Abstract
Zinc deficiency is a frequent complication of alcohol abuse for multiple reasons including poor intake, increased excretion, internal redistribution, and altered transporters. Zinc deficiency has been postulated to play a role in the development/progression of alcoholic liver disease (ALD). This study aimed to relate serum zinc levels with alcohol intake, serum albumin concentration, and markers of inflammation and liver injury. One hundred and eight male and female very heavy drinking (≥10 drinks/day) individuals without clinical evidence of alcoholic liver disease (ALD) were grouped by serum zinc concentration: normal zinc group (zinc level > 71 mcg/dL) included 67 patients, and low zinc group (zinc level <71 mcg/dL) included 41 patients. Data were collected on demographics, drinking history in last 90 days (heavy drinking days, HDD90 and total drinks, TD90) & Lifetime Drinking history (LTDH), and clinical/laboratory assessments. Our data show that in a very well-characterized, chronically heavy-drinking population without clinical evidence of liver disease, about 40% of subjects had low serum zinc levels. Frequency of heavy drinking days (HDD90) was significantly higher in the low zinc group. Total drinks in past 90 days, LTDH, and HDD90 showed significant associations with low zinc levels. The group with the low serum zinc had a higher AST/ALT ratio (good marker of alcoholic liver disease). Those in the low-zinc group had the lower albumin levels, a marker of hepatic synthetic function, and the highest CRP level, a biomarker of inflammation.
Keywords: Alcohol, C-reactive protein, Drinking history, Liver injury, Zinc
1. Introduction
Alcoholic liver disease (ALD), a significant medical problem worldwide, manifests itself as a spectrum of disease, namely steatosis, inflammation, fibrosis, cirrhosis and, in some cases, hepatocellular carcinoma, and it has been attributed to heavy and chronic alcohol consumption [1, 2]. Several clinical events and molecular mechanisms participate in the onset of the ALD [3–5] and many of these mechanisms of liver injury depend upon nutritional status (including minerals) and nutrient interactions with chronic or heavy alcohol drinking [6–11].
Chronic alcohol intake can lead to the deficiency of several micronutrients including zinc [10, 12] that could contribute to ALD development and progression. This has been shown in experimental animals [13]. Reports on altered zinc metabolism in liver disease, linked with organ/tissue/cell metabolic dysfunction, such as abnormal dark adaptation in ALD, have been documented for over a half-century [14–17].
Several proteins synthesized in the liver could be affected by alcohol-induced liver injury. C-reactive protein (CRP) is an hepatic acute phase protein that increases with infections or inflammation [18]. Reductions in CRP levels have been observed with light and moderate alcohol drinking [19]; however the relation of CRP and heavy drinking has not been investigated thoroughly. Albumin is a major binding protein for zinc, and albumin levels usually decrease with liver disease due to decreased synthesis and/or vascular leakage with inflammation [20].
Drinking profile and nutrition are risk factors that are thought to be involved in ALD development and progression [21–24]. Heavy drinking is primarily estimated as an historical measure assessed by collecting drinking history [25], and can be estimated by the use of tests such as serum Carbohydrate-Deficient Transferrin (CDT) [26].
In this study, we evaluated the interactions of drinking patterns and serum zinc, albumin and CRP concentrations on the development of liver injury (as demonstrated by ALT, AST, AST:ALT ratio, and bilirubin levels) in a cohort of very heavy drinkers without evidence of clinical ALD.
2. Patients and Methods
2.1 Patient population and enrollment
This study was conducted under a larger protocol study approved by the Institutional Review Board of the NIAAA (ClinicalTrials.gov identifier # NCT00106106), and all patients signed Informed Consent documents prior to entering the study. One hundred and eight male and female patients, aged 21 – 65, were included in this study (Table 1). Patients had a diagnosis of alcohol dependence according to the DSM-IV, based on the alcohol dependence module of the SCID I-interview, and they had alcohol withdrawal symptoms. Patients were excluded from the study if they were diagnosed with severe psychiatric illness, were suicidal or violent, or had agitation requiring immediate clinical treatment. Patients with other clinically significant psychiatric illnesses (unless stable, and not requiring medication such as antidepressants, lithium, neuroleptics, naltrexone, acamprosate, disulfiram, benzodiazepines or antiepileptic compounds within the last four weeks) were also excluded. Illnesses such as advanced lung disease, unstable cardiovascular disease, renal failure (creatinine clearance < 30 ml/min), advanced liver disease (hepatocellular carcinoma, clinically evident alcoholic hepatitis, cirrhosis), or HIV were other exclusionary criteria. Day of assessment exclusionary criteria included: (1) pregnancy (negative test required) or ongoing breastfeeding; and/or (2) a positive urine screen for any illicit drug. Importantly, these patients had no clinical signs of alcoholic liver disease.
Table 1.
Demographic and drinking history assessment in AD patients by zinc level and sex. P-value in the last column resulted from the comparison between normal zinc and low zinc groups. Data presented as Mean±SD. Statistical significance set at P ≤ 0.05.
| Measures | Normal Zinc | Low Zinc | p-value for normal vs. low zinc | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Males (50) |
Females (17) |
Total (67) |
Males (21) |
Females (20) |
Total (41) |
||
| Age (years) |
38.9 ± 11.1# | 40.8 ± 12.0 | 39.4 ± 11.3 | 44.5 ± 9.3# | 45.3 ± 8.3 | 44.9 ± 8.8 | 0.025 |
| BMI (kg/m2) |
26.5 ±3.5 | 25.1 ± 5.7 | 26.1 ± 4.2 | 26.2 ± 4.9 | 26.7 ± 7.1 | 26.4 ± 6.0 | NS |
|
| |||||||
| Drinking Profile (History/Lab) | |||||||
|
| |||||||
| TD90
|
1076±585 | 901±688 | 1029±614 | 1187±529 | 990±510 | 1066±573 | NS |
| HDD90
|
67.5 ± 26.5 | 64.7±17.6 | 66.7 ± 24.3 | 71.6 ± 19.7 | 74.1 ± 20.9 | 72.6 ± 19.9 | 0.030 |
| AvgDPD90
|
14.5 ± 6.9 | 12.4 ± 7.1 | 13.9 ± 7.0 | 15.3 ± 7.2 | 13.1 ± 6.1 | 14.4 ± 6.8 | NS |
| NDD90
|
72.5 ± 23.0 | 68.7 ± 17.4 | 71.4 ± 21.6 | 74.2 ± 19.4 | 75.9 ± 19.8 | 74.9 ± 19.3 | NS |
| LTDH
|
14.2 ± 9.6 | 13.0 ± 9.8 | 13.9 ± 9.6 | 15.9 ± 9.5 | 11.8 ± 8.1 | 14.0 ± 9.0 | NS |
| CDT | 0.102 ± 0.14 | 0.072 ± 0.05 | 0.094 ± 0.12 | 0.124 ± 0.09 | 0.084 ± 0.08 | 0.104 ± 0.09 | NS |
Note:
significant difference between normal versus low zinc for males, p=0.050; BMI: Body mass index; TD90: Total drinks in 90 days; HDD90: heavy drinking days in last 90 days; AvgDPD90: Average drinks per drinking day in last 90 days; NDD90: number of drinking days in last 90 days; LTDH: lifetime drinking history; CDT: Carbohydrate Deficient Transferrin (Unit in ratio: 0.00 – 0.010 ≤ normal [for alcohol abuse]).
2.2 Specimen sampling and clinical data
On the day of evaluation, blood samples were collected for serum trace metals (including zinc), as well as a comprehensive metabolic chemistry panel (including albumin concentration). Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), AST:ALT ratio, and total bilirubin levels were used to assess liver injury. Samples were also assayed for the marker of inflammation, C-reactive protein (CRP), and carbohydrate deficient transferrin (CDT), a biomarker of alcohol intake.
Heavy drinking is defined as 15 drinks or more per week for males and eight drinks or more per week for females (https://www.cdc.gov/alcohol/faqs.htm). All patients in this study qualified as heavy drinkers per the Center of Disease Control recommendation. Time-line follow-back past 90 days (TLFB90) questionnaire [23] is a validated and well-established instrument to collect self-reported data on total number of drinks for each day in the past 90 days. Other recent drinking measures that were derived from the TLFB90 questionnaire include: Total drinks in the past 90 days (TD90), Number of drinking days in the past 90 days (NDD90), Drinks per drinking day in the past 90 Days (DPD90), Average drinks per drinking day in the past 90 days (AvgDD90), and Heavy drinking days in the past 90 Days (HDD90). We also used the lifetime drinking history (LTDH) questionnaire and the number of years as other drinking measures of evaluation in this study (https://pubs.niaaa.nih.gov/publications/AssessingAlcohol/measures.htm).
The standard lower limit of normal for serum zinc is 71 mcg/dL. Patients were grouped by serum zinc levels, ≥ 71 mcg/dL as normal; and <71 mcg/dL as the zinc deficient/low group. To evaluate the relevance of the degree of zinc deficiency in the clinical determination of ALD, we further separated the low zinc patients into two sub-groups; borderline (61-70 mcg/dL) and deficient <61 mcg/dL). We used “Controlling Nutritional Status Test” (CONUT) data to establish nutritional status [27]. Patients did not show any clinical evidence of liver disease, and overt clinical liver disease was an exclusion criteria in the study.
2.3 Statistical Analysis
Two-way analysis of variance (ANOVA) was used to assess whether there were significant differences between males and females within each zinc group, as well as whether there were significant differences between the normal zinc and low zinc groups for each sex. One-way ANOVA was used to examine significant differences between the normal zinc and low zinc groups when sex was not a significant variable. Multiple regression models were used to examine which drinking markers were significantly associated with zinc levels, and which laboratory markers were associated with zinc level when controlling for drinking history. Step-down variable selection was applied to obtain a parsimonious model for interpretation. Drinking history measures were included as covariates, as applicable. SPSS 24.0 (IBM Chicago, IL) and statistical software R (https://www.r-project.org/) and Microsoft Excel 2016 (MS Corp, Redmond WA) were used for data analyses. A test was considered significant if p<0.05 was observed. Levels of markers are shown as Mean±SD in the tables and as Mean±SE in the figures.
3. Results
3.1 Patient description
An approximately equal number of male (n=21) and female (n=20) patients were in the low zinc group (41 patients); however, there were more males than females in the normal zinc group (67 patients). Mean age was significantly higher in low zinc group; in particular, the age for males in normal zinc group was significantly lower than for those in the low zinc group. BMI was not different between the two groups, nor between males and females (Table 1). We did not find any statistically significant differences between males and females in the demographic and drinking profiles for the patients within or across the groups. Patients were not clinically malnourished as assessed by the CONUT.
3.2 Association of serum zinc and drinking markers
HDD90, a measure of heavy drinking history over the previous 90 days, was significantly higher in the low zinc group. However, there were no significant differences between groups on other drinking measures (see Table 1). When we used a multiple regression model to examine which drinking markers were associated with zinc level, we found that drinking markers impacted zinc level differently in the low zinc group and the normal zinc group. Step-down variable selection procedure was used, and NDD90 was the only drinking marker left in the model which was significantly associated with zinc level in both the low and normal zinc groups. We also found that NDD90 was highly correlated with HDD90 (correlation coefficient = 0.90), moderately correlated with TD90 (correlation coefficient = 0.56), and not correlated with LTDH (correlation coefficient = 0.08). In the low zinc group, serum zinc level was significantly associated with NDD90, HDD90, TD90, and LTDH (Fig. 1). On the other hand, in the normal zinc group, serum zinc was modestly, but significantly associated with NDD90 and HDD90, but not with TD90 or LTDH. Sex and BMI were not significantly associated with zinc level. There were no other significant associations between zinc and the TLFB drinking history marker or carbohydrate deficient transferrin (CDT) in either group.
Figure 1.
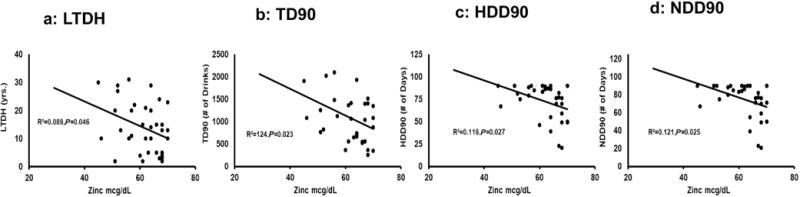
Association of zinc level with drinking markers in low zinc group. Significant correlations were noted among these variables in individuals with low serum zinc levels (Figures 1a-1d). Limited or no significant associations were found between serum zinc levels and drinking markers among subjects with normal serum zinc levels (not shown). Statistical significance was set at p ≤ 0.05.
3.3 Association of serum zinc and albumin
Serum albumin in the low zinc group was significantly lower than in the normal zinc group (Fig. 2a). When we evaluated the association of albumin with zinc, we found a highly significant association in the overall population as well as in the normal zinc group where both albumin and zinc were within the normal ranges, and a less robust association in the low zinc group (Fig. 2b-d). There was a significant lowering in albumin (p=0.027) in females compared to their male counterparts in the low zinc group. With LTDH as a covariate, the significance in this difference further augmented to p=0.014.
Figure 2.

Serum albumin by zinc level. Fig. 2a: Significant lowering of albumin in the low zinc group, p = 0.007 compared to the normal zinc group. Fig. 2b: Overall zinc vs. albumin across all the patients shows a close association of albumin and zinc. Fig. 2c: albumin correlation in the low zinc group. Fig. 2d: Albumin correlation in the normal zinc group. Statistical significance was set at p ≤ 0.05; ** p ≤ 0.01.
3.4 Association of serum zinc and C-reactive protein (CRP)
CRP was significantly elevated in the low zinc group (Table 2), and there was a significant association between serum zinc and CRP levels in the low zinc group (Fig. 3). However, no such association between serum zinc and CRP levels was found in the normal zinc group, even with adjusting for the drinking makers, HDD90 or NDD90 as independent variables. When we ran the analysis with log transformed data, the results were not changed. In the low zinc group, we further established the association between CRP and liver injury markers. CRP had a significant association with the AST:ALT ratio, adjusted R2=0.216, p=0.001, and the association was augmented to an adjusted R2=0.267, p=0.004 with HDD90 as an additional independent variable. Total bilirubin and CRP showed a significant association as well, adjusted R2=0.229, p=0.001 in low zinc patients.
Table 2.
Serum zinc, laboratory markers of Liver injury, and markers of nutritional state in alcohol dependent patients. Data are presented as Mean±SD. Statistical significance set at P ≤ 0.05. NS: Not significant.
| Measures | Normal Zinc | Low Zinc | p – value for normal vs. low zinc | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Males | Females | Total | Males | Females | Total | ||
| Zinc | 84.1 ± 9.9 | 86.8 ± 26.0 | 84.8 ± 15.4 | 61.4 ± 8.02 | 60.3 ± 9.4 | 60.9 ± 8.6 | Not Applicable |
|
| |||||||
| Laboratory markers of Liver-injury | |||||||
|
| |||||||
| AST
|
69.8 ± 79.8 | 68.7 ± 84.4 | 69.5 ± 80.3 | 86.8 ± 59.0 | 117.6 ± 122.2 | 101.8 ± 95.3 | 0.062 |
| ALT
|
57.1 ± 39.9 | 49.3 ± 55.3 | 55.1 ± 44.0 | 63.7 ± 35.3 | 70.1 ± 77.1 | 66.8 ± 58.8 | NS |
| AST:ALT ratio
|
1.1 ± 074 | 1.3 ± 0.4 | 1.15 ± 0.6 | 1.3 ± 0.5 | 1.6 ± 0.6 | 1.45 ± 0.6 | 0.016 |
| INR
|
0.989 ± 0.17 | 0.966 ± 0.04 | 0.984 ± 0.16 | 1.003 ± 0.12 | 0.999 ± 0.08 | 1.001 ± 0.10 | NS |
| Bilirubin Total
|
0.808 ± 0.74 | 0.547 ± 0.18 | 0.742 ± 0.66 | 0.781 ± 0.70 | 0.825 ± 0.76 | 0.802 ± 0.72 | NS |
| Albumin
|
3.9 ± 0.4 | 3.8 ± 0.5 | 3.9 ± 0.4 | 3.8 ± 0.3@ | 3.5 ± 0.3@ | 3.6 ± 0.3 | 0.007 |
| CRP | 1.5 ± 2.3 | 0.5 ± 0.2 | 1.3 ± 2.1 | 2.6 ± 4.3 | 3.5 ± 7.7 | 3.1 ± 6.1 | 0.037 |
|
| |||||||
| Nutritional Evaluation (CONUT) | |||||||
|
| |||||||
| CONUT | 1.06 ± 0.9 | 0.88 ± 1.1 | 1.01 ± 0.9 | 1.24 ± 1.5 | 1.45 ± 1.2 | 1.34 ± 1.3 | NS |
Note:
significant lowering in albumin level in females compared to males in low zinc group, p=0.027. Serum zinc: normal > 70 [Unit: mcg/dL]; AST: Aspartate Aminotransferase, normal ≤40 [Unit: U/L]; ALT: Alanine Aminotransferase, normal ≤41 [Unit: U/L]; INR: international normalized ratio, normal range: 0.8 – 1.2 (in absence of anticoagulation therapy); Bilirubin Total: normal ≤ 1.2 [Unit: mcmol/L]; CRP: C - reactive protein, normal < 5.0, [Unit: mg/L]; Albumin: normal 3.5 – 5.2 [Unit: g/dL]. Units and levels derived from NIH department of Laboratory Medicine guidelines.
Figure 3.
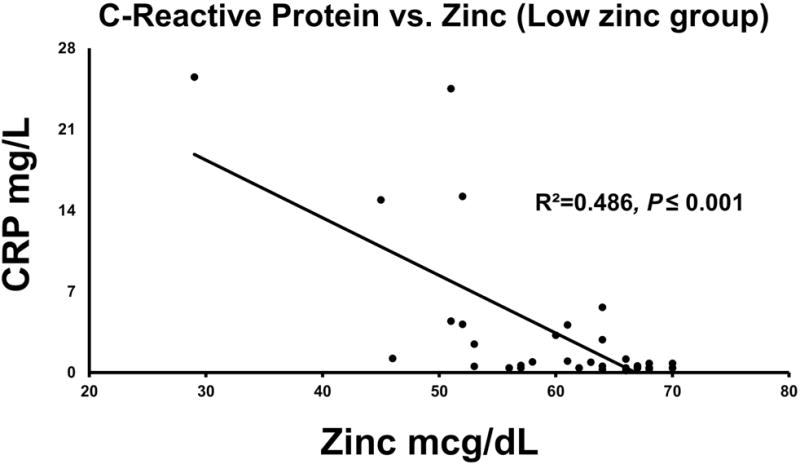
Association of zinc levels with CRP levels in the low zinc group, with a high effect of the association. Statistical significance was set at p ≤ 0.05.
3.5 Evaluation of liver injury markers in relation to zinc and drinking profile
AST levels were numerically greater in the low zinc group than in the normal zinc group, but this did not reach statistical significance. However, when we evaluated the AST:ALT ratio (a robust marker of ALD), we found a significant elevation of this ratio in the low zinc group, p=0.016 (Fig. 4a). In a multiple regression analysis, AST, total bilirubin, and CRP together showed a highly significant main effect with zinc, p≤ 0.001.
Figure 4.
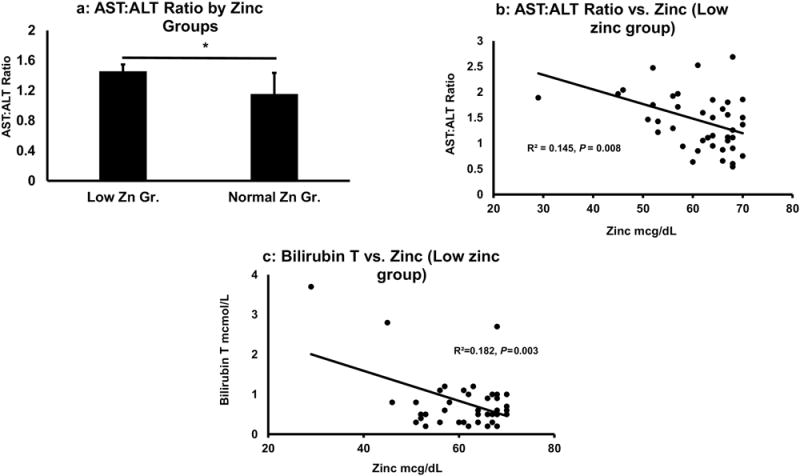
Association of zinc level with AST:ALT ratio and bilirubin levels. Fig. 4a: AST:ALT ratio was significantly higher in low zinc group, p = 0.016. Fig. 4b: Association of zinc and AST:ALT ratio in low zinc group. Fig. 4c: Association of Total bilirubin and zinc were significantly associated in low zinc group. Statistical significance was set at * p ≤ 0.05.
We then evaluated the association of zinc and the AST:ALT ratio by zinc group, and we found that there was no significant association between AST:ALT and zinc in the normal zinc group. However, there was a significant association between zinc and the AST:ALT ratio (Fig. 4b) in the low zinc group, and that association became stronger when co-varied with NDD90. There was a significant association found between zinc level and total bilirubin in the low zinc group (Fig. 4c), which was not observed in the normal zinc group. We further evaluated other markers of liver injury–the acute inflammatory marker, CRP, and drinking history markers–by the level of zinc deficiency (borderline low versus deficient [61-70 mcg/dL vs. <61 mcg/dL]) that might show augmented liver injury (Table 3). We found that there were statistically significantly higher AST:ALT ratio, higher CRP levels, and higher drinking history markers in the zinc deficient group vs. the borderline group (Table 3).
Table 3.
Sensitivity of zinc level with liver injury progression and inflammation in AD patients who exhibit liver injury. Significant differences were identified in liver injury and drinking markers in zinc deficient group of AD patients.
| Markers | Borderline (70-61) | Deficient (≤60) | Effect & p-value |
|---|---|---|---|
| Liver injury progression and inflammation | |||
|
| |||
|
AST/ALT Ratio
|
1.3±0.5 (26) | 1.7±0.6 (15) | R2=0.118; p=0.028 |
| CRP | 0.954±1.3 (25) | 6.6±8.9 (15) | R2=0.206; p=0.003 |
|
| |||
| Drinking Markers | |||
|
| |||
|
LTDH
|
11.86±7.9 (22) | 17.61±9.9 (13) | R2=0.097; p=0.068 |
|
TD90
|
904.1±456.7 (23) | 1403±661.5 (11) | R2=0.171; p=0.015 |
| NDD90 | 70.4±21.6 (23) | 84.4±7.6 (11) | R2=0.119; p=0.046 |
Serum zinc: normal > 70 [Unit: mcg/dL]; ]; CRP: C - reactive protein, normal < 5.0 [Unit: mg/L]; LTDH: lifetime drinking history;TD90: Total drinks past 90 days; NDD90: number of drinking days in last 90 days.
4. Discussion
In this study, we showed that 38% (41 subjects) of the 108 heavy drinking subjects admitted to a treatment program for alcohol use disorder (AUD) without clinical evidence of alcoholic hepatitis had low serum zinc concentrations. Drinking history profile has been validated and used extensively to understand the variability in alcohol intake by the rate and level of drinking; however correlations of drinking measures and zinc status have not been reported to date. TLFB90 has been shown to be a highly reliable measure to assess drinking history (high convergent validity), with responses from different groups of the drinking population (quantity-frequency) and various markers of drinking (grid measures) [28], as well as satisfactory reliability (consistency in responses over a longer period of time) [29]. HDD90, a measure of heavy drinking history, was significantly higher in the low zinc group. However, there were no significant between group differences in other drinking measures. This suggests that episodic heavy drinking (as assessed by the HDD90) may be important in the development of zinc deficiency. When zinc was in the normal range, markers of drinking history were either not significantly associated with zinc level, or their association was very weak. However, we showed significant associations between low serum zinc and specific markers of heavy alcohol intake, namely with LTDH, TD90, HDD90 and NDD90, and these results support the concept that specific patterns of drinking and their independent and collective presentation (primarily NDD90, HDD90) are associated with zinc deficiency.
A decrease in the serum zinc concentration is well documented after systemic inflammation or infection as part of the acute-phase response [30, 31]. During the acute-phase or stress response, zinc is redistributed to specific organs and cellular compartments, especially the liver, leading to a decrease in the serum zinc concentration [32]. CRP is an acute phase protein that is elevated with infection/inflammation. Interestingly, moderate alcohol consumption has been associated with a lowering of CRP [33, 34], suggesting that moderate drinking may have an anti-inflammatory effect. In this study, patients in the normal zinc group also had normal concentrations of CRP, and there was no association between zinc and CRP. However, in the low-zinc group, CRP concentrations were elevated, and there was a strong inverse correlation between elevated CRP levels and decreased serum zinc levels. This finding suggests that the decrease in the serum zinc concentration may be due, at least in part, to systemic inflammation and the acute-phase response.
Serum albumin is a negative acute-phase reactant, and serum albumin level decreases with acute inflammation [20]. Serum albumin is the major zinc binding protein in the blood. Two main mechanisms for hypoalbuminemia are decreased hepatic production of albumin and vascular leakage [35]. Zinc has been shown to attenuate vascular permeability in in vitro studies [36]. We showed significant correlations between albumin and zinc in the total population (as has been also reported previously [37]), as well as in the normal zinc group in this study. The less robust correlation in the low zinc group could reflect a dissociation of zinc from serum albumin and redistribution of zinc due to inflammation in the acute-phase response. Females tended to have lower albumin levels compared to their male counterparts in the low zinc group.
Serum AST and ALT were numerically but not statistically higher in the low zinc group, but importantly, the AST:ALT ratio was significantly elevated in the low zinc group. The AST:ALT ratio of ≥ 2 is often used as a biomarker of alcoholic hepatitis [38]. In our study, the AST:ALT ratio may indicate the development/progression of liver injury and it has a relation to the level of serum zinc (Fig. 4a & 4b) in these heavy drinking patients. Increased liver injury in the low zinc group and an association between bilirubin and serum zinc is also shown in Fig. 4c with low serum zinc correlating strongly with increases in the serum bilirubin. These translational human data are consistent with animal studies showing that zinc deficiency occurs in early experimental ALD.
There are limitations to the study. While we had a moderately large study population of 108 patients, we could not identify sex-based differences between groups based on zinc levels. This may be due to inadequate sample size. Effect sizes in this study were mostly moderate, again indicative of the patient sample size in our study. This was not a longitudinal study and we could not correlate changes in zinc status with improvement or worsening of liver injury. Zinc status was only assessed by serum zinc levels, but this is the main indicator of zinc status used clinically. Our results clearly support the involvement of zinc in ALD development. Drinking history markers showed significant correlations, but with mild effect, suggesting that they may play an important role but that there may be other cofactors involved. These other cofactors could include metabolic factors, genetics, ethnicity, etc. Determining these cofactors was beyond the scope of this study.
In conclusion, this study showed that almost 40% of very heavy drinking alcohol-dependent patients had low serum zinc concentrations. The low-zinc group had more evidence of liver injury (AST, ALT, AST:ALT, bilirubin), lower serum albumin, and a strong inverse correlation between low zinc and C-reactive protein. To our knowledge, this is the first study evaluating zinc status in a large cohort of subjects with AUD who had very well characterized drinking histories and no clinical evidence for advanced liver disease. These data provide further support for a role of zinc deficiency in the early stages/development of human ALD.
Highlights.
Markers of very heavy drinking and zinc deficiency were associated with liver injury
A close association of zinc deficiency and low albumin was observed in subjects with liver injury (as defined by AST, ALT and bilirubin)
Elevated C-reactive protein was significantly associated with zinc deficiency in subjects with liver injury (as defined by AST, ALT and bilirubin)
The acute phase inflammatory response is one possible mechanism for the observed hypozincemia in alcohol abuse
The degree of zinc deficiency correlated with markers of progressive alcoholic liver disease
Acknowledgments
Authors acknowledge clinical staff of LCTS NIAAA for the patient support. Authors thank Ms. Marion McClain for editing the manuscript.
FINANCIAL/GRANT SUPPORT: NIH grants: U01AA021901, U01AA021893-01, U01AA022489-01A1, R01AA023681-01 (CJM), K23AA018339 (MCC), ZIA-AA000466 (VAR), Z99-AA999999 (VV) and the VA (CJM). Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113226 (CJM), and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number P50AA024337 (CJM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AD
Alcohol Dependent
- ALD
Alcoholic Liver Disease
- ALT
Alanine Aminotransferases
- AST
Aspartate Aminotransferases
- CDT
Carbohydrate-deficient transferrin
- CRP
C - reactive protein
- Gr. 1
Group 1 with normal serum zinc level
- Gr. 2
Group 2 with low zinc level
- LTDH
Lifetime Drinking History
- TLFB
Timeline Followback (Total drinks in the past 90 days [TD90], Number of drinking days in the past 90 days [NDD90], Drinks per drinking day in the past 90 Days [DPD90], Average drinks per drinking day in the past 90 days [AvgDD90], and Heavy drinking days in the past 90 Days [HDD90])
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST and DISCLOSURES: All authors have no disclosures to make. All authors have no conflict of interests.
Trial registration: ClinicalTrials.gov identifier # NCT00106106
Author Contribution: CJM and VV are responsible for the study concept and design. VV and MLS participated in data collection and assembly. MK, VV performed statistical analysis. CJM, VV, MK, and MCC interpreted the statistical analysis and determined the clinical outcomes. VV, MCC and NL drafted the manuscript. CJM, VAR, DTG, and MLS provided scientific contribution and revised the manuscript critically. Study supervision was performed by DTG. Project funding was supported by CJM, VAR, and VV. All authors critically reviewed content and approved final version for publication.
INFORMED CONSENT: Informed consent was obtained from every patient enrolled in the study protocol.
References
- 1.O’Shea RS, Dasarathy S, McCullough AJ, Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249–64. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 5.Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005;26:391–404. doi: 10.1016/j.mam.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.George J, Ganesh HK, Acharya S, Bandgar TR, Shivane V, Karvat A, et al. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol. 2009;15:3516–22. doi: 10.3748/wjg.15.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220–31. [PMC free article] [PubMed] [Google Scholar]
- 8.McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35:815–20. doi: 10.1111/j.1530-0277.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalor BC, France MW, Powell D, Adams PH, Counihan TB. Bone and mineral metabolism and chronic alcohol abuse. Q J Med. 1986;59:497–511. [PubMed] [Google Scholar]
- 10.McClain CJ, Marsano L, Burk RF, Bacon B. Trace metals in liver disease. Semin Liver Dis. 1991;11:321–39. doi: 10.1055/s-2008-1040450. [DOI] [PubMed] [Google Scholar]
- 11.Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis: is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol. 1996;11:417–21. doi: 10.1111/j.1440-1746.1996.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 12.McClain CJ, Antonow DR, Cohen DA, Shedlofsky SI. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–9. doi: 10.1111/j.1530-0277.1986.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One. 2013;8:e76522. doi: 10.1371/journal.pone.0076522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallee BL, Wacker WE, Bartholomay AF, Hoch FL. Zinc metabolism in hepatic dysfunction. II. Correlation of metabolic patterns with biochemical findings. N Engl J Med. 1957;257:1055–65. doi: 10.1056/NEJM195711282572201. [DOI] [PubMed] [Google Scholar]
- 15.Morrison SA, Russell RM, Carney EA, Oaks EV. Zinc deficiency: a cause of abnormal dark adaptation in cirrhotics. Am J Clin Nutr. 1978;31:276–81. doi: 10.1093/ajcn/31.2.276. [DOI] [PubMed] [Google Scholar]
- 16.McClain CJ, Van Thiel DH, Parker S, Badzin LK, Gilbert H. Alterations in zinc, vitamin A, and retinol-binding protein in chronic alcoholics: a possible mechanism for night blindness and hypogonadism. Alcohol Clin Exp Res. 1979;3:135–41. doi: 10.1111/j.1530-0277.1979.tb05287.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27:8–20. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–7. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 20.Hennig B, Honchel R, Goldblum SE, McClain CJ. Tumor necrosis factor-mediated hypoalbuminemia in rabbits. J Nutr. 1988;118:1586–90. doi: 10.1093/jn/118.12.1586. [DOI] [PubMed] [Google Scholar]
- 21.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–87. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barve S, Chen SY, Kirpich I, Watson WH, McClain C. Development, Prevention, and Treatment of Alcohol-Induced Organ Injury: The Role of Nutrition. Alcohol Res. 2017;38:289–302. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–90. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB, Connors GJ, Agrawal S. Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcohol Clin Exp Res. 2003;27:1661–6. doi: 10.1097/01.ALC.0000091227.26627.75. [DOI] [PubMed] [Google Scholar]
- 26.Anton RF, Stout RL, Roberts JS, Allen JP. The effect of drinking intensity and frequency on serum carbohydrate-deficient transferrin and gamma-glutamyl transferase levels in outpatient alcoholics. Alcohol Clin Exp Res. 1998;22:1456–62. doi: 10.1111/j.1530-0277.1998.tb03935.x. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima K, Ueno Y, Kawagishi N, Kondo Y, Inoue J, Kakazu E, et al. The nutritional index ‘CONUT’ is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J Exp Med. 2011;224:215–9. doi: 10.1620/tjem.224.215. [DOI] [PubMed] [Google Scholar]
- 28.Grant KA, Tonigan JS, Miller WR. Comparison of three alcohol consumption measures: a concurrent validity study. J Stud Alcohol. 1995;56:168–72. doi: 10.15288/jsa.1995.56.168. [DOI] [PubMed] [Google Scholar]
- 29.Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58:358–64. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- 30.Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr. 1985;5:341–63. doi: 10.1146/annurev.nu.05.070185.002013. [DOI] [PubMed] [Google Scholar]
- 31.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol. 1997;272:E952–6. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- 32.Albergoni V. Physiological properties of copper and zinc. In: KD R, R M, JRJ S, GP V, editors. Copper and Zinc in Inflammatory and Degenerative Diseases. Dordrecht: Springer; 1998. pp. 7–17. [Google Scholar]
- 33.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–7. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 34.Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. 2002;56:1130–6. doi: 10.1038/sj.ejcn.1601459. [DOI] [PubMed] [Google Scholar]
- 35.Mendez CM, McClain CJ, Marsano LS. Albumin therapy in clinical practice. Nutr Clin Pract. 2005;20:314–20. doi: 10.1177/0115426505020003314. [DOI] [PubMed] [Google Scholar]
- 36.Hennig B, Wang Y, Ramasamy S, McClain CJ. Zinc protects against tumor necrosis factor-induced disruption of porcine endothelial cell monolayer integrity. J Nutr. 1993;123:1003–9. doi: 10.1093/jn/123.6.1003. [DOI] [PubMed] [Google Scholar]
- 37.Bates J, McClain CJ. The effect of severe zinc deficiency on serum levels of albumin, transferrin, and prealbumin in man. Am J Clin Nutr. 1981;34:1655–60. doi: 10.1093/ajcn/34.9.1655. [DOI] [PubMed] [Google Scholar]
- 38.Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845–54. doi: 10.1111/apt.12279. [DOI] [PubMed] [Google Scholar]


