Abstract
MicroRNAs (miRNAs) have been reported to be involved in tumor metastasis. In this study, we investigated the function of miR-506 in the metastasis of human hepatocellular carcinoma (HCC). We found that miR-506 is significantly downregulated in the primary tissue of metastatic HCC and in highly metastatic HCC cell lines. Overexpression of miR-506 suppressed HCC cell migration, invasion, and metastasis both in vitro and in vivo. Furthermore, miR-506 was found to specifically target the 3’ untranslated region (3’-UTR) of interleukin 8 (IL8) mRNA. Spearman’s correlation analysis revealed that miR-506 expression inversely correlated with IL8 mRNA and protein expression in HCC tissue samples. IL8 treatment reversed miR-506-induced suppression of HCC cell migration and invasiveness. Thus, miR-506 acts as a tumor suppressor that may inhibit the migration, invasiveness, and metastasis of HCC cells by targeting IL8.
Keywords: miR-506, IL8, invasion, metastasis, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) ranks fifth in incidence and third in cancer mortality worldwide and brings about a heavy healthcare-related economic burden worldwide [1]. Despite promising treatment strategies, the outcomes of patients with HCC remain poor. The overall recurrence rate among patients with HCC reaches 70% [2]. Most patients with HCC die of metastasis, which has been a consistent problem in tumor prognosis and therapy. Hence, there is an urgent need for determining the exact mechanisms of metastasis and for improving the current therapeutic strategies against HCC.
MicroRNAs (miRNAs) are a class of small noncoding RNA molecules (18-25 nucleotides in length) that negatively regulate expression of protein-coding genes at the post-transcriptional level through binding to the 3’ untranslated region (UTR) [3]. Increasing evidence shows that miRNAs are aberrantly expressed in various human cancers and can act as tumor suppressors or oncogenic agents [4]. To date, several deregulated miRNAs have been shown to promote or suppress cell growth, invasiveness, and metastasis of HCC, e.g., miR-26a [5], miR-187-3p [6], and miR-331-3p [7]. Nevertheless, the relation between miRNAs and HCC metastasis remains elusive.
MiR-506 has been reported to be downregulated and to serve as a potential tumor suppressor in several cancer types including pancreatic cancer [8], gastric cancer [9], cervical cancer [10], and breast cancer [11]. MiR-506 is also a reliable marker of the chemotherapy response and survival in serous [12]. Regarding its function, miR-506 can suppress proliferation and migration of gastric cancer [9], breast cancer [11], and HCC cells [13]. Of note, miR-506 levels are negatively associated with lymph node invasion and metastasis, and this miRNA may suppress the invasiveness and metastasis of colon cancer [14]. Nevertheless, the roles and mechanism of action of miR-506 in HCC metastasis remain largely unknown.
In this study, we carried out a series of in vitro and in vivo assays and found that weaker expression of miR-506 in HCCs is associated with a more aggressive tumor phenotype. Elevated miR-506 expression significantly suppressed cell migration, invasion, and epithelial-mesenchymal transition (EMT) in vitro. Moreover, miR-506 overexpression suppressed the growth of HCC-derived xenograft tumors and lung metastasis of HCC cells in vivo. Finally, our data revealed that miR-506 may inhibit HCC tumor growth and metastasis by reducing the expression of its target gene IL8.
Materials and methods
Cell lines and human tissue samples
Human HCC cell lines (Huh7, HepG2, HCCLM3, and MHCC97H), and normal hepatic cell line LO2 were obtained from the Cell Bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences (Shanghai, China). These cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% of fetal bovine serum and 1% of a penicillin/streptomycin solution (Sigma, St. Louis, MO, USA) in a humidified incubator at 37°C and 5% CO2. Thirty samples of human HCC and their matching adjacent noncancerous tissues were collected from patients that underwent a liver resection at the Eastern Hepatobiliary Surgery Hospital (Shanghai, China). Another 10 metastatic tissue samples were also obtained from this hospital. After the resection, these tissue samples were immediately frozen in liquid nitrogen and stored at -80°C for later RNA extraction or formalin fixing and paraffin embedding for immunohistochemistry. All the human tissue samples were obtained with informed consent, and the protocol was approved by the Ethics Committee of the Eastern Hepatobiliary Surgery Hospital.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNAs containing miRNAs were isolated with the miRNeasy Mini Kit (Qiagen, Valencia, CA, USA), and RNA concentrations were determined on a NanoDrop 2000. MiRNAs or mRNAs were reverse-transcribed to generate cDNA using the One Step PrimeScript miRNA cDNA Synthesis Kit (Takara, Dalian, China) or oligo-dT primers, respectively. qRT-PCR analyses were performed by means of the Mir-XTM miRNA qRT-PCR SYBR® Kit (Takara) for miR-506 and the TB GreenTM Fast qPCR Mix (Takara) for IL8. U6 was analyzed as an internal control for miRNA, whereas GAPDH served as an internal control for mRNA. Relative quantification analysis was conducted by the 2-ΔΔCT method.
Cell transfection
miR-506 mimic and its negative control (miR-ctrl), and miR-506 inhibitor (anti-miR-506) and its negative control (NC) were purchased from RiboBio (Guangdong, China). Cells (5 × 105/well) were seeded at 60% confluence in a 6-well plate. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transfect the cells with the miR-506 mimic or miR-ctrl. After 48 h of transfection, the cells were collected for the following experiments.
Wound-healing assay
Cells transfected with the miR-506 mimics or anti-miR-506 were seeded in 6-well plates and allowed to grow to ~95% confluence. A linear wound was created with a 200 μL sterile pipette tip, and the cells were washed with the serum-free medium to remove floating ones. The wounded monolayers were photographed at 0 and 48 h by means of an inverted microscope to assess the rate of gap closure [15].
Transwell and three-dimensional (3D) spheroid invasion assays
For the Transwell invasion assay, 105 cells were seeded in the upper chambers of 24-well Transwell plates (Corning Costar, Lowell, MA, USA) precoated with Matrigel (BD Biosciences, Franklin, NJ, USA). The medium containing 20% of fetal bovine serum was added to the lower compartment as a chemoattractant. After incubation for 48 h, the cells on the upper surface of the filters were removed with a cotton swab. Invading cells on the bottom were fixed and stained with 0.5% crystal violet and photographed in five random visual fields under a microscope. A 3D spheroid invasion assay was performed as previously described [16]. In brief, 2 × 103 transfected cells were mixed with 20% Matrigel and seeded in 24-well plates coated with 100% Matrigel (BD Biosciences). The medium was changed every other day. Ten days later, tumor cell outgrowth was visualized by microscopy.
Detection of target proteins of miR-506
The concentration of IL8 in the culture medium was detected by an enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, USA). Total protein was isolated from HCC cells and subjected to western blot analysis as described elsewhere [17]. The primary antibodies were anti-E-cadherin, anti-vimentin, and anti-β-actin antibodies (Proteintech, Wuhan, China).
Vector construction and luciferase reporter assay
The wild-type (wt) 3’-UTR of the IL8 sequence containing a putative binding site for miR-506 was inserted into the pmirGLO vector (Promega, Madison, WI, USA). The mutant (mt) 3’-UTR of IL8 was created by mutating the seed region of the miR-506-binding site using the QuikChange Lightning Mutagenesis Kit (Stratagene, Santa Clara, CA, USA). For the luciferase reporter assay, HEK293 cells were cotransfected with the luciferase reporters with either miR-506 mimics or miR-ctrl. After 48 h, the cells were harvested, and luciferase activity was measured by means of the Dual-Luciferase Reporter Assay System (Promega). Renilla luciferase served for normalization.
In vivo tumor growth and metastasis assay
Six-week-old BALB/c nude mice were acquired from Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China) and were housed and maintained under specific pathogen-free conditions according to the guidelines. All the animal experiments were approved by the Scientific Investigation Board of the Second Military Medical University (Shanghai, China). HCCLM3 cells (2 × 106) infected with lentiviral vectors expressing the miR-506 (LV-miR-506) or negative control (LV-NC) (Hanbio, Shanghai, China) were inoculated subcutaneously into the flanks of nude mice (five mice per group). The mice were closely monitored for tumor growth, and tumor size was measured every 5 days. Tumor volume (V) was calculated via the formula V = 0.5 × length × width2. After 30 days, the mice were euthanized, and tumors were collected and weighed. As a metastasis model, 106 lentivirus-infected HCCLM3 cells in 0.1 mL of PBS were injected into nude mice via the tail vain (six mice per group). After 6 weeks, the mice were euthanized, and the lungs were excised, embedded in paraffin, and stained with hematoxylin and eosin (H&E). The metastatic nodules were counted microscopically to assess the development of pulmonary metastases.
Immunohistochemical (IHC) analysis
This analysis was carried out as previously described [18]. Briefly, the paraffin-embedded specimens were deparaffinized and rehydrated, then processed for antigen recovery and endogenous-peroxidase blocking. After blocking with goat serum, the slides were incubated with primary antibodies against Ki67, vimentin, E-cadherin, and IL8 (Proteintech) at 4°C overnight and treated with a horseradish peroxidase (HRP)-conjugated secondary antibody, then incubated with 3,3’-diaminobenzidine (DAB) for color development. The array was counterstained with hematoxylin and was examined under a microscope. Staining intensity was scored visually by two experienced pathologists independently as follows: 0 = no staining, 1 = weak staining, 2 = moderate straining, and 3 = strong staining. Five visual fields with tumor cells were randomly selected and scored based on the percentage of positively stained cells (0-100%). The final IHC score was calculated by multiplying the intensity score with the percentage of positive cells [19].
Statistical analysis
Numerical data were presented as mean ± SD. All the statistical analyses were conducted in the SPSS software, version 18.0. The significance of the differences in variables was assessed by Student’s t test. The association between miR-506 and IL8 expression was evaluated by Spearman’s correlation analysis. Data with P values < 0.05 were considered significant.
Results
MiR-506 is frequently downregulated in HCC
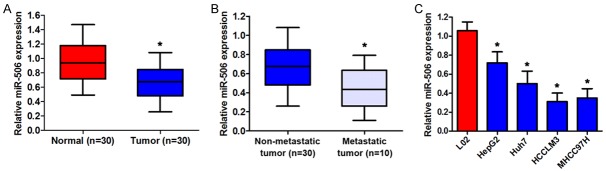
In this study, we first determined the expression level of miR-506 in 30 pairs of HCC samples and matching adjacent nontumorous tissues by qRT-PCR. As shown in Figure 1A, the expression level of miR-506 was significantly lower in HCC tissues compared to the corresponding adjacent normal tissues. In addition, miR-506 expression was significantly lower in metastatic-HCC tissue samples than in nonmetastatic-HCC tissue samples (Figure 1B). We also measured miR-506 levels in four HCC cell lines with different metastatic potentials: HepG2, Huh7, HCCLM3, and MHCC97H. The results showed that miR-506 expression levels were lower in all four HCC cell lines than in the normal hepatic cell line LO2. This was especially true for the highly metastatic HCC cell lines HCCLM3 and MHCC97H, which were used in the subsequent experiments (Figure 1C). The results above suggest that miR-506 may participate in HCC metastasis.
Figure 1.
The expression of miR-506 in HCC tissue samples and cell lines. A. Expression levels of miR-506 were measured by qRT-PCR in 30 pairs of samples of HCC and adjacent nontumorous tissue. B. Expression levels of miR-506 in nonmetastatic (n = 30) and metastatic (n = 10) tumors. C. qRT-PCR analysis of miR-506 expression in four human HCC cell lines, with the human hepatocyte LO2 cell line as a control. The average miR-506 expression was normalized to U6 expression. *P < 0.05.
MiR-506 suppresses HCC cell migration, invasion, and EMT in vitro
Next, we explored the potential involvement of miR-506 in the regulation of HCC metastasis. We transfected miR-506 mimics or the control (miR-ctrl) into HCCLM3 and MHCC97H cells. The wound-healing assay revealed that miR-506 overexpression suppressed cell migration of HCC cell lines (Figure 2A). Similarly, the invasion ability of HCC cells decreased after miR-506 overexpression in the Transwell and Matrigel-based 3D invasion assays (Figure 2B, 2C). In addition, we evaluated the expression of EMT markers in HCC cells by western blotting. The level of epithelial marker E-cadherin increased and that of mesenchymal marker vimentin decreased after overexpression of miR-506 (Figure 2D), suggesting that miR-506 inhibits the EMT of HCC cells. In contrast, miR-506 knockdown using anti-miR-506 in HepG2 cells significantly increased cell migration and invasion, and promoted EMT compared to the negative control (Figure 2E-G). These observations suggest that miR-506 suppresses HCC cell migration, invasion, and EMT in vitro.
Figure 2.
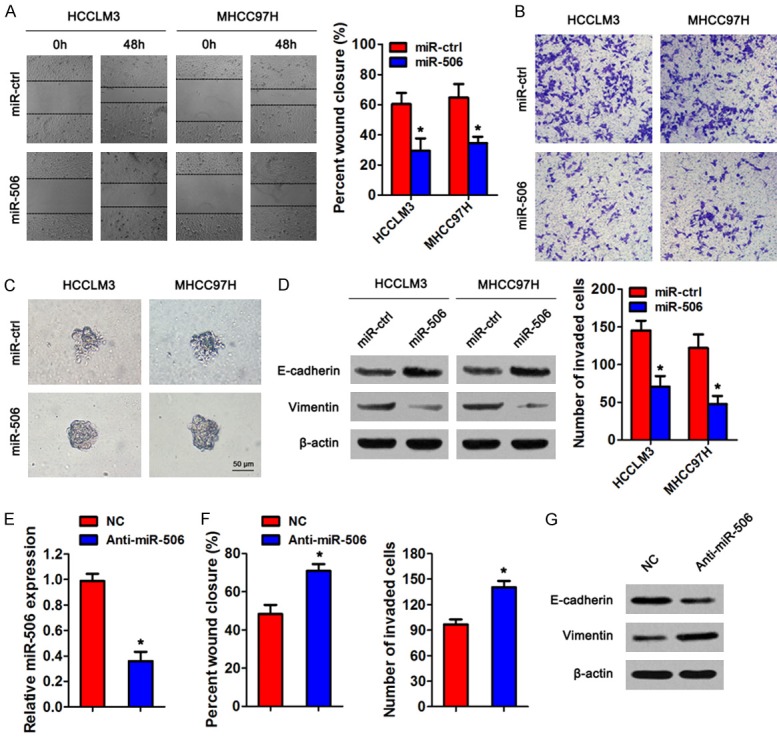
MiR-506 suppresses cell migration, invasion and EMT in vitro. HCCLM3 and MHCC97H cells were transiently transfected with miR-506 mimics or miR-ctrl, respectively. A. A wound-healing assay was performed to detect the cell migration ability. B. Cell invasion was determined by a Transwell assay. C. Representative micrographs of the indicated cultured cells in a Matrigel-based 3D spheroid invasion assay. D. Western blot analysis of EMT markers E-cadherin and vimentin in HCCLM3 and MHCC97H cells transfected with miR-506 mimics or miR-ctrl. β-Actin served as an internal control. E. qRT-PCR analysis of miR-506 expression in HepG2 cells transfected with anti-miR-506 or negative control. F. Wound-healing assay and Transwell invasion assay. G. Western blot analysis of EMT markers in the transfected HepG2 cells. *P < 0.05.
Overexpression of miR-506 inhibits tumor growth and metastasis in vivo
We then determined the influence of miR-506 on the tumorigenic potential and metastasis in vivo. HCCLM3 cells infected with a lentiviral vector expressing miR-506 or negative control were subcutaneously injected into the flanks of nude mice. The mice were euthanized after 30 days, and the tumors were collected. The results revealed that the mice injected with miR-506-overexpressing tumor cells had significantly smaller tumor volumes and tumor weight than did the mice injected with the control tumor cells (Figure 3A, 3B). We next detected the expression levels of Ki67, E-cadherin, and vimentin in the tumors processed by IHC staining. As Figure 3C indicates, the expression of Ki67 in the groups with miR-506 overexpression was significantly lower relative to the negative control. In addition, epithelial marker E-cadherin expression increased, whereas mesenchymal maker vimentin expression decreased after miR-506 overexpression. To further elucidate the effect of miR-506 on tu-mor metastasis in vivo, lentivirus-infected HCCLM3 cells were injected into nude mice through the tail vein. At 6 weeks after the injection, we found that miR-506 overexpression significantly decreased the number of metastatic nodules in lungs (Figure 3D). These findings suggest that miR-506 suppresses the growth of xenograft tumors and lung metastasis derived from HCC cells.
Figure 3.
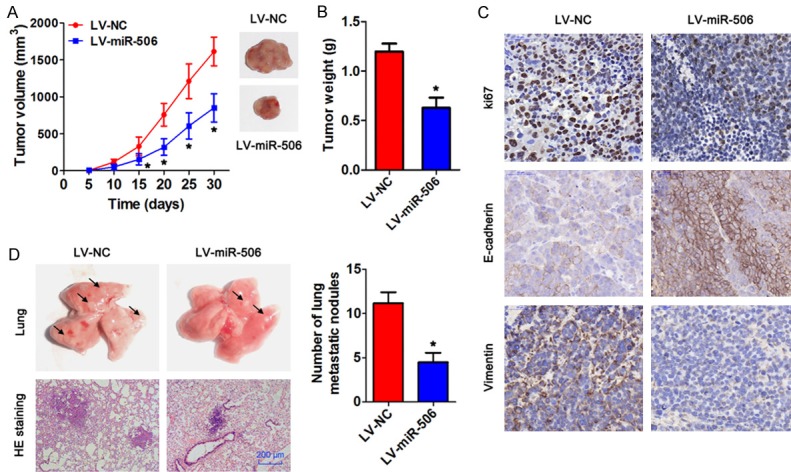
MiR-506 inhibits tumor growth and metastasis in vivo. HCCLM3 cells infected with lentivirus LV-miR-506 or LV-miR-NC were intravenously injected into nude mice (n = 5), and the tumors were collected after 30 days. A. The volume of the xenograft tumors. B. The weight of the xenograft tumors. C. IHC staining of Ki67, E-cadherin, and vimentin in subcutaneous tumors. Bar = 50 μm. D. Representative H&E-stained sections of the lung tissues collected from the LV-NC and LV-miR-506 groups are shown in the left panel. Quantitative analysis was carried out by counting the metastatic nodules in five randomly selected visual fields. Bar = 200 μm. *P < 0.05.
IL8 expression was directly targeted by miR-506
To further investigate the mechanism of miR-506 action in the regulation of HCC metastasis, the miRanda software was employed to predict the potential targets of miR-506. IL8 was identified as a potential effector of miR-506 according to the putative binding site in the 3’-UTR of IL8 mRNA (Figure 4A). To test whether miR-506 directly targets IL8 3’-UTR, we inserted the target region sequence of the IL8 3’-UTR (wt) or the mutated sequence (mt) into a luciferase reporter vector and coexpressed one of these vectors with either miR-506 mimics or miR-ctrl in HEK293 cells. As shown in Figure 4B, overexpression of miR-506 resulted in a significant decrease in the luciferase activity of the construct containing the wt 3’-UTR of IL8 but had no effect on the mt 3’-UTR reporter. In addition, miR-506 overexpression significantly suppressed the mRNA expression of IL8 in HCCLM3 and MHCC97H cells and reduced IL8 protein levels in the culture supernatant as compared to their respective controls (Figure 4C, 4D). Accordingly, subcutaneous tumors formed by miR-506-overexpressing HCCLM3 cells showed less IL8 staining (Figure 4E). We next investigated the relation between miR-506 expression and IL8 mRNA and protein expression in HCC tissues by Spearman’s correlation analysis. The results revealed that miR-506 expression inversely correlated with IL8 expression in HCC tissues (Figure 4F, 4G). Taken together, these data indicated that miR-506 represses IL8 expression via its specific seed region in the 3’-UTR of IL8 mRNA.
Figure 4.
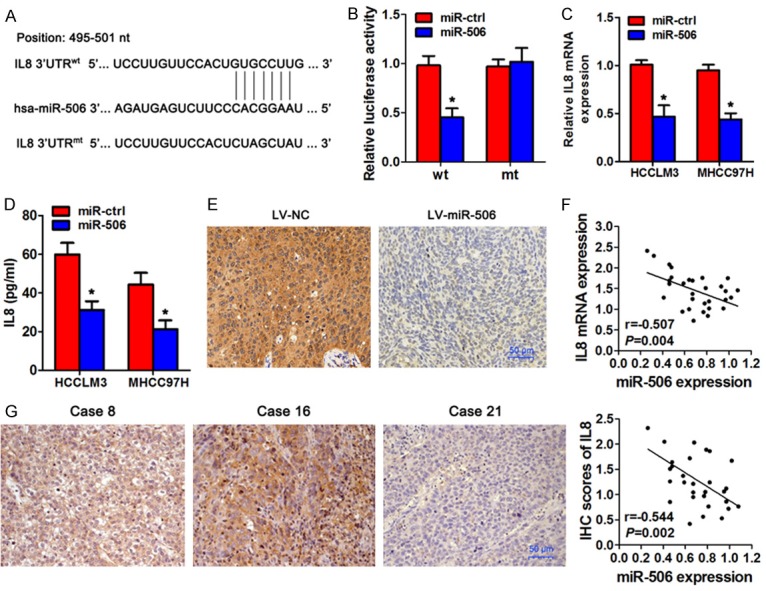
IL8 is a direct target gene of miR-506 in HCC cells. A. A schematic diagram of miR-506-binding sites in the IL8 3’-UTR. The sequence of miR-506 was compared with wild-type (wt) or mutant (mt) putative target sites in the 3’-UTR of IL8. B. Relative luciferase activity in HEK293T cells cotransfected with miR-506 or miR-ctrl and the wt or mt IL8 3’-UTR luciferase reporter construct. C. qRT-PCR analyses of IL8 expression in miR-506-overexpressing HCCLM3 and MHCC97H cells. D. IL8 protein levels were quantified by an ELISA in HCCLM3 and MHCC97H cells and in the cell culture supernatant after transient transfection with a miR-506 mimic. E. IHC staining of IL8 in subcutaneous tumors. F. The correlation between miR-506 levels and IL8 mRNA levels in 30 HCC tissue samples. G. The correlation between miR-506 levels and IL8 protein levels in 30 HCC tissue samples. *P < 0.05.
IL8 attenuates the effects of miR-506 on cell migration, invasion, and EMT
To determine whether miR-506 functionally targets IL8 3’-UTR, we added 100 pg/mL recombinant IL8 protein to the culture medium of HCC cells to simulate overexpression of IL8. As presented in Figure 5A, 5B, treatment with IL8 significantly attenuated the miR-506-induced suppression of HCC cell invasion and migration. Furthermore, IL8 treatment reversed the upregulation of E-cadherin as well as downregulation of vimentin caused by miR-506 overexpression (Figure 5C). Therefore, IL8 was a direct target gene through which miR-506 suppressed the EMT process and invasiveness of HCC cells.
Figure 5.
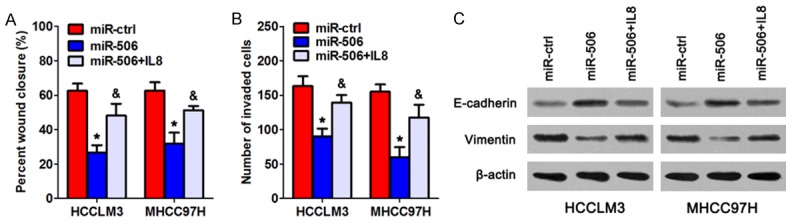
IL8 attenuates the effects of miR-506 on migration, invasiveness, and EMT of HCC cells. HCCLM3 and MHCC97H cells transfected with miR-506 mimics or miR-ctrl were treated with 100 pg/mL recombinant IL8 protein. A. A wound-healing assay was performed to assess the cell migration ability. B. A Transwell invasion assay was conducted to evaluate cell invasion. C. The expression levels of E-cadherin and vimentin were assessed by western blotting. β-actin served as an internal control. *P < 0.05 as compared with the miR-ctrl group; &P < 0.05 as compared with the miR-506 group.
Discussion
In this study, we analyzed the biological role of miR-506 in human HCC metastasis. We found that miR-506 is significantly downregulated in metastatic HCC tissues and cell lines compared with nonmetastatic HCCs and HCC cells with a low metastatic potential. Overexpression of miR-506 remarkably inhibited the migration and invasiveness of HCC cells in vitro and metastasis in vivo. In terms of the mechanism, we identified IL8 as a direct and functional target gene of miR-506. This finding deepens our understanding of the mechanisms underlying HCC metastasis.
MiR-506 is a member of the family of X chromosome-linked miRNAs, which was first identified by Bentwich and colleagues [20]. MiR-506 usually acts as a tumor suppressor by regulating tumor cell characteristics, including proliferation, cell cycle distribution, and apoptosis. Moreover, several lines of evidence indicate that miR-506 may perform novel functions in tumor invasion and metastasis. For example, Zhang et al. have demonstrated that downregulation of miR-506 is associated with colon cancer metastasis, whereas miR-506 suppresses tumor cell invasion and metastasis [14]. Li et al. have reported that miR-506 overexpression inhibits gastric cancer cell migration, invasion, and EMT [9]. In our study, we found that miR-506 expression levels were low in HCC cell lines and HCC tissue samples. Weak expression of miR-506 is highly associated with distant metastasis and has been shown to be a strong and an independent predictor of shorter disease-free survival among patients with HCC. We confirmed that restoration of miR-506 expression suppresses HCC cell migration and invasion in vitro and inhibits the growth of xenograft tumors and lung metastasis in vivo.
EMT is a critical process in tumor invasion and metastasis [21]. Epithelial cells lose their epithelial adherence, polarity, and cell-cell contacts, and these changes facilitate cell motility and invasion [22]. In our study, we demonstrated that ectopic expression of miR-506 significantly increases E-cadherin expression and concomitantly decreases vimentin expression in HCC cells, suggesting that miR-506 inhibits HCC metastasis by suppressing EMT.
Previous studies have revealed that miR-506 can regulate the expression of sphingosine kinase 1 (SPHK1) [23], snail family zinc finger 2 (SNAI2) [24], and zeste homolog 2 (EZH2) [14]. Here, we identified IL8 as a direct and functional target gene of miR-506. IL8 is well known as an inflammatory chemoattractant and contributes to the formation of a tumor microenvironment and to the progression of cancer. Thus, IL8 is involved in mobilization of immature myeloid cells and acceleration of tumorigenesis [25]. Studies indicate that high levels of IL8 contribute to poor prognosis of HCC, and IL8 promotes inflammation and the invasive and metastatic abilities of HCC cell lines [26]. In our study, administration IL8 reversed the miR-506-mediated suppression of migration, invasion, and EMT of HCC cells. Moreover, miR-506 expression negatively correlated with IL8 expression in human HCC specimens. These data indicate that IL8 is a direct target gene and that the IL8 protein attenuates the effects of miR-506 on the migration, invasiveness, and EMT of HCC cells.
In conclusion, our experiments indicate that miR-506 suppresses the migration, invasiveness, and metastasis of HCC cells and specifically targets the 3’-UTR region of IL8 mRNA. These findings suggest that miR-506 may function as a tumor suppressor and could be a therapeutic target in HCC.
Acknowledgements
This work was supported by the Shanghai Health and Family Planning Commission Foundation (20154Y0210).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Di Maio M, De Maio E, Perrone F, Pignata S, Daniele B. Hepatocellular carcinoma: systemic treatments. J Clin Gastroenterol. 2002;35:S109–114. doi: 10.1097/00004836-200211002-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Wijnhoven B, Michael M, Watson D. MicroRNAs and cancer. Br J Surg. 2007;94:23–30. doi: 10.1002/bjs.5673. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Liang L, Zhang X, Jia H, Qin Y, Zhu X, Gao X, Qiao P, Zheng Y, Sheng Y, Wei J, Zhou H, Ren N, Ye Q, Dong Q, Qin L. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–70. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 6.Dou C, Liu Z, Xu M, Jia Y, Wang Y, Li Q, Yang W, Zheng X, Tu K, Liu Q. miR-187-3p inhibits the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett. 2016;381:380–90. doi: 10.1016/j.canlet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Chang R, Yang H, Fang F, Xu J, Yang L. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology. 2014;60:1251–63. doi: 10.1002/hep.27221. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Zhang Q, Zhuo Z, Wu S, Xu Y, Zou L, Gan L, Tan K, Xia H, Liu Z, Gao Y. Enhanced homing of CXCR-4 modified bone marrow-derived mesenchymal stem cells to acute kidney injury tissues by micro-bubble-mediated ultrasound exposure. Ultrasound Med Biol. 2016;42:539–48. doi: 10.1016/j.ultrasmedbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Guo W, Xiong M, Han H, Chen J, Mao D, Tang B, Yu H, Zeng Y. Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med. 2015;36:1205–14. doi: 10.3892/ijmm.2015.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen S, Lin Y, Yu Y, Cao S, Zhang R, Yang X, Li J, Zhang Y, Wang Y, Ma M, Sun W, Lou X, Wang J, Teng Y, Zhang Z. miR-506 acts as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2015;34:717–25. doi: 10.1038/onc.2014.9. [DOI] [PubMed] [Google Scholar]
- 11.Sun G, Liu Y, Wang K, Xu Z. miR-506 regulates breast cancer cell metastasis by targeting IQGAP1. Int J Oncol. 2015;47:1963–70. doi: 10.3892/ijo.2015.3161. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang D, Parker Kerrigan BC, Shmulevich I, Chen K, Sood AK, Xue F, Zhang W. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol. 2014;233:308–18. doi: 10.1002/path.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai W, Huang H, Hu M, Wang S, He H, Chen N, Li M. microRNA-506 regulates proliferation, migration and invasion in hepatocellular carcinoma by targeting F-spondin 1 (SPON1) Am J Cancer Res. 2015;5:2697–707. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Lin C, Liao G, Liu S, Ding J, Tang F, Wang Z, Liang X, Li B, Wei Y, Huang Q, Li X, Tang B. MicroRNA-506 suppresses tumor proliferation and metastasis in colon cancer by directly targeting the oncogene EZH2. Oncotarget. 2015;6:32586–601. doi: 10.18632/oncotarget.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Zhang C, Liu L, Yi C, Lu S, Zhou X, Zhang Z, Peng Y, Yang Y, Yun J. miR-720 inhibits tumor invasion and migration in breast cancer by targeting TWIST1. Carcinogenesis. 2014;35:469–78. doi: 10.1093/carcin/bgt330. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Du C, Yao J, Wu B, Duan Y, Zhou L, Xu D, Zhou F, Gu L, Zhou H, Sun Y. C/EBPalpha suppresses lung adenocarcinoma cell invasion and migration by inhibiting beta-catenin. Cell Physiol Biochem. 2017;42:1779–88. doi: 10.1159/000479457. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Zou K, Yu L, Zhao W, Lu Y, Mao J, Wang B, Wang L, Fan S, Song B, Li L. MicroRNA-140 inhibits the epithelial-mesenchymal transition and metastasis in colorectal cancer. Mol Ther Nucleic Acids. 2018;10:426–37. doi: 10.1016/j.omtn.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Liu F, Cai Y, Rong X, Chen J, Zheng D, Chen L, Zhang J, Luo R, Zhao P, Ruan J. MiR-661 promotes tumor invasion and metastasis by directly inhibiting RB1 in non small cell lung cancer. Mol Cancer. 2017;16:122. doi: 10.1186/s12943-017-0698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–70. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 20.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–70. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 21.Thiery J, Sleeman J. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 22.Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wu H, Li W, Yin L, Guo S, Xu X, Ouyang Y, Zhao Z, Liu S, Tian Y, Tian Z, Ju J, Ni B, Wang H. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene. 2016;35:5501–14. doi: 10.1038/onc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot C, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, Cogdell D, Nykter M, Broaddus R, Rodriguez-Aguayo C, Lopez-Berestein G, Liu J, Shmulevich I, Sood A, Chen K, Zhang W. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell. 2013;23:186–99. doi: 10.1016/j.ccr.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarogoulidis P, Katsikogianni F, Tsiouda T, Sakkas A, Katsikogiannis N, Zarogoulidis K. Interleukin-8 and interleukin-17 for cancer. Cancer Invest. 2014;32:197–205. doi: 10.3109/07357907.2014.898156. [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, Wu K, Xia L. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149:1053–67. doi: 10.1053/j.gastro.2015.05.058. [DOI] [PubMed] [Google Scholar]