Abstract
Repeated pairings of heroin and a context results in Pavlovian associations which manifest as heroin-conditioned appetitive responses and peripheral immunomodulation upon re-exposure to heroin-paired conditioned stimuli (CS). The dorsal hippocampus (DH) plays a key role in the neurocircuitry governing these context-heroin associations. Within the DH, signaling of the pro-inflammatory cytokine interleukin-lβ (IL-lβ) is required for the expression of heroin-conditioned peripheral immunomodulation. However, the role of signaling via IL-1 receptor type 1 (IL-1R1) has not been examined. Furthermore, it has not been evaluated whether the involvement of IL-1 in associative learning extends to classically conditioned appetitive behaviors, such as conditioned place preference (CPP). The first set of experiments investigated whether DH IL-1R1 signaling during CS re-exposure modulates heroin-conditioned immunomodulation and CPP. The second set of experiments employed chemogenetic techniques to examine whether DH astroglial signaling during CS re-exposure alters the same responses. This line of investigation is based on previous research indicating that astrocytes facilitate hippocampal-dependent learning and memory through the expression of IL-1 β protein and IL-1R1. Interestingly, IL-1R1 antagonism disrupted heroin-conditioned suppression of peripheral immune parameters but failed to alter heroin-CPP. Similarly, chemogenetic activation of Gi-signaling in DH astrocytes attenuated heroin-conditioned peripheral immunomodulation, but failed to alter heroin-CPP. Collectively our data show that both IL-1R1 stimulation and astrocyte signaling in the DH are critically involved in the expression of heroin-conditioned immunomodulation, but not heroin-CPP. As such these findings strongly suggest hippocampal neural immune signaling differentially regulate Pavlovian immunomodulatory and appetitive behaviors.
Keywords: dorsal hippocampus, heroin, IL-1, learning, CPP, immune conditioning, DREADDs, IL-1 receptor antagonist, opioid
1. Introduction
Repeated pairings between environmental stimuli and the subjective and physiological effects of heroin result in robust associative learning. The consequent stimulus control over physiology and behavior is integral to heroin addiction, and has detrimental health consequences that represent a growing public health concern. Heroin-associated contextual stimuli can act as conditioned stimuli (CS) that trigger Pavlovian appetitive conditioned responses, including conditioned place preference (CPP) (Tzschentke, 1998). Additionally, drug-paired contextual stimuli can act as discriminative stimuli or occasion setters that signal drug availability and thus engender drug-seeking behavior in instrumental paradigms (Crombag et al., 2008; Fuchs et al., 2008). Regardless of the specific role of the contextual stimulus, the hippocampus is essential for context-drug associative learning (Kutlu and Gould, 2016). In particular, the dorsal hippocampus (DH) plays a critical role in drug-induced CPP (Corrigall and Linseman, 1988; Meyers et al., 2003; Xia et al., 2017) as well as context-induced drug-seeking behaviors (Fuchs et al., 2005; Fuchs Rita et al., 2007; Ge et al., 2017; Xie et al., 2010).
In addition to heroin-conditioned appetitive responses, heroin-associated contextual stimuli can elicit the immunomodulatory effects induced by opioids (Lysle and Ijames, 2002). Heroin and other opioids negatively alter host immunity (McCarthy et al., 2001; Wang et al., 2011). Following repeated context-heroin pairings, exposure to the heroin-paired CS is sufficient to evoke heroin-conditioned suppression of lipopolysaccharide (LPS)-induced peripheral immune parameters (Lysle and Ijames, 2002). We have characterized this heroin-conditioned peripheral immunomodulation as classically conditioned response that follows the principles of learning (Szczytkowski and Lysle, 2007). Consequently, it is mediated through DH-dependent processes. GABA agonist-induced DH inactivation during CS exposure significantly disrupts heroin-conditioned suppression of LPS-induced peripheral indices of nitric oxide (NO) production (Szczytkowski et al., 2013). Thus, the DH is an essential component of the neural circuitry governing the retrieval or utilization of the context-heroin association that controls host immunity.
The role of the pro-inflammatory cytokine, interleukin-lβ (IL-lβ), in hippocampal-dependent learning and memory has been well established (Goshen et al., 2007; Jones et al., 2015), with evidence to suggest it’s involvement in the development and maintenance of long-term potentiation (Donzis and Tronson, 2014; Yirmiya and Goshen, 2011). We have determined IL-lβ within the DH is required for the expression of heroin-conditioned immunomodulation. siRNA-mediated knock-down of IL-lβ within the DH during CS exposure blocked the heroin-conditioned suppression of LPS-induced peripheral immune measures (Szczytkowski et al., 2013). Interestingly, similar to IL-lβ itself, stimulation of IL-1 receptor type 1 (IL-1R1) has been implicated in hippocampal-dependent learning and memory. Genetic knockouts of hippocampal IL-1R1 show profound deficits in learning tasks and long-term potentiation (Ben Menachem-Zidon et ah, 2011). Thus, it is likely that IL-lβ-dependent memory mechanisms occur through IL-1R1 stimulation and subsequent signaling cascades. Furthermore, we have shown that acquisition of the context-heroin association required for conditioned immunomodulation is mediated through DH IL-1R1 (Lebonville et al., 2016). However, it is unclear whether DH IL-1R1 signaling is involved in the expression of heroin-conditioned immunomodulation. Additionally, it is yet to be determined if heroin-conditioned appetitive responses are governed through DH IL-1-dependent mechanisms. To further our understanding, the first set of experiments in the present study examined the effects of DH IL-1R1 antagonism during CS exposure on the expression of heroin-conditioned suppression of peripheral indices of NO production and heroin-CPP.
The neural immune system is a vastly complex network involving multiple cell types and signaling molecules. These components function in concert to produce persistent adaptations in neural communication (Yirmiya and Goshen, 2011). Relevant to our model, astrocyte activity has been implicated in both mechanisms of learning and memory (Ben Achour and Pascual, 2010; Ota et al., 2013) and substance use disorders (Lacagnina et al., 2018; Miguel-Hidalgo, 2009; Scofield and Kalivas, 2014). Astrocytes can directly alter neuronal function and synaptic plasticity through the release of gliotransmitters (Haydon and Carmignoto, 2006) and cytokines (Lacagnina et al., 2018; Santello and Volterra, 2012). Interestingly, astroglia have been shown to support hippocampal-dependent learning and memory through the expression of IL-lβ (Jones et al., 2017) and IL-1R1 (Ben Menachem-Zidon et al., 2011). While a mechanistic link between astrocyte activity and subsequent IL-lß release has not yet been confirmed, astrocytes may be a critical cell population involved in mediating heroin-conditioned immunomodulation. Moreover, the role of hippocampal astroglia in heroin-conditioned appetitive responses is presently unknown. Thus, the second set of experiments in the present study examined the role of DH astroglial activity in heroin-conditioned immunomodulatory and appetitive responses. We employed chemogenetic techniques to evaluate the importance of DH astroglial signaling during exposure to heroin-paired contextual stimuli. An adeno-associated viral construct was used to selectively target DH astroglia and express G-coupled designer receptors exclusively activated by designer drugs (DREADDs) in this cell population. DREADDs are mutated muscarinic receptors that no longer respond to endogenous ligands and instead are activated by clozapine-N-oxide (CNO) (Roth, 2016). CNO-induced stimulation of astroglial G-signaling will attenuate induction of cyclic adenosine monophosphate (cAMP) (Jones et al, In Press) and have distinct functional outcomes for cellular activity. Overall, the present study investigated hippocampal neural immune signaling, by way of both IL-1R1 and astroglial signaling, in two Pavlovian procedures: heroin-conditioned immunomodulation and heroin-CPP.
2. Materials and Methods
2.1. Animals
Male Lewis rats (~225–250 g) were purchased from Charles Rivers Laboratories (Kingston, NY). All rats were individually housed on a 12-hour reversed light-dark cycle. Animals were handled regularly prior to and throughout experimental procedures. Animals received ad libitum home cage access to food and water. All procedures were conducted in compliance with regulations by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
2.2. Drug Administration
Heroin (diacetylmorphine, National Institute on Drug Abuse Drug Supply Program, Bethesda, MD) was dissolved in 0.9% sterile saline. Heroin was stored at 4°C until use at room temperature. In all experiments, heroin was administered subcutaneously at a dose of 1 mg/kg. This dose was selected based on prior research showing that it induces conditioning and alters endotoxin-induced indices of NO production (Lysle and How, 2000; Lysle and Ijames, 2002; Szczytkowski and Lysle, 2007). Human recombinant interleukin-1 receptor antagonist (IL-1RA; Genscript, Piscataway, NJ) was reconstituted in 0.9% sterile saline vehicle to a concentration of 2.5 μg/μL and stored at −20°C until use at room temperature. In Experiments 1 and 2, intra-DH IL-1RA (1.25 pg/0.5–0.6 pL per hemisphere) was infused bilaterally at a rate of 0.25 μL/min. Clozapine-A-oxide (CNO; Sigma, St. Louis, MO or the National Institutes of Health, Bethesda, MD) was dissolved in a vehicle of 0.9% sterile saline with 0.5% dimethyl sulfoxide (DMSO). In Experiments 3 and 4, CNO (3 mg/kg) or vehicle was administered subcutaneously. Lipopolysaccharide (LPS; derived from E. coli, serotype 055:B5, Sigma) was dissolved in 0.9% sterile, pyrogen-free saline. In Experiments 1 and 3, LPS (1 mg/kg) was administered subcutaneously. This LPS dose produces sickness behavior and induces measures of NO production.
2.3. Surgical Procedures
Animals were fully anesthetized with a 1 mg/kg intraperitoneal injection of ketamine hydrochloride (100 mg/mL) mixed with xylazine (100 mg/mL) in a 9:1 (vol:vol) ratio.
2.3.1. Cannulation Surgeries for IL-1RA Experiments
Guide cannulae (26 gauge, Plastics One, Roanoke, VA) were directed bilaterally at the DH (AP −3.4 mm, ML ±3.1 mm, DV −2.2 mm, relative to bregma, 15° angle laterally, (Paxinos and Watson, 2006)). Cannulae were secured to the skull with screws, cyanoacrylate adhesive gel, and dental acrylic. Dummy injectors (.008/.2 mm no projection, Plastics One) were inserted into the guide cannulae to prevent occlusion. Animals were given one week for post-operative recovery and were handled regularly during this time.
2.3.2. Virus Infusion Surgeries for the GFAP-hM4Di Experiments
An astroglial G-coupled DREADD virus (AAV8-GFAP-hM4D(Gi)-mCherry) was infused into the DH. The DREADD construct was packaged into an adeno-associated virus (AAV) by the University of North Carolina at Chapel Hill Vector Core (Chapel Hill, North Carolina). Injectors (33 Gauge, Plastics One) were directed bilaterally at the DH (AP −3.4 mm, ML ±3.1 mm, DV −3.2 mm, relative to bregma, 15° angle laterally, (Paxinos and Watson, 2006)). Purified viruses were obtained pre-dialyzed (350 mM NaCl, 5% D-sorbitol in PBS) and were microinjected at a viral titer of 2.0 × 1012 particles/mL (Experiment 3) or 9.8 × 1012 particles/mL (Experiment 4). Virus infusions of 0.7 μL per hemisphere were delivered bilaterally at a rate of 0.05–0.1 μL/min, At the end of the infusion, injectors were left in place for 10–15 min to allow for diffusion away from the injection site. Following virus infusion surgeries, animals remained in their home cage for three weeks to allow for post-operative recovery and astroglial DREADD expression.
2.4. Heroin-conditioning and Testing
For Experiments 1 and 2, animals were habituated to the intracranial infusion procedure forty-eight hours prior to testing. The injectors (33 gauge, Plastics One) were inserted into the guide cannulae and were left in place for 90 sec.
2.4.1. Heroin-conditioned Immunomodulation (Experiments 1 & 3)
The heroin-conditioning paradigm employed here has been described previously (Szczytkowski et al., 2011; Szczytkowski et al., 2013). Briefly, all animals received five 1-h pairings of heroin with a conditioning chamber (conditioned stimulus, CS). The conditioning chambers (BRS/LVE, Laurel, MD; H 26.7 cm x D 24.1 cm x W 30.5 cm) were located in a room separate from the vivarium. The chambers contained metal grid flooring and cedar bedding to create an environment with different olfactory, tactile, and visual characteristics relative to the home cage. The chambers were enclosed within sound- and light-attenuating chambers (H 36.8 cm x D 34.3 cm x W 50.8 cm) with a house fan to mask background noise. Heroin-conditioning sessions took place during the dark phase of the light cycle and were separated by 48 h. Following the last conditioning session, animals remained undisturbed in their home cage for 6 days. Animals were randomly assigned to four groups according to a 2 (CS or home cage) x 2 (drug or vehicle) between-subjects design. In Experiment 1, animals received bilateral intra-DH infusions of either IL-1RA or vehicle. At the end of the infusion, injectors were left in place for 1 min to allow for drug diffusion away from the injection site. For Experiment 3, animals received either an injection of CNO or vehicle. Thirty minutes after drug treatment, the animals were re-exposed to the heroin-paired context (CS) for 1 h in the absence of heroin or remained in their home cage. Immediately after the CS exposure or equivalent home cage stay, the animals were injected with LPS and placed into their home cages until tissue collection, 6 h later.
2.4.2. Heroin-conditioned Place Preference (Experiments 2 & 4)
The conditioned place preference (CPP) apparatus was located in a room separate from the vivarium. A three-chambered apparatus was used, with the two large chambers containing distinct olfactory, visual, and tactile cues from home cage, as well as each other. Animals were habituated to the CPP apparatus. During habituation to the CPP apparatus, baseline test, and each subsequent CPP test, animals were given free access to all three chambers for 15 min in a heroinfree state. Behavior within the apparatus during test sessions was video recorded using a Sony Handycam (HDR-CX455, 9.2 megapixels). The time spent in each side of the apparatus was scored manually by an experimenter blind to treatment assignment. Twenty-four hours after habituation, a pre-conditioning baseline CPP test was conducted to determine unconditioned side preferences. Using a biased conditioning procedure, heroin was paired with the initially nonpreferred side of the apparatus. Saline-conditioned controls were included to test for unconditioned drift in side preference that might occur with repeated exposure to the apparatus.
Assignment to heroin- and saline-conditioned groups, as well as to the order of heroin and saline conditioning sessions, was counterbalanced based on unconditioned side preferences. Animals received a heroin or saline injection and were confined to one side for 30 min. The next day animals were injected with the opposite treatment and confined to the opposite side for 30 min. Conditioning continued as an alternating regimen across a total of 10 daily sessions. Animals then received a CPP test. After heroin CPP was confirmed, as indicated by significantly increased time spent on the heroin-paired side during the CPP test relative to the baseline test, animals were assigned to treatment groups, counterbalanced based by initial and post-training preferences. In Experiment 2, animals received bilateral intra-DH infusions of IL-1RA or vehicle 30 min prior to an IL-1RA test session. In Experiment 4, animals received two CNO test sessions, 24 h apart, with CNO or vehicle administered 30 min prior to Test 1, and the opposite treatment administered prior to Test 2. There were no statistical differences between these two tests, thus data across CNO test days were combined to increase power. Data are presented for both experiments as time (sec) spent in the heroin-paired side during IL-1RA (Experiment 2) or CNO (Experiment 4) tests and as change in time spent in the heroin-paired side during IL-1RA or CNO tests relative to baseline Additionally, CPP score is reported and is defined as the time spent in the heroin-paired side minus that in the saline-paired side.
2.5. Tissue Collection and Histology
Animals were sacrificed via cervical dislocation (Experiments 1–3) or transcardial perfusion (Experiment 4). In studies examining the effects of heroin-conditioned immunomodulation (Experiments 1 and 3), samples of spleen and blood plasma were collected 6 h following LPS injection to assess indices of NO production. Spleen tissue for RNA extraction was divided into −100 mg samples which were stored in RNAlater (Ambion, ThermoFisher Scientific, Waltham, MA). To verify proper cannula placements in Experiments 1 and 2, Alcian blue dye was infused via the cannula post-mortem. Afterwards, brain tissue was extracted and fixed in 4% paraformaldehyde for 48 h, cryoprotected in 30% sucrose in 0.1 M phosphate buffer (PB, pH = 7.4), and stored at 4°C. All brain tissue was frozen and sectioned into 40 pm coronal slices via cryostat (CM3050 S, Leica, Buffalo Grove, IL) or freezing microtome (SM 2000R, Leica). Animals with cannula placement outside of the DH were removed from analysis. To evaluate DH- and astroglial-specificity of hM4D(Gi)-mCherry expression in Experiments 3 and 4, brain tissue was post-fixed in 4% paraformaldehyde for 48 h, cryoprotected in 30% sucrose in 0.1 M PB (pH = 7.4), and stored at 4°C until sectioned. Sections were labeled using standard immunohistochemistry (IHC) methods as described in section 2.6. All tissue sections were analyzed by an experimenter blind to treatment group.
2.6. Immunohistochemistry
To verify cell-type specificity of GFAP-hM4Di(Gi)-mCherry expression in Experiments 3 and 4, sections were washed three times for 10 min in 0.1 M PB (pH = 7.4) and incubated in 5% Normal Goat Serum (NGS; Vector Laboratories, Burlingame, CA) and 0.5% Triton-X100 for 60 min at room temperature. Tissue was then incubated overnight at 4°C in 5% NGS, 0.5% Triton-X100, and primary antibody, mouse anti-GFAP (1:1000, ThermoFisher Scientific, Waltham, MA, Cat# MS-1376P) or mouse anti-NeuN (1:1000, Millipore, Burlington, MA, Cat# MAB377). The next day, tissue was washed three times for 10 min in 0.1 M PB (pH = 7.4) and then incubated at room temperature in 5% NGS, 0.5% Triton-X100, and secondary antibody for 2 h. Secondary antibodies used for visualization were conjugated with Alexa-Fluor dyes (Alexa-488, 1:1000, Invitrogen, ThermoFisher Scientific, Cat#A-l 1001). Tissue was then washed three times for 10 min in 0.1 M PB (pH = 7.4), mounted onto SuperFrost Plus slides (ThermoFisher Scientific), and coverslipped using Vectashield HardSet mounting medium (Vector Laboratories). Slides were stored at 4°C until time of analysis. Specificity of each primary antibody was verified in control experiments.
2.7. Microscopy
In order to verify DREADD DH- and astroglial-specificity, mCherry expression was carefully examined by an experimenter blind to treatment group. DH sections were visualized using confocal microscopy (Zeiss LSM800, Jena, Germany) and representative images for publication were acquired using 1024 × 1024 frame size, 16-bit image resolution, and frame average of 4. Laser lines that excite at 488 nm and 561 nm were used to visualize AlexaFluor-488 and mCherry respectively. Images were deconvolved using Bitplane AutoQuant X3 (10 iterations), and exported to Biplane Imaris Software (Zurich, Switzerland). mCherry was expected to be expressed bilaterally throughout the DH, selectively within the DH, and specifically in DH astrocytes. Animals with non-DH and/or non-astrocyte specific mCherry expression were removed from data analysis.
2.8. RT-qPCR mRNA Analysis
2.8.1. RNA Extraction and cDNA synthesis
Messenger RNA (mRNA) was extracted to assess measures of NO in the spleen. Spleen tissue was homogenized in 1 mL of cold TriReagent (Molecular Research Center, Cincinnati, OH) using a bead homogenizer (Precellys Instruments, Montigny-le-Bretonneux, France). Tissue was centrifuged, and the homogenate transferred to a second tube. Next, the samples were shaken and incubated with BCP at room temperature and centrifuged for phase separation. The aqueous layer was thoroughly mixed with isopropanol, incubated at room temperature, and samples were centrifuged to form the RNA pellet. The pellet was then washed three times in 75% ethanol and air dried to remove residual ethanol. The RNA pellet was reconstituted in warm RNase-free water. Absorbance for samples diluted (1:20) in lxTE (pH = 7.5) was assessed using spectrophotometer (Epoch™, BioTek Instruments Inc., Winooski, VT). Sample mRNA concentrations were read using the Take3 Application and Gen5 Software for Nucleic Acid Quantification (BioTek Instruments Inc.), and A260/280 ratios were assessed to ensure purity.
Sample mRNA input concentration was equalized using PCR-grade water. cDNA was synthesized using the Advantage for RT-PCR Kit (ClonTech, Takara, Mountain View, CA) following the manufacturer’s protocol and using the Veriti 96 Well Fast Thermal Cycler (Applied Biosystems, ThermoFisher Scientific). A subset of undiluted cDNA samples were pooled together, and five serial 1:10 dilutions were made to test qPCR reaction efficency. The remaining original sample was then diluted 1:5 in PCR-grade water for qPCR.
2.8.2. qPCR Quantification of Splenic iNOS Gene Expression
qPCR was performed using the TaqMan™Fast Advanced Master Mix Kit (Applied Biosystems, ThermoFisher Scientific) according to the manufacturer’s instructions. Reactions were carried out in triplicate on a 384-well plate, with each individual reaction containing 1.5 μL of cDNA pooled or sample cDNA. In order to assess indices of NO production, levels of splenic inducible nitric oxide synthase (iNOS) gene expression were analyzed. NO is produced by iNOS in response to inflammatory stimuli (Nathan and Shiloh, 2000). Thus, two different genes were analyzed by using the TaqMan™ Gene Expression Assays (FAM): inducible nitric oxide synthase 2 (ÎNOS/NOS2, Assay ID: Rn00561646_ml, ThermoFisher Scientific) and 60S ribosomal protein L13a (Rpll3a, reference gene, Assay ID: Rn01475911_gl; ThermoFisher Scientific). A no template control was run to ensure purity of these reactions. Plates were run in the QuantStudio™ 6 Flex RealTime PCR System (Applied Biosystems, ThermoFisher Scientific). Data were collected using the QuantStudio™ RealTime PCR Software with a PCR Run Method as follows: 50°C for 2 min for PCR product contamination degradation, hold at 95°C for 20 sec for polymerase activation, and 45 PCR cycles of 95° C for 1 sec and 60° C for 20 sec with data collection at the end of each cycle. Data were analyzed using the Comparative CT (ΔΔΟΤ) Method. iNOS CT data were normalized to the reference gene (Rpl13a), and then normalized to an the overall average of reference normalized values.
2.9. Nitrate/nitrite Assay
As NO is degraded quickly, degradation products in plasma can be analyzed in combination with iNOS expression as indices of NO production. Plasma nitrate/nitrite concentrations were assessed using the Griess reagent assay as described previously (Szczytkowski and Lysle, 2007). Briefly, plasma was diluted in dH2O and incubated with nitrate reductase (1.0 U/mL), 0.31 Μ PB (pH = 7.5), 0.86 mM NADPH (Sigma-Aldrich Inc., Milwaukee, WI), and 0.11 mM flavin adenine dinucleotide in a 96-well plate for 90 min at room temperature in the dark. Following incubation, Griess reagent (1:1 (vokvol) solution 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(l-napthyl)ethylenediamine dihydrochloride in distilled H2O) was added to the samples and allowed to develop at room temperature. Absorbance was assessed at 550 nm using a spectrophotometer (Epoch™, BioTek Instruments Inc). Reactions were carried out in triplicate. The total micromolar concentration of nitrite was determined for each sample based on a concurrently run standard curve.
2.10. Statistical Analysis
Data for each experiment herein was analyzed using 2×2 analysis of variance (ANOVA) in SPSS Statistics (IBM, Armonk, NY). Planned contrasts were made using a two-tailed independent samples t-test with homogeneity of variance determined using Levene’s Test. For Experiments 1 and 3, we tested planned comparisons between CS-exposed and corresponding home cage control groups, as well as differences between CS-exposed groups themselves. For analysis of RT-qPCR, ΔΔΟΤ values were analyzed, although the linearly transformed were used to display the data graphically. For Experiments 2 and 4, we tested a planned contrast between the heroin-conditioned groups, at IL-1RA (Experiment 2) or CNO (Experiment 4) test, for time spent in heroin-paired side, change in time spent in heroin-paired side relative to baseline, and CPP score. Initial verification of acquired CPP was performed using an independent t-test comparing heroin-conditioned to saline-conditioned animals. Statistically significant outliers were detected using Grubb’s test and removed from analysis. Alpha was set at p = 0.05.
3. Results
3.1. Experiment 1: Intra-DH IL-1RA disrupts heroin-conditioned immunomodulation
Experiment 1 examined the role of DH IL-1R1 signaling in the expression of heroin-conditioned immunomodulation (for experimental timeline see Fig 1A). Cannula placements were carefully assessed to ensure on target bilateral location in the DH (see Fig 1B).
Figure 1:
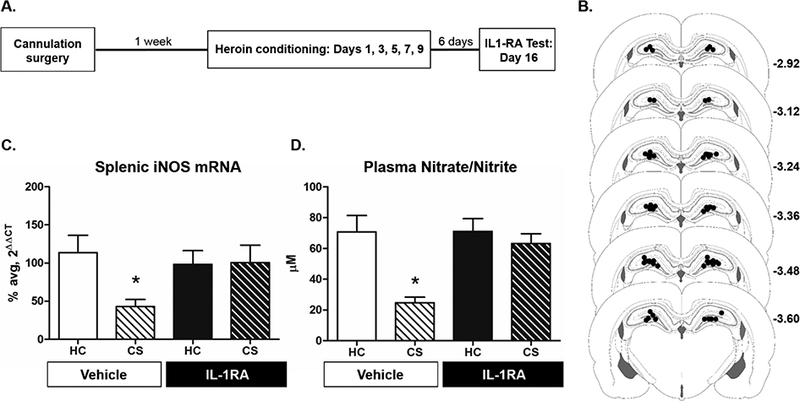
Intra-DH IL-1RA disrupts heroin-conditioned suppression of peripheral indices of NO production. The experimental timeline (A) and cannula placements (B) are depicted for Experiment 1. Black dots indicate the ventral most point of the injector. Coordinates represent distance from bregma based on the rat brain atlas of Paxinos and Watson (2006). Intra-DH administration of IL-1RA significantly disrupted heroin-conditioned LPS-induced splenic iNOS mRNA expression (C) and plasma nitrate/nitrite concentration (D). Group sizes were n = 7–8 for the final analysis of splenic iNOS mRNA expression and plasma nitrate/nitrate concentration. * represents statistically significant difference relative to respective home cage control group (p < 0.05).
Intra-DH IL-1RA administration prior to CS exposure significantly inhibited heroin-conditioned suppression of splenic iNOS mRNA levels (Fig. 1C). A 2×2 ANOVA of mRNA levels revealed no significant differences between groups for splenic Rpl13a mRNA levels (F(3,26) = 0.38, p = 0.768), validating Rpll3a as an appropriate reference gene. For splenic iNOS mRNA levels, a 2×2 ANOVA revealed a significant CS exposure main effect (F(1,26) = 5.31, p = 0.029), but no main effect of IL-1RA treatment (F(l,26) = 2.59, p = 0.120). Notably, there was a very strong trend for CS exposure x IL-1RA treatment interaction (F ( 1,26) = 4.06, p = 0.054). Planned contrasts revealed that, in the vehicle-treated groups, CS exposure reduced splenic iNOS mRNA levels relative to home cage exposure (p < 0.05). Intra-DH IL-1RA administration restored splenic iNOS mRNA levels such that CS exposed animals did not differ from IL-IRA-treated home cage controls (p = 0.840), and were significantly higher than vehicle-treated CS-exposed animals (p < 0.05).
Similar to splenic iNOS mRNA levels, intra-DH IL-1RA administration prior to CS exposure significantly inhibited heroin-conditioned suppression of plasma nitrate/nitrite concentration (Fig. 1D). A 2×2 ANOVA of plasma nitrate/nitrite concentration revealed a significant CS exposure x IL-1RA treatment interaction (F(1,26) = 6.21, p < 0.05). Planned contrasts revealed that in vehicle groups, CS exposure reduced plasma nitrate/nitrite concentration relative to home cage control exposure (p < 0.05). Intra-DH IL-1RA administration restored plasma nitrate/nitrite concentration such that concentration in IL-1RA treated, CS-exposed groups did not differ relative to IL-1RA-treated home cage controls (p = 0.457), and were significantly higher than the vehicle-treated CS-exposed group (p < 0.05).
3.2. Experiment 2: Intra-DH IL-1RA fails to alter the expression of heroin-CPP
Experiment 2 investigated the importance of DH IL-1R1 signaling in the expression of heroin-CPP (see experimental timeline Fig. 2A). Cannula placements were carefully assessed to ensure on target bilateral location in the DH (see Fig 2B). All animals acquired heroin-CPP, and during the CPP test session (data not shown), heroin-conditioned animals spent significantly more time in the heroin-paired side than saline-conditioned animals (t(18.579) = −4.94, p < 0.05), verifying the effectiveness of the biased conditioning procedure.
Figure 2:
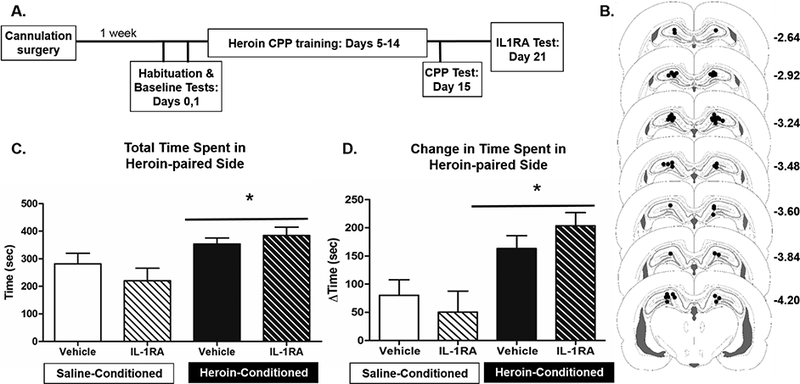
Intra-DH IL-1RA fails to alter heroin-CPP. The experimental timeline (A) and cannula placements (B) for Experiment 2 are shown. Black dots indicate the ventral most point of the injector. Coordinates represent distance from bregma based on the rat brain atlas of Paxinos and Watson (2006). Intra-DH administration of IL-1RA failed to alter total time spent in the heroin-paired side (C) and change in time spent in the heroin-paired side relative to pre-conditioning baseline (D). Group sizes were n = 4–5 for each saline-conditioned group and n = 7–9 for each heroin-conditioned group in the final analysis of heroin-CPP measures. * represents a main effect of heroin-conditioning, with bar indicating no statistical difference between heroin-conditioned groups (p < 0.05).
Intra-DH IL-1RA or vehicle prior to test of CPP (IL-1RA test) failed to alter the expression of heroin CPP (Fig. 2C and 2D). Consistent with this, a 2×2 ANOVA of time spent in the heroin-paired side during IL-1RA test revealed only a significant main effect of conditioning (F(1, 21) = 11.38, p < 0.05), with no significant main effect of IL-1RA treatment (F(1, 21) = 0.20, p = 0.662) nor interaction (F(1, 21) = 1.74, p = 0.202). Similarly, a 2×2 ANOVA of change in time spent in the heroin-paired chamber at IL-1RA test relative to baseline indicated a significant main effect of conditioning (F(1, 20) = 17.35, p < 0.05) with no significant main effect of IL-1RA treatment (F(1, 20) = 0.03, p = 0.856) nor interaction (F(1, 20) = 1.53, p = 0.231). Finally, a 2×2 ANOVA of CPP scores (data not shown) at IL-1RA test revealed a significant main effect of conditioning (F(1, 21) = 5.44, p < 0.05), with no significant main effect of IL-1RA treatment (F(1, 21) = 1.47, p = 0.238) nor interaction (F(1, 21) = 1.16, p = 0.294). A planned contrast between the heroin-conditioned groups revealed no significant effect of IL-1RA treatment on total time spent in the heroin-paired side (p = 0.474), change in time relative to baseline (p = 0.259), or CPP score (p = 0.911).
3.3. Experiment 3: Stimulation of astroglial Gi-signaling in the DH disrupts heroin-conditioned immunomodulation
Experiment 3 examined the role of DH astrocyte signaling in the expression of heroin-conditioned suppression of LPS-induced indices of NO production (see timeline in Fig. 3A). DREADD expression, as indicated by mCherry, was observed throughout the DH (Fig.3B). Furthermore, hM4Di-mCherry expression was restricted to astrocytes (Fig. 4).
Figure 3:
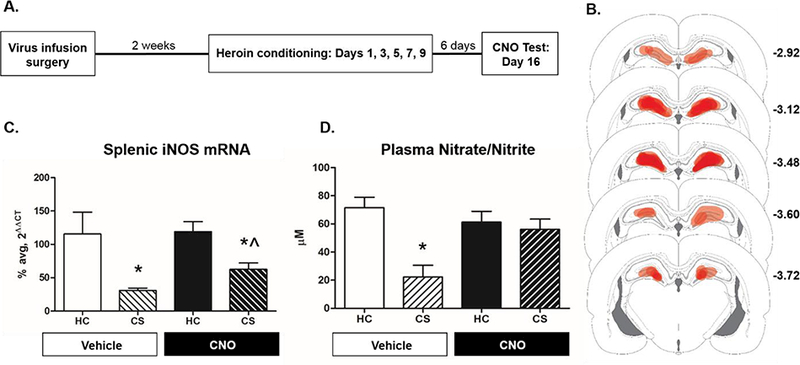
Activation of astroglial Gi-coupled signaling in the DH disrupts heroin-conditioned suppression of peripheral indices of NO production. For Experiment 3, the timeline is depicted (A) as well as the spread of GFAP-hM4D(Gi) as indicated by mCherry expression throughout the DH (B). Darker red areas are indicative of denser mCherry expression, with coordinates indicating distance from bregma based on Paxinos and Watson (2006). CNO administration significantly attenuated heroin-conditioned LPS-induced splenic iNOS mRNA expression (C) and completely blocked heroin-conditioned LPS-induced plasma nitrate/nitrite concentration (D). Group sizes were n = 5–6 in the final analysis for splenic iNOS mRNA expression and plasma nitrate/nitrite concentration. * represents statistically significant differences relative to respective home cage control group and Λ denotes statistical significance from CS-exposed counterpart (p < 0.05).
Figure 4:
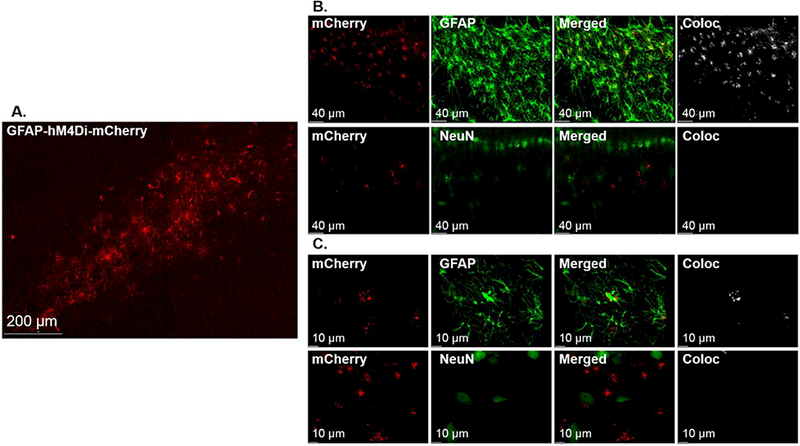
GFAP-hM4Di-mCherry is selectively expressed in DH astrocytes. A representative confocal 10X tile image depicts robust mCherry expression and spread throughout the DH (A). Representative confocal images at 20X demonstrating the mCherry tag is colocalized with astroglial marker GFAP (AlexaFlour-488; top row) but not with neuronal marker NeuN (AlexaFlour-488, bottom row) (B). Representative oil-immersion 63X images demonstrating that mCherry fluorescence is colocalized with astroglial marker, GFAP (Alexa-488; top row), but not with neuronal marker, NeuN (Alexa-488, bottom row) (C). Background signal was subtracted out using Bitplane Imaris Software and Adobe Photoshop.
CNO-induced stimulation of DH astroglial Gi-signaling attenuated heroin-conditioned splenic iNOS mRNA suppression (Fig. 3C). A 2×2 ANOVA of splenic Rpl13A mRNA levels revealed no significant differences between the groups (F(3,18) = 1.48, p = 0.252), validating Rpl13A as a reference gene. A 2×2 ANOVA of splenic iNOS mRNA levels revealed significant main effects of CS exposure (F(1,18) = 30.96, p < 0.05) and CNO treatment (F(1,18) = 6.05, p < 0.05), but no CS exposure by CNO treatment interaction (F(1,18) = 2.57, p = 0.127). Planned contrasts revealed that CS exposure significantly reduced splenic iNOS mRNA levels relative to home cage controls in the vehicle-treated (p < 0.05). CNO treatment partially attenuated heroin-conditioned suppression of splenic iNOS mRNA expression in that CNO-treated CS-exposed iNOS mRNA expression was reduced relative to CNO-treated home cage controls (p < 0.05), but was higher than vehicle-treated CS-exposed animals (p < 0.05). Thus, stimulation of DH astroglial Gi-signaling significantly increased splenic iNOS gene expression, yet does not completely restore mRNA levels to those of control animals.
In contrast to splenic iNOS mRNA levels, CNO-induced stimulation of DH astroglial Gi-signaling completely inhibited heroin-conditioned suppression of plasma nitrate/nitrite concentration (Fig. 3D). A 2×2 ANOVA of nitrate/nitrite concentration revealed a significant CNO treatment x CS exposure interaction (F(1,18) = 8.05, p < 0.05). Planned contrasts revealed that, in vehicle-treated groups, CS exposure reduced plasma nitrate/nitrite concentrations relative to home cage controls (p<0.05), indicating expression of heroin-conditioned immunomodulation. CNO-induced stimulation of Gi-signaling in DH astrocytes restored plasma nitrate/nitrite concentrations, such that concentrations for the CS-exposed group did not significantly differ from CNO-treated home cage controls (p = 0.646) and were higher than vehicle-treated CS-exposed animals (p < 0.05).
3.4. Experiment 4: Stimulation of astroglial Gi-signaling in the DH fails to alter heroin-conditioned place preference
Experiment 4 investigated the role of astrocyte signaling in the expression of heroin-CPP (see timeline in Fig. 5A). DREADD expression, as indicated by mCherry, was observed throughout the DH (Fig.5B). As in Experiment 2, all animals acquired CPP. Heroin-conditioned animals spent significantly more time in the heroin-paired side than saline-conditioned animals during the CPP test session (t(12.47) = −3.22, p < 0.05), verifying the effectiveness of the biased conditioning procedure. CPP data were collapsed across experimental CNO test days 1 and 2.
Figure 5:
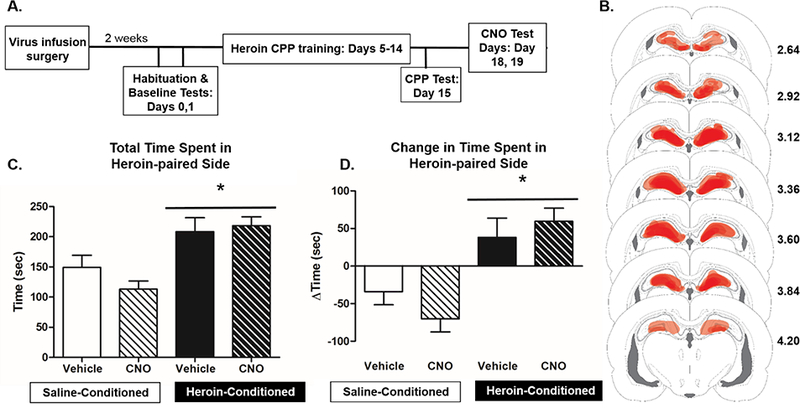
Activation of astroglial Gi-coupled signaling in the DH fails to alter heroin-CPP. For Experiment 4, the experimental timeline shown (A) as well as the spread of GFAP-hM4D(Gi) as indicated by mCherry expression throughout the DH (B). Darker red areas are indicative of denser mCherry expression with coordinates indicating distance from bregma based on Paxinos and Watson (2006). CNO administration fails to disrupt total time spent in the heroin-paired side (C) or change in time spent in the heroin-paired side relative to a pre-conditioning baseline (D). Group sizes were n = 6 for each saline-conditioned group and n = 8–9 for each heroin-conditioned group in the final analysis of heroin-CPP measures. * represents a main effect of heroin-conditioning, with bar indicating no statistical difference between heroin-conditioned groups (p < 0.05).
CNO-induced stimulation of DH astroglial Gi-signaling failed to alter heroin-CPP at CNO test relative to controls (Fig. 5C and 5D). Specifically, a 2×2 ANOVA for total time spent in the heroin-paired chamber at CNO test revealed a significant main effect of conditioning (F(1, 26) = 17.63, p < 0.05) with no significant main effect of CNO treatment (F(1, 26) = 0.44, p = 0.511) nor interaction (F(1, 26) = 1.37, p = 0.253). Similarly, the 2×2 ANOVA for change in time spent in the heroin-paired side relative to baseline indicated a significant main effect of heroin conditioning (F(1, 24) = 23.28, p < 0.05) with no significant main effect of CNO treatment (F(1, 24) = 0.12, p = 0.729) nor interaction (F(1, 24) = 1.88, p = 0.183). Finally, a 2×2 ANOVA of CPP scores on CNO test day (data not shown) also revealed a significant main effect of conditioning (F(1, 26) = 16.18, p < 0.05), with no significant main effect of CNO treatment (F(1, 26) = 0.12, p = 0.736) nor interaction (F(1, 26) = 3.91, p = 0.059). Thus, heroin-conditioned animals spent significantly more total time, time relative to baseline, and time relative to saline-paired side in the heroin-paired side independent of CNO treatment. Planned contrasts between the heroin-conditioned groups revealed no differences between these groups regardless of CNO treatment for total time spent in the heroin-paired side (p = 0.694), for change in time relative to baseline (p = 0.444), and for CPP score (p = 0.207).
4. Discussion
Through associative learning, contextual stimuli can come to elicit heroin-conditioned responses, including CPP and immunomodulation. The DH plays a critical role in contextual learning and memory, and has been implicated in both opioid-conditioned reward (Corrigall and Linseman, 1988) and -conditioned immunomodulation (Szczytkowski et al., 2013). In addition, neural immune signaling, in terms of both gliotransmission and cytokine signaling, is essential in learning and memory processes (Ben Achour and Pascual, 2010; Donzis and Tronson, 2014; Santello and Volterra, 2012; Yirmiya and Goshen, 2011) and in some drug-conditioned responses and instrumental behaviors relevant for drug addiction (Haydon et al., 2009; Lacagnina et al., 2018; Scofield and Kalivas, 2014). Astrocytes, for example, have an established involvement in the IL-1R1 signaling required for some forms of learning and memory (Ben Menachem-Zidon et al., 2011). Findings in the present study significantly extend these lines of research by demonstrating that DH neural immune signaling plays a causal and selective role in heroin-conditioned immunomodulation, but not in heroin-CPP. Specifically, we show that undisturbed DH IL-1R1-mediated signaling and DH astroglial signaling during CS exposure are necessary for heroin-conditioned suppression LPS-induced of indices of NO production. Conversely, manipulations of the same signaling pathways failed to disrupt measures of heroin-CPP under the present experimental parameters. Together, these data suggest that divergent mechanisms within the DH govern heroin-conditioned peripheral immunomodulation and heroin-conditioned appetitive behavior.
Our current findings further our understanding of the IL-1 signaling mechanisms mediating heroin-conditioned immunomodulation. In a previous study (Szczytkowski et al., 2013), our laboratory established IL-lβ expression is necessary for hippocampal-dependent heroin-conditioned immune responses. Consistent with this, we have demonstrated sustained, inducible knockdown of DH IL-lβ mRNA expression prior to CS exposure disrupts heroin-conditioned suppression of peripheral modulators, including indices of NO production (Szczytkowski et al., 2013). Our current findings complement these findings by demonstrating IL-1R1-mediated signaling in the DH is necessary for the expression of heroin-conditioned immunomodulation. IL-1 signaling is complex and very tightly regulated through decoy receptors (i.e. IL-1R2) and endogenous receptor antagonists (Boraschi and Tagliabue, 2013). IL-lβ is capable of targeting the active receptor complex of IL-1R1 and IL-1R accessory protein (IL-1RAP) to induce activation of the nuclear factor-κΒ (NF-κΒ) and mitogen-activated protein kinase (MAPK) pathways (Sims and Smith, 2010). Within the hippocampus, both astrocytes and microglia are capable of producing and responding to IL-lβ signaling (Friedman, 2001; Hanisch, 2002), indicating either or both of these cell types could facilitate the IL-1 signaling required for heroin-conditioned immunomodulation. While the second set of experiments in the present study strongly suggest astroglia mediate heroin-conditioned immunomodulation, the additional role of DH microglial involvement in this conditioned response should be investigated.
Both pyramidal neurons and astrocytes within the hippocampal can express IL-1R1, and IL-lß administration triggers receptor upregulation of this receptor in both cell types (Friedman, 2001). Interestingly, IL-lß stimulation of IL-1R1 has distinct signaling consequences for each cell population. In hippocampal astrocytes, IL-lβ action at IL-1R1 evokes NF-κΒ signaling cascades (Srinivasan et al., 2004) and thus elicits the transcription of pro-inflammatory factors, including IL-lβ and other cytokines, serving as a potential positive feedback loop for IL-lβ expression. Conversely, in hippocampal neurons, IL-lβ stimulation of IL-1R1 elicits MAPK activation and CREB induction (Srinivasan et al., 2004). Since we have established both the importance of DH IL-lß expression and IL-1R1 signaling in heroin-conditioned immunomodulation, IL-1R1 antagonism in the present study likely impaired heroin-conditioned immunomodulation by interfering with the action of IL-lβ in both DH pyramidal neurons and astrocytes. Consistent with this, both GABA-agonist induced neuronal inactivation (Szczytkowski et al., 2013) and astroglial Gi-signaling disrupt the expression of heroin-conditioned immunomodulation. Moreover, astroglial Gi-signaling attenuates cAMP induction (Jones et al., In Press). As converging evidence suggests that activity of NF-κΒ is modulated by cAMP induction (Gerlo et al., 2011), it is possible astroglial G-signaling attenuates IL-lβ production in hippocampal astrocytes. Future experiments should be aimed at testing the relationship between astrocyte activity and subsequent IL-1 signaling.
The current study strongly suggests that DH astroglial signaling is a critical component in the expression of heroin-conditioned immunomodulation, but not heroin-CPP. The absence of effects on heroin-CPP were surprising given the established role of astroglial activity in addiction (Scofield and Kalivas, 2014). Specifically, prior research has shown that chemogenetic manipulation of astroglial Gq-signaling in the nucleus accumbens (NAc) core ameliorates the ability of cocaine-conditioned stimuli to elicit drug-seeking behaviors (Scofield Michael et al., 2015). Although there is a functional projection from the DH to the NAc core (Peleg-Raibstein and Feldon, 2006), the current study targeted DH astroglial Gi-signaling in vivo during exposure to heroin-paired stimuli. While the DH is critical for encoding context-drug associations (Xia et al., 2017), it is the connection from the ventral hippocampus to the NAc shell that drives context-induced heroin-seeking behaviors (Bossert et al., 2016). It is possible that chemogenetic manipulation of ventral hippocampal astroglia would yield downstream consequences for heroin-conditioned appetitive responses. The current data suggest astroglial involvement varies across conditioned appetitive behaviors as a function of evoked signaling pathway, target brain region, animal model, and drug of abuse.
The neuroimmune system is both impacted by opioid administration and serves as a key regulator of opioid-induced responses. Opioids produce alterations in hippocampal GFAP and IL-lβ protein expression that are attenuated through anti-inflammatory compounds, including ibudilast (Hutchinson et al., 2009). At the same time, ibudilast administration reduces opioid withdrawal and simultaneously increases antinociception (Hutchinson et al., 2009). These findings indicate the neural immune system differentially regulates opioid-induced responses depending on the type of response in question. Consistent with this, the current study establishes a divergence in mechanism governing heroin-conditioned responses.
The data demonstrating astroglial Gi-signaling disrupts heroin-conditioned immunomodulation are in line with recent findings demonstrating that modulation of DH astroglial signaling directly alters hippocampal-dependent mechanisms of learning and memory (Adamsky et al., 2018). In addition to the canonical gliotransmitters (i.e. D-serine, glutamate, ATP), glial-derived cytokines critically have been shown to modulate hippocampal synaptic plasticity (Santello and Volterra, 2012). Notably, chemogenetic stimulation of astroglial Gi-signaling did not fully restore LPS-induced NO measures. It is possible that astrocytes are not the only cellular component involved in the expression of heroin-conditioned immunomodulation. Consistent with this, astroglial-mediated neuronal alterations improve hippocampal-dependent memory, while neuronal activation alone impairs it (Adamsky et al., 2018). Furthermore, we have established a role for hippocampal neurons (Szczytkowski et al., 2013) in addition to astrocytes in heroin-conditioned immunomodulation. Thus, the possibility of astrocyte-neuron interplay, and the specific mechanisms involved in heroin-conditioned immunomodulation, will merit further investigation.
In the current set of experiments we employed a 2×2 statistical design in which all animals received intra-DH infusions of AAV8-GFAP-hM4D(Gi)-mCherry. Thus, DREADD expression was present in all animals and transfection alone could not account for group differences in heroin-conditioned immunomodulation or heroin-CPP. Furthermore, all animals were thoroughly examined for site- and cell-type-specific expression which did not differ across groups. Although there have been recent concerns of CNO effects alone irrespective of DREADD expression (Gomez et al., 2017), other groups report no effect of CNO administration alone during experiments involving astroglial chemogenetic techniques (Adamsky et al., 2018; Bull et al., 2014; Scofield Michael et al., 2015). While we do not presently report use of a control DREADD, CNO did not alter any of the current measures relative to vehicle in home cage controls. Thus, effects on reported measures were likely induced by astroglial Gi-signaling pathway manipulation, specifically. Importantly, we have recently demonstrated CNO attenuates LPS-induced cAMP expression in mCherry-positive DH astrocytes using the same viral construct (Jones et al., In Press). Thus, confirming CNO exerts its effects through the stimulation of Gi-signaling cascades and the inhibition of downstream cAMP within DH astrocytes.
In summary, the present study suggests that divergent mechanisms within the DH regulate Pavlovian heroin-conditioned responses. The current findings suggest that neural immune mechanisms in the DH regulate conditioned immunomodulatory, but not conditioned appetitive, effects of heroin, thorough IL-1R1 and astroglial signaling. The immunomodulatory effects of heroin can exacerbate infectious and other disease progression in addicts (Ninkovic and Roy, 2013; Wang et al., 2011). Since immunomodulation can become conditioned to environmental stimuli over the course of chronic heroin use, the detrimental health effects of heroin may persist in heroin-associated environments even after cessation of drug use. This suggests that interference with specific neural immune substrates that maintain heroin-conditioned immunomodulation may be a promising therapeutic target for harm reduction in heroin use disorders.
Highlights:
DH IL-1 receptor antagonism disrupts heroin-conditioned peripheral immunomodulation
DH IL-1 receptor antagonism fails to alter heroin-conditioned place preference
DH astroglial Gi-signaling disrupts heroin-conditioned peripheral immunomodulation
DH astroglial Gi-signaling fails to alter heroin-conditioned place preference
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA034721 and DA007244. C.L. Lebonville was supported by National Science Foundation Graduate Research Fellowship DGE-1144081.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, London M, Goshen I, 2018. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell. [DOI] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O, 2010. Glia: The many ways to modulate synaptic plasticity. Neurochemistry International 57, 440–445. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O., Avital A, Ben-Menahem Y, Goshen I, Kreisel T, Shmueli EM, Segal M, Ben Hur T, Yirmiya R, 2011. Astrocytes support hippocampal-dependent memory and long-term potentiation via interleukin-1 signaling. Brain, Behavior, and Immunity 25, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Boraschi D, Tagliabue A, 2013. The interleukin-1 receptor family. Seminars in Immunology 25, 394–407. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Adhikary S, St Laurent R, Marchant NJ, Wang H-L, Morales M, Shaham Y, 2016. Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology 233, 1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KCC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS, 2014. Rat Nucleus Accumbens Core Astrocytes Modulate Reward and the Motivation to Self-Administer Ethanol after Abstinence. Neuropsychopharmacology 39, 2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Linseman MA, 1988. Conditioned place preference produced by intra-hippocampal morphine. Pharmacology Biochemistry and Behavior 30, 787–789. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y, 2008. Context-induced relapse to drug seeking: a review. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC, 2014. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiology of learning and memory 0, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ, 2001. Cytokines Regulate Expression of the Type 1 Interleukin-1 Receptor in Rat Hippocampal Neurons and Glia. Experimental Neurology 168, 23–31. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE, 2005. The Role of the Dorsomedial Prefrontal Cortex, Basolateral Amygdala, and Dorsal Hippocampus in Contextual Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology 30, 296. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X, 2008. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug discovery today. Disease models 5, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Rita A., Eaddy Jessica L., Su ZI, Bell Guinevere H, 2007. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience 26, 487–498. [DOI] [PubMed] [Google Scholar]
- Ge F, Wang N, Cui C, Li Y, Liu Y, Ma Y, Liu S, Zhang H, Sun X, 2017. Glutamatergic Projections from the Entorhinal Cortex to Dorsal Dentate Gyrus Mediate Context-Induced Reinstatement of Heroin Seeking. Neuropsychopharmacology 42, 1860–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlo S, Kooijman R, Beck IM, Kolmus K, Spooren A, Haegeman G, 2011. Cyclic AMP: a selective modulator of NF-κΒ action. Cellular and Molecular Life Sciences 68, 3823–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannais RF, Pomper MG, Bond A, Michaelides M, 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R, 2007. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32, 1106–1115. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, 2002. Microglia as a source and target of cytokines. Glia 40, 140–155. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Blendy J, Moss SJ, Jackson FR, 2009. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology 56, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G, 2006. Astrocyte Control of Synaptic Transmission and Neurovascular Coupling. Physiological Reviews 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW, 2009. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain, behavior, and immunity 23, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Barrus D, Lysle DT, 2015. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology 40, 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Paniccia JE, Baientine ME, Reissner KJ, Lysle DT, 2017. Hippocampal interleukin-1 mediates stress-enhanced fear learning: A potential role for astrocyte-derived interleukin-1β. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Paniccia JE, Lebonville CL, Reissner KJ, Lysle DT, In Press. Chemogenetic manipulation of dorsal hippocampal astrocytes protects against the development of stress-enhanced fear learning. Neuroscience [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ, 2016. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learning & Memory 23, 515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD, 2018. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology 42, 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebonville CL, Jones ME, Hutson LW, Cooper LB, Fuchs RA, Lysle DT, 2016. Acquisition of heroin conditioned immunosuppression requires IL-1 signaling in the dorsal hippocampus. Brain Behav Immun 56, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysle DT, How T, 2000. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharmacology 46, 181–192. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Ijames SG, 2002. Heroin-associated environmental stimuli modulate the expression of inducible nitric oxide synthase in the rat. Psychopharmacology (Berl) 164, 416–422. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ, 2001. Opioids, opioid receptors, and the immune response. Drug and Alcohol Dependence 62, 111–123. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL, 2003. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. NeuroReport 14, 2127–2131. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, 2009. The Role of Glial Cells in Drug Abuse. Current drug abuse reviews 2, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU, 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proceedings of the National Academy of Sciences 97, 8841–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Roy S, 2013. Role of the mu opioid receptor in opioid modulation of immune function. Amino acids 45, 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, Hallock RM, 2013. The Role of Astrocytes in the Regulation of Synaptic Plasticity and Memory Formation. Neural Plasticity 2013, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2006. The Rat Brain in Stereotaxic Coordinates Sixth Edition by. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J., 2006. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology 51, 947–957. [DOI] [PubMed] [Google Scholar]
- Roth BL, 2016. DREADDs for Neuroscientists. Neuron 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Volterra A, 2012. TNFα in synaptic function: switching gears. Trends in Neurosciences 35, 638–647. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW, 2014. As trocytic Dysfunction and Addiction: Consequences of Impaired Glutamate Homeostasis. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 20, 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield Michael D., Boger Heather A., Smith Rachel J., Li H, Haydon Philip G., Kalivas Peter W., 2015. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biological psychiatry 78, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Smith DE, 2010. The IL-1 family: regulators of immunity. Nature Reviews Immunology 10, 89. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Yen J-H, Joseph DJ, Friedman W, 2004. Cell Type-Specific Interleukin-1β Signaling in the CNS. The Journal of Neuroscience 24, 6482–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Fuchs RA, Lysle DT, 2011. Ventral tegmental area-basolateral amygdala-nucleus accumbens shell neurocircuitry controls the expression of heroin-conditioned immunomodulation. J Neuroimmunol 237, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lebonville C, Hutson L, Fuchs RA, Lysle DT, 2013. Heroin-induced conditioned immunomodulation requires expression of IL-1beta in the dorsal hippocampus. Brain Behav Immun 30, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT, 2007. Conditioned effects of heroin on the expression of inducible nitric oxide synthase in the rat are susceptible to extinction and latent inhibition. Psychopharmacology 191, 879–889. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, 1998. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology 56, 613–672. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang T, Ho W-Z, 2011. Opioids and HIV/HCV Infection. Journal of neuroimmune pharmacology : the official journal of the Society on Neuroimmune Pharmacology 6, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Nygard SK, Sobczak GG, Hourguettes NJ, Bruchas MR, 2017. Dorsal-CA1 Hippocampal Neuronal Ensembles Encode Nicotine-Reward Contextual Associations. Cell Reports 19, 2143–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA, 2010. Effects of mGluRl Antagonism in the Dorsal Hippocampus on Drug Context-induced Reinstatement of Cocaine-seeking Behavior in Rats. Psychopharmacology 208, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I, 2011. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity 25, 181–213. [DOI] [PubMed] [Google Scholar]


