Abstract
Reproduction and fertility are regulated via hormones of the hypothalamic-pituitary-gonadal (HPG) axis. Control of this reproductive axis occurs at all levels, including the brain and pituitary, and allows for promotion or inhibition of gonadal sex steroid secretion and function. In addition to guiding proper gonadal development and function, gonadal sex steroids also act in negative and positive feedback loops to regulate reproductive circuitry in the brain, including kisspeptin neurons, thereby modulating overall HPG axis status. Additional regulation is also provided by sex steroids made within the brain, including neuroprogestins. Furthermore, as reproduction and survival need to be coordinated and balanced, the HPG axis is able to modulate—and be modulated by—stress hormone signaling, including cortiscosterone, from the hypothalamic-pituitary-adrenal (HPA) axis. This review covers recent data related to the neural, hormonal, and stress regulation of the HPG axis and emerging interactions between the HPG and HPA axes, focusing on actions at the level of the brain and pituitary.
Keywords: reproduction, GnRH, kisspeptin, stress, estradiol
1. Introduction
Reproduction is necessary for the continuation of species, and reproductive success is dependent on many neuropeptide and hormonal systems working in concert to regulate gonadal function and sexual behavior. Reproduction is governed by the hypothalamic-pituitary-gonadal (HPG) axis. The hypothalamic control of reproduction is coordinated through the release of gonadotropin-releasing hormone (GnRH). GnRH, typically secreted in pulses, stimulates secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), from the anterior pituitary gonadotropes. These hormones function at the gonads to stimulate the production of gametes and promote gonadal release of sex steroids [i.e., testosterone (T), estradiol (primarily 17β-estradiol, E2), and progesterone (P4)]. Along with guiding reproductive function in the peripheral tissues, these gonadal steroids can also feedback and modulate upstream HPG components. Each level of the HPG axis is tightly regulated but can be modulated to influence reproductive status. This review will focus on neural and hormonal factors controlling and modulating HPG axis activity, as well as how the HPG axis can influence and be influenced by stress signaling.
2. The Neuroendocrine Reproductive Axis and its Upstream Control by Kisspeptin and RFRP-3
Within the brain, an interconnected network of neurons regulates the pulsatile release of GnRH; however, many of the key mechanisms and factors involved in the regulation of GnRH release, including modulation of GnRH pulsatile and surge modes of secretion, remain poorly-defined. Within the past decade, the neuropeptides kisspeptin and RFRP-3 have been shown to have potent stimulatory or inhibitory actions on GnRH secretion in mammals, thereby modulating reproductive status.
The Kiss1 gene encodes the neuropeptide kisspeptin, which potently stimulates GnRH secretion1. Early experiments determined that exogenous kisspeptin treatment of rodents and other species, including humans, robustly increases circulating LH and FSH levels (reviewed in 1,2). Subsequent studies from numerous species have now provided a wealth of information supporting the model that hypothalamic-derived kisspeptin directly activates GnRH neurons via kisspeptin receptor (Kiss1R) to stimulate the reproductive axis (reviewed in 1,2). In a wide range of mammals, including rodents, sheep, and primates, Kiss1 mRNA or kisspeptin protein has been detected primarily in two discrete regions of the hypothalamus, the preoptic area [POA; which in rodents includes the morphological continuum comprising the anteroventral periventricular nucleus and neighboring periventricular nucleus (AVPV/PeN)] and, more caudally, the arcuate nucleus (ARC; analogous to the primate infundibular nucleus) (reviewed in 3,4).
In adulthood, the secretion of GnRH is regulated by positive and negative feedback actions of gonadal sex steroids. Importantly, GnRH neurons do not express estrogen receptor α (ERα) or the androgen receptor (AR), the receptors that mediate sex steroid feedback, indicating that other sex steroid-sensitive circuits “upstream” of GnRH neurons receive and relay sex steroid feedback signals to GnRH cells3. Recent evidence suggests that hypothalamic kisspeptin neurons, which express ERα and AR, are these upstream sex steroid-sensitive neurons. Kisspeptin neurons in the ARC are likely involved in promoting pulsatile GnRH and LH secretion and responding to sex steroid hormone negative feedback1,2. In contrast, kisspeptin neurons in the AVPV/PeN are thought to be involved in mediating E2-positive feedback which triggers the preovulatory LH surge in females5. Supporting this hypothesis, AVPV/PeN Kiss1 gene expression is greater in females than males (males do not show an LH surge), Kiss1 neurons in the AVPV/PeN express ERα and show increased neuronal activation exclusively during the LH surge, and Kiss1 knockout (KO) mice and Kiss1R KO mice do not exhibit an LH surge even with exogenous E2 treatment3,5.
In contrast to kisspeptin, another neuropeptide, RFamide-related peptide-3 (RFRP-3), has potent inhibitory actions on LH secretion in many mammalian species6–8. RFRP-3 is encoded by the Rfrp gene and is the mammalian orthologue of avian gonadotropin-inhibiting hormone (GnIH). RFRP-3 neurons are found exclusively in the dorsal-medial nucleus of the hypothalamus (DMN) of rodents7,8 and may be involved in influencing the onset of puberty, the onset of cyclicity in seasonally reproductive mammals, and the suppression of GnRH-induced release of gonadotropins9,10. RFRP-3 has been functionally shown to inhibit the electrical firing of some GnRH and ARC kisspeptin neurons11–13 suggesting that RFRP-3 may inhibit the reproductive axis, in part, by signaling to kisspeptin and/or GnRH populations. Interestingly, both RFRP-3 and kisspeptin neurons are influenced by stress hormone regulation, as will be discussed further below.
3. GnRH Regulation of Pituitary Gonadotropin Production
The gonadotrope is a target for many different endocrine inputs; yet, none of these is more fundamental than GnRH. The unique phenotype of gonadotropes is defined by expression of the GnRH receptor (GnRHR) on the plasma membrane and the synthesis and secretion of LH and FSH. It is well established that GnRH induces the release of stored LH secretory granules into the systemic circulation14,15. Increased GnRH pulse frequency and amplitude favor LH secretion, while a slower frequency favors FSH secretion16,17. This elegant mechanism is particularly important for female reproduction, where increases in GnRH pulsatility are required for the LH surge and, thus, ovulation.
GnRH binding to its cognate membrane receptor leads to an increase in GnRHR numbers on the plasma membrane and increased expression of the different gonadotropin subunits. The GnRHR is a member of the rhodopsin like family of seven transmembrane domain G protein coupled receptors (GPCR)18. Hormone binding the GnRHR activates Gαq/11 which initiates multiple phospholipase activities leading to formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol19,20. Receptor activation also increases intracellular calcium concentrations through release of intracellular stores and opening of voltage gated L-type calcium channels, which underlie activation of protein kinase C (PKC) isoforms21.
Transcription of the LHβ gene is highly sensitive to stimulation by GnRH. The proximal 140 base pairs (bp) of the LHβ gene promoter is highly conserved across species, but the GnRH responsive promoter regions vary between species. The proximal 140bp consist of tandem copies of DNA binding motifs for the early growth response protein 1 (Egr-1) and steroidogenic factor 1 (SF-1) transcription factors22,23. Sandwiched between the tandem Egr and gonadotrope specific element (GSE) motifs, is the binding site for Pitx1, a paired-like homeodomain transcription factor24. Data from transgenic mice suggest that the GSE and the Pitx1 elements are required for GnRH induced LHβ gene promoter activity23,25. Additionally, GnRH stimulation results in an increase in Egr-1 mRNA and protein, which is tightly linked to an increase in LHβ synthesis26.
GnRH also induces epigenetic mechanisms to regulate LHβ gene expression. Lim et al. showed that the gene encoding LHβ, Lhb, is occupied by histone deacetylases (HDACs) in αT3-1 cells resulting in transcriptional silencing27. In this cell line, GnRH stimulated the export of class IIa HDACs resulting in increased acetylation of gonadotropin genes, while in LβT2 cells, another gonadotrope derived cell line, GnRH treatment enhanced the association of the histone acetyltransferase p300 with the Lhb gene28. Chromatin organizing the β-subunit gene promoters also contains low levels of trimethylated histone H3 lysine 4 (H3K4me3). GnRH stimulation increases this modification resulting in chromatin decondensation by menin and the menin-mixed-lineage leukemia (MLL) 1/2 methyl transferase complex29. Work by Khan et al. shows that GnRH also induces the citrullination of histone H3 arginine residues 2, 8, and 17 to stimulate expression of the Lhb gene30. Thus, GnRH stimulation initiates both epigenetic and transcriptional mechanisms to increase LHβ gene expression in gonadotrope cells.
4. Estrogen Modulation of Reproductive Hypothalamic Circuits
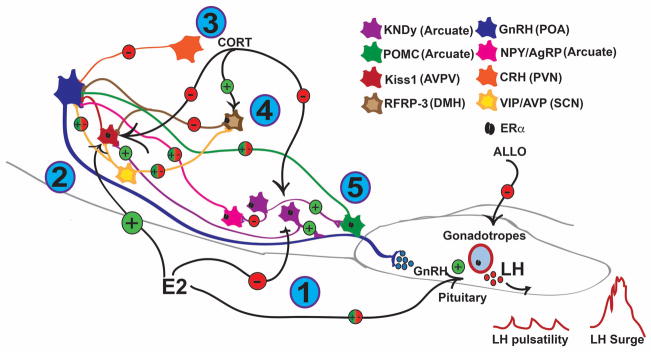
Estrogens, primarily E2, feedback to the brain to control the pulsatile release of GnRH into the primary portal plexus through bimodal interactions with the hypothalamic neurocircuitry that modulate GnRH neurons. These neuronal circuits include kisspeptin neurons, both in the AVPV and the ARC nuclei, vasoactive intestinal peptide (VIP) and arginine vasopressin (AVP) neurons of the suprachiasmatic nucleus (SCN), RFRP-3 neurons of the DMN, corticotropin-releasing hormone (CRH) neurons of the paraventricular nucleus (PVN), and the melanocortin (proopiomelanocortin (POMC) or neuropeptide Y and agouti-related peptide (NPY/AgRP)) neurons of the ARC31–33. These hypothalamic circuits collectively express the range of ER and employ both nuclear-initiated estrogen signaling (through estrogen response element (ERE)-dependent and ERE-independent pathways) and membrane-initiated estrogen signaling (through palmitoylated nuclear ER (α/β) and E2-responsive GPCR)34.
Nuclear-initiated ERα and ERβ signaling controls gene expression primarily through direct binding to DNA via an ERE. After ligand-dependent receptor activation, ER disassociates from molecular chaperones, dimerizes, and binds to coactivators or corepressors to initiate or suppress transcription34. Alternatively, ERα and ERβ can modulate transcription through protein-protein interactions with other transcription factors interacting with DNA in non-ERE promoter sites.35 Membrane-associated ERα and ERβ can also activate signaling cascades to modulate cell physiology and control gene expression including phosphatidylinositol 3′-kinase (PI3K), phospholipase C (PLC), MAPK and protein kinase pathways (PKA, PKC, etc.)36 . A second putative, Gq-coupled, E2-responsive GPCR (Gq-mER) controls PLC-mediated signaling in a wide variety of hypothalamic neurosecretory neurons36,37.
ERα has been identified as the key ER mediating the negative and positive feedback of E2. ERα KO mice are infertile and do not have a normal estrous cycle or the proestrus surge of LH, while ERβ KO mice are subfertile with regular estrous cycles38. E2 also modulates GABAergic tone of GABA neurons in the POA, and specific deletion of ERα in hypothalamic GABA neurons produces infertile female mice due to a failure to generate an LH surge and ovulation39. However, GnRH neurons from both sexes, and from many species, express ERβ but not ERα31. Indeed, membrane-initiated signaling via ERβ activates a PLC-mediated signaling cascade leading to retrograde endocannabinoid signaling to reduce the frequency of presynaptic GABA release and spontaneous postsynaptic currents40. E2 at high physiological concentrations enhance GnRH firing frequency through modulation of the after-hyperpolarization potential via a membrane-mediated ERβ-PKA pathway in mice41. Furthermore, activation of GPER by its selective ligand STX in mouse GnRH neurons controlled the expression and activity of cation channels involved in burst firing and membrane hyperpolarization42,43, indicating the E2 has multiple pathways and receptors to control GnRH neuronal excitability, gene expression, and peptide release. ER expression across the various cell types of the hypothalamic-pituitary circuit are summarized in table 1.
Table 1.
Estrogen receptor expression in the hypothalamic-pituitary circuits
| Cell Type | ERα | ERβ | GPER1 | Gq-mER |
|---|---|---|---|---|
| GnRH | − | + | + | + |
| Kiss1 | + | + | + | ND |
| KNDy | + | + | + | ND |
| POMC | + | − | ND | + |
| NPY/AgRP | + | + | ND | + |
| CRH | − | + | + | + |
| RFRP-3 | + | − | ND | ND |
| SCN - AVP | − | − | ND | ND |
| SCN - VIP | − | − | ND | ND |
| Gonadotropes | + | + | + | ND |
+: expression; -: no expression; ND: no data
As previously discussed, ARC-derived kisspeptin is the primary “gatekeeper” of GnRH pulsatility. Both populations of kisspeptin neurons express ERα (with low ERβ expression) but respond differentially to E2 through ERE-dependent and ERE-independent mechanisms44,45. In females, ARC kisspeptin expression is suppressed through ERα-mediated, ERE-independent mechanisms or de-repressed through a hormone response element44,46. Neurokinin B and dynorphin, co-expressed with kisspeptin (KNDy neurons) in the ARC nucleus and vital components of the GnRH pulse generator, are also downregulated by E2 but through ERα-mediated, ERE-dependent mechanisms44,45. In AVPV/PeN kisspeptin neurons, Kiss1 mRNA expression and kisspeptin synthesis are stimulated by E2 through ERα-mediated, ERE-dependent mechanisms45. This E2-induced expression of AVPV/PeN kisspeptin occurs with an increase in kisspeptin neuronal activation and peptide release onto GnRH neurons to drive the pre-ovulatory surge of GnRH and LH3,5.
In rodents, neurons of the SCN, the body’s central circadian clock, play a prominent role in the promotion and timing of the LH surge initiated at the beginning of the dark cycle. These SCN neurons synthesize either AVP and project to AVPV/PeN kisspeptin and RFRP-3 neurons or synthesize VIP and project to GnRH and RFRP-3 neurons, thereby controlling the timing of the LH surge47. Neither AVP nor VIP neurons of the SCN express detectable ERα or ERβ protein in the mouse48. However, E2 restores AVP-induced activation of kisspeptin neurons in the AVPV/PeN in ovariectomized (OVX) female mice49,50, and E2 perfusion can rapidly depolarize SCN neurons by increasing the frequency of excitatory postsynaptic currents leading to an increase in firing frequency51. Elevated E2 levels preceding the surge of GnRH and LH activate SCN neurons to accelerate the downstream release of kisspeptin and simultaneously decrease the inhibition of GnRH secretion provided by RFRP-332,52.
E2 also impacts the inhibitory hypothalamic inputs, including DMN RFRP-3 neurons. E2 reduces RFRP-3 expression in the DMN of mice, although only ~20% of RFRP-3 neurons express detectable levels of ERα protein and no detectable ERβ53. In the hamster, E2 reduces RFRP-3 neuronal activation, perhaps to promote downstream GnRH surge secretion, with ~40% of RFRP-3 neurons in the female hamster expressing ERα7. The low expression of ERα and ERβ in RFRP-3 neurons suggests that membrane-initiated estrogen signaling may be key to the regulation of these neurons, as has recently been characterized in ARC NPY neurons54. However, this has yet to be studied in RFRP-3 neurons.
Several other populations of hypothalamic neurons also can excite or inhibit GnRH neurons and mediate E2’s influence on the pulsatile and surge release of LH. One such population is CRH neurons of the PVN, which are the master control neurons of the stress response. CRH intracerebroventricular (ICV) administration suppresses both LH pulsatility and the LH surge, and this suppression is enhanced by E255, potentially through CRH innervation of the locus coeruleus56. However, recent evidence indicates that CRH directly modulates GnRH excitability in a dose-dependent and receptor-specific manner under the influence of E257. CRH neurons are also activated by membrane-initiated E2 signaling through the Gq-mER to suppress the M-current and augment excitatory postsynaptic current amplitude58. Another population of neurons modulated by E2 is the melanocortin neurons of the ARC nucleus that express POMC or NPY and AgRP. The two neuropeptides produced by POMC neurons, α-MSH and β-endorphin, differentially control GnRH excitability and release. α-MSH activation of the melanocortin 4 (MC4) receptor increases GnRH action potential firing while β-endorphin signaling through μ-opioid receptors inhibit GnRH excitability33,59. Furthermore, NPY can excite and inhibit GnRH neurons through the Y1 and Y4 receptors, respectively, and AgRP, an endogenous agonist to MC4 receptors, can also excite and inhibit GnRH neurons33,60. Both POMC and NPY neurons express ERα protein, although at strikingly different levels54,61. Furthermore, both neurons express the Gq-mER, which disinhibits GABA and μ-opioid tone in POMC neurons and augments GABAergic tone in NPY neurons54. The distinct E2-induced responses in the melanocortin neurons potentially amplify E2’s role in the negative feedback on the HPG axis.
5. Neuroprogesterone Control of the Reproductive Axis
Emerging evidence indicates that steroid hormones synthesized in the brain, termed “neurosteroids,” have impact on the reproductive axis. In some brain areas, locally derived pregnenalone is converted to P4 via 3β-hydroxysteroid dehydrogenase (3β-HSD) which is then metabolized to allopregnenalone (ALLO), the main active derivate, by 5α-reductase and 3α-HSD62. Both 5α-reductase and 3α-HSD are expressed in the cortex, hippocampus, and amygdala62, supporting ALLO’s potential ability to modulate behavior.
Neurosteroids, particularly P4 and ALLO, have been associated with mood and anxiety disorders63. While P4 may exert behavioral effects through the classical nuclear P4 receptor (PR) mechanism64, most of P4’s effect may correspond to P4 conversion to ALLO63. ALLO interacts with the GABAA receptors as an allosteric modulator63,65 and enhances GABA-mediated inhibition at GABAA receptors, and, at high doses, ALLO acts as a GABAA receptor agonist. Additionally in the last decade, a new pathway of ALLO action through membrane P4 receptors (mPR α and β) was discovered allowing for rapid responses65. ALLO and its stereoisomer, pregnanolone, can also be sulphated allowing them to modulate glutamatergic neurotransmission through NMDA receptors63. It is under debate whether sulphated neurosteroids reach neurophysiological levels to exert endogenous modulatory effects on glutamatergic neurotransmission63. The mechanisms of actions of ALLO need more clarification due to the differences found between receptors and areas of action.
ALLO interaction with GABAA receptors contributes to ALLO’s anxiolytic, neuroprotective, neuromodulatory, and anti-gonadotropic properties66–68. In serotonergic neurons, ALLO, not P4, potentiated GABAA receptor response to agonists69. Serotonin signalling disruption is associated with mood disorders thereby suggesting an association between ALLO and serotonergic signalling. This effect of ALLO on serotonin signalling may be pertinent for disorders linked to reproductive hormone fluctuations such as premenstrual dysphoric disorder and postpartum depression69.
ALLO fluctuations during the estrous cycle have a strong impact on reproductive function70,71. ALLO levels decrease in the hypothalamus during proestrus, when ovulation occurs, which supports a role for ALLO in modulating the HPG axis to control ovulation70. One ICV administered dose of ALLO on the morning of proestrus has an inhibitory effect on both ovulation rate and sexual receptivity71,72. When ALLO ICV injection was coupled with a GABAA receptor antagonist, sexual receptivity was recovered, suggesting that some ALLO action involves GABAA signalling71,72. Furthermore, Giuliani et al. observed increased GnRH release from hypothalamic slices following treatment with ALLO which was absent when slices were also treated with an NMDA receptor antagonist. This suggests that ALLO may also modulate NMDA receptor activity to alter HPG axis activity73.
ALLO signalling can also influence development of the ovary. Rats treated with a single ICV injection of ALLO on the morning of proestrus showed ovarian disorders, such as follicular atresia, ovarian cysts, and delayed luteolysis, an apoptotic process of the corpus luteum71. ALLO treatment also caused alterations in both follicular and luteal dynamics during the estrous cycle74.
6. Stress and Reproduction: Bidirectional Interactions
Stress signaling is regulated by the hypothalamic-pituitary-adrenal (HPA) axis. Upon exposure to a stressor, the PVN is activated to release CRH which then stimulates release of adrenocorticotropic hormone (ACTH) from the pituitary. ACTH, in turn, stimulates release of glucocorticoids (CORT; corticosterone in rodents, cortisol in humans) from the adrenals which then provide negative feedback back to the brain in a classic homeostatic feedback loop to fine-tune HPA axis signaling. The HPG and the HPA axes are tightly linked to balance reproduction and survival.
6a. Influence of Stress on Reproductive Status and Brain Circuitry
Stress, including psychological stress, can negatively impact reproduction in many mammalian species, including humans. In females, stress can disrupt ovarian cyclicity as well as upstream gonadotropin synthesis and secretion75,76. For example, restraint stress, a commonly-used model of psychosocial stress, reduces LH and FSH in rodents and other species77,78. In multiple species, psychosocial stress exposure or treatment with exogenous CORT inhibited the pulsatile release of LH79–81 which is assumed to reflect inhibition of upstream GnRH pulsatile secretion. To date, little is known about underlying mechanisms for stress suppression of GnRH and downstream LH secretion, especially at the level of the brain. Given the importance of ARC kisspeptin neurons in directing pulsatile GnRH secretion, AVPV/PeN kisspeptin neurons in generating the LH surge, and RFRP-3 neurons in inhibiting GnRH and LH output, it is possible that stress alters downstream GnRH and LH secretion via upstream actions on kisspeptin and/or RFRP-3 neurons.
The E2-induced surge mode of GnRH and LH secretion (i.e., positive feedback), which governs ovulation in females, is vulnerable to the effects of stress. Isolation-restraint stress can block the LH surge in rats, and in sheep and monkeys, the LH surge can be delayed or blocked by stress82,83. Despite this evidence, the fundamental mechanisms that result in disrupted surge LH secretion are not well understood. In a recent collaboration between the Breen and Kauffman labs, exogenous CORT treatment of gonad-intact female mice arrested cyclicity in diestrus84. A separate cohort of OVX female mice were treated with an LH surge-inducing E2 implant, as well as a CORT or cholesterol (control) pellet, and assessed two days later for LH levels on the evening of the anticipated LH surge. All cholesterol-treated females showed a clear LH surge, whereas LH levels were undetectable in CORT-treated females84. Brain analyses revealed that CORT robustly suppressed the percentage of AVPV/PeN Kiss1 cells co-expressing cfos, as well as reduced the number of AVPV/PeN Kiss1-expressing cells, compared to expression in control brains84. Collectively, these findings in female mice support the hypothesis that physiological stress-levels of CORT disrupt ovarian cyclicity, in part, through disruption of positive feedback mechanisms at hypothalamic kisspeptin circuits which are known to be necessary for LH surge generation.
The effects of stress on LH pulsatility in mice, whose small size posed a decades-long technical challenge for assaying LH in repeated blood samples, was recently tested for the first time when a new LH assay and blood sampling methodology was invented. OVX female mice were either not stressed (controls) or exposed to 90 min of restraint stress, during which time serial blood samples were collected every 5 minutes to measure pulsatile LH levels. LH pulse frequency and interpulse intervals were significantly and rapidly decreased in stressed female mice compared to controls, with no effects on LH pulse amplitude85. Next, to determine the effects of acute restraint stress on reproductive neuropeptides known to regulate GnRH neurons, OVX female mice were separated into control (no stress) or 45, 90, or 180-min restraint stressed groups. Acute restraint stress had no effect on Kiss1 mRNA levels, though there was a significant decrease in Kiss1 neuronal activation at all time points, suggesting a decrease in stimulatory kisspeptin input to GnRH neurons85. Rfrp neuronal activation in female mice was significantly increased after 45 min of restraint stress but not following longer stress durations, suggesting that increased RFRP-3 signaling, known to inhibit GnRH and LH, may occur rapidly following acute stress85. Furthermore, Rfrp mRNA levels in female mice were elevated after 180 min of restraint stress, perhaps as a compensatory response to replenish RFRP-3 stores that were reduced when these neurons were more active during initial stress exposure85.
Collectively, the data described in this section suggest that stress-induced suppression of the reproductive axis in female mice may be due, at least in part, to regulation at levels upstream of GnRH neurons, either directly or indirectly, through ARC kisspeptin and/or DMN RFRP-3 neurons. Supporting the mouse data, in rats, 5 hours of restraint stress increased hypothalamic Rfrp expression and decreased mean LH secretion via a pathway dependent on elevated CORT77. The specific underlying neural, hormonal, and cellular mechanisms by which stress alters RFRP-3 and kisspeptin neurons remain to be determined.
6b. Influence of Reproductive Hormones on the HPA axis
Anxiety and mood disorders are associated with HPA axis dysregulation and show large gender disparities, with women being more likely to experience these disorders86. This gender disparity in prevalence supports a role for gonadal steroids, E2, P4, and T, in modulating the HPA axis. Through these gonadal steroids, the HPG axis can exert a modulatory effect over the HPA axis (for review see 63,86).
Numerous studies find that basal CORT levels are higher in females than males and that following restraint stress, glucocorticoid release was higher in females86, suggesting that E2 increases HPA axis reactivity. This is further supported by observations that during the estrus phase of the estrous cycle, when E2 levels are lowest, CORT response to stress is reduced compared to during proestrus when E2 and P4 levels are elevated87. Additionally, OVX females showed reduced levels of CORT release following restraint stress86, and gonadectomized (GDX) males treated with E288 or estradiol benozoate (EB)89 showed elevated CORT release following acute restraint stress compared to control animals. While these data suggest that E2 increases HPA axis reactivity, the effects of E2 were not always consistent86.
This inconsistency of E2’s effect on the HPA axis relates to E2 activity at ERα and ERβ. Signaling through these 2 receptors yield opposing effects on HPA axis reactivity, with ERα increasing and ERβ inhibiting HPA axis reactivity (vide infra). Both ER receptors are found throughout the brain and with overlapping expression in numerous areas important in HPA axis regulation86. Male GDX rats treated with an ERα agonist showed increased HPA axis reactivity following acute stress88. When rats were treated with EB or an ERα agonist near the PVN, glucocorticoid receptor (GR)-mediated suppression of CORT release following acute restraint stress was inhibited, and this inhibition was not seen when animals were treated with an ERβ agonist. These results suggest that the GR-induced negative feedback of the HPA axis was impaired by ERα on GABAergic neurons in the peri-PVN area90. ERα is also expressed in other GABAergic neurons that project into the PVN, suggesting that ERα may reduce inhibitory input into the PVN, thereby reducing negative feedback from the glucocorticoids90.
Rats88,89 and mice91 treated with an ERβ selective agonist showed decreased stress-induced ACTH and CORT release compared to vehicle-treated animals. This reduction in HPA axis reactivity was also seen when ERβ in the PVN were selectively activated88. Within the PVN, ERβ is expressed in a large population of oxytocin neurons, in approximately half of CRH neurons, and a small population of AVP neurons92. Oxytocin has been shown to reduce HPA axis reactivity, and E2, via ERβ signaling, increases oxytocin mRNA levels (for review see 93). Additionally, ERβ has been shown to bind to the promoter of all three neuropeptides86. The mechanism through which ERβ reduces HPA axis reactivity has yet to be determined but could be through modulation of these PVN neuropeptides.
Progesterone and ALLO have also been implicated in HPA axis modulation and in human psychopathology (for review see 63). Stress increases brain and plasma P4 and ALLO concentrations, and increased concentrations may allow for termination of HPA axis signaling63. The effects of P4 on HPA axis reactivity are thought to be primarily due to conversion of P4 to ALLO rather than through intracellular PR signaling63. As previously discussed, ALLO binds to GABAA receptors to promote inhibition63; however, ALLO administration may also reduce HPA axis reactivity through reduction of CRH and AVP gene transcription63.
While E2 increases HPA axis reactivity, T has been found to reduce HPA axis reactivity86. T binds to AR, which is expressed in hypothalamic reproductive areas, such as the ARC, VMN and POA nuclei86. GDX male rats showed elevated CORT release following stressor exposure compared to gonad-intact males94. This elevated CORT release was reduced with administration of T or DHT, a T metabolite94. Additionally, treatment with DHT reduced CORT release following restraint stress88,89. T treatment reduced CORT release in response to stressors when administered into brain areas that synapse onto the PVN86 as well as when DHT was administered just above the PVN88. However, the effect of DHT within the PVN is not solely through AR signaling, as AR antagonist treatment does not completely abolish the effect of T on reducing HPA axis reactivity88. DHT can be metabolized to 3β-diol which is an ERβ specific ligand thereby contributing to DHT’s inhibition of HPA axis reactivity88.
6c. Betamethasone Effects on the HPG Axis in Development and Adulthood
During intrauterine development, the hormonal systems that regulate the HPG and HPA axes plays a crucial role in the growth and development of fetal tissues95. In the third trimester of pregnancy, an increase in fetal glucocorticoid concentration occurs95. This increase provides a number of modifications in several tissues and organs essential for fetal survival, including lung maturation and pulmonary surfactant production96. With preterm births, these changes have yet to occur as endogenous concentrations of glucocorticoids are still low. Therefore, antenatal therapy using glucocorticoids, especially betamethasone, is critical to promote lung maturation in these scenarios97.
In rodents, a critical window of study is between GD 12 to GD 1998–100. During this period of intrauterine growth, important changes in fetal development are occurring, such as the gonadogenesis, the establishment of the HPG axis and the morphogenesis of reproductive ducts and glands (epididymis, vas deferens, seminal vesicle and prostate)98–100. Thus, glucocorticoid exposure in this critical window of development becomes important, as any disturbance can lead to irreversible damage on the reproductive tract95,101. The increase in glucocorticoid concentration with antenatal therapy directly interferes in the control of the HPG axis, leading to a decrease in GnRH release and, consequently, a decrease in FSH and LH102,103. In addition, the increase in glucocorticoid concentrations interfere with serum T concentration since it inhibits T biosynthesis leading to decreased rates of T secretion from Leydig cells104,105.
The secretion of T, especially in the final periods of fetal development, is fundamental for the processes of masculinization and defeminization of the hypothalamus. Pereira et al. observed that intrauterine exposure to hydrocortisone during this critical window of fetal development interfered with these two processes98, and Pereira & Piffer observed an incomplete masculinization of the hypothalamus followed by damage of seminal vesicle contractility in adult rats exposed in utero to hydrocortisone99. Piffer et al. observed changes in serum T concentrations, sperm production and sexual preference of adult males following intrauterine betamethasone exposure100. These findings demonstrate the impact of glucocorticoid exposure on the masculinization process of the hypothalamus. Borges et al. reinforced these findings and also demonstrated that the impact of fetal programming promoted by betamethasone exposure was seen at birth, as low pup weight, at puberty, as reduced serum T levels and delayed puberty onset, and at adulthood, as direct effects on gonadal morphology and function, sperm quality and fertility106–108. Indeed, in utero betamethasone exposure confirmed its effects acting mainly on the HPG axis, since the same pattern of hormonal alteration was observed in both, male and female pups, as well as in the following two generations106–109.
Pedrana et al. also observed that in utero betamethasone exposure directly affected the development of sheep testes, decreasing the size of the testicular cords and interstitial space, suggesting an impact on Leydig cells, since they express GR110. Reduction of the seminiferous tubules diameter as well as reduced T production were also observed by Borges et al107,108. Moreover, the intrauterine exposure to betamethasone promoted drastic alterations in the seminiferous tubule morphology, compromising its cytoarchitecture and spermatogenesis108.
Overall, treatment with glucocorticoids during a critical window of development promotes alterations to the reproductive system, for both, male and female offspring, which continued into adulthood. Additional studies should be conducted to clarify, based on the exposure periods, how the reproductive organs can be reprogrammed throughout development, especially considering the critical periods of organogenesis and the establishment of the HPG axis.
7. Conclusions
Altogether, we can see that the HPG axis is an elegantly-regulated endocrine system that allows for successful reproduction and species propagation. Many components, including neural and hormonal factors, contribute to the proper regulation of the HPG axis and provide many interesting avenues for future research. The complex interactions between these hormonal and neuronal systems are summarized in Figure 1. As reproduction and survival are necessary for life, it follows that the two systems promoting these aspects, the HPG and HPA axes respectively, would be interlinked. Through further study of the HPG axis and the components regulating it, we can better understand factors contributing to reproductive maladies and health disorders.
Figure 1.
Acknowledgments
We thank the International Workshop in Neuroendocrinology (IWNE) 2017. This work was supported by NIH grants R21 ES027119 (TAR), R01 HD090161 and P50 HD012303 (ASK), NSF grant IOS-1457226 (ASK), FAPESP - The State of São Paulo Research Foundation grants 2012/25350-1 and 2014/13660-1 (CSB), and National Council for Scientific and Technological Development grant 308842/2013-8 (CSB). The authors do not have any conflicts of interest to disclose.
References
- 1.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–43. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324(1–2):51–63. doi: 10.1016/j.mce.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57(2):277–87. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013:78427–62. doi: 10.1007/978-1-4614-6199-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131–43. doi: 10.1111/j.1365-2826.2011.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–40. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 7.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poling MC, Kauffman AS. Regulation and Function of RFRP-3 (GnIH) Neurons during Postnatal Development. Front Endocrinol (Lausanne) 2015:6150. doi: 10.3389/fendo.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwasa T, Matsuzaki T, Murakami M, Kinouchi R, Osugi T, Gereltsetseg G, Yoshida S, Irahara M, Tsutsui K. Developmental changes in the mammalian gonadotropin-inhibitory hormone (GnIH) ortholog RFamide-related peptide (RFRP) and its cognate receptor GPR147 in the rat hypothalamus. Int J Dev Neurosci. 2012;30(1):31–7. doi: 10.1016/j.ijdevneu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30(30):10205–19. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–11. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 14.Brinkley HJ. Endocrine signaling and female reproduction. Biol Reprod. 1981;24(1):22–43. doi: 10.1095/biolreprod24.1.22. [DOI] [PubMed] [Google Scholar]
- 15.Desjardins C. Endocrine signaling and male reproduction. Biol Reprod. 1981;24(1):1–21. doi: 10.1095/biolreprod24.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Clarke IJ, Cummins JT, Findlay JK, Burman KJ, Doughton BW. Effects on plasma luteinizing hormone and follicle-stimulating hormone of varying the frequency and amplitude of gonadotropin-releasing hormone pulses in ovariectomized ewes with hypothalamo-pituitary disconnection. Neuroendocrinology. 1984;39(3):214–21. doi: 10.1159/000123982. [DOI] [PubMed] [Google Scholar]
- 17.Wu FC, Irby DC, Clarke IJ, Cummins JT, de Kretser DM. Effects of gonadotropin-releasing hormone pulse-frequency modulation on luteinizing hormone, follicle-stimulating hormone and testosterone secretion in hypothalamo/pituitary-disconnected rams. Biol Reprod. 1987;37(3):501–10. doi: 10.1095/biolreprod37.3.501. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6(7):1163–9. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- 19.Poulin B, Rich N, Mitev Y, Gautron JP, Kordon C, Enjalbert A, Drouva SV. Differential involvement of calcium channels and protein kinase-C activity in GnRH-induced phospholipase-C, -A2 and -D activation in a gonadotrope cell line (alpha T3-1) Mol Cell Endocrinol. 1996;122(1):33–50. doi: 10.1016/0303-7207(96)03868-3. [DOI] [PubMed] [Google Scholar]
- 20.Shah BH, Milligan G. The gonadotrophin-releasing hormone receptor of alpha T3-1 pituitary cells regulates cellular levels of both of the phosphoinositidase C-linked G proteins, Gq alpha and G11 alpha, equally. Mol Pharmacol. 1994;46(1):1–7. [PubMed] [Google Scholar]
- 21.Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18(1):46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol. 1999;19(4):2567–76. doi: 10.1128/mcb.19.4.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keri RA, Nilson JH. A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone beta subunit promoter in gonadotropes of transgenic mice. J Biol Chem. 1996;271(18):10782–5. doi: 10.1074/jbc.271.18.10782. [DOI] [PubMed] [Google Scholar]
- 24.Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol Endocrinol. 2001;15(5):734–46. doi: 10.1210/mend.15.5.0628. [DOI] [PubMed] [Google Scholar]
- 25.Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone beta gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996;271(12):6645–50. doi: 10.1074/jbc.271.12.6645. [DOI] [PubMed] [Google Scholar]
- 26.Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274(20):13870–6. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Luo M, Koh M, Yang M, bin Abdul Kadir MN, Tan JH, Ye Z, Wang W, Melamed P. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27(11):4105–20. doi: 10.1128/MCB.00248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouillet JF, Sonnenberg-Hirche C, Yan X, Sadovsky Y. p300 regulates the synergy of steroidogenic factor-1 and early growth response-1 in activating luteinizing hormone-beta subunit gene. J Biol Chem. 2004;279(9):7832–9. doi: 10.1074/jbc.M312574200. [DOI] [PubMed] [Google Scholar]
- 29.Wijeweera A, Haj M, Feldman A, Pnueli L, Luo Z, Melamed P. Gonadotropin gene transcription is activated by menin-mediated effects on the chromatin. Biochim Biophys Acta. 2015;1849(3):328–41. doi: 10.1016/j.bbagrm.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Khan SA, Edwards BS, Muth A, Thompson PR, Cherrington BD, Navratil AM. GnRH Stimulates Peptidylarginine Deiminase Catalyzed Histone Citrullination in Gonadotrope Cells. Mol Endocrinol. 2016;30(10):1081–91. doi: 10.1210/me.2016-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radovick S, Levine JE, Wolfe A. Estrogenic regulation of the GnRH neuron. Front Endocrinol (Lausanne) 2012:352. doi: 10.3389/fendo.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo KA, La JL, Stephens SB, Poling MC, Padgaonkar NA, Jennings KJ, Piekarski DJ, Kauffman AS, Kriegsfeld LJ. Circadian Control of the Female Reproductive Axis Through Gated Responsiveness of the RFRP-3 System to VIP Signaling. Endocrinology. 2015;156(7):2608–18. doi: 10.1210/en.2014-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153(11):5587–99. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- 34.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30(3):315–27. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 36.Ronnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25(3):165–77. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- 37.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–55. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 39.Cheong RY, Czieselsky K, Porteous R, Herbison AE. Expression of ESR1 in Glutamatergic and GABAergic Neurons Is Essential for Normal Puberty Onset, Estrogen Feedback, and Fertility in Female Mice. J Neurosci. 2015;35(43):14533–43. doi: 10.1523/JNEUROSCI.1776-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balint F, Liposits Z, Farkas I. Estrogen Receptor Beta and 2-arachidonoylglycerol Mediate the Suppressive Effects of Estradiol on Frequency of Postsynaptic Currents in Gonadotropin-Releasing Hormone Neurons of Metestrous Mice: An Acute Slice Electrophysiological Study. Front Cell Neurosci. 2016:1077. doi: 10.3389/fncel.2016.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29(17):5616–27. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C, Bosch MA, Rick EA, Kelly MJ, Ronnekleiv OK. 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29(34):10552–62. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27(38):10153–64. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang JA, Mamounis KJ, Yasrebi A, Roepke TA. Regulation of gene expression by 17beta-estradiol in the arcuate nucleus of the mouse through ERE-dependent and ERE-independent mechanisms. Steroids. 2016:107128–38. doi: 10.1016/j.steroids.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–5. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang JA, Stires H, Belden WJ, Roepke TA. The Arcuate Estrogen-Regulated Transcriptome: Estrogen Response Element-Dependent and -Independent Signaling of ERalpha in Female Mice. Endocrinology. 2017;158(3):612–26. doi: 10.1210/en.2016-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152(2):595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20(11):1270–7. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 49.Piet R, Fraissenon A, Boehm U, Herbison AE. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J Neurosci. 2015;35(17):6881–92. doi: 10.1523/JNEUROSCI.4587-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kallo I. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 2010;22(9):1032–9. doi: 10.1111/j.1365-2826.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- 51.Fatehi M, Fatehi-Hassanabad Z. Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology. 2008;33(6):1354–64. doi: 10.1038/sj.npp.1301523. [DOI] [PubMed] [Google Scholar]
- 52.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–71. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molnar CS, Kallo I, Liposits Z, Hrabovszky E. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology. 2011;152(4):1684–90. doi: 10.1210/en.2010-1418. [DOI] [PubMed] [Google Scholar]
- 54.Smith AW, Bosch MA, Wagner EJ, Ronnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17beta-estradiol. Am J Physiol Endocrinol Metab. 2013;305(5):E632–40. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cates PS, Li XF, O’Byrne KT. The influence of 17beta-oestradiol on corticotrophin-releasing hormone induced suppression of luteinising hormone pulses and the role of CRH in hypoglycaemic stress-induced suppression of pulsatile LH secretion in the female rat. Stress. 2004;7(2):113–8. doi: 10.1080/1025389042000218988. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell JC, Li XF, Breen L, Thalabard JC, O’Byrne KT. The role of the locus coeruleus in corticotropin-releasing hormone and stress-induced suppression of pulsatile luteinizing hormone secretion in the female rat. Endocrinology. 2005;146(1):323–31. doi: 10.1210/en.2004-1053. [DOI] [PubMed] [Google Scholar]
- 57.Phumsatitpong C, Moenter SM. Estradiol-Dependent Stimulation and Suppression of Gonadotropin-Releasing Hormone Neuron Firing Activity by Corticotropin-Releasing Hormone in Female Mice. Endocrinology. 2018;159(1):414–25. doi: 10.1210/en.2017-00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu P, Liu J, Yasrebi A, Gotthardt JD, Bello NT, Pang ZP, Roepke TA. Gq Protein-Coupled Membrane-Initiated Estrogen Signaling Rapidly Excites Corticotropin-Releasing Hormone Neurons in the Hypothalamic Paraventricular Nucleus in Female Mice. Endocrinology. 2016;157(9):3604–20. doi: 10.1210/en.2016-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC., Jr Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153(5):2408–19. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151(6):2736–46. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roepke TA, Malyala A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148(10):4937–51. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- 62.Locci A, Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol. 2017;174(19):3226–41. doi: 10.1111/bph.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirth MM. Beyond the HPA Axis: Progesterone-Derived Neuroactive Steroids in Human Stress and Emotion. Front Endocrinol (Lausanne) 2011:219. doi: 10.3389/fendo.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charles NJ, Thomas P, Lange CA. Expression of membrane progesterone receptors (mPR/PAQR) in ovarian cancer cells: implications for progesterone-induced signaling events. Horm Cancer. 2010;1(4):167–76. doi: 10.1007/s12672-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Micevych P, Sinchak K. The Neurosteroid Progesterone Underlies Estrogen Positive Feedback of the LH Surge. Front Endocrinol (Lausanne) 2011:290. doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laconi MR, Casteller G, Gargiulo PA, Bregonzio C, Cabrera RJ. The anxiolytic effect of allopregnanolone is associated with gonadal hormonal status in female rats. Eur J Pharmacol. 2001;417(1–2):111–6. doi: 10.1016/s0014-2999(01)00865-2. [DOI] [PubMed] [Google Scholar]
- 67.Concas A, Mostallino MC, Perra C, Lener R, Roscetti G, Barbaccia ML, Purdy RH, Biggio G. Functional correlation between allopregnanolone and [35S]-TBPS binding in the brain of rats exposed to isoniazid, pentylenetetrazol or stress. Br J Pharmacol. 1996;118(4):839–46. doi: 10.1111/j.1476-5381.1996.tb15476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88(10):4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaura V, Ingram CD, Gartside SE, Young AH, Judge SJ. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol. 2007;17(2):108–15. doi: 10.1016/j.euroneuro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Genazzani AR, Palumbo MA, de Micheroux AA, Artini PG, Criscuolo M, Ficarra G, Guo AL, Benelli A, Bertolini A, Petraglia F, et al. Evidence for a role for the neurosteroid allopregnanolone in the modulation of reproductive function in female rats. Eur J Endocrinol. 1995;133(3):375–80. doi: 10.1530/eje.0.1330375. [DOI] [PubMed] [Google Scholar]
- 71.Laconi MR, Chavez C, Cavicchia JC, Foscolo M, Sosa Z, Yunes RF, Cabrera RJ. Allopregnanolone alters the luteinizing hormone, prolactin, and progesterone serum levels interfering with the regression and apoptosis in rat corpus luteum. Horm Metab Res. 2012;44(8):632–8. doi: 10.1055/s-0032-1314834. [DOI] [PubMed] [Google Scholar]
- 72.Pelegrina LT, Escudero C, Garcia S, Cabrera R, Laconi MR. Pharmacological effect of one icv dose of Allogregnanolone in the female rat: behavioral profile. Brazilian Journal of Biological Sciences. 2015;2(3):39–50. [Google Scholar]
- 73.Giuliani FA, Yunes R, Mohn CE, Laconi M, Rettori V, Cabrera R. Allopregnanolone induces LHRH and glutamate release through NMDA receptor modulation. Endocrine. 2011;40(1):21–6. doi: 10.1007/s12020-011-9451-8. [DOI] [PubMed] [Google Scholar]
- 74.Sabaliauskas N, Shen H, Molla J, Gong QH, Kuver A, Aoki C, Smith SS. Neurosteroid effects at alpha4betadelta GABAA receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 2015:1621170–86. doi: 10.1016/j.brainres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHbeta-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26(10):1716–31. doi: 10.1210/me.2011-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagenmaker ER, Moenter SM. Exposure to Acute Psychosocial Stress Disrupts the Luteinizing Hormone Surge Independent of Estrous Cycle Alterations in Female Mice. Endocrinology. 2017;158(8):2593–602. doi: 10.1210/en.2017-00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106(27):11324–9. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orr TE, Mann DR. Effects of restraint stress on plasma LH and testosterone concentrations, Leydig cell LH/hCG receptors, and in vitro testicular steroidogenesis in adult rats. Horm Behav. 1990;24(3):324–41. doi: 10.1016/0018-506x(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 79.Saketos M, Sharma N, Santoro NF. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49(6):1270–6. doi: 10.1095/biolreprod49.6.1270. [DOI] [PubMed] [Google Scholar]
- 80.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O’Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21(1):20–9. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 81.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ. Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology. 2005;146(4):2107–15. doi: 10.1210/en.2004-1457. [DOI] [PubMed] [Google Scholar]
- 82.Roozendaal MM, Swarts JJ, van Maanen JC, Wiegant VM, Mattheij JA. Inhibition of the LH surge in cyclic rats by stress is not mediated by opioids. Life Sci. 1997;60(10):735–42. doi: 10.1016/s0024-3205(96)00652-2. [DOI] [PubMed] [Google Scholar]
- 83.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293(1):E270–6. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- 84.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice. Endocrinology. 2016;157(3):1187–99. doi: 10.1210/en.2015-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang JA, Song CI, Hughes J, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS, Breen KM. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology. 2017 doi: 10.1210/en.2017-00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35(2):197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 88.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16(3):272–8. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 90.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–95. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK. Anxiolytic effects and neuroanatomical targets of estrogen receptor-beta (ERbeta) activation by a selective ERbeta agonist in female mice. Endocrinology. 2012;153(2):837–46. doi: 10.1210/en.2011-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oyola MG, Thompson MK, Handa AZ, Handa RJ. Distribution and chemical composition of estrogen receptor beta neurons in the paraventricular nucleus of the female and male mouse hypothalamus. J Comp Neurol. 2017;525(17):3666–82. doi: 10.1002/cne.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Acevedo-Rodriguez A, Mani SK, Handa RJ. Oxytocin and Estrogen Receptor beta in the Brain: An Overview. Front Endocrinol (Lausanne) 2015:6160. doi: 10.3389/fendo.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55(1):117–24. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 95.Manojlović-Stojanoski MNN, Milošević V. Prenatal Glucocorticoids: Short-Term Benefits and Long-Term Risks. In: Qian X, editor. Glucocorticoids - New Recognition of Our Familiar Friend. 2012. [Google Scholar]
- 96.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3(6):479–88. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 97.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127(5):515–26. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 98.Pereira OCM, Arena AC, Yasuhara F, Kempinas WG. Effects of prenatal hydrocortisone acetate exposure on fertility and sexual behavior in male rats. Regul Toxicol Pharm. 2003;38(1):36–42. doi: 10.1016/s0273-2300(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 99.Pereira OC, Piffer RC. Puberty installation and adrenergic response of seminal vesicle from rats exposed prenatally to hydrocortisone. Life Sci. 2005;77(12):1381–90. doi: 10.1016/j.lfs.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Piffer RC, Garcia PC, Pereira OC. Adult partner preference and sexual behavior of male rats exposed prenatally to betamethasone. Physiol Behav. 2009;98(1–2):163–7. doi: 10.1016/j.physbeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34(10):841–5. doi: 10.1016/j.placenta.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 102.Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin subunit mRNA levels. Mol Cell Endocrinol. 2006;256(1–2):40–8. doi: 10.1016/j.mce.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 104.Hardy MP, Gao HB, Dong Q, Ge R, Wang Q, Chai WR, Feng X, Sottas C. Stress hormone and male reproductive function. Cell Tissue Res. 2005;322(1):147–53. doi: 10.1007/s00441-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 105.Ward IL, Weisz J. Differential-Effects of Maternal Stress on Circulating Levels of Corticosterone, Progesterone, and Testosterone in Male and Female Rat Fetuses and Their Mothers. Endocrinology. 1984;114(5):1635–44. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- 106.Borges CD, Dias AF, Silva PV, Rosa JL, Guerra MT, Silva RF, Kiguti LR, Pupo AS, Kempinas WG. Long-term adverse effects on reproductive function in male rats exposed prenatally to the glucocorticoid betamethasone. Toxicology. 2017:37615–22. doi: 10.1016/j.tox.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Borges CDS, Pacheco TL, da Silva KP, Fernandes FH, Gregory M, Pupo AS, Salvadori DMF, Cyr DG, Kempinas WG. Betamethasone causes intergenerational reproductive impairment in male rats. Reprod Toxicol. 2017:71108–17. doi: 10.1016/j.reprotox.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 108.Borges CS, Dias AF, Rosa JL, Silva PV, Silva RF, Barros AL, Sanabria M, Guerra MT, Gregory M, Cyr DG, De GKW. Alterations in male rats following in utero exposure to betamethasone suggests changes in reproductive programming. Reproductive toxicology. 2016:63125–34. doi: 10.1016/j.reprotox.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 109.Borges CS, Pacheco TL, Guerra MT, Barros AL, Silva PV, Missassi G, da Silva KP, Anselmo-Franci JA, Pupo AS, Kempinas WG. Reproductive disorders in female rats after prenatal exposure to betamethasone. J Appl Toxicol. 2017;37(9):1065–72. doi: 10.1002/jat.3457. [DOI] [PubMed] [Google Scholar]
- 110.Pedrana G, Sloboda DM, Perez W, Newnham JP, Bielli A, Martin GB. Effects of pre-natal glucocorticoids on testicular development in sheep. Anat Histol Embryol. 2008;37(5):352–8. doi: 10.1111/j.1439-0264.2008.00853.x. [DOI] [PubMed] [Google Scholar]