Abstract
Glutamine is a major nutrient for cancer cells during rapid proliferation. Alanine-serine-cysteine (ASC) transporter 2 (ASCT2; SLC1A5) mediates glutamine uptake in a variety of cancer cells. We previously reported that KM8094, a novel anti-ASCT2 humanized monoclonal antibody, possesses anti-tumor efficacy in gastric cancer patient-derived xenografts. The aim of this study was to investigate the molecular mechanism underlying the effect of KM8094 and to further substantiate the preclinical feasibility of using KM8094 as a potential therapeutic agent against gastric cancer. First, ASCT2 was found to be highly expressed in cancer tissues derived from gastric cancer patients by an immunohistochemical analysis. Next, we performed in vitro studies using multiple gastric cancer cell lines and observed that several gastric cancer cells expressing ASCT2 showed glutamine-dependent cell growth, which was repressed by KM8094. We found that KM8094 inhibited the glutamine uptake, leading to the reduction of glutathione (GSH) level and the elevation of oxidative stress. KM8094 suppressed the cell cycle progression and increased the apoptosis. Furthermore, KM8094 exerted antibody dependent cellular cytotoxicity (ADCC) against human gastric cancer cells in vitro. Finally, in vivo studies revealed that KM8094 suppressed tumor growth in several gastric cancer xenografts. This effect was enhanced by docetaxel, one of the agents commonly used in gastric cancer therapy. Thus, our findings suggest that KM8094 is a potential new therapeutic agent for gastric cancer expressing ASCT2, which blocks the cellular glutamine metabolism and possesses ADCC activity.
Keywords: ADCC, ASCT2, gastric cancer, glutamine metabolism, glutamine transporter, oxidative stress
Introduction
Glutamine (Gln) is an amino acid required in large quantities by the human body [1,2]. It is a critical nutrient for the biosynthesis of proteins, lipids, and nucleotides, and for ATP generation and redox homeostasis [3-7]. Thus, glutamine is considered to be a critical substrate for cell growth, proliferation, and survival. Particularly, cancer cells have high demand for glutamine, many of which cannot grow without exogenous glutamine supply in culture [8,9]. Therefore, targeting glutamine metabolism is assumed to be a potent and attractive strategy for cancer therapy.
Alanine-serine-cysteine (ASC) amino acid transporter 2 (ASCT2; SLC1A5) is a Na+-coupled neutral amino acid transporter and is known as the major glutamine transporter in cancer cells [10-12]. Indeed, the expression level of ASCT2 is up-regulated in a variety of cancerous tissues, such as breast, prostate, melanoma, colorectal, pancreatic, tongue, and lung cancers [13-26] and several studies have shown that the overexpression of ASCT2 is associated with poor prognosis in some cancers, including those of lung, tongue, breast, and colon [16,17,19,21,22]. Furthermore, because blocking of ASCT2 can prevent glutamine uptake and tumor cell growth in many cancer cells, it can be assumed that ASCT2 acts as a potential therapeutic target [21,24,25,27,28].
Gastric cancer is the second leading cause of global cancer mortalities, with an overall 5-year survival rate of approximately 20% [29,30]. Because no typical signs indicative of gastric cancer are detected, most gastric cancer patients are diagnosed at advanced stages of the disease and, thus, have limited treatment options. Therefore, finding of more beneficial therapeutic strategies against gastric cancer is urgently required. Moreover, in addition to the standard cytotoxic regimens, recent progress in molecular targeting therapies using monoclonal antibodies, such as trastuzumab, ramucirumab, and pembrolizumab, has shown that these are clinically promising therapeutic strategies [31,32].
Recently, the development of an anti-ASCT2 monoclonal antibody by Kyowa Hakko Kirin Co., Ltd (Japan), which inhibits glutamine uptake, has been reported [33]. Moreover, we have previously elucidated that the defucosylated humanized derivative (IgG1) of this antibody, KM8094, possesses anti-tumor efficacy in gastric cancer patient-derived xenografts [34]. In this study, we investigated the molecular mechanism underlying the effect of KM8094 in gastric cancer cells and further evaluated the preclinical feasibility of using KM8094 as a therapeutic agent against gastric cancer.
Materials and methods
Chemicals and reagents
A defucosylated humanized anti-human ASCT2 monoclonal antibody KM8094 (IgG1) was produced by Kyowa Hakko Kirin Co., Ltd (Japan). KM8094 is a humanized derivative antibody of KM4008, a mouse monoclonal antibody, which specifically binds to the natural configuration of an extracellular domain of ASCT2 [33]. A negative control monoclonal antibody, KM8047 (defucosylated human anti-dinitrophenol antibody IgG1), was also produced by Kyowa Hakko Kirin Co., Ltd. Other chemicals and reagents were of the highest grade and were purchased from local commercial sources.
Cell lines and cell culture
Three human gastric cancer cell lines, namely SNU-16, AGS, and NCI-N87, were obtained from the American Type Culture Collection. Four cell lines, OCUM-1, MKN28, MKN1, and NUGC-4, were obtained from the Japanese Collection of Research Bioresources Cell Bank (Japan). MKN45 was obtained from RIKEN BioResource Research Center (Japan). Three cell lines, HSC-39, HSC-43, and HSC-44, were kindly provided by Kazuto Nishio (Kinki University, Japan). Six lines, HSC-40A, HSC-58, HSC-59, HSC-60, 60As6, and HSC-64, were kindly provided by Gokichi Yanagihara (Yasuda Women’s University, Japan). All the cancer cell lines, except for AGS, were cultured in RPMI-1640 (Life Technologies) supplemented with 10% fetal bovine serum (FBS). AGS was cultured in Ham’s F-12K (Kaighn’s) (Life Technologies) in the presence of 10% FBS.
Clinical samples
Sections of human gastric cancer tissues (N = 10) were prepared from frozen tissue block provided by Kanagawa Cancer Research and Information Association (Japan). Human peripheral blood mononuclear cells (PBMCs) were isolated from the blood of gastric cancer (GC) patients (N = 10, from the National University Health System, Singapore (NUHS)) and healthy donors (N = 13). Human PBMCs from healthy donors were either obtained commercially from Stemcell Technologies (Singapore) or obtained from donors from the National University of Singapore Hospital (NUH, Singapore). All donors gave informed written consent prior to the sampling procedure. Experiments for clinical samples were conducted in accordance with the approval by the Research Ethics Review Committee in Kyowa Hakko Kirin Co., Ltd.
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) was conducted using frozen sections of human gastric cancer tissues. The frozen sections (approximately 6 μm) were fixed in 4% paraformaldehyde and then endogenous peroxidase was quenched. After avidin/biotin blocking, the sections were incubated with 10 μg/mL KM8094 or KM8047 (control article) biotin conjugate for 1 h at room temperature. Anti-biotin antibody (VECTASTAIN Kit, Vector Laboratory) was applied for 30 min at room temperature and the color was then developed using the chromogenic substrate, prepared from DAB tablet (FUJIFILM Wako Pure Chemical Industries), for 4 min at room temperature. The counterstaining was done with hematoxylin. The immunohistochemical expression was assessed under a microscope. The staining specificity was judged when staining intensity in biotin-KM8094-stained sections exceeded that in KM8047-stained sections at an equivalent concentration. The staining intensity was graded according to the following criteria: negative, ±; very faint, +; light, 2+; light-medium, 3+; moderate, 4+; dark. The staining site was graded as follows: M; Membranous, C; Cytoplasmic. The staining frequency was graded as follows: Negative; no labeled cell, Very rare; <25%, Rare; ≥25% and <50%, Occasional; ≥50% and <75%, Frequent; ≥75%.
Analysis of ASCT2 expression in gastric cancer cells by flow cytometry
The expression level of ASCT2 in the gastric cancer cells was evaluated by flow cytometry (Cytomics FC500, BECKMAN COULTER, USA) using 10 μg/mL KM8094 or KM8047 (control article) and 10 μg/mL PE-conjugated goat anti-human IgG monoclonal antibody (Southern Biotech) as a secondary antibody.
Glutamine dependent cell growth assay
The cells were plated at 2 × 103 cells/well in 96-well flat-bottomed plates and were cultured in DMEM high glucose containing 10% dialyzed-FBS in the presence of 0, 0.2, or 2 mM glutamine (Nacalai). After incubation for 24, 72, and 96 h at 37°C, viable cells were detected with CellTiter-Glo Luminescent Cell Viability Assay (Promega), according to the manufacturer’s instructions.
siRNA transfection
The siRNA-mediated knockdown experiments were carried out with Stealth RNAi siRNA (10 nM for SNU-16 and 40 nM for MKN28; Thermo Fisher Scientific). The sequence of ASCT2 siRNA was as follows: 5’-GGUCCUGUACCGUCCUCAAUGUAGA-3’. The Stealth RNAi siRNA negative control duplex was used as the control siRNA. The cells were transfected with the annealed siRNAs for 48 to 144 h using Lipofectamine RNAi MAX reagent (Invitrogen), according to the manufacturer’s protocol.
Quantitative RT-PCR analysis
Total RNA was extracted from the cultured cells using Maxwell 16 LEV simply RNA Cells Kit (Promega) and was subjected to quantitative RT-PCR analysis. Taqman RT-PCR assays were conducted using a 7500 Fast Real-Time PCR System (Applied Biosystems). The probe-primer mixes specific for human ASCT2 and β-actin were purchased from Applied Biosystems. Twenty microliter reactions containing 10 μL 10 × TaqmanTM Universal PCR Master Mix (Applied Biosystems), a probe-primer mix, and 5 μL of cDNA (diluted 10-1 in water) were prepared in triplicate. A probe-primer mix specific for β-actin was used as an internal control.
Glutamine uptake assay
The cells were plated at 1 × 105 cells/well in a 96-well round-bottomed plates and were preincubated in HBSS/HEPES for 15 min at 37°C. Thereafter, 14C-glutamine (PerkinElmer) was added to the cells and the cells were incubated for 15 min at 37°C. After the excess 14C-glutamine was washed out, the cells were lysed in 0.1% NP-40-buffer (Invitrogen) and the amount of 14C-glutamine taken into the cells was measured by microplate scintillation counter, TopCount NXT (PerkinElmer).
Cell growth assay
The cells were plated at 2 × 103 cells/well in 96-well flat-bottomed plates and were cultured in DMEM high glucose supplemented with 10% dialyzed-FBS and 0.2 mM glutamine (Nacalai). After incubation for 24 h at 37°C, KM8094 or KM8047 was added at 100 μg/mL to the cells. Some experiments were carried out in the presence of 5 mM N-acetylcysteine (NAC) (Sigma-Aldrich). After incubation for 72 h at 37°C, viable cells were detected with CellTiter-Glo Luminescent Cell Viability Assay (Promega) or Cell Counting Kit-8 (Dojindo Molecular Technologies) according to the manufacturer’s instructions. The cell growth was calculated from sample counts (value) as follows: % cell growth = 100 × [(A72-B72) - (A0-B0)/(C72-B72) - (A0-B0)] (A72, value for KM8094-treated cells for 72 h; B72, value of blank at 72 h; A0, value for non-treated cells at 0 h; B0, value of blank at 0 h; C72, value for KM8047-treated cells at 72 h).
Analysis of cell cycle distribution by flow cytometry
The SNU-16 cells were treated with 10 μg/mL KM8094 or vehicle (10 mM sodium L-glutamate, 262 mM D-sorbitol, 0.05 mg/mL polysorbate 80, pH 5.7) in 0.2 mM glutamine medium for 24 h. The cells were then harvested, fixed, and stained with PI/RNase (BD PharmingenTM). The cellular DNA content was analyzed using flow cytometry (FACS Calibur, BD Bioscience, USA) and was displayed as histogram. The DNA content at each cell cycle phase was analyzed using ModFit software (Verity Software House).
Detection of apoptosis by Annexin V-FITC/PI double staining
The SNU-16 cells were treated with 10 μg/mL KM8094 or vehicle (10 mM sodium L-glutamate, 262 mM D-sorbitol, 0.05 mg/mL polysorbate 80, pH 5.7) in 0.2 mM glutamine medium for 72 h. The cells were harvested and stained with propidium iodide (ThermoFisher Scientific) and FITC Annexin V (BD PharmingenTM) for 15 min and examined by flow cytometry. The percentages of early apoptotic cells (Annexin V+, PI-) and late apoptotic cells (Annexin V+, PI+) were analyzed using Flowjo (Tree Star, USA).
Metabolomics analysis
The SNU-16 cells were cultured at 2 × 104 cells/mL in DMEM high glucose supplemented with 10% dialyzed-FBS and 0.2 mM glutamine (Nacalai). After incubation for 24 h at 37°C, KM8094 or vehicle was added at 10 μg/mL to the cells and incubated for 8 h. The cells were harvested and washed with 5% D(-)- Mannitol (FUJIFILM Wako Pure Chemical Corporation). Thereafter, 1 mL methanol (LC/MS) (FUJIFILM Wako Pure Chemical Corporation) containing the internal standard solution (HMT) was added to the cells. The metabolome measurements were carried out a facility provided by Human Metabolome Technologies (HMT, Japan).
LC-MS/MS measurement of stable isotope labeled glutamine (13C-Gln)
Glutamine, labeled with 13C5, was purchased from Cambridge Isotope Labs. In this experiment, HBSS/HEPES containing 10% dialyzed-FBS and 4.5 g/L glucose (Sigma-Aldrich) was used as an assay buffer. The cells were cultured in DMEM high glucose supplemented with 10% dialyzed-FBS and 0.2 mM glutamine (Nacalai). The following day, the cells were harvested, plated at 3 × 105 cells/well in a 96-well round-bottomed plate, and preincubated in the assay buffer with 0.2 mM glutamine for 15 min at 37°C. After the incubation, the medium was exchanged with the assay buffer containing 0.3 mg/mL KM8094 and 0.2 mM 13C5 glutamine. The cells were then incubated for 10 and 90 min at 37°C. After incubation, the medium was removed and the cells were washed with ice-cold HBSS for five times. The cells were collected and metabolite fractions were extracted using 5% trichloroacetic acid (Nacalai) and analyzed by LC-MS/MS. The LC-MS/MS system consisted of an Acquity UPLC (Waters) coupled to a triple quadrupole tandem mass spectrometer API4000 (Applied Biosystems/MDS SCIEX) with an electrospray ionization ion source operating in positive mode. The concentration of each metabolite was normalized on the basis of total number of viable cells.
ROS measurement
The reactive oxygen species (ROS) was measured using ROS-GloTM H2O2 Assay kit (Promega), according to the manufacturer’s instructions. The cells were plated at 2.4 × 103 cells/well in 96-well white plates in DMEM high glucose containing 10% dialyzed-FBS and 0.2 mM glutamine. After overnight incubation at 37°C, KM8094 or KM8047 was added at 10, 100, and 1000 μg/mL with or without 5 mM N-acetylcysteine (Sigma-Aldrich) to the cells for 24 h at 37°C. H2O2 substrate was added at 25 µM to the cells, 6 h before the end of the incubation. After incubation, ROS-GloTM Detection Solution was added to the wells for 20 min at room temperature and luminescence was measured by Top Count.
Antibody-dependent cellular cytotoxicity assay
The antibody-dependent cellular cytotoxicity (ADCC) of KM8094 against the ASCT2-positive human gastric cancer cell line (SNU-16) was examined using human PBMCs, either from gastric cancer patients or healthy controls, as effector cells. The cytotoxicity induced by KM8094 was measured by the calcein-AM release assay as mentioned below. The target SNU-16 cells were labeled with 25 µg/mL calcein-AM (Life Technologies), washed, and plated in triplicate at 104 cells/well in round-bottomed 96-well plates. 100 ng/mL of KM8094 and effector cells were added at effector-to-target ratios (E/T) of 10:1 and 20:1, in a final volume of 150 μL/well and incubated for 4 h at 37°C in 5% CO2. The cells were also plated in triplicate wells for spontaneous (SPON) and maximum release (MAX). The cell lysis buffer was added to the target cells, 45 min before the end of the 4-h incubation period to obtain the maximum release of calcein-AM. After 4 h, 50 μL of the culture supernatant was transferred to a 96-well Perkin Elmer Optiplate and fluorescence was read on a Varioskan Flash reader (Thermo Scientific, at 490 nm excitation/515 nm emission) as fluorescent units (FU). The percentage of cytotoxicity (CTX) was calculated according to the formula mentioned below. ADCC (%) was calculated as CTX with KM8094 (%) - CTX without KM8094 (%). The CTX was calculated as follows: CTX (%) = 100 × [FU (sample) - (FU (effector SPON) + FU (target SPON))]/[FU (target MAX) - FU (target SPON)].
In vivo anti-tumor effects on xenograft transplantation
Animal experiments in this study were conducted in accordance with the approval of Institutional Animal Care and Use Committee (IACUC) in Kyowa Hakko Kirin Co., Ltd. C.B-17/Icr-scid/scidJcl male mice were purchased from CLEA Japan, Inc. Anti-asialo GM1 (FUJIFILM Wako Pure Chemical Corporation) was injected intraperitoneally into 6-14-week-old mice (0.3 mg/head), following which, 5 × 106 or 10 × 106 cells suspended in DPBS with or without 50% Matrigel (BD Bioscience) were implanted subcutaneously into the right flank of the mice. For experiments of KM8094 monotherapy, tumors were propagated to about 100 mm3 before therapy and 10 mice were selected and divided into two groups such that the mean tumor volume in each group was comparable (five mice per group). The first day of drug administration was set as day 0. KM8094 (10 mg/kg) or saline (vehicle control) (Otsuka) was administered from tail vein twice weekly for 2 weeks for SNU-16 xenograft model or once weekly for 2 weeks for the other xenograft models. For experiments of KM8094 combination therapy with docetaxel, tumors were propagated to about 600 mm3 before therapy and 40 mice were selected and divided into eight groups such that the mean tumor volume in each group was comparable (five mice per group). KM8094 (10 mg/kg) or saline was administered from tail vein on day 0 and 8. Docetaxel (5 or 10 mg/kg) was administered through vein on day 0. The dosing volume was 5 mL/kg body weight. The body weight and tumor volume of the mice were measured twice a week.
The tumor volume was calculated as follows: tumor volume = DL × DS × DS × 1/2 (DL, long diameter; DS, short diameter). The areas under the curves (AUC) were calculated for each tumor growth curve and statistically analyzed using the SAS software program (Release 9.2, SAS Institute). The significant differences between the KM8094-treated group and the vehicle-treated group were analyzed by Student’s t-test or Aspin-Welch t-test. In this test, P<0.05 was considered to be significant.
Results
ASCT2 is highly expressed in gastric cancer tissues
To assess the expression of ASCT2 in gastric cancer, we conducted immunohistochemical analysis of gastric cancer tissues diagnosed with different histologic subtypes using KM8094 as an anti-human ASCT2 antibody. The specificity of KM8094 for ASCT2 was previously described [34]. In 9 out of 10 (90%) gastric cancer tissues, the ASCT2 signals were detected along the plasma membrane and/or in the cytoplasm (Figure 1A). Whereas ASCT2 signals were also detected in tumor adjacent normal stomach tissues, the intensity and signal frequency in the cancer tissues tended to be higher than those in the normal tissues (Table 1). This result showed that ASCT2 is highly expressed in gastric cancer tissues. Similarly, we found that ASCT2 was expressed on the cell-surface in several human gastric cancer cell lines by flow cytometry (Figure 1B).
Figure 1.
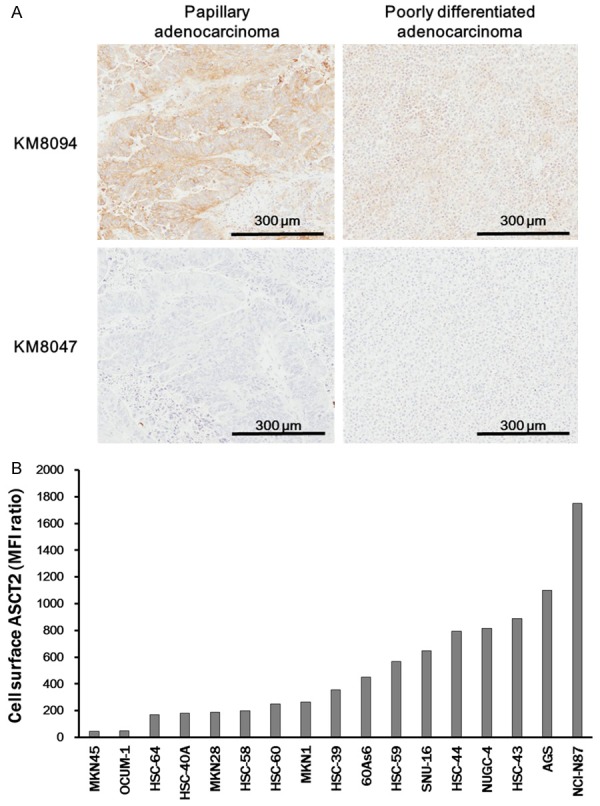
ASCT2 expression in gastric cancer tumors and gastric cancer cell lines. A. Gastric cancer tumor sections from patients were subjected to immunohistochemical staining with biotinylated KM8094 or KM8047 (control antibody) and its secondary antibody. Scale bars, 300 μm. B. Seventeen lines of human gastric cancer cells were subjected to flow cytometric analysis with KM8094 or KM8047. The MFI ratio was calculated according to the following formula: MFI ratio = (S/C, where S is MFI of cells stained by KM8094, C is the MFI of cells stained by KM8047 (MFI: mean fluorescent intensity).
Table 1.
Immunoreactivity of KM8094 on human gastric cancer and normal tissues
| Specimen No. | Tumor type | Cell location | |
|---|---|---|---|
|
| |||
| Tumor cell | Non-tumor cell* | ||
| 1 | Papillary adenocarcinoma | 2+ M, C Frequent | + C Frequent |
| 2 | Papillary adenocarcinoma, Tubular adenocarcinoma | 2+ M, C Frequent | + C Frequent |
| 3 | Moderately differentiated > well differentiated tubular adenocarcinoma | + M, C Frequent | + C Frequent |
| 4 | Moderately differentiated tubular adenocarcinoma | + M, C Frequent | + C Frequent |
| 5 | Poorly differentiated adenocarcinoma (non-solid type) | + C Frequent | + C Frequent |
| 6 | Poorly differentiated adenocarcinoma (solid type) | 2+ C Frequent | MV |
| 7 | Poorly differentiated adenocarcinoma (solid type > non-solid type), signet ring cell carcinoma ,well differentiated tubular adenocarcinoma | + C Frequent | 2+ C Frequent |
| 8 | Signet ring cell carcinoma | - | + C Frequent |
| 9 | Poorly differentiated adenocarcinoma (non-solid type), signet ring cell carcinoma | ± C Frequent | + C Frequent |
| 10 | Poorly differentiated adenocarcinoma (non-solid type) | 2+ C Frequent | + C Frequent |
Criteria of intensity: -; negative, ±; very faint, +; light, 2+; light-medium, 3+; moderate, 4+; dark. Site of staining: M; membranous, C; cytoplasmic. Criteria of staining frequency: Negative; no labeled cell, Very rare; <25%, Rare; ≥25% and <50%, Occasional; ≥50% and <75%, Frequent; ≥75%. MV: Missing Value.
Tumor adjacent normal mucosa, basal side.
KM8094 inhibits glutamine dependent cell growth in gastric cancer cells expressing ASCT2
To investigate the effect of glutamine availability on the growth of gastric cancer cells expressing ASCT2, we cultured seven gastric cancer cell lines (HSC-39, MKN-1, SNU-16, HSC-40A, MKN28, 60As6, and HSC-60) expressing ASCT2 in media supplemented with different concentrations of glutamine for 96 h. While the cell growth was induced in the presence of 0.2 or 2 mM glutamine, it was clearly decreased under glutamine-deprived condition (Figure 2A). This result suggests that glutamine is necessary for cell growth in these gastric cancer cell lines.
Figure 2.
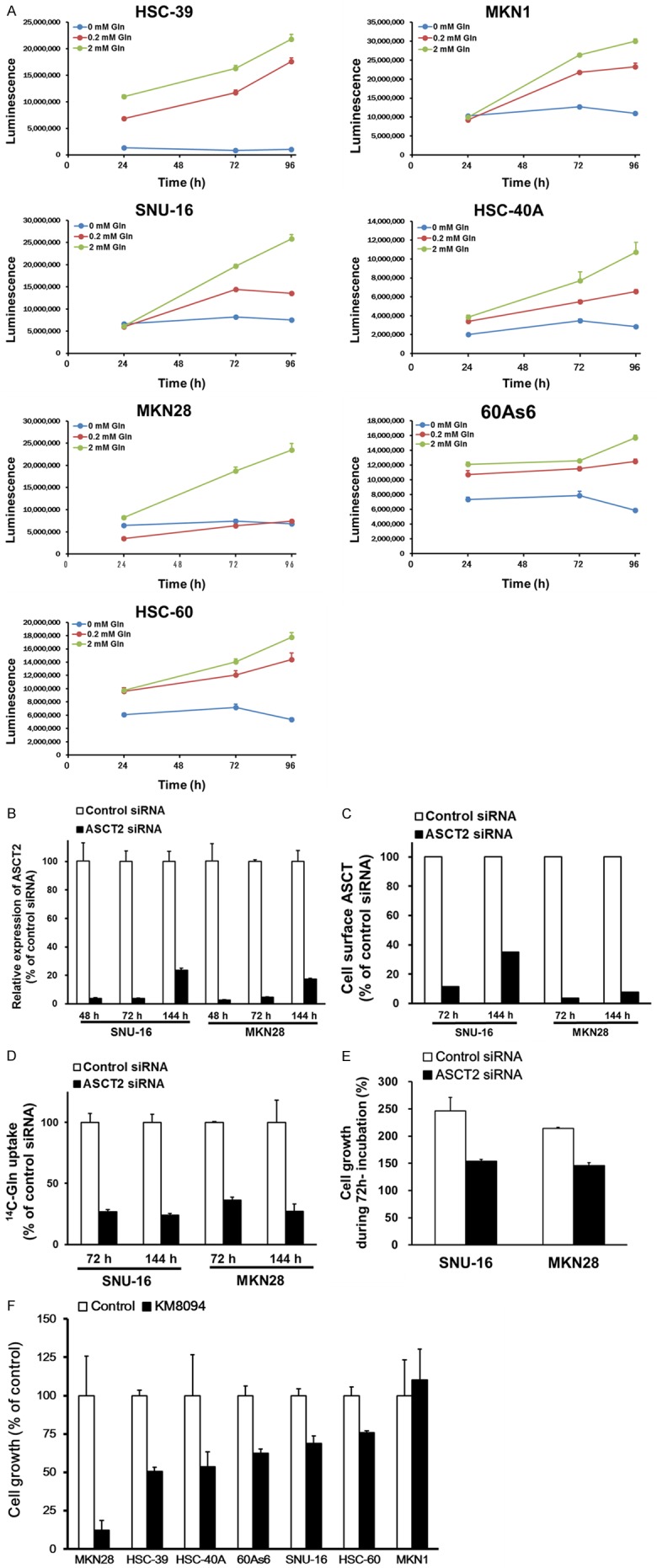
KM8094 suppresses glutamine dependent cell growth in gastric cancer cells. (A) Seven lines of gastric cancer cells (HSC-39, MKN-1, SNU-16, HSC-40A, MKN28, 60As6, and HSC-60) were incubated in the medium containing of 0, 0.2, or 2 mM Gln for 96 h. Time course of growth for each cell line was assessed. Data are presented as means + SD of triplicate readings. (B-E) Effect of ASCT2 knockdown on SNU-16 and MKN28 cells. Cells were transfected with ASCT2 siRNA or nonspecific siRNA control. (B, C) Expression of ASCT2 mRNA (B) and protein (C) in SNU-16 and MKN28 cells after 48, 72, and 144 h of siRNA treatment. (D) After 72 and 144 h of siRNA treatment, the transfected cells were harvested and incubated with 14C-Glutamine for 15 min. The intracellular 14C-Glutamine level was assessed. Data are presented as means + SD of triplicate measurements. (E) After 48 h of siRNA treatment, the transfected cells were incubated in the medium containing 0.2 mM Gln for 72 h and the cell growth was assessed. Data are presented as means + SD of triplicate measurements. (F) Cells were incubated with 10 μg/mL KM8094 or KM8047 (control antibody) in the medium containing of 0.2 mM Gln for 72 h, after which the cell growth was assessed. Data are means + SD from three independent experiments.
Because previous studies have demonstrated that blockade of ASCT2 in ASCT2-expressing cancer cells reduces the glutamine uptake and cell growth in various cancer cell lines [21,24,25,27,28], we assessed whether the down-regulation of ASCT2 in gastric cancer cells could repress glutamine uptake and cell growth by using siRNA. As expected, the down-regulation of ASCT2 resulted in the inhibition of glutamine uptake and cell growth in the gastric cancer cells (Figure 2B-E). These results suggest that glutamine transported by ASCT2 is necessary for the growth of gastric cancer cells. Next, to examine whether KM8094 can suppress the growth of gastric cancer cells, we treated the ASCT2-expressing gastric cancer cell lines, which grew in glutamine-dependent manner as shown in Figure 2A, with KM8094 for 72 h. We observed that KM8094 suppressed the cell growth in MKN28, HSC-39, HSC-40A, 60As6, SNU-16, and HSC-60 cells whereas the suppression activity was not observed in MKN1 (Figure 2F). These results indicate that KM8094 exerts inhibitory activity on in vitro cell growth in some of the human gastric cancer cells expressing ASCT2.
KM8094 induces cell cycle arrest and apoptosis
To assess the effect of KM8094 on cell-cycle progression and apoptosis in gastric cancer cells, we treated the SNU-16 cell line, which expresses ASCT2 and is moderately sensitive to KM8094 in cell growth inhibition, with KM8094. In the cell cycle analysis, we observed an increased percentage of cells in the G1-phase and decreased percentage of cells at S- and G2/M-phases after 24 h of the KM8094 treatment, when compared to the vehicle treatment (Figure 3A). The apoptosis of cells was detected by Annexin V-FITC/PI staining after treatment of SNU-16 cells with KM8094 for 72 h. The percentage of apoptotic cells was increased upon treatment with KM8094 compared to that in the control cells treated with the vehicle (Figure 3B).
Figure 3.
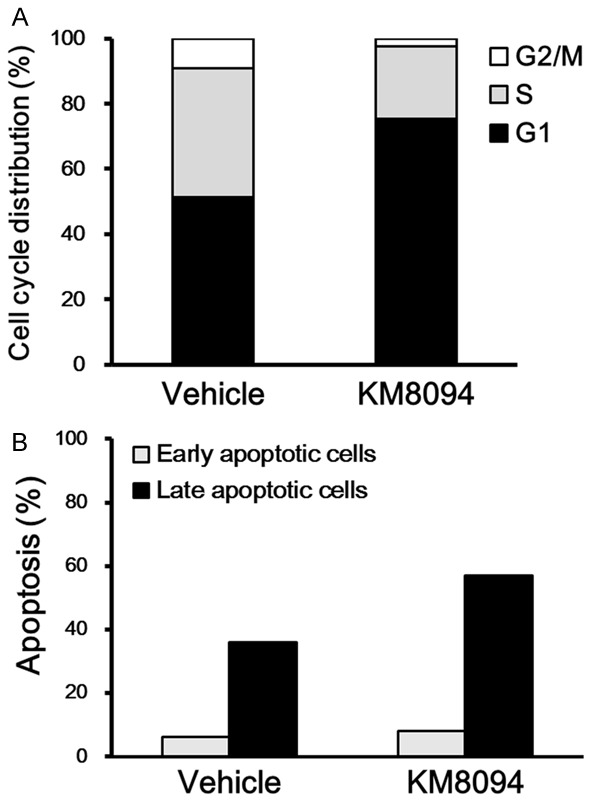
KM8094 induces cell cycle arrest in G1 phase and apoptosis in gastric cancer cells. A. Cell cycle analysis of SNU-16 cells treated with 10 μg/mL KM8094 or vehicle in the 0.2 mM Gln medium for 24 h. The cell cycle distribution was determined by flow cytometry. Histogram represents the percentage of cell populations in each phase of the cell cycle. B. Apoptosis analysis of SNU-16 cells treated with 10 μg/mL KM8094 or vehicle in the 0.2 mM Gln medium for 72 h. The apoptosis of cells was assessed by flow cytometry. Histogram represents the percentage of early apoptotic (Annexin V+, PI-) and late apoptotic (Annexin V+, PI+) cells.
Inhibitory activity of KM8094 on cell growth is mediated by oxidative stress
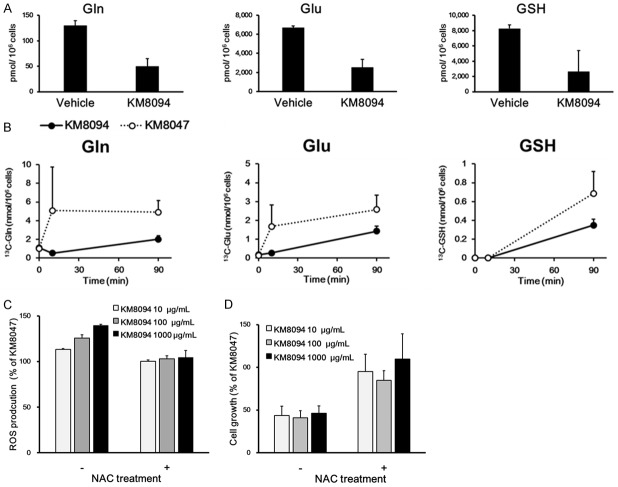
To investigate the mechanism of action of KM8094 in the gastric cancer cells, we first quantified the intracellular metabolites in the SNU-16 cells after 8-h treatment with KM8094. The results of our shotgun metabolic data revealed that the contents of glutamate and glutathione (GSH), as well as glutamine, in the cells were reduced by the KM8094 treatment (Figure 4A). GSH, a tripeptide of Glu, Cys, and Gly, is a major anti-oxidant molecule that neutralizes ROS. Glutamine is imported into cells and is converted to glutamate, which is consequently used to synthesize GSH. Thus, glutamine regulates ROS homeostasis to maintain cell survival [3]. We, therefore, examined whether KM8094 can affect the oxidative and reductive pathways in gastric cancer cells. The SNU-16 cells were treated with KM8094 in the presence of 13C-Glutamine for 90 min and the isotopic label present in the intracellular glutamine, glutamate, and GSH was quantified. KM8094 decreased the glutamine-derived 13C label in glutamate and GSH compared with that in the cells treated with the negative control antibody, indicating that KM8094 inhibits glutamine uptake followed by suppression of GSH production (Figure 4B).
Figure 4.
KM8094 represses glutathione (GSH) synthesis and enhances the intracellular ROS level in gastric cancer cells. A. Metabolite abundance in SNU-16 cells treated with 10 μg/mL KM8094 or vehicle in the 0.2 mM Gln medium for 8 h. B. Intracellular concentrations of 13C-Gln, 13C-Glu, and 13C-GSH in SNU-16 cells treated with 300 μg/mL KM8094 or KM8047 in the 0.2 mM 13C-Gln medium for 10 and 90 min. The 13C-labeled metabolite was measured by LC-MS/MS. Each point represents the mean + SD of triplicate experiments. The 13C-GSH concentrations at 0 and 10 min were below the lower limit of quantification. C. Relative ROS levels in SNU-16 cells treated with 10, 100, and 1000 μg/mL KM8094 or KM8047 in either the presence or absence of 5 mM NAC for 24 h. Data are presented as means + SD of triplicate experiments. D. Relative cell growth of SNU-16 cells treated with 10, 100, and 1000 μg/mL KM8094 or KM8047 either in the presence or absence of 5 mM NAC for 72 h. Data are presented as means + SD of three independent experiments.
To assess whether KM8094 directly increase the intracellular ROS, we treated SNU-16 cells with KM8094 for 24 h and measured the intracellular ROS levels. The SNU-16 cells treated with KM8094 exhibited an increase in intracellular ROS level in a concentration-dependent manner (Figure 4C). Notably, this increase in intracellular ROS level by KM8094 was rescued by supplementing with the antioxidant, NAC [35]. In addition, supplementation with NAC abrogated the inhibition of cell growth by KM8094 in SNU-16 cells (Figure 4D). These results indicate that the inhibition of cell growth by KM8094 in gastric cancer cells is mediated by oxidative stress.
Antibody dependent cellular cytotoxicity of KM8094 in gastric cancer cells
Because KM8094 is a defucosylated-IgG1 antibody, which has enhanced ADCC, we evaluated whether KM8094 exerts ADCC in human gastric cancer cells. We cultured the SNU-16 cells with PBMCs from healthy controls and gastric cancer patients in the presence of KM8094. KM8094 exhibited ADCC when PBMCs from either healthy controls or gastric cancer patients were used, indicating that it exerts not only inhibitory activity on the transporter function of ASCT2 but also causes ADCC in human gastric cancer cells (Figure 5).
Figure 5.
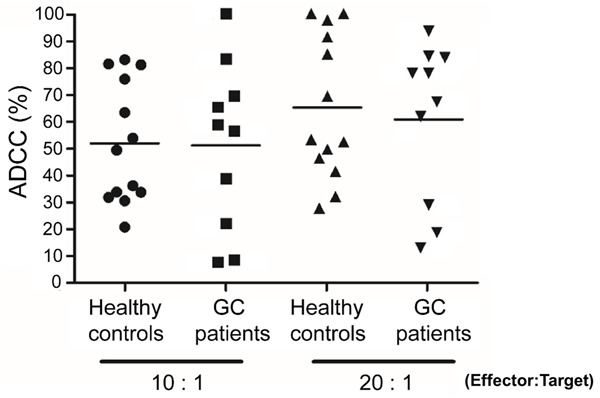
KM8094 exerts in vitro ADCC against gastric cancer cells with human peripheral blood mononuclear cells from healthy control donors or gastric cancer patients. The graph represents individual data points from 13 healthy control donors and 10 gastric cancer donors. SNU-16 cells and human PBMCs from either healthy controls or gastric cancer (GC) patients were incubated with 100 ng/ml KM8094, at E/T ratios of 10:1 and 20:1. ADCC was determined as described in Materials and Methods. All the experiments were performed in triplicate and the percentage of cytotoxicity is presented as the mean. The black bars represent the mean ADCC (%) in each group.
KM8094 represses the in vivo tumor growth in gastric cancer xenografts and exerts synergistic effect with docetaxel
Based on our previous report indicating that KM8094 suppresses the tumor growth in some gastric cancer patient-derived xenograft models expressing ASCT2 [34], we further assessed the in vivo anti-tumor effect of KM8094 using several gastric cancer xenografts. We developed seven gastric cancer xenograft models, in which HSC-39, MKN-1, SNU-16, HSC-40A, MKN28, 60As6, and HSC-60 cells were subcutaneously injected into the flank of SCID mice, respectively, and KM8094 (10 mg/kg, i.v.) was administrated twice weekly for 2 weeks for the SNU-16 xenograft model or once weekly for 2 weeks for the other xenograft models. The subcutaneous tumors of HSC-39, HSC-40A, 60As6, SNU-16, MKN28, and HSC-60 displayed significantly decreased growth upon KM8094 treatment whereas those of MKN1 did not (Figure 6A). These results suggest that KM8094 exerts inhibitory activity not only on in vitro cell growth but also on in vivo tumor growth in several gastric cancer cell lines.
Figure 6.
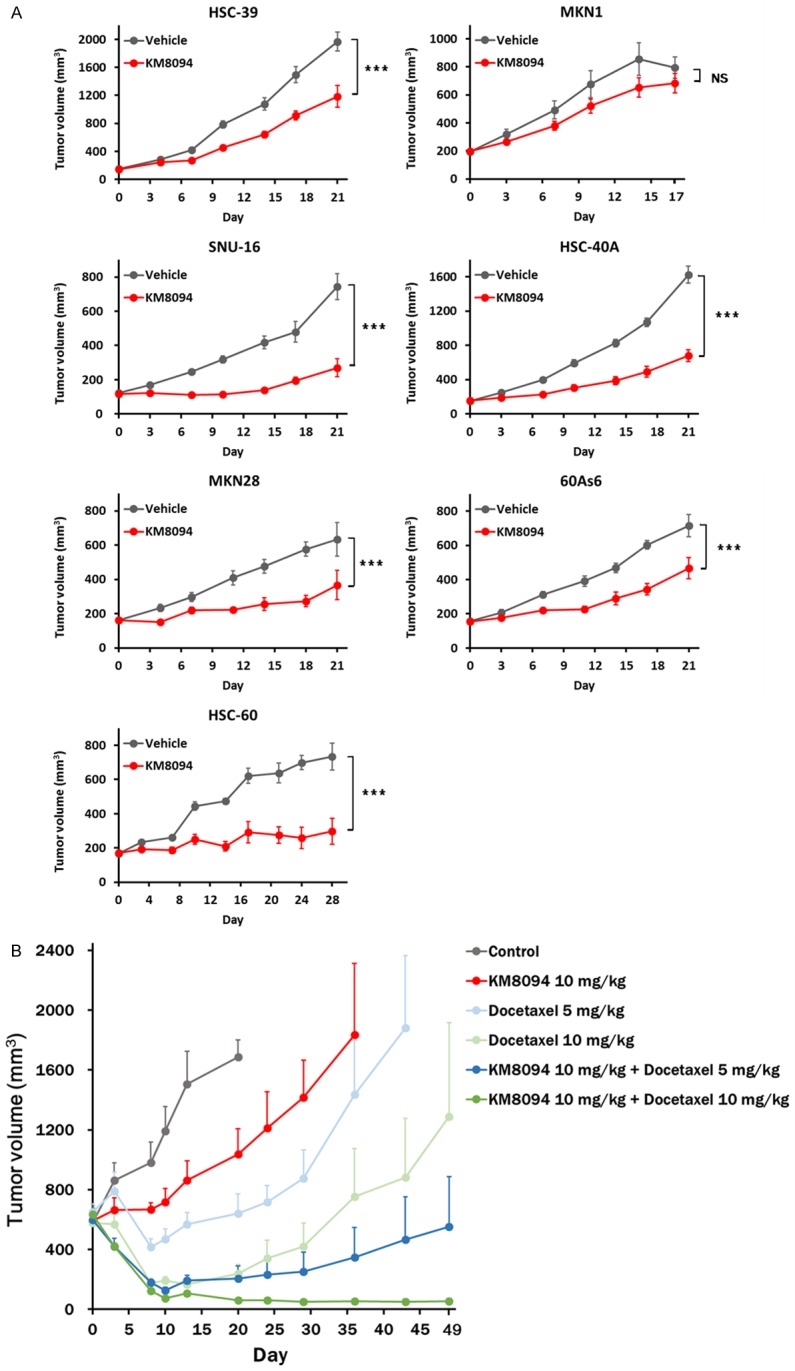
KM8094 represses in vivo tumor growth in gastric cancer xenograft models. A. SCID mice bearing xenografts from the gastric cancer cell lines (HSC-39, MKN-1, SNU-16, HSC-40A, MKN28, 60As6, and HSC-60) were treated with KM8094 (10 mg per kg body weight) or vehicle intravenously on day 0 and 7 (MKN28, HSC-39, HSC-40A, 60As6, HSC-60, and MKN1) or on day 0, 3, 7, and 10 (SNU-16). The tumor volumes were measured. N = 5 mice per group. Values are means ± SE. The p values on AUC for each tumor growth curve were determined by Student’s t-test. Asterisks denote the p values as follows: *P<0.05; **P<0.01; ***P<0.005, NS, not significant. B. SCID mice bearing SNU-16 xenografts were treated with KM8094 (10 mg per kg body weight) on day 0 and 8, docetaxel (5 or 10 mg per kg body weight) on day 0, or a combination of the two. The tumor volumes were measured. Mice were euthanized by cervical dislocation when tumor volume measurements first exceeded 10% of body weight (approximately 3000 mm3) or mice became moribund. N = 5 mice per group. Values are means + SE.
Docetaxel is a well-known agent for gastric cancer therapy [36]. To acquire more insights into the clinical potential of KM8094, we finally assessed the anti-tumor effect of KM8094 in combination with docetaxel in the SNU-16 xenograft model. The combination therapy showed enhanced tumor growth inhibition compared with the case when either of the agents was used alone (Figure 6B).
Discussion
Glutamine is a critical amino acid for growth and survival of cancer cells [3]. Several studies have indicated that ASCT2 is a primary glutamine transporter in cancer cells and its expression is upregulated in a variety of cancer types [13]. Therefore, targeting of ASCT2 to inhibit the cellular glutamine uptake could be a potent therapy for prevention of tumor cell growth. Gastric cancer is one of major causes of cancer death, worldwide [30,31]. Because the therapeutic effects of current chemotherapeutic regimens are limited, there is an unmet need for cancer therapy [32]. A novel anti-ASCT2 monoclonal antibody with a neutralizing activity against glutamine uptake has been reported [33]. In addition, we previously demonstrated that the humanized derivative (defucosylated-IgG1) of this antibody, KM8094, has antitumor efficacy in gastric cancer patient-derived xenograft (PDX) mouse models [34]. However, the molecular mechanism underlying the action of KM8094 in gastric cancer cells has not been fully elucidated. In this study, we evaluated the anti-tumor efficacy of KM8094 in vitro and in vivo using several gastric cancer cells and investigated the underlying molecular mechanism. KM8094 inhibited the growth of gastric cancer cells, mediated by ASCT2-dependent glutamine uptake in vitro. KM8094 suppressed the glutamine uptake and GSH synthesis, elevated oxidative stress, and induced apoptosis and cell cycle arrest. In addition, we found that KM8094 exerted ADCC activity against the SNU-16 cells. These results indicate that the molecular mechanisms underlying the action of KM8094 involves the inhibition of glutamine uptake followed by induction of oxidative stress and ADCC activity. Furthermore, we observed that KM8094 enhanced the in vivo antitumor efficacy of docetaxel, a conventional chemotherapeutic agent in gastric cancer xenograft models. Altogether, our findings suggest that KM8094 can be a potent therapeutic agent for gastric cancer by blocking cellular glutamine metabolism and ADCC activity.
In the immunohistochemistry experiments, ASCT2 expression was observed in the gastric cancer tissues (Figure 1A). Based on comparison of the staining intensity and frequency, the expression tended to be higher in gastric cancer tissues than in normal gastric tissues. Several studies have reported that ASCT2 is upregulated in multiple cancer types, and some of them have also reported that the expression of ASCT2 is correlated with poor prognosis. We showed that inhibition of ASCT2-mediated glutamine uptake by knockdown of ASCT2 in SNU-16 and MKN28 cells suppressed the cell growth (Figure 2B-E). These results indicate that ASCT2 can be a potential therapeutic target in gastric cancer.
KM8094 inhibited the in vitro growth of several ASCT2-expressing gastric cancer cells (Figure 2F). It has been reported that expression level of ASCT2 in NSCLC correlates with the sensitivity to ASCT2 blockade [27]. However, we did not observe any correlation between the ASCT2 expression levels and KM8094 sensitivity. Because there are several glutamine transporters functioning in cancer cells, such as SLC6A14, SLC38A1, and SLC38A2 [37,38], our results indicate that KM8094-resistant gastric cancer cells may prefer such transporters in glutamine uptake rather than ASCT2. Otherwise, it can be considered that the expression patterns of enzymes related to glutaminolysis, such as glutaminase (GLS) and glutamine synthetase (GS), are different between the KM8094-sensitive and resistant cells. Glutamine is intracellularly converted into glutamate by GLS and is synthesized by GS in the cell [39,40]. Therefore, the expression levels of these enzymes can affect the glutamine dependence. Indeed, a recent study reported that high expression of GS causes resistance of an ASCT2 inhibitor, benzylserine, in cultured gastric cancer cells and the ASCT2 expression level is not associated with the sensitivity [41]. Oncogenes and tumor suppressors, such as c-myc and retinoblastoma protein (Rb), regulate glutamine metabolism [42,43]. C-myc facilitates GLS1 expression to maintain TCA cycle and ROS homeostasis in cancer cells. Rb, a tumor suppressor protein, has been reported to suppress GLS1 expression. Thus, the expression or functional status of these molecules affects glutamine metabolism and sensitivity against ASCT2 inhibitor. Further studies are needed to elucidate the factors affecting KM8094 sensitivity in gastric cancer.
We identified that KM8094 has anti-tumor efficacy against several gastric cancer cells in vitro and/or in vivo, but there was no clear correlation between the in vitro and in vivo sensitivity to KM8094 in the gastric cancer cells tested. Since KM8094 is a potelligent antibody which has been shown to exert ADCC in SCID mice [44-46], ADCC as well as the neutralizing activity might contribute to the anti-tumor efficacy of KM8094 in vivo. Considering that sensitivities to ADCC might be different among cell lines, it is, therefore, not surprising that the KM8094-sensitivity observed in vivo did not correlate with that in vitro.
As a consequence of the inhibition of glutamine uptake by KM8094, intracellular levels of glutamate and GSH, which were derived from glutamine, were suppressed. Considering that GSH is an antioxidant, the elevation of ROS level observed in the KM8094 treated-SNU16 cells may be resulted from the reduction of GSH (Figure 4). Because KM8094 induced apoptosis and cell cycle arrest in the SNU-16 cells (Figure 3), it can be considered that the in vitro anti-tumor effect of KM8094 is due to apoptosis or cell cycle arrest in response to elevated intracellular oxidative stress. Indeed, KM8094-induced inhibition of cell growth was canceled by treatment with a ROS scavenger, NAC. The induction of cell death and oxidative stress upon inhibition of ASCT2 transporter is consistent with the findings in NSCLC and CRC [21,27,47].
Given the fact that KM8094 is a potelligent antibody, we evaluated the ADCC activity of KM8094 against SNU-16 cells with PBMCs from healthy volunteers and gastric cancer patients. KM8094 showed ADCC activity against the SNU-16 cells (Figure 5). Thus, in addition to the inhibitory activity of glutamine uptake, it is expected that ADCC can further contribute to the anti-tumor efficacy of KM8094. The ASCT2-targeting agents, benzylserine, γ-L-glutamyl-p-nitroanilide (GPNA), and V-9302 have preclinically been shown to inhibit ASCT2-mediated glutamine uptake and suppress tumor growth [25,27,47]. Whereas the compounds mentioned above can only inhibit the function of ASCT2 transporter, KM8094 can not only inhibit the ASCT2 function but can also exert ADCC.
In summary, we demonstrated that KM8094 might be a potent therapeutic agent against gastric cancer for blocking cellular glutamine metabolism and ADCC.
Acknowledgements
We thank Drs. N. Shiraishi, S. Soga, T. Takahashi, K. Nakamura, and M. Koike for excellent support and helpful suggestion, and K. Masunaga for in vivo technical assistance. This research was funded by Kyowa Hakko Kirin, Co. Ltd.
Disclosure of conflict of interest
None.
References
- 1.Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36:693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- 2.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 3.Deberardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. 2012;23:362–369. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 9.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 11.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–3625. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasa M, Wang HS, Okada A. Characterization of L-glutamine transport by a human neuroblastoma cell line. AJP Cell Physiol. 2002;282:C1246–C1253. doi: 10.1152/ajpcell.00324.2001. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O’Toole SA, Rasko JE, Holst J. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–3208. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang F, Zhao Y, Zhao J, Wu S, Jiang Y, Ma H, Zhang T. Upregulated SLC1A5 promotes cell growth and survival in colorectal cancer. Int J Clin Exp Pathol. 2014;7:6006–6014. [PMC free article] [PubMed] [Google Scholar]
- 16.Witte D, Ali N, Carlson N, Younes M. Overexpression of the neutral amino acid transporter ASCT2 in human colorectal adenocarcinoma. Anticancer Res. 2002;22:2555–2557. [PubMed] [Google Scholar]
- 17.Toyoda M, Kaira K, Ohshima Y, Ishioka NS, Shino M, Sakakura K, Takayasu Y, Takahashi K, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Oyama T, Chikamatsu K. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer. 2014;110:2506–2513. doi: 10.1038/bjc.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte ML, Hight MR, Ayers GD, Liu Q, Shyr Y, Washington MK, Manning HC. Non-invasive glutamine PET reflects pharmacological inhibition of BRAFV600E in vivo. Mol Imaging Biol. 2017;19:421–428. doi: 10.1007/s11307-016-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M, Oyama T, Takeyoshi I. ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer. 2014;110:2030–2039. doi: 10.1038/bjc.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Kim DH, Jung WH, Koo JS. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer. 2013;20:339–348. doi: 10.1530/ERC-12-0398. [DOI] [PubMed] [Google Scholar]
- 21.Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, Clark JE, Alborn WE, Eisenberg R, Massion PP. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon YJ, Khelifa S, Ratnikov B, Scott DA, Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill LM, Jiang T, Rimm DL, Cardiff RD, Mills GB, Smith JW, Osterman AL, Kluger Y, Ronai ZA. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell. 2015;27:354–369. doi: 10.1016/j.ccell.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Younes M, Frolov A, Wheeler TM, Scardino P, Ohori M AG. Expression of neutral amino acid transporter ASCT2 in human prostate. Anticancer Res. 2003;4:3413–3418. [PubMed] [Google Scholar]
- 24.Wang Q, Hardie RA, Hoy AJ, Van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ, Metierre C, Pinello N, Otte NJ, Lehman ML, Gleave M, Nelson CC, Bailey CG, Ritchie W, Rasko JE, Holst J. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236:278–289. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Beaumont KA, Otte NJ, Font J, Bailey CG, Van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM, Jormakka M, Haass NK, Rasko JE, Holst J. Targeting glutamine transport to suppress melanoma cell growth. Int J Cancer. 2014;135:1060–1071. doi: 10.1002/ijc.28749. [DOI] [PubMed] [Google Scholar]
- 26.Kaira K, Sunose Y, Arakawa K, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Oyama T, Takeyoshi I. Clinicopathological significance of ASC amino acid transporter-2 expression in pancreatic ductal carcinoma. Histopathology. 2015;66:234–243. doi: 10.1111/his.12464. [DOI] [PubMed] [Google Scholar]
- 27.Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, Ji X, Harris FT, Harris BK, Boyd KL, Chen H, Eisenberg R, Massion PP. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer. 2015;137:1587–1597. doi: 10.1002/ijc.29535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M, Schmitt A, Poulain L, Green AS, Uzunov M, Kosmider O, Radford-Weiss I, Moura IC, Auberger P, Ifrah N, Bardet V, Chapuis N, Lacombe C, Mayeux P, Tamburini J, Bouscary D. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122:3521–3532. doi: 10.1182/blood-2013-03-493163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma R, Sharma PC. Next generation sequencing-based emerging trends in molecular biology of gastric cancer. Am J Cancer Res. 2018;8:207–225. [PMC free article] [PubMed] [Google Scholar]
- 30.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cidon EU, Ellis SG, Inam Y, Adeleke S, Zarif S, Geldart T. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers (Basel) 2013;5:64–91. doi: 10.3390/cancers5010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kankeu Fonkoua L, Yee N. Molecular characterization of gastric carcinoma: therapeutic implications for biomarkers and targets. Biomedicines. 2018;6:32. doi: 10.3390/biomedicines6010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki M, Toki H, Furuya A, Ando H. Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochem Biophys Res Commun. 2017;482:651–657. doi: 10.1016/j.bbrc.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 34.Kasai N, Sasakawa A, Hosomi K, Poh TW, Chua BL, Yong WP, So J, Chan SL, Soong R, Kono K, Ishii T, Yamano K. Anti-tumor efficacy evaluation of a novel monoclonal antibody targeting neutral amino acid transporter ASCT2 using patient-derived xenograft mouse models of gastric cancer. Am J Transl Res. 2017;9:3399–3410. [PMC free article] [PubMed] [Google Scholar]
- 35.Sun SY. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther. 2010;9:109–110. doi: 10.4161/cbt.9.2.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol. 2014;20:3905–3915. doi: 10.3748/wjg.v20.i14.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cha Y, Kim ES, Koo J. Amino acid transporters and glutamine metabolism in breast cancer. Int J Mol Sci. 2018;19:907. doi: 10.3390/ijms19030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhutia YD, Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863:2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukey MJ, Wilson KF, Cerione RA. Therapeutic strategies impacting cancer cell glutamine metabolism. Future Med Chem. 2013;5:1685–1700. doi: 10.4155/fmc.13.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9:3884–3886. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- 41.Ye J, Huang Q, Xu J, Huang J, Wang J, Zhong W, Chen W, Lin X, Lin X. Targeting of glutamine transporter ASCT2 and glutamine synthetase suppresses gastric cancer cell growth. J Cancer Res Clin Oncol. 2018;144:821–833. doi: 10.1007/s00432-018-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Cui H. Targeting glutamine induces apoptosis: a cancer therapy approach. Int J Mol Sci. 2015;16:22830–22855. doi: 10.3390/ijms160922830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG, Dean DC, Clem BF. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33:556–566. doi: 10.1038/onc.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 45.Nimmerjahn F, Ravetch JV. Immunology: divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 46.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35:932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, Kondo J, Coffey RJ, Johnson MO, Rathmell JC, Sharick JT, Skala MC, Smith JA, Berlin J, Washington MK, Nickels ML, Manning HC. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24:194–202. doi: 10.1038/nm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]