Abstract
Over the course of an animal’s lifespan, there is a protracted breakdown in basic homeostatic functions. Stressors (both psychological and physiological) can accelerate this process and compromise multiple homeostatic mechanisms. For example, both stress and aging can modulate neuroinflammatory function and cause a primed phenotype resulting in a heightened neuroinflammatory profile upon immune activation. Microglia, the brain’s resident myeloid cell, produce “silent” immune machinery in response to stress and aging that does not cause immediate immune activation; rather, these changes prime the cell for a subsequent immune insult. Primed microglia exhibit a hyperinflammatory response upon immune activation that can exacerbate pathology. In this review, we will explore parallels between stress- and aging-induced neuroinflammatory priming. First, we will provide a background on the basic principles of neuroimmunology. Next, we will discuss evidence that neuroinflammatory responses become primed in the context of both stress and aging. We will also describe cell-specific contributions to neuroinflammatory priming with a focus on microglia. Finally, common mechanisms underlying priming in the context of stress and aging will be discussed: these mechanisms include glucocorticoid signaling; accumulation of danger signals; dis-inhibition of microglia; and breakdown of circadian rhythms. Overall, there are multifarious parallels between stress- and aging-elicited neuroinflammatory priming, suggesting that stress may promote a form of premature aging. Further unravelling mechanisms underlying priming could lead to improved treatments for buffering against stress- and aging-elicited behavioral pathologies.
I. Background
The concept that stress and aging are interrelated has pervaded the gerontology literature in two main forms (Sapolsky et al., 1986). First, aging activates stress response pathways in a maladaptive, chronic manner, leading to reduced adaptivity to stress in senescence. Consequently, physiological systems in aged animals inadequately respond to stressors (Haigis and Yankner, 2010). For example, aged and young animals typically show comparable thermoregulation at baseline, but aged animals exhibit thermoregulatory impairments when heat- or cold-challenged (Degroot and Kenney, 2007; Tournissac et al., 2017). Second, chronic stress accelerates the aging process. Selye (Selye and Tuchweber, 1976) and later Sapolsky (Sapolsky et al., 1986) postulated that prolonged stress prematurely depletes an organisms energy reserves, accelerating the onset of senescence. Experimental evidence supports this theory. For example, hypocortisolism, which can develop from chronic stress exposure, is associated with shortened telomere length, a hallmark of aging (Wikgren et al., 2012).
The aging process is characterized by a protracted breakdown in basic homeostatic functions over the course of the lifespan. Stress can accelerate this process and compromise multiple homeostatic mechanisms. Of special interest here, aging and stress both have profound effects on neuroimmune regulation. For example, young animals maintain an adaptive balance between pro- and anti-inflammatory mechanisms in the brain: the healthy adult immune system is not activated at baseline but is poised for an effective immune response to infection or damage. In contrast, with aging, this balance shifts towards a potentially pathological sensitized neuroimmune state at baseline (Fonken et al., 2016b). Similarly, exposure to acute and chronic stressors can release inhibitory mechanisms that regulate immune cell activity in the central nervous system (CNS), leading to heightened neuroinflammatory responses to immune challenge (Frank et al., 2018).
This shift towards a baseline sensitized inflammatory phenotype has been termed neuroinflammatory “priming”. Priming is defined as a process whereby an antecedent condition or prior exposure to a stimulus potentiates the immune response to a subsequent condition or stimulus. In particular, priming of the neuroinflammatory response is attributed to microglia, the major CNS immune cell. Primed microglia exhibit a much stronger response to an inflammatory stimulus than that observed in stimulus-naïve microglia. Indeed, aging and stressors (both physiological and psychological) cause microglia to develop a primed phenotype, resulting in a protracted neuroinflammatory profile upon immune activation.
Here, we will explore the common mechanisms that mediate stress and aging induced neuroinflammatory priming. First, we will provide a brief background on some basic principles of neuroimmunology. Next, we will discuss cell specific contributions to neuroinflammatory priming with a focus on microglia. We will then explore evidence that neuroinflammatory responses become primed in the context of stress and aging with a focus on the common mechanisms that underlie priming in these two conditions. Finally, we will conclude by discussing the translational relevance of neuroinflammatory priming.
II. Key principles of neuroimmunology
Del Rio-Hortega provided a comprehensive characterization of the brain’s non-neuronal cellular components about 100 years ago. In particular, del Rio-Hortega was the first to identify and accurately assess the origin and role of microglia; he proposed that these cells were of mesodermal origin and helped clear debris during CNS pathology (Somjen, 1988). A number of diverse functional roles have since been attributed to microglia. We now know that glial cells are present in healthy unstimulated brain and regulate basic homeostatic functions including synaptic development and pruning (Stogsdill and Eroglu, 2017; Wu et al., 2015), neuronal migration (Deverman and Patterson, 2009), and progenitor cell differentiation (Gonzalez-Perez et al., 2010). Microglia form and function are tightly regulated in the CNS to help regain homeostasis following activation. However, prolonged or exaggerated immune activation can cause maladaptive neuroinflammatory changes, or as del Rio-Hortega described, cause microglia to take on the phenotype of “voracious monsters”. This introductory section will review the basic constituents of the brain’s immune system and describe how immune activity is communicated to the CNS.
IIA. Immune to brain signaling
Neuroimmune activation can be induced by signals originating both inside and outside the CNS. Peripheral immune stimulation dramatically alters neural activity and induction of cytokines in the CNS (Dantzer et al., 2008). Exposure to pathogens or injury activates pattern recognition receptors (PRRs), that function as ‘danger’ sensors on peripheral innate immune cells, to initiate inflammatory cascades. PRRs bind both pathogen-associated molecular patterns (PAMPs), which are evolutionarily conserved molecular motifs from microbial organisms, as well as danger-associated molecular patterns (DAMPs), which are endogenous cellular products that are released following injury or stress. DAMPS include proteins, purine metabolites, DNA, and RNAs that are either typically found inside the cell (so once extracellular they signal damage) or are mediators that are secreted actively to drive inflammation (reviewed in (Schaefer, 2014). In response to PRR activation, innate immune cells including macrophages, monocytes, and neutrophils secrete inflammatory mediators such as pro-inflammatory cytokines [(e.g. interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and interferon γ (IFNγ )]. The blood-brain barrier (BBB) generally prevents peripheral pathogens from entering the brain and directly activating CNS immune cells (Banks, 2015); however, inflammatory signals can be communicated from the periphery to the CNS through various pathways, including neural (e.g. vagus) and blood-borne (e.g. circulating PAMPs reach the circumventricular organs) routes (reviewed in (Dantzer et al., 2008).
IIB. Immune response in the CNS
In response to peripheral cytokines, a variety of changes occur in brain including de novo production of cytokines, chemokines, reactive oxygen species and secondary messengers that propagate CNS inflammation (Hansen et al., 1998; van Dam et al., 1992). These mediators are produced by resident CNS glia (microglia and astrocytes), endothelial cells, and peripherally-derived immune cells. The detection and propagation of an immune signal throughout the CNS causes a suite of behavioral and physiological modifications, collectively known as the sickness response (Dantzer et al., 2008). Behavioral changes associated with increased inflammatory molecules within the CNS include reduced food and water intake, decreased exploration and social behavior, hyperalgesia (pain hypersensitivity) and global changes in mood and cognition. Overall, the sickness response represents a shift in the motivational state of an organism and is considered highly adaptive and critical for host defense (Hart, 1988). This response, however, can become pathological when immune cell activation in the CNS is exaggerated or persists long-term, such as in neurodegenerative diseases.
In addition to immune activation originating peripherally, CNS-localized injury and disease states can also lead to pathological persistent neuroinflammation. For example, spinal cord injury causes a robust CNS immune response with activation of CNS glia and recruitment of peripheral inflammatory cells to the lesion site (Popovich et al., 1997). Unfortunately, after CNS injury, the persistent inflammatory response can become increasingly pro-inflammatory over time, rather than repairing and resolving the injury, as in the periphery (Gaudet et al., 2017; Kigerl et al., 2009). Indeed, multiple CNS pathologies can be caused or exacerbated by neuroinflammation, including multiple sclerosis, ischemic stroke, Parkinson’s disease, and Alzheimer’s disease (Dendrou et al., 2015; Perry and Holmes, 2014; Ritzel et al., 2016).
IIC. Dynamic changes in microglia phenotype
Microglia are phagocytic myeloid cells that are distributed throughout the brain parenchyma and are the major source of cytokines and other inflammatory molecules in the CNS (Ransohoff and Perry, 2009). Microglia functions mirror those of tissue-resident macrophages but differ in a few key ways. Other types of myeloid cells in the brain, including meningeal, choroid plexus, and perivascular macrophages (Butovsky et al., 2014; Gautier et al., 2012), typically reside outside the parenchyma and have distinct developmental origins. Microglia are derived from progenitors in the yolk sac that colonize the brain around embryonic day 8 in mice (Alliot et al., 1999). Unlike monocytes, which continuously renew from bone marrow hematopoietic stem cells, microglia persist throughout adulthood by a self-renewal process (Ajami et al., 2007; Askew et al., 2017; Tay et al., 2017). Microglia numbers are tightly maintained in the adult brain by spatial and temporal coupling of proliferation and apoptosis (Askew et al., 2017). Undefined local signals cause microglia to develop region-specific phenotypes (variations in anatomical features, lysosome content, membrane properties, and transcriptomic profile) during the second postnatal week (De Biase et al., 2017). These region-specific phenotypes can cause microglia from different brain regions to demonstrate functional differences. For example, Grabert et al found that microglia isolated from the cerebellum, as compared to the cortex, have enhanced phagocytic activity ex vivo (Grabert et al., 2016). Microglia continuously assess their surrounding microenvironment via extension, withdrawal, and reformation of long cellular processes (Nimmerjahn et al., 2005). This active surveying of the microenvironment likely serves a housekeeping function, enabling microglia to effectively monitor and clear accumulated metabolic products and tissue debris.
Various homeostatic perturbations in the CNS can trigger microglia activation. Microglia that detect a homeostatic disturbance or “danger” signal can undergo rapid morphological and functional changes in order to: (1) phagocytose microbes and cellular debris; (2) migrate or proliferate to increase density in specific regions (Tay et al., 2017); and (3) produce and secrete inflammatory molecules (Yirmiya et al., 2015). Phenotypic shifts in microglia activity occur across a broad spectrum in the sense that microglia can synthesize a number of both pro- and anti-inflammatory cytokines and release other molecular mediators (e.g. DAMPs).
Microglial activation in the adult CNS is tightly controlled by endogenous signaling. Notably, unstimulated microglia express much lower levels of cytoplasmic and cell surface molecules than do other types of tissue-resident macrophages, gating their transition to a pro-inflammatory state (Gautier et al., 2012). Exposure to neuronal cell-surface and soluble factors maintain microglia in a comparatively quiescent immunophenotype compared to other myeloid cells (Biber et al., 2007). The tight regulation of microglia by molecular factors in the CNS likely evolved to prevent microglia from damaging brain tissue, which is less susceptible to infection, but has more limited capacity for repair and regeneration than peripheral tissue.
III. Characteristics of neuroinflammatory priming
Before discussing priming phenomena in detail, it is important to consider the conceptual distinction between neuroinflammatory or in vivo priming and microglial or ex vivo priming. In the neuroimmune literature, this distinction is sometimes blurred, which might lead to erroneous conclusions. As mentioned above, in the context of immune-related phenomena, priming is a process whereby an antecedent condition or prior exposure to a stimulus potentiates the immune response to a subsequent stimulus. Thus, neuroinflammatory priming necessarily involves examining the effects of an antecedent condition such as aging on the immune response in the brain to a subsequent stimulus such as intra-peritoneal injection of the bacterium E. coli., which elicits a robust pro-inflammatory response in the brain via immune-to-brain signaling (see section IIA). As explored in detail below, the neuroinflammatory response to E. coli is potentiated in aged animals compared to young animals, which demonstrates that aging serves as an antecedent priming condition (Barrientos et al., 2010). However, because E. coli was administered in vivo, determination of the cellular substrate(s) primed by aging is not possible under these experimental conditions. For example, the aging-induced potentiation of the neuroinflammatory response might be driven through enhanced immune-to-brain signaling via E. coli signaling at primed peripheral immune substrates (e.g. macrophages, monocytes and dendritic cells). Thus, microglia may be simply exhibiting a heightened pro-inflammatory response to increased peripheral pro-inflammatory drive via either neural (i.e. vagal) or humoral pathways. To definitively determine whether a cellular substrate is primed by an antecedent condition, it is necessary to directly expose a purified cell type to an immune challenge ex vivo. For example, highly pure hippocampal microglia were isolated from aged and young animals, and directly exposed to lipopolysaccharide (LPS; a cell wall component of gram-negative bacteria; E. coli) ex vivo. Microglia from aged animals exhibited a potentiated pro-inflammatory response to LPS compared to the response observed in microglia from young animals (Frank et al., 2010b) demonstrating that aging does induce a primed activation state in hippocampal microglia (Fig 1). Thus, it is important to consider the possible cellular components contributing to a primed neuroinflammatory response.
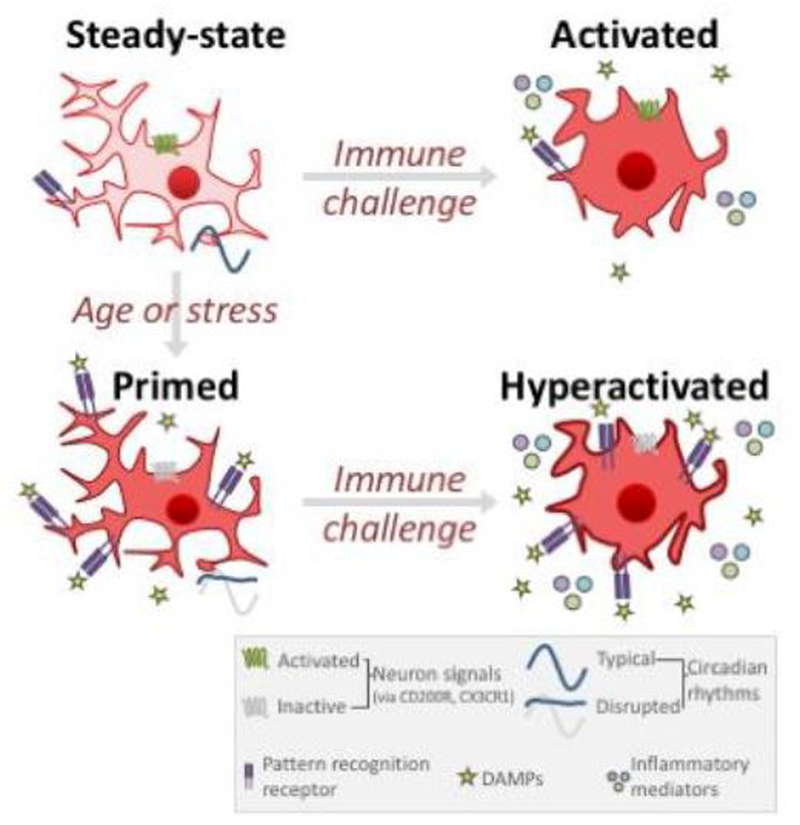
Figure 1. Microglial priming during aging and stress.
In the healthy CNS, a steady-state inactive microglial phenotype is associated with limited pattern recognition receptor expression (and low extracellular damage-associated molecular pattern (DAMP) expression), activation of neuron-microglia signaling pathways, and typical daily rhythms in circadian and inflammatory genes. During aging or after stress, microglia develop a “primed” phenotype that does not increase effector function but sensitizes the cells to subsequent stimulation. Primed microglia upregulate pattern recognition receptors, have more DAMPs in the extracellular environment, and de-activated neuron-microglia signaling (dis-inhibition of microglial). When primed microglia experience an immune challenge, the cell responds with an exaggerated inflammatory response that can be prolonged and can exacerbate pathology. Processes shown here represent key common mechanisms driving microglia priming; please see text for more details.
IIIA. Microglia priming
Microglia have multiple roles in the CNS and functional and morphological changes occur across a broad spectrum of activation states resulting in varying immunophenotypes and cytokine profiles (De Biase et al., 2017; Grabert et al., 2016; Ransohoff and Perry, 2009). For instance, microglia challenged with an initial minor immune stimulus can become primed – that is, predisposed to activation (Frank et al., 2016; Perry and Holmes, 2014) (Fig 2). Primed microglia maintain an intermediate inflammatory state characterized by shortened processes and expression of cell surface markers similar to activated microglia, but without producing appreciable secretion of inflammatory cytokines (Frank et al., 2007). Importantly, priming makes microglia susceptible to subsequent inflammatory stimuli. That is, primed microglia exhibit an exaggerated and/or prolonged inflammatory response to a subsequent immune challenge (Frank et al., 2007; Frank et al., 2010b). This secondary immune stimulus can arise from either peripheral or central sources of inflammation. For example, in rodents exhibiting microglia priming, injury to the brain or periphery can induce both prolonged neuroinflammatory and behavioral changes (Barrientos et al., 2012; Fenn et al., 2014a).
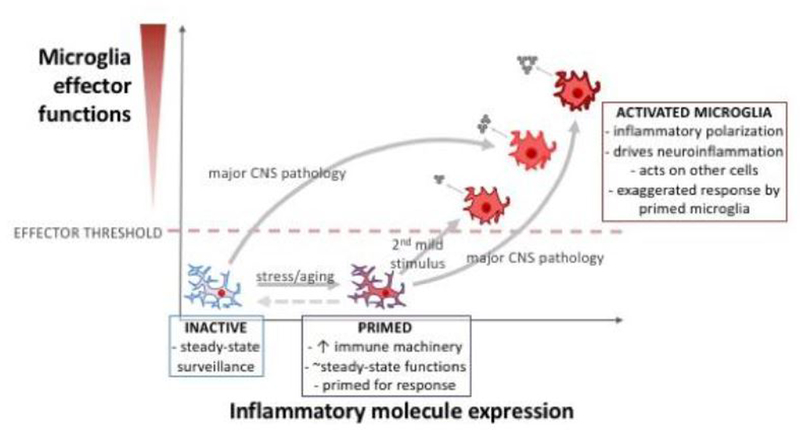
Figure 2. Microglial activation occurs along a continuum.
In healthy adult CNS tissue, microglia exist in an inactive state: these microglia survey the CNS microenvironment for infection or damage, and are involved in homeostatic regulation (e.g., synapse modulation, phagocytosis of normal debris, etc.). Following certain physiological challenges (such as stress or aging), microglia can take on a “primed” phenotype. Primed microglia modulate immune machinery involved in an inflammatory response (e.g., increased MHC II, NOD-like receptors, purinergic receptors; see text), but do not substantially increase their inflammatory effector phenotype (i.e., without further stimulus, primed microglia do not release inflammatory molecules). However, if microglia in a primed state are subjected to a stimulus, these primed microglia mount an exaggerated activated response that is maladaptive and can exacerbate pathology. Major CNS pathology (e.g., trauma or ischemia) can directly cause microglia to develop a strongly activated state, but primed microglia may fail to resolve the inflammatory response and continue to produce and secrete inflammatory molecules. By better understanding underlying mechanisms of microglia priming and activation, we may develop effective treatments that dampen aberrant microglial inflammatory phenotypes (dashed arrows).
Microglia priming is a general process that is identified/revealed by the activity of the cell in response to an ex vivo secondary immune stimulus. Furthermore, whether distinct priming stimuli produce different phenotypes and molecular signatures in microglia is largely unknown. However, there are stereotypic changes in immunophenotype that may indicate that microglia are primed in the CNS, including upregulated expression of inflammatory pathway genes such as major histocompatibility complex II, toll-like receptors, NOD-like receptors, and purinergic receptors (Fonken et al., 2016a; Frank et al., 2006; Weber et al., 2015) and down-regulated expression of receptors in key anti-inflammatory pathways such as CD200R and CX3CR1 (Corona et al., 2010; Fonken et al., 2016c; Frank et al., 2017; Wynne et al., 2010).
IIIB. Other cell types contribute to neuroinflammatory priming
The CNS microenvironment strictly controls microglia activation state. Microglia communicate with other CNS cells, including neurons, astrocytes, and oligodendrocytes (Perry and Holmes, 2014) (summarized in Fig 3). Furthermore, soluble factors released by other cells can act to repress (e.g., TGF-β (Butovsky et al., 2014)) or activate (e.g., DAMPs (Venereau et al., 2015)) microglia. Additionally, the recruitment of other cells into the CNS can induce microglia activation and/or directly affect neuroinflammation.
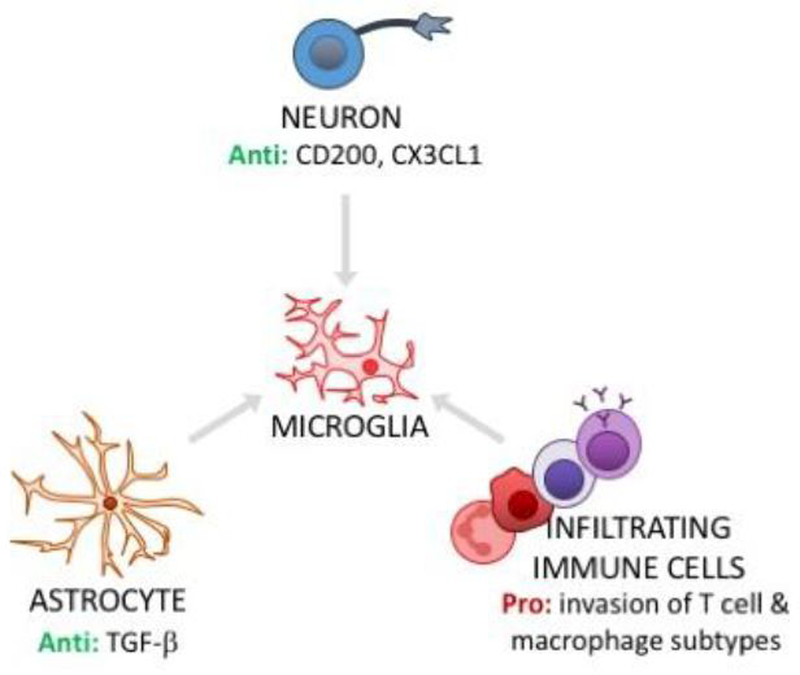
Figure 3. Microglial phenotype is regulated by other cells in the CNS.
In healthy CNS, steady-state microglial quiescence is maintained by signaling with neurons (neuron CD200L to microglia CD200R; neuron CX3CL1 to microglia CX3CR1) and astrocytes (e.g., TGF-β signaling limits microglial activation). Dysregulation of these cell-cell interactions can contribute to microglial priming. In addition, hematogenous immune cells - which typically have no/very limited access to the CNS - can invade CNS tissue in stress and aging. Pro-inflammatory subtypes of infiltrating cells, such as macrophages, can signal to microglia to elicit priming and/or activation. Anti = anti-inflammatory actions; Pro = pro-inflammatory actions.
a. Neuron-microglia interactions
Microglia-neuron interactions tightly regulate the phenotype and function of microglia. Under healthy conditions, several neuronally expressed ligands interact with inhibitory receptors on microglia to repress microglia activation. These microglia-neuron signaling dyads include CD200-CD200 receptor (CD200R) (Hoek et al., 2000), CX3CL1-CX3CR1 (Cardona et al., 2006), and CD47-SIRP-α (a “don’t eat me” signal also expressed on other cell types) (Jaiswal et al., 2009). Neuronal stress or damage can cause down-regulation of these neuronally expressed ligands leading to dis-inhibition of microglia. For example, CD200 is a membrane glycoprotein that is expressed throughout the CNS on neurons, endothelial cells, and oligodendrocytes (Koning et al., 2009). CD200R is exclusively expressed on microglia and other myeloid cells in the CNS (Cardona et al., 2006). Interactions between CD200 and its receptor inhibit myeloid cell activity by initiating anti-inflammatory intracellular signaling cascades (Jenmalm et al., 2006) (described in more detail in section VC).
b. Microglia-astrocyte interactions
Astrocytes actively regulate microglia morphology and function and can both promote and inhibit microglia activity. Astrocytic inhibitory regulation of microglia involves the release of diffusible signals. For example, adding conditioned media from astrocytes to cultured microglia increases antioxidant activity (hemeoxygenase-1 gene and protein expression) and reduces IFN-γ-induced reactive oxygen species in microglia (Min et al., 2006). Astrocyte-derived factors promote microglia health and survival (Bohlen et al., 2017). Transforming growth factor β (TGF-β) is one such diffusible signal that can contribute to anti-inflammatory regulation of microglia by astrocytes (Brionne et al., 2003). TGF-β is synthesized and released by most cell types, including astrocytes, and is required for the development of normal adult-like microglia (Bialas and Stevens, 2013; Butovsky et al., 2014; Schachtrup et al., 2015). TGF-β can reduce the production of reactive oxygen species and cytokines in microglia (Herrera-Molina and von Bernhardi, 2005; Huang et al., 2010), likely via an NF-κB dependent mechanism (Noh et al., 2016).
There are also bi-directional pro-inflammatory interactions between astrocytes and microglia, such that activated microglia promote astrocyte activation and vice versa (Liu et al., 2011). For example, classically activated pro-inflammatory microglia release cytokines that act on astrocytic toll-like receptors to elicit “reactive” astrocytes (Farina et al., 2005). Reactive astrocytes become hypertrophic, upregulate intermediate filaments, can proliferate, and secrete cytokines and other mediators (Karimi-Abdolrezaee and Billakanti, 2012). Once reactive, astrocytes can lose the ability to promote neuronal survival, outgrowth, synaptogenesis and phagocytosis, and induce death of neurons and oligodendrocytes (Liddelow et al., 2017). Importantly, neuronal death can then lead to the release of DAMPs such as high mobility group box-1 (HMGB1), which in turn can contribute to microglia activation (Scaffidi et al., 2002). Astrocytes can further propagate inflammatory signals through the release of DAMPs such as adenosine triphosphate (ATP), which are detected by proximal microglia (Davalos et al., 2005).
Two recent studies by Norden et al. highlight the possibility that astrocytes contribute to priming of microglia responses (Norden et al., 2014; Norden et al., 2016). Astrocytes isolated from aged mice had reduced expression of growth factors and the receptor for the anti-inflammatory cytokine IL-10. Following an immune challenge (intraperitoneal LPS), aged astrocytes appeared insensitive to IL-10, which resulted in a failure to induce TGF-β and resolve microglia activation (Norden et al., 2016). Further, the blockade of TGF-β signaling in the brain of healthy adult mice at the time of an immune challenge (intraperitoneal LPS) increased pro-inflammatory cytokine gene expression (IL-1β, TNF-α, IL-6) in a coronal brain slice and produced an exaggerated sickness responses (social withdrawal) (Norden et al., 2014). Therefore, astrocytes can collaborate with microglia to resolve – or exacerbate – neuroinflammatory priming.
c. Infiltrating immune cells
Peripheral immune cells can infilitrate the CNS and induce microglia priming. In the healthy adult CNS, the BBB tightly regulates the entry of leukocytes (e.g. monocytes, B cells, and T cells) into the brain parenchyma. Immune cell migration across the BBB is typically controlled by the expression of chemokines and adhesion molecules. Under pathological conditions, BBB disruption and/or upregulation of chemokines and adhesion molecules can induce trafficking of peripheral immune cells into the brain (Banks and Erickson, 2010).
There are age-associated increases in the number of peripheral immune cells in the CNS (Gemechu and Bentivoglio, 2012). Both an increase in BBB permeability and an upregulation of chemokines may contribute to the increase in peripheral immune cells in the aged brain (Blau et al., 2012; Gemechu and Bentivoglio, 2012). In particular, a gradual increase in T-cell recruitment to the brain with aging is thought to activate local myeloid cell populations (Stichel and Luebbert, 2007), although see (Ritzel et al., 2016). The influx of T cells into the brain parenchyma and choroid plexus is also exaggerated following a central immune challenge (intracerebroventricular TNFα or IFNγ) in aged animals (Xu et al., 2010). The recruitment of specific populations of macrophages to the CNS with aging may also contribute to microglia priming. For example, following spinal cord injury, there is increased recruitment of potentially harmful IL-4Rα- macrophages to the CNS in aged as compared to adult mice, which may inhibit CNS repair (Fenn et al., 2014b).
Stress-induced priming of microglia can also be induced by the recruitment of peripheral myeloid cells into the CNS (McKim et al., 2016; McKim et al., 2017; Wohleb et al., 2013). Following social-defeat stress in mice, there is an exposure dependent upregulation of mRNA expression of the chemokines CXCL1 and CXCL2 and adhesion molecule E-selectin that corresponds with the recruitment of myeloid cells into the CNS (Sawicki et al., 2015). Blockade of trafficking of these inflammatory myeloid cells into the CNS can prevent elevated neuroinflammatory changes, microglia priming, and behavioral deficits in stressed mice (McKim et al., 2016; McKim et al., 2017; Wohleb et al., 2013). Of note, recruitment of peripheral myeloid cells into the brain following stress might be specific to the type and severity of the stressor and may depend on peripheral injury to the animal (i.e., if stressor model includes tissue damage (Lehmann et al., 2016)). For example, Lehmann et al demonstrated that social defeat stress induces region-specific microglia proliferation but not recruitment of myeloid cells to the CNS when tissue damage is prevented.
In some contexts, neuroinflammatory priming may occur in the absence of enhanced glial activation. For example, Ritzel et al recently found that the brain of aged (18–22 mos) C57BL/6J mice had increased T cell prevalence and a positive correlation between T cell and microglia numbers. This increase in immune cell number was associated with a primed phenotype following an ischemic insult: aged animals displayed an enhanced inflammatory signature and increased damage following experimental stroke. However, the presence of T cells was associated with a more anti-inflammatory/phagocytic phenotype in microglia, suggesting that the primed inflammatory response in this model of aging may be directly due to activation of T cells in the CNS (Ritzel et al., 2016).
IV. Neuroinflammatory priming elicited by stress and aging
IVA. Neuroinflammatory priming in the context of aging
A steady decline in homeostatic function occurs with aging. Indeed, reduced function with aging manifests at all levels of an organism, from the molecular level – e.g., shortening telomeres increases DNA lability and susceptibility to aging-related disease (Blackburn et al., 2015) – to the behavioral level – e.g., declining memory performance (Nyberg et al., 2012). In parallel, aging is associated with dysregulation of the immune and neuroimmune systems.
The neuroimmune system shows a gradual shift from a balance between pro- and anti-inflammatory functions in adulthood towards a primed neuroinflammatory environment in aging. This contrasts with the effect of aging on peripheral myeloid cells: macrophages show a senescent diminished inflammatory capacity with aging (Rawji et al., 2016). Indeed, peripheral immune challenges (e.g., intraperitoneal E. coli, LPS, surgery, etc.) induce blunted or comparable inflammatory responses in the periphery of aged as compared to young rats but produce an enhanced inflammatory profile in the CNS (Barrientos et al., 2009; Barrientos et al., 2012). For example, one intraperitoneal injection of E. coli induces a transient (< 24h) neuroinflammatory response in young rats. In contrast, aged rats display elevations in hippocampal IL-1β gene and protein expression for up to 8 days following the exact same dose of E. coli (Barrientos et al., 2009). That is, the amount of E. coli was not increased to compensate for the greater body weight of the older rats, so the neuroinflammatory difference between young and aging subjects is likely underestimated by this conservative dosing procedure. Interestingly, this enhanced immune response is specifically segregated to the CNS as initial peripheral cytokine responses are actually elevated in young as compared to aged rats (Barrientos et al., 2009). Sterile peripheral immune challenges (such as a laparotomy procedure) also induce prolonged elevations in hippocampal IL-1β in aged, as compared to young rats (Barrientos et al., 2012). Aged mice show similarly exaggerated neuroinflammatory changes in response to immune challenges (Chen et al., 2008; Godbout et al., 2005; Huang et al., 2008). For example, peripheral LPS induces enhanced IL-1β and IL-6 gene and protein expression in the brain of aged as compared to young mice (Chen et al., 2008; Godbout et al., 2005). In addition to peripheral immune challenges inducing heightened neuroinflammatory responses, direct injury to the CNS also exacerbates inflammatory responses in aged rodents. For example, spinal cord injury, traumatic brain injury, and ischemic stroke are all associated with increased neuroimmune activation in aged as compared to young rodents (Dinapoli et al., 2010; Fenn et al., 2014b; Kumar et al., 2013; Manwani et al., 2011; Onyszchuk et al., 2008; Zhang et al., 2016).
Importantly, microglia priming contributes to these heightened neuroinflammatory responses in aged mice and rats (Chen et al., 2008; Frank et al., 2010b; Huang et al., 2008). For example, following ischemic stroke, microglia activation, as measured by increased density of microglia immunoreactive for ionized calcium binding adapter molecule-1 (Iba1), is more robust in the brain of aged as compared to young mice (Manwani et al., 2011). The increase in microglia activation is also apparent in vitro, outside the influence of other CNS cells, suggesting that it is due to an intrinsic change in cellular phenotype: microglia isolated from the hippocampus of aged (versus young) rats and challenged ex vivo with LPS display exaggerated upregulation in pro-inflammatory cytokine gene expression (Frank et al., 2010b).
However, aging-related microglia priming may vary regionally as immunohistochemical analyses reveals that aged C57/BL6 mice have greater upregulation of markers of microglia activation (CD11b, CD68, CD11c, F4/80, and FcyRI) in the white matter as compared to the grey matter (Hart et al., 2012). This may indicate that microglia are more prone to activation in the white versus the grey matter with age. Further evidence that microglia priming may be unique to specific regions of the aged brain comes from a study by Sierra et al. Microglia that were isolated from the whole brain of aged mice did not exhibit enhanced cytokine gene expression (TNFα, IL-1β, IL-6, IL-12, etc) following an intraperitoneal LPS challenge (Sierra et al., 2007). Microglia isolated from aged animals did have a modest increase in basal cytokine expression, but no age by LPS interaction was observed, indicating a lack of priming. This finding suggests that not all microglia in the aged CNS become primed with age, but rather it is possible that only microglia in vulnerable regions, such as the hippocampus, take on a primed phenotype. Indeed, Grabert et al demonstrated that the “ageing” of microglia varies in a region-specific manner. A principal component analysis of the transcriptome of microglia purified from either the cerebellum, cortex, hippocampus, or striatum of mice at 4, 12, and 22 months of age showed an age-dependent progression suggesting an interaction between age and brain region on global gene activity. This parallels the age-associated in vivo neuroinflammatory profile that follows an immune challenge: enhanced cytokine responses to peripheral immune challenge are only found in specific regions of the CNS (Barrientos et al., 2009).
There are important functional implications associated with neuroinflammatory priming in the aged brain. Aged mice and rats show prolonged cognitive and affective behavioral changes in response to peripheral immune stimulation such as LPS, E. coli, and surgery (Barrientos et al., 2012; Fonken et al., 2016a; Godbout et al., 2005) and CNS injuries including traumatic brain injury and spinal cord injury (Fenn et al., 2014b; Onyszchuk et al., 2008). The behavioral deficits follow a similar time course to the inflammatory profile in aged rats (Barrientos et al., 2009) and are likely due to the enhanced cytokine production in the CNS following immune stimulation. Indeed, central administration of IL-1 receptor antagonist (IL-1RA), which blocks the pro-inflammatory cascade initiated by IL-1β(Basu et al., 2004), attenuates the prolonged behavioral deficits following peripheral immune challenges in aged animals (Abraham and Johnson, 2009; Barrientos et al., 2012; Frank et al., 2010a). IL-1β has been designated the “master” cytokine (Basu et al., 2004) because of its pleiotropic role in the induction and propagation of the central inflammatory response and behavioral sickness response (Goshen and Yirmiya, 2009).
IVB. Stress and neuroinflammatory priming
Exposure to acute psychological and physiological stressors can rapidly alter the inflammatory milieu of the brain. Stressors can transiently induce pro-inflammatory cytokines in stress-reactive areas of the brain such as the hippocampus and hypothalamus (Deak et al., 2003; Johnson et al., 2005; Nguyen et al., 2000; O’Connor et al., 2003) and this increase in cytokines appears dependent on catecholaminergic signaling (Johnson et al., 2005). The initial pro-inflammatory response to stress typically resolves within a few hours (Nguyen et al., 2000). However, in addition to this transient neuroinflammatory response with associated molecular and behavioral effects, stress can induce a prolonged primed neuroimmune phenotype (de Pablos et al., 2014; Espinosa-Oliva et al., 2011; Fonken et al., 2018; Fonken et al., 2016c; Frank et al., 2007; Frank et al., 2018b; Frank et al., 2012; Johnson et al., 2002; Johnson et al., 2003; Munhoz et al., 2006; Wohleb et al., 2012; Wohleb et al., 2011). For example, following a single session of inescapable tail shock stress, rats exhibit elevated hippocampal IL-1β production in response to intra-peritoneal LPS (Johnson et al., 2002; Johnson et al., 2003). Although LPS is commonly used as the immune challenge, stress also potentiates pro-inflammatory cytokine responses in the CNS following other peripheral and central immune challenges such as surgery or ischemic injury (Karelina et al., 2009; Norman et al., 2010; Wang et al., 2017; Weil et al., 2008). Potentiated neuroinflammatory responses follow a diverse array of stressors including chronic variable stress (de Pablos et al., 2014; Espinosa-Oliva et al., 2011; Yue et al., 2017), social defeat (Wohleb et al., 2012), stress from shipping/travel (Holder and Blaustein, 2017), social isolation (Gaudier-Diaz et al., 2017), chronic sleep restriction (Bellesi et al., 2017), and inescapable tail shock (Frank et al., 2007; Johnson et al., 2002) suggesting that neuroinflammatory priming may be a conserved feature of the stress response. The degree of neuroimmune activation may vary across stressors. For example, a meta-analysis revealed 20 to 200% increases in Iba1 immunoreactivity in the hippocampus in response to different stress models (Calcia et al., 2016).
Stress-induced neuroinflammatory priming is associated with exaggerated behavioral responses to an immune challenge. Indeed, prior stressor exposure enhances LPS-induced sickness responses including elevated depressive and anxiety-like behaviors, fever, and hyperalgesia (Fonken et al., 2018; Johnson et al., 2003; Loram et al., 2011; Wohleb et al., 2012). Stress can also exaggerate behavioral deficits following CNS injury. For example, social isolation stress prior to global cerebral ischemic injury was associated with enhanced CNS cytokine gene expression, greater neuronal loss, and increased depressive-like behavior (Norman et al., 2010). Social isolation also increased damage following focal cerebral ischemia (middle cerebral artery occlusion; MCAO). However, stress induced increases in damage following MCAO were associated with reduced CNS IL-6 gene and protein expression (Karelina et al., 2009).
Stress can also prime the microglia response to an ex vivo immune challenge. Prior exposure to stress potentiates the pro-inflammatory response to LPS in hippocampal microglia isolated from male rodents (Fonken et al., 2018; Frank et al., 2017; Frank et al., 2012; Weber et al., 2015; Wohleb et al., 2011). Interestingly, the phenomenon of stress-induced priming of microglia differs between males and females. Similar to males, female rats exhibit potentiated behavioral and neuroinflammatory responses to stress. Microglia isolated from male and female rats post-stress also show comparable changes in phagocytic activity ex vivo (Fonken et al, 2018). However, pro-inflammatory microglia priming ex vivo is not evident in microglia isolated from female rats following stress: microglia isolated from female rats following tail shock stress do not exhibit enhanced cytokine mRNA expression following ex vivo challenge with LPS. This finding, along with other work, suggests that distinct cellular substrates of stress-elicited neuroinflammation might exist in females and males (Fonken et al., 2018; Pyter et al., 2013; Wohleb et al., 2018).
Stressor exposure may also induce a primed neuroinflammatory environment by increasing microglial proliferation in vivo (Kreisel et al., 2013; Lehmann et al., 2016; Nair and Bonneau, 2006). That is, stress may increase the pro-inflammatory response because it leads to an increase in the number of microglia that respond to a challenge. While this is a possible explanation for the neuroinflammatory priming effects of stress, increased proliferation is not responsible for the priming of microglia by stress observed in male rats as these studies controlled for the number of microglia exposed to immune challenge ex vivo.
The immunological and behavioral effects of stress require activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS). HPA axis activation in response to physiological and psychological stressors culminates in the release of glucocorticoids (GCs; predominately cortisol in humans and corticosterone in mice and rats) from the adrenal gland (reviewed in (Bellavance and Rivest, 2014)). GCs are released into the general circulation and are detected throughout the body and brain. For example, inescapable tailshock in rats induces profound increases in serum GC levels (Fleshner et al., 1995) as well as hippocampal GC levels (Frank et al., 2012). GCs are potent immunomodulatory molecules that act on both innate and adaptive immune cells due to the ubiquitous expression of glucocorticoid receptors (GRs) (Bellavance and Rivest, 2014; Cain and Cidlowski, 2017). GCs have powerful and well-characterized anti-inflammatory actions in a number of contexts (reviewed in (Cain and Cidlowski, 2017), but can paradoxically also contribute to neuroinflammatory priming in the context of aging and stress (described in section VA). The SNS, and in particular norepinephrine, also plays a crucial role in mediating neuroimmune changes in response to stress (Johnson et al., 2005; McKim et al., 2016; Porterfield et al., 2012; Powell et al., 2013; Wohleb et al., 2011). This conclusion stems from findings that pharmacological inhibition of noradrenergic signaling during stress (e.g. inescapable tailshock and repeated social defeat) can prevent microglia from developing a primed phenotype (Johnson et al., 2005; McKim et al., 2016; Porterfield et al., 2012; Wohleb et al., 2011). A considerable number of studies have demonstrated that stress-induced noradrenergic and GC signaling co-regulate one another in the brain (Herman et al., 2003), but the interactive effects of these stress systems in the context of neuroimmune priming has largely been unexplored (Lowrance et al., 2016). Future studies should further elucidate how the HPA axis and the SNS interact to produce stress- and aging-elicited neuroinflammatory priming.
Importantly, aging and stress can also act additively to induce neuroinflammatory priming, in that aged animals have exaggerated stress-induced cytokine production and behavioral deficits (Buchanan et al., 2008). It is possible that this compounded aging-stress priming effect could represent hyper-activation of a conserved priming pathway and/or coincident activation of parallel priming pathways.
V. Mechanisms underlying neuroinflammatory priming: common features of stress and aging
As described above, the changes in neuroinflammatory phenotype in response to aging and stress share several common features: exaggerated sickness response following an immune challenge, increased hippocampal cytokine production, microglia priming, etc. Thus, it follows that there are likely common mechanisms that mediate priming in the context of stress and aging. In this section, we discuss mediators that contribute to the shared neuroinflammatory pathways underlying stress and aging (Fig 4).
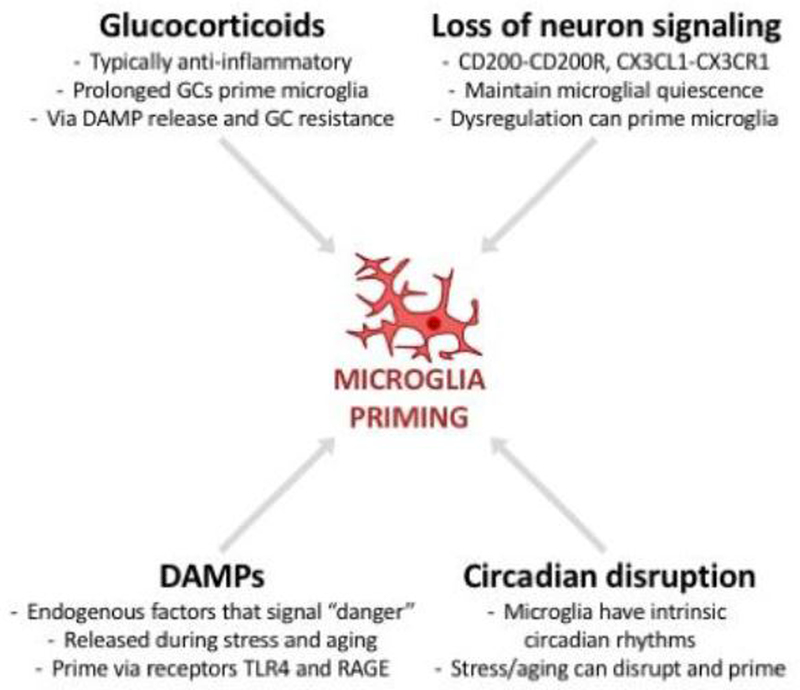
Figure 4. Microglial priming is elicited by common mechanisms in aging and stress.
Microglial priming can be caused by intense and/or prolonged glucocorticoid signaling, by excess release of damage-associated molecular patterns (DAMPs), by loss of neuron signaling (which typically helps maintain microglia homeostasis), and by circadian disruption. Aging and stress can elicit priming by any or all of these mechanisms.
VA. Glucocorticoid signaling
GCs have traditionally been regarded as potent anti-inflammatory molecules (Cain and Cidlowski, 2017 provide an excellent comprehensive review of the pleiotropic actions of GCs on the immune system). Indeed, GCs are used therapeutically to suppress inflammation in a number of conditions including arthritis, asthma, eczema, etc. (Cain and Cidlowski, 2017). However, the clinical application of GCs has outpaced our understanding of the mechanisms that mediate GC actions, which has led, in some cases, to the inappropriate use of GCs. Clinical use of GCs has been called into question for several conditions including cardiac arrest (Jastremski et al., 1989; Sapolsky and Pulsinelli, 1985), spinal cord injury (Hurlbert et al., 2013), and Crohn’s disease (Irving et al., 2007). A number of paradoxical results with GCs (Busillo et al., 2011; Dhabhar and McEwen, 1999; Frank et al., 2010c; Frank et al., 2012; Hermoso et al., 2004; Lim et al., 2007; MacPherson et al., 2005) suggest that GCs do not universally exert anti-inflammatory actions (examples follow). Both the timing (Barber et al., 1993; Frank et al., 2010c) and dose (Dhabhar and McEwen, 1999; Lim et al., 2007) of GC administration influence whether GCs exert antiinflammatory or pro-inflammatory actions. For example, humans administered GCs concomitant with LPS show reduced cytokine responses; however, GCs administered 12 h or 1 week prior to LPS enhance cytokine responses (Barber et al., 1993). Similarly, in rats, administering GCs (2.5 mg/kg subcutaneous corticosterone) 1 h following peripheral LPS suppresses IL-1β gene and protein increases in both liver and hippocampus. However, when GCs are administered 2 or 24 h prior to the immune challenge, the hippocampal IL-1β induction by LPS is potentiated (Frank et al, 2010c). Dose of GCs also critically controls their immunomodulatory activity. For example, Dhabar and McEwen demonstrated that a high pharmacological dose of corticosterone (40 mg/kg intraperitoneal) suppresses skin delayed-type hypersensitivity responses (DTH; cell-mediated immune measure), whereas a lower physiological dose of GCs (5 mg/kg) enhanced DTH responses.
Studies in both rodents and humans support the hypothesis that GCs generally promote brain aging (Landfield et al., 1981; Lupien et al., 1998). Here, we propose that GCs may more specifically prime neuroinflammatory responses in the aged brain. Barrientos et al recently demonstrated that aging selectively alters the circadian rhythm of GCs in the hippocampus. In adult rats, GCs are typically highest at the beginning of the active phase, and lowest at the beginning of the inactive phase. Aged rats exhibit increases in hippocampal corticosterone during the inactive phase of the circadian cycle (Barrientos et al., 2015). The hippocampal corticosterone values were not reflected in blood corticosterone, highlighting the importance of evaluating brain regional changes in GCs. Importantly, dysregulated GCs in the aged CNS are a critical cause of neuroinflammatory priming. For example, blockade of GC action in the brain with an intracisternal magna injection of the GC receptor antagonist mifepristone prevents the ex vivo microglial priming produced by aging (Barrientos et al., 2015). Consistent with these findings, prophylactic mifepristone treatment also prevented prolonged hippocampal-dependent deficits in learning and memory following infection. Interestingly, exercise equalized corticosterone concentrations in the hippocampus of young and aged rats and also prevented aspects of age-related neuroinflammatory priming (Barrientos et al., 2011; Barrientos et al., 2015).
GCs are also critically involved in the neuroinflammatory response to stress. Various physiological and psychological stimuli activate the HPA axis, resulting in the production of GCs. GCs are a proximate signal through which stress primes inflammatory responses in rodents (Frank et al., 2013). Indeed, both acute and chronic administration of exogenous GCs can induce neuroinflammatory priming in rats (Fonken et al., 2018; Frank et al., 2014; Frank et al., 2010c; Kelly et al., 2012; Munhoz et al., 2010). For example, a single subcutaneous injection of corticosterone, at a dose (2.5 mg/kg) that mimics the serum rise in GCs produced by tail shock stress (Fleshner et al., 1995), induces heightened hippocampal cytokine gene and protein expression to later immune challenge with LPS in Sprague Dawley rats (Fonken et al., 2018; Frank et al., 2010c). In addition, priming induced by GCs is reflected in microglia isolated from male, but not female rats (Fonken et al., 2018). Establishing causality, pharmacological inhibition of GC signaling in rats blocks the stress-induced exaggeration of the neuroinflammatory response to LPS following both chronic (chronic variable stress) (de Pablos et al., 2006; Espinosa-Oliva et al., 2011) and acute (tail shock stress) stressors (Frank et al., 2012). Similarly, blockade of the stress-induced GC response via adrenalectomy in rats prevents neuroinflammatory and microglia priming in response to acute stress (Frank et al., 2012).
Several possible mechanisms that underlie GC-elicited neuroinflammatory priming have been explored. First, GCs may act through disruption of CD200 signaling (described below). In support, ex vivo treatment of microglia isolated from adult male rats with corticosterone downregulates CD200R (Fonken et al., 2016c). Second, neuroinflammatory priming may occur through the induction of GC resistance. Peripheral macrophages from mice that have undergone social disruption stress become hyposensitive to GCs and show potentiated inflammatory responses to an ex vivo LPS challenge (Avitsur et al., 2001; Stark et al., 2001), suggesting that a similar process may occur in microglia. GC resistance is mediated by diminished GR function in macrophages as evidenced by decreased nuclear translocation of GR following stress, which diminishes the inhibitory effect of GCs on the pro-inflammatory transcription factor NF-κB (Quan et al., 2003). GR downregulation is also detected in brain macrophages following stress and is dependent on the release of adrenal GCs (McKim et al., 2017; Niraula et al., 2018). However, microglia, isolated from animals that received chronic social disruption stress, failed to show GC resistance ex vivo (Wohleb et al., 2011). Consistent with a primed response, GC resistance following stress requires immune stimulation (i.e. injury during social defeat or LPS) (Foertsch et al., 2017). Reports on GR changes in the aged brain are conflicting (Barrientos et al., 2015; Murphy et al., 2002), and glial specific changes in GR have not been evaluated.
Finally, GCs are capable of inducing key components of the inflammasome, a multiprotein complex that is involved in the processing and maturation of IL-1β (as well as IL-18). In particular, GCs prime the NLRP3 inflammasome through upregulation of NLRP3 expression in myeloid cells in vitro (Busillo et al., 2011). NLRP3 inflammasome formation and activation requires a 2-step process. The first step involves increased NLRP3 gene transcription, translation and protein production. This has been called the “priming” step, and is thought to involve the ligation of TLR4 (Hornung and Latz, 2010). Once sufficient NLRP3 protein has been formed, then a second activation signal is needed that leads NLRP3 to interact with the adaptor protein, apopotosis-associated speck-like protein containing a CARD (ASC), which then recruits pro-caspase-1 through its caspase recruitment domain. This assembly of proteins is considered the inflammasome, and once formed triggers the activation/cleavage of pro-caspase 1. Mature, active caspase-1 then acts to cleave pro-IL-lβ into mature IL-1β protein. The NLRP3 inflammasome is also unusual in that it responds to a very broad range of signals as the second event that produces inflammasome formation/activation (e.g. ATP, K+ efflux, β-amyloid, silica, uric acid crystals, ROS; (Elliott and Sutterwala, 2015). Chronic GC administration up-regulates NLRP3 expression in hippocampus concomitant with priming of the microglial pro-inflammatory response ex vivo (Frank et al., 2014). Taken together, these findings suggest that GC-induced neuroinflammatory priming might involve a diverse array of mechanisms of action.
VB. Accumulation of danger signals
Innate immune cells can be activated in the absence of infectious processes (i.e. sterile inflammation) by endogenous molecules, which have been termed danger associated molecular patterns (DAMPs) or alarmins (Schaefer, 2014). DAMPs signal through PRRs (e.g. TLR4) expressed by innate immune cells to activate signal transduction pathways such as NF-kB, which are pivotal in eliciting a pro-inflammatory immune response. Interestingly, in many instances PAMPs such as LPS use these same PRRs to induce a pro-inflammatory response, suggesting that DAMPs may prime these pathways to subsequent PAMP exposure (Bianchi, 2007). A number of DAMPs have been characterized including heat shock proteins, uric acid, S100 proteins, IL-lα and high mobility group box-1 (HMGB1), which are released under a variety of sterile inflammatory conditions (Bianchi, 2007). In the CNS, the neuroinflammatory effects of DAMPs have been characterized in ischemia (Yang et al., 2010), traumatic CNS injury (Corps et al., 2015), chronic pain states (Agalave et al., 2014), seizure (Vezzani, 2014), ethanol exposure (Szabo and Lippai, 2014) and methamphetamine exposure (Frank et al., 2015a). Of particular relevance here, DAMPs have also been found to play a pivotal role in the aging- and stress-induced priming of neuroinflammatory processes.
Our laboratory has recently implicated the DAMP HMGB1 in stress- and age-elicited neuroinflammatory priming (Fonken et al., 2016a; Weber et al., 2015). HMGB1 is a chromatin-binding factor localized to the nucleus that is released from the nucleus, and subsequently the cell, under pathological conditions (e.g. sepsis, stroke, Alzheimer’s disease) (Lotze and Tracey, 2005). HMGB1 is released in the brain both actively by microglia and passively by necrotic or damaged cells (Scaffidi et al., 2002). Extracellular HMGB1 interacts with the key immune receptors TLR2 and 4 and the receptor for advanced glycation end products (RAGE), thereby driving priming and/or pro-inflammatory responses (Yang and Tracey, 2010). Importantly, extracellular HMGB1 does not necessarily induce a pro-inflammatory cytokine response in the brain; rather, HMGB1 can potentiate the effects of a subsequent neuroinflammatory challenge (Weber et al., 2015).
Aged rats exhibit basal elevations in hippocampal and cerebrospinal fluid HMGB1, the latter suggesting increased extracellular release of HMGB1 (Fonken et al., 2016a). As noted above, aged rats do not exhibit an increase in basal brain cytokines, but following an immune challenge (E. coli) they display prolonged elevations in hippocampal cytokines and greater microglial reactivity. This phenotype is associated with age-related increases in HMGB1, and indeed, HMGB1 is required for age-related neuroinflammatory priming. Blockade of the actions of HMGB1 by CNS administration of a competitive antagonist, Box A, can prevent aging-induced neuroinflammatory priming and associated behavioral changes (Fonken et al., 2016a). Similarly, in an aged rat model of post-operative cognitive decline, systemic HMGB1 neutralization prevented neuroinflammatory and behavioral pathologies (Terrando et al., 2016). Aged rats that received anti-HMGB1 treatment exhibited a reduction in hippocampal microglia activation (Terrando et al., 2016). Interestingly, it appears that microglia in the aged brain attempt to compensate for this age-related upregulation in endogenous danger signals as microglia from aged rats downregulate genes involved in sensing endogenous ligands such as DAMPs, but not other foreign debris (Hickman et al., 2013).
As with aging, we have recently found that acute stressor exposure (tail shock stress) increases levels of HMGB1 in hippocampus (Frank et al., 2018a; Frank et al., 2018b; Weber et al., 2015) as well as amygdala of rats (Frank et al., 2018a), findings replicated in other stress paradigms (Cheng et al., 2016; Franklin et al., 2018; Lian et al., 2017). Further, acute stress increases the release of HMGB1 ex vivo from hippocampal microglia (Weber et al., 2015). HMGB1 likely plays a mediating role in stress-induced priming as central administration of a competitive antagonist to HMGB1 (Box A) before stress exposure blocks stress-induced priming of the microglial pro-inflammatory response to ex vivo LPS treatment (Weber et al., 2015). Furthermore, central administration of HMGB1 mimics the impact of stressor exposure and potentiates the neuroinflammatory, behavioral, as well as the microglia pro-inflammatory response to a subsequent immune challenge (Frank et al., 2015b). Similar effects of HMGB1 were observed in vitro. As noted above, HMGB1 signals through a number of innate immune receptors including RAGE. A recent study showed that stress-induced anhedonia, as assessed by reduced sucrose preference, was blocked in RAGE KO mice, suggesting that HMGB1 (or another RAGE receptor ligand) might contribute to the anhedonic effects of stress (Franklin et al., 2018). Consistent with this idea, we recently found that central administration of box A blocks the anxiogenic effects (i.e. decrements in juvenile social exploration) of inescapable tailshock (unpublished observations), suggesting that stress-induced HMGB1 is necessary for the immediate behavioral effects of stress.
In addition to increased production/secretion of DAMPs, impairments in paravascular clearance may contribute to the pathological accumulations of DAMPs in the brain following stress and aging. Indeed, the brain’s glymphatic system is critical for removing extracellular solutes from the brain parenchyma and subsequently the cerebrospinal fluid (Aspelund et al., 2015; Iliff et al., 2012; Louveau et al., 2015). With aging, loss of perivascular aquaporin-4, a critical component of the glymphatic system, is associated with a decline in cerebrospinal fluid clearance and accumulation of DAMPs such as amalyoid β (Iliff et al., 2012; Kress et al., 2014; Zeppenfeld et al., 2017). Voluntary exercise, which can diminish the maladaptive consequences of aging and stress, can promote glymphatic clearance and reduce the accumulation of amyloid plaques (He et al., 2017). Whether stressors can also dampen effectiveness of the glymphatic system remains unknown.
VC. Dis-inhibition of microglia
Exposure to neuronal cell-surface and soluble factors maintain microglia in a comparatively quiescent immunophenotype compared to other myeloid cells (Biber et al., 2007). For example, ‘off’ signals including the neuronally derived factors CD200 and fractalkine (CX3CL1) are constitutively expressed at high levels in healthy brain and signal through their cognate receptors on microglia, CX3CR1 and CD200R1, to inhibit activation. As discussed above, exposure to PAMPs or DAMPs amplifies release of ‘on’ signals, which can override or dis-inhibit these ‘off’ signals and lead to the pro-inflammatory shift in microglia (Katsumoto et al., 2014).
a. CD200:CD200R
Downregulation of CD200R contributes to microglia priming in the contexts of both stress (Fonken et al., 2016c; Frank et al., 2017) and aging (Cox et al., 2012). CD200 is a membrane glycoprotein that is ubiquitously expressed on neurons, endothelial cells, and oligodendrocytes (Koning et al., 2009). The cognate receptor for CD200, CD200R, is exclusively expressed on microglia and other myeloid cells in the CNS (Cardona et al., 2006). Ligation of CD200R by CD200 inhibits microglia activation and constitutively dampens myeloid cell activity (Jenmalm et al., 2006). Unlike most inhibitory receptors, the CD200R does not contain an immunoreceptor tyrosine-based inhibitory motif; rather, stimulation by CD200 leads to the phosphorylation of a cytoplasmic tyrosine residues which recruits the adaptor protein downstream of tyrosine kinase 2 (Vaine and Soberman, 2014). Disruption of this anti-inflammatory CD200:CD200R signaling potentiates microglial responses to immune stimuli (Costello et al., 2011; Denieffe et al., 2013; Hoek et al., 2000).
Alterations in the CD200:CD200R signaling dyad occur in aging and following stress. CD200 is downregulated in the hippocampus of aged rats (Cox et al., 2012; Frank et al., 2006). Administration of a soluble fragment of CD200 (CD200Fc; a CD200R agonist) into the hippocampus of aged rats decreases markers of microglia activation including MHCII immunoreactivity and CD40 gene expression (Cox et al., 2012). Stress-induced neuroinflammatory priming is associated with suppressed CD200R mRNA expression across sub-regions of the hippocampus (CA1, CA3, and dentate gyrus) as well as in isolated hippocampal microglia (Frank et al., 2017). CD200R protein is also reduced in whole hippocampus following tail shock stress (Frank et al., 2017). Moreover, administration of CD200Fc into the cisterna magna prior to tail shock stress exposure prevents ex vivo stress-induced microglia priming. Intriguingly, changes in CD200:CD200R signaling are also implicated in pathological neuroinflammatory responses in other context including Alzheimer’s disease (Varnum et al., 2015; Walker et al., 2017), CNS injury (Cohen et al., 2017), and neuropathic pain (Hernangomez et al., 2016).
b. CX3CL1:CX3CR1
The CX3CL1:CX3CR1 signaling axis has features similar to CD200:CD200R regulation of microglia. Complementary expression of the ligand CX3CL1 (a.k.a. fractalkine) in neurons and the receptor CX3CR1 in microglia serves to regulate the activation of microglia (Cardona et al., 2006; Harrison et al., 1998; Mizutani et al., 2012). Constitutive expression and release of CX3CL1 by neurons inhibits microglia from developing a pro-inflammatory phenotype. Blocking the actions of CX3CL1 exaggerates pro-inflammatory responses to immune stimulation (Zujovic et al., 2001), whereas treatment with soluble CX3CL1 inhibits LPS-induced cytokine production (Lyons et al., 2009). Furthermore, mice lacking CX3CR1 have exaggerated neuroinflammatory and behavioral responses to an immune challenge (Corona et al., 2010). Counter-intuitively, deletion of CX3CR1 can have protective effects following CNS injury (Freria et al., 2017; Mattison et al., 2013). However, as noted in the study by Mattison et al, one limitation to using global genetic knockout models is that compensation for gene deletions may occur during development. Thus, to determine whether CX3CR1 removal is truly neuroprotective, future studies could use conditional deletion or receptor antagonist strategies.
Changes in CX3CL1 signaling have been implicated in neuroinflammatory priming. For example, CX3CL1 expression is reduced in the brain of aged rodents, an effect that is accompanied by evidence of age-related microglia priming (Bachstetter et al., 2011; Lyons et al., 2009; Wynne et al., 2010). Administering soluble or membrane-bound CX3CL1 attenuates primed immune responses in the aged rats, including blocking age-related evidence of microglial priming indicated by increased MHCII and CD40 mRNA expression (Lyons et al., 2009), deficits in LTP (Lyons et al., 2009), and the decline in neurogenesis (Bachstetter et al., 2011). CX3CL1 signaling has also been implicated in neuroinflammatory priming in the context of stress, albeit in an opposite role than expected. Stress-induced exacerbations in neuroinflammation are not apparent in CX3CR1 knockout mice and affective behavioral changes associated with stress are reduced (Hellwig et al., 2016; Milior et al., 2016; Rimmerman et al., 2017; Winkler et al., 2017). It is possible that the lack of stress induced neuroinflammation in global CX3CR1 knockout is due to a compensatory upregulation in another anti-inflammatory pathway. Overall, CX3CR1 knockouts may show a primed phenotype at baseline as they are more sensitive to LPS-induced neuroinflammation and sickness behaviors (Corona et al., 2010).
VD. Breakdown of circadian rhythms
Anticipating predictable daily events and adjusting physiology and behavior accordingly is adaptive. The circadian system synchronizes organisms to daily fluctuations in their external environment and temporally organizes internal systems to maintain homeostatic balance. For example, there are time of day variations in the risk for an organism encountering infection and injury and the circadian system regulates multiple immunological processes including the release of immunomodulatory hormones, immune cell trafficking, and cellular cytokine production (Scheiermann et al., 2013). Indeed, the time at which an immune challenge occurs can modulate the severity of the immune response (Halberg et al., 1960; Marpegan et al., 2009; Spengler et al., 2012). For example, mortality following a bacterial challenge varies depending on the time of immunostimulation (Halberg et al., 1960). Circadian regulation of immune cells includes direct regulation of microglia (Fonken et al., 2015; Nakazato et al., 2017). Indeed, we demonstrated that microglia possess intrinsic circadian clock mechanisms and display altered immune potential over the course of the day, with peak immune activation occurring during the light phase (Fonken et al., 2015). As might be expected, disruption of the circadian system can lead to heightened peripheral and central inflammatory responses (Castanon-Cervantes et al., 2010; Fonken and Nelson, 2013; Wright et al., 2015). Indeed, circadian disruption by nighttime light exposure results in heightened microglia cytokine mRNA expression in response to a peripheral immune challenge (Fonken and Nelson, 2013). Importantly, the circadian system has notable changes in response to both aging and stress that can impact neuroimmune activity.
Aging is associated with decreased amplitude and precision of behavioral, metabolic, and other endocrine rhythms (Bonaconsa et al., 2014; Nakamura et al., 2015; Smith et al., 2005; Valentinuzzi et al., 1997). Furthermore, disruption in rhythms in immune activity also occurs in aged animals (Fonken et al., 2016b; Sato et al., 2014). Diurnal rhythms in the microglial activation signature are observed in young, but not aged rats. While microglia from young rats display diurnal differences in microglia activation potential both in vivo and ex vivo, with dampened microglial pro-inflammatory cytokine responses during the rats’ active phase, microglia from aged rats exhibit constitutively elevated microglia activation potential. Changes in microglia activation parallel the intensity of neuroinflammatory responses in young and aged rats. For example, hippocampal IL-1β protein induction in response to intraperitoneal E. coli depends on time of day in young, but not aged rats. The erosion of this potentially beneficial diurnal fluctuation in inflammatory response may contribute to the protracted neuroinflammatory responses that are observed in aged animals. Moreover, changes in circadian clock genes/proteins are implicated in the aging process. For example, disruption of the core clock gene BMAL1 causes pre-mature aging (Kondratov et al., 2006) and neurodegeneration (Kress et al., 2018; Musiek et al., 2013), although see (Yang et al., 2016). Interestingly, strategies that strengthen circadian rhythms, such as exercise (Droste et al., 2009; Edgar and Dement, 1991; Leise et al., 2013), can prevent neuroinflammatory and microglia priming (Barrientos et al., 2011).
The circadian and stress response systems have a unique interrelated relationship. Both systems are critical for enabling acclimatization to environment challenges: while the circadian system is important for adjusting physiology and behavior to predictable daily events, the stress system exists to maintain homeostasis in response to unpredictable challenges. Responses to stressors and susceptibility to stress-related comorbidities are regulated by the circadian system (Cohen et al., 2015). For example, the time of day at which a stressor or inflammatory challenge occurs significantly defines the magnitude of the stress-elicited neuroinflammatory response (Fonken et al., 2016c; Johnson et al., 2003). Indeed, while tail shock stress induces neuroinflammatory priming when the stressor occurs during the middle of the light (rest) phase in rats, priming is significantly blunted during the animals’ active phase (Fonken et al., 2016c).
Reciprocally, stress has profound effects on circadian rhythms. GCs show robust circadian oscillations that are driven by both the central clock in the SCN and local adrenal clocks (Oster et al., 2006). GCs are an important entrainment factor for extra-SCN circadian rhythms. Stress both directly induces GCs and causes longer-term dysregulation of circadian rhythms in GCs. As discussed above (IVa), microglia are sensitive to daily or stress-induced changes in GC concentrations. Thus, the disruption of circadian rhythms with stress has the potential to dysregulate immune cells, which are sensitive to daily or stress-induced changes in GC concentrations (Borniger et al., 2017).
VE. Mechanism interactions
In section V, we introduced several mechanisms by which stress and aging can contribute to neuroinflammatory priming. An important remaining question is how do these mechanisms interact? Are distinct forms of neuroinflammatory priming induced by GCs versus danger signals versus dis-inhibition of microglia? Or are all of these elements involved at different points in the same signaling cascade that leads to neuroinflammatory priming? Here we will present evidence that the mechanisms described above can interact to induce neuroinflammatory priming.
As already noted, GCs are critical for inducing neuroinflammatory priming in a number of different stress models (de Pablos et al., 2006; Espinosa-Oliva et al., 2011; Frank et al., 2012; Wang et al., 2017) and likely are at the top of the signaling cascade that induces neuroinflammatory priming. The rapid synthesis and release of GCs in response to stressors may induce the release of the danger signal HMGB1 via disinhibition (reduced CD200:CD200R signaling) of microglia. In two recent papers from our group (Fonken et al., 2016c; Frank et al., 2018a), we demonstrated that stress (and GCs) might induce neuroinflammatory and microglia priming via downregulating CD200R signaling. Exposure to tail shock stress reduced CD200R gene and protein expression in the hippocampus. Furthermore, CAAT/enhancer binding protein (C/EBP)β, a transcription factor that binds to the CD200R and inhibits its transcription (Dentesano et al., 2012), was potently upregulated by stress exposure at both the gene and protein expression level (Frank et al., 2018a). Microglia isolated from adult rats also showed a dose dependent downregulation in CD200R in response to ex vivo treatment with corticosterone suggesting that GCs may act directly on microglia to target reduced CD200R (Fonken et al., 2016c). Intriguingly, the administration of CD200Fc into the cisterna magna immediately prior to stress prevented stress-induced elevations in hippocampal HMGB1 protein, suggesting that microglia disinhibition may cause increased HMGB1 synthesis in microglia (Frank et al., 2018a). Furthermore, a recent paper by Wang et al highlights the importance of GCs in CD200:CD200R signaling: reductions in CD200 in the hippocampus of rats following chronic unpredictable stress were prevented by pharmacological blockade of GC signaling produced by intraperitoneal injection of RU486 during the CUS protocol (Wang et al., 2017). Although, note that this study evaluated CD200 rather than CD200R. Taken together, these studies suggest that several of the mechanisms described above can interact to induce neuroinflammatory priming.
VI. Neuroinflammatory priming in human aging and stress
This review has primarily focused on neuroinflammatory priming in animal models of aging and stress. The majority of work that investigates neuroinflammatory priming is conducted in animal models due to the difficulty of studying this phenomenon in humans. For example, there are serious ethical considerations when performing an immune challenge in humans, although some studies use low dose LPS, viral challenges, or measure inflammation following vaccination. Additionally, measuring neuroimmune changes following an immune challenge is not feasible in humans. However, there is indirect evidence (evidence of neuroimmune changes, studies on peripheral immune cell priming, etc.) that suggests that neuroinflammatory priming may occur in humans and contribute to pathology. Thus, here we will provide a brief discussion of evidence suggesting that neuroinflammatory priming may occur in humans in the context of aging and stress.
Extensive innate immune gene upregulation accompanies brain aging. For example, a microarray study by Cribbs et al, which profiled immune/inflammation-related genes in young (29 to 59) and aged ((60 to 99) human brain tissue, revealed that age was associated with increased expression of genes involved in complement (C1q, C3, C5, etc.), TLR (TLR2, TLR4, etc.), and inflammasome (caspase-1) pathways (Cribbs et al., 2012). The authors concluded that conditions in the aged human brain are comparable to conditions following a chronic injury (Cribbs et al., 2012). Morphologically, microglia take on a dystrophic phenotype in the aged human brain that is distinct from an activated phenotype (Streit et al., 2004). Furthermore, the transcriptomic and proteomic profile of human microglia changes with aging. Aged human microglia display downregulated genes involved in anti-inflammatory signaling (e.g. the TGFβ signaling pathway gene set) and upregulated genes involved in neurodegeneration and amyloid pathology (Olah et al., 2018). Taken together, these studies suggest a primed inflammatory signature may develop in the aged brain.
Neuroimmune changes with aging may underlie vulnerability to age-ass ociated pathologies such as cognitive decline and neurodegenerative diseases. Indeed, age is the most salient risk factor for postoperative cognitive decline (POCD) (Monk et al., 2008; Steinmetz et al., 2009), a disorder characterized by impairments in memory, concentration, and information processing that can occur following surgery. Systemic inflammation positively correlates with POCD, suggesting that changes in immune activation,at least in the periphery, may contribute to the development of this condition (Burkhart et al., 2010; Hudetz et al., 2011). Other inflammatory insults can also induce cognitive decline in aged individuals. For example, in elderly individuals with Alzheimer’s disease, an inflammatory insults such as periodontitis, is associated with cognitive decline (Ide et al., 2016). However, cognitive decline is not always associated with increased inflammation in the aged population (Hajjar et al., 2018; Knezevic et al., 2017).
Stress and GCs are also associated with primed immune cell responses in humans. Several studies have characterized the effects of stress on peripheral immune cell populations. For example, Powell et al, demonstrated that stress may induce monocyte proliferation in humans and mice (microglia are the CNS myeloid cell and monocytes are the blood myeloid cell). Indeed, chronic social stress defined by socioeconomic status in humans and repeated social defeat stress in mice was associated with selective up-regulation of a subpopulation of immature pro-inflammatory monocytes identified with a transcriptome analysis of the circulating leukocyte pool (Powell et al., 2013). Stress may also cause peripheral immune cells to become hyper-responsive by inducing GC receptor resistance. Cohen et al, demonstrated that stressful life events were associated with GC receptor resistance in peripheral lymphocytes (Cohen et al., 2012). GC resistance positively correlated with nasal pro-inflammatory cytokine expression following a cold virus challenge (Cohen et al., 2012). Furthermore, the treatment of peripheral blood mononuclear cells from healthy donors with GCs can upregulate expression of chemokine (CCR1 and CCR2) and cytokine (IL-1R1,TNFR, IFNγR1) receptors and complement family (C1q, C3, C5) genes assessed with DNA microarray and qPCR, while repressing the expression of adaptive immune-related genes (Galon et al., 2002). In a random double-blind study, Yeager and colleagues demonstrated that prior treatment (24h prior) of human volunteers with intravenous infusion of hydrocortisone enhanced plasma concentrations of the pro-inflammatory cytokine IL-6 at 3h and 4h post-LPS treatment (Yeager et al., 2009). Notably, LPS-induced plasma levels of the anti-inflammatory cytokine IL-10 were reduced by prior hydrocortisone treatment.
Importantly, stress-induced changes in innate immune activity are likely not limited to the periphery. Stress is a predisposing factor for the development of neuropsychiatric disorders (major depressive disorder, generalized anxiety disorder, post-traumatic stress disorder, etc.) and there is evidence that enhanced neuroimmune activation may occur in a subset of patients with neuropsychiatric disorders (Dantzer et al., 2008). For example, elevated microglia density in limbic brain structures has been found in brains after suicide (Schnieder et al., 2014; Steiner et al., 2008). Moreover, myeloid cells in the brains of depressed individuals that committed suicide show a more primed phenotype (Torres-Platas et al., 2014). Consistent with these findings, imaging studies reveal that major depressive episodes are associated with increased translocator protein density, which is increased in activated microglia (Setiawan et al., 2015).
VII. Conclusions
Neuroinflammatory priming is a common mechanism linking pathology caused by stress and by aging. Homeostatic disruptions can cause direct and immediate neuroinflammatory changes, such as increases in pro-inflammatory cytokines. This pro-inflammatory response, however, is typically brief and so cannot account for long-term changes that depend on CNS immune processes. However, here we focus on a long-term and more derivative change. Through both peripheral and central signaling, CNS cells – particularly microglia – can become primed. After stress or during aging, primed microglia produce new “silent” immune machinery that does not cause immediate immune activation, but rather, this immune machinery primes the cell for a subsequent immune insult and a hyperinflammatory response that can exacerbate pathology (Fig 1 and 2). Remarkable parallels exist between stress- and aging-elicited microglial priming. Microglia priming by stress or aging can be elicited by glucocorticoid signaling, by excess release of endogenous DAMPs, by dysregulated neuron-microglia homeostatic signaling (e.g., CD200L-CD200R, CX3CL1-CX3CR1), and by disrupted circadian rhythms (Fig 4). Accumulating research highlights the probable relevance of microglial activation in stress and aging in human brains. Therefore, optimizing neuroinflammatory responses could have broad benefits for organismal responses to stress, aging, and other CNS disorders. Future research will reveal more details regarding substrates that underlie microglial priming, how priming occurs in females versus males, and how to manipulate priming so that microglia produce effective inflammatory responses while minimizing pathology.
Highlights.
Stress and aging can prime neuroinflammatory processes
Priming can cause pathological neuroinflammation following an immune challenge
Silent immune machinery produced by microglia in stress and aging may cause priming
Here we discuss common mechanisms of priming induced by stress and aging
Acknowledgements
L.K.F. was supported by a NARSAD Young Investigator Grant sponsored by the NY Women’s Committee. M.G.F. and S.F.M. were supported by NIH grant R01MH108523. A.D.G. was supported by Craig H. Neilsen Foundation (382497) and the Wings for Life Foundation (Project number 139).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Johnson RW, 2009. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun 23, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI, 2014. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 155, 1802–1813. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM, 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience 10, 1538–1543. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B, 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117, 145–152. [DOI] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnar Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D, 2017. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep 18, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K, 2015. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF, 2001. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav 39, 247–257. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C, 2011. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging 32, 2030–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, 2015. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun 44, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Erickson MA, 2010. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 37, 26–32. [DOI] [PubMed] [Google Scholar]
- Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF, 1993. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol 150, 1999–2006. [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF, 2011. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci 31, 11578–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF, 2009. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun 23, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF, 2010. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis 1, 212–231. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF, 2012. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci 32, 14641–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA, Maier SF, 2015. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol Aging 36, 1483–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW, 2004. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res 78, 151–156. [DOI] [PubMed] [Google Scholar]
- Bellavance MA, Rivest S, 2014. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front Immunol 5, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, Cirelli C, 2017. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J Neurosci 37, 5263–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, Stevens B, 2013. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nature neuroscience 16, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bianchi ME, 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81, 1–5. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW, 2007. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 30, 596–602. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J, 2015. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Blau CW, Cowley TR, O’Sullivan J, Grehan B, Browne TC, Kelly L, Birch A, Murphy N, Kelly AM, Kerskens CM, Lynch MA, 2012. The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol Aging 33, 1005 e1023–1035. [DOI] [PubMed] [Google Scholar]
- Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA, 2017. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 94, 759–773 e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaconsa M, Malpeli G, Montaruli A, Carandente F, Grassi-Zucconi G, Bentivoglio M, 2014. Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Experimental gerontology 55, 70–79. [DOI] [PubMed] [Google Scholar]
- Borniger JC, Cisse YM, Surbhi, Nelson RJ, 2017. Reciprocal regulation of circadian rhythms and immune function. Current Sleep Medicine Reports 3, 93–103. [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, Wyss-Coray T, 2003. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145. [DOI] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW, 2008. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology 33, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart CS, Dell-Kuster S, Gamberini M, Moeckli A, Grapow M, Filipovic M, Seeberger MD, Monsch AU, Strebel SP, Steiner LA, 2010. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 24, 555–559. [DOI] [PubMed] [Google Scholar]
- Busillo JM, Azzam KM, Cidlowski JA, 2011. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. The Journal of biological chemistry 286, 38703–38713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL, 2014. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA, 2017. Immune regulation by glucocorticoids. Nat Rev Immunol 17, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD, 2016. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 233, 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM, 2006. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience 9, 917–924. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ, 2010. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185, 5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW, 2008. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22, 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, Jope RS, Beurel E, 2016. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Ben-Yehuda H, Porat Z, Raposo C, Gordon S, Schwartz M, 2017. Newly Formed Endothelial Cells Regulate Myeloid Cell Activity Following Spinal Cord Injury via Expression of CD200 Ligand. J Neurosci 37, 972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB, 2012. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A 109, 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Vainer E, Matar MA, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA, Cohen H, 2015. Diurnal Fluctuations in HPA and Neuropeptide Y-ergic Systems Underlie Differences in Vulnerability to Traumatic Stress Responses at Different Zeitgeber Times. Neuropsychopharmacology 40, 774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP, 2010. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps KN, Roth TL, McGavern DB, 2015. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Lyons A, Denieffe S, Browne TC, Cox FF, Lynch MA, 2011. Long term potentiation is impaired in membrane glycoprotein CD200-deficient mice: a role for Toll-like receptor activation. The Journal of biological chemistry 286, 34722–34732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox FF, Carney D, Miller AM, Lynch MA, 2012. CD200 fusion protein decreases microglial activation in the hippocampus of aged rats. Brain Behav Immun 26, 789–796. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW, 2012. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation 9, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB, 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience 8, 752–758. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, Zhang HY, Liu QR, Shen H, Xi ZX, Goldman D, Bonci A, 2017. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 95, 341–356 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Munoz MF, Machado A, Venero JL, 2014. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A, 2006. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci 26, 5709–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, 2003. Exposure to forced swim stress does not alter central production of IL-1. Brain Res 972, 53–63. [DOI] [PubMed] [Google Scholar]
- Degroot DW, Kenney WL, 2007. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol 292, R103–108. [DOI] [PubMed] [Google Scholar]
- Dendrou CA, Fugger L, Friese MA, 2015. Immunopathology of multiple sclerosis. Nat Rev Immunol 15, 545–558. [DOI] [PubMed] [Google Scholar]
- Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA, 2013. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun 34, 86–97. [DOI] [PubMed] [Google Scholar]
- Dentesano G, Straccia M, Ejarque-Ortiz A, Tusell JM, Serratosa J, Saura J, Sola C, 2012. Inhibition of CD200R1 expression by C/EBP beta in reactive microglial cells. J Neuroinflammation 9, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH, 2009. Cytokines and CNS development. Neuron 64, 61–78. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, 1999. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A 96, 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, Rosen CL, Huber JD, O’Callaghan JP, 2010. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience 170, 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Collins A, Lightman SL, Linthorst AC, Reul JM, 2009. Distinct, time-dependent effects of voluntary exercise on circadian and ultradian rhythms and stress responses of free corticosterone in the rat hippocampus. Endocrinology 150, 4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, 1991. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol 261, R928–933. [DOI] [PubMed] [Google Scholar]
- Elliott EI, Sutterwala FS, 2015. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265, 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J, 2011. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging 32, 85–102. [DOI] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E, 2005. Preferential expression and function of Toll-like receptor 3 in human astrocytes. Journal of neuroimmunology 159, 12–19. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP, 2014a. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry 76, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP, 2014b. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci 34, 8904–8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF, 1995. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology 136, 5336–5342. [DOI] [PubMed] [Google Scholar]
- Foertsch S, Fuchsl AM, Faller SD, Holzer H, Langgartner D, Messmann J, Strauss G, Reber SO, 2017. Splenic glucocorticoid resistance following psychosocial stress requires physical injury. Scientific reports 7, 15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Gaudet AD, D’Angelo HM, Daut RA, Hampson EC, Ayala MT, Watkins LR, Maier SF, 2018. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF, 2015. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 45, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF, 2016a. The Alarmin HMGB1 Mediates Age-Induced Neuroinflammatory Priming. J Neurosci 36, 7946–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitt MM, Gaudet AD, Barrientos RM, Watkins LR, Maier SF, 2016b. Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol Aging 47, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ, 2013. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun 34, 159–163. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Weber MD, Daut RA, Kitt MM, Frank MG, Watkins LR, Maier SF, 2016c. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology 66, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF, 2015a. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF, 2007. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun 21, 47–59. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF, 2006. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging 27, 717–722. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF, 2010a. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun 24, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF, 2010b. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. Journal of neuroimmunology 226, 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Annis JL, Watkins LR, Maier SF, 2017. Stress disinhibits microglia via down-regulation of CD200R: a mechanism of neuroinflammatory priming. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Annis JL, Watkins LR, Maier SF, 2018a. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain Behav Immun 69, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Heinze JD, Schmidt D, Watkins LR, Maier SF, Lowry CA, 2018b. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF, 2014. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology 40, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF, 2010c. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun 24, 19–30. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF, 2012. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun 26, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF, 2013. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF, 2015b. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun 55, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF, 2016. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol Stress 4, 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TC, Wohleb ES, Zhang Y, Fogaca M, Hare B, Duman RS, 2018. Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior. Biol Psychiatry 83, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, Popovich PG, 2017. Deletion of the Fractalkine Receptor, CX3CR1, Improves Endogenous Repair, Axon Sprouting, and Synaptogenesis after Spinal Cord Injury in Mice. J Neurosci 37, 3568–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O’Shea JJ, Chrousos GP, Bornstein SR, 2002. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 16, 61–71. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG, 2017. MicroRNAs: Roles in Regulating Neuroinflammation. Neuroscientist, 1073858417721150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudier-Diaz MM, Zhang N, Haines AH, Surbhi, Zhou M, DeVries AC, 2017. Social interaction modulates the neuroinflammatory response to global cerebral ischemia in male mice. Brain Res 1673, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Immunological Genome C, 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology 13, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemechu JM, Bentivoglio M, 2012. T Cell Recruitment in the Brain during Normal Aging. Front Cell Neurosci 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW, 2005. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J 19, 1329–1331. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Jauregui-Huerta F, Galvez-Contreras AY, 2010. Immune system modulates the function of adult neural stem cells. Curr Immunol Rev 6, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R, 2009. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30, 30–45. [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW, 2016. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nature neuroscience 19, 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA, 2010. The aging stress response. Mol Cell 40, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Hayek SS, Goldstein FC, Martin G, Jones DP, Quyyumi A, 2018. Oxidative stress predicts cognitive decline with aging in healthy adults: an observational study. J Neuroinflammation 15, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Johnson EA, Brown BW, Bittner JJ, 1960. Susceptibility rhythm to E. coli endotoxin and bioassay. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine 103, 142–144. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Taishi P, Chen Z, Krueger JM, 1998. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. J Neurosci 18, 2247–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L, 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 95, 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AD, Wyttenbach A, Perry VH, Teeling JL, 2012. Age related changes in microglial phenotype vary between CNS regions: grey versus white matter differences. Brain Behav Immun 26, 754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL, 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12, 123–137. [DOI] [PubMed] [Google Scholar]
- He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, Pei Z, Xu GQ, Lan Y, 2017. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci 10, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig S, Brioschi S, Dieni S, Frings L, Masuch A, Blank T, Biber K, 2016. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav Immun 55, 126–137. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE, 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24, 151–180. [DOI] [PubMed] [Google Scholar]
- Hermoso MA, Matsuguchi T, Smoak K, Cidlowski JA, 2004. Glucocorticoids and tumor necrosis factor alpha cooperatively regulate toll-like receptor 2 gene expression. Mol Cell Biol 24, 4743–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernangomez M, Klusakova I, Joukal M, Hradilova-Svizenska I, Guaza C, Dubovy P, 2016. CD200R1 agonist attenuates glial activation, inflammatory reactions, and hypersensitivity immediately after its intrathecal application in a rat neuropathic pain model. J Neuroinflammation 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Molina R, von Bernhardi R, 2005. Transforming growth factor-beta 1 produced by hippocampal cells modulates microglial reactivity in culture. Neurobiol Dis 19, 229–236. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J, 2013. The microglial sensome revealed by direct RNA sequencing. Nature neuroscience 16, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD, 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290, 1768–1771. [DOI] [PubMed] [Google Scholar]
- Holder MK, Blaustein JD, 2017. Developmental time course and effects of immunostressors that alter hormone-responsive behavior on microglia in the peripubertal and adult female mouse brain. PLoS One 12, e0171381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Latz E, 2010. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 40, 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Yen FC, Shie FS, Pan CM, Shiao YJ, Yang CN, Huang FL, Sung YJ, Tsay HJ, 2010. TGF-beta1 blockade of microglial chemotaxis toward Abeta aggregates involves SMAD signaling and down-regulation of CCL5. J Neuroinflammation 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP, 2008. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging 29, 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS, 2011. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth 25, 1–9. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Ryken TC, Theodore N, 2013. Pharmacological therapy for acute spinal cord injury. Neurosurgery 72 Suppl 2, 93–105. [DOI] [PubMed] [Google Scholar]
- Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P, Raybould R, Thomas R, Puenter U, Teeling J, Perry VH, Holmes C, 2016. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS One 11, e0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving PM, Gearry RB, Sparrow MP, Gibson PR, 2007. Review article: appropriate use of corticosteroids in Crohn’s disease. Aliment Pharmacol Ther 26, 313–329. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL, 2009. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastremski M, Sutton-Tyrrell K, Vaagenes P, Abramson N, Heiselman D, Safar P, 1989. Glucocorticoid treatment does not improve neurological recovery following cardiac arrest. Brain Resuscitation Clinical Trial I Study Group. JAMA 262, 3427–3430. [PubMed] [Google Scholar]
- Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD, 2006. Regulation of myeloid cell function through the CD200 receptor. J Immunol 176, 191–199. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M, 2005. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135, 1295–1307. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF, 2002. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun 16, 461–476. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF, 2003. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol 284, R422–432. [DOI] [PubMed] [Google Scholar]
- Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC, 2009. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A 106, 5895–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Billakanti R, 2012. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol 46, 251–264. [DOI] [PubMed] [Google Scholar]
- Katsumoto A, Lu H, Miranda AS, Ransohoff RM, 2014. Ontogeny and functions of central nervous system macrophages. J Immunol 193, 2615–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, Miller DB, Bowyer JF, O’Callaghan JP, 2012. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. Journal of neurochemistry 122, 995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG, 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29, 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic D, Verhoeff NPL, Hafizi S, Strafella AP, Graff-Guerrero A, Rajji T, Pollock BG, Houle S, Rusjan PM, Mizrahi R, 2017. Imaging microglial activation and amyloid burden in amnestic mild cognitive impairment. J Cereb Blood Flow Metab, 271678X17741395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP, 2006. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes & development 20, 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I, 2009. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol 68, 159–167. [DOI] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R, 2013. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Molecular psychiatry. [DOI] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M, 2014. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, Musiek ES, 2018. Regulation of amyloid-beta dynamics and pathology by the circadian clock. The Journal of experimental medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ, 2013. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34, 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Baskin RK, Pitler TA, 1981. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science 214, 581–584. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Cooper HA, Maric D, Herkenham M, 2016. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation 13, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise TL, Harrington ME, Molyneux PC, Song I, Queenan H, Zimmerman E, Lall GS, Biello SM, 2013. Voluntary exercise can strengthen the circadian system in aged mice. Age (Dordr) 35, 2137–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y, Yang YY, Peng W, Zhang T, Zhou JR, Jiang CL, Wang YX, 2017. Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid-HMGB1. Brain Behav Immun 59, 322–332. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Muller N, Herold MJ, van den Brandt J, Reichardt HM, 2007. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 122, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J, 2011. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci 89, 141–146. [DOI] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR, 2011. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun 25, 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ, 2005. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5, 331–342. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J, 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrance SA, Ionadi A, McKay E, Douglas X, Johnson JD, 2016. Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology 68, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ, 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature neuroscience 1, 69–73. [DOI] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, Lynch MA, 2009. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem 110, 1547–1556. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R, 2005. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol 194, 376–383. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD, 2011. Functional recovery in aging mice after experimental stroke. Brain Behav Immun 25, 1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA, 2009. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int 26, 1430–1442. [DOI] [PubMed] [Google Scholar]
- Mattison HA, Nie H, Gao H, Zhou H, Hong JS, Zhang J, 2013. Suppressed pro-inflammatory response of microglia in CX3CR1 knockout mice. Journal of neuroimmunology 257, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF, 2016. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP, 2017. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, Deflorio C, Lau ro C, Alboni S, Limatola C, Branchi I, Tremblay ME, Maggi L, 2016. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav Immun 55, 114–125. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yang MS, Kim SU, Jou I, Joe EH, 2006. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci 26, 1880–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE, 2012. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol 188, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS, 2008. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108, 18–30. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C, 2006. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26, 3813–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM, 2010. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci 30, 13690–13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EK, Spencer RL, Sipe KJ, Herman JP, 2002. Decrements in nuclear glucocorticoid receptor (GR) protein levels and DNA binding in aged rat hippocampus. Endocrinology 143, 1362–1370. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, Fitzgerald GA, 2013. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. The Journal of clinical investigation 123, 5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH, 2006. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol 171, 72–85. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, B lock GD, Age-Related Changes in the Circadian System Unmasked by Constant Conditions(1,2,3). eNeuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato R, Hotta S, Yamada D, Kou M, Nakamura S, Takahata Y, Tei H, Numano R, Hida A, Shimba S, Mieda M, Hinoi E, Yoneda Y, Takarada T, 2017. The intrinsic microglial clock system regulates interleukin-6 expression. Glia 65, 198–208. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF, 2000. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res 859, 193–201. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F, 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Niraula A, Wang Y, Godbout JP, Sheridan JF, 2018. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J Neurosci 38, 2328–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh MY, Lim SM, Oh KW, Cho KA, Park J, Kim KS, Lee SJ, Kwon MS, Kim SH, Mesenchymal Stem Cells Modulate the Functional Properties of Microglia via TGF-beta Secretion. Stem Cells Transl Med 5, 1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP, 2014. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 62, 881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Walker FR, Godbout JP, 2016. Insensitivity of astrocytes to interleukin 10 signaling following peripheral immune challenge results in prolonged microglial activation in the aged brain. Neurobiol Aging 44, 22–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC, 2010. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci U S A 107, 16342–16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L, 2012. Memory aging and brain maintenance. Trends Cogn Sci 16, 292–305. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF, 2003. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res 991, 123–132. [DOI] [PubMed] [Google Scholar]
- Olah M, Patrick E, Villani AC, Xu J, White CC, Ryan KJ, Piehowski P, Kapasi A, Nejad P, Cimpean M, Connor S, Yung CJ, Frangieh M, McHenry A, Elyaman W, Petyuk V, Schneider JA, Bennett DA, De Jager PL, Bradshaw EM, 2018. A transcriptomic atlas of aged human microglia. Nat Commun 9, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM, 2008. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma 25, 153–171. [DOI] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G, 2006. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell metabolism 4, 163–173. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C, 2014. Microglial priming in neurodegenerative disease. Nat Rev Neurol 10, 217–224. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT, 1997. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. The Journal of comparative neurology 377, 443–464. [DOI] [PubMed] [Google Scholar]
- Porterfield VM, Gabella KM, Simmons MA, Johnson JD, 2012. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1beta production. Brain Behav Immun 26, 1249–1255. [DOI] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW, 2013. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN, 2013. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun 30, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF, 2003. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. Journal of neuroimmunology 137, 51–58. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH, 2009. Microglial physiology: unique stimuli, specialized responses. Annual review of immunology 27, 119–145. [DOI] [PubMed] [Google Scholar]
- Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW, 2016. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139, 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmerman N, Schottlender N, Reshef R, Dan-Goor N, Yirmiya R, 2017. The hippocampal transcriptomic signature of stress resilience in mice with microglial fractalkine receptor (CX3CR1) deficiency. Brain Behav Immun 61, 184–196. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, Jellison ER, McCullough LD, 2016. Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J Immunol 196, 3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS, 1986. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine reviews 7, 284–301. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA, 1985. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science 229, 1397–1400. [DOI] [PubMed] [Google Scholar]
- Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T, 2014. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol 192, 407–417. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, McKim DB, Wohleb ES, Jarrett BL, Reader BF, Norden DM, Godbout JP, Sheridan JF, 2015. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience 302, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME, 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195. [DOI] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Mammadzada K, Khan AS, Carlton PM, Perez A, Christian F, Le Moan N, Vagena E, Baeza-Raja B, Rafalski V, Chan JP, Nitschke R, Houslay MD, Ellisman MH, Wyss-Coray T, Palop JJ, Akassoglou K, 2015. Nuclear pore complex remodeling by p75(NTR) cleavage controls TGF-beta signaling and astrocyte functions. Nature neuroscience 18, 1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, 2014. Complexity of danger: the diverse nature of damage-associated molecular patterns. The Journal of biological chemistry 289, 35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Frenette PS, 2013. Circadian control of the immune system. Nat Rev Immunol 13, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieder TP, Trencevska I, Rosoklija G, Stankov A, Mann JJ, Smiley J, Dwork AJ, 2014. Microglia of prefrontal white matter in suicide. J Neuropathol Exp Neurol 73, 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H, Tuchweber B, 1976. Stress in relation to aging and disease In: Everitt A, Burgess J (Eds.), Hypothalamus, Pituitary and Aging. Charles C Thomas, Springfield, IL, pp. 557–573. [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH, 2015. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K, 2007. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424. [DOI] [PubMed] [Google Scholar]
- Smith RG, Betancourt L, Sun Y, 2005. Molecular endocrinology and physiology of the aging central nervous system. Endocrine reviews 26, 203–250. [DOI] [PubMed] [Google Scholar]
- Somjen GG, 1988. Nervenkitt: notes on the history of the concept of neuroglia. Glia 1, 2–9. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP, 2012. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci U S A 109, E2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF, 2001. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol 280, R1799–1805. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B, 2008. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42, 151–157. [DOI] [PubMed] [Google Scholar]
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Group I, 2009. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110, 548–555. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Luebbert H, 2007. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging 28, 1507–1521. [DOI] [PubMed] [Google Scholar]
- Stogsdill JA, Eroglu C, 2017. The interplay between neurons and glia in synapse development and plasticity. Curr Opin Neurobiol 42, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL, 2004. Dystrophic microglia in the aging human brain. Glia 45, 208–212. [DOI] [PubMed] [Google Scholar]
- Szabo G, Lippai D, 2014. Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol 118, 359–380. [DOI] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Datta, M. Sagar, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O, Prinz M, 2017. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nature neuroscience 20, 793–803. [DOI] [PubMed] [Google Scholar]
- Terrando N, Yang T, Wang X, Fang J, Cao M, Andersson U, Erlandsson HH, Ouyang W, Tong J, 2016. Systemic HMGB1 Neutralization Prevents Postoperative Neurocognitive Dysfunction in Aged Rats. Front Immunol 7, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N, 2014. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Tournissac M, Vandal M, Francois A, Planel E, Calon F, 2017. Old age potentiates cold-induced tau phosphorylation: linking thermoregulatory deficit with Alzheimer’s disease. Neurobiol Aging 50, 25–29. [DOI] [PubMed] [Google Scholar]
- Vaine CA, Soberman RJ, 2014. The CD200-CD200R1 inhibitory signaling pathway: immune regulation and host-pathogen interactions. Adv Immunol 121, 191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW, 1997. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol 273, R1957–1964. [DOI] [PubMed] [Google Scholar]
- van Dam AM, Brouns M, Louisse S, Berkenbosch F, 1992. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res 588, 291–296. [DOI] [PubMed] [Google Scholar]
- Varnum MM, Kiyota T, Ingraham KL, Ikezu S, Ikezu T, 2015. The anti-inflammatory glycoprotein, CD200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of Alzheimer’s disease. Neurobiol Aging 36, 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venereau E, Ceriotti C, Bianchi ME, 2015. DAMPs from Cell Death to New Life. Front Immunol 6, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, 2014. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy currents / American Epilepsy Society 14, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Tang TM, Adler CH, Caviness JN, Sabbagh MN, Serrano GE, Sue LI, Beach TG, 2017. Changes in CD200 and intercellular adhesion molecule-1 (ICAM-1) levels in brains of Lewy body disorder cases are associated with amounts of Alzheimer’s pathology not alpha-synuclein pathology. Neurobiol Aging 54, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ma H, Li Z, Gao Y, Cao X, Jiang Y, Zhou Y, Liu S, 2017. Chronic unpredictable stress exacerbates surgery-induced sickness behavior and neuroinflammatory responses via glucocorticoids secretion in adult rats. PLoS One 12, e0183077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF, 2015. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci 35, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Norman GJ, Barker JM, Su AJ, Nelson RJ, Devries AC, 2008. Social isolation potentiates cell death and inflammatory responses after global ischemia. Mol Psychiatry 13, 913–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF, 2012. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry 71, 294–300. [DOI] [PubMed] [Google Scholar]
- Winkler Z, Kuti D, Ferenczi S, Gulyas K, Polyak A, Kovacs KJ, 2017. Impaired microglia fractalkine signaling affects stress reaction and coping style in mice. Behav Brain Res 334, 119–128. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP, 2012. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF, 2011. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31, 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF, 2013. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, Duman RS, 2018. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biol Psychiatry 83, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP Jr., Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA, 2015. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B, 2015. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol 36, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP, 2010. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun 24, 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YZ, Nygard M, Kristensson K, Bentivoglio M, 2010. Regulation of cytokine signaling and T-cell recruitment in the aging mouse brain in response to central inflammatory challenge. Brain Behav Immun 24, 138–152. [DOI] [PubMed] [Google Scholar]
- Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G, FitzGerald GA, 2016. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med 8, 324ra316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Tracey KJ, 2010. Targeting HMGB1 in inflammation. Biochimica et biophysica acta 1799, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC, 2010. Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Frontiers in bioscience 2, 1081–1091. [DOI] [PubMed] [Google Scholar]
- Yeager MP, Rassias AJ, Pioli PA, Beach ML, Wardwell K, Collins JE, Lee HK, Guyre PM, 2009. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Critical care medicine 37, 2727–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R, 2015. Depression as a microglial disease. Trends Neurosci 38, 637–658. [DOI] [PubMed] [Google Scholar]
- Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang Y, Yu J, 2017. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation 14, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, Iliff JJ, 2017. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol 74, 91–99. [DOI] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, McVicar AL, Gensel JC, 2016. Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol Aging 47, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V, 2001. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. Journal of neuroimmunology 115, 135–143. [DOI] [PubMed] [Google Scholar]