Introduction
Pediatric obsessive-compulsive disorder (OCD) is a disabling psychiatric illness that affects between 1 to 3% of children and significantly interferes in their academic, social, and familial functioning (Douglass et al., 1995; Zohar, 1999). Fortunately, cognitive behavioral therapy (CBT) is an efficacious treatment for this debilitating condition (Freeman et al., 2014a; Watson and Rees, 2008). Much research has demonstrated that CBT outperforms non-active (e.g., waitlist; Bolton et al., 2011) and active (e.g., treatment-as-usual; relaxation training; Freeman et al., 2014b; Lewin et al., 2014) comparison conditions and the reduction in obsessive-compulsive symptom severity persists for up to seven-years following treatment (O’Leary et al., 2009). However, up to 60% of children receiving CBT for OCD do not fully remit (POTS, 2004).
To improve the response rate and accelerate treatment response, researchers have attempted to augment CBT with cognitive enhancers (Farrell et al., 2013; Storch et al., 2010b, 2016), such as D-cycloserine (DCS), an NMDA partial agonist thought to enhance extinction learning (Ledgerwood et al., 2003; Ressler et al., 2004). Results of the largest trial to date demonstrated that DCS augmentation of CBT for pediatric OCD did not expedite treatment gains, nor was it more effective than CBT monotherapy (Storch et al., 2016). However, as demonstrated in adults with OCD (Andersson et al., 2015; Otto et al., 2016), certain baseline factors may moderate patients’ response to DCS. Given the need to direct children with OCD towards treatments with the highest likelihood of success, the current study aims to identify the conditions under which DCS augmentation works best (i.e., moderators) and the baseline characteristics associated with outcome, irrespective of treatment type (i.e., predictors).
Identifying the baseline factors that moderate DCS augmentation of CBT for pediatric OCD could facilitate the allocation and optimization of treatment to most efficiently meet patients’ needs. Additionally, a more personalized approach to treatment might ultimately lead to better clinical outcomes and can inform future randomized clinical trials (e.g., moderators can be included in stratification algorithms to maximize power). However, given the methodological requirements needed to facilitate a successful examination of the predictors and moderators of DCS augmentation (i.e., large sample size, two or more treatment arms for moderation analyses; Kraemer et al., 2002), little to no research has evaluated this important question in pediatric OCD. Therefore, our research questions were informed by past research on (a) the predictors and moderators of CBT for pediatric OCD and (b) DCS augmentation of CBT in adults. Multiple studies have examined predictors of CBT for pediatric OCD (Turner et al., 2018); however, only OCD symptom severity (Garcia et al., 2010; Ginsburg et al., 2008; Rudy et al., 2014; Torp et al., 2015) and degree of family accommodation (Garcia et al., 2010; Ginsburg et al., 2008; Merlo et al., 2009; Rudy et al., 2014) have been consistently examined. A recent review of the literature (Caporino and Storch, 2016) indicated that symptom severity and family accommodation do not uniformly predict outcome across studies. Other demographic and psychosocial variables have been identified as predictors, such as comorbid internalizing or externalizing disorders (Garcia et al., 2010; Storch et al., 2008; Torp et al., 2015;), age (Torp et al., 2015), functional impairment (Garcia et al., 2010), treatment expectations (Lewin et al., 2011), and gender (Rudy et al., 2014). Like OCD symptom severity and family accommodation, these psychosocial factors have not been consistently supported (Caporino and Storch, 2016; Turner et al., 2018). The conflicting findings may be attributed to variation in CBT approach (i.e., family vs. individual) or frequency of sessions (i.e., weekly vs. daily intensive; Caporino and Storch, 2016; McGuire et al., 2015). Additional research using a large sample size and methodologically rigorous design are needed to clarify discordant findings.
Although only a limited number of pediatric studies have compared CBT to other active treatment conditions, two baseline patient factors have emerged as significant moderators. First, children who have a first-degree relative with OCD do poorly in CBT monotherapy when compared to children with no family history (i.e., sporadic cases; Garcia et al., 2010, Turner et al., 2018). Second, children with a comorbid tic disorder respond more favorably to CBT monotherapy compared to those receiving other active treatments (see meta-analysis for review; McGuire et al., 2015, Turner et al., 2018). Similar to the predictor findings, these two moderators have not been consistently replicated (Caporino and Storch, 2016).
To the authors’ knowledge, there is only one established baseline moderator of DCS augmentation of CBT for OCD in adults – antidepressant use (Andersson et al., 2015). Specifically, DCS emerged as a superior augmentation agent compared to placebo for those who were free of antidepressant medication (Andersson et al., 2015). However, Storch and colleagues (2016) demonstrated that concomitant antidepressants did not moderate outcomes in DCS augmentation of CBT for pediatric OCD. Moreover, a recent meta-analysis on DCS augmentation of CBT for anxiety, OCD, and posttraumatic stress disorders did not find that antidepressants moderated the effects of DCS. In fact, none of the initially specified moderators were associated with treatment outcomes (Mataix-Cols et al., 2017). Thus, the research results are inconsistent. It remains plausible that the moderating effect of an antidepressant may be better explained by other patient factors that led to initiation of medication treatment, such as degree of psychosocial impairment or comorbid anxiety disorder. Therefore, it is crucial to conduct further research to identify theoretically-grounded baseline patient factors, associated with DCS response in OCD.
The current study examines the moderators of DCS augmentation of CBT for children aged 7–17 years with OCD who were randomized to either DCS + CBT or placebo + CBT (see Storch et al., 2016 for study design). Given the study’s methodological rigor, multimodal design, large well-characterized sample, and the fact that all patients received CBT, data will also be used to clarify historically conflicted findings regarding the predictors of CBT for pediatric OCD.
Due to the conflicting findings regarding predictors of CBT for pediatric OCD, we adopted an exploratory approach. Specifically, we examined the degree to which demographics (age, gender, race, ethnicity), OCD-specific features (symptom severity, insight, OCD symptom dimensions, family accommodation, impairment), comorbidity (number of comorbidities, a comorbid anxiety, depression, or tic disorder, anxiety symptom severity, depression symptom severity, and externalizing behavior symptom severity), and treatment-related factors (treatment alliance at randomization) predicted response. Additionally, we examined how theoretically- and empirically-grounded variables interact with DCS augmentation to moderate outcome. As described above, only tics and family history have emerged as moderators in the literature. While we did not collect data on family history in this trial, we examined tics. Given that tic comorbidity has led to promising moderator results in previous studies, we decided to explore other comorbidities as moderators (specifically those that had been associated with positive outcomes as predictors). In addition, we explored age and gender, which emerged as predictors in previous studies. Other variables that were included as predictors in the current trial were not examined as moderators either due to limited power (e.g., in the case of race or ethnicity) or because there was no theoretical or empirical justification for why they would be affected by DCS augmentation/enhanced extinction learning. Previous studies of pediatric OCD treatments predominantly focus on the dimensional outcome of the reduction in OCD symptom severity and are only rarely focused on symptom remission (i.e. when a patient has only minimal symptoms, McGuire et al. 2015). To address this limitation, we also examined moderators and predictors of symptom remission.
Methods
Description of the Parent Study
Data came from a two-site randomized controlled trial of DCS augmentation of CBT for pediatric OCD, described by Storch and colleagues (2016). Eligible patients were enrolled at Massachusetts General Hospital (MGH) in Boston, MA and University of South Florida (USF) in St. Petersburg, FL. Both sites obtained approval from their respective Institutional Review Boards and parents provided written consent and children provided assent. Inclusion criteria were: (a) current and primary DSM-IV-TR OCD diagnosis, as determined by the Schedule for Affective Disorders and Schizophrenia for School-Age Children– Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997) and (b) score of at least 16 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS; Scahill et al., 1997). Exclusion criteria included: (a) initiation or increase of an antidepressant medication 12 or 8 weeks, respectively, prior to enrollment, (b) initiation or increase of an antipsychotic medication 6 weeks prior to enrollment, (c) epilepsy, renal insufficiency, current or lifetime substance abuse, weight less than 22.5 kg, or DCS allergy, (d) inability to swallow study medication, (e) active suicidality or suicide attempt within past year, (f) currently pregnant or engaging in unprotected sex (for females only), and (g) comorbid psychosis, bipolar disorder, autistic disorder, anorexia nervosa, or non-OCD primary hoarding symptoms.
Eligible participants were screened by a trained independent evaluator (IE) with a master’s degree or higher, who administered the KSADS-PL (Kaufman et al., 1997) and the CY-BOCS (Scahill et al., 1997) to the parent and child. The IE also completed the Clinical Global Impression scale-Severity (CGI-Severity) rating at baseline. Children who met inclusion criteria began a 10-session cognitive behavioral treatment at a rate of two sessions per week for the first two weeks. The first three sessions included psychoeducation, cognitive interventions, and hierarchy development. Prior to the fourth CBT session, a computer-generated randomization program assigned patients to either DCS + CBT or placebo + CBT at a 1:1 ratio, using a double-blind approach. The remainder of treatment (sessions 4–10) was conducted weekly and involved exposure with response prevention exercises specific to the patient’s symptom profile. Between-session homework assignments paralleled the exercises conducted in session. Depending upon the patient’s treatment assignment, either DCS or a matching placebo pill was taken under study supervision 1 hour prior to all exposure sessions. All patients were re-administered the CY-BOCS and CGI-Severity at study visits 3, 5, 7, 8, 9, 11 and within one week following the final CBT session (study visit 12).
Sample
Two hundred and six children and adolescents were enrolled. One hundred and forty-two (M = 12.79, SD = 2.99; range 7–17-years-old) were randomized to either DCS + CBT (n = 70) or placebo + CBT (n = 72; for full consort diagram, please refer to Storch et al., 2016). Demographic characteristics are provided in Table 1.
Table 1.
Demographic and Pre-treatment Characteristics
| Variable | Total N = 142 |
DCS+CBT N = 70 |
Placebo+CBT N = 72 |
Inferential Statistics |
|---|---|---|---|---|
| Age Mean (SD) | 12.79 (2.99) | 13.05 (2.93) | 12.54 (3.04) |
F(1, 139) = 1.03, p = .31 |
| Gender (% Female) | 53.5% | 60.0% | 47.2% | χ2 (1) = 2.33, p = .127 |
| Race/Ethnicity (% Caucasian) |
88.7% | 87.1% | 90.3% | χ2 (1) = 0.35, p = .555 |
| Mean Comorbidities (SD) | 2.12 (2.49) | 2.09 (2.09) | 2.15 (2.85) |
F(1, 140) = 0.02, p = .902 |
| Previous Comorbidities | ||||
| Depression | 20.4% | 22.9% | 18.1% | χ2 (1) = 0.50, p = .478 |
| Any Anxiety Disorder | 43.0% | 42.9% | 43.1% | χ2 (1) = 0.01, p = .981 |
| Any Tic Disorder | 13.6% | 11.6% | 15.5% | χ2 (1) = 0.45, p = .501 |
| Current Comorbidities | ||||
| Depression | 14.9% | 15.9% | 13.9% | χ2 (1) = 0.12, p = .732 |
| Any Anxiety Disorder | 36.7% | 37.7% | 35.7% | χ2 (1) = 0.06, p = .810 |
| Any TIC Disorder | 9.2% | 7.2% | 11.1% | χ2 (1) = 0.63, p = .428 |
| CYBOCS | 25.27 (5.98) | 26.24 (6.01) | 24.33 (5.85) | F(1, 139) = 3.67, p = .058 |
| CYBOCS-Item 11 | 1.13 (1.03) | 1.13 (1.10) | 1.13 (0.96) | F (1, 139) = 0.01, p = .955) |
| Family Accommodation | 15.09 (8.79) | 15.17 (8.75) | 15.03 (8.88) | F (1, 139) = .01, p = .922 |
| CBCL-Externalizing | 8.54 (7.25) | 8.09 (6.90) | 8.97 (7.59) | F (1, 132) = 0.49, p = .485 |
| MASC total | 48.66 (21.12) | 53.00 (22.39) | 44.25 (18.92) | F (1, 129) = 5.83, p = .017 |
| COIS-Child | 14.28 (10.26) | 14.63 (10.58) | 13.94 (9.99) | F (1, 129) = 0.15, p = .701 |
| TASCC total | 3.16 (.57) | 3.17 (.52) | 3.15 (.61) | F (1, 127) = 0.08, p = .779 |
| CDRS | 26.64 (9.13) | 27.56 (10.21) | 25.76 (7.91) |
F (1, 136) = 1.35, p = .218 |
Outcome Measures
Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS; Scahill et al., 1997).
The CY-BOCS is a 10-item semi-structured clinician administered measure that assesses obsessions and compulsions. Each item is rated on a scale from 0–4, yielding an additive total score of 40. IEs, trained to agreement on administration and blinded to treatment condition, administered the CY-BOCS at baseline, and at study visit 3, 5, 7, 8, 9, 11, and 12 (post-treatment).
CGI-Severity (CGI-S; Guy, 1976).
The CGI-S is a one-item index of symptom severity that ranges from 0 (“no illness”) to 6 (“extremely severe”). The CGI-S was completed by IEs on the same administration schedule as the CY-BOCS.
Moderators and Predictors
All moderator and predictor variables were assessed at baseline, prior to randomization.
Demographics and Clinical Information.
The following variables were assessed via parent/self-report questionnaire: age, gender, race, ethnicity, and medication status.
Schedule for Affective Disorders and Schizophrenia for School-Age Children– Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997).
The KSADS-PL is a semi-structured clinician administered diagnostic interview that assesses a range of child psychopathology.
Insight into obsessive-compulsive symptoms was assessed using item 11 on the CY-BOCS (CY-BOCS; Scahill et al., 1997). This item is rated on a 5-point Likert scale from 0 (“Excellent”) to 4 (“Absent”)
Family Accommodation Scale (FAS; Calvocoressi et al., 1995; 1999).
The FAS is a 13-item clinician-administered interview that assesses family’s accommodation of the child’s OCD symptoms. Items (e.g., degree of reassurance seeking, modification of family activities) are scored on a 5-point Likert type scale. Higher scores reflect greater accommodation.
Multidimensional Anxiety Scale for Children (MASC; March et al., 1997).
The MASC is a 39-item self-report questionnaire that assesses anxiety symptom severity. Each item is rated on a 4-point Likert scale, ranging from 0 (“never true”) to 3 (“often true”).
Child Obsessive-Compulsive Impact Scale–Child (COIS-C; Piacentini et al., 2007).
The COIS-C is a 21 item, child-report measure that assesses the impairment caused by the OCD symptoms. Items assess the degree of psychosocial impairment due to OCD in three domains - school, social, and home - and are rated on a Likert scale from 0 (“not at all”) to 3 (“very much”).
Children’s Depression Rating Scale-Revised (CDRS-R; Poznanski and Mokros, 1996).
The CDRS is a 17-item semi-structured interview that assesses children’s depression symptom severity. The scale assesses the cognitive, somatic, affective, and psychomotor symptoms associated with depression. Total scores range from 17 (no symptoms) to 113 (extremely depressed).
Therapist Alliance Scale–Child (TAS-C; Shirk and Saiz, 1992).
The TAS-C is an 8-item measure of the youth’s affect toward the therapist. The TAS-C was administered prior to randomization at Week 4.
Child Behavior Checklist (CBCL; Achenbach, 2001).
The CBCL is a 113-item parent-rated measure of child psychopathology. Each item is rated from 0 (“not true”) to 2 (“very or often true”). For the purposes of this study, the CBCL was used to assess comorbid externalizing symptoms (e.g., oppositional, inattentive).
Analytic Plan
Analysis of the primary and secondary outcomes was conducted using linear mixed models with treatment group (DCS, placebo) as a between subjects variable and time as the within groups factor, as well as their interaction (Hofmann et al., 2015; Littell et al., 2006). Each variable that was considered as a potential moderator was included as a main effect in the model and as an interaction with treatment group and time. If statistically significant, this effect would provide evidence that the moderator impacted the nature of longitudinal changes by treatment group. For the predictor analyses, each measure was included as a main effect and interacted with time. The parent study was powered to detect 20% differences in slopes between the two treatment arms. For the moderator analysis, Monte Carlo simulation estimates using Mplus (Muthén and Muthén, 2014; Muthén and Muthén, 2002) indicate a power of .77 to detect a medium-size effect of the variable within each treatment arm. For the predictor analysis, Monte Carlo simulation using Mplus indicated a power of .87 to detect a small to medium-sized effect of the predictor on the longitudinal slope. The analyses with continuous outcomes described above were replicated using a categorical outcome of remission status based upon CY-BOCS scores and the empirically validated cut-point of 12 (Storch et al., 2010a).
Results
Sample Characteristics
Demographic characteristics by treatment group are shown in Table 1. There were no differences in child’s age as a function of treatment group, recruitment site or the group by site interaction. Child gender and race/ethnicity were comparable across treatment groups. There were no group differences in average number of comorbid conditions and the interaction between group and site was not statistically significant. However, there was a main effect of site (F (1, 137) = 6.10, p = .005) with children at MGH (M = 2.71, SD = 3.21) having more comorbid conditions at baseline as compared to USF (M = 1.54, SD = 1.29). Finally, there were no group differences in current or previous diagnosis of depression, anxiety disorders or tic disorders.
In Table 1, means and standard deviations for the measures at visit 1 are provided, along with their statistical test information. Treatment group differences were observed for the MASC-total score with the DCS group exhibiting higher scores than the placebo. None of the other main effects of group were statistically significant.
Moderator Analyses
The outcomes of interest for the moderator analyses were CY-BOCS total score and the CGI-S. In addition, we examined remission (i.e., CY-BOCS score of 12 or below) as an outcome. The moderators of interest were age, gender, current history and previous history of depression, anxiety and tics. Across all of the analyses, significant improvements were observed for all outcome measures (all p’s < .05), but the treatment group by time by moderator term was not statistically significant for any of the outcome/moderator pairs. The same pattern of results was observed when the categorical outcome of remission status was used as the dependent variable.
Predictors of Treatment Response
The analyses described above indicated that there were no statistically significant moderators of change in the outcomes as a function of treatment group. In the next set of analyses, we collapse across treatment group to examine predictors of response for the group as a whole. The outcomes of interest for the predictor analyses were CY-BOCS total score and the CGI-S, as well as remission (assessed with a CY-BOCS score of 12 or below). The predictors of interest were age, gender, race, ethnicity, OCD symptom severity (CY-BOCS), insight (item 11 on the CY-BOCS), family accommodation (FAS), impairment (COIS-C), comorbidity (number of comorbidities, a comorbid anxiety, depression, or tic disorder), anxiety symptom severity (MASC), depression symptom severity (CDRS-R), externalizing behavior symptom severity (CBCL) and treatment alliance (TAS) (see Table 1).
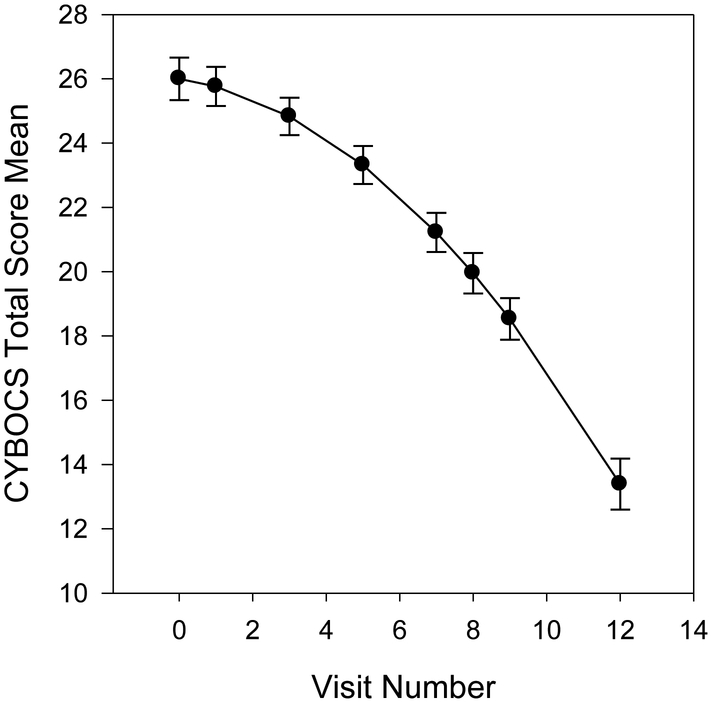
CY-BOCS Total.
Results indicated that a model including a linear and quadratic effect for time provided the best fit to the data. The overall trajectory is shown in Figure 1. At baseline, CY-BOCS total scores were almost 26 points (𝛽 = 25.98, SE = .47, p < .001) and the linear effect of time was not statistically significant, but the quadratic effect was significant and negative (𝛽 = −0.17, SE = .09, p = .066; 𝛽 = −0.07, SE = .01, p < .001, respectively). Among the predictors, the CY-BOCS total score was higher for patients who were one point higher on the following: FAS (𝛽 = 0.17, SE = .05, p < .001), MASC (𝛽 = 0.02, SE = .002, p < .001), COISC (𝛽 = 0.19, SE = .045, p < .001), CDRS (𝛽 = 0.20, SE = .05, p < .001), and the CBCL-externalizing (𝛽 = 0.14, SE = .06, p = .024). The insight item from the CYBOCS was not related to scores at study onset. None of the examined predictors (including insight) were associated with longitudinal changes.
Figure 1.
Changes in CY-BOCS Total Score over the Course of Treatment
CGI-Severity.
Results indicated that a model including a linear and quadratic effect for time provided the best fit to the data. At baseline, CGI-Severity scores were over 3 points (𝛽 = 3.34, SE = .07, p < .001) and the linear and quadratic effects of time were both statistically significant (𝛽 = 0.06, SE = .01, p < .001; 𝛽 = −0.01, SE = .001, p < .001, respectively). Among the predictors, higher scores at baseline were related to a previous history of depression (𝛽 = 0.44, SE = .18, p < .05), higher FAS scores (𝛽 = 0.03, SE = .01, p < .001), higher COISC scores (𝛽 = 0.03, SE = .01, p < .001), higher CDRS scores (𝛽 = 0.04, SE = .01, p < .001), and the CBCL-externalizing (𝛽 = 0.02, SE = .01, p = .022). The insight item from the CYBOCS was not related to scores at study onset (𝛽 = 0.04, SE = .07, p = .588), but was related to linear (𝛽 = −0.03, SE = .01, p = .038), and quadratic changes over time (𝛽 = 0.002, SE = .001, p = .022). Lower scores on the item at baseline (i.e., better insight) was associated with more improvement longitudinally. None of the additional predictors were related to longitudinal changes.
Dichotomous remission as an outcome.
Several predictor variables were associated with a decreased likelihood of remitting, including higher FAS scores (OR = .94, 95% CI = .91 - .98), higher COISC scores (OR = .95, 95% CI = .92 - .99), higher CDRS scores (OR = .96, 95% CI = .92 - .99), and higher CBCL-externalizing scores (OR = .93, 95% CI = .88 - .98). MASC scores were unrelated to remission (OR = .99, 95% CI = .98 – 1.01).
Discussion
The current study sought to identify both baseline moderators of DCS augmentation of CBT for pediatric OCD, and predictors of response in this large and well-characterized sample. Identifying moderators and predictors of CBT for pediatric OCD could optimize treatment to more efficiently meet patients’ needs, inform stratification algorithms in future randomized clinical trials, and align with the personalization of psychiatric medicine. The clinical characteristics reported by patients in the current study parallel that of other large treatment-seeking samples of children with OCD (e.g., POTS, 2004). At baseline, patients reported moderately severe OCD symptoms and those with greater OCD symptom severity also reported greater psychosocial impairment caused by OCD, as well as greater depression, anxiety and externalizing symptom severity. Additionally, a greater baseline obsession total score, compulsion total score, and overall degree of illness were associated with a historical depression diagnosis.
Our study showed that no demographic or clinical variables moderated the effects of DCS augmentation. These findings are consistent with a large meta-analysis that included 21 trials with a range of disorders (but only 4 pediatric trials), which found no reliable moderators of DCS response (Mataix-Cols et al., 2017). The absence of significant moderators of DCS augmentation, however, is in contrast to research on adults with OCD. Indeed, Andersson and colleagues (2015) found that antidepressant use interacted with DCS augmentation in adults with OCD receiving CBT; however, when baseline concomitant antidepressant use was examined as a moderator in this parent study (Storch et al., 2016), it was not found to diminish the effectiveness of DCS augmentation. It may be that patients’ prior history of antidepressant use interfered with the effects of DCS and Hofmann (2016) suggests that this should be examined in future research.
While no demographic or clinical variables moderated the effects of DCS augmentation; several of the baseline patient factors we assessed in the current study predicted CBT outcomes. Importantly, our predictor analyses indicated that better insight at baseline was associated with more improvement longitudinally on the CGI-S, a clinician- rated summary measure of illness severity. Our results validate the experience of OCD clinicians, who know how challenging it can be to encourage OCD patients to give up rituals when patients are completely convinced that their behaviors are reasonable and realistically connected to the prevention of negative outcomes. Thus, having better insight at the beginning of treatment might make it more likely that patients engage in therapy, which ultimately will have positive effects on their illness severity and their ability to function. However, insight at baseline was not associated with longitudinal changes on the CY-BOCS. One might argue that the discrepant findings are likely due the differences between the two measures, specifically, the CY-BOCS has been criticized for low sensitivity at the higher (i.e., initial) scores (Geller and March, 2012) .
Also, several predictor variables were associated with a decreased likelihood of remitting, including higher family accommodation scores, higher impairment scores, higher depression scores, and higher externalizing scores. While our findings are consistent with research showing that these variables predict treatment outcome, most previous studies examined treatment outcome dimensionally (i.e., as changes in OCD symptom severity, e.g., Garcia et al., 2010; Piacentini et al., 2002; Torp et al., 2015). “Remission”- a categorical outcome, has rarely been examined (e.g., Rudy et al., 2014). Our findings on the prediction of remission are important as they can guide clinical decision making. Specifically, clinicians might be able to improve treatment outcomes by incorporating interventions that address these problem areas in personalized treatment plans. Thus, therapists might benefit from treatment manuals that not only include core interventions (such as exposure and response prevention for OCD) but also a range of treatment modules that therapists can choose from depending on needs of the specific child or family they are working with. Such modules might include interventions for training parents of children with externaling disorders, interventions for parents who excessively accommodate children’s OCD symptoms, or other interventions for children who also suffer from depression. The inclusion of interventions focused on enhancing insight might also be beneficial.
While we found several categorical predictors for remission, none of the dimensional predictors (other than insight predicting reducing symptom severity measured with the CGI-S, as described above) reached significance. One of the most surprising null results was that of initial OCD symptom severity. Despite much research demonstrating that greater baseline symptom severity is associated with poorer CBT outcomes (e.g., Garcia et al., 2010; Ginsburg et al., 2008; Rudy et al., 2014; Torp et al., 2015), our results indicate that pediatric patients with OCD, regardless of initial symptom severity, improve with CBT. Several other baseline characteristics (e.g., demographics, psychiatric comorbidity) that had previously been identified as a predictor of CBT outcome for children with OCD were not significant in our study. However, despite our non-significant results, it would be premature to conclude that all youngsters who embody these characteristics will certainly improve with CBT and that there is no need for future research on predictors, moderators, or personalization of treatment for these children. It is important to recognize that the treatment response in the current trial was very high, specifically 72.2% (placebo) and 82.9% (DCS, Storch et al, 2016) which made it less likely for us to identify predictors and moderators that might shed light on personalization approaches to treatment.
An important limitation of this study is that we have not examined all relevant variables. For example, although a family history of OCD has been shown to moderate the effectiveness of CBT for pediatric OCD (Garcia et al., 2010), a systematic assessment of patients’ family history was not conducted in the current study. We therefore could not include this variable in study analyses. Similarly, variables such cognitive profile that had emerged as predictors in previous adult or pediatric CBT for OCD studies (Deallocate et al. 2012; Flessner et al 2010) were not examined, but should be included in future research. In addition to examining clinical and neuropsychological measures, future studies might also assess neural or neurocognitive predictors and moderators. For example, a recent functional magnetic resonance imaging on adults with OCD showed that pre-treatment functional connectivity patterns within the default mode network and visual network significantly predicted post-treatment OCD severity and these networks were stronger predictors than pretreatment clinical scores (Reggente et al, 2018). Moreover, the current study only examined how baseline factors moderated DCS augmentation and recent research demonstrates that exposure-related processes may be relevant moderators. Specifically, the benefits of DCS may be contingent upon the variability of self-reported fear during exposure exercises. Past research has demonstrated that for those with social anxiety disorder, greater habituation (i.e., lower fear post-exposure) was associated with greater benefit from DCS augmentation, whereas those who reported limited habituation (i.e., higher fear post-exposure) experienced less benefit from DCS (e.g., Hofmann et al., 2013; Smits, et al., 2013a, 2013b). Patients’ subjective anxiety ratings during exposure sessions were not recorded in the current study and therefore, the interaction of habituation processes and DCS augmentation could not be examined. Given the importance of habituation processes to DCS augmentation, Otto and colleagues (2016) suggest administering DCS at the end of an exposure session if the patient reports significant habituation. Indeed, selective post-session administration of DCS was found to be beneficial for adults with acrophobia (Tart et al., 2013). In children with OCD, Mataix-Cols and colleagues (2014) found that post-session DCS administration did not significantly affect CBT outcomes; however, DCS was administered after every session, not selectively based on habituation patterns. Thus, we need to expand on the type of moderators (and predictors) we are considering in pediatric OCD and we might even need to examine how a certain combination of characteristics (positive family history of OCD and poor treatment expectations) at baseline may significantly predict outcome or interact with DCS augmentation.
In summary, while CBT for pediatric OCD is considered a powerful treatment, not everyone responds. To maximize clinical benefits, additional research is needed on predictors and moderators so clinicians and researchers can tailor existing treatments to patients’ needs, as well as develop new treatments for patients who do not have a satisfactory treatment response.
References
- Achenbach TM, Escola M, 2001. Manual for the ASEBA School-age Forms and Profiles. Burlington: University of Vermont Research Center for Children, Youth, and Families. [Google Scholar]
- Andersson E, Hedman E, Enander J, Radu Djurfeldt D, Ljótsson B, Cervenka S, Isung J, Svanborg C, Mataix-Cols D, Kaldo V, Andersson G, Lindefors N, Rück C, 2015. D-Cycloserine vs Placebo as Adjunct to Cognitive Behavioral Therapy for Obsessive-Compulsive Disorder and Interaction With Antidepressants: A Randomized Clinical Trial. JAMA Psychiatry 72, 659–667. [DOI] [PubMed] [Google Scholar]
- Bolton D, Williams T, Perrin S, Atkinson L, Gallop C, Waite P, Salkovskis P, 2011. Randomized controlled trial of full and brief cognitive- behavior therapy and wait-list for pediatric obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry 52, 1269–1278. [DOI] [PubMed] [Google Scholar]
- Calvocoressi L, Lewis B, Harris M, Trufan SJ, Goodman WK, McDougle CJ, Price LH, 1995. Family accommodation in obsessive-compulsive disorder. Am. J. Psychiatry 152, 441–3. [DOI] [PubMed] [Google Scholar]
- Calvocoressi L, Mazure CM, Kasl SV, Skolnick J, Fisk D, Vegso SJ, Van Noppen BL, Price LH, 1999. Family accommodation of obsessive-compulsive symptoms: instrument development and assessment of family behavior. J. Nerv. Ment. Dis. 187, 636–642. [DOI] [PubMed] [Google Scholar]
- Caporino N, Storch EA, 2016. Personalizing the Treatment of Pediatric Obsessive-Compulsive Disorder: Evidence for Predictors and Moderators of Treatment Outcomes. Child and Developmental Psychiatry 3, 73–85. [Google Scholar]
- D’alcante CC, Diniz JB, Fossaluza V, Batistuzzo MC, Lopes AC, Shavitt RG, ... & Hoexter MQ (2012). Neuropsychological predictors of response to randomized treatment in obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 39(2), 310–317. [DOI] [PubMed] [Google Scholar]
- Douglass HM, Moffit TE, Dar R, McGee R, Silva P, 1995. Obsessive-compulsive disorder in a birth cohort of 18-year-olds: prevalence and predictors. J. Am. Acad. Child Adolesc. Psychiatry 34, 1424–1431. [DOI] [PubMed] [Google Scholar]
- Farrell LJ, Waters AM, Boschen MJ, Hattingh L, McConnell H, Milliner EL, Collings N, Zimmer-Gembeck M, Shelton D, Ollendick TH, Testa C, Storch EA, 2013. Difficult-to-treat pediatric obsessive-compulsive disorder: Feasibility and preliminary results of a randomized pilot trial of D-cycloserine-augmented behavior therapy. Depress. Anxiety 30, 723–731. [DOI] [PubMed] [Google Scholar]
- Flessner CA, Allgair A, Garcia A, Freeman J, Sapyta J, Franklin ME, ... & J. March (2010). The impact of neuropsychological functioning on treatment outcome in pediatric obsessive–compulsive disorder. Depression and anxiety, 27(4), 365–371. [DOI] [PubMed] [Google Scholar]
- Freeman J, Garcia A, Frank H, Benito K, Conelea C, Walther M, Edmunds J, 2014a. Evidence base update for psychosocial treatments for pediatric obsessive-compulsive disorder. J. Clin. Child Adolesc. Psychol. 43, 7–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Sapyta J, Garcia A, Compton S, Khanna M, Flessner C, FitzGerald D, Mauro C, Dingfelder R, Benito K, Harrison J, Curry J, Foa E, March J, Moore P, Franklin M, 2014b. Family-based treatment of early childhood obsessive-compulsive disorder: The pediatric obsessive-compulsive disorder treatment study for young children (POTS Jr)- randomized clinical trial. JAMA Psychiatry 71, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Sapyta JJ, Moore PS, Freeman JB, Franklin ME, March JS, Foa EB, 2010. Predictors and moderators of treatment outcome in the Pediatric Obsessive-Compulsive Treatment Study (POTS I). J. Am. Acad. Child Psy. 49, 1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA, March J Practice Parameter for the Assessment and Treatment of Children and Adolescents With Obsessive-Compulsive Disorder. J. Am. Acad. Child Adolesc. Psychiatry, 2012;51(1):98 –113. [DOI] [PubMed] [Google Scholar]
- Ginsburg GS, Kingery JN, Drake KL, Grados MA, 2008. Predictors of treatment response in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Psy. 47, 868–78. [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. Clinical global impressions scale ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare/NIMH; Pub no. (AMD) 76–338. Rockville, MD, USA. [Google Scholar]
- Hofmann SG, 2016. Schrödinger’s Cat and d-Cycloserine to Augment Exposure Therapy—Both Are Alive and Dead. JAMA Psychiatry 73, 771–772. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Carpenter JK, Otto MW, Rosenfield D, Smits JAJ, Pollack MH, 2015. Dose timing of d-cycloserine to augment cognitive behavioral therapy for social anxiety: Study design and rationale. Contemp. Clin. Trials 43, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, Rosenfield D, Simon N, Otto MW, Meuret AE, Marques L, Fang A, Tart C, Pollack MH, 2013. D-cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry, 170, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS, 2002. Mediators and moderators of treatment effects in randomized clinical trials. Arch. Gen. Psychiatry 59, 877–883. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J, 2003. Effects of D-cycloserine on the extinction of conditioned freezing. Behavioral Neuroscience 117, 341–349. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Park JM, Jones AM, Crawford EA, De Nadai AS, Menzel J, Arnold EB, Murphy TK, Storch EA, 2014. Family-based exposure and response prevention therapy for preschool-aged children with obsessive-compulsive disorder: a pilot randomized controlled trial. Behavior Research and Therapy 56, 30–38. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Peris TS, Bergman R, McCracken JT, Piacentini J, 2011. The role of treatment expectancy in youth receiving exposure-based CBT for obsessive-compulsive disorder. Behav. Res. Ther. 49, 536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Milliken GA, Wolfinger RD, Schabenberger O, 2006. SAS for Mixed Models; Second Edition SAS Institute. [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK, 1997. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry 36, 554–565. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Fernández de la Cruz L, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, Frumento P, de Kleine RA, Difede J, Dunlop BW, Farrell LJ, Geller D, Gerardi M, Guastella AJ, Hofmann SG, Hendriks GJ, Kushner MG, Lee FS, Lenze EJ, Levinson CA, McConnell H, Otto MW, Plag J, Pollack MH, Ressler KJ, Rodebaugh TL, Rothbaum BO, Scheeringa MS, Siewert-Siegmund A, Smits JAJ, Storch EA, Strohle A, Tart CD, Tolin DF, van Minnen A, Waters AM, Weems CF, Wilhelm S, Wyka K, Davis M, Ruck C, and the DCS Anxiety Consortium, 2017. D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: A systematic review and meta-analysis. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2016.3955. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Turner C, Monzani B, Isomura K, Murphy C, Krebs G, Heyman I, 2014. Cognitive-behavioural therapy with post-session Dcycloserine augmentation for paediatric obsessive-compulsive disorder: Pilot randomised controlled trial. Br. J. Psychiatry 204, 77–78. [DOI] [PubMed] [Google Scholar]
- Merlo LJ, Lehmkuhl HD, Geffken GR, Storch EA, 2009. Decreased family accommodation associated with improved therapy outcome in pediatric obsessive-compulsive disorder. J. Consult. Clin. Psychol. 77, 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA, 2015. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: Moderators of treatment efficacy, response, and remission. Depress. Anxiety 32, 580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO, Muthén L Mplus Users Guide (v. 7). Los Angeles, CA: Muthén & Muthén; 2014. [Google Scholar]
- Muthén LK, Muthén BO How to use a Monte Carlo study to decide on sample size and determine power. Structural Equation Modeling. 2002;9(4):599–620. [Google Scholar]
- O’Leary EMM, Barrett P, Fjermestad KW, 2009. Cognitive-behavioral family treatment for childhood obsessive-compulsive disorder: A 7-year follow-up study. Journal of Anxiety Disorders 23, 973–978. [DOI] [PubMed] [Google Scholar]
- Otto MW, Kredlow MA, Smits JAJ, Hofmann SG, Tolin DF, de Kleine RA, van Minnen A, Evins AE, Pollack MH, 2016. Enhancement of Psychosocial Treatment with D-Cycloserine: Models, Moderators, and Future Directions. Biological Psychiatry 80, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatric OCD Treatment Study Team (POTS), 2004. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder. JAMA 292, 1969–1976. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bergman RL, Jacobs C, McCracken JT, & Kretchman J (2002). Open trial of cognitive behavior therapy for childhood obsessive–compulsive disorder. Journal of anxiety disorders, 16(2), 207–219. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Peris TS, Bergman RL,Chang S, Jaffer M, 2007. Functional impairment in childhood OCD: development and psychometrics properties of the Child Obsessive-Compulsive Impact Scale–Revised (COIS-R). J. Clin. Child Adolesc. Psychol 36, 645–653. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB, 1996. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Reggente N, Moody TD, Morfini F, Sheen C, Rissman J, O’Neill J, Feusner JD, 2018. Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive-compulsive disorder. PNAS, 115, 2222–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M, 2004. Cognitive enhancers as adjuncts to psychotherapy. Use of D-Cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry 61, 1136–1142. [DOI] [PubMed] [Google Scholar]
- Rudy BM, Lewin AB, Geffken GR, Murphy TK, Storch EA, 2014. Predictors of treatment response to intensive cognitive-behavioral therapy for pediatric obsessive-compulsive disorder. Psychiatry Res. 220, 433–40. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF, 1997. Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and Validity. Journal of the American Academy of Child & Adolescent Psychiatry 36, 844–852. [DOI] [PubMed] [Google Scholar]
- Shirk S, Saiz C, 1992. The therapeutic alliance in child therapy: clinical, empirical and developmental perspectives. Development and Psychopathology 4, 713–728. [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, Simon NM, Pollack MH, Hofmann SG, 2013a. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J. Psychiatr. Res. 47, 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, Pollack MH, Tart CD, 2013b. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: Evidence from the treatment of height phobia. Biological Psychiatry 73, 1054–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Lewin AB, De Nadai AS, Murphy TK. Defining treatment response an remission in obsessive-compulsive disorder: a signal detection analysis of the Children’s Yale-Brown Obsessive Compulsive Scale. J Am Acad Child Adolesc Psychiatry. 2010a. July;49(7):708–17. doi: 10.1016/j.jaac.2010.04.005. Epub 2010 Jun 2. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Larson MJ, Geffken GR, Lehmkuhl HD, Jacob ML, Murphy TK, Goodman WK, 2008. Impact of comorbidity on cognitive-behavioral therapy response in pediatric obsessive- compulsive disorder. J. Am. Acad. Child Psy. 47, 583–92. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, Micco JA, Sprich S, Wilhelm S, Geller DA, 2010b. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol. Psychiatry 68, 1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Wilhelm S, Sprich S, Henin A, Micco J, Small BJ, McGuire J, Mutch PJ, Lewin AB, Murphy TK, Geller DA, 2016. Efficacy of augmentation of cognitive behavior therapy with weight-adjusted D-cycloserine vs placebo in pediatric obsessive-compulsive disorder: A randomized clinical trial. JAMA Psychiatry 73, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tart CD, Handelsman PR, Deboer LB, Rosenfield D, Pollack MH, Hofmann SG, Powers MB, Otto MW, Smits JA, 2013. Augmentation of exposure therapy with post-session administration of D-cycloserine. J. Psychiatr. Res. 47, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp NC, Dahl K, Skarphedinsson G, Compton S, Thomsen PH, Weidle B, Hybel K, Valderhaug R, Melin K, Nissen JB, Ivarsson T, 2015. Predictors associated with improved cognitive-behavioral therapy outcome in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Psy. 54, 200–207. [DOI] [PubMed] [Google Scholar]
- Turner C, O’Gorman B, Nair A, O’Kearney R. Moderators and predictors of response to cognitive behaviour therapy for pediatric obsessive-compulsive disorder: A systematic review. Psychiatry Res. 2018. March;261:50–60. doi: 10.1016/j.psychres.2017.12.034. Epub 2017 Dec 20. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- Watson HJ, Rees CS, 2008. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J. Child. Psychol. Psychiatry 49, 489–498. [DOI] [PubMed] [Google Scholar]
- Zohar AH, 1999. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child. Adolesc. Psychiatr. Clin. N. Am. 8, 445–460. [PubMed] [Google Scholar]