stress response and promotes coping with stress. For example, the mPFC is part of a neural circuit that inhibits neurons in the paraventricular nucleus of the hypothalamus and supports extinction of the neuroendocrine stress response (Radley et al., 2009; Radley and Sawchenko, 2011). The mPFC is also critical for the ability of environmental enrichment to promote stress resistance, as lesioning of the IL prior to environmental enrichment eliminates resistance to the effects of social defeat stress in mice (Lehmann and Herkenham, 2011). In squirrel monkeys, exposure to mild maternal separation early in life (i.e. stress inoculation) leads to decreased anxiety, increased novel object exploration, and diminished stress-induced cortisol levels (Parker et al., 2004; Parker et al., 2007). Importantly, monkeys exposed to this mild maternal separation also exhibit increased grey and white matter in the mPFC compared to non-separated controls, indicating that the process of coping with mild early life stress increases myelination and volume of the mPFC (Katz et al., 2009). In addition, we have shown previously that dominant hamsters not only display reduced CD compared to subordinates, but they also have increased defeat-induced neural activation in the IL (Morrison et al., 2012). Furthermore, pharmacological inactivation of the mPFC prior to social defeat reinstates normal levels of CD in dominant hamsters (Morrison et al., 2013). In sum, neural activity within the mPFC is necessary for resistance to the behavioral and physiological effects of stress.
The mPFC also has a well-established role in PTSD. Neuroimaging studies have revealed that the vmPFC is one area of hypoactivation in subjects with PTSD relative to controls (Bremner et al., 1999; Bremner et al., 1999; Hayes et al., 2012). Additionally, when the mPFC was hypoactivated, greater amygdala activation was observed in PTSD patients (Hayes et al., 2012; Rauch et al., 2000; Shin et al., 2004). Further, PTSD is often conceptualized as a deficit in fear extinction (Milad et al., 2008; Orr et al., 2000; Wessa and Flor, 2007), and studies on the mechanisms of fear extinction have also implicated the mPFC and its connection with the amygdala (Hefner et al., 2008; Linnman et al., 2012; Phelps et al., 2004).
Pyramidal neurons in the IL send glutamatergic projections to both the basolateral amygdala (BLA) and central amygdala (CeA) (Gabbott et al., 2005; McDonald et al., 1996; Vertes, 2004), and these glutamatergic inputs inhibit the BLA and CeA via GABAergic interneurons (Ehrlich et al., 2009). Furthermore, the IL sends robust projections to the intercalated (ITC) GABAergic neurons located between the BLA and CeA, as does the PL, albeit to a lesser extent (McDonald et al., 1996; Pinard et al., 2012). These ITC cells provide feed-forward inhibition to the CeA in response to glutamatergic input into the BLA (Paré and Smith, 1993) and are required for the expression of fear extinction (Likhtik et al., 2008). It is also well established that neural activity specifically within the IL is required for the formation of an extinction memory (Milad and Quirk, 2002; Milad et al., 2004). More specifically, an IL-to-BLA pathway has also been shown to regulate the decrease in conditioned fear that results from extinction training (Herry et al., 2010; Sierra-Mercado et al., 2011).
Pyramidal neurons in the PL also send robust projections to the BLA and CeA (Gabbott et al., 2005; McDonald et al., 1996; Vertes, 2004). However, the role of PL-to-amygdala projections in stress-related behavior is somewhat mixed. Following intra-PL cholecystokinin (CCK) administration to induce anxiety, optogenetic activation of a PL-to-BLA projection was able to decreased anxiety in an elevated plus maze (Vialou et al., 2014). Other studies suggest that neural activity in a PL-to-BLA pathway promotes fear expression. Specifically, BLA-projecting neurons within the PL were selectively activated during the renewal of fear (Orsini et al., 2011). Activity within PL-to-lateral amygdala neurons may also promote fear expression over retrieval of an extinction memory (Knapska et al., 2012). Additionally, ventral hippocampus (vHPC) neurons projecting to the BLA have been shown to express increased c-Fos during fear renewal (Jin and Maren, 2015; Orsini et al., 2011).
While multiple cortical and limbic afferents to the amygdala modulate fear and anxiety, it is unclear which projections modulate status-dependent differences in stress vulnerability. In this study, we predicted that acute social defeat would lead to greater activation of IL-to-BLA, but not a PL-to-BLA or vHPC-to-BLA, neural projections in dominant hamsters compared to subordinates and controls.
Experimental Procedures
Subjects
Subjects were male Syrian hamsters (3–4 months old, 120–180 g) obtained from our breeding colony that is derived from animals purchased from Charles River Laboratories. Older hamsters (>6 months, >190g) were individually housed and used as resident aggressors for social defeat stress. All animals were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Food and water were available ad libitum. Cages were not changed for one week prior to dominant–subordinate encounters to allow individuals to scent mark their territory. Subjects were handled several times one week prior to dominant–subordinate encounters to habituate them to the stress of human handling. Animals were housed in a temperature controlled colony room (21 ± 2°C) and kept on a 14:10 h light:dark cycle to maintain reproductive condition. All behavioral protocols were performed during the first 3 h of the dark phase of their cycle. All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Dominant-subordinate encounters
To allow animals to establish social dominance, 32 subjects were weight-matched into resident-intruder dyads and paired in daily social encounters on Days 1–9 and 12–16. Subjects were randomly assigned as a resident or intruder, and all dominance encounters occurred in the resident’s home cage. Encounters were 10 min in duration prior to the establishment of dominance relationships, while all subsequent encounters were 5 min. Dominant and subordinate animals were identified by the direction of agonistic behavior within each dyad. Thirty-one control hamsters (18 social status controls and 13 no defeat controls) did not receive dominance encounters.
Stereotaxic surgery and injection of retrograde tracer
On Day 10 all animals were anesthetized with isoflurane, mounted in a stereotaxic instrument, and received a unilateral injection of the retrograde tracer cholera toxin B (CTB; List Biological Laboratories; 1% solution) into the BLA. Dominance encounters were paused for stereotaxic surgery on Day 10 because previous research indicates maximal retrograde transport of CTB 7 days post-injection (Been and Petrulis, 2011; Coolen and Wood, 1998). The stereotaxic coordinates were 0.6 mm posterior and 3.95 mm lateral to bregma and 6.5 mm below dura. The side of the injection was counterbalanced across all experimental groups. Pressure injections were used to deposit 50 nL of CTB solution over a 5 min period, and the syringe was left in place for 10 min following the injection to minimize flow of the solution up the needle tract. Animals were given 48 hrs to recover from surgery before resuming daily dominance encounters.
Acute social defeat stress
Acute social defeat stress consisted of subjects being placed in the home cages of three separate resident aggressors on Day 17. Resident aggressors were also prescreened to ensure that they reliably attacked and defeated intruders. Subjects (dominants, subordinates, and social status controls) were exposed to three resident aggressors in consecutive 5-min aggressive encounters at 5-min inter-trial intervals. The first defeat episode began when the subject submitted to an attack from the resident aggressor. Subjects submitted immediately in the second and third defeat episodes. No defeat control animals were placed in the empty home cages of three separate resident aggressors for three 5-min exposures to control for the novel environment and olfactory cues associated with social defeat stress. Social defeats were digitally recorded for behavioral analysis and monitored closely in real time. The number of attacks received during social defeat stress, the duration of aggressive behavior received during social defeat stress, as well as whether or not subjects fought back during the first defeat were scored by an observer blind to treatment conditions. This observer achieved 90% agreement on aggressive behavior in a subset of existing video files used for reliability training. Aggressive encounters were carefully monitored for wounding and animals that received minor scratches were treated with antiseptic solution.
Histology and immunohistochemistry
60 min after social defeat stress or control procedures, all animals were anesthetized with isoflurane and transcardially perfused with 100 ml of 0.1 M phosphate buffered solution (PB; pH 7.4) followed by 100 ml of 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde for 24 h, followed by 0.1 M PB/30% sucrose solution for 48 h, and then were stored in cryoprotectant, all at 4 °C. A consecutive series of 40 μm coronal sections were cut submerged in PB on a vibrating microtome, collected in 9 vials, and stored as free-floating sections in cryoprotectant at 4 °C. The collected sections were processed for either CTB (sections containing the amygdala complex) or c-Fos + CTB (sections containing the vmPFC or vHPC) immunoreactivity (IR). After immunohistochemistry, all sections were washed with distilled H2O prior to being mounted on glass microscope slides. After air-drying for 48 hrs, sections were dehydrated using a series of alcohols, cleared with citrosolv, and coverslipped using DPX mountant (Sigma-Aldrich). All vmPFC or vHPC tissue was processed simultaneously, while injection sites on amygdala sections were processed by cohort.
For CTB labeling, sections were rinsed in five 10 min washes in a phosphate buffered Triton solution (0.2% PB-Tx; 0.2% Triton X-100 in 0.1 M PB, pH 7.4) before each incubation, which were conducted at room temperature (RT) unless otherwise stated. Sections were quenched for endogenous peroxidase activity in a 0.3% hydrogen peroxide and 30% methanol solution for 25 min. Sections were then incubated in 5% horse serum (HS) in 0.2% PB-Tx for 30 min to block non-specific binding. Next, sections were incubated 1 hr in 5% HS in 0.2% PB-Tx with CTB primary antibody at 1:40,000 concentration (goat anti-CTB, List Biological Laboratories). Sections were then incubated in the primary antibody solution for an additional 48 hrs at 4 °C. Following incubation in the CTB primary antibody solution, the sections were rinsed in 0.2% PB-Tx and then incubated for 1 hr in 0.2% PB-Tx with biotinylated horse, anti-goat IgG antibody at 1:200 concentration (Vector Laboratories: BA-9500). Sections were then incubated for 1 hr in 0.2% PB-Tx with an avidin-biotin complex (ABC Kit, Vector Laboratories: PK-4000), and the peroxidase reaction with visualized using a 10–15 min incubation in 50% 3,3’-diaminobenzidine (DAB tablet, Sigma-Aldrich: D5905) and hydrogen peroxide dissolved in PB. Finally, sections were rinsed in five 10 min PB washes.
For c-Fos + CTB labeling, sections were first rinsed in three 5 min washes in PB (all rinses and incubations were conducted at RT unless otherwise stated). Then sections were treated with freshly prepared 0.5% sodium borohydride in PB for 30 min. Sections were next rinsed in six 2 min washes in PB. Then, sections were blocked in a phosphate buffered Triton solution (0.5% PB-Tx; 0.5% Triton X-100 in 0.1 M PB, pH 7.4) for 10, 30, and 10 min. Sections were next incubated 1 hr in 5% HS in 0.5% PB-Tx with primary antibody at 1:40,000 concentration (goat anti-CTB, List Biological Laboratories). Then sections were incubated in the primary antibody solution for an additional 48 hrs at 4 °C. Next, sections were rinsed in four 15 min washes in 0.5% PB-Tx (as were all subsequent washes prior to each incubation unless otherwise stated). Sections were then incubated for 1 hr in 0.5% PB-Tx with biotinylated horse, anti-goat IgG antibody at 1:200 concentration (Vector Laboratories: BA-9500), incubated for 1 hr in 0.5% PB-Tx with an avidin-biotin complex (ABC Kit, Vector Laboratories: PK-4000), and then the peroxidase reaction was visualized using a 5–10 min incubation in 5% 3,3’-diaminobenzidine (DAB tablet, Sigma-Aldrich: D5905) and hydrogen peroxide dissolved in PB. Tissue was then dunked a few times in a fresh 0.5% PB-Tx rinse before being rinsed in three 10 min washes in 0.5% PB-Tx. Next, sections were incubated for 15 min in 5% Avidin-D (Vector Laboratories: SP-2001) in 0.5% PB-Tx. Sections were then dunked a few times in a fresh 0.5% PB-Tx rinse. Next, sections were incubated overnight at RT in c-Fos primary antibody at 1:5,000 concentration (rabbit anti-c-Fos, Santa Cruz: sc-52) with 5% Biotin blocker (Vector Laboratories: SP-2001) and 5% HS in 0.5% PB-Tx. Following incubation in the c-Fos primary antibody solution, the sections were rinsed in 0.5% PB-Tx and then incubated for 1 hr in 0.5% PB-Tx with biotinylated horse, anti-rabbit IgG antibody at 1:200 concentration (Vector Laboratories: BA-1100). Sections were then incubated for 1 hr in 0.5% PB-Tx with an avidin-biotin complex (ABC Kit, Vector Laboratories: PK-4000), and the peroxidase reaction was visualized using a 10–20 min incubation in 25% 3,3’-diaminobenzidine (DAB tablet, Sigma-Aldrich: D5905) with ammonium nickel sulfate hexahydrate and hydrogen peroxide dissolved in PB. Sections were then dunked a few times in a fresh 0.5% PB-Tx rinse, rinsed in two 10 min 0.5% PB-Tx washes, and finally washed one time for 10 min in PB.
Immunohistochemistry analysis
Localization of CTB injection sites and quantification of c-Fos, CTB, and c-Fos + CTB-IR was performed in real-time using an Olympus BX51 microscope. Localization of CTB injection sites in the amygdala was performed at 2× magnification, while quantifications in the IL, PL, and vHPC were performed at 20× magnification. Double-labeling for c-Fos + CTB-IR was also verified at 40× magnification in real time. Cell counts were limited to the area within defined boxes, and two boxes at 20× magnification per tissue section were analyzed and captured using Olympus DP Controller. The box sizes used for quantification at 20× magnification were 439 μm × 330 μm (width × height), resulting in a total tissue amount of 439 μm × 660 μm in the PL and IL and 878 × 330 μm in the vHPC.
CTB-IR was indicated by brown cytoplasmic staining and c-Fos-IR was indicated by black nuclear straining. Importantly, c-Fos + CTB-IR was clearly indicated by black nuclear staining with brown cytoplasmic staining in a center-surround manner, respectively. Quantification in the IL and PL was centered on layers II/III of the cortex, as this is where the majority of CTB-IR cells were observed. Furthermore, for the vHPC, preliminary studies indicated that defeat-induced c-Fos expression in Syrian hamsters is localized to the most ventral part of the CA1 region of the vHPC; therefore we focused our quantifications in this area. For each brain region, we quantified 3–8 tissue sections per individual along a rostral-caudal axis, and quantifications were performed by observers blind to treatment condition. We manually counted IR cells and applied a standard criteria for determining which cells showed staining above background levels. In addition, we established inter-rater reliability on select images as 90% agreement on total c-Fos, CTB, and c-Fos + CTB-IR.
Statistical analysis
Data were analyzed with Student’s t-tests or one-way ANOVAs followed by Tukey’s post-hoc tests, where appropriate. No differences were found between right and left CTB injections, and data were thus pooled for analysis. In addition, chi-square tests were used to analyze the proportion of animals that fought back during social defeat. Statistical significance was set at p < 0.05. Data are reported as mean ± SEM, except where noted.
Results
Behavioral Data
On average, dominance relationships were established on Day 1.41 (SD = 0.57) and the direction of aggression remained consistent throughout the dyadic encounters (Table 1). Unfortunately, the social defeat videos for some animals (n = 7) were lost due to camera malfunctions. In those animals we were able to quantify, there were no significant differences between dominant, subordinate, and social status control hamsters in the number of attacks received or the duration of aggressive behavior received during social defeat stress (F(2, 40) = 0.060, p = 0.942, F(2, 40) = 0.401, p = 0.673, respectively). We also found that dominant animals (10/12) were more likely to fight back against the resident aggressor during the first social defeat episode compared to subordinates (3/13) (χ2 = 9.077, df = 1, p = 0.003), but not social status controls (11/18) (χ2 = 1.693, df = 1, p = 0.193). In those animals we were unable to quantify from videos, we verified from our handwritten notes that observers trained in aggressive behavior reliability took, they too received an adequate amount of aggression during all three defeats.
Table 1.
Agonistic behavior during the maintenance of dominance relationships.
| Social status | Submissive behavior (sec) (mean ± SEM) |
Aggressive behavior (sec) (mean ± SEM) |
||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 1 | Day 7 | Day 14 | |
| Subordinate | 229.45 ± 39.17 | 204.76 ± 13.66 | 201.41 ± 17.46 | ___ | ___ | ___ |
| Dominant | ___ | ___ | ___ | 177.04 ± 28.31 | 134.39 ± 22.83 | 152.20 ± 27.08 |
Subjects were weight-matched in resident-intruder dyads and paired daily in social encounters for 14 days. Day 1 encounters were 600 sec in duration, while days 7 and 14 encounters were 300 sec in duration. Dominants displayed high rates of aggression throughout all 14 days, while subordinates maintained high rates of submissive behavior. No dominant animals exhibited submissive behavior and no subordinates showed aggression.
Immunohistochemistry Data
Localization of CTB Injection Sites in the Amygdala
A representative CTB injection site is shown in Figure 1A, as well as a schematic illustration of maximal and minimal infusions (Fig. 1B; atlas image was adapted from (Morin and Wood, 2001). For some animals, injections spread from the BLA into the CeA (BLA/CeA), while others had injections completely confined to the BLA or CeA. Because we had a substantially larger sample size of animals with BLA/CeA injections (n = 5–9 per group) compared to BLA injections (n = 2–4 per group), we analyzed c-Fos + CTB-IR in animals with both BLA and BLA/CeA injections combined and then confirmed these findings in animals with BLA injections. Because the sample size of animals with CeA injections was very small (n = 0–4 per group), we did not quantify c-Fos + CTB-IR but, rather, quantified CTB-IR in these animals to investigate neuroanatomical difference in inputs to the CeA and BLA.
Figure 1. Localization of CTB injection sites in the amygdala.
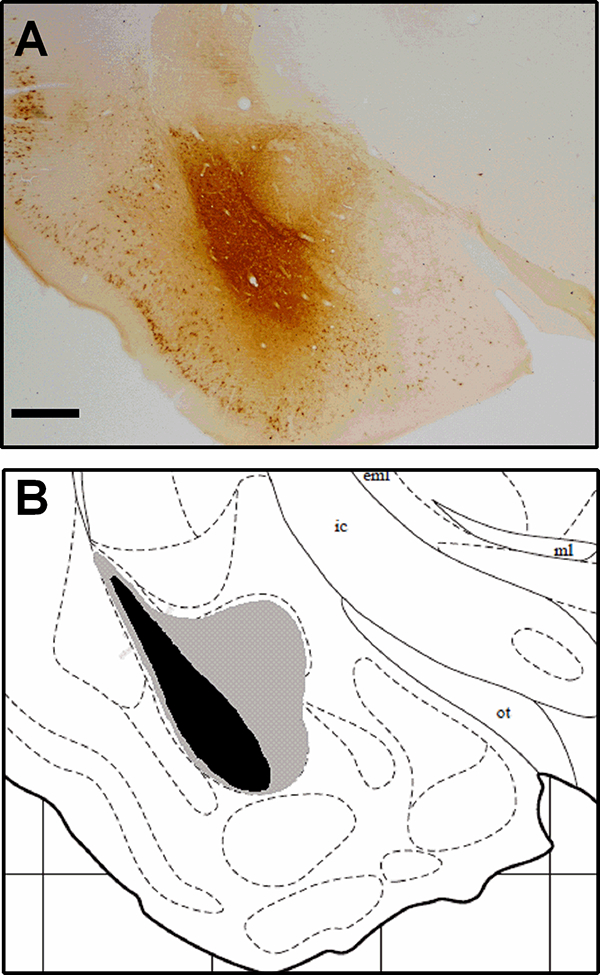
A) Photomicrograph from representative image of CTB immunohistochemistry in the amygdala at 2× magnification. Scale bar = 500μm. B) Schematic images of maximal and minimal CTB spread; gray indicates hamsters with maximal CTB spread, and black indicates hamsters with the smallest injection of CTB. Atlas image was adapted from Morin and Wood (2001).
Quantifications in the PL, IL, and vHPC
Immunoreactivity for c-Fos, CTB, and c-Fos + CTB positive cells was assessed in the PL, IL, and vHPC for animals that received CTB injections into the BLA and BLA/CeA. Representative photomicrographs of c-Fos positive cells co-localized with CTB positive cells in the vmPFC and vHPC are shown in Figure 2. Additionally, representative photomicrographs of c-Fos + CTB dual immunohistochemistry in the IL across all four experimental groups are shown in Figure 3. Because variation in CTB injection volume and degree of retrograde transport might result in variation in CTB-IR, we calculated the proportion of double-labeled cells as the number of c-Fos + CTB-IR cells divided by the total number of CTB-IR cells × 100 for each animal.
Figure 2. Co-localization of c-Fos and CTB.
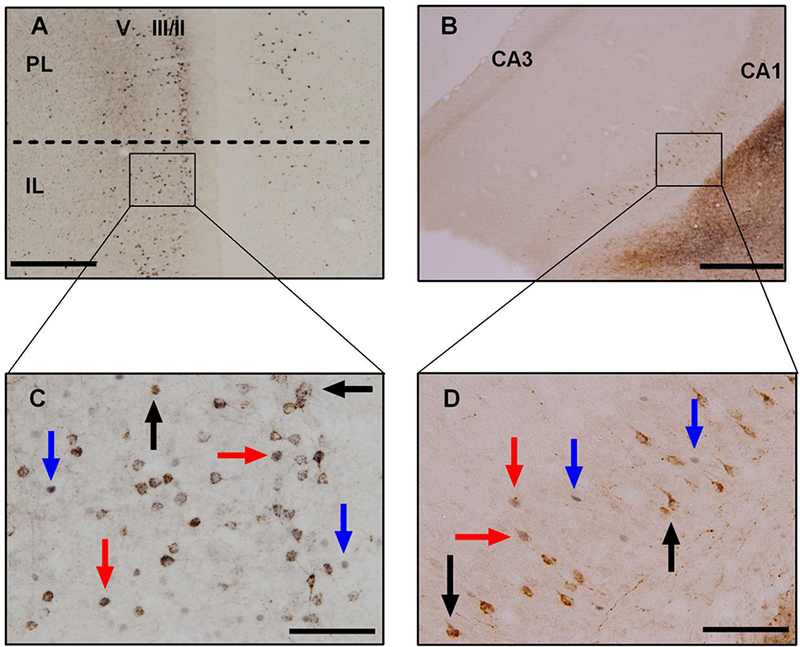
Photomicrographs from representative images of c-Fos + CTB dual immunohistochemistry in A) the IL at 4× magnification, in B) the vHPC at 4× magnification, in C)the IL at 20× magnification, and finally in D) the vHPC at 20× magnification. Cells with brown cytoplasmic staining (black arrows) are CTB positive, cells with black nuclear staining (blue arrows) are c-Fos positive, and cells with black nuclear staining surrounded by brown cytoplasmic staining (red arrows) are c-Fos + CTB positive. Scale bar = 500 μm at 4× magnification, while scale bar = 100 μm at 20× magnification.
Figure 3. Comparison of c-Fos + CTB staining in the IL.
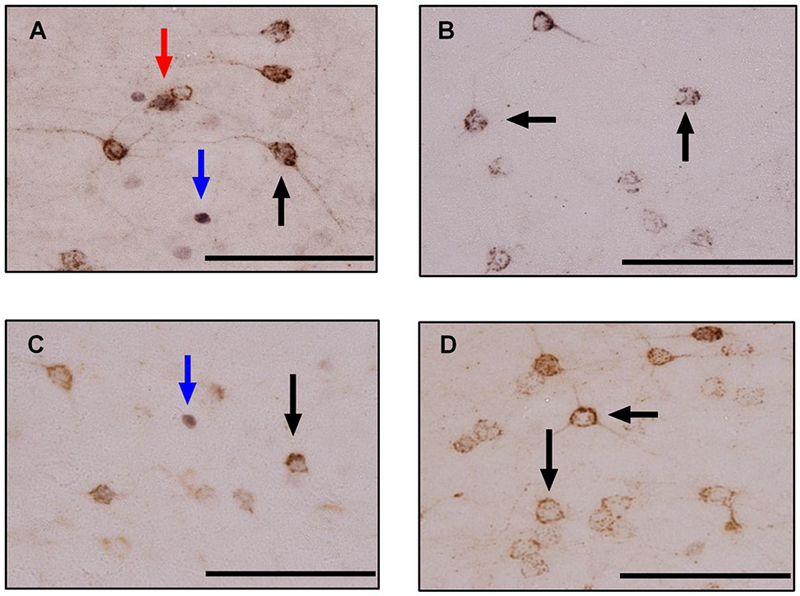
Photomicrographs of c-Fos + CTB dual immunohistochemistry at 40× magnification from a representative A) dominant, B) subordinate, C) social status control, and D) no defeat control. Cells with brown cytoplasmic staining (black arrows) are CTB positive, cells with black nuclear staining (blue arrows) are c-Fos positive, and cells with black nuclear staining surrounded by brown cytoplasmic staining (red arrows) are c-Fos + CTB positive. Scale bar = 100 μm.
In the PL, we found significant differences in the expression of c-Fos-IR (Fig. 4A; F(3, 40) = 13.611, p < 0.001); dominants had significantly greater c-Fos-IR compared to subordinates, social status controls, and no defeat controls (p < 0.001, p = 0.001, and p < 0.001, respectively). There were no significant differences in CTB-IR across groups (Fig. 4B; F(3, 40) = 0.521, p = 0.670), suggesting that the efferent projections themselves are not affected by dominance status. However, there were significant differences in the proportion of c-Fos + CTB-IR (Fig. 4C; F(3, 40) = 6.236, p = 0.001), such that dominants had a significantly greater proportion of double-labeled cells compared to subordinates and no defeat controls (p = 0.004 and p = 0.002, respectively).
Figure 4. Dominance status modulates neural activity in both PL-to-BLA/CeA and IL-to-BLA/CeA projections, but not in a vHPC-to-BLA/CeA projection.
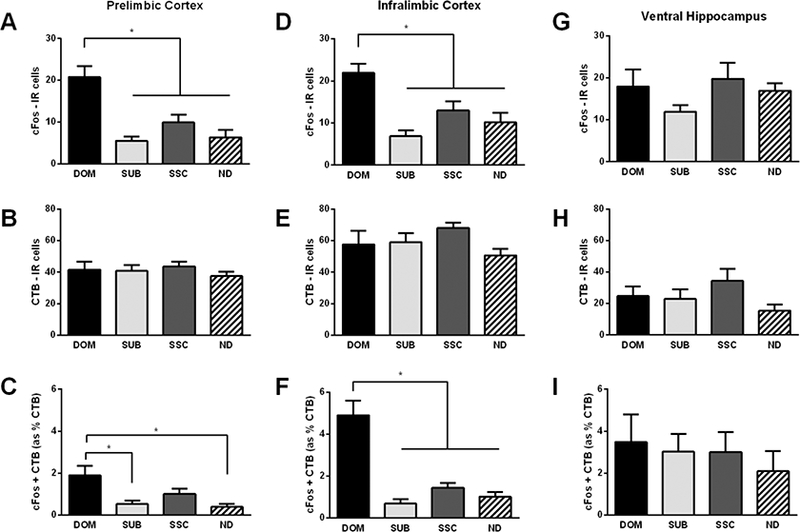
In the PL, A) dominants (DOM, n = 9) display significantly greater c-Fos-IR compared to subordinates (SUB, n = 12), social status controls (SSC, n = 12), and no defeat controls (ND, n = 11). B) No significant differences were found in CTB-IR. C) Dominants display a significantly greater proportion of double-labeled c-Fos + CTB-IR compared to subordinates and no defeat controls. In the IL, D) dominants (n = 9) display significantly greater c-Fos-IR compared to subordinates (n = 12), social status controls (n = 12), and no defeat controls (n = 11). E) No significant differences were found in CTB-IR. F) Dominants display a significantly greater proportion of double-labeled c-Fos + CTB-IR compared to subordinates, social status controls, and no defeat controls. In the vHPC, no significant differences were found in G) c-Fos-IR (DOM, n = 7; SUB n = 12; SSC, n = 12, and ND, n = 12), H) CTB-IR, or I) the proportion of double-labeled c-Fos + CTB-IR. The above data includes animals with both BLA and BLA/CeA hits. Data are shown as mean ± SEM. An asterisk indicates a significant difference between groups as determined by a Tukey’s post hoc test (*p < 0.05).
In the IL, we also found significant differences in the expression of c-Fos-IR (Fig. 4D; F(3, 40) = 9.556, p < 0.001). Specifically, dominants had greater c-Fos-IR compared to subordinates (p < 0.001), social status controls (p = 0.019), and no defeat controls (p = 0.002). As expected, there were no significant differences in the amount of CTB-IR (Fig. 4E; F(3, 40) = 1.847, p = 0.154). Importantly, significant differences were observed in the proportion of double-labeled c-Fos + CTB-IR cells (Fig 4F; F(3, 40 = 27.648, p < 0.001). Specifically, dominants had a greater proportion of these double-labeled cells compared to subordinates, social status controls, and no defeat controls (all p’s < 0.001). Together, these data suggest that dominant hamsters not only activate the PL and IL significantly more than subordinates, they specifically activate both PL-to-amygdala and IL-to-amygdala neuronal projections during social defeat stress.
In the vHPC, we found no significant differences in c-Fos-IR (Fig. 4G; F(3, 39) = 1.501, p = 0.230). Additionally, no significant differences in CTB-IR were noted (Fig. 4H; F(3, 39) = 1.722, p = 0.178). Furthermore, there were no significant differences observed in the proportion of double-labeled c-Fos +CTB cells in the vHPC (Fig. 4I; F(3, 39) = 0.325, p = 0.808).
To determine whether status-dependent differences in neural activity are found specifically in axonal projections to the BLA, we quantified the proportion of double-labeled c-Fos + CTB-IR cells in animals with injections confined to the BLA. We found significant differences in the proportion of double-labeled c-Fos + CTB-IR cells in the IL (F(3, 9) = 15.66, p = 0.0006). More specifically, dominants (3.783 ± 0.513) had a significantly higher proportion of double-labeled c-Fos + CTB-IR cells compared to subordinates (0.237 ± 0.273; p < 0.001), social status controls (1.258 ± 0.616; p = 0.009), and no defeat controls (0.0 ± 0.0; p < 0.001). However, in the PL, these proportions did not reach significance (F(3, 9) = 2.627, p = 0.114) with dominants, subordinates, social status controls, and no defeat controls displaying proportions of 1.302 ± 0.461, 0.0 ± 0.0, 0.838 ± 0.518, and 0.272 ± 0.272, respectively. In the vHPC, on the other hand, no significant differences in the proportion of double-labeled cells were noted (F(3, 8) = 0.615, p = 0.624), and dominants, subordinates, social status controls, and no defeat controls displayed proportions of 5.902 ± 2.521, 3.520 ± 1.481, 2.497 ± 0.980, and 2.930 ± 2.930, respectively.
To examine the relative size of the efferent projections from the IL and PL we compared the number of CTB-IR cells in animals with injections confined to the BLA and CeA. Those subjects with injections confined to the BLA did not differ in CTB-IR in the IL (F(3, 9) = 0.690, p = 0.581) or PL (F(3, 9) = 0.671, p = 0.591); therefore, these subjects were collapsed. In the IL, when those subjects with injections confined to the BLA (57.48 ± 4.363) were compared to those subjects with CeA injections (23.06 ± 2.904), CeA animals showed significantly lower CTB-IR (t(17) = 5.065, p < 0.0001). Furthermore, in the PL, CeA animals (12.56 ± 2.981) showed significantly lower CTB-IR compared to BLA animals (34.52 ± 3.306; t(17) = 4.132, p = 0.0007). This finding indicates that there are fewer efferent projections from both the IL and PL to the CeA compared to the BLA. More specifically, there were approximately three times as many IL cells projecting to the BLA compared to the CeA and two times as many PL cells projecting to the BLA compared to the CeA.
There is an association between latency to assume a subordinate posture and stress susceptibility in rats, as measured by hypothalamic-pituitary-adrenal dysfunction and immobility in a forced swim test (Wood et al., 2010). Therefore, we performed several correlations to determine if latency to submit in the first defeat episode was correlated with the proportion of c-Fos + CTB-IR in the PL, IL, and vHPC. However, these correlations were non-significant. Specifically, the latency to submit was not significantly correlated with the proportion of double-labeled cells in the PL (r = 0.205, p = 0.295), IL (r = 0.052, p = 0.795), or vHPC (r = 0.077, p = 0.681). We also analyzed dominant hamsters only, but again found no significant correlations between latency to submit and the proportion of double-labeled cells in the PL (r=0.234, n = 10, p = 0.516), IL (r = .298, p = .402), or vHPC (r = −.457, p = 0.362). Overall, it does not appear that the proportion of c-Fos + CTB-IR cells is associated with the latency to assume a subordinate position during the first defeat episode.
Discussion
The present study reveals that mPFC projections to the BLA are activated by dominant hamsters during acute social defeat stress. Specifically, dominant hamsters selectively activate an IL-to-BLA neuronal projection compared to subordinates and controls. Contrary to our expectations, dominant hamsters also activate a PL-to-BLA/CeA neural projection during social defeat stress significantly more than subordinates. However, no effect of dominance status was found in defeat-induced neural activity within a vHPC-to-BLA/CeA projection. We also confirmed previous findings that dominant hamsters, in general, show increased c-Fos-IR in the PL and IL, but not vHPC, compared to subordinates and no defeat controls. This study extends our previous knowledge by demonstrating that defeat-induced neural activity in the mPFC of dominant hamsters is specifically associated with activity in IL efferents to the BLA and PL efferents to the BLA/CeA. Furthermore, these findings are consistent with the hypothesis that activity within this neuronal projection is responsible for status-dependent differences in the CD response. However, given that a low proportion of mPFC cells were double labeled with c-Fos and CTB, there are likely other downstream projections from the IL and PL regulating the effect of dominance status on the CD response.
The double-labeling results in the PL-to-BLA/CeA projection were somewhat surprising, given the PL’s well-established role in fear expression. For instance, inactivation of the PL impairs fear expression (Corcoran and Quirk, 2007; Sierra-Mercado et al., 2011), while stimulation of the PL increases fear expression (Vidal-Gonzalez et al., 2006). Moreover, renewal and suppression of fear are associated with different patterns of c-Fos expression in the PL and IL, as well as differences in the proportion of double-labeled c-Fos + CTB cells in PL-to-BLA and IL-to-BLA projections. Specifically, more c-Fos in a PL-to-BLA projection is associated with fear renewal and more c-Fos in an IL-to-BLA projection is associated with the recall of extinction (Orsini et al., 2011). Similarly, in PSD-95:Venus transgenic rats, more activity in an IL-to-lateral amygdala projection is associated with retrieval of an extinction memory, and activity in a PL-to-lateral amygdala projection is associated with the renewal of fear (Knapska et al., 2012). However, our study does not address the expression and extinction of conditioned fear and the neural circuitry regulating responses to social defeat may not be identical to conditioned fear. Brains were collected after social defeat stress and not during CD testing, which means that c-Fos-IR represents neural processes associated with the acquisition of defeat-induced changes in behavior. Also, the IL-to-BLA projection was approximately twice as strong as the PL-to-BLA projection. In addition, optogenetic activation of a PL-to-BLA neural projection has been shown to reduce CCK-induced generalized anxiety in an elevated plus maze (Vialou et al., 2014).
Neural activity in the mPFC has been shown to play an important role in the immunizing effect of a brief exposure to controllable tailshock on the development of learned helplessness. More specifically, pharmacological activation of the vmPFC during a controllable tailshock blocks the immunizing effect (Amat et al., 2006), while activation of the vmPFC during an inescapable tailshock promotes immunization, even in the absence of controllability (Amat et al., 2008). Furthermore, when NMDA receptors and the extracellular signal-regulated kinase cascade are blocked, specifically within the PL, it prevents the immunizing effect of stress controllability, which suggests that activity within the PL is necessary for this form of stress resilience (Christianson et al., 2014). With these studies taken into account, it isn’t surprising that dominant hamsters have elevated c-Fos expression in the PL. Although the present data suggest dominant hamsters are activating a PL-to-amygdala projection, it is possible that they are also activing PL efferents to the dorsal raphe nucleus (DRN). For instance, prior experience with escapable tailshock increases c-Fos expression in PL neurons that project to the DRN (Baratta et al., 2009). Furthermore, chemogenetic inhibition of a PL-to-DRN projection prevents ketamine-induced resistance to learned helplessness in female rats (Dolzani et al., 2018). Also, optogenetic activation of a PL-to-nucleus accumbens projection reverses CCK-induced social avoidance and increases hedonic behavior in a sucrose preference test (Vialou et al., 2014). In short, there are several downstream projections from the PL that regulate stress-related behavior, and, at present, it isn’t clear whether these projections function independently to modulate stress-related behavior.
An IL-to-BLA neural circuit may promote the extinction of conditioned fear via several mechanisms. For example, stimulation of IL neurons activates local circuit interneurons within the BLA and suppresses conditioned fear responses (Cho et al., 2013; Rosenkranz et al., 2003). Also, changes in the synaptic efficacy of IL projections to the BLA control the extinction of conditioned fear (Cho et al., 2013; Knapska et al., 2012). IL neurons also project to the GABAergic ITC cells located on the border of the BLA and CeA and thus inhibit output neurons in the CeA (Amir et al., 2011; Likhtik et al., 2008; Pinard et al., 2012). Similarly, inactivation of the IL cortex has been shown to reduce activity of ITC neurons and impair the extinction of conditioned fear (Amano et al., 2010). Finally, chemogenetic inhibition of an IL-to-BLA projection also impairs extinction retrieval (Bloodgood et al., 2018). While efferent projections from the IL cortex suppress BLA activity and regulate the extinction of fear-related memories, the role of this neural pathway in stress resistance is less well known. The present study extends the literature of this projection beyond fear conditioning and indicates that these IL projections to the BLA are also linked to the reduced CD response observed in dominant hamsters, although we cannot, at this time, rule out a role for ITC cells in the CD response.
Considering the vHPC, optogenetic inhibition of BLA inputs to the vHPC in mice have been shown to reduce anxiety-related behaviors in an elevated plus maze and open field arena (Felix-Ortiz et al., 2013). Activation of this same pathway also increases social behavior in a resident-intruder test (Felix-Ortiz and Tye, 2014). Importantly, fear renewal procedures in rats activate vHPC inputs to the BLA (Jin and Maren, 2015). However, it does not appear that neural activity within a vHPC-to-amygdala projection is associated with vulnerability to CD in subordinate hamsters. It is noteworthy that the vHPC is necessary for the acquisition of CD, as pharmacological inactivation of the vHPC with muscimol prior to social defeat reduces submissive behavior in a social interaction test 24 hrs later (Markham et al., 2010). Thus, it was surprising that social defeat stress did not increase c-Fos expression in social status controls compared to no defeat controls. However, lack of an effect of dominance status in the vHPC is consistent with the possibility that status-dependent differences in defeat-induced social avoidance are not mediated by circuits promoting the CD response.
The current study is not without some limitations and considerations. First, the proportion of double labeled cells in the IL and PL is, admittedly, somewhat low. However, this relatively low proportion of double-labeled cells is comparable to that observed in an IL-to-BLA pathway during fear extinction (Orsini et al., 2011). Been and Petrulis (2011) found that c-Fos and another immediate early gene, Egr1, show low, but significant, levels of co-localization with CTB-positive cells in medial amygdala neurons that send projections to the bed nucleus of the stria terminalis. One possibility for these findings is that because c-Fos expression primarily reflects NMDA receptor and cAMP response element binding protein (CREB) activity (Flavell and Greenberg, 2008), it underestimates total activity within neural substrates. Another, similar possibility is that criteria for identifying double-labeled cells may also underestimate neural activity within a circuit. Further, dominant hamsters show increased c-Fos expression in many cells that do not project to the amygdala, and it is essential to determine how these cells fit into a neural circuit that modulates status-dependent differences in responses in social defeat. At any rate, an important consideration for the field is how activity in relatively discrete pathways acts within complex neural circuits to drive behavior.
In conclusion, we show here that hamsters with a dominant social status display increased defeat-induced c-Fos expression in the IL and PL, but not vHPC. Further, we show that dominant hamsters selectively activate IL neurons that project to the BLA during social defeat stress. Activity in these projections may contribute to the reduced CD response observed in dominant hamsters and contribute to status-dependent differences in coping with social stress. Ultimately, this project extends our understanding of the neural circuits underlying resistance to social stress, which is an important step towards delineating a circuit-based approach for the prevention and treatment of stress-related psychopathologies.
Highlights.
The retrograde tracer cholera toxin B (CTB) effectively labeled PL, IL, and vHPC efferents to the amygdala in Syrian hamsters
Dominant hamsters have elevated c-Fos expression in the PL and IL, but not vHPC, during acute social defeat stress
Dominants selectively activate PL and IL, but not vHPC, neural projections to the amygdala during acute social defeat stress
Acknowledgements
We would like to thank our team of undergraduate and graduate students for their daily support and technical assistance, most notably Annie Loewen. We would also like to thank Dr. Alex Osmand for his assistance optimizing the dual-label immunohistochemistry protocol. This research was supported by National Institutes of Health grant MH107007 to MAC.
Abbreviations
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- CD
conditioned defeat
- CeA
central amygdala
- CREB
cAMP response element binding protein
- CTB
cholera toxin B
- DRN
dorsal raphe nucleus
- HS
horse serum
- IL
infralimbic
- IR
immunoreactivity
- ITC
intercalated
- mPFC
vmedial prefrontal cortex
- NMDA
N-methyl-D-aspartate receptor
- PB
phosphate buffered solution
- PB-Tx
phosphate buffered Triton solution
- PL
prelimbic
- PTSD
post-traumatic stress disorder
- SD
standard deviation
- SEM
standard error of the mean
- vHPC
ventral hippocampus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have no potential conflicts of interest to report.
References
- Abelson JL, Khan S, Liberzon I, Young EA (2007), HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety 24:66–76. [DOI] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D (2010), Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF (2008), Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience 154:1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF (2006), Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci 26:13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D (2011), Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105:3054–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF (2009), Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. European Journal of Neuroscience 30:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A (2011), Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Hormones and behavior 59:536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood DW, Sugam JA, Holmes A, Kash TL (2018), Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Translational psychiatry 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS (1999), Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry 156:1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS (1999), Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry 45:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY (2013), Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80:1491–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, Watkins LR, Maier SF (2014), Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front Behav Neurosci 8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Wood RI (1998), Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. Journal of Comparative Neurology 399:189–209. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ (2007), Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience 27:840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW (2004), Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2002), Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51:68–80. [DOI] [PubMed] [Google Scholar]
- Dolzani S, Baratta M, Moss J, Leslie N, Tilden S, Sørensen A, Watkins L, Lin Y, et al. (2018), Inhibition of a Descending Prefrontal Circuit Prevents Ketamine-Induced Stress Resilience in Females. Eneuro 5:ENEURO. 0025–0018.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A (2009), Amygdala inhibitory circuits and the control of fear memory. Neuron 62:757–771. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM (2013), BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM (2014), Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. Journal of Neuroscience 34:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME (2008), Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31:563–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005), Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005), Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology 492:145–177. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM (2012), Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of mood & anxiety disorders 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson R-M, Saksida LM, Bussey TJ, Singewald N, et al. (2008), Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. Journal of Neuroscience 28:8074–8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008), The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A (2010), Neuronal circuits of fear extinction. European Journal of Neuroscience 31:599–612. [DOI] [PubMed] [Google Scholar]
- Huhman KL (2006), Social conflict models: can they inform us about human psychopathology? Hormones and behavior 50:640–646. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM (2003), Conditioned defeat in male and female Syrian hamsters. Hormones and behavior 44:293–299. [DOI] [PubMed] [Google Scholar]
- Jin J, Maren S (2015), Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Scientific reports 5:8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, Buckmaster CL, Bammer R, et al. (2009), Prefrontal plasticity and stress inoculation-induced resilience. Dev Neurosci 31:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, et al. (2012), Functional anatomy of neural circuits regulating fear and extinction. Proceedings of the National Academy of Sciences 109:17093–17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, et al. (2012), Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci U S A 109:17093–17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M (2011), Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D (2008), Amygdala intercalated neurons are required for expression of fear extinction. Nature 454:642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D (2008), Amygdala intercalated neurons are required for expression of fear extinction. Nature 454:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR (2012), Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. American Journal of Psychiatry 169:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL (2010), Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learning & memory 17:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A, Mascagni F, Guo L (1996), Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71:55–75. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M (2007), Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry 191:387–392. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008), Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of psychiatric research 42:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002), Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk G (2004), Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral neuroscience 118:389. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI (2001) The Golden Hamster Brain. Academic Press, San Diego. [Google Scholar]
- Morrison KE, Bader LR, Clinard CT, Gerhard DM, Gross SE, Cooper MA (2014), Maintenance of dominance status is necessary for resistance to social defeat stress in Syrian hamsters. Behavioural brain research 270:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Bader LR, McLaughlin CN, Cooper MA (2013), Defeat-induced activation of the ventral medial prefrontal cortex is necessary for resistance to conditioned defeat. Behavioural brain research 243:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Curry DW, Cooper MA (2012), Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience 210:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Swallows CL, Cooper MA (2011), Effects of dominance status on conditioned defeat and expression of 5-HT1A and 5-HT2A receptors. Physiology & behavior 104:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010), Animal models of neuropsychiatric disorders. Nature neuroscience 13:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK (2000), De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of abnormal psychology 109:290. [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S (2011), Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. Journal of Neuroscience 31:17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y (1993), The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57:1077–1090. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM (2004), Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry 61:933–941. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Rainwater KL, Buckmaster CL, Schatzberg AF, Lindley SE, Lyons DM (2007), Early life stress and novelty seeking behavior in adolescent monkeys. Psychoneuroendocrinology 32:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004), Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, McDonald AJ (2012), Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, McDonald AJ (2012), Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE (2009), A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29:7330–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE (2011), A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci 31:9683–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK (2000), Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological psychiatry 47:769–776. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA (2003), The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci 23:11054–11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, et al. (2004), Regional cerebral blood flow in the amygdala and medial prefrontalcortex during traumatic imagery in male and female vietnam veterans with ptsd. Archives of general psychiatry 61:168–176. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ (2011), Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ (2011), Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004), Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, et al. (2014), Prefrontal cortical circuit for depression-and anxiety-related behaviors mediated by cholecystokinin: Role of ΔFosB. The Journal of Neuroscience 34:3878–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ (2006), Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & memory 13:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS (2006), Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci 9:534–542. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H (2007), Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. American Journal of Psychiatry 164:1684–1692. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, & Bhatnagar S (2010). Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology, 151(4), 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]


