Abstract
Cancer stem cells (CSCs) that closely correlated with tumor growth, metastasis, provide a plausible explanation for chemoresistance and cancer relapse. CSCs are usually isolated and enriched from carcinoma cells, which is inconvenient, low-efficient, and even unreliable. Here, we converted mouse induced pluripotent stem cells (miPSCs) into prostate cancer stem-like cells with carcinoma microenvironment following exposure to conditioned medium (CM) derived from RM9, a mouse prostate cancer cell line. These transformed cells, termed as miPS-RM9CM, displayed CSCs properties, including spheroids morphology and expression of both stemness genes and cancer stem cells surface markers, such as Oct3/4, Sox2, Nanog, Klf-4, c-Myc, CD44, and CD133. In addition, in vivo transplantation experiment was performed to confirm the tumorigenicity. Furthermore, we used the model to assess conventional chemotherapeutic agent, docetaxel. The results showed that miPS-RM9CM cells exhibited increased resistance to docetaxel, however, high susceptibility to the cancer cell stemness inhibitor I (BBI-608). Our current study demonstrates that CM from cultured RM9 cells play a crucial role in the determination of cell fate from miPSCs to cancer stem-like cells and provide a potentially valuable system for the study of CSCs.
Keywords: Induced pluripotent stem cell, prostate cancer, cancer stem cell, conditioned medium, tumor microenvironment, chemoresistance
Introduction
Prostate cancer (PCa) is the second most common cancer and one of the leading causes of cancer death among men worldwide [1]. The current standard practices for the treatment of prostate cancers include surgical resection, radiotherapy, and chemotherapy. However, these treatments often result in poor outcome due to early metastasis and further post-operative recurrence [2]. Recent studies come to implicate that CSCs are the source of cancer occurrence, development and recurrence [3,4]. CSCs or tumor-initiating cells are a subset of cancer cells and characterized by self-renew and differentiation potential. In solid tumors, the minority population of CSCs appears to locate in the tumor mass involved in tumor initiation and progression. Furthermore, accumulating evidence show that CSCs are the major contributor for chemoresistance [5]. Conventional chemotherapeutic agents are considered to target differentiated cells, while CSCs appear to possess resistance to their toxicity [6]. Therefore, identifying the mechanisms that hamper CSCs stemness may help to improve the efficacy of chemotherapy.
Induced pluripotent stem cells (iPSCs) are generated by introducing transcription factors (Oct3/4, Sox2, Klf4, c-Myc, and Nanog) into ordinary somatic cells and show the same morphology, pluripotency, and differentiation potential similar to embryonic stem cells [7]. CSCs display great flexibility and reversible switch between stem and non-stem cell states. Chen, L et al. reported that Nanog positive miPSCs could acquire characters of CSCs when cultured in the conditioned culture medium derived from mouse Lewis lung carcinoma, which is a mimic of tumor microenvironment [8]. In another study, tumor-derived extracellular vesicles (tEVs) secreted by Lewis lung carcinoma cells facilitate the process of transforming miPSCs into CSCs [9]. Taken together, these studies suggest a role for secreted factor(s) from tumor microenvironment in the determination of stem cell fate and these factors promote the formation of CSCs from miPSCs, despite the mechanism underlying the conversion remains unclear.
CSCs are usually isolated and enriched from carcinoma cells, which is inconvenient, low-efficient, and the success rate of cancer cells into CSCs was much lower. In this study, we investigated whether CM derived from RM9 could transform miPSCs into cancer stem-like cells easily and efficiently. These results imply the possibility for formations of CSCs by CM and could represent a valuable model for the study of CSCs in vitro.
Material and methods
Cell culture and reagents
miPSCs expressed GFP gene in control of Nanog promoter were obtained from Riken Cell Bank (Kyoto, Japan). The cells were maintained on feeder layers of mitomycin-C-treated MEF cells (Reprocell, Japan) in ES-101B complete ES cell media with 15% FBS and LIF (ES-101-B, Millipore, USA). Mice prostate cancer cell line RM9 was kindly provided by Dr. T.C. Thompson (Baylor College of Medicine, Houston, TX, USA) and maintained in RPMI-1640 medium (Gibco, Invitrogen, Carlsbad, USA) supplemented with 10% FBS at 37°C in a humidified incubator.
The CM from RM9 were gained as described [8]. In brief, culture supernatants were collected from confluent dishes and centrifuged for 5 mins at 1000 rpm to remove cells and large debris. The miPSCs were induced to differentiate with CM and miPSCs medium (1:1), and the medium was changed every two days for 42 days. Cells morphology were photographed by random field selection under a fluorescence microscope (IX-71, Olympus, Tokyo, Japan).
Antibodies and reagents
Antibodies against Oct 4A (#83932), Sox2 (#14962), Nanog (#8822), Klf-4 (#4038), β-actin (#4970), and anti-rabbit IgG HRP-linked antibody (#7076) were purchased from CST (Billerica, MA, USA), antibody against CD133, was from Proteintech (18470-1-AP, Chicago, USA), CD44 from Abcam (ab157107, MA, USA).
Cancer cell stemness inhibitor I (BBI-608) was obtained from BioVision (Mountain View, CA). Docetaxel was purchased from Sanofi (#87424, Tokyo, Japan).
Western blot
Cells were collected and lysed in lysis buffer (#78410, Thermo Scientific). After centrifugal separation, the protein concentrations were measured using BCATM Protein Assay Kit (Thermo Scientific, Rockford, USA). Protein samples were added to 10% SDS/gel polyacrylamide (Bio-Rad) for electrophoresis and transferred onto PVDF membrane. And then, the membrane was blocked with 5% non-fat milk for 1 h. The primary antibodies were incubated overnight at 4°C and secondary antibodies for 1 h at room temperature. Bands were visualized with enhanced chemiluminescent (ECL) kit.
RNA extraction and RT-PCR
Total RNA was extracted using the RNeasy Plus Mini Kit (QIAGEN). In total, RNA (1 μg) was reverse-transcribed into first strand cDNA with the PrimeScript™ RT reagent Kit and gDNA Eraser (Takara). PCR amplifications for OCT3/4, SOX2, NANOG, KLF-4, C-MYC, and GAPDH were performed using primer sequences as Table 1. The PCR conditions were as follows: 3 min at 98°C and 30 cycles of 96°C for 30 sec, 65°C for 30 sec and 72°C for 30 sec. After the final cycle, the reaction was amplified at 72°C for 3 min. All the reactions were performed in three independent experiments, and the GAPDH gene was used as the internal control. All transcripts were confirmed using 3% agarose gel electrophoresis.
Table 1.
The primers used in the RT-PCR analysis
| Primer name | Primer sequence |
|---|---|
| OCT3/4 | Forward: 5’-CTGAGGGCCAGGCAGGAGCACGAG-3’ |
| Reverse: 5’-CTGTAGGGAGGGCTTCGGGCACTT-3’ | |
| SOX2 | Forward: 5’-GGTTACCTCTTCCTCCCACTCCAG-3’ |
| Reverse: 5’-TCACATGTGCGACAGGGGCAG-3’ | |
| NANOG | Forward: 5’-AGGGTCTGCTACTGAGATGCTCTG-3’ |
| Reverse: 5’-CAACCACTGGTTTTTCTGCCACCG-3’ | |
| KLF-4 | Forward: 5’-AGTGTGACAGGGCCTTTTCCAGGT-3’ |
| Reverse: 5’-AAGCTGACTTGCTGGGAACTTGACC-3’ | |
| C-MYC | Forward: 5’-CAGAGGAGGAACGAGCTGAAGCGC-3’ |
| Reverse: 5’-TTATGCACCAGAGTTTCGAAGCTGTTCG-3’ | |
| GAPDH | Forward: 5’-CCGCATCTTCTTGTGCAGTG-3’ |
| Reverse: 5’-CTGTGGTCATGAGCCCTTCC-3’ |
Evaluation of tumorigenicity in vivo
Male C57BL/6 mice (6-8 weeks) were obtained from Japan SLC, Inc. and kept under SPF environment. All studies involving animals were approved by the Ethics Committee of Okayama University. Tumorigenicity was determined by subcutaneously (s.c.) injecting different density of miPSCs and miPS-RM9CM cells, the cells were suspended in 100 μL DMEM containing 10% FBS and respectively injected into the flanks of 6-week-old C57BL/6 mice. The left side was injected s.c. with 103 cells and the right side was injected s.c. with 105 cells. Tumor growth was then monitored weekly for up to 2 weeks after transplantation. Mice were sacrificed, and the tumor was resected for histologic analysis. The tumor was embedded, and one section every 4 μm was stained with H&E according to the standard protocols.
Drug treatment assays
miPS-RM9CM cells and miPSCs were treated with increasing concentrations of BBI608 (0, 0.5 and 2 μM) or docetaxel (0, 1 and 5 nM) for 24 hours. Cells morphology were observed and photographed under a fluorescence microscope and then harvested the cells for RNA and protein to evaluate the expression of Oct4, Sox2, Nanog, Klf4, CD44, and CD133.
Results
Induction of miPS-RM9CM cells by conditioned medium derived from mouse prostate cancer RM9 cells
We investigated whether CM from mouse prostate cancer cell line RM9 could stimulate the transformation of miPSCs into CSCs. Experiment scheme for the induction of miPSCs was shown in Figure 1A. The miPSCs exposed to CM for 42 days by changing half of the medium every two days, were observed on MEF cells cultured dishes. Nanog-GFP miPSCs harbored a GFP reporter gene, which allowed us to distinguish self-renewing undifferentiated cells. GFP positive cells were abundant and displayed enlarged and flattened spheroid morphology (Figure 1B).
Figure 1.
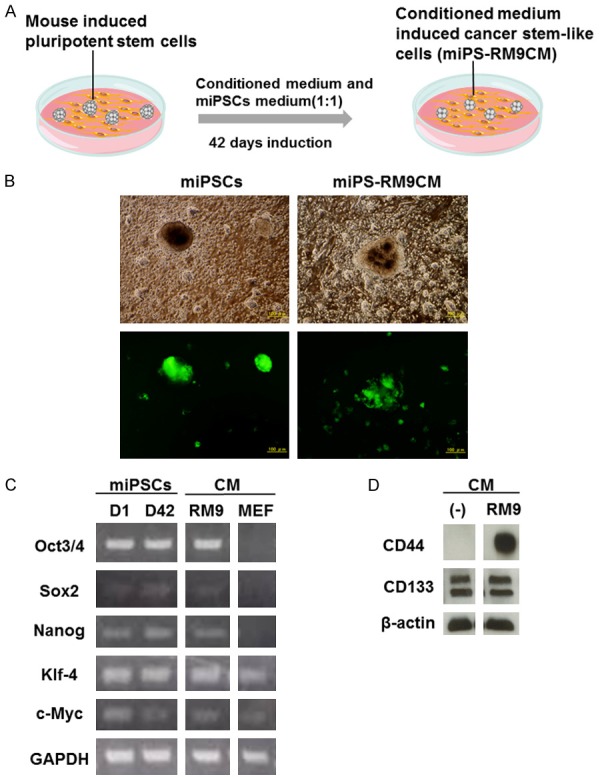
Functional analysis of induced cancer stem-like miPS-RM9CM cells. A. Experiment scheme for the induction of miPS-RM9CM cells B. Representative images of miPSCs and miPS-RM9CM cells with GFP expression. Both the miPSCs and miPS-RM9CM cells displayed spheroid morphology under a fluorescence microscope. Scale bar: 100 μm. C. RT-PCR analysis of transcription factors, including Oct3/4, Sox-2, Nanog, Klf-4, c-Myc in miPSCs and miPS-RM9CM cells before and after induction using the conditioned medium (CM) derived from RM9 cell. The expression levels of transcription factors in MEF cells was used as a control. D. Cancer stem cell surface marker, CD133 and CD44 expression in miPS-RM9CM cells were analyzed by western blot. GAPDH and β-actin served as a loading control. Results were representative of three independent experiments.
Expression of the transcription factors in transformed cells
Transcription factors such as Oct-4, Sox-2, Nanog, Klf-4, and c-Myc have been implicated in pluripotency. To determine if miPS-RM9CM have the characteristics of stemness, we examined the level of the transcription factors by reverse transcription PCR (RT-PCR). As shown in Figure 1C, both original miPSCs and miPS-RM9CM cells expressed the transcription factors, Oct 3/4, Sox-2, Nanog, Klf-4, c-Myc, no significant difference was found in miPSCs versus miPS-RMCM cells. The results imply that miPS-RM9CM still maintain stem cell property. The expression levels of transcription factors in MEF cells were used as a control. To further study the transformation of miPSCs into CSCs, we investigated CSCs surface markers expression on both cells. We observed the expression of CD44 in miPS-RM9CM cells was at a higher level when compared with the original miPSCs. No significant differences in the CD133 expression were found between two kinds of cell types (Figure 1D).
In vivo tumor-initiating properties of miPS-RM9CM
To assess the tumor-forming ability of miPSCs and miPS-RM9CM cells in vivo, we subcutaneously injected these cells into C57BL/6 mice and monitored for 2 weeks respectively. All mice were induced tumorigenesis after injection of miPS-RM9CM, 100,000 cells per mouse (5/5 mice, 100%; Figure 2A). The tumor-formation frequency of miPS-RM9CM (100,000 cells) was significantly high. However, no tumor was formed when injected with the number of 1000 and 100,000 miPSCs (Figure 2A). These results suggest that miPS-RM9CM exhibit high degree of tumorigenicity with rapid growth in vivo. Furthermore, histological examinations of tumor tissues revealed that subcutaneous tumors from miPS-RM9CM cells displayed typical malignant phenotypes such as atypia and pathological mitotic figure. In addition, apoptotic bodies were observed in some grown cells (Figure 2B).
Figure 2.
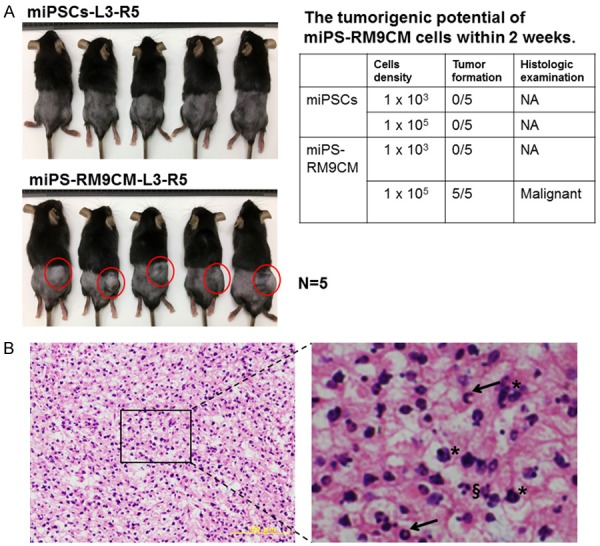
In vivo tumorigenic potential assay. A. Different density of miPSCs or miPS-RM9CM cells were subcutaneously injected into C57BL/6 mice. L3 represented the left flank of mice injected with 103 cells and R5 represented the right flank injected with 105 cells. The number of tumor formation within 2 weeks were counted in the right table. NA: not applicable. B. Representative H&E staining sections of subcutaneous xenograft tumor from miPS-RM9CM cells. The tumors exhibited typical malignant phenotype with the different characteristic of atypia (asterisks), and pathological mitotic figure (arrows), and apoptotic bodies (§) were observed in some grown cells. Scale bar: 100 μm. Results were representative of three independent experiments.
Cancer stem cell inhibitor, BBI608 downregulates stemness in the miPS-RM9CM cells
BBI608, a cancer stemness inhibitor, prevents self-renewal and promotes cell death in CSCs. Formation of spheres is one of the main characteristics of cancer stemness. Interestingly, even with high-dose BBI608 treatment, the change in the morphology of miPSCs was not significant (Figure 3A). Contrarily, we found that BBI608 treatment attenuates the sphere-forming ability of miPS-RM9CM cells in a dose-dependent manner (Figure 3B). In addition, we used the established model to track docetaxel therapeutic effect on CSCs. miPS-RM9CM cells still maintained the intact spheroids morphology after docetaxel treatment. At the protein level, BBI608 dramatically downregulated the expression of Oct-4A, Sox-2, Nanog in miPS-RM9CM cells in a dose-dependent manner but not in miPSCs (Figure 4A). In contrast, docetaxel had no effect on the expression level of these factors in miPS-RM9CM cells and miPSCs (Figure 4B). It is noteworthy that after BBI608 treatment, the decrease in transcription activities of Oct-4, Sox-2, Nanog, and Klf-4 was also consistent with their protein levels. The expression of transcription factors in miPS-RM9CM cells was inhibited significantly by BBI608 (Figure 4C). Nevertheless, docetaxel did not affect the expression of these factors in miPS-RM9CM cells (Figure 4D). We also detected the expression level of cancer stem cell surface marker by western blot analysis, BBI608 markedly inhibited the expression of CD133 and CD44 in miPS-RM9CM cells (Figure 4E).
Figure 3.
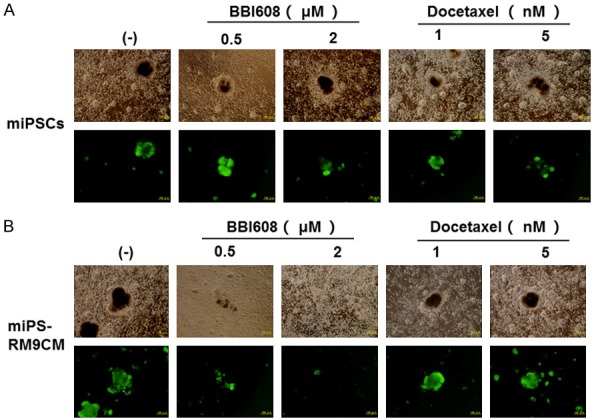
BBI608 treatment attenuated the sphere-forming ability of miPS-RM9CM cells in a dose-dependent manner. A. Representative morphology of miPSCs with BBI608 or docetaxel treatment in different doses for 24 h. B. Representative morphology of miPSC-RM9CM with BBI608 or docetaxel treatment in different doses for 24 h. Scale bar: 100 μm.
Figure 4.
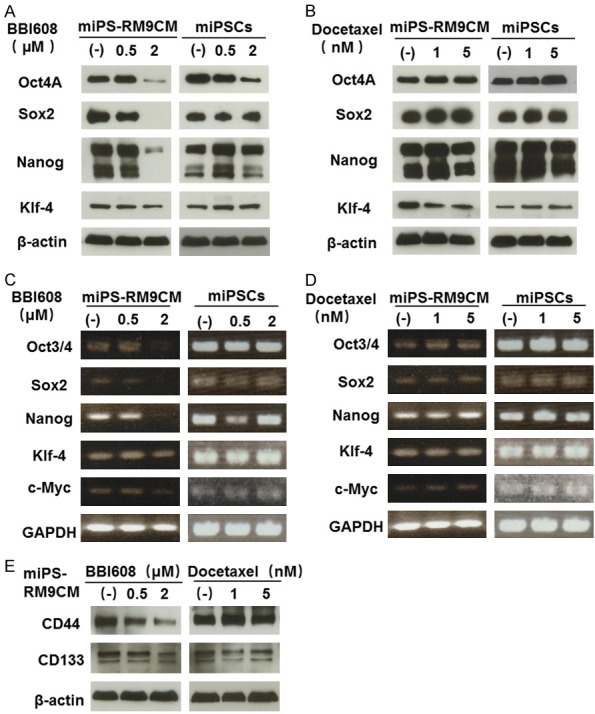
Protein and gene expression analysis of stemness factor and CSC surface marker in miPSCs and miPS-RM9CM cells treated with BBI608 and docetaxel. A and B. Western blot analysis of Oct4A, Sox2, Nanog and Klf-4 expression in miPSCs and miPS-RM9CM cells with BBI608 or docetaxel treatment for 24 h. C and D. RT-PCR analysis of Oct3/4, Sox2, Nanog and Klf-4 expression in miPSCs and miPS-RM9CM cells with BBI608 or docetaxel treatment for 24 h. GAPDH served as a loading control. E. The expression of CD133 and CD44 in miPS-RM9CM cells with BBI608 or docetaxel treatment for 24 h. β-actin was used as a loading control. Results are representative of three independent experiments.
Discussion
We have demonstrated in the study that CM derived from mouse prostate cancer RM9 cell induced the transformation of miPSCs into cancer stem-like cells. These transformed cells are characterized by spheroids morphology, pluripotency, high tumorigenic potential and chemotherapeutic resistance. In addition, cancer stem-like cells can be converted from miPSCs cells efficiently and help us design new therapeutic strategies targeting CSCs.
iPSCs display the same characteristics of embryonic stem cells (ESCs) such as morphology, immortal cell growth, marker expression, self-renewal and differentiation into multiple lineages [10]. It is believed that under the influence of appropriate environments, iPSCs could differentiate into a various mature cell including cardiomyocyte [11], neurons [12], hepatocytes [13], and trophoblast [14]. Despite these studies indicate that iPSCs possess great value for regeneration therapy, there is a long way to go in transplanting into patients. The main concern is that iPSCs into nude mice are likely to generate teratomas and lead to malignant transformation [15-17].
The stem cell niche, a particular microenvironment, regulate the balance between self-renewal and differentiation in stem cell [18]. The molecular cross-talk signals and factors in microenvironment make cell fate decisions easy in an unclear manner [19]. When cultured with newborn bovine serum, ES cells could differentiate into CSC-like cells [20], suggesting that abnormal niche signals or factors have a critical influence on normal stem cells. Our study showed that CSCs could stem from normal stem cells by conditioned medium. Despite not familiar with the specific mechanisms of dominating transformation procedure, several studies attribute it to genetic instability in these cells, which may explain miPSCs is susceptible to the soluble factor (s) in the tumor microenvironment [8,9,21]. Exosomes are small extracellular vesicles that bear proteins and nucleic acids. They serve as pathways of intercellular communication and play a key role in cell growth and malignant transformation [22-24]. Exosomes have been identified as mediators to modulate tumor micro-environment [25-27]. It would be worthwhile to explore the role of the exosomes released from RM9 cells during the transformation of miPSCs into cancer stem-like cells.
The transcription network regulating pluripotency in stem cell is considered to play a crucial role in the tumor-initiating ability of cancer cells. Nanog has been shown to initiate CSC-like characteristics and contributes to oncogenesis in several cancers [28,29]. BORA-SINGHA et al. demonstrate that Oct4 regulate self-renewal through activation of the downstream signal pathway in cancer-stem like cells [30]. One study by Boumahdi (2014) also argues that Sox2 deletion in squamous cell cancer result in tumor regression and decrease the ability of tumor formation in vivo [31]. Overall, this evidence indicated that there is a positive association between these stemness factors and cancer stem cells. Our results showed that no difference was found in the expression of Oct4, Sox2, Nanog, Klf4 and c-Myc in miPS-RM9CM cells when compared with miPSCs, which suggests that miPS-RM9CM are maintained in the relatively undifferentiated state as those of miPSCs and high expression of these factors may be responsible for high self-renewal ability of miPS-RM9CM cells. CD44 have been identified as a negative cell surface marker for pluripotent stem cell and is found to overexpress in CSCs originated from prostate cancer cells [32,33]. In the current study, miPS-RM9CM expressed a high level of CD44, indicating that miPS-RM9CM cells had undergone malignant transformation and acquired CSCs cell surface marker after induction of CM.
It is generally true that CSCs have high resistance capacity to routine drug therapy [34,35]. Our previous study has reported that BBI608 downregulated the expression of stemness factors in prostate cancer stem cells and increased the sensitivity of prostate cancer cells to docetaxel [36]. In the study, we observed that miPS-RM9CM exhibited resistance to the chemotherapeutic compound, docetaxel, but high sensitivity to cancer cell stemness inhibitor, BBI608, suggesting that comparing with miPSCs cells, miPS-RM9CM cells possess characteristic associated with CSCs, especially the ability of drug resistance.
In our study, we demonstrated that prostate cancer stem-like cells can be efficiently induced from miPSCs by the conditioned medium derived from prostate cancer cell line and provide a valuable model for the future study of CSCs.
Acknowledgements
Thanks to Dr. Shunai Li for the technical assistant with RT-PCR experiment and helpful advice. This study was funded by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17K11138) and the Science and Technology Planning Project of the Guangdong Province (2016A020215109), the Guangdong Natural Science Foundation (No. 2015A030313291) and China Scholarship Council (No. 201508210184).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Poortmans P, Bossi A, Vandeputte K, Bosset M, Miralbell R, Maingon P, Boehmer D, Budiharto T, Symon Z, van den Bergh AC, Scrase C, Van Poppel H, Bolla M EORTC Radiation Oncology Group. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84:121–127. doi: 10.1016/j.radonc.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Park M, Jang H, Hyun H, Lim W. Chemoresistance to 5-FU inhibited by 635 nm LED irradiation in CD133+ KB cell line. Lasers Med Sci. 2018;33:57–66. doi: 10.1007/s10103-017-2335-2. [DOI] [PubMed] [Google Scholar]
- 7.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Kasai T, Li Y, Sugii Y, Jin G, Okada M, Vaidyanath A, Mizutani A, Satoh A, Kudoh T, Hendrix MJ, Salomon DS, Fu L, Seno M. A model of cancer stem cells derived from mouse induced pluripotent stem cells. PLoS One. 2012;7:e33544. doi: 10.1371/journal.pone.0033544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan T, Mizutani A, Chen L, Takaki M, Hiramoto Y, Matsuda S, Shigehiro T, Kasai T, Kudoh T, Murakami H, Masuda J, Hendrix MJ, Strizzi L, Salomon DS, Fu L, Seno M. Characterization of cancer stem-like cells derived from mouse induced pluripotent stem cells transformed by tumor-derived extracellular vesicles. J Cancer. 2014;5:572–584. doi: 10.7150/jca.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JH, Wilson GF, Soerens AG, Koonce CH, Yu JY, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research. 2009;104:E30–E41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 13.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 15.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hubner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Scholer HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 19.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 20.Fujimori H, Shikanai M, Teraoka H, Masutani M, Yoshioka K. Induction of cancerous stem cells during embryonic stem cell differentiation. J Biol Chem. 2012;287:36777–36791. doi: 10.1074/jbc.M112.372557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasai T, Chen L, Mizutani A, Kudoh T, Murakami H, Fu L, Seno M. Cancer stem cells converted from pluripotent stem cells and the cancerous niche. J Stem Cells Regen Med. 2014;10:2–7. doi: 10.46582/jsrm.1001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 24.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013;91:431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim EE, Babaei-Jadidi R, Saadeddin A, Spencer-Dene B, Hossaini S, Abuzinadah M, Li N, Fadhil W, Ilyas M, Bonnet D, Nateri AS. Embryonic NANOG activity defines colorectal cancer stem cells and modulates through AP1- and TCF-dependent mechanisms. Stem Cells. 2012;30:2076–2087. doi: 10.1002/stem.1182. [DOI] [PubMed] [Google Scholar]
- 29.Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, Repass J, Zaehres H, Shen JJ, Tang DG. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, Chellappan S. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells. 2015;33:1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohee S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 32.Quintanilla RH, Asprer JST, Vaz C, Tanavde V, Lakshmipathy U. CD44 Is a negative cell surface marker for pluripotent stem cell identification during human fibroblast reprogramming. PLoS One. 2014:9. doi: 10.1371/journal.pone.0085419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang LL, Jiao M, Li L, Wu DP, Wu KJ, Li X, Zhu GD, Dang Q, Wang XY, Hsieh JT, He DL. Tumorspheres derived from prostate cancer cells possess chemoresistant and cancer stem cell properties. J Cancer Res Clin Oncol. 2012;138:675–686. doi: 10.1007/s00432-011-1146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 35.Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901–1906. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Jin Z, Zhou H, Ou X, Xu Y, Li H, Liu C, Li B. Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med. 2016;5:1251–1258. doi: 10.1002/cam4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


