Abstract
Exposure to stressors induces anxiety- and depressive-like behaviors, which are mediated, in part, by neuroinflammatory processes. Recent findings demonstrate that treatment with the immunoregulatory and anti-inflammatory bacterium, Mycobacterium vaccae (M. vaccae), attenuates stress-induced exaggeration of peripheral inflammation and stress-induced anxiety-like behavioral responses. However, the effects of M. vaccae on neuroimmune processes have largely been unexplored. In the present study, we examined the effect of M. vaccae NCTC11659 on neuroimmune regulation, stress-induced neuroinflammatory processes and anxiety-like behavior. Adult male rats were immunized 3x with a heat-killed preparation of M. vaccae (0.1 mg, s.c.) or vehicle. M. vaccae induced an anti-inflammatory immunophenotype in hippocampus (increased interleukin (Il)4, Cd200r1, and Mrc1 mRNA expression) and increased IL4 protein 8 d after the last immunization. Central administration of recombinant IL4 recapitulated the effects of M. vaccae on Cd200r1 and Mrc1 mRNA expression. M. vaccae reduced basal levels of genes (Nlrp3 and Nfkbia) involved in microglial priming; thus, we explored the effects of M. vaccae on stress-induced hippocampal microglial priming and HMGB1, which mediates priming. We found that M. vaccae blocked stress-induced decreases in Cd200r1, increases in the alarmin HMGB1, and priming of the microglial response to immune challenge. Furthermore, M. vaccae prevented stress-induced increases in anxiety-like behavior. The present findings suggest that M. vaccae enhances immunomodulation in the CNS and mitigates the neuroinflammatory and behavioral effects of stress, which may underpin its capacity to impart a stress resilient phenotype.
Keywords: stress, M. vaccae, microglia, immunoregulation, neuroinflammation, alarmin
1. Introduction
Anxiety and trauma-related disorders are the most commonly occurring of all mental disorders, with estimated lifetime prevalence as high as 25% (Kessler, 2002). In addition to high prevalence, these disorders have an early age at onset, have high chronicity, and involve substantial role impairment (Kessler, 2002). Furthermore, anxiety and trauma-related disorders have significant psychiatric comorbidities including major depression, non-affective psychosis, and alcohol and drug abuse/dependence. It is estimated that the annual total cost of anxiety and trauma-related disorders in the U.S. is between $42 billion (Greenberg et al., 1999) and $47 billion (DuPont et al., 1996).
Recent studies suggest that chronic inflammation contributes to risk of anxiety disorders, trauma, and stress-related disorders, as well as affective disorders (Eraly et al., 2014; Miller and Raison, 2016; Rohleder, 2014). Indeed, it has been proposed that an enhanced stress-induced inflammatory immune activation plays a causal role in the development of these disorders (Khandaker et al., 2014; Kivimaki et al., 2014; Pervanidou et al., 2007). This hypothesis is supported by preclinical studies demonstrating that interleukin (IL)-6, a pleiotropic cytokine released in association with inflammatory responses, is predictive for subsequent development of anxiety and depressive-like symptoms (Hodes et al., 2014). Furthermore, lower numbers of regulatory T cells (Treg) have been found in individuals with a diagnosis of anxiety, trauma, and stress-related disorders such as posttraumatic stress disorder (PTSD) (Sommershof et al., 2009) as well as in major depression (Li et al., 2010). Some evidence is accumulating to support improved outcomes following anti-inflammatory treatment in individuals with major depressive disorder (Dean et al., 2017; Kohler et al., 2014; Raison et al., 2013).
Increases in chronic low-grade inflammation in modern urban societies have been attributed in part to reduced immunoregulation secondary to decreases in microbial exposures, as proposed by the hygiene hypothesis (Rook and Lowry, 2008), “Old Friends” hypothesis (Rook et al., 2015; Rook et al., 2013, 2014), and biodiversity hypothesis (von Hertzen et al., 2015). Immunoregulation refers to a balanced expression of effector T cells (i.e., Th1, Th2, and Th17 cells) and regulatory T cells (Treg) that produce anti-inflammatory cytokines such as IL-10 and transforming growth factor beta (TGFβ)(Lowry et al., 2016; Rook, 2013). Throughout human evolution, “Old Friends” needed to be tolerated by the immune system, as they were either part of host physiology (human microbiota), were harmless but inevitably contaminating air, food and water (environmental microbiota), or caused severe tissue damage when attacked by the host immune system (e.g., helminthic parasites). Mycobacterium vaccae (M. vaccae) is a saprophytic bacterium found in soil, water, and mud (Hoisington et al., 2015), and is considered an “Old Friend” with potent immunoregulatory effects (Rook et al., 2004).
Given the evidence for reduced immunoregulation and chronic low-grade inflammation in anxiety and trauma-related disorders, microbial interventions that increase Treg, promote immunoregulation, and increase anti-inflammatory signaling may have value in the prevention or treatment of these disorders. M. vaccae increases induction of Treg and production of anti-inflammatory cytokines, including IL-10 and TGFβ (Reber et al., 2016b; Zuany-Amorim et al., 2002). Furthermore, immunization with M. vaccae in mice prevents development of a PTSD-like syndrome, stress-induced colitis, chemically induced colitis in a model of inflammatory bowel disease, stress-induced exaggeration of proinflammatory cytokine secretion from freshly isolated and stimulated mesenteric lymph node cells, and anxiety-like behaviors (Reber et al., 2016a; Reber et al., 2016b).
These findings suggest that treatment with immunoregulatory and anti-inflammatory agents can promote a peripheral anti-inflammatory immunophenotype and buffer organisms against the proinflammatory effects of stress. However, the effects of M. vaccae on stress-induced neuroinflammation have not been studied. Because peripheral immune signals are communicated to the CNS, we explored the possibility that immunization with a heat-killed preparation of M. vaccae might induce an anti-inflammatory immunophenotype in the central nervous system (CNS) and thus mitigate the neuroinflammatory and behavioral effects of stress exposure (Frank et al., 2015b, 2016).
Exposure to acute and chronic stressors induces proinflammatory cytokines in the CNS (Goshen and Yirmiya, 2009) as well as sensitization of these inflammatory processes to subsequent immune challenges (Frank et al., 2016). For example, a number of studies have found that prior exposure to either acute or chronic stressors potentiates the neuroinflammatory and microglial proinflammatory response to subsequent immune challenges (de Pablos et al., 2006; Espinosa-Oliva et al., 2011; Frank et al., 2007; Johnson et al., 2003; Johnson et al., 2004; Munhoz et al., 2006; Wohleb et al., 2011). These findings suggest that stressors might sensitize or prime microglia, which are considered a key substrate of this stress-induced phenomenon (Frank et al., 2015b). Also, neuroinflammatory processes have been found to play a mediating role in the depressogenic and anxiogenic effects of stress, and induction of neuroinflammatory processes is sufficient to recapitulate these behavioral effects of stress (Goshen and Yirmiya, 2009). In light of M. vaccae's effects on peripheral anti-inflammatory processes as well as its anxiolytic effects, we examined the effects of immunization with a heat-killed preparation of M. vaccae on an anti-inflammatory immunophenotype in the CNS as well as stress-induced microglial sensitization in association with stress-induced anxiety-like behavior.
2. Materials and Methods
2.1. Animals
Adult male Sprague-Dawley rats (60–90 d old; Envigo) were pair-housed with food and water available ad libitum. The colony was maintained at 22 °C on a 12 h light/dark cycle (lights on at 07:00 h). All experimental procedures were conducted in accord with the University of Colorado Boulder Institutional Animal Care and Use Committee.
2.2. Mycobacterium vaccae (M. vaccae) treatment
M. vaccae is a saprophytic environmental mycobacterium (Rook et al., 2004), which we have used previously to mitigate stress-induced anxiety-like behavior and peripheral proinflammatory responses (Reber et al., 2016b). Briefly, whole heat-killed M. vaccae (strain NCTC 11659, batch ENG 1; Bio Elpida; 10 mg/ml) was suspended in sterile borate-buffered saline (BBS) to yield a final concentration of 1 mg/ml. Sterile BBS served as the vehicle control.
Consistent with our prior work with M. vaccae (Reber et al., 2016b), experimental animals received 3x subcutaneous (s.c.) immunizations with 0.1 mg whole heat-killed M. vaccae suspension (0.1 ml) or vehicle (0.1 ml). Injections occurred at -21 d, -14 d and -7 d prior to stress exposure. Fig. 1 provides a timeline of M. vaccae treatment relative to stress exposure, behavioral testing and tissue/microglia collection.
Fig. 1. Timeline of experimental treatments.
This schematic depicts the timing of M. vaccae treatment relative to stress exposure (IS; inescapable tailshock), behavioral testing (JSE; juvenile social exploration) and tissue collection.
2.3. Recombinant rat IL4 (rIL4) treatment
Vehicle (sterile 1x PBS + 0.1% BSA) or rIL4 (100 ng dissolved in vehicle; R & D Systems, cat. no. 504-RL) was injected intra-cisterna magna (i.c.m.; 5 μl total volume). Two and 24 h post-injection, hippocampus was collected for gene expression analysis of IL4-sensitive target genes. The dose of rIL4 used here was based on findings of Lyons and colleagues (Lyons et al., 2007), who demonstrated that intracerebroventricular (i.c.v.) administration of this dose of rIL4 was sufficient to induce robust expression of IL4-sensitive genes in hippocampus. We have demonstrated that i.c.m. injections of substances reach distal target regions in the CNS (i.e., hippocampus) consistent with more typical i.c.v. procedures, and this procedure produces no detectable inflammatory responses (Frank et al., 2012a). Rats were anesthetized with 5% isoflurane in oxygen and then maintained on 3% isoflurane during the brief procedure (~3 min). The dorsal aspect of the skull was shaved and swabbed with 70% EtOH. A sterile 27-gauge needle attached via sterile PE50 tubing to a 25 μl Hamilton syringe was inserted transcutaneously at midline between the base of the skull and first vertebrae into the cisterna magna (verified by withdrawing 2 μl of clear CSF), and drug was injected over a 30 s period. After injection, the needle was left in place for 30 s to allow for diffusion of drug.
2.4. Inescapable tailshock (IS)
Details of the stressor protocol have been published previously and this protocol reliably potentiates proinflammatory cytokine responses in the hippocampus after peripheral immune challenge (Johnson et al., 2003) and sensitizes ex vivo lipopolysaccharide (LPS)-induced proinflammatory cytokine secretion from isolated hippocampal microglia (Frank et al., 2007). Briefly, animals were placed in Plexiglas® tubes (23.4 cm in length x 7 cm in diameter) and exposed to 100 1.6 mA, 5-s tailshocks with a variable inter-trial interval (ITI) ranging from 30 – 90 s (average ITI = 60 s). All IS treatments occurred between 09:00 and 11:00 h. IS animals were returned to their home cages immediately after termination of shock. Home cage control (HCC) animals remained undisturbed in their home cages.
2.5. Tissue and blood collection
Animals were given a lethal dose of sodium pentobarbital. Cardiac blood was collected by cardiac puncture after opening the thoracic cavity. Transcardial perfusion was then performed with ice-cold saline (0.9%) for 3 min to remove peripheral immune leukocytes from the CNS vasculature. Brain was rapidly extracted, placed on ice and brain regions bilaterally dissected with each hemisphere designated for either protein or mRNA analysis. Hippocampus was collected given our prior findings of robust stress-induced priming effects in this region (Frank et al., 2015b) and amygdala was also collected as a stress-sensitive limbic structure (Vecchiarelli et al., 2016). Choroid plexus was removed from hippocampus prior to tissue processing. For experiments involving measurement of in vivo cytokine mRNA expression and protein, hippocampus and amygdala were flash frozen in liquid nitrogen and stored at -80° C. For experiments involving measurement of IL4 mRNA expression and protein in dorsal, intermediate, and ventral hippocampus, hippocampus was dissected and each hemisphere was trisected into these distinct subregions based on the method of Lee et al. (Lee et al., 2017). Hippocampal subregions were flash frozen in liquid nitrogen and stored at -80° C. For experiments involving ex vivo LPS stimulation of isolated hippocampal microglia, hippocampal microglia were immediately isolated.
2.6. Serum corticosterone (CORT) assay
Blood was collected in untreated Eppendorf tubes, centrifuged (10 min, 14,000 x g, 4° C) and serum collected. CORT was measured in duplicate using a competitive immunoassay (Enzo Life Science) as described in the manufacturer’s protocol (intra-assay mean %CV = 7.7; inter-assay mean %CV = 9.7).
2.7. Ex vivo immune stimulation of hippocampal microglia with LPS
Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006). We have previously shown (Frank et al., 2006) that this microglia isolation procedure yields highly pure microglia (Iba-1+/Cd163-/Gfap- mRNA). In the present experiments, immunophenotype and purity of microglia were assessed using real time RT-PCR. Microglia were suspended in DMEM+10% FBS and microglia concentration was determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 1 x 104 cells/100 μl and 100 μl added to individual wells of a 96-well v-bottom plate. LPS (E. coli serotype O111:B4; Sigma-Aldrich; cat. no. L3012) was utilized to challenge microglia ex vivo as we have previously determined the optimal in vitro conditions under which LPS stimulates a microglial proinflammatory cytokine response (Frank et al., 2006). Cells were incubated with LPS (1, 10, and 100 ng/ml) or media alone for 2 h at 37 °C, 5% CO2. The plate was centrifuged at 1000 x g for 10 min at 4 °C to pellet cells and cells were then washed 1x in ice-cold PBS and centrifuged at 1000 x g for 10 min at 4 °C. Cell lysis/homogenization and cDNA synthesis were performed according to the manufacturer’s protocol using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen).
2.8. Real time RT-PCR measurement of gene expression
Total RNA was isolated from whole hippocampus and amygdala utilizing a standard method of phenol:chloroform extraction (Chomczynski and Sacchi, 1987). For detailed descriptions of RNA isolation, cDNA synthesis and PCR amplification protocols refer to prior publication (Frank et al., 2007). cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using the Operon Oligo Analysis Tool (http://www.operon.com/technical/toolkit.aspx) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul et al., 1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analyses. All primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA (see Table 1 for primer description and sequences).
Table 1.
Primer Specifications.
| Gene Symbol | Primer Sequence 5′ → 3′ | Function |
|---|---|---|
| Actb | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (housekeeping gene) |
| Arg1 | F: CTACCTGCTGGGAAGGAAG R: GTCCTGAAAGTAGCCCTGTC |
IL4-sensitive gene |
| Cd3e | F: AAAGCCAGAGTGTGCGAGAA R: CCTTCCTTTTCTTGCTCCAG |
Epsilon chain of the T-cell receptor-CD3 complex |
| Cd163 | F: GTAGTAGTCATTCAACCCTCAC R: CGGCTTACAGTTTCCTCAAG |
Hemoglobin receptor expressed by macrophages, but not microglia |
| Cd200 | F: CTCTCTATGTACAGCCCATAG R: GGGAGTGACTCTCAGTACTAT |
Neuronal antigen that binds CD200R1 to inhibit microglial function |
| Cd200r1 | F: TAGAGGGGGTGACCAATTAT R: TACATTTTCTGCAGCCACTG |
Cognate receptor for CD200 that inhibits microglial function |
| Gfap | F: AGATCCGAGAAACCAGCCTG R: CCTTAATGACCTCGCCATCC |
Astrocyte antigen |
| Iba1 | F: GGCAATGGAGATATCGATAT R: AGAATCATTCTCAAGATGGC |
Microglia/macrophage antigen |
| Il1b | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| Il4 | F: GAACTCACTGAGAAGCTGCA R: GAAGTGCAGGACTGCAAGTA |
Anti-inflammatory cytokine in the CNS |
| Il6 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| Il10 | F: GGACTTTAAGGGTTACTTGGG R: AGAAATCGATGACAGCGTCG |
Anti-inflammatory cytokine |
| Il13 | F: AGACCAGAAGACTTCCCTGT R: TCAATATCCTCTGGGTCCTG |
Anti-inflammatory cytokine |
| Mrc1 | F: GGGGTTGTTGCTGTTGATGT R: GCTCGAAACGGAAAAGGTTC |
Receptor for mannose that is induced by IL4 |
| Nts | F: TGCATCGAAGGTCAGCAAAG R: TCCTTTTCGCAACAAGGTCG |
A highly enriched mRNA in dorsal hippocampus |
| Nfkbia | F: CACCAACTACAACGGCCACA R: GCTCCTGAGCGTTGACATCA |
Induced by NFκB to inhibit NFκB function |
| Nlrp3 | F: AGAAGCTGGGGTTGGTGAATT R: GTTGTCTAACTCCAGCATCTG |
Inflammasome component mediating caspase-1/IL1B activation |
| Nr2f2 | F: TGTTCACCTCAGATGCCTGT R: AGGGAGACGAAGCAAAAGCT |
A highly enriched mRNA in ventral hippocampus |
| Tgfb1 | F: TACTGCTTCAGCTCCACAGA R: TGTCCAGGCTCCAAATGTAG |
Anti-inflammatory cytokine |
| Tnf | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
Abbreviations: Actb, beta actin; Arg1, arginase 1; Cd, cluster of differentiation; Cd200r1, CD200 receptor 1; Gfap, glial fibrillary acidic protein; Il, interleukin; Iba-1, ionized calcium-binding adaptor molecule-1; Mrc1, mannose receptor, C type 1; Nfkbia, nuclear factor kappa light chain enhancer of activated B cells inhibitor alpha; Nlrp3, NACHT domain-, leucine-rich repeat-, and PYD-containing protein 3; Nr2f2, nuclear receptor subfamily 2, group F, member 2; Nts, neurotensin; Tgfb1, transforming growth factor-β1; Tnf, tumor necrosis factor.
PCR amplification of cDNA was performed using the QuantiTect SYBR Green PCR Kit (Qiagen). Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad). Relative gene expression was determined by taking the expression ratio of the gene of interest to β-actin.
2.9. Enzyme-linked immunosorbent assay (ELISA)
Hippocampus was sonicated in a mixture containing extraction buffer (Invitrogen; Cat. No. FNN0071) and protease inhibitors (Sigma-Aldrich; Cat. No. P2714). Ice-cold tissue sonicates were centrifuged at 14,000 rpm for 10 min at 4 °C. The supernatant was removed and the total protein concentration for each sample was quantified using the Bradford method. High Mobility Group Box 1 (HMGB1; LifeSpan Biosciences, Inc.; cat. no. LS-F4039), IL1B (R & D Systems; cat. no. RLB00) and IL4 (R & D Systems; cat. no. R4000) protein was measured using a standard colorimetric sandwich ELISA according to the manufacturer's instructions. Protein was quantified as pg/mg total protein.
2.10. Juvenile social exploration (JSE)
Stress exposure (IS) produces robust decrements in JSE (Christianson et al., 2008), which is a widely used and validated measure of anxiety (File and Seth, 2003) and is sensitive to the neuroinflammatory effects of stress (Goshen and Yirmiya, 2009). Here, JSE was measured 24 h prior to (baseline) and 24 h after IS (test). Each experimental subject was transferred to a novel cage with shaved wood bedding in a dimly lit room (40 lx). After a 15-min habituation period, a 28–32 day-old juvenile male rat was introduced to the subject's cage for 5 min. Exploratory behaviors of the adult (sniffing, pinning, licking and allo-grooming of the juvenile) were timed by an observer blind to treatment condition. After the test, the juvenile was removed and the experimental adult rat was returned to its homecage. Although juvenile stimulus rats were reused for multiple tests, the adult was never re-tested with the same juvenile. For each animal, JSE test data were quantified as a percent of baseline JSE.
2.11. Statistical analysis and data presentation
All data are presented as mean + s.e.m. Statistical analyses consisted of Student’s t-tests or ANOVA followed by post hoc tests (Newman-Keuls) using Prism 5 (Graphpad Software, Inc.). Threshold for statistical significance was set at two-tailed α = 0.05. Sample sizes are provided in figure captions. Area under the LPS concentration curve (AUC) was computed to capture the cumulative effect of stress and M. vaccae treatment on the cytokine response to LPS ex vivo.
3. Results
3.1. Effect of M. vaccae on hippocampal and amygdalar anti-inflammatory mediators and markers of alternative activation
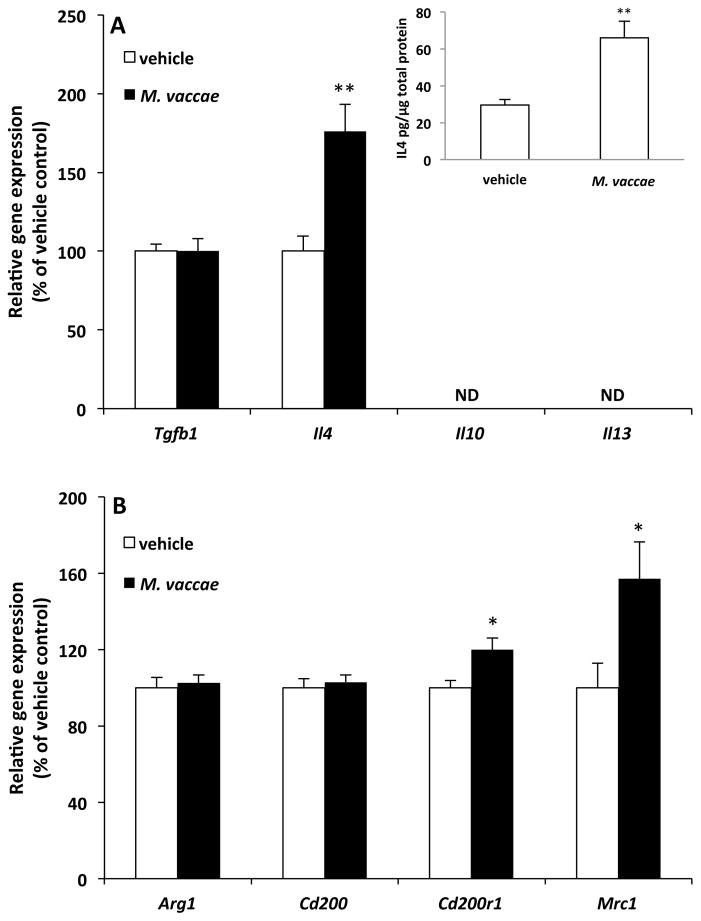
M. vaccae treatment has been found to increase peripheral levels of anti-inflammatory cytokines including IL10 and transforming growth factor beta (TGFB1) (Rook et al., 2004). Therefore, we conducted an initial investigation into whether M. vaccae treatment induces an anti-inflammatory immunophenotype in the CNS independent of stress exposure. Here, the effect of M. vaccae on the gene expression of several anti-inflammatory mediators (Il4, Il10, Il13, and Tgfb1) was examined. As depicted in Fig. 2A, M. vaccae treatment increased expression of Il4 (t = 3.61, df = 18, p = 0.002) in hippocampus; however, all other analytes were either not affected by M. vaccae treatment (Tgfb1) or not detected due to low expression levels (Il10, Il13). M. vaccae also increased IL4 protein levels in hippocampus (contralateral hemisphere) of the same animals (see Fig. 2A inset; t = 2.86, df = 22, p = 0.008). Interestingly, M. vaccae failed to affect the expression level of these analytes in the amygdala (Suppl. Fig. 1A).
Fig. 2. Effect of M. vaccae on hippocampal anti-inflammatory mediators and markers of alternative activation.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight d after the third injection, gene expression of (A) anti-inflammatory mediators and (B) markers of alternative macrophage activation were measured in hippocampus. Data are presented as the mean + s.e.m. N = 9–12 animals per experimental group. Significant M. vaccae effects compared to vehicle, * p < 0.05, ** p < 0.01. ND = non-detected.
IL4 is considered a powerful stimulus of alternative macrophage activation (Ransohoff and Perry, 2009), also termed a wound healing macrophage phenotype (Mosser and Edwards, 2008). IL4 has been found to induce markers of alternative macrophage activation including arginase 1 (ARG1) (Ransohoff and Cardona, 2010), the mannose receptor (MRC1) (Ambarus et al., 2012; Ransohoff and Cardona, 2010) and CD200R1 (Ambarus et al., 2012). In addition, IL4 is a stimulus of neuronal CD200 expression (Lyons et al., 2007), which is thought to constrain microglial activation via ligation of CD200R1 (Gorczynski, 2005). Therefore, we examined the effect of M. vaccae on these IL4-sensitive antigens in hippocampus. As depicted in Fig. 2B, M. vaccae treatment increased expression of Cd200r1 (t = 2.71, df = 22, p = 0.01) as well as Mrc1 (t = 2.48, df = 22, p = 0.02), but failed to alter expression of Cd200 and arg1. In the amygdala, M. vaccae failed to significantly alter the expression levels of any of these IL4-sensitive targets (Suppl. Fig. 1B), which is consistent with the lack of an M. vaccae effect on amygdalar IL4 as well as other anti-inflammatory cytokines. Because M. vaccae failed to induce an anti-inflammatory milieu in amygdala, subsequent experiments excluded amygdala analyses and focused solely on M. vaccae's effects in hippocampus.
3.2. Effect of M. vaccae on hippocampal proinflammatory mediators
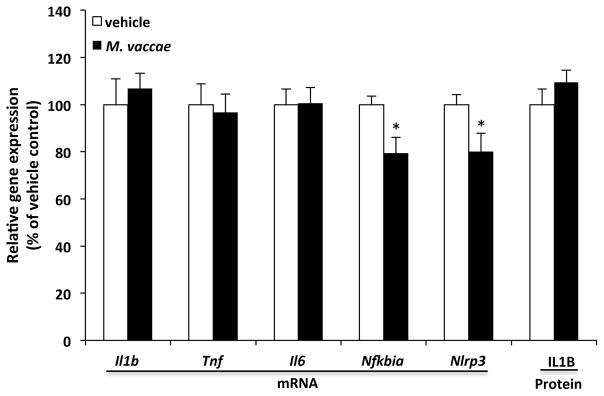
Under basal conditions, IL4 largely fails to modulate expression of proinflammatory cytokines; however, it attenuates the proinflammatory response to immune challenge (Awad et al., 2017; Edwards et al., 2006). Interestingly, IL4 is capable of down-regulating basal mRNA levels of NLR family pyrin domain containing 3 (Nlrp3) (Niebuhr et al., 2014), which is an inflammasome component that mediates the processing of pro-IL1B into its mature, bioactive form (Leemans et al., 2011). Here, we tested whether basal levels of proinflammatory mediators in the hippocampus were altered in the context of increased hippocampal IL4. We found that M. vaccae treatment failed to affect basal gene expression of proinflammatory cytokines (Il1b, Il6, and Tnf) as well as IL1B protein, but reduced expression of Nlrp3 (t = 2.25, df = 22, p = 0.035) and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (Nfkbia) (t = 2.71, df = 22, p = 0.013) (Fig. 3).
Fig. 3. Effect of M. vaccae on hippocampal proinflammatory mediators.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight d after the third injection, gene expression of hippocampal proinflammatory mediators was measured. Data are presented as the mean + s.e.m. N = 9–12 animals per experimental group. Significant M. vaccae effects compared to vehicle, * p < 0.05.
3.3. Effect of rIL4 on the hippocampal immunophenotype
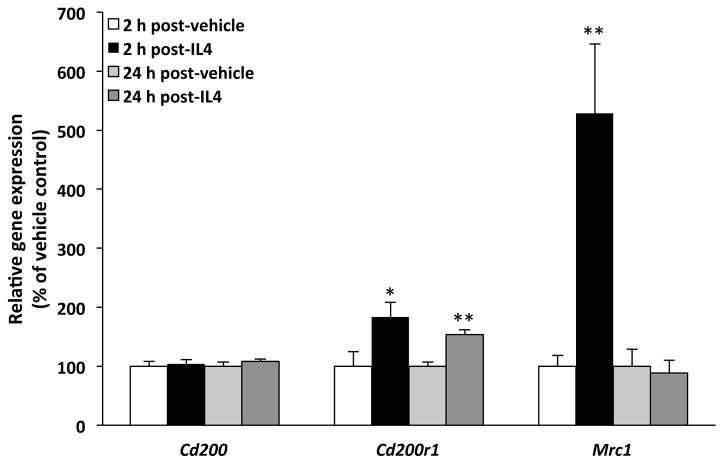
The data presented thus far suggest that IL4 might play a pivotal role in M. vaccae's anti-inflammatory effects in the CNS. To address this possibility, we examined whether administration of rIL4 is sufficient to recapitulate the anti-inflammatory effects of M. vaccae. Here, rIL4 was administered i.c.m. and the effect on M. vaccae-sensitive antigens (Cd200, Cd200r1, Mrc1) was then examined in hippocampus 2 h and 24 h post-injection. As depicted in Fig. 4, rIL4 induced a significant increase in Mrc1 at 2 h post-injection (t = 3.09, df = 12, p = 0.009) and Cd200r1 at 2 h (t = 2.7, df = 12, p = 0.04) and 24 h post-injection (t = 5.1, df = 6, p = 0.002), but failed to significantly alter expression of Cd200 at either timepoint, thus recapitulating the effects of M. vaccae.
Fig. 4. Effect of recombinant interleukin 4 (rIL4) on the hippocampal immunophenotype.
Animals received injections of either vehicle or rIL4 (100 ng, i.c.m.). IL4-sensitive target genes were measured in hippocampus at 2 h (N = 6–8 per group) or 24 h post-injection (N = 4 per group). Data are presented as the mean + s.e.m. Significant M. vaccae effects compared to vehicle at each timepoint post-injection, * p < 0.05, ** p < 0.01.
3.4. Effect of M. vaccae on stress-induced microglial priming
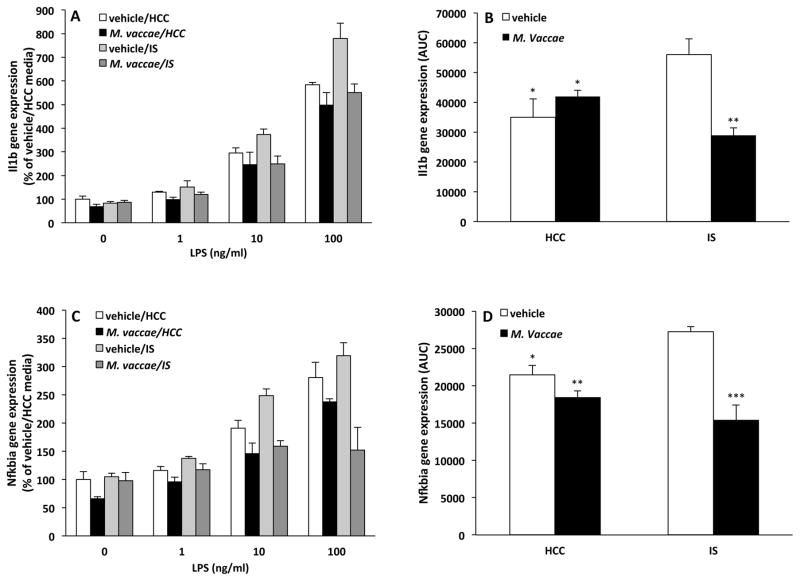
NLRP3 and NFKBIA signaling have been implicated in stress-induced microglial priming (Weber et al., 2015). Given the effects of M. vaccae on basal expression of these genes, we examined the possibility that M. vaccae's anti-inflammatory effects may buffer the CNS against the neuroinflammatory priming effects of stress. Exposure to acute stress sensitizes microglia to proinflammatory immune challenges ex vivo such that acute stress exposure potentiates the microglial proinflammatory cytokine response to subsequent immune challenges (e.g. LPS). Here, animals were exposed to stress 7 d after the final M. vaccae injection. Consistent with our prior studies (Frank et al., 2007), hippocampal microglia were isolated 24 h after termination of the stressor and challenged with LPS ex vivo. Depicted in Fig. 5A/C are data showing the proinflammatory response of microglia to several concentrations of LPS in vitro and the differential effects of stress and M. vaccae treatment. Area-under-the-curve (AUC) analysis (Fig. 5B/D) was used to represent the overall magnitude of the proinflammatory response across LPS concentrations and to examine the interaction between stress and M. vaccae treatment on this response. The interaction between stress and M. vaccae treatment was significant for Il1b (interaction effect, F = 15.48, df = 1, 12, p = 0.002; Fig. 5B) and Nfkbia (interaction effect, F = 10.75, df = 1, 12, p = 0.007; Fig. 5D), while the interaction between these treatments on Il6 and Tnf failed to attain significance (data not shown). Post hoc comparisons show that in vehicle-treated animals, stress potentiated the Il1b (p < 0.05; Fig. 5B) and Nfkbia (p < 0.05; Fig. 5D) responses to LPS compared to HCC animals. In IS-exposed animals, M. vaccae treatment significantly reduced the Il1b (p < 0.05; Fig. 5B) and Nfkbia (p < 0.05; Fig. 5D) responses compared to vehicle treatment, thus blunting the proinflammatory response to levels comparable to HCC animals.
Fig. 5. Effect of M. vaccae on stress-induced microglial priming.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Seven d after the third injection, animals were exposed to stress (IS; inescapable tailshock) or served as home cage controls (HCCs). 24 h post-IS, hippocampal microglia were isolated from all animals and exposed to several concentrations of LPS (0, 1, 10 and 100 ng/ml) for 2 h. (A and C) the cytokine response (Il1b and Nfkbia) at each concentration of LPS was captured and (B and D) the overall magnitude of the cytokine response (area-under-the-curve; AUC) computed. N = 4/experimental group. Data are presented as the mean + s.e.m. For the AUC data, the vehicle/IS treatment group significantly differed from all other treatment groups, * p < 0.05, ** p < 0.01, *** p < 0.001.
3.5. Effect of M. vaccae on basal and stress-induced serum CORT levels
It is important to consider the possibility that M. vaccae's effect on microglial priming could be due to M. vaccae's effects on basal as well as stress-induced levels of glucocorticoids, which have profound anti-inflammatory effects (Cain and Cidlowski, 2017) as well as paradoxical effects on neuroinflammatory processes (Sorrells et al., 2009). Serum CORT levels were measured 24 h after IS exposure in animals immunized with M. vaccae. Consistent with our prior work (Johnson et al., 2003), IS induced a persistent elevation in serum CORT 24 h after termination of the stressor independent of M. vaccae treatment (Fig. 6; main effect of IS, F = 29.88, df = 1, 39, p < 0.0001). In addition, M. vaccae treatment failed to alter basal levels of serum CORT in HCC animals.
Fig. 6. Effect of M. vaccae on basal and stress-induced serum corticosterone (CORT) levels.
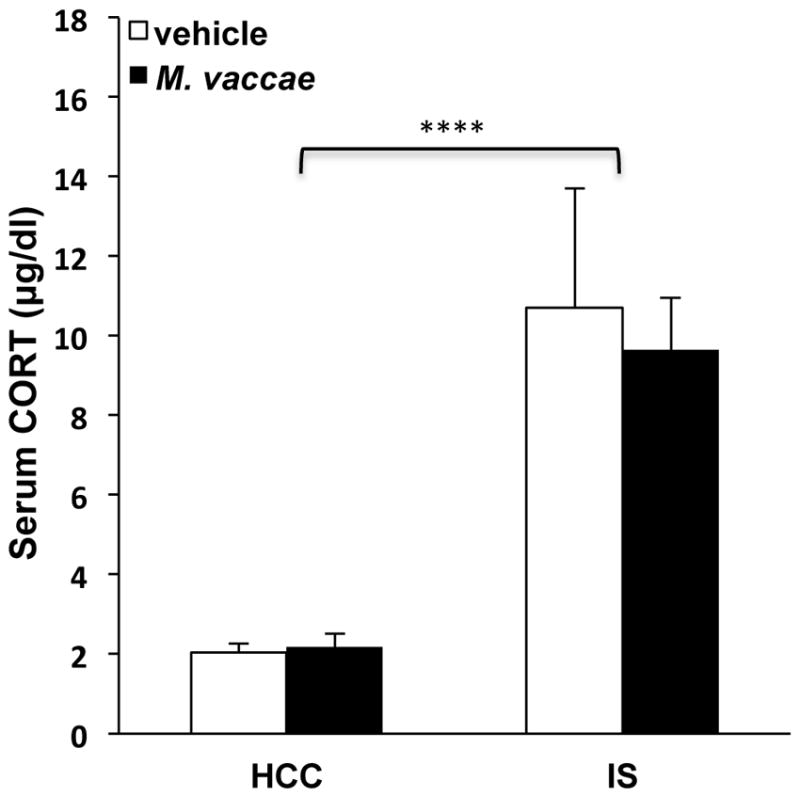
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Seven d after the third injection, animals were exposed to stress (IS; inescapable tailshock) or served as home cage controls (HCCs). 24 h post-IS, serum CORT levels were measured. N = 9–12 animals per group. Data are presented as the mean + s.e.m. IS independent of M. vaccae treatment increased serum CORT compared to HCC, **** p < 0.0001.
3.6. Effect of M. vaccae on stress-induced reductions in Cd200r1
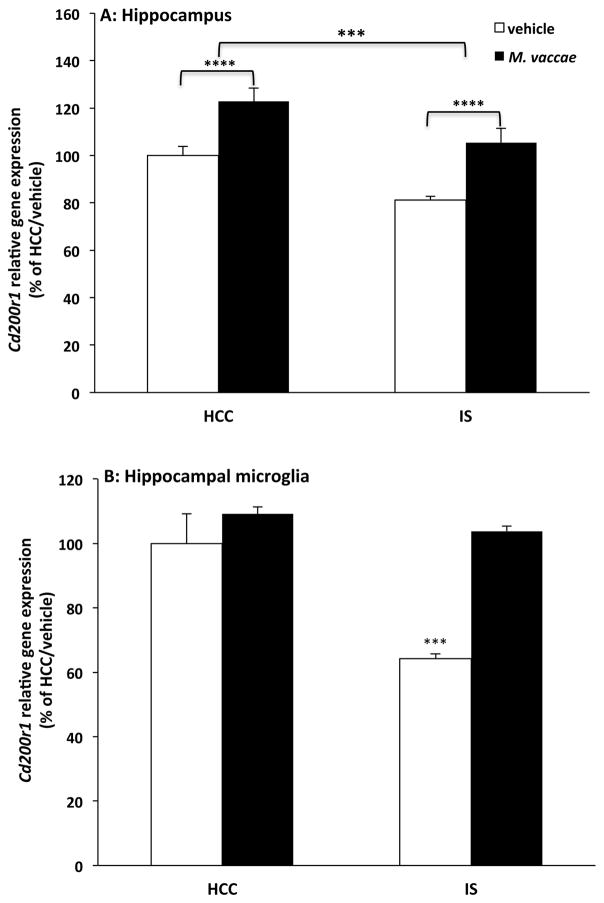
Recent evidence from our laboratory demonstrates that exposure to IS reduces Cd200r1 expression 0 h and 24 h after termination of the stressor (Frank et al., 2017). Further, we found that IS disrupts CD200:CD200R1 signaling, thereby leading to microglial disinhibition and priming of microglial proinflammatory responses ex vivo (Frank et al., 2017). Considering that M. vaccae induces hippocampal Cd200r1 expression, we explored the possibility that M. vaccae treatment might prevent the stress-induced reductions in Cd200r1 gene expression. Consistent with our prior findings (Frank et al., 2017), we found that stress reduced expression of Cd200r1 compared to HCC animals (Fig. 7A; main effect of IS, F = 14.68, df = 1, 41, p < 0.001) and that M. vaccae treatment increased Cd200r1 expression compared to vehicle treatment (main effect of M. vaccae, F = 24.47, df = 1, 41, p < 0.0001), thus preventing stress-induced reductions in Cd200r1 expression. To examine whether these effects of stress and M. vaccae on Cd200r1 extended to microglia, hippocampal microglia were isolated 24 h after stress exposure in animals treated with vehicle or M. vaccae. Similar to our observations in whole hippocampus, stress induced a reduction in microglial Cd200r1 expression, which was prevented by M. vaccae immunization (Fig. 7B; interaction effect, F = 9.82, df = 1, 12, p < 0.01). Post hoc comparisons demonstrated that in vehicle-treated animals, exposure to IS reduced Cd200r1 expression compared to HCCs (p < 0.001); however, in IS-exposed animals, M. vaccae treatment abrogated this effect of IS on Cd200r1 compared to vehicle treatment (p < 0.001).
Fig. 7. Effect of M. vaccae on stress-induced reductions in Cd200r1.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Seven d after the third injection, animals were exposed to stress (IS; inescapable tailshock) or served as home cage controls (HCCs). Twenty-four h post-IS, Cd200r1 expression was measured in (A) whole hippocampal tissue (N = 10–12 animals per group) and (B) hippocampal microglia ex vivo (N = 4 animals per group). Data are presented as the mean + s.e.m. In panel (A), main effect of HCC vs IS, *** p < 0.001; main effect of vehicle vs M. vaccae, **** p < 0.0001. In panel (B), the vehicle/IS treatment group significantly differed from all other treatment groups, *** p < 0.001.
3.7. Effect of M. vaccae on stress-induced HMGB1
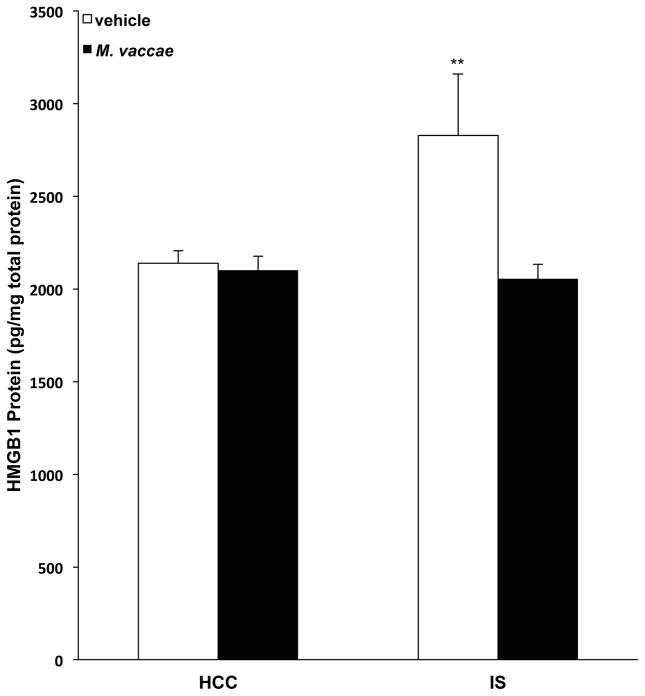
Alarmins are host biomolecules that can initiate and perpetuate a noninfectious inflammatory response, often in response to cell or tissue damage, or immune activation (Yang et al., 2017). We have previously found that stress induces the alarmin HMGB1 in hippocampus and increases release of HMGB1 protein from hippocampal microglia ex vivo. In addition, we found that HMGB1 mediates stress-induced priming of microglial proinflammatory responses assessed ex vivo (Weber et al., 2015). Interestingly, we also found that HMGB1 induces a primed phenotype in primary microglia, which was characterized by up-regulation of Nlrp3 and Nfkbia, but not proinflammatory cytokines (Frank et al., 2015a). Furthermore, we found that stress-induced increases in HMGB1 are a consequence of disrupted CD200:CD200R1 signaling (Frank et al., 2017). Considering the effects of M. vaccae on Nlrp3, Nfkbia, and Cd200r1 (in the absence of any effects on proinflammatory cytokines) as well as its effects on stress-induced microglial priming, we explored the possibility that M. vaccae might modulate stress-induced HMGB1. Consistent with our prior findings (Weber et al., 2015), exposure to IS resulted in a significant upregulation of hippocampal HMGB1 protein levels compared to levels in HCC animals; however, this effect of IS was blocked by prior immunization with M. vaccae (Fig. 8; interaction effect, F = 5.19, df = 1, 39, p = 0.03). Post hoc comparisons demonstrate that HMGB1 levels in vehicle-treated IS animals were significantly increased compared to levels observed in vehicle-treated HCC (p < 0.01), M. vaccae-treated HCC (p < 0.01) and M. vaccae-treated IS (p < 0.01) animals.
Fig. 8. Effect of M. vaccae on stress-induced HMGB1.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Seven d after the third injection, animals were exposed to stress (IS; inescapable tailshock) or served as home cage controls (HCCs). Twenty-four h post-IS, hippocampal HMGB1 protein levels were measured. N = 9–12 animals per group. Data are presented as the mean + s.e.m. The vehicle/IS treatment group significantly differed from all other treatment groups, ** p < 0.01.
3.8. Effect of M. vaccae on stress-induced anxiety-like behavior
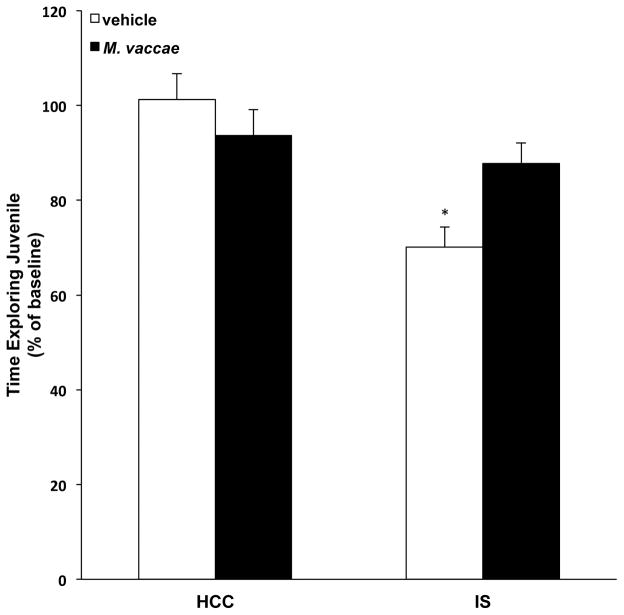
Exposure to acute and chronic stressors induces anxiety-like behavior in a number of behavioral tests including the juvenile social exploration (JSE) test, which is blocked by central administration of anti-inflammatory mediators such as IL1 receptor antagonist (Goshen and Yirmiya, 2009). Likewise, central administration of rIL4 blocks LPS-induced anxiety-like behavior (Bluthe et al., 2002). Therefore, given M. vaccae's induction of an anti-inflammatory milieu in the CNS, we examined whether M. vaccae would mitigate the effects of stress on anxiety-like behavior. As depicted in Fig. 9, M. vaccae treatment differentially modulated the effect of IS on JSE (interaction effect, F = 5.41, df = 1, 42, p = 0.02). Consistent with our prior findings (Christianson et al., 2008), IS reduced JSE 24h after stress exposure in vehicle-treated animals (p < 0.05). However, M. vaccae treatment blunted this effect of IS on JSE compared to vehicle treatment (p < 0.05).
Fig. 9. Effect of M. vaccae on stress-induced anxiety-like behavior.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Six d after the third injection, baseline juvenile social exploration (JSE) was measured in all animals. Twenty-four h after baseline testing, animals were exposed to stress (IS; inescapable tailshock) or served as home cage controls (HCCs). Twenty-four h post-IS, JSE was measured. Data are presented as a percent of baseline JSE. N = 10–12 animals per group. Data are presented as the mean + s.e.m. The IS/vehicle treatment group significantly differed from all other groups, * p < 0.05.
3.9. Effect of M. vaccae on IL4 mRNA and protein expression in subregions of the hippocampus
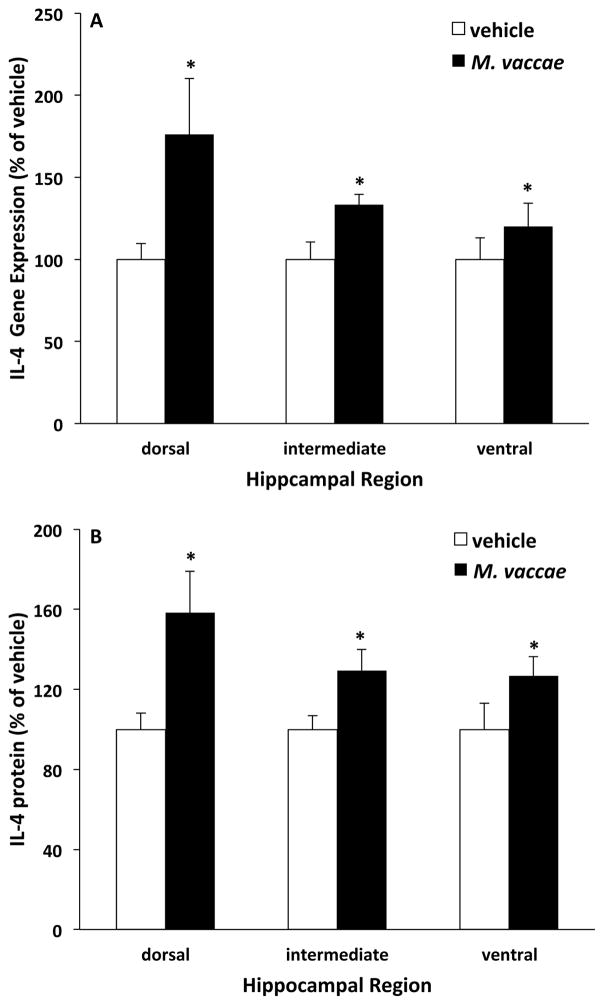
A number of studies now suggest that there is an anatomical segregation of hippocampal function (for review see (Fanselow and Dong, 2010)); such that, the dorsal hippocampus mediates spatial navigation and contextual memory (Holt and Maren, 1999; Kim and Fanselow, 1992), while the ventral hippocampus plays a role in fear and anxiety (Kjelstrup et al., 2002). Indeed, the ventral hippocampus selectively provides input to the amygdala (Maren and Fanselow, 1995), a brain structure critical for mediating fear and anxiety responses (Janak and Tye, 2015). Furthermore, the anti-inflammatory cytokine IL4 has been found to regulate learning and memory (Derecki et al., 2010) as well as depressive- and anxiety-like behavior (Wachholz et al., 2017). Therefore, given the effect of M. vaccae on IL4 (section 3.1.) and stress-elicited anxiety-like behavior (section 3.8.), we explored the possibility that M. vaccae might differentially induce IL4 in the ventral versus the dorsal hippocampus. Animals received vehicle or M. vaccae as outlined in Fig. 1 and, 8 days post-injection, hippocampus was trisected into dorsal, intermediate, and ventral subregions according to the experimental approach described in Lee et al. (Lee et al., 2017). Interestingly, a number of genes are differentially expressed at high levels in rat dorsal versus ventral hippocampus (Lee et al., 2017). For example, neurotensin (Nts) is highly expressed in dorsal relative to ventral hippocampus, while Nr2f2 is highly expressed in ventral relative to dorsal hippocampus. Therefore, we initially assessed these mRNAs in dorsal, intermediate and ventral hippocampus to verify that our trisection of hippocampus resulted in the anatomical segregation of the hippocampus into the desired subregions. Consistent with the findings of Lee and colleagues, we found that Nts was highly expressed in dorsal versus intermediate and ventral sub-regions (F = 52.99, df = 2, 14, p < 0.0001)(Suppl. Fig. 2A), while Nr2f2 was highly expressed in ventral versus intermediate and dorsal subregions (F = 108.2, df = 2, 14, p < 0.0001)(Suppl. Fig. 2B). We also assessed expression of Cd3e, which Lee et al. found to be the most enriched transcript in dorsal hippocampus compared to ventral hippocampus and is a component of the T cell receptor-CD3 complex. Indeed, we replicated the findings of Lee et al. and found that dorsal hippocampus exhibited high levels of Cd3e expression compared to intermediate and ventral hippocampus (F = 189.2, df = 2, 14, p < 0.0001)(Suppl. Fig. 2C). Interestingly, M. vaccae treatment increased Cd3e expression only in dorsal hippocampus (brain region x M. vaccae interaction, F = 6.24, df = 2, 14, p < 0.01). These findings support that our trisection method resulted in the anatomical segregation of the hippocampus into dorsal, intermediate, and ventral subregions. Therefore, we next examined the effects of M. vaccae on IL4 in these subregions. Consistent with our initial findings (section 3.1.), M. vaccae treatment induced IL4 mRNA (main effect of M. vaccae, F = 8.17, df = 1, 14, p < 0.05) and protein (main effect of M. vaccae, F = 7.58, df = 1, 14, p < 0.05) and did so irrespective of hippocampal subregion (Fig. 10). It should be noted that dorsal hippocampus tended to show a greater effect of M. vaccae on IL4; however, the interaction between M. vaccae and hippocampal subregion was not significant for either mRNA or protein.
Fig. 10. Effect of M. vaccae on IL4 mRNA and protein expression in subregions of the hippocampus.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight d after the third injection, (A) IL4 mRNA expression and (B) IL4 protein levels were measured in dorsal, intermediate, and ventral hippocampus. Data are presented as the mean + s.e.m. N = 8 animals per experimental group. Significant main effect of M. vaccae compared to vehicle, * p < 0.05.
4. Discussion
A number of studies have demonstrated that microbial-based interventions can mitigate the effects of stress on anxiety- and depressive-like behavior (Bharwani et al., 2017; Bravo et al., 2011; Desbonnet et al., 2010; Liang et al., 2015; Marin et al., 2017; Reber et al., 2016b). In light of 1) the pivotal role neuroinflammatory processes play in these behavioral effects of stress, 2) the effect of microbial-based interventions on peripheral immunoregulation, and 3) communication of peripheral immune signals to the CNS, we explored the possibility that immunization with a heat-killed preparation of the immunoregulatory and anti-inflammatory bacterium, M. vaccae, might mitigate the effects of stress via prevention of stress-induced neuroinflammation.
Indeed, we found that treatment with the immunoregulatory and anti-inflammatory bacterium, M. vaccae, induced a distinct immunophenotype in hippocampus characterized by increased anti-inflammatory cytokines (Il4 mRNA and IL4 protein) and IL4-sensitive antigens including Cd200r1 and the mannose receptor (Mrc1). These immunophenotypic changes resemble, in large part, an alternative macrophage activation state, which is characterized by low proinflammatory cytokine production and enhanced tissue repair function (Ransohoff and Perry, 2009). Particularly noteworthy is the effect of M. vaccae on Cd200r1, which plays a prominent role in microglial immunomodulation. In the CNS, CD200R1 is expressed almost exclusively on microglia as well as other CNS macrophages (Koning et al., 2009; Wright et al., 2000). In the CNS microenvironment, microglia are maintained in a surveillant or quiescent state of activation through several inhibitory signaling dyads (Hoarau et al., 2011; Ransohoff and Cardona, 2010) including the CD200:CD200R1 dyad. CD200R1 inhibits myeloid cell function via engagement of its ligand CD200 (Gorczynski, 2005), which is expressed at high levels in the CNS on neurons, endothelial cells and oligodendrocytes. Interestingly, disruption of CD200:CD200R1 signaling potentiates the proinflammatory response of microglia to immune stimuli (Costello et al., 2011; Denieffe et al., 2013) and has been implicated in neuroinflammatory processes observed in aging (Lyons et al., 2007), neuropathic pain (Hernangomez et al., 2016) and Alzheimer’s disease (Walker et al., 2009). Furthermore, we found that central administration of recombinant IL4 increased hippocampal Cd200r1 and Mrc1 expression, thus recapitulating the effect of M. vaccae on these genes. Taken together, the parallel effects of M. vaccae and recombinant IL4 on Cd200r1 suggest that endogenous IL4 might mediate the effects of M. vaccae on Cd200r1; however, additional studies are still required to definitively address the causality of this relationship. It is important to consider that this effect of M. vaccae on IL4, Cd200r1 and Mrc1 was observed 8 days after the last M. vaccae immunization. Of course, the onset of these immunophenotypic changes relative to M. vaccae treatment is unclear. It is worth noting, though, that the peripheral anti-inflammatory effects of M. vaccae have been found to last up to 12 weeks after M. vaccae immunization (Zuany-Amorim et al., 2002), which raises the intriguing possibility that M. vaccae might induce long-term anti-inflammatory changes in the CNS.
An important consideration concerns mechanisms by which peripheral M. vaccae immunization generates an anti-inflammatory milieu in the CNS. Potential mechanisms might include meningeal immunity, through which T cells in the meningeal compartment modulate CNS function (Walsh et al., 2014), movement of lipophilic M. vaccae-derived metabolites, such as triacylglycerols, long-chain saturated fatty acid polyesters, or their fatty acid derivatives (Agusti et al., 2008) across the blood-brain barrier, and movement of alternatively activated dendritic cells (following phagocytosis of M. vaccae) into the CNS (Sagar et al., 2012). In addition, M. vaccae's peripheral anti-inflammatory effects might alter immune-to-brain signaling via modulation of humoral or neural (e.g. vagal) routes of communication to the CNS (Sarkar et al., 2016). As a part of examining the effects of M. vaccae on anatomical subregions of the hippocampus (see below), we found that M. vaccae selectively increased expression of the T cell antigen Cd3e in the dorsal hippocampus, suggesting the possibility that M. vaccae might induce influx of T cells, which are a source of IL4, into the dorsal hippocampus. This possible mechanism of M. vaccae's anti-inflammatory effects in the hippocampus is currently being explored.
The effect of M. vaccae on Cd200r1 suggests that myeloid cells in the CNS might be under greater inhibitory control via enhanced CD200 engagement. Therefore, we examined the effect of M. vaccae on several genes involved in neuroinflammatory processes. We found that basal levels of proinflammatory cytokines (Il1b, Il6, and Tnf) were not affected by M. vaccae treatment despite increased Cd200r1 expression. However, these effects are consistent with Denieffe and colleagues who found that disrupted CD200:CD200R1 signaling in CD200 knockout mice does not alter basal cytokine levels, but primes microglia to subsequent challenges (Denieffe et al., 2013). Interestingly, we found that M. vaccae reduced expression of Nlrp3 and Nfkbia, which have been implicated in stress-induced priming of microglia (Weber et al., 2015). Therefore, we examined the effect of M. vaccae on this priming phenomenon. Consistent with prior findings (Frank et al., 2016), exposure to an acute stressor potentiated the microglial proinflammatory response to an immune challenge ex vivo; however, treatment with M. vaccae abrogated this priming effect of stress. It is important to note that M. vaccae failed to alter stress-induced serum glucocorticoid levels, which are largely anti-inflammatory (Cain and Cidlowski, 2017), but also exhibit paradoxical effects on neuroinflammatory processes (Sorrells et al., 2009). This suggests that M. vaccae's effect on stress-induced microglial priming is not due to modulation of either basal or stress-induced glucocorticoid levels. However, it is important to note that stress-induced glucocorticoids were measured at one time-point post-stress (24h), thus we cannot exclude the possibility that M. vaccae modulated the glucocorticoid response at a time-point more proximal to stress exposure. In addition, the present findings do not exclude the possibility that M. vaccae might have altered glucocorticoid receptor expression or induced a glucocorticoid resistant phenotype.
Several studies have examined potential mediators of stress-induced neuroinflammatory and microglial priming (Frank et al., 2012b; Johnson et al., 2005; Johnson et al., 2004; Weber et al., 2015; Wohleb et al., 2011). We have recently found that exposure to acute stress reduces hippocampal expression of Cd200r1 resulting in disrupted CD200:CD200R1 signaling and priming of microglia (Frank et al., 2017) . In addition, exposure to acute stress increases levels of the danger-associated molecular pattern HMGB1 in hippocampus (Weber et al., 2015), a finding replicated in alternate stress paradigms (Cheng et al., 2016; Lian et al., 2017). We have found that this stress-induced increase in HMGB1 is a consequence of disrupted CD200:CD200R1 signaling (Frank et al., 2017) and that HMGB1 mediates the effects of stress on microglial priming (Weber et al., 2015). Also, HMGB1 is sufficient to prime microglia in vivo and in vitro (Frank et al., 2015a). Therefore, we examined the effect of M. vaccae on stress-induced reductions in Cd200r1 expression and increases in HMGB1. Here, we reproduced our prior findings demonstrating that acute stress downregulates hippocampal and microglial Cd200r1 expression concomitant with up-regulating HMGB1 levels in hippocampus. Consistent with its effect on stress-induced microglial priming, M. vaccae immunization abrogated these effects of stress on hippocampal Cd200r1 and HMGB1 levels. This result suggests that M. vaccae's effect on microglial priming might be a consequence of its capacity to up-regulate CD200R1 expression, thereby increasing microglial inhibitory drive and blocking induction of HMGB1.
Taken together, the effects of M. vaccae on anti-inflammatory mediators, microglial priming, Cd200r1, and the alarmin HMGB1 suggest that M. vaccae skews the CNS microenvironment towards an anti-inflammatory or alternatively activated immunophenotype. We have previously found that central anti-inflammatory treatment (i.e, IL1RA) blocks the effects of stress on depressive-like behavior (Maier and Watkins, 1995). Therefore, we tested whether M. vaccae might mitigate the behavioral effects of stress using the juvenile social exploration (JSE) test, which is a highly sensitive measure of the sickness response to proinflammatory challenges and stress (Goshen and Yirmiya, 2009). Consistent with prior findings (Christianson et al., 2008), exposure to acute stress reduced JSE 24 h after stress. However, treatment with M. vaccae blocked this behavioral effect of stress. This effect of M. vaccae immunization on IS-induced decrements in JSE is consistent with its anxiolytic effects in animals exposed to a chronic subordinate colony housing stressor (Reber et al., 2016b); the studies by Reber et al. (2016) found no effect of M. vaccae on measures of locomotor activity, suggesting that these effects of M. vaccae are not due to an overall decrease in activity. While the present effects of M. vaccae on behavior occurred in parallel with effects on anti-inflammatory mediators and neuroinflammatory processes, additional studies are clearly warranted to explore causal relationships. Despite this limitation, the current studies clearly demonstrate that microbial- or microbiome-based interventions have potential for prevention of stress-induced microglial priming, stress-induced exaggeration of neuroinflammation, and their behavioral sequelae, including anxiety-like behavioral responses.
Accumulating evidence now suggests that there is an anatomical segregation of hippocampal function (for review see (Fanselow and Dong, 2010)). The dorsal subregion is thought to play a role in spatial memory, while the ventral subregion plays a role in affective responses (e.g. anxiety/fear responses). Considering that M. vaccae mitigated the anxiogenic effect of stress, we explored the possibility that M. vaccae might differentially induce an anti-inflammatory effect across subregions of the hippocampus. Consistent with our initial findings, M. vaccae induced IL4 mRNA and protein; however, this effect occurred independent of hippocampal subregion. These findings suggest that M. vaccae might exert effects on both dorsal hippocampus-mediated behavior (i.e. contextual memory) as well as ventral hippocampus-mediated behavior (i.e. anxiety-like behavior). It is worth noting that IL4, via T helper 2 cells in the CNS, is thought to play a critical role in learning and memory (Gadani et al., 2012).
Prior studies have demonstrated that M. vaccae induces long-lasting stress resilience (Reber et al., 2016b) via enhanced peripheral immunoregulation. The present study extends these findings by demonstrating that M. vaccae enhances anti-inflammatory mechanisms in the CNS and mitigates the neuroinflammatory and behavioral effects of stress. These findings suggest the possibility that M. vaccae's induction of an anti-inflammatory milieu in the CNS may underpin, in part, its capacity to impart a stress resilient phenotype.
Supplementary Material
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight days after the third injection, gene expression of (A) anti-inflammatory mediators and (B) markers of alternative macrophage activation were measured in amygdala. Data are presented as the mean + s.e.m. N = 9–12 animals per experimental group. ND = non-detected.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight d after the third injection, gene expression of (A) Nts, (B) Nr2f2 and (C) Cd3e was measured in dorsal, intermediate, and ventral hippocampus. Data are presented as the mean + s.e.m. N = 8 animals per experimental group. (A) Dorsal versus ventral and intermediate hippocampus, *** p < 0.001. (B) Ventral versus dorsal and intermediate hippocampus, *** p < 0.001. (C) Dorsal versus ventral and intermediate hippocampus, *** p < 0.001. Vehicle versus M. vaccae in dorsal hippocampus, * p < 0.05.
M. vaccae immunization induced an anti-inflammatory milieu in the hippocampus.
M. vaccae immunization blocked stress-induced decreases in CD200R1.
M. vaccae immunization blocked stress-induced increases in the alarmin HMGB1.
M. vaccae prevented stress-induced priming of the microglia proinflammatory response.
M. vaccae immunization blocked stress-induced increases in anxiety-like behavior.
Acknowledgments
We are grateful for the technical support of Jared D. Heinze. This work was supported by grants from the National Institutes of Health to M.G.F. and S.F.M. (R01MH108523), to L.K.F. (F32AG048672), and to C.A.L., M.G.F., S.F.M., and L.K.F. (R21MH116263). C.A.L. is supported by the Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (grant number N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development (VA-ORD) RR&D Small Projects in Rehabilitation Research (SPiRE) (I21) (grant number 1 I21 RX002232-01), Colorado Clinical & Translational Sciences Institute (CCTSI) Center for Neuroscience (grant number CNSTT-15-145), the Colorado Department of Public Health and Environment (CDPHE; grant number DCEED-3510), and the Alfred P. Sloan Foundation (grant number G-2016-7077). Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agusti G, Astola O, Rodriguez-Guell E, Julian E, Luquin M. Surface spreading motility shown by a group of phylogenetically related, rapidly growing pigmented mycobacteria suggests that motility is a common property of mycobacterial species but is restricted to smooth colonies. Journal of bacteriology. 2008;190:6894–6902. doi: 10.1128/JB.00572-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. Journal of immunological methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Awad F, Assrawi E, Jumeau C, Georgin-Lavialle S, Cobret L, Duquesnoy P, Piterboth W, Thomas L, Stankovic-Stojanovic K, Louvrier C, Giurgea I, Grateau G, Amselem S, Karabina SA. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PloS one. 2017;12:e0175336. doi: 10.1371/journal.pone.0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC medicine. 2017;15:7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Lestage J, Rees G, Bristow A, Dantzer R. Dual effect of central injection of recombinant rat interleukin-4 on lipopolysaccharide-induced sickness behavior in rats. Neuropsychopharmacology. 2002;26:86–93. doi: 10.1016/S0893-133X(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, Jope RS, Beurel E. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Lyons A, Denieffe S, Browne TC, Cox FF, Lynch MA. Long term potentiation is impaired in membrane glycoprotein CD200-deficient mice: a role for Toll-like receptor activation. J Biol Chem. 2011;286:34722–34732. doi: 10.1074/jbc.M111.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean OM, Kanchanatawan B, Ashton M, Mohebbi M, Ng CH, Maes M, Berk L, Sughondhabirom A, Tangwongchai S, Singh AB, McKenzie H, Smith DJ, Malhi GS, Dowling N, Berk M. Adjunctive minocycline treatment for major depressive disorder: A proof of concept trial. The Australian and New Zealand journal of psychiatry. 2017;51:829–840. doi: 10.1177/0004867417709357. [DOI] [PubMed] [Google Scholar]
- Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun. 2013;34:86–97. doi: 10.1016/j.bbi.2013.07.174. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. The Journal of experimental medicine. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- DuPont RL, Rice DP, Miller LS, Shiraki SS, Rowland CR, Harwood HJ. Economic costs of anxiety disorders. Anxiety. 1996;2:167–172. doi: 10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O'Connor DT, Baker DG Marine Resiliency Study, T. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging. 2011;32:85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF. IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. Journal of neuroimmunology. 2012a;252:33–39. doi: 10.1016/j.jneuroim.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Annis JL, Watkins LR, Maier SF. Stress disinhibits microglia via down-regulation of CD200R: a mechanism of neuroinflammatory priming. Brain, behavior, and immunity. 2017 doi: 10.1016/j.bbi.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain, behavior, and immunity. 2012b;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun. 2015a;55:215–224. doi: 10.1016/j.bbi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015b;48:1–7. doi: 10.1016/j.bbi.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiology of stress. 2016;4:62–70. doi: 10.1016/j.ynstr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Current opinion in investigational drugs. 2005;6:483–488. [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. The Journal of clinical psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Hernangomez M, Klusakova I, Joukal M, Hradilova-Svizenska I, Guaza C, Dubovy P. CD200R1 agonist attenuates glial activation, inflammatory reactions, and hypersensitivity immediately after its intrathecal application in a rat neuropathic pain model. J Neuroinflammation. 2016;13:43. doi: 10.1186/s12974-016-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, Das T, Thon-Hon GV, Kumar S, Neal JW, Gasque P. Activation and control of CNS innate immune responses in health and diseases: a balancing act finely tuned by neuroimmune regulators (NIReg) CNS & neurological disorders drug targets. 2011;10:25–43. doi: 10.2174/187152711794488601. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoisington AJ, Brenner LA, Kinney KA, Postolache TT, Lowry CA. The microbiome of the built environment and mental health. Microbiome. 2015;3:60. doi: 10.1186/s40168-015-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Greenberg PE. The economic burden of anxiety and stress disorders. Neuropsychopharmacology - 5th Generation of Progress. 2002:981–992. [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Shipley MJ, Batty GD, Hamer M, Akbaraly TN, Kumari M, Jokela M, Virtanen M, Lowe GD, Ebmeier KP, Brunner EJ, Singh-Manoux A. Long-term inflammation increases risk of common mental disorder: a cohort study. Molecular psychiatry. 2014;19:149–150. doi: 10.1038/mp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol. 2009;68:159–167. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- Lee AR, Kim JH, Cho E, Kim M, Park M. Dorsal and Ventral Hippocampus Differentiate in Functional Pathways and Differentially Associate with Neurological Disease-Related Genes during Postnatal Development. Frontiers in molecular neuroscience. 2017;10:331. doi: 10.3389/fnmol.2017.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunological reviews. 2011;243:152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, Yang H. Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord. 2010;124:68–75. doi: 10.1016/j.jad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y, Yang YY, Peng W, Zhang T, Zhou JR, Jiang CL, Wang YX. Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid-HMGB1. Brain Behav Immun. 2017;59:322–332. doi: 10.1016/j.bbi.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE, Jr, Yamashita PS, Fox JH, Reber SO, Brenner LA, Hoisington AJ, Postolache TT, Kinney KA, Marciani D, Hernandez M, Hemmings SM, Malan-Muller S, Wright KP, Knight R, Raison CL, Rook GA. The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health. Current environmental health reports. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–282. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Scientific reports. 2017;7:43859. doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr M, Baumert K, Heratizadeh A, Satzger I, Werfel T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy. 2014;69:1058–1067. doi: 10.1111/all.12428. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–999. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reber SO, Langgartner D, Foertsch S, Postolache TT, Brenner LA, Guendel H, Lowry CA. Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment-2016 Curt Richter Award Paper. Psychoneuroendocrinology. 2016a;74:221–230. doi: 10.1016/j.psyneuen.2016.08.031. [DOI] [PubMed] [Google Scholar]
- Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, Lowe KR, Wheeler KJ, Fox JH, Hassell JE, Jr, Greenwood BN, Jansch C, Lechner A, Schmidt D, Uschold-Schmidt N, Fuchsl AM, Langgartner D, Walker FR, Hale MW, Lopez Perez G, Van Treuren W, Gonzalez A, Halweg-Edwards AL, Fleshner M, Raison CL, Rook GA, Peddada SD, Knight R, Lowry CA. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A. 2016b;113:E3130–3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci U S A. 2013;110:18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer seminars in immunopathology. 2004;25:237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29:150–158. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, Raison CL. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res. 2015;1617:47–62. doi: 10.1016/j.brainres.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA. Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evolution, medicine, and public health. 2013;2013:14–17. doi: 10.1093/emph/eos005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv Exp Med Biol. 2014;817:319–356. doi: 10.1007/978-1-4939-0897-4_15. [DOI] [PubMed] [Google Scholar]
- Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P. Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 2012;7:74–94. doi: 10.1007/s11481-011-9302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends in neurosciences. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun. 2009;23:1117–1124. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli HA, Gandhi CP, Gray JM, Morena M, Hassan KI, Hill MN. Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain Behav Immun. 2016;51:70–91. doi: 10.1016/j.bbi.2015.07.026. [DOI] [PubMed] [Google Scholar]
- von Hertzen L, Beutler B, Bienenstock J, Blaser M, Cani PD, Eriksson J, Farkkila M, Haahtela T, Hanski I, Jenmalm MC, Kere J, Knip M, Kontula K, Koskenvuo M, Ling C, Mandrup-Poulsen T, von Mutius E, Makela MJ, Paunio T, Pershagen G, Renz H, Rook G, Saarela M, Vaarala O, Veldhoen M, de Vos WM. Helsinki alert of biodiversity and health. Annals of medicine. 2015;47:218–225. doi: 10.3109/07853890.2015.1010226. [DOI] [PubMed] [Google Scholar]
- Wachholz S, Knorr A, Mengert L, Plumper J, Sommer R, Juckel G, Friebe A. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. 2017;326:165–172. doi: 10.1016/j.bbr.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer's disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology. 2014;141:340–344. doi: 10.1111/imm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- Yang, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. 2017;280:41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nature medicine. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight days after the third injection, gene expression of (A) anti-inflammatory mediators and (B) markers of alternative macrophage activation were measured in amygdala. Data are presented as the mean + s.e.m. N = 9–12 animals per experimental group. ND = non-detected.
Animals received 3 injections of either vehicle or M. vaccae (0.1 mg, s.c.). Eight d after the third injection, gene expression of (A) Nts, (B) Nr2f2 and (C) Cd3e was measured in dorsal, intermediate, and ventral hippocampus. Data are presented as the mean + s.e.m. N = 8 animals per experimental group. (A) Dorsal versus ventral and intermediate hippocampus, *** p < 0.001. (B) Ventral versus dorsal and intermediate hippocampus, *** p < 0.001. (C) Dorsal versus ventral and intermediate hippocampus, *** p < 0.001. Vehicle versus M. vaccae in dorsal hippocampus, * p < 0.05.