Abstract
Recent evidence suggests that cancer stem cells (CSCs), a small population of cancer cells that are highly tumourigenic, capable of self-renewal and have the ability to differentiate into cells that constitute the tumor, are the “drivers” of local recurrence and metastatic spread and may be associated with resistant to conventional therapy. The objectives of the study are to identify and characterize two head and neck cancer cell lines with regard CD44high/CD133high/CD117high profile (CSCs) and CD44low/CD133low/CD117low profile (Non-CSCs); to investigate the influence of chemotherapy treatment in CSCs and compare with Non-CSCs; to evaluate CD44 and EGFR gene expression in CSCs. Fluorescent-activated cell sorting (FACS) using specific cell surface marker combination (CD44, CD117 and CD133) was performed to isolate CSCs of Non-CSCs from cell lines. The Wound Healing assay was performed to confirm the presence of CSCs. After, the CSCs subpopulation and Non-CSCs were cultured and exposed for 24 h to Cetuximab and Paclitaxel treatment, separately. Cell proliferation was determined by MTS assay. CD44 and EGFR gene expression was quantified by quantitative real time PCR (qPCR) using TaqMan® Assay in both subpopulations. CSCs subpopulation untreated were considered as relative expression control. We firstly characterized CSCs in HN13 and HEP-2 cell lines with CD44, CD133 and CD117 biomarkers. We treated CSCs and Non-CSCs subpopulations with Cetuximab and Paclitaxel treatment and found that CSCs subpopulations demonstrated more resistance to Paclitaxel chemoterapy, when compared with Non-CSCs subpopulations of oral cancer cell line. These CSCs subpopulations presented up-regulation of CD44 gene and down-regulation of EGFR gene in oral cancer cell line, and down-regulation of CD44 gene and up-regulation of EGFR gene in laryngeal cancer cell line when compared with Non-CSCs subpopulations. We conclude that the combination of CD44, CD133 and CD117 biomarkers have stem cell properties in both cell lines. CSCs has ability to resist to Paclitaxel treatment in oral cancer cell line. CSCs present high expression of CD44 gene and down expression of EGFR gene in oral cancer cell line. CSCs in laryngeal cell line present down expression of CD44 gene and high expression of EGFR gene when compared with cells without characteristics of cancer stem cells.
Keywords: Cancer stem cells, chemotherapy, head and neck neoplasias, gene, expression, CD44, EGFR, cell line
Introduction
Head and neck cancer (HNC) is an aggressive disease that accounts for more than 500,000 cases each year worldwide [1]. The high prevalence of the disease is due to high rates of recurrence and metastasis. Furthermore the rate of success in treatment still remains low [2-4]. The treatment options for HNC depend of tumoral stage and can be surgery, radiotherapy and/or chemoterapy [5]. Treatment for HNC in early stage (stage I and II) generally involves single-modality therapy: Surgery or radiotherapy. However, patients with HNC locally advanced (stage III and IV A/B) are treated with chemoradiotherapy with or without chemotherapy [3,7].
Chemotherapy treatment has improved in the last years but the supportive care for patients in treatment has increased because still there are many collateral effects as mucositis, skin desquamation, depression, fatigue, nausea, vomiting and others. Furthermore some patients have no answer for chemotherapy treatment compared to other patients with the same tumoral stage and the overall survival rate remains low [2,8-10]. The fact can be associated with the presence of cancer stem cell (CSC) in tumor [11,12].
CSC are defined as a small subpopulation of cells located within the tumor mass with high capacity of tumorigenic potential, self-renewal properties and slow growth cycle which is responsible to resistance to therapies that firstly target cancer cells that present faster growth [13-15]. The identification of CSC can provide interesting data regarding new therapeutic approaches in HNC and they may be identified through molecular biomarkers as CD44, CD117 and CD133 [16-18].
In the current study, the aim was to identify and separate cancer stem cells through CD44, CD133 and CD117 biomarkers in two subpopulations of head and neck cancer cell lines (HN13 and HEP-2 cell lines): CD44high/CD133high/CD117high (CSCs) and CD44low/CD133low/CD117low (Non-CSCs), to verify if these biomarkers have stem cell properties; to compare effectiveness of Cetuximab and Paclitaxel treatment in CSCs and Non-CSCs subpopulations of HN13 and HEP-2 cell lines, and to evaluate CD44 and EGFR gene expression in the CSCs subpopulations.
Material and methods
Cell line and culture conditions
HN13 (squamous cell carcinoma of oral cancer cell line) and HEP-2 (laryngeal cancer cell line) cells were cultured in D-MEN (Sigma-Aldrich, St. Louis, MO) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 1 mM L-glutamine, 100 units/mL penicillin, and 100 lg/mL streptomycin (all reagents were from Invitrogen, Grand Island, NY).
Flow cytometry (Indetification and isolation of CSCs)
The trypsinized cells were resuspended, incubated with monoclonal antibodies for 30 min at 4°C, washed twice with phosphate buffered saline (PBS). The antibodies utilized were CD44-phycoerythrin (PE), CD117-fluorescein isothiocyanate (FITC) and CD133-allophycocyanin (APC). Fluorescent-activated cell sorting (FACS) of live cells was used to separate subpopulations of HN13 and HEP-2 subpopulation of cells using specific cell surface biomarkers combinations (CD44/PE, CD117/FITC and CD133/APC) with BD FACSAria Fusion equipament (BD Biosciences).
The subpopulation of sorted cell lines were classified based on the expressions of CD44/CD117/CD133 in combination as: CD44high/CD133high/CD117high: presence of CSCs and CD44low/CD133low/CD117low profile (Non-CSCs). CSCs and Non-CSCs were resuspended in D-MEN for further experiments.
Wound healing assay
For confirmation of presence of CSCs, the CSCs and Non-CSCs subpopulations cells were plated at a density of 2 × 106 cells/wells and cultured until they reached confluence. A diametric scratch was created using a pipette tip and washed with PBS 3 times. The cells were photographed in microscope (OLYMPUS - CKX61/40 × objective lens) in three pre-marked spots as 0 h. Images were then acquired at 24 h in the same spots for comparison.
Drug sensitivity and MTS assay
CSCs and Non-CSCs subpopulations were plated at a density of 2 × 106 cells/well in six well plates. Cetuximab (CT), Paclitxel (P) chemotherapeutic agents at 0.06 mg/ml and 0.05 mg/ml concentrations, respectively, were added in the CSCs and Non-CSCs subpopulations [19,20]. The cultures were incubated at 37°C for 24 h. The proliferation of cell lines were measured at OD 490 nm using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS, Promega, Madison, WI, USA). The experiments were repeated two times. The results were expressed as percentage relative to the control cells. The chemotherapeutics evaluated are widely utilized in patients with oral cancer, so they were included in the study.
Real-time quantitative RT-PCR
RNA isolation was performed using Trizol (Invitrogen) according to manufacturers’ manuals. The concentration of RNA utilized was 2 ug (Picodrop Equipament). For cDNA synthesis, 1 ug RNA was used with primers by High capacity cDNA kit (Applied Biosystem®) according manufacturer’s protocol. Genetic expression in all samples was evaluated by quantitative RT-PCR (qRT-PCR) with StepOnePlusTM Equipament (Applied Biosystems).
A polymerase chain reaction (PCR) was realized with 10 μL of Taqman Universal PCR Master Mix (Applied Biosystems), 80 nmol/L of primer, 2 nmol/L probe and 2 μL of cDNA. The cycling conditions were: 95°C for initial denaturation by 20 s, 40 cycles of 95°C for denaturation by 0,3 seconds, 60°C for annealing by 1 min and 72°C for extension by 30 seconds. TaqMan® Gene Expression Assay was pre-optimized PCR primer and probe sets for qRT-PCR formulated at 20 × concentration. Specific primers were utilized for quantification of genes evaluated through TaqMan® Custom Array Plate. Two reference genes (b-actin and Glyceraldehyde-3-phosphate dehydrogenase-GAPDH) and 2 target genes (CD44 and EGFR) were utilized. All reactions were realized in duplicate to better PCR specificity. Gene expression was normalized with β-actin and GAPDH genes. Gene expression of CD44 and EGFR genes were compared in CSCs and Non-CSCs and it was calculated by delta threshold cycle (Ct) method according to mathematical following formula: Expression level of target gene = 2-(Delta Ct) × 1,000 Delta Ct = Ct of target gene - (Mean Ct of β-actin and GAPDH genes).
Results
Identification and isolation of CSCs and Non-CSCs subpopulations in cell lines
The subpopulation of sorted HN13 cell line with CD44high/CD133high/CD117high (CSCs) was detected in 0.7% and isolated of Non-CSCs. The expression of CD44, CD117 and CD133 were 0.1%, 0.4% and 0.2%, respectively.
The subpopulation of sorted HEP2 cell line with CD44high/CD133high/CD117high (CSCs) was detected in 0.8% and isolated of Non-CSCs. The expression of CD44, CD117 and CD133 were 0.3%, 0.4% and 0.1%, respectively.
Confirmation of presence of CSCs
After sorting, CSCs and Non-CSCs subpopulations were then collected and cultured separately under the same conditions, as described above. As shown in Figures 1 and 2, CSCs demonstrated increased invasive capacity as compared with Non-CSCs subpopulations after 24 hours in both cell lines. In CSC HN13 the migration was 92% and in Non-CSC HN13 was 53%. In CSC HEP-2 the migration was 94% and in Non-CSC HEP-2 was 13%.
Figure 1.
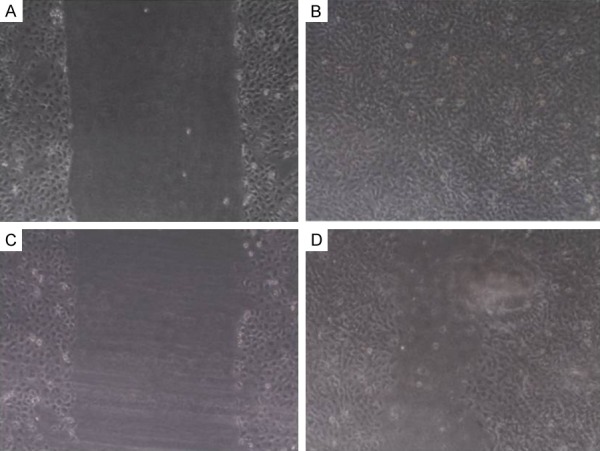
Cell Migration of CSCs and Non-CSCs subpopulations of HN13 cell line seeded in 6-well plates and cultured for 24 h. A. CSCs subpopulations of HN13: A diametric scratch using a pipette tip was made at 0 h; B. CSCs subpopulations of HN13 after 24 h; C. Non-CSCs subpopulations of HN13: A diametric scratch using a pipette tip was made at 0 h; D. Non-CSCs subpopulations of HN13 after 24 h.
Figure 2.
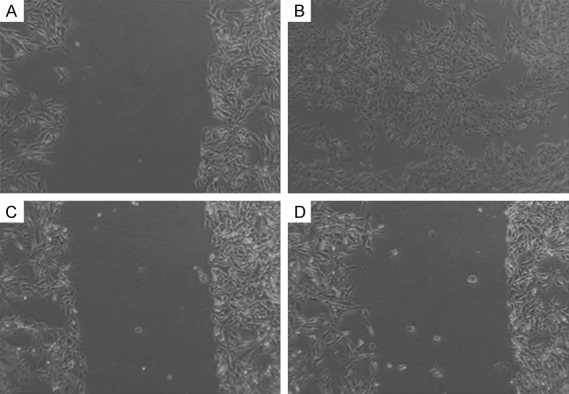
Cell Migration of CSCs and Non-CSCs subpopulations of HEP-2 cell line seeded in 6-well plates and cultured for 24 h. A. CSCs subpopulations of HEP-2: A diametric scratch using a pipette tip was made at 0 h; B. CSCs subpopulations of HEP-2 after 24 h; C. Non-CSCs subpopulations of HEP-2: A diametric scratch using a pipette tip was made at 0 h; D. Non-CSCs subpopulations of HEP-2 after 24 h.
Drug sensitivity of CSCs after treatment
Both CSCs and Non-CSCs subpopulations of cell lines were treated with Cetuximab and Paclitaxel agents, and then cell proliferation was assessed using MTS assay. As shown in Figure 3, CSCs subpopulation cells demonstrated more cell proliferation when compared with Non-CSCs subpopulation in HN13 and HEP-2 cell lines.
Figure 3.
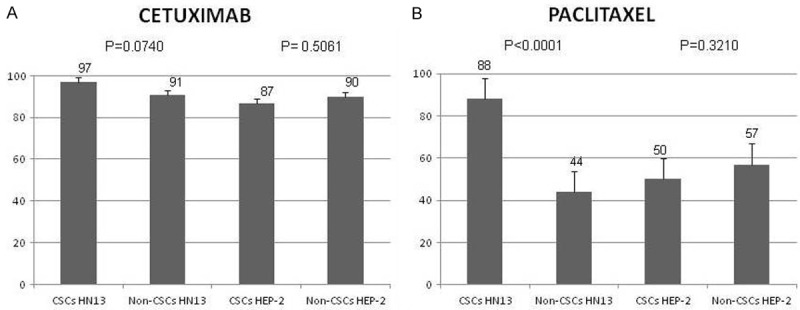
Cell proliferation of CSCs and Non-CSCs subpopulations of HN13 and HEP-2 cell lines treated with chemotherapies after 24 hours. A. CSCs and Non-CSCs subpopulations of HN13 and HEP-2 cell lines treated with Cetuximab. B. CSCs and Non-CSCs subpopulations of HN13 and HEP-2 cell lines treated with Paclitaxel chemotherapeutic.
Expression of genes related to stem cell and cancer drug resistance in Non-CSCs and CSCs subpopulations
To exanimate the difference in the expression of genes related to stem cell and cancer drug resistance between Non-CSCs and CSCs subpopulations cells, we used delta threshold cycle (Ct) method according to mathematical following formula: Expression level of target gene = 2-(Delta Ct) × 1,000 Delta Ct = Ct of target gene - (Mean Ct of β-actin and GAPDH genes). Regarding to HN13 cell line, we found that CD44 gene presented up-regulation (rate < 2.0) in CSCs when compared with Non-CSCs and, EGFR gene presented down-regulation (rate > 2.0) in CSCs when compared with Non-CSCs. For HEP-2 cell line the results showed that CD44 gene presented down-regulation (rate < 2.0) in CSCs when compared with Non-CSCs and, EGFR gene presented up-regulation (rate > 2.0) in CSCs when compared with Non-CSCs (Table 1).
Table 1.
CD44 and EGFR gene expression in CSCs HN13 and CSCs HEP-2 cell lines
| Gene symbol | Gene description | GenBank | Fold change | |||
|---|---|---|---|---|---|---|
|
|
||||||
| CSCs HN13 | Non-CSCs HN13 | CSCs HEP-2 | Non-CSCs HEP-2 | |||
| CD44 | The protein encoded by this gene is a cell-surface glycoprotein involved in cell-cell interactions, cell adhesion and migration. | NM_000610.3 | 102.775859 | 1 (REF) | 0.65892 | 1 (REF) |
| EGFR | EGFR and its ligands are cell signaling molecules involved in diverse cellular functions, including cell proliferation, differentiation, motility, and survival, and in tissue development | NM_001346897.1 | 0.741344907 | 1 (REF) | 7.55986 | 1 (REF) |
Discussion
We firstly characterized CSCs in two head and neck cell lines with CD44, CD133 and CD117 biomarkers. So we treated CSCs and Non-CSCs subpopulations with Cetuximab and Paclitaxel chemoterapies and found that CSCs subpopulations demonstrated more resistance to Paclitaxel, as compared with Non-CSCs subpopulations in HN13 cell line. These HN13 CSCs subpopulations presented up-regulation of CD44 gene and down-regulation of EGFR gene when compared with Non-CSCs subpopulations while HEP-2 CSCs presented down-regulation of CD44 gene and up-regulation of EGFR gene when compared with Non-CSCs subpopulations.
Regarding to characterization, the culture condition was capable of expanding CD44high/CD133high/CD117high cells from HN13 and HEP-2 cell lines. CD44 biomarker is a cell surface hyaluronan receptor protein involved in cell adhesion, cell-cell interactions and cell proliferation besides being receptor for hyaluronic acid [21,22]. CD44 was firstly identified in head and neck cancer in 2007 by Prince and collaborators and found that positive CD44 cells initiated tumor growth with high tumorigenic potential and differentiation capacity when compared with negative CD44 cells confirming that positive CD44 population of human head and neck cancer has properties of cancer stem cells and head and neck cancer contain a subpopulation of CSC, which was confirmed in our study in HNC cell lines [16].
CD133 biomarker is a cell-surface glycoprotein comprising five trans-membrane domains associated with cell membrane topology organization. It is often expressed on adult stem cells with function of maintaining stem cell properties by suppressing differentiation [23,24]. CD133 also has been identified human tongue, laryngeal and bucal cancer cell lines with ability of tumorigenic, power of cell proliferation and differentiation when compared to CD133-subpopulations, now we confirmed the identification of CD133+ cells in oral cancer cell line [25-28].
CD117 biomarker is a transmembrane receptor for MGF (mast cell growth factor, also known as stem cell factor) with cellular function not entirely known, however studies show that CD117 promotes the proliferation, survival, and metastasis of tumor cells and has been regarded as a cancer stem cell biomarker, but is not yet evaluated in oral cancer. We found CD117 high in oral cancer cell line, however more studies is needed to evaluate the importance of this biomarker is cancer stem cells development [30-32].
Regarding to treatment of CSCs and Non-CSCs subpopulations with Cetuximab and Paclitaxel we found more resistance to Paclitaxel chemoterapy. As compared with Non-CSC subpopulations in both cell lines suggesting that CD44high/CD133high/CD117high cells should be considered as targets in future therapies with Paclitaxel.
This is the first study that isolates cancer stem cells of head and neck cancer cell lines through of CD44/CD133/CD117 biomarkers in combination and evaluated the cancer treatment with Cetuximabe and Paclitaxel chemotherapies to single-modality treatment. Literature studies already evaluated these biomarkers alone and found that CD44high/CD133high/CD117high cells besides presenting stem cell properties also has ability to resist chemotherapeutic agents in cancer treatment, including head and neck cancer. Furthermore CSCs often have enhanced telomerase and DNA repair activities, as well as, membrane bound ATP-binding cassette transporters (ABC “drug” transporters) whose normal functions are to exclude xenobiotics, as chemotherapies [33-36].
Cetuximab is a monoclonal antibody binding the epidermal growth factor receptor (EGFR) on both normal and tumor cells. It is a functional antagonist of the EGF and TGF ligands and is thus inhibitors of the EGFR-dependent signaling pathways leading to inhibition of cancer cell division in the G1 phase and metastatic spread because of the lack of transcription factors [37]. In our study we found the Cetuximab is not effective in CSCs subpopulation of head and neck cancer cell lines. There is a suggestion of pathways activated in head and neck cells by EGFR increase the migratory potential of cells and interfere with their sensitivity to single-modality treatment with cetuximab, as our study [38,39].
Paclitaxel chemotherapy is a mitotic inhibitor used in cancer chemotherapy that interferes with the normal function of microtubule growth. It binds to the β subunit of tubulin, that is the “building block” of mictotubules, and the binding of paclitaxel locks these building blocks. The resulting microtubule/paclitaxel complex affects cell function leading to mitotic arrest, prevention of cell division, and eventually apoptosis [40]. In our study the Paclitaxel is not effective in CSCs subpopulation of oral cancer cell line. Studies show that mesenchymal stem cells have been shown to be highly resistant to the cytotoxic effects of Paclitaxel and other chemotherapeutic agents due to regulation of the cell cycle [41,42].
Besides that we found high expression of CD44 gene in HN13 CSCs and down expression of CD44 gene in HEP-2 CSCs suggesting that the exact influence of CD44 gene expression in resistant to chemotherapy is not entirely clear. The mechanistic origins can be associated with DNA repair, resistance to apoptosis, low mitotic rate, and increased tolerance of DNA damage [48,49] According literature data the high expression of CD44 has been identified in treatment resistant in cancer with CSCs properties, including head and neck cancer, as our study [34,43-47]. The high expression of CD44 gene in CSCs and resistance treatment can be explained due to association of this gene with cell-cell interactions, cell adhesion and migration that is increased in CSCs.
We also found down expression of CD44 in laryngeal cancer cell line, reports confirmed that levels of CD44 expression are linked to stem cell properties [50,51]. The HEP-2 cell line presented decreased rate of population expansion with cancer stem cell characteristics which may justify this finding. However several signalling pathways can be associated with CSCs survival and therapies that target such pathways might be therapeutically effective [52].
Regarding to EGFR gene expression, our study found that the HN13 CSCs showed down expression of EGFR and HEP-2 CSCs showed high expression of EGFR. The EGFR is found in surface of cells to which epidermal growth factor (EGF) binds. When EGF attaches to EGFR, it activates tyrosine kinase activity, triggering reactions that cause the cells to grow and multiply this way activates a wide variety of intracellular cascades and induces the regulation of target genes, leading to a specific cellular response [53,54].
The blocking EGFR signaling has provided less therapeutic benefit and this may be related to the presence of sub-populations of CSCs and heterogeneity of tumours [55,56]. Literature data confirm that head and neck patient tumors express EGFR (~98%), however only approximately 15-20% of patients respond positively and benefit from treatment [57,58]. Our results suggest that 80-85% of patients may present tumor with CSCs and, consequently, alterations in EGFR expression, what can contribute to treatment resistance but the mechanisms are still unclear and need to be further studied in another cell lines and primary tumor.
In conclusion, our results show that the combination of CD44, CD133 and CD117 biomarkers have stem cell properties and ability to resist Paclitaxel chemoterapy. CSCs present high expression of CD44 gene and down expression of EGFR gene in oral cancer cell line. CSCs in laryngeal cell line presents down expression of CD44 gene and high expression of EGFR gene when compared with cells without characteristics of cancer stem cells.
Acknowledgements
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (N° 2015/04403-8) (N° 2014/15009-6), National Council for Scientific and Technological Development (CNPq), Capes (Coordination for the Improvement of Higher Level) and FAMERP/FUNFARME.
Disclosure of conflict of interest
None.
References
- 1.Epidemiology and risk factors for head and neck câncer. UptoDate. 2015. Topic 3390 http://www.uptodate.com/contents/epidemiology-and-risk-factors-for-head-and-neck-cancer; Stenson KM., Brockstein BE., Ross ME.; 2015 [Acessed 01 March 2018.
- 2.Galbiatti AL, Padovani-Junior JA, Maníglia JV, Rodrigues CD, Pavarino ÉC, Goloni-Bertollo EM. Head and neck cancer: causes, prevention and treatment. Braz J Otorhinolaryngol. 2013;79:239–47. doi: 10.5935/1808-8694.20130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyawali B, Shimokata T, Honda K, Ando Y. Chemotherapy in locally advanced head and neck squamous cell carcinoma. Cancer Treat Rev. 2016;44:10–6. doi: 10.1016/j.ctrv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Han G, Xu C, Yu D. Mechanisms correlated with chemotherapy resistance in tongue cancers. J Cancer Res Ther. 2018;14:1–5. doi: 10.4103/jcrt.JCRT_763_17. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers. https://www.nccn.org/professionals/physician_gls/default.aspx Vol. 2, 2008. [Acessed in 28 Fev 2018]
- 6.Gyawali B, Shimokata T, Honda K, Ando Y. Chemotherapy in locally advanced head and neck squamous cell carcinoma. Cancer Treat Rev. 2016;44:10–6. doi: 10.1016/j.ctrv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Sharma N, Annigeri RG. Translational research in oral cancer: a challenging key step in moving from bench to bedside. J Cancer Res Ther. 2018;14:255–259. doi: 10.4103/0973-1482.183556. [DOI] [PubMed] [Google Scholar]
- 8.Mason H, DeRubeis MB, Burke N, Shannon M, Karsies D, Wolf G, Eisbruch A, Worden F. Symptom management during and after treatment with concurrent chemoradiotherapyfor oropharyngeal cancer: a review of the literature and areas for future research. World J Clin Oncol. 2016;7:220–6. doi: 10.5306/wjco.v7.i2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal L, Ben Aharon I, Limon D, Cohen E, Popovtzer A. Role of induction chemotherapy prior to chemoradiation in head and neck squamous cellcancer-systematic review and meta-analysis. Cancer J. 2017;23:79–83. doi: 10.1097/PPO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 10.Oral Complications of Chemotherapy and Head/Neck Radiation (PDQ®)-Health Professional Version. https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq. 2018. [Acessed in 28 Fev 2018]
- 11.Tinhofer I, Saki M, Niehr F, Keilholz U, Budach V. Cancer stem cell characteristics of circulating tumor cells. Int J Radiat Biol. 2014;12:1–6. doi: 10.3109/09553002.2014.886798. [DOI] [PubMed] [Google Scholar]
- 12.Almeida LO, Guimarães DM, Squarize CH, Castilho RM. Profiling the behavior of distinct populations of head and neck cancer stem cells. Cancers (Basel) 2016:8. doi: 10.3390/cancers8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 14.Méry B, Guy JB, Espenel S, Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magné N. Targeting head and neck tumoral stem cells: from biological aspects to therapeutic perspectives. World J Stem Cells. 2016;8:13–21. doi: 10.4252/wjsc.v8.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeishi S, Nakayama KI. To wake up cancer stem cells, or to let them sleep, that is the question. Cancer Sci. 2016;107:875–81. doi: 10.1111/cas.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mărgăritescu C, Pirici D, Simionescu C, Stepan A. The utility of CD44, CD117 and CD133 in identification of cancer stem cells (CSC) in oral squamous cell carcinomas (OSCC) Rom J Morphol Embryol. 2011;52(Suppl):985–93. [PubMed] [Google Scholar]
- 18.Baillie R, Tan ST, Itinteang T. Cancer stem cells in oral cavity squamous cell carcinoma: a review. Front Oncol. 2017;7:112. doi: 10.3389/fonc.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tekade RK, D’Emanuele A, Elhissi A, Agrawal A, Jain A, Arafat BT, Jain NK. Extraction and RP-HPLC determination of taxol in rat plasma, cell culture and quality control samples. J Biomed Res. 2013;27:394–405. doi: 10.7555/JBR.27.20120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simple M, Suresh A, Das D, Kuriakose MA. Cancer stem cells and field cancerization of oral squamous cell carcinoma. Oral Oncol. 2015;51:643–51. doi: 10.1016/j.oraloncology.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 22.RefSeq-PUBMED- CD44 gene. https://www.ncbi.nlm.nih.gov/gene/960; 2018. [Acessed in 28 Fev 2018]
- 23.Silva Galbiatti-Dias AL, Pavarino ÉC, Kawasaki-Oyama RS, Maniglia JV, Maniglia EJ, Goloni Bertollo EM. Cancer stem cells in head and neck cancer: a mini review. Cell Mol Biol (Noisy-le-grand) 2015;61:39–43. [PubMed] [Google Scholar]
- 24.RefSeq-PUBMED- CD133 gene. https://www.ncbi.nlm.nih.gov/gene/8842; 2018. [Acessed in 28 Fev 2018]
- 25.Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133+ cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010;289:151–60. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Wei X, Wang J, He J, Ma B, Chen J. Biological characteristics of CD133(+) cancer stem cells derived from human laryngeal carcinoma cell line. Int J Clin Exp Med. 2014;7:2453–62. [PMC free article] [PubMed] [Google Scholar]
- 27.Mannelli G, Magnelli L, Deganello A, Busoni M, Meccariello G, Parrinello G, Gallo O. Detection of putative stem cell markers, CD44/CD133, in primary and lymph nodemetastases in head and neck squamous cell carcinomas. A preliminaryimmunohistochemical and in vitro study. Clin Otolaryngol. 2015;40:312–20. doi: 10.1111/coa.12368. [DOI] [PubMed] [Google Scholar]
- 28.Yu CC, Hu FW, Yu CH, Chou MY. Targeting CD133 in the enhancement of chemosensitivity in oral squamous cell carcinoma-derived side population cancer stem cells. Head Neck. 2016;38(Suppl 1):E231–8. doi: 10.1002/hed.23975. [DOI] [PubMed] [Google Scholar]
- 29.RefSeq-PUBMED- CD117 gene. https://www.ncbi.nlm.nih.gov/gene/3815; 2018. [Acessed in 28 Fev 2018]
- 30.Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31:2521–2534. doi: 10.1038/onc.2011.429. [DOI] [PubMed] [Google Scholar]
- 31.Tang YL, Fan YL, Jiang J, Li KD, Zheng M, Chen W, Ma XR, Geng N, Chen QM, Chen Y, Liang XH. C-kit induces epithelial-mesenchymal transition and contributes to salivary adenoid cystic cancer progression. Oncotarget. 2014;5:1491–501. doi: 10.18632/oncotarget.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Xu H, Qian C. c-kit-positive adipose tissue-derived mesenchymal stem cells promote the growth and angiogenesis of breast cancer. Biomed Res Int. 2017:7407168. doi: 10.1155/2017/7407168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabrò E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto A, Chikamatsu K, Sakakura K, Hatsushika K, Takahashi G, Masuyama K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:633–639. doi: 10.1016/j.oraloncology.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Han R, Huangfu H, Gao W, Zhang C, Jin Y, Li Z, Wang B. Neoplasms stem cells play an important role in resistance of laryngeal squamous cancer to chemoradiotherapy. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:400–5. [PubMed] [Google Scholar]
- 36.Moro M, Bertolini G, Pastorino U, Roz L, Sozzi G. Combination treatment with all-trans retinoic acid prevents cisplatin-induced enrichment of CD133+ tumor-initiating cells and reveals heterogeneity of cancer stem cell compartment in lung cancer. J Thorac Oncol. 2015;10:1027–36. doi: 10.1097/JTO.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mechanism of action - Cetuximab. https://www.drugbank.ca/drugs/DB00002., 2018. [Acessed in 01 March 2018]
- 38.Holz C, Niehr F, Boyko M, Hristozova T, Distel L, Budach V, Tinhofer I. Epithelial-mesenchymal-transition induced by EGFR activation interferes with cell migration and response to irradiation and cetuximab in head and neck cancer cells. Radiother Oncol. 2011;101:158–164. doi: 10.1016/j.radonc.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 39.Braig F, Kriegs M, Voigtlaender M, Habel B, Grob T, Biskup K, Blanchard V, Sack M, Thalhammer A, Ben Batalla I, Braren I, Laban S, Danielczyk A, Goletz S, Jakubowicz E, Märkl B, Trepel M, Knecht R, Riecken K, Fehse B, Loges S, Bokemeyer C, Binder M. Cetuximab resistance in head and neck cancer is mediated by EGFR-K521 polymorphism. Cancer Res. 2017;77:1188–1199. doi: 10.1158/0008-5472.CAN-16-0754. [DOI] [PubMed] [Google Scholar]
- 40.Mechanism of action - Paclitaxel. https://www.drugbank.ca/drugs/DB01229. 2018. [Acessed in 01 March 2018]
- 41.Polioudaki H, Kastrinaki MC, Papadaki HA, Theodoropoulos PA. Microtubule-interacting drugs induce moderate and reversible damage to human bone marrow mesenchymal stem cells. Cell Prolif. 2009;42:434–47. doi: 10.1111/j.1365-2184.2009.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moitra K, Lou H, Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin Pharmacol Ther. 2011;89:491–502. doi: 10.1038/clpt.2011.14. [DOI] [PubMed] [Google Scholar]
- 43.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944–8950. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 44.La Fleur L, Johansson AC, Roberg K. A CD44high/EGFRlow subpopulation within head and neck cancer cell lines shows anepithelial-mesenchymal transition phenotype and resistance to treatment. PLoS One. 2012;7:e44071. doi: 10.1371/journal.pone.0044071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgerald TL, McCubrey JA. Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv Biol Regul. 2014;56:45–50. doi: 10.1016/j.jbior.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Cheng CC, Chou KF, Wu CW, Su NW, Peng CL, Su YW, Chang J, Ho AS, Lin HC, Chen CG, Yang BL, Chang YC, Chiang YW, Lim KH, Chang YF. EGFR-mediated interleukin enhancer-binding factor 3 contributes to formation and survival of cancer stem-like tumorspheres as a therapeutic target against EGFR-positive non-small cell lung cancer. Lung Cancer. 2018;116:80–89. doi: 10.1016/j.lungcan.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Zhou J, Hu Q, Zhang X, Zheng J, Xie B, Xu Z, Zhang W. Sensitivity to chemotherapeutics of NSCLC cells with acquired resistance to EGFR-TKIs is mediated by T790M mutation or epithelial-mesenchymal transition. Oncol Rep. 2018;39:1783–1792. doi: 10.3892/or.2018.6242. [DOI] [PubMed] [Google Scholar]
- 48.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 49.Richard V, Nair MG, Santhosh Kumar TR, Pillai MR. Side population cells as prototype of chemoresistant, tumor-initiating cells. Biomed Res Int. 2013:517237. doi: 10.1155/2013/517237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, Costea DE, Mackenzie IC. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011;71:5317–26. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 51.Kidwai F, Costea DE, Hutchison I, Mackenzie I. The effects of CD44 down-regulation on stem cell properties of head and neck cancer cell lines. J Oral Pathol Med. 2013;42:682–90. doi: 10.1111/jop.12076. [DOI] [PubMed] [Google Scholar]
- 52.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–8. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 53.Aichler M, Motschmann M, Jütting U, Luber B, Becker K, Ott K, Lordick F, Langer R, Feith M, Siewert JR, Walch A. Epidermal growth factor receptor (EGFR) is an independent adverse prognostic factor in esophageal adenocarcinoma patients treated with cisplatin-based neoadjuvant chemotherapy. Oncotarget. 2014;5:6620–32. doi: 10.18632/oncotarget.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fogli S, Polini B, Re MD, Petrini I, Passaro A, Crucitta S, Rofi E, Danesi R. EGFR-TKIs in non-small-cell lung cancer: focus on clinical pharmacology and mechanisms of resistance. Pharmacogenomics. 2018;19:727–740. doi: 10.2217/pgs-2018-0038. [DOI] [PubMed] [Google Scholar]
- 55.Echarri MJ, Lopez-Martin A, Hitt R. Targeted therapy in locally advanced and recurrent/metastatic head and neck squamous cell carcinoma (LA-R/M HNSCC) Cancers (Basel) 2016:8. doi: 10.3390/cancers8030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boesch M, Sopper S, Zeimet AG, Reimer D, Gastl G, Ludewig B, Wolf D. Heterogeneity of cancer stem cells: rationale for targeting the stem cell niche. Biochim Biophys Acta. 2016;1866:276–289. doi: 10.1016/j.bbcan.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 58.Bardelli A, Janne PA. The road to resistance: EGFR mutation and cetuximab. Nat Med. 2012;18:199–200. doi: 10.1038/nm.2646. [DOI] [PubMed] [Google Scholar]


