Abstract
Hepatic fibrosis is characterized by abnormal accumulation of extracellular matrix (ECM) that can lead to ductopenia, cirrhosis, and even malignant transformation. In this review, we examine cholestatic liver diseases characterized by extensive biliary fibrosis such as Primary Sclerosing Cholangitis (PSC), Primary Biliary Cholangitis (PBC), Polycystic Liver Disease (PLD), and MDR2−/− and BDL mouse models. Following biliary injury, cholangiocytes, the epithelial cells that line the bile ducts, become reactive and adopt a neuroendocrine phenotype in which they secrete and respond to neurohormones and neuropeptides in an autocrine and paracrine fashion. Emerging evidence indicates that cholangiocytes influence and respond to changes in the ECM and stromal cells in the microenvironment. For example, activated myofibroblasts and hepatic stellate cells are major drivers of collagen deposition and biliary fibrosis. Additionally, the liver is richly innervated with adrenergic, cholinergic, and peptidergic fibers that release neurohormones and peptides to maintain homeostasis and can be deranged in disease states. This review summarizes how cholangiocytes interact with their surrounding environment, with particular focus on how autonomic and sensory regulation affects fibrotic pathophysiology.
Introduction
Cholangiocytes are specialized epithelial cells lining the intra and extrahepatic bile ducts that function to modify bile composition through water and electrolyte secretion and absorption and to detoxify xenobiotics. Bile is initially secreted by the apical surface of hepatocytes into specialized interstitial spaces called bile canaliculi that are sealed by tight junctions. Bile flows towards the portal tracts where it first enters the small intrahepatic biliary tree lined with “small” cholangiocytes (~ 8 μm) at the Canals of Herring. The biliary tree becomes progressively larger to form large intrahepatic bile ducts, lined by “large” cholangiocytes (~ 15 μm), and ultimately extrahepatic bile ducts empties bile into the duodenum 1, 2. Large and small cholangiocytes, lining large and small bile ducts, respectively, exhibit distinct morphological, functional, and proliferative features that vary based on the disease state 1–3.
Disease targeting cholangiocytes, termed cholangiopathies, lead to cholestasis, increased biliary pressure, biliary fibrosis, and chronic inflammation that can trigger cirrhosis or malignant transformation 4. Cholestasis refers to the accumulation of bile in hepatic tissue following intrahepatic or extrahepatic biliary obstruction. Extrahepatic obstruction can be caused by gallstones, pancreatic ductal adenocarcinoma, strictures, or cholangiocarcinoma, whereas intrahepatic biliary diseases include Primary Biliary Cholangitis (PBC), Primary Sclerosing Cholangitis (PSC), and polycystic liver disease (PLD) 5. Cholangiopathies are commonly characterized by four main stages of disease. Disease progression begins with portal hepatitis and inflammation with bile duct destruction. This is followed by periportal hepatitis and biliary proliferation, which can progress to fibrous septa or bridging necrosis in the liver and eventually stage four, cirrhosis 6. During late stages of disease, the balance between proliferation and cholangiocyte death is disturbed, leading to ductopenia and further biliary fibrosis 7. However chronic inflammation can also induce cholangiocyte hyperplasia resulting in an increased risk for malignant transformation, especially in primary sclerosing cholangitis 4. Symptoms of cholangiopathies are reflective of the cholestatic process and eventual loss of liver function including fatigue, pruritus, portal hypertension and xanthomas 6. Current treatment options are limited to ursodeoxycholic acid supplementation, providing the best improvement in PBC patients7.
Cirrhosis is end-stage, irreversible liver scarring and the leading cause of liver failure for which the current mainstay of treatment is liver transplant. There is about an 80% mortality rate without transplant once full hepatic failure ensues 5,8. Major consequences of cirrhosis include portal hypertension and its effects such as ascites, variceal bleeding, hepatic encephalopathy and renal failure 9. Liver Fibrosis or scar formation is characterized by abnormal accumulation of extracellular matrix and further progression to cirrhosis involves diffuse scarring with dense fibrous septations around regenerating hepatocytes 5. The onset of liver fibrosis begins with injury or insult to either the hepatic parenchyma or the biliary epithelium, as seen in cholestatic disease. In the face of chronic injury hepatocyte regeneration is no longer sufficient and ductular proliferation of intrahepatic bile ducts takes place. An increased population of cholangiocytes accumulates at the border of the bile ducts and hepatic parenchyma, contributing to the progression of liver fibrosis through the recruitment of fibrogenic cells 7,5. The etiology of injury plays a role in the cirrhotic pattern, with biliary fibrosis inducing toxic bile acid accumulation that leads to inflammation and activation of cholangiocytes and myofibroblasts that cause a portal-portal fibrotic picture 9.
The aim of this paper is to summarize the new and current research on the proliferative, neuroendocrine and secretory effects of cholangiocytes during cholestatic liver injury and the role they may play in initiating liver fibrosis.
Pathobiology of Liver Fibrosis
Microenvironment
The anatomical features and the microenvironment surrounding cholangiocytes play an important role in its pathophysiology. Bile ductules are formed by 4–5 ~8 μm diameter cuboidal-shaped small cholangiocytes 1, 3, whereas large, columnar-shaped cholangiocytes (~15 μm diameter), make up the increasingly larger bile ducts 1, 3. The apical side of cholangiocytes facing the lumen possess single, primary cilia that is used to both sense the composition of passing bile and to physically help push bile along 5. Additionally, cholangiocytes are linked by tight junctions to prevent backflow of water, solutes, and/or toxins. The epithelial barrier can become leaky over the course of injury and lead to the regurgitation of toxic substances back into the hepatic parenchyma.
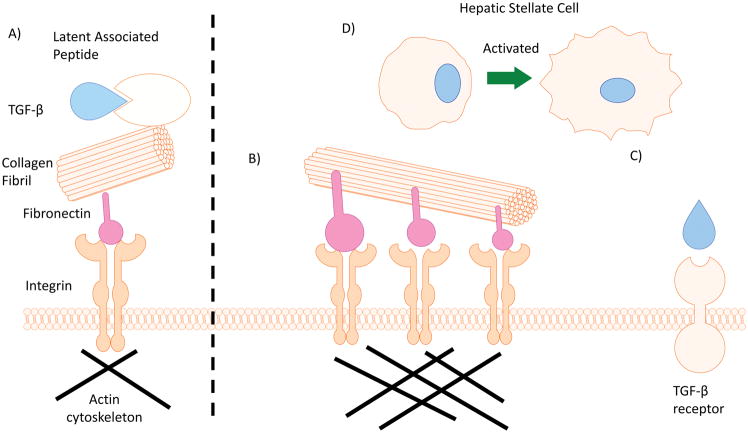
Cholangiocytes sense and respond to their surrounding microenvironment to maintain homeostasis. Biliary epithelium sits atop a basement membrane that separates it from a matrix of proteoglycans and fibrous proteins produced and maintained by portal mesenchymal and fibroblast cells 10. Proteoglycan fibers hold a large amount of water taking on a gel-like consistency that gives tissue the ability to withstand compressive forces. In addition, they also store signaling ligands for TGF-β and Wingless (Wnt) pathways that are released following injury or changes in the microenvironment such as mechanical stress or proteolytic cleavage 11. In contrast, fibrous proteins are more rigid and provide resistance to stretching forces. The major family of fibrous proteins found in the extracellular matrix is collagen (which causes fibrosis when overproduced in disease states) however other fibrous proteins are important as well including fibronectin, elastin, and laminin 10. While collagen and elastin provide tensility and strength to biliary architecture, a specialized membrane-embedded surface receptor, integrin, links cytoskeleton machinery to the ECM 12. Integrins are responsible for both attaching to and migrating through the ECM as well as initiating intracellular signaling in response to extracellular stimuli. Integrins can group together to form focal adhesions to anchor cells in the ECM. However, focal adhesion kinases (FAK) can cross phosphorylate integrin-fibronectin associated structures and cause dissociation. Focal complexes are temporary, sparsely located integrin complexes that form when the cell is migrating through the ECM. The main type of integrin expressed in biliary epithelium is αvβ6 subtype (Figure 1) 13.
Figure 1.
Cholangiocyte physiology
The main physiological role of cholangiocytes is to modify the passage of bile with water and electrolytes and to nullify xenobiotics and microbiota 7, 14. Bile salts are secreted by salt transporters on the apical portion of cholangiocytes and are recycled via the enterohepatic system 15. Bacteria can be recycled from the large intestine along with modified bile acids and come into contact with cholangiocytes, which express toll-like receptors and pathogen-associated molecular patterns 16. In response, cholangiocytes secrete large amounts of IgA and pro-inflammatory cytokines to recruit inflammatory cells and clear unhealthy bacterial presence 16. Following food intake, the hormone secretin is released from S cells of the duodenum as well as cholangiocytes themselves 17. Table 1 provides a complete list of signaling in cholangiocyte pathophysiology.
Table 1.
A list of extracellular hepatic neurohormone, neuropeptides, and autonomic innervation signaling with corresponding changes in intracellular signaling and functional outcome.
| Extracellular Signal | Intracellular Signal | Functional Outcome |
|---|---|---|
| Secretin | ↑ cAMP | ↑ large cholangiocyte proliferation/fibrosis/ductular secretion |
| ↑ Ca2+ | ↑ small cholangiocyte proliferation | |
| Gastrin | ↓ cAMP | ↓ ductular secretion |
| TGR5/FXR | ↑ cAMP | ↑ ductular secretion |
| α1 | ↑ PLC/Ca2+ | ↑ proliferation/activation of HSCs and small cholangiocytes |
| α2 | ↓ cAMP | |
| β 1–3 | ↑ cAMP | ↑ in lammation/fibrosis |
| AT1 | ↑ PKA/ERK1/2/pCREB | ↑ cholangiocyte proliferation |
| AVP | ↑ cAMP | ↑ cholangiocyte proliferation |
| M3 | ↑ cAMP | ↑ cholangiocyte proliferation |
| ↑LJ3K/MEK | ↑ HSC proliferation | |
| α7nAChR | ↑ Ca2+/ERK1/2 | ↑ cholangiocyte proliferation |
| Melatonin-MT1 | ↓ cAMP/PKA | ↓ proliferation/ductular secretion |
| α-CGRP | ↑ cAMP/PKA/CREB | ↓ in lammation |
| SP | ↑ cAMP/PKA/CREB | ↑ cholangiocyte proliferation/fibrosis ↓ apoptosis |
| NPY | - | ↑ HSC/myo ibroblast proliferation/activation |
| IP3/Ca2+/PKC | ↓ cholangiocyte proliferation | |
| H1 | IP3/CAMK1/CREB | ↑ small cholangiocyte proliferation |
| H2 | ↑ cAMP | ↑ large cholangiocyte proliferation |
In response, large cholangiocytes, but not small, have enhanced intracellular cAMP level and activated the apical AE2 channel which exchanges bicarbonate anions into the lumen for chloride anion. This layer of bicarbonate anions is referred to as an “umbrella” and protects the biliary epithelium from the harsh acidity and other toxic components of bile acids 18. Coincidentally, this mechanism can also be stimulated by acetylcholine released from vagal innervation. Secretin also downregulates miR-125b and let7a, which reduces the expression of VEGF and NGF, respectively, to increase biliary proliferation 17. Secretin also drives TGF-β-1/TGF-β-1R autocrine signaling loop in MDR2−/− mouse models and human PSC cases 19. The actions of secretin are countered by somatostatin, which downregulates cAMP, and gastrin, which downregulates expression of secretin receptor itself.
Much of these processes are regulated by bile acid sensing within the cholangiocytes. Surface receptor TGR5, and nuclear farnesoid X receptor (FXR) sense accumulation of bile acids and respond accordingly. TGR5 responds to bile acid sensing by increasing intracellular cAMP thus bolstering the bicarbonate umbrella, by modulating cholangiocyte proliferation and by exerting anti-inflammatory effects 20,21. FXR agonism has similar outcomes. TGR5 agonism and increased cAMP signaling is one mechanism of cholangiocyte hyperproliferation following cholestatic liver injury 22. For example, TGR5 is overexpressed in polycystic liver disease (PLD) and blocking it with an antagonist reduces proliferation and cyst size 22. This outcome is logical as somatostatin, which counteracts secretin signaling and is a cAMP downregulator, is the only currently available therapy for PLD.
Proliferation
Following cholestatic liver injury, cholangiocytes become reactive and adopt a neuroendocrine-like phenotype by secreting and responding to a number of peptides in both autocrine and paracrine fashions. Almost all models of biliary injury trigger cholangiocyte proliferation, a process deemed “ductular reaction.” There are multiple cell types involved in this process, and the type of liver injury determines how ductular reaction takes shape. For example, following bile-duct ligation (BDL) a mouse model for extrahepatic cholestasis, large cholangiocytes respond by undergoing mitosis and proliferating while small cholangiocytes transdifferentiate into large cholangiocytes. Distinct signaling mechanisms regulate these disparate outcomes. In this model, large cholangiocytes respond to increased secretin-secretin receptor cognate interactions to boost intracellular cAMP levels and trigger the PKA/Src/MEK/ERK1/1 pathway 23. In contrast, small cholangiocytes are characterized by activation of the IP3/Ca2+/calmodulin pathway 24. Since new ducts arise from pre-existing ducts, growth is usually constrained to portal areas of injury. However, some cholangiopathies, including PSC and PBC, can involve progenitor cell populations to grow de novo bile ducts. These progenitor cells, sometimes termed “oval” cells, are located in the Canals of Herring where hepatocytes that form bile canaliculi meet with small cholangiocytes of the terminal bile ductules.
The Neurohormonal and Mechanical Basis for Hepatic Fibrosis
A key step in the initiation of liver fibrosis is activation of cells that transform into myofibroblasts 9. In certain models of biliary injury, activated cholangiocytes can recruit inflammatory cells and myofibroblasts to the site of injury where they secrete ECM components and pro-inflammatory, pro-fibrotic cytokines that causes scarring and fibrosis 5, 8. Several sources of myofibroblasts have been identified, including hepatic stellate cells (HSCs) and portal fibroblasts. While epithelial cells (i.e. cholangiocytes) may not undergo epithelial to mesenchymal transition (EMT) based on some reports, they can assume a pro-fibrogenic, non-cuboidal phenotype 25.
TGF-β plays a major role in fibrosis, proliferation, EMT, and ECM turnover. TGF-β lies latent within the extracellular matrix until it is activated by mechanical stress and/or proteolytic cleavage in response to injury or cellular signaling. Once it binds to its cognate receptor (TGF-β receptor), it induces phosphorylation of its associated transcription factor, SMAD. Phosphorylated SMADs then translocate to the nucleus where it controls gene expression. A direct outcome of TGF-β signaling is increased expression, synthesis, and deposition of ECM such as collagen, fibronectin, and proteoglycans. Furthermore, TGF-β inhibits matrix metalloproteinases (MMP) and increases the activity of tissue inhibitors of proteinases (TIMPS) to decrease ECM breakdown. 5,8 An imbalance between MMPs and TIMPS is a major characteristic of fibrosis, with increased TIMP activity greatly increasing ECM deposition.
Recently, the role of mechanical-induced stress and TGF-β activation, and subsequent fibrosis, has been clarified through its relationship with αvβ6 integrin. As mentioned above, integrin-fibronectin complexes can be activated by increased mechanical stress. Increased binding affinity of αvβ6 integrin associates with latency-associated peptide, which releases latent TGF-β and triggers SMAD phosphorylation and nuclear translocation. Wang et al demonstrated that αvβ6 integrin expression is highly upregulated in cholangiocytes following acute biliary obstruction, and that lack of αvβ6 integrin expression ameliorated fibrotic phenotype. Additionally, αvβ6 integrin is implicated in potential cholangiocyte EMT, presumably towards pro-fibrotic fibroblasts 13,26.
Angiogenesis and sinusoidal remodeling also accompanies fibrogenesis with an increase in pro-angiogenic molecules such as vascular endothelial growth factor (VEGF). Angiogenesis occurs along with ductular reaction in order to meet the increased nutritional needs of the proliferating epithelial cells. When cholangiocytes proliferate, VEGF-induced angiogenesis of the peribiliary plexus (PBP) takes place. Cholangiocytes secrete VEGF, allowing for an autocrine regulation of cholangiocyte proliferation and a paracrine regulation of PBP proliferation. 27 VEGF also plays a role in fibrosis, acting to activate HSC and collagen production. Other players in liver fibrosis include Kupffer cells and inflammatory cells such as lymphocytes and mast cells that release pro-fibrogenic molecules 5.
Studies have demonstrated that it is possible to attenuate liver fibrosis, depending on the level of progression. 9 Scar formation can be reversed if the insulting stimulus is removed. HSCs can cease to drive fibrotic reaction if they are inactivated by apoptosis, senescence, or reversion back to their pre-myofibroblast phenotype and by the degradation of collagen via matrix metalloproteinases. 5,8,25. Future therapies aimed at inhibiting pro-fibrotic and/or stimulating anti-fibrotic pathways have the potential to ameliorate difficult-to-treat diseases such as PSC or PBC and pre-empt the need for liver transplantation.
The Role of the Neuroendocrine System
Structure and Function of Hepatic Innervation
The unique neural environment of the hepatobiliary system has been extensively studied in the setting of hepatic fibrosis. The liver contains both efferent and afferent nerves that can be influenced by catecholamines, acetylcholine and neuropeptides. Furthermore, these nerves possess specific locations and differing densities throughout the hepatic architecture. Efferent nerves originate from hypothalamic nuclei and control autonomic output to the liver. Afferent sensory nerves travel either via the vagal pathway to sense and relay information about circulating cytokines and metabolites, or the lower thoracic pathway to relay pain information to the CNS. 28 The nerves follow the pathway of hepatic vasculature within the portal triad. Thus, both hepatic innervation and blood supply are in close contact with biliary microenvironment and ECM. Human liver sections have shown increased intralobular innervation compared to non- human subjects 29.
The autonomic nervous system plays a complex role in liver function including glucose metabolism, fluid balance, regulation of blood and bile flow as well as hepatic regeneration and fibrosis 30. It functions as a two-neuron system consisting of a preganglionic and postganglionic neuron. Sympathetic and parasympathetic nerves both release acetylcholine from preganglionic neurons that synapse with nicotinic acetylcholine receptors on postganglionic nerves.
Sympathetic Nervous System
The sympathetic efferent autonomic innervation originates from the celiac or mesenteric ganglion and release norepinephrine in the liver that interacts with adrenergic receptors. Adrenergic receptors consist of α-1, α-2, β-1, β-2 and β-3 subtypes, each with different receptor mechanisms and downstream effects. A-1 receptors are G protein coupled receptors that activate phospholipase C and intracellular calcium signaling. A-2 receptors inhibit adenylyl cyclase and lower intracellular cAMP levels whereas β-1, β-2 and β-3 receptors stimulate adenylyl cyclase and intracellular cAMP levels.
The sympathetic nervous system and its role in hepatic fibrosis have been well characterized. HSCs express adrenergic receptors and secrete and respond to catecholamines, such as norepinephrine, in an autocrine fashion to increase proliferation 31. α-1 adrenergic receptor stimulation increases collagen deposition from HSCs, presumably in a calcium signaling-dependent manner 28. The total number of HSCs and activated HSCs, as well as liver fibrosis progression, was reduced by chemical sympathectomy and treatment with adrenergic blocking drugs32,33. Furthermore, cholangiocytes express all adrenergic receptor subtypes and activation of α-1 receptors by catecholamines stimulate the growth of small cholangiocytes by activating the IP3/Ca2+/calmodulin pathway 34.
The “Local RAS”
The sympathetic nervous system is amplified during times of stress with many downstream effects, including influences on the renin-angiotensin-aldosterone system (RAS) due to its strict control over renin production. The RAS is responsible for regulating blood pressure and maintaining fluid homeostasis. Decreases in kidney perfusion or direct sympathetic stimulation via B2 adrenergic receptors are potent stimuli for renin release. Renin converts angiotensinogen to angiotensin I in the liver, with subsequent conversion to angiotensin II via the ACE converting enzyme in the lungs. Angiotensin II has many important downstream effects, including regulation of fluid balance by stimulating aldosterone release, increasing tubular reabsorption of sodium, stimulating release of arginine vasopressin and acting as a vasoconstrictor 35. Angiotensin II mediates it effects through two receptor types, AT1 and AT2. The regulatory mechanisms of fluid balance including sodium balance, vasopressin and aldosterone release, and vasoconstriction are mediated by the AT1 receptor. The AT1 receptor has also been implicated to be involved in pro-fibrogenic mechanisms such as cell proliferation, hypertrophy and endothelial dysfunction 36.
The fibrotic effects of the RAS are mediated by a “local RAS” consisting of functional components of the RAS system found to be up-regulated in organs undergoing fibrotic change. In the liver, RAS system components are expressed by hepatic stellate and Kupffer cells. Activation of the AT1 receptor in hepatic stellate cells increases TGF-β1 expression and consequently hepatic fibrosis 37. Cholangiocytes express local RAS components as well and its expression is increased in the cholestatic BDL mouse model. Biliary mass, proliferation, and fibrosis increases following AngII treatment but is reduced with losartan treatment, an AT1R antagonist. In vitro, AT1 receptor activation increases cholangiocyte proliferation in a PKA/ERK1/2/pCREB-dependent fashion38. In MDR2−/− mice, a model of PSC, treatment with propranolol, a non-selective β-adrenergic blocker, decreased hepatic inflammation and fibrosis, including expression of angiotensin 39.
AVP
Arginine vasopressin (AVP), a neurohormone released by the posterior pituitary, is increased in times of pain, stress, trauma, and increased concentrations of angiotensin II. The main role of AVP is to maintain fluid homeostasis in response to changing plasma osmolarity and effective arterial volume 35. Its effects are regulated by two G protein coupled receptors V1 and V2. V2 receptor is upregulated in liver samples from both BDL mice and human polycystic disease. Furthermore, in vitro stimulation of AVP increased cellular proliferation and cAMP levels in both small cholangiocytes and cell lines taken from cystic biliary epithelium 40. Given that the biliary epithelium in both BDL mouse models and human polycystic liver disease experience increased mechanical stress, and AVP functions in part to regulate the mechanical properties in its target organ, the role of AVP in response to increased biliary mechanical stress remains to be elucidated.
Parasympathetic Nervous System
The parasympathetic nervous system signals the liver through the vagus nerve and releases acetylcholine that reacts with both nicotinic and muscarinic acetylcholine receptors. Muscarinic acetylcholine receptors are metabotropic G-protein coupled receptors composed of five subtypes, M1–M5, which vary in tissue location 41. Nicotinic acetylcholine receptors (nAChR) are ionotropic receptors composed of a 4 subunits around a central pore 42. Cholangiocytes primarily express M3 muscarinic receptors and α7 nicotinic receptors (α7nAChR) 43. Acetylcholine is quickly degraded by acetylcholinesterase upon release. Nicotinic receptors, but not muscarinic receptors, can also be stimulated by nicotine, the principal addictive component of tobacco smoke. In contrast to acetylcholine, nicotine cannot be degraded by acetylcholinesterase and has an approximate half-life of two hours. In addition, nicotine’s lipophilic profile allows for its accumulation in tissue 44. Cholinergic stimulation appears to have a pro-proliferation, pro-survival impact on biliary growth. BDL mice who underwent vagotomy exhibited decreased biliary mass and M3 muscarinic receptor expression as well increased cholangiocyte apoptosis. In addition, deactivated cAMP signaling leads to a decrease in ductal secretion 45. HSCs also express muscarinic receptors and activated HSCs upregulate the M3 subtype. HSCs secrete and respond to acetylcholine in an autocrine and paracrine fashion to increase their proliferation and expression of fibrotic markers albeit in a PI3-K and MEK dependent fashion 46.
Nicotine has been shown to be involved in many pro-fibrotic mechanisms, including initial damage to epithelium and contributing to the development of fibrosis through recruiting inflammatory mediators, producing reactive oxygen species and activating the cells responsible for collagen deposition 47. Nicotine has been shown to up-regulate fibrosis in the hearts of rat embryos and decrease cardiac function as seen by decreased blood volume pumped from the left ventricle and ejection fraction 48. Female mice treated with nicotine showed significantly increased heart and liver weight with fat deposition around portal veins and increased necrosis, congestion and fibrosis 49. Nicotine also causes increased activation of HSCs and pro-fibrotic players such as TGF-B and collagen 1-α-2, correlating with increased liver fibrosis 50.
Nicotinic receptors can be classified into neuronal and muscle subtypes. Genome studies demonstrated that neuronal subtypes have been found in non-neuronal tissues including the lung, liver, colon and intestine 51. The neuronal α7nAChR is expressed in macrophages, HSCs, and cholangiocytes and is implicated in inflammation and fibrosis 52,50. Nicotine administration to xenograft cholangiocarcinoma (CCA) mouse models increased CCA proliferation and fibrosis in an α7nAChR-dependent manner 53. Furthermore, chronic nicotine exposure in rats increased biliary proliferation and fibrosis also in an α7nAChR-dependent manner. Specific α7-nAChR agonism acts through the Ca2+/ERK1/2 pathway 54. More studies need to be done in order to categorize other nicotine receptors found in the hepatobiliary system and their functions, as well as whether inhibition of the α 7-nAChR can attenuate proliferation and fibrosis.
The Role of Neuropeptides and Neurohormones
During cholestatic injury, cholangiocytes secrete and respond to neuropeptides and neurohormones, adopting a neuroendocrine phenotype. This is demonstrated by cholangiocyte expression of neuroendocrine cell markers such as chromogranin A and S-100. Neuropeptides are short chain polypeptides that function as neurotransmitters or neurohormones. They can be secreted from peptidergic nerves but are often secreted along with epinephrine or acetylcholine from adrenergic and cholinergic fibers, respectively. Nerve fibers that contain peptides are found in association with hepatic vasculature and bile ducts, including the portal vein and hepatic artery. 29 Neuropeptides serve many functions including vasoconstriction, vasodilation and functioning in efferent sensory pathways with current research focusing on their role in cholangiocyte proliferation. The role these markers play in proliferation, fibrosis, and progression of cholestatic disease is a subject of active research 55.
Melatonin
Melatonin is a neurohormone synthesized in the pineal gland by serotonin N-acetyltransferase (AANAT). It is secreted in bile in high concentrations, indicating a role in the hepatobiliary system. It is thought that it provides antioxidant effects in the hepatobiliary tract by neutralizing reactive oxygen species 56. Synthesis of melatonin is normally increased after prolonged exposure to darkness. In mouse models of BDL and PSC, prolonged exposure to darkness increased melatonin synthesis to reduce biliary hyperplasia and liver fibrosis 57,58. Directly enhancing levels of AANAT and melatonin also attenuate liver fibrosis, with decreased biliary proliferation, improved liver morphology and a decrease in pro-fibrotic cytokines 57. Melatonin acts on melatonin receptor type I (MT1) and downregulates cAMP/PKA levels to reduce biliary proliferation and ductular secretion 59. The role of other melatonin receptors (MT2 and MT3) on biliary pathophysiology remains unclear. Melatonin modulates angiogenesis following cholestatic liver injury and biliary proliferation through control of VEGF levels. Increased angiogenesis plays a crucial role in cholangiocyte proliferation, as increased mass leads to increased nutritional needs. The proliferation of the peribiliary plexus meets these needs by responding to pro-angiogenic factors secreted by proliferating cholangiocytes, such as VEGF. Melatonin plays an anti-angiogenic role, as increases in VEGF were seen when AANAT expression was decreased 60. Furthermore, the reduction in angiogenesis is paralleled by decreased liver fibrosis as well. In mouse models of PSC, miR200b antagonizes AANAT and melatonin expression leading to increased biliary proliferation, angiogenesis, and fibrosis. Overexpressing AANAT or inhibiting miR-200b ameliorated these processes 58.
A-CGRP
A-calcitonin gene-related peptide (α-CGRP) is a 37-amino acid neuropeptide that acts through G protein coupled receptor calcitonin-like receptor (CLR) to upregulate cAMP/PKA/CREB activity. It is most commonly found in capsaicin-sensitive dorsal root ganglia of spinal afferent nerve pathway and thus plays a role in sensing pain. It is also released into peripheral organs in response to stimuli to regulate vasodilation and inflammatory cytokines. In the liver, CGRP-positive nerves are located primarily in the periportal areas in close complex with hepatic vasculature and biliary tree. CLR expression was demonstrated in hepatocytes, Kupffer cells, and B-lymphocytes. CGRP−/− mice exhibited increased hepatic damage in immune-mediated liver injury. Exogenous α-CGRP administration rescued this phenotype and reduced hepatic levels of IL-6 and TNF-α 61.
Additionally, CLR expression was measured in isolated cholangiocytes, and α-CGRP was found in cholangiocyte supernatant, implying the prospect of α-CGRP regulation of biliary growth in an autocrine and paracrine loop. Paradoxically, both exogenous administration of CGRP and CGRP−/− mice exhibited decreased cholangiocyte proliferation following BDL 62. This is in line with previous reports that α-CGRP stimulation can have opposite effects on macrophage activation 61. CGRP plasma levels increase following BDL, and pretreatment of in vitro cholangiocytes with CGRP attenuated the pro-proliferative effects of BDL supernatant 62. While CGRP plays a role in cholangiocyte proliferation, it clearly must also work in concert with other neuropeptides.
Substance P
Substance P (SP) co-localizes with CGRP within the spinal afferent nerve pathway and the periportal nerve plexus and is often released alongside CGRP to mediate hepatic vasodilation 63. SP is part of the tachykinin family and specifically binds to the tachykinin receptor neurokinin-1 (NK-1R). SP is elevated following cholestatic liver injury and drives large cholangiocyte proliferation in a cAMP/PKA/ERK1/2 dependent manner. Loss of NK-1R reduced biliary hyperplasia and expression of fibrotic markers following BDL while increasing biliary apoptosis 64. Normal NK-1R−/− mice also exhibited increased hepatocyte apoptosis and elevated serum transaminases indicating liver damage. Similarly to CGRP, Kupffer cells express NK-1R and respond to SP stimulation by secreting increased levels of IL-6 and TNF-α 65. The SP/NK-1R axis is activated in human PSC samples and is a driver of liver fibrosis in the setting of cholestatic disease. While SP may increase biliary proliferation and ductular mass in normal setting, activating the SP/NK-1R axis in cholestatic settings results in increased large cholangiocyte senescence. Senescence is the process by which cells enter growth arrest and secrete inflammatory cytokines and fibrogenic markers. Additionally, NK-1R is expressed on HSCs and secrete and respond to SP in an autocrine/paracrine fashion to drive proliferation and activation while decreasing senescence. Blocking NK-1R reversed large cholangiocyte senescence while provoking HSC senescence and reduced hepatic fibrosis 66.
Neuropeptide Y (NPY)
NPY is a neuropeptide that is richly expressed in hepatic nerves and counteracts the vasodilatory effects of SP and CGRP 67. Cholangiocytes and HSCs express at least some of the Y1–Y5 receptors. NPY and Y2R cognate interaction activates HSCs into a myofibroblastic state and increases proliferation and production of fibrogenic factors 31,68. While cholangiocytes secrete NPY into their supernatant, NPY actually has an anti-proliferative effect on biliary epithelium. NPY secretion decreases following BDL and exogenous NPY administration decreased biliary duct mass 69. Additionally, NPY is upregulated in CCA models and slows tumor growth and invasion in a IP3/Ca2+/PKC-dependent manner 70.
Histamine
Histamine, an aminergic molecule, is produced by histidine decarboxylase (HDC) in pre-formed granules and most abundantly carried by mast cells. Mast cell activation includes, but is not limited by, G-protein coupled histamine receptors 1–4 (H1–4R) binding which causes intracellular signaling changes and degranulation, or release, of histamine. Small cholangiocytes proliferate following H1R activation in a IP3/CaMK I/CREB dependent pathway whereas large cholangiocytes proliferate following H2R activation through cAMP upregulation. H3–4R are thought to be anti-proliferative. Following cholestatic liver injury, mast cells are recruited to the liver and hepatic and serum histamine levels increase. Animal knockout models or cromolyn sodium treatment that reduces mast cell recruitment and histamine levels reduces biliary growth, fibrosis, liver damage and angiogenesis71. It is important to note that mast cells release other factors such as TGF-β1 and VEGF that can affect biliary growth, fibrosis, and angiogenesis72.
Intrahepatic Cholangiopathies
Primary biliary cholangitis is an autoimmune cholangiopathy most common in females over the age of 40 that is characterized by only small intrahepatic bile duct destruction. 5 Presence of antimitochondrial antibodies and reactive t-lymphocytes supports an autoimmune etiology with the characteristic presence of self-reactive cytotoxic T-cells against pyruvate dehydrogenase complex antigens. 73 It is characterized histologically by the florid duct lesion, which involves destruction of interlobular bile ducts often accompanied by granulomas or the absence of bile ducts. 5 Diagnosis requires at least two positive diagnostic criteria, which include a persistent increase in alkaline phosphatase levels for greater than 6 months, a positive anti-mitochondrial antibody titer, or a positive liver biopsy.74
In contrast, primary sclerosing cholangitis is diagnosed mostly in men in the third through fifth decades of life. Etiology is unclear but studies have shown that both genetic and environmental factors play a role. The strong association between ulcerative colitis and primary sclerosing cholangitis supports a theory that alterations in the normal gut microbiome may be involved. 75 It is characterized histologically by large duct inflammation that involves infiltration by neutrophils and lymphocytes leading to the development of strictures and eventual scarring. Small duct involvement is often characterized by concentric onion skin fibrosis. 5 Fibropolycystic liver disease is a type of congenital cholangiopathy that includes congenital hepatic fibrosis, biliary hamartomas, autosomal dominant polycystic kidney disease, Caroli disease and choledochal cysts. All are characterized by abnormal embryologic development of the cell layer surrounding the portal vein, which give rise to biliary ducts. Consequences include cholangitis, portal hypertension or possible obstruction. 76 Choledochal cysts often predispose to obstructive liver disease such as gallstones, strictures or stenosis and are characterized by congenital dilation of the common bile duct and increased pressure across the biliary tree that can present with symptoms such as jaundice or biliary colic 5.
Conclusion and future perspectives
This review focuses on the role of sensory innervation in cholestatic liver disease and the pathophysiology of liver fibrosis. It highlights the complex interplay of cholangiocyte proliferation, fibrosis, and angiogenesis as well its interaction with the surrounding ECM and stromal cell types. The role of mechanical stress in extrahepatic biliary obstruction and PLD remain an area of active research and have the potential to shed new insights into these disease processes. Furthermore, how hepatic innervation and neurohormonal/peptide signaling affects cholangiopathies could lay the groundwork for comprehensive therapies in the future. Deeper understanding of these overlapping and often redundant pathways is necessary in order to make progress on disease management.
Acknowledgments
This work was supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award to Dr. Alpini (5I01BX000574), a VA Merit Award (5I01BX002192) to Dr. Glaser, a VA Merit Award (1I01BX001724) to Dr. Meng from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and the NIH grants DK110035, DK107310, DK76898, DK115184, DK054811 to Drs. Alpini, Meng and Glaser. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–43. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti A, Bassotti C, Rapino K, Marucci L, Jezequel AM. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol. 1996;24:335–42. doi: 10.1016/s0168-8278(96)80014-6. [DOI] [PubMed] [Google Scholar]
- 3.Glaser S, Gaudio E, Rao A, Pierce L, Onori P, Franchitto A, Francis H, Dostal DE, Venter J, DeMorrow S, Mancinelli R, Carpino G, Alvaro D, Kopriva S, Savage J, Alpini G. Morphological and Functional Heterogeneity of the Mouse Intrahepatic Biliary Epithelium. Laboratory investigation; a journal of technical methods and pathology. 2009;89:456–69. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SM. The Crucial Role of Cholangiocytes in Cholangiopathies. Gut and Liver. 2012;6:295–304. doi: 10.5009/gnl.2012.6.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar V, Abbas AK, Aster JC. Pathologic Basis of Disease. 9. Philadelphia, PA: Elselvier Saunders; 2015. [Google Scholar]
- 6.Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology (Baltimore, Md) 2000;31:1005–13. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 7.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–96. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–41. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bottcher K, Pinzani M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Advanced drug delivery reviews. 2017 doi: 10.1016/j.addr.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Wells RG. Cellular Sources of Extracellular Matrix in Hepatic Fibrosis. Clinics in liver disease. 2008;12:759, viii. doi: 10.1016/j.cld.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piersma B, Bank RA, Boersema M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Frontiers in Medicine. 2015;2:59. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nejjari M, Hafdi Z, Gouysse G, Fiorentino M, Beatrix O, Dumortier J, Pourreyron C, Barozzi C, D’Errico A, Grigioni WF, Scoazec JY. Expression, regulation, and function of alpha V integrins in hepatocellular carcinoma: an in vivo and in vitro study. Hepatology (Baltimore, Md) 2002;36:418–26. doi: 10.1053/jhep.2002.34611. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of αvβ6 Integrin in Acute Biliary Fibrosis. Hepatology (Baltimore, Md) 2007;46:1404–12. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–78. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–42. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Chen X-M, O’Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunology and cell biology. 2008;86:497–505. doi: 10.1038/icb.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, McDaniel K, Marzioni M, Invernizzi P, Ueno Y, Lai JM, Huang L, Standeford H, Alvaro D, Gaudio E, Franchitto A, Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–808. e12. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3)(−) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology (Baltimore, Md) 2010;52:1489–96. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 19.Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, Marzioni M, Alvaro D, Franchitto A, Gaudio E, Glaser S, Alpini G. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-beta1 biliary secretion in mice. Hepatology (Baltimore, Md) 2016;64:865–79. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masyuk TV, Masyuk AI, LaRusso NF. TGR5 in the Cholangiociliopathies. Digestive diseases (Basel, Switzerland) 2015;33:420–5. doi: 10.1159/000371696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keitel V, Reich M, Haussinger D. TGR5: pathogenetic role and/or therapeutic target in fibrosing cholangitis? Clinical reviews in allergy & immunology. 2015;48:218–25. doi: 10.1007/s12016-014-8443-x. [DOI] [PubMed] [Google Scholar]
- 22.Masyuk TV, Masyuk AI, Lorenzo Pisarello M, Howard BN, Huang BQ, Lee PY, Fung X, Sergienko E, Ardecky RJ, Chung TDY, Pinkerton AB, LaRusso NF. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Galphas signaling. Hepatology (Baltimore, Md) 2017 doi: 10.1002/hep.29284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–37. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, DeMorrow S, Carpino G, Kopriva S, White M, Fava G, Alvaro D, Alpini G. After Damage of Large Bile Ducts by Gamma-Aminobutyric Acid, Small Ducts Replenish the Biliary Tree by Amplification of Calcium-Dependent Signaling and de Novo Acquisition of Large Cholangiocyte Phenotypes. The American Journal of Pathology. 2010;176:1790–800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin D, Koyama Y, Brenner D, Kisseleva T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation; research in biological diversity. 2016;92:84–92. doi: 10.1016/j.diff.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of Integrin Alphavbeta6 on Cholangiocytes Blocks Tgfbeta Activation and Retards Biliary Fibrosis Progression. Gastroenterology. 2008;135:660–70. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Liang Y, Kong F, Qiu J, Liu X, Chen A, Luxon BA, Wu HW, Wang Y. Vascular endothelial growth factor promotes the activation of hepatic stellate cells in chronic schistosomiasis. Immunol Cell Biol. 2017;95:399–407. doi: 10.1038/icb.2016.109. [DOI] [PubMed] [Google Scholar]
- 28.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004;280:808–20. doi: 10.1002/ar.a.20086. [DOI] [PubMed] [Google Scholar]
- 29.McCuskey RS. Anatomy of efferent hepatic nerves. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004;280:821–6. doi: 10.1002/ar.a.20087. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno K, Ueno Y. Autonomic Nervous System and the Liver. Hepatology research : the official journal of the Japan Society of Hepatology. 2016 doi: 10.1111/hepr.12760. [DOI] [PubMed] [Google Scholar]
- 31.Oben JA, Roskams T, Yang S, Lin H, Sinelli N, Torbenson M, Smedh U, Moran TH, Li Z, Huang J, Thomas SA, Diehl AM. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004;53:438–45. doi: 10.1136/gut.2003.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mende S, Schulte S, Strack I, Hunt H, Odenthal M, Pryymachuck G, Quasdorff M, Demir M, Nierhoff D, Dienes HP, Goeser T, Steffen HM, Tox U. Telmisartan plus propranolol improves liver fibrosis and bile duct proliferation in the PSC-like Abcb4−/− mouse model. Digestive diseases and sciences. 2013;58:1271–81. doi: 10.1007/s10620-012-2499-3. [DOI] [PubMed] [Google Scholar]
- 33.Tian X, Zhao C, Guo J, Xie S, Yin F, Huo X, Zhang X. Carvedilol Attenuates the Progression of Hepatic Fibrosis Induced by Bile Duct Ligation. BioMed research international. 2017;2017:4612769. doi: 10.1155/2017/4612769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alpini G, Franchitto A, DeMorrow S, Onori P, Gaudio E, Wise C, Francis H, Venter J, Kopriva S, Mancinelli R, Carpino G, Stagnitti F, Ueno Y, Han Y, Meng F, Glaser S. Activation of Alpha(1)-Adrenergic Receptors Stimulate the Growth of Small Mouse Cholangiocytes via Ca(2+)-dependent Activation of NFAT2 and SP1. Hepatology (Baltimore, Md) 2011;53:628–39. doi: 10.1002/hep.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhoades RA, Bell DR. Autonomic Nervous System Medical Physiology: Principles for Clinical Medicine. 4. Baltimore, MD: Wolters Kluwer Business; 2013. pp. 311–4. [Google Scholar]
- 36.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood pressure. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 37.Simões e Silva AC, Miranda AS, Rocha NP, Teixeira AL. Renin angiotensin system in liver diseases: Friend or foe? World Journal of Gastroenterology. 2017;23:3396–406. doi: 10.3748/wjg.v23.i19.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afroze SH, Munshi MK, Martinez AK, Uddin M, Gergely M, Szynkarski C, Guerrier M, Nizamutdinov D, Dostal D, Glaser S. Activation of the renin-angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. American journal of physiology Gastrointestinal and liver physiology. 2015;308:G691–701. doi: 10.1152/ajpgi.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strack I, Schulte S, Varnholt H, Schievenbusch S, Tox U, Wendland K, Steffen HM, Drebber U, Dienes HP, Odenthal M. beta-Adrenoceptor blockade in sclerosing cholangitis of Mdr2 knockout mice: antifibrotic effects in a model of nonsinusoidal fibrosis. Lab Invest. 2011;91:252–61. doi: 10.1038/labinvest.2010.162. [DOI] [PubMed] [Google Scholar]
- 40.Mancinelli R, Franchitto A, Glaser S, Vetuschi A, Venter J, Sferra R, Pannarale L, Olivero F, Carpino G, Alpini G, Onori P, Gaudio E. Vasopressin regulates the growth of the biliary epithelium in polycystic liver disease. Lab Invest. 2016;96:1147–55. doi: 10.1038/labinvest.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eglen RM. Muscarinic receptor subtype pharmacology and physiology. Progress in medicinal chemistry. 2005;43:105–36. doi: 10.1016/S0079-6468(05)43004-0. [DOI] [PubMed] [Google Scholar]
- 42.Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiological Reviews. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marzioni M, Fava G, Benedetti A. Nervous and Neuroendocrine regulation of the pathophysiology of cholestasis and of biliary carcinogenesis. World Journal of Gastroenterology. 2006;12:3471–80. doi: 10.3748/wjg.v12.i22.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. The AAPS journal. 2006;7:E885–94. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeSage G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–9. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 46.Morgan ML, Sigala B, Soeda J, Cordero P, Nguyen V, McKee C, Mouraliderane A, Vinciguerra M, Oben JA. Acetylcholine induces fibrogenic effects via M2/M3 acetylcholine receptors in non-alcoholic steatohepatitis and in primary human hepatic stellate cells. Journal of gastroenterology and hepatology. 2016;31:475–83. doi: 10.1111/jgh.13085. [DOI] [PubMed] [Google Scholar]
- 47.Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. The FASEB Journal. 2012;26:4778–87. doi: 10.1096/fj.12-206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu F, Zheng A, Qian J, Li Y, Wu L, Yang J, Gao X. Prenatal nicotine exposure results in the myocardial fibrosis in the adult male offspring rats. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2016;68:445–50. doi: 10.1016/j.etp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Iranloye BO, Bolarinwa AF. Effect of nicotine administration on weight and histology of some vital visceral organs in female albino rats. Nigerian journal of physiological sciences : official publication of the Physiological Society of Nigeria. 2009;24:7–12. doi: 10.4314/njps.v24i1.46374. [DOI] [PubMed] [Google Scholar]
- 50.Soeda J, Morgan M, McKee C, Mouralidarane A, Lin C, Roskams T, Oben JA. Nicotine induces fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on hepatic stellate cells. Biochemical and Biophysical Research Communications. 2012;417:17–22. doi: 10.1016/j.bbrc.2011.10.151. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B, Madden P, Gu J, Xing X, Sankar S, Flynn J, Kroll K, Wang T. Uncovering the transcriptomic and epigenomic landscape of nicotinic receptor genes in non-neuronal tissues. BMC genomics. 2017;18:439. doi: 10.1186/s12864-017-3813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 53.Martinez AK, Jensen K, Hall C, O’Brien A, Ehrlich L, White T, Meng F, Zhou T, Greene J, Jr, Bernuzzi F, Invernizzi P, Dostal DE, Lairmore T, Alpini G, Glaser SS. Nicotine Promotes Cholangiocarcinoma Growth in Xenograft Mice. The American Journal of Pathology. 2017;187:1093–105. doi: 10.1016/j.ajpath.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Jensen K, Afroze S, Ueno Y, Rahal K, Frenzel A, Sterling M, Guerrier M, Nizamutdinov D, Dostal DE, Meng F, Glaser SS. Chronic nicotine exposure stimulates biliary growth and fibrosis in normal rats. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2013;45:754–61. doi: 10.1016/j.dld.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–31. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Reiter RJ, Rosales-Corral SA, Manchester LC, Liu X, Tan DX. Melatonin in the biliary tract and liver: health implications. Current pharmaceutical design. 2014;20:4788–801. doi: 10.2174/1381612819666131119105826. [DOI] [PubMed] [Google Scholar]
- 57.Han Y, Onori P, Meng F, DeMorrow S, Venter J, Francis H, Franchitto A, Ray D, Kennedy L, Greene J, Renzi A, Mancinelli R, Gaudio E, Glaser S, Alpini G. Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis. American journal of physiology Gastrointestinal and liver physiology. 2014;307:G894–904. doi: 10.1152/ajpgi.00288.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu N, Meng F, Zhou T, Han Y, Kennedy L, Venter J, Francis H, DeMorrow S, Onori P, Invernizzi P, Bernuzzi F, Mancinelli R, Gaudio E, Franchitto A, Glaser S, Alpini G. Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation. The FASEB Journal. 2017 doi: 10.1096/fj.201700097R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Renzi A, Glaser S, Demorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, White M, Francis H, Han Y, Alvaro D, Gaudio E, Carpino G, Ueno Y, Onori P, Alpini G. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. American journal of physiology Gastrointestinal and liver physiology. 2011;301:G634–43. doi: 10.1152/ajpgi.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Renzi A, Mancinelli R, Onori P, Franchitto A, Alpini G, Glaser S, Gaudio E. Inhibition of the liver expression of arylalkylamine N-acetyltransferase increases the expression of angiogenic factors in cholangiocytes. Hepatobiliary surgery and nutrition. 2014;3:4–10. doi: 10.3978/j.issn.2304-3881.2014.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroeger I, Erhardt A, Abt D, Fischer M, Biburger M, Rau T, Neuhuber WL, Tiegs G. The neuropeptide calcitonin gene-related peptide (CGRP) prevents inflammatory liver injury in mice. Journal of Hepatology. 51:342–53. doi: 10.1016/j.jhep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Glaser SS, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, Francis HL, Dickerson IM, DiPette DJ, Supowit SC, Alpini GD. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest. 2007;87:914–26. doi: 10.1038/labinvest.3700602. [DOI] [PubMed] [Google Scholar]
- 63.Biernat J, Pawlik WW, Sendur R, Dembinski A, Brzozowski T, Konturek SJ. Role of afferent nerves and sensory peptides in the mediation of hepatic artery buffer response. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2005;56:133–45. [PubMed] [Google Scholar]
- 64.Glaser S, Gaudio E, Renzi A, Mancinelli R, Ueno Y, Venter J, White M, Kopriva S, Chiasson V, DeMorrow S, Francis H, Meng F, Marzioni M, Franchitto A, Alvaro D, Supowit S, DiPette DJ, Onori P, Alpini G. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. American journal of physiology Gastrointestinal and liver physiology. 2011;301:G297–305. doi: 10.1152/ajpgi.00418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang YAN, Yan M, Zhang H, Wang X. Substance P participates in immune-mediated hepatic injury induced by concanavalin A in mice and stimulates cytokine synthesis in Kupffer cells. Experimental and Therapeutic Medicine. 2013;6:459–64. doi: 10.3892/etm.2013.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan Y, Meng F, Wu N, Zhou T, Venter J, Francis H, Kennedy L, Glaser T, Bernuzzi F, Invernizzi P, Glaser S, Huang Q, Alpini G. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology (Baltimore, Md) 2017 doi: 10.1002/hep.29138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corder R, Withrington PG. The actions of neuropeptide Y and peptide YY on the hepatic arterial and portal vascular beds of the anaesthetized dog. British journal of pharmacology. 1988;94:1149–56. doi: 10.1111/j.1476-5381.1988.tb11633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oben JA, Yang S, Lin H, Ono M, Diehl AM. Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells. Biochem Biophys Res Commun. 2003;302:685–90. doi: 10.1016/s0006-291x(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 69.DeMorrow S, Meng F, Venter J, Leyva-Illades D, Francis H, Frampton G, Pae HY, Quinn M, Onori P, Glaser S, McDaniel K, Mancinelli R, Gaudio E, Alpini G, Franchitto A. Neuropeptide Y inhibits biliary hyperplasia of cholestatic rats by paracrine and autocrine mechanisms. American journal of physiology Gastrointestinal and liver physiology. 2013;305:G250–7. doi: 10.1152/ajpgi.00140.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeMorrow S, Onori P, Venter J, Invernizzi P, Frampton G, White M, Franchitto A, Kopriva S, Bernuzzi F, Francis H, Coufal M, Glaser S, Fava G, Meng F, Alvaro D, Carpino G, Gaudio E, Alpini G. Neuropeptide Y inhibits cholangiocarcinoma cell growth and invasion. American journal of physiology Cell physiology. 2011;300:C1078–89. doi: 10.1152/ajpcell.00358.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hargrove L, Kennedy L, Demieville J, Jones H, Meng F, DeMorrow S, Karstens W, Madeka T, Greene J, Jr, Francis H. Bile duct ligation-induced biliary hyperplasia, hepatic injury, and fibrosis are reduced in mast cell-deficient KitW-sh mice. Hepatology (Baltimore, Md) 2017;65:1991–2004. doi: 10.1002/hep.29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Francis H, Meng F, Gaudio E, Alpini G. Histamine regulation of biliary proliferation. J Hepatol. 2012;56:1204–6. doi: 10.1016/j.jhep.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones DE. Pathogenesis of primary biliary cirrhosis. J Hepatol. 2003;39:639–48. doi: 10.1016/s0168-8278(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 74.Invernizzi P, Floreani A, Carbone M, Marzioni M, Craxi A, Muratori L, Vespasiani Gentilucci U, Gardini I, Gasbarrini A, Kruger P, Mennini FS, Ronco V, Lanati E, Canonico PL, Alvaro D. Primary Biliary Cholangitis: advances in management and treatment of the disease. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2017 doi: 10.1016/j.dld.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. The New England journal of medicine. 2016;375:1161–70. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brancatelli G, Federle MP, Vilgrain V, Vullierme MP, Marin D, Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics : a review publication of the Radiological Society of North America, Inc. 2005;25:659–70. doi: 10.1148/rg.253045114. [DOI] [PubMed] [Google Scholar]