Abstract
Background:
Many pregnant women in the United States have suboptimal vitamin D, but the impact on infant development is unclear. Moreover, no pregnancy-specific vitamin D recommendations have been widely accepted.
Aims:
Given the ubiquitous expression of vitamin D receptors in the brain, we investigated the association between early prenatal plasma 25-hydroxyvitamin D [25(OH)D] concentrations and children’s social and emotional development in the Newborn Epigenetic Study, a prospective study of pregnancies from 2009 to 2011 in Durham, North Carolina.
Methods:
We measured 25(OH)D concentrations in 1st or 2nd trimester plasma samples and categorized 25(OH)D concentrations into quartiles. Covariates were derived from maternal questionnaires. Mothers completed the Infant Toddler Social-Emotional Development Assessment when children were 12–24 months of age. We used multivariable linear regression to evaluate associations between 25(OH)D and specific behavior scores, adjusted for season of blood draw, maternal age, education, parity, smoking, marital status, pre-pregnancy BMI, and infant gender. We investigated effect-measure modification by race/ethnicity.
Results:
Of the 218 mother-infant pairs with complete data, Black mothers had much lower 25(OH)D concentrations as compared to White and Hispanic mothers. After adjustment, lower prenatal 25(OH)D was associated with slightly higher (less favorable) Internalizing scores among White children, but lower (more favorable) Internalizing scores among Black and Hispanic children. Lower prenatal 25(OH)D also appears to be associated with higher (less favorable) Dysregulation scores, though only among White and Hispanic children.
Conclusions:
Though imprecise, preliminary results warrant further investigation regarding a role for prenatal vitamin D on children’s early social and emotional development.
Keywords: Epidemiology, 25(OH)D, neurodevelopment, prenatal nutrition
1. Introduction
Approximately half of pregnant women in the United States have suboptimal vitamin D levels, and black women are disproportionally affected [1–4]. Vitamin D is a prohormone derived from both diet and UVB light exposure and plays an essential role in bone development. Recent epidemiologic studies suggest that insufficient prenatal vitamin D increases risk of adverse pregnancy outcomes, including preeclampsia, pregnancy loss, and fetal growth restriction [5–8]. However, very little research has focused on the role of prenatal vitamin D in neurodevelopmental processes of young children. Although the exact mechanism through which vitamin D might affect neurodevelopment is unclear, vitamin D receptors are widespread in human neurons and glial cells, justifying the desire to examine the effect of vitamin D on neurocognitive function in children [9, 10]. Moreover, the bioactive forms of vitamin D play important roles in modulating inflammation and immune response, which are known to affect fetal brain development [11, 12].
Current guidelines for vitamin D sufficiency are inconsistent among governing bodies (IOM, Endocrine Society, etc.) and were historically developed for bone health [13]. Moreover, no pregnancy-specific guidelines have been widely implemented. Understanding the effect of prenatal vitamin D on infant neurodevelopment is essential to informing pregnancy-specific guidelines for vitamin D supplementation.
Pre-clinical studies in rodents show that vitamin D plays an important role in fetal brain development, placental inflammation, and regulation of serotonin [14–18]. A growing body of epidemiologic studies on prenatal vitamin D and offspring neurodevelopment have produced mixed results. Vitamin D deficiency was associated with language delays in an Australian cohort (ages 5 and 10), but not with cognitive delays in an American cohort (ages 4 and 7) [19, 20]. A recent cohort study also found reduced risk of Attention Deficit/Hyperactivity Disorder symptoms in children whose mothers had higher levels of vitamin D during pregnancy [21].
No studies to our knowledge have investigated the association of prenatal vitamin D and social-emotional developmental outcomes during infancy. Clarifying the link between prenatal vitamin D status and neurodevelopment outcomes in offspring could help inform etiology of childhood neurodevelopmental problems as well as inform prevention strategies to reduce the prevalence of such conditions. To address this gap, we investigated early pregnancy plasma 25(OH)D concentrations and subsequent scores on the Infant Toddler Social Emotional Assessment (ITSEA) among offspring at age 1 year in a racially-diverse sample.
Recent epidemiologic studies suggest the etiologic effects of vitamin D on health outcomes differ by race/ethnicity [6, 8, 22, 23]. Moreover, a recent study reported that effects of prenatal vitamin D concentrations on birth outcomes is modified by race/ethnicity [24]. Therefore, we additionally investigated if the relationship between early prenatal vitamin D concentrations and infant social-emotional development scores was modified by race/ethnicity.
2. Materials and Methods
2.1. Study population
The Newborn Epigenetic Study (NEST) is a prospective study of women and their children in Durham, NC, USA. Details on the NEST study design are available elsewhere [25]. In brief, between 2009 and 2012, 1700 pregnant women were recruited at their first prenatal visit from three Duke Maternal Fetal Medicine prenatal clinics. Women were eligible to participate if they were 18 years of age or older, HIV negative, and planned to continue prenatal care in the aforementioned clinics. Participating mothers completed a detailed interview at the time of enrollment, provided a blood sample during 1st or 2nd trimester (median: 11.3 weeks gestation, IQR: 8.9–15.6), and provided access to medical records for delivery information. Mothers were followed 1 year post-partum to collect information on their health and the health and development of their children. Mother-infant dyads were eligible for the present study if they had both prenatal 25(OH)D data and had completed the ITSEA when the child was one year old. The NEST study was approved by the Duke University IRB and written consent was obtained from all mothers participating as study subjects [25].
2.2. Vitamin D measurement
Vitamin D is derived from diet and from endogenous cutaneous synthesis that requires sunlight exposure [26]. The 25(OH)D biomarker reflects the total bioavailable vitamin D, regardless of how it was derived. In humans, the two key vitamin D compounds are ergocalciferol (D2) and cholecalciferol (D3), which together comprise 25(OH)D in blood. A single-sample blood draw from mothers during 1st or 2nd trimester was used to assay the 25(OH)D biomarker. Resources allowed us to assay 25(OH)D only for samples collected in the first two years of the NEST study. To determine plasma concentrations of 25(OH)D, we used the commercially-available immunodiagnostic system (IDS Fountain Hills AZ) enzyme immunoassay (cat AC57F1) (Craft Technologies, Inc, Wilson, NC, USA). Twenty-five microliters of plasma were used to perform the immunoassay, as per manufacturer instructions. This assay has a 96-well microplate coated with a sheep anti-human 25(OH)D antibody. The kit provides calibrants to create the standard curve and two controls. The colorimetric reaction was read at 450 nm using a Molecular Devices Versamax Tunable microplate reader, serial number BO2478, and data was analyzed using the Softmax Pro version 3.1.
2.3. ITSEA scores
Figure 1 provides the directionality and potential range of scores for each ITSEA domain to provide context when interpreting effect estimates. Mothers returned the completed the ITSEA questionnaire about their infants at the 1-year Well Child visit or by mail. The ITSEA is a validated measure of social and emotional development for children aged 12–36 months [27]. The assessment is composed of 66 behaviors that can be problematic if they occur too frequently or infrequently. Mothers rate each behavior as 0 (not true/rarely), 1 (somewhat true, sometimes) or 2 (very true/often). Scores are then calculated for four primary domains of behavior: externalizing, internalizing, dysregulation, and social-emotional competence. Previous studies suggest high test-retest reliability and criterion validity for these ITSEA domains [27, 28]. The ITSEA also has two domain scores that potentially reflect early signs of Autism Spectrum Disorder (ASD) symptoms: ASD problem behavior and ASD social competence. These domain scores tend to identify social traits similar to those targeted in the Modified Checklist for Autism in Toddlers (M-CHAT), an ASD screening tool, but to date have not been used to screen or diagnose ASD [29, 30] (see Figure 1).
Figure 1.
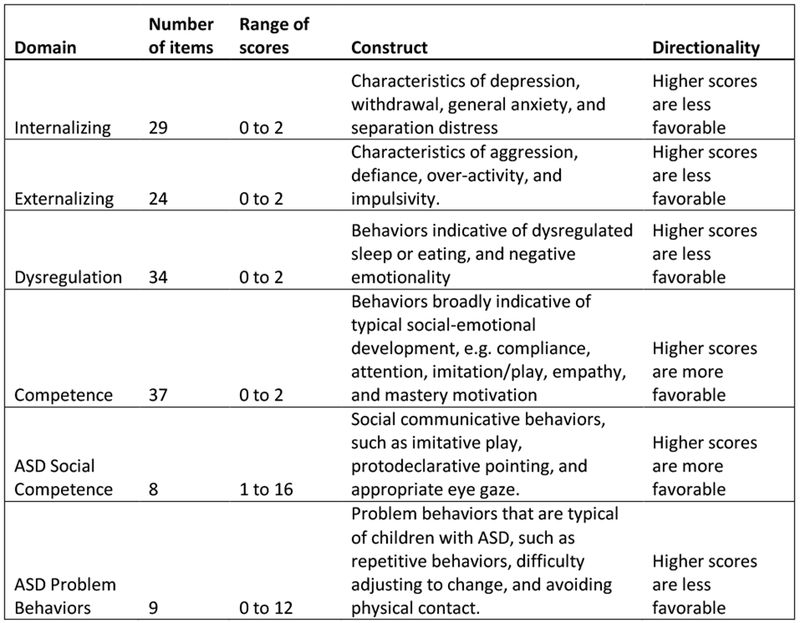
Description of ITSEA Domain Scores and Constructs
2.4. Covariates
At enrollment, mothers completed a detailed questionnaire providing demographic and lifestyle factors. Information on gestational age and birth weight were collected from medical records.
2.5. Statistical Analysis
Current recommendations for vitamin D were developed for bone health; cut-points to indicate insufficiency or deficiency for other outcomes like neurodevelopment are unknown. Moreover, no pregnancy-specific cut-points have been widely accepted. Therefore, we categorized 25(OH)D concentrations into quartiles to best fit the exploratory nature of this analysis (see Figure 2). Regression coefficients and 95% confidence intervals were estimated using multiple linear regression in SAS 9.3 (SAS Institute, Cary, NC). A directed acyclic graph was used to determine potential confounders [31]. All results are adjusted for season of blood draw (winter, spring, summer, or fall), self-identified race/ethnicity (White, Black, Hispanic), maternal education (<high school or high school graduate/GED, some college or college graduate), maternal age at delivery (<34, ≥34), marital status at enrollment (unmarried, married), baseline prenatal vitamin use (yes, no), parity (nulliparous, non-nulliparous), pre-pregnancy BMI (<25 kg/m2, ≥25 kg/m2), and smoking during pregnancy (never, ever). Results are also adjusted for infant age at assessment (continuous) and infant sex (male, female) since ITSEA scores are expected to differ by age and sex. Note that birthweight and gestational age at birth are hypothesized to be on the causal pathway and are therefore not included in the adjustment set. Potential confounders were evaluated for assumptions of linearity and dose-response to minimize model misspecification. We also conducted analyses stratified by race/ethnicity (White, Black, Hispanic) to assess potential effect-measure modification.
Figure 2.
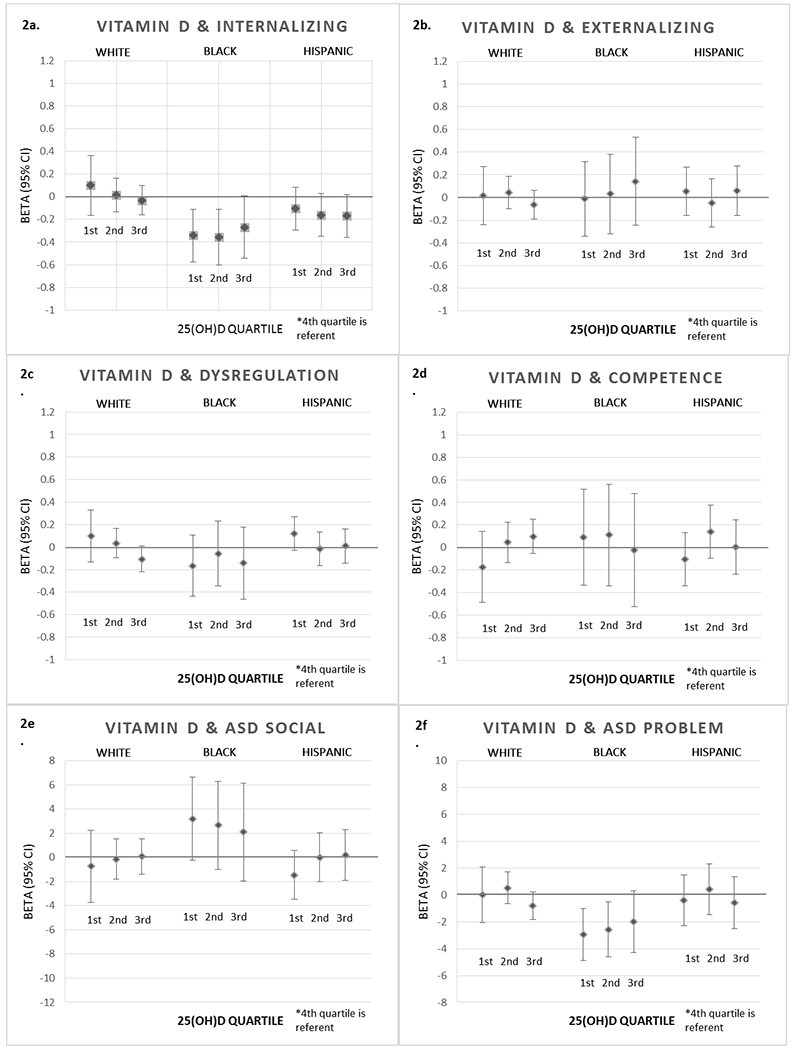
Prenatal Vitamin D Quartiles and ITSEA Scores at Age 1 in the NEST Cohort
*Results adjusted for maternal education, maternal age, parity, pre-pregnancy BMI, prenatal vitamin use, smoking during pregnancy, marital status, infant gender, infant age at assessment, and season of blood draw.
*4th quartile of 25(OH)D is referent for all estimates.
3. Results
3.1. Study population
In sum, 218 mother-infant dyads were included in this analysis. The mother-infant dyads eligible for this analysis were similar demographically to the larger NEST cohort in distributions of race/ethnicity and all measured confounders [25]. Moreover, the study population is similar demographically to the population of Durham women of reproductive age [32]. Most infants were assessed at 12 months, but about 20% of infants were assessed between 13–24 months (mean age=14.3 months, SD=3.3 months). The study population included 36% who self-identified as White, 30% Black, and 34% Hispanic mothers. Mothers varied greatly in educational attainment and marital status. Over half of mothers were overweight or obese at last menstrual period (see Table 1).
Table 1.
Baseline Characteristics of Study Population by Vitamin D Quartile
| Characteristic | 1st quartile (n=56) |
2nd quartile (n=55) |
3rd quartile (n=51) |
4th quartile (n=56) |
||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Race | ||||||||
| White | 3 | 3.8 | 16 | 20.3 | 24 | 30.4 | 36 | 45.6 |
| Black | 35 | 53 | 18 | 27.3 | 8 | 12.1 | 5 | 7.6 |
| Hispanic | 18 | 24.6 | 21 | 28.8 | 19 | 26 | 15 | 20.6 |
| Education | ||||||||
| < High school grad | 20 | 35.7 | 18 | 32.7 | 12 | 32.7 | 10 | 17.9 |
| High school grad/GED | 12 | 21.4 | 13 | 23.6 | 6 | 11.8 | 11 | 20 |
| Some college | 11 | 19.6 | 7 | 12.7 | 4 | 7.8 | 4 | 7.1 |
| College graduate | 13 | 23.2 | 16 | 29.1 | 29 | 56.9 | 31 | 55.4 |
| Marital status | ||||||||
| Married | 15 | 26.8 | 18 | 32.7 | 31 | 60.8 | 37 | 66.1 |
| Not married | 41 | 73.2 | 37 | 67.3 | 20 | 39.2 | 19 | 33.9 |
| Parity | ||||||||
| Nulliparious | 21 | 37.5 | 23 | 41.8 | 25 | 49 | 18 | 32.1 |
| Non-nulliparious | 35 | 62.5 | 32 | 58.2 | 26 | 51 | 38 | 67.9 |
| Pre-pregnancy BMI | ||||||||
| Underweight: <18.5 | 2 | 3.6 | 2 | 3.6 | 3 | 5.9 | 3 | 5.4 |
| Normal: 18.5-24.9 | 11 | 19.6 | 10 | 18.2 | 26 | 51 | 30 | 53.4 |
| Overweight: 25.0-29.9 | 20 | 35.7 | 22 | 40 | 16 | 31.4 | 12 | 21.4 |
| Obese: ≥30 | 23 | 41.1 | 21 | 38.2 | 6 | 11.8 | 11 | 19.6 |
| Smoking during pregnancy | ||||||||
| Ever | 9 | 16.1 | 4 | 7.3 | 2 | 3.9 | 5 | 8.9 |
| Never | 44 | 78.6 | 49 | 89.1 | 49 | 96.1 | 50 | 89.3 |
| No response | 3 | 5.3 | 2 | 3.6 | 0 | 0 | 0 | 0 |
| Prenatal vitamin use | ||||||||
| Yes | 34 | 60.7 | 31 | 56.4 | 30 | 58.8 | 37 | 66.1 |
| No | 22 | 39.3 | 22 | 40 | 21 | 41.2 | 19 | 33.9 |
| No response | 0 | 0 | 2 | 3.6 | 0 | 0 | 0 | 0 |
CI = confidence interval, SD = standard deviation, BMI = body mass index
3.2. Vitamin D
The study population mean plasma 25(OH)D concentration was 41.5 nmol/L (SD: 14.3 nmol/L). Prenatal plasma 25(OH)D concentrations varied greatly by race/ethnicity. Black women had a much lower 25(OH)D distribution, compared to White and Hispanic women. Hispanic women had a slightly lower 25(OH)D distribution compared to White women. Women with lower 25(OH)D concentrations were more likely to be unmarried, less educated, and overweight or obese compared with women with higher 25(OH)D concentrations (see Table 1).
3.3. ITSEA scores
The study population means for each ITSEA outcome were: Internalizing: 0.46 (SD: 0.25); Externalizing: 0.34 (SD: 0.25); Dysregulation: 0.33 (SD: 0.22); Competence: 1.08 (SD: 0.37); ASD problem behavior: 2.86 (SD: 2.59); ASD social competence: 10.74 (SD: 2.98). Note that each ITSEA outcome differs in directionality and range of scores (see Figure 1). Women with higher educational attainment had infants with more favorable scores, especially on the Externalizing, Dysregulation, and ASD Problem Behavior domains. Favorable scores were slightly associated with marital status, but not with maternal age, prenatal vitamin use, or parity. Older infants were slightly more likely to have favorable scores on the Internalizing, Externalizing, and Dysregulation domains.
3.4. Associations between prenatal vitamin D concentrations and ITSEA scores
Race/ethnicity strongly modified the association between prenatal vitamin D concentrations and ITSEA scores, thus we present race-specific results despite imprecision due to small sample size in each racial/ethnic category. A few patterns appeared for the relationship between prenatal 25(OH)D concentrations and specific domain scores. After adjustment, lower prenatal 25(OH)D was associated with higher (less favorable) Internalizing scores among White infants, but the opposite association was observed among Black and Hispanic infants. The lowest 25(OH)D quartile appears associated with higher (less favorable) Dysregulation scores, though only among White and Hispanic children. No strong patterns emerged with prenatal 25(OH)D and Externalizing or Competence scores. Among Black infants only, lower prenatal 25(OH)D was associated with higher (more favorable) ASD Social Competence scores and lower (more favorable) ASD Problem scores (see Figure 2). Among White and Hispanic infants, the lowest 25(OH)D quartile was associated with lower (less favorable) ASD Social Competence scores.
4. Discussion
There is increasing recognition that social-emotional developmental delays in infancy, though difficult to measure, are important indicators of later neurodevelopmental issues in children [27, 28]. Most research on neurodevelopment focuses on motor, cognitive, and language development. Less attention has been paid to social-emotional development, which encompasses essential skills like handling upsetting situations, lack of aggressive behaviors, and interacting with peers [27, 28].
We examined the association between prenatal vitamin D levels and subsequent social-emotional developmental in 1-year-old infants in a racially-diverse pregnancy cohort in Durham, NC. Due to the explorative nature of this study and number of effect estimates presented, we focus on the overall pattern of results rather than individual estimates.
Results indicate a possible adverse association between low prenatal 25(OH)D concentrations and some aspects of social-emotional development, but not all (see Figure 2). Lower quartiles of 25(OH)D were associated with less favorable scores on the following ITSEA domains: Dysregulation (e.g. dysregulated sleep or eating) and ASD Social Competence (e.g. imitative play, eye contact). However, these adverse associations were observed only among White and Hispanic infants. Among Black infants, lower quartiles of 25(OH)D were associated with more favorable scores on the following ITSEA domains: Internalizing behaviors (e.g. anxiety, withdrawal), ASD Social Competence (e.g. imitative play, eye contact), and ASD Problem Behavior (e.g. repetitive behavior, difficulty adjusting to change). These results among Black infants are counter to our hypothesis; it is unclear why lower prenatal 25(OH)D would be associated with more favorable ITSEA scores. It is possible that unmeasured confounding or small samples sizes contribute to these counter-intuitive results. Note that the ASD-related scores are relatively new measures, and while they identify certain constructs of ASD, they are not validated as screening or diagnostic tools for ASD. The racial and ethnic diversity of the cohort allowed for investigation of effect-measure modification by race, but estimates are imprecise.
These results are somewhat consistent with the small epidemiologic literature on prenatal vitamin D and neurodevelopment: vitamin D is potentially associated with some aspects of neurodevelopment, but not all. A recent analysis of a large US pregnancy cohort from 1959–73 evaluated the relationship between prenatal vitamin D and a host of neurodevelopmental outcomes at ages 4 and 7. Crude associations with Bayley mental scores, Stanford-Binet IQ, WRAT academic scores, internalizing behavior, and externalizing behaviors were attenuated to null upon confounder adjustment [20]. In a large Australian pregnancy cohort from 1989–91, maternal vitamin D was associated with language delay at ages 5 and 10, but not with emotional development, which is inconsistent with our findings on emotional development [19]. However, the Australian cohort consisted of only White women, whereas our findings are from a racially-diverse cohort. Darker skin reduces vitamin D synthesis via sunlight, so 25(OH)D concentrations can vary greatly by race [33]. A Spanish pregnancy cohort from 2003–08 revealed an association between low prenatal vitamin D and ADHD-like symptoms [21]. ADHD characteristics track with the Externalizing ITSEA domain, for which we did not find an association with vitamin D. However, Morales et al. used a clinical verification of the outcome and measured ADHD-like symptoms when children were older. All aforementioned studies had similar methods for vitamin D measurement, but the timing of measurement during pregnancy and child age at neurodevelopmental assessment differed across studies.
Recent pre-clinical studies have revealed a few potential mechanisms for vitamin D’s role in brain development [9, 10, 14–18]. In mice models, vitamin D plays a role in brain cellular differentiation, proliferation, and apoptosis [18]. Vitamin D also regulates serotonin, a neurotransmitter that can act as a mood-regulator and is often disrupted in psychiatric conditions like ADHD and ASD [17]. Another potential mechanism is that vitamin D reduces general inflammation in the intrauterine environment, potentially reducing risk of adverse effects of inflammation on fetal brain development [16]. Animal research shows that vitamin D3 supplementation in pregnant mice greatly reduces placental inflammation [14, 15]. While animal studies have elucidated multiple potential mechanisms for vitamin D’s role in brain development, comparable studies in humans have yet to be conducted and mechanisms in humans remain unknown.
Our method of exposure assessment – plasma measurement of 25(OH)D – incorporates both food and UVB light sources. The plasma 25(OH)D biomarker has a half-life of approximately 15 days [34, 35]. However, we only had one biomarker per mother, which may not accurately reflect each mother’s vitamin D levels during the etiologically relevant window in pregnancy. Moreover, the etiologically relevant window in pregnancy for which exposure to vitamin D could impact neurodevelopment is unknown. Future studies would benefit from multiple 25(OH)D measurement over the course of pregnancy, or methods to impute the pattern of 25(OH)D over the course of pregnancy, to better ascertain true exposure. Additionally, recent studies suggest that vitamin D receptor (VDR) and vitamin D binding protein (VDBR) genes play in important role in synthesizing bioavailable vitamin D. Future studies could include measures of VDR and VDBR, in addition to circulating 25(OH)D measures, to better assess how much vitamin D is truly bioavailable.
While this study population is racially and ethnically diverse, our ability to evaluate the effect of vitamin D by race was limited. Since 25(OH)D among Black women was heavily skewed towards low concentrations, and 25(OH)D among White women were skewed towards high concentrations, exposure variability within a given racial group was limited. For example, very few Black women fell in the 4th quartile of 25(OH)D concentrations, thus greatly reducing precision of estimates comparing low to high 25(OH)D concentrations in this racial group. Future studies would benefit from greater vitamin D variability within racial groups, which could result from a larger sample size.
Though we controlled for important known confounders, these results could still be confounded by unmeasured factors. For example, broader nutritional status, socio-economic factors, or other lifestyle factors could confound this association. Maternal education and marital status could account for some of this confounding, but not all. Future studies should consider measuring and adjusting for broader social and economic confounders of this relationship.
5. Conclusions
Prenatal vitamin D is associated with some, but not all, measures of socio-emotional development among infants. Effects vary greatly by maternal race/ethnicity and sub-type of socio-emotional development. Understanding the role of prenatal vitamin D on fetal brain development is essential for guiding clinical recommendations for vitamin D supplementation among pregnant women. Since current recommendations for vitamin D are controversial and not specific to pregnant women, further research is needed to support evidence-based vitamin D recommendations for this population.
Brief Rationale:
Many pregnant women in the United States have suboptimal vitamin D, but the impact on infant development is unclear. We investigated the association between early prenatal plasma 25-hydroxyvitamin D [25(OH)D] concentrations and children’s social and emotional development in a prospective pregnancy cohort in Durham, North Carolina. Suboptimal vitamin D was associated with less favorable Internalizing and Dysregulation scores, though effects differed by race/ethnicity. Though future research is needed, results may influence guidelines for vitamin D sufficiency during pregnancy.
Acknowledgements:
We would like to thank Rachel Maguire (NEST data manager) and all the NEST participants for their contributions to this research.
Funding Details: This work was supported by the National Institute of Environmental Health Science (NIEHS grant R01ES016772, Hoyo PI) and the National Institute of Child Health and Development (NICHD T32 Reproductive, Perinatal, Pediatric Training Grant).
Sources of funding: NIEHS grant R01ES016772 (Hoyo PI); NICHD T32 Reproductive, Perinatal, Pediatric Epidemiology Training Grant
Appendix
Table 2.
Prenatal Vitamin D Quartiles and ITSEA Scores at Age 1 in the NEST Cohort
| ITSEA Domain | Maternal Race | Vitamin D Quartile | Beta Estimate | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Internalizing | White | 1st | 0.099 | −0.164 | 0.362 |
| 2nd | 0.016 | −0.132 | 0.164 | ||
| 3rd | −0.032 | −0.162 | 0.097 | ||
| Black | 1st | −0.342 | −0.574 | −0.110 | |
| 2nd | −0.358 | −0.604 | −0.112 | ||
| 3rd | −0.269 | −0.543 | 0.006 | ||
| Hispanic | 1st | −0.105 | −0.293 | 0.084 | |
| 2nd | −0.162 | −0.350 | 0.027 | ||
| 3rd | −0.171 | −0.362 | 0.020 | ||
| Externalizing | White | 1st | 0.018 | −0.239 | 0.274 |
| 2nd | 0.043 | −0.101 | 0.188 | ||
| 3rd | −0.065 | −0.192 | 0.061 | ||
| Black | 1st | −0.013 | −0.344 | 0.317 | |
| 2nd | 0.031 | −0.320 | 0.381 | ||
| 3rd | 0.143 | −0.247 | 0.533 | ||
| Hispanic | 1st | 0.054 | −0.162 | 0.269 | |
| 2nd | −0.049 | −0.264 | 0.167 | ||
| 3rd | 0.059 | −0.159 | 0.227 | ||
| Dysregulation | White | 1st | 0.101 | −0.131 | 0.332 |
| 2nd | 0.035 | −0.096 | 0.165 | ||
| 3rd | −0.106 | −0.220 | 0.009 | ||
| Black | 1st | −0.166 | −0.438 | 0.105 | |
| 2nd | −0.058 | −0.345 | 0.230 | ||
| 3rd | −0.143 | −0.463 | 0.178 | ||
| Hispanic | 1st | 0.121 | −0.028 | 0.270 | |
| 2nd | −0.016 | −0.165 | 0.133 | ||
| 3rd | 0.010 | −0.141 | 0.160 | ||
| Competence | White | 1st | −0.172 | −0.486 | 0.141 |
| 2nd | 0.045 | −0.131 | 0.222 | ||
| 3rd | 0.099 | −0.055 | 0.253 | ||
| Black | 1st | 0.090 | −0.336 | 0.516 | |
| 2nd | 0.110 | −0.341 | 0.562 | ||
| 3rd | −0.023 | −0.526 | 0.480 | ||
| Hispanic | 1st | −0.103 | −0.340 | 0.134 | |
| 2nd | 0.140 | −0.097 | 0.377 | ||
| 3rd | 0.005 | −0.234 | 0.245 | ||
| ASD Social Competence | White | 1st | −0.736 | −3.717 | 2.244 |
| 2nd | −0.151 | −1.831 | 1.530 | ||
| 3rd | 0.080 | −1.389 | 1.548 | ||
| Black | 1st | 3.191 | −0.237 | 6.619 | |
| 2nd | 2.657 | −0.975 | 6.289 | ||
| 3rd | 2.096 | −1.951 | 6.144 | ||
| Hispanic | 1st | −1.453 | −3.482 | 0.577 | |
| 2nd | −0.004 | −2.030 | 2.022 | ||
| 3rd | 0.183 | −1.918 | 2.284 | ||
| ASD Problem Behavior | White | 1st | 0.018 | −2.066 | 2.101 |
| 2nd | 0.529 | −0.645 | 1.704 | ||
| 3rd | −0.804 | −1.830 | −0.804 | ||
| Black | 1st | −2.936 | −4.881 | −0.991 | |
| 2nd | −2.570 | −4.631 | −0.510 | ||
| 3rd | −1.996 | −4.292 | 0.300 | ||
| Hispanic | 1st | −0.413 | −2.307 | 1.481 | |
| 2nd | 0.407 | −1.486 | 2.300 | ||
| 3rd | −0.574 | −2.491 | 1.343 | ||
Results adjusted for maternal education, maternal age, parity, pre-pregnancy BMI, prenatal vitamin use, smoking during pregnancy, marital status, infant gender, infant age at assessment, and season of blood draw.
4th quartile of 25(OH)D is referent for all estimates.
Footnotes
Declaration of Interest Statement: Authors have no conflicts of interest to report.
Conflict of Interest statement: Authors declare no conflicts of interest.
References
- 1.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA: Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 2002, 76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM: High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007, 137(2):447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF: Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007, 46(1):42–44. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C: Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol 2010, 202(5):429 e421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen LB, Jorgensen JS, Jensen TK, Dalgard C, Barington T, Nielsen J, Beck-Nielsen SS, Husby S, Abrahamsen B, Lamont RF et al. : Vitamin D insufficiency is associated with increased risk of first-trimester miscarriage in the Odense Child Cohort. Am J Clin Nutr 2015, 102(3):633–638. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar LM, Simhan HN, Catov JM, Roberts JM, Platt RW, Diesel JC, Klebanoff MA: Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold DL, Enquobahrie DA, Qiu C, Huang J, Grote N, VanderStoep A, Williams MA: Early pregnancy maternal vitamin D concentrations and risk of gestational diabetes mellitus. Paediatr Perinat Epidemiol 2015, 29(3):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, Marazita ML, Simhan HN: Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr 2010, 140(5):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soni M, Kos K, Lang IA, Jones K, Melzer D, Llewellyn DJ: Vitamin D and cognitive function. Scand J Clin Lab Invest Suppl 2012, 243:79–82. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland MK, Somerville MJ, Yoong LK, Bergeron C, Haussler MR, McLachlan DR: Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28k mRNA levels. Brain Res Mol Brain Res 1992, 13(3):239–250. [DOI] [PubMed] [Google Scholar]
- 11.Stolp HB: Neuropoietic cytokines in normal brain development and neurodevelopmental disorders. Mol Cell Neurosci 2013, 53:63–68. [DOI] [PubMed] [Google Scholar]
- 12.Keunen K, van Elburg RM, van Bel F, Benders MJ: Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res 2015, 77(1-2):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxmen A: Nutrition advice: the vitamin D-lemma. Nature 2011, 475(7354):23–25. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn JA, Hewison M, Wagner CL, Bulmer JN, Kilby MD: Immunological role of vitamin D at the maternal-fetal interface. J Endocrinol 2015, 224(3):R107–121. [DOI] [PubMed] [Google Scholar]
- 15.Chen YH, Yu Z, Fu L, Wang H, Chen X, Zhang C, Lv ZM, Xu DX: Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci Rep 2015, 5:10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, Equils O, Hewison M: Vitamin D and the regulation of placental inflammation. J Immunol 2011, 186(10):5968–5974. [DOI] [PubMed] [Google Scholar]
- 17.Patrick RP, Ames BN: Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J 2015, 29(6):2207–2222. [DOI] [PubMed] [Google Scholar]
- 18.Eyles DW, Burne TH, McGrath JJ: Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 2013, 34(1):47–64. [DOI] [PubMed] [Google Scholar]
- 19.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH: Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 2012, 129(3):485–493. [DOI] [PubMed] [Google Scholar]
- 20.Keim SA, Bodnar LM, Klebanoff MA: Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol 2014, 28(5):434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales E, Julvez J, Torrent M, Ballester F, Rodriguez-Bernal CL, Andiarena A, Vegas O, Castilla AM, Rodriguez-Dehli C, Tardon A et al. : Vitamin D in Pregnancy and Attention Deficit Hyperactivity Disorder-like Symptoms in Childhood. Epidemiology 2015, 26(4):458–465. [DOI] [PubMed] [Google Scholar]
- 22.Burris HH, Thomas A, Zera CA, McElrath TF: Prenatal vitamin use and vitamin D status during pregnancy, differences by race and overweight status. J Perinatol 2015, 35(4):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michos ED, Misialek JR, Selvin E, Folsom AR, Pankow JS, Post WS, Lutsey PL: 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. (1879-1484 (Electronic)). [DOI] [PMC free article] [PubMed]
- 24.Tian Y, Holzman C, Siega-Riz AM, Williams MA, Dole N, Enquobahrie DA, Ferre CD: Maternal Serum 25-Hydroxyvitamin D Concentrations during Pregnancy and Infant Birthweight for Gestational Age: a Three-Cohort Study. Paediatr Perinat Epidemiol 2016, 30(2):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, Kurtzberg J, Jirtle RL, Murphy SK: Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 2011, 11(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacis MM, Fortin CN, Zarek SM, Mumford SL, Segars JH: Vitamin D and assisted reproduction: should vitamin D be routinely screened and repleted prior to ART? A systematic review. J Assist Reprod Genet 2015, 32(3):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter AS, Briggs-Gowan MJ, Jones SM, Little TD: The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. J Abnorm Child Psychol 2003, 31(5):495–514. [DOI] [PubMed] [Google Scholar]
- 28.Briggs-Gowan MJ, Carter AS, Bosson-Heenan J, Guyer AE, Horwitz SM: Are infant-toddler social-emotional and behavioral problems transient? J Am Acad Child Adolesc Psychiatry 2006, 45(7):849–858. [DOI] [PubMed] [Google Scholar]
- 29.Gardner LM, Murphy L, Campbell JM, Tylavsky F, Palmer FB, Graff JC: Screening accuracy for risk of autism spectrum disorder using the Brief Infant-Toddler Social and Emotional Assessment (BITSEA). Research in Autism Spectrum Disorders 2013, 7(5):591–600. [Google Scholar]
- 30.Kruizinga I, Visser JC, van Batenburg-Eddes T, Carter AS, Jansen W, Raat H: Screening for autism spectrum disorders with the brief infant-toddler social and emotional assessment. PLoS One 2014, 9(5):e97630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland S, Pearl J, Robins JM: Causal diagrams for epidemiologic research. Epidemiology 1999, 10(1):37–48. [PubMed] [Google Scholar]
- 32.Services NCDoHaH: 2011 County Health Data Book. North Carolina Community Health Assessment Process; In.; 2011. [Google Scholar]
- 33.Harris SS: Vitamin D and African Americans. J Nutr 2006, 136(4):1126–1129. [DOI] [PubMed] [Google Scholar]
- 34.Jones G: Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008, 88(2):582S–586S. [DOI] [PubMed] [Google Scholar]
- 35.Mallah EM, Hamad MF, Elmanaseer MA, Qinna NA, Idkaidek NM, Arafat TA, Matalka KZ: Plasma concentrations of 25-hydroxyvitamin D among Jordanians: Effect of biological and habitual factors on vitamin D status. BMC Clin Pathol 2011, 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]


