Abstract
In addition to direct oncolysis, oncolytic viruses trigger immunogenic cell death (ICD) and primes antitumor immunity. We have previously shown that oncolytic Newcastle disease virus (NDV), strain FMW (NDV/FMW), induces apoptosis and/or autophagy in cancer cells. In this study, we investigated whether oncolytic NDV can induce ICD in lung cancer cells and whether apoptosis or autophagy plays a role in NDV-triggered ICD. To this end, we examined cell surface expression of calreticulin (CRT) on NDV-infected lung cancer cells and measured ICD determinants, high mobility group box 1 (HMGB1), heat shock protein 70/90 (HSP70/90) and ATP in supernatants following viral infection. Flow cytometric analysis using anti-CRT antibody and PI staining of NDV-infected lung cancer cells showed an increase in the number of viable (propidium iodide-negative) cells, suggesting the induction of CRT exposure upon NDV infection. In addition, confocal and immunoblot analysis using anti-CRT antibody showed that an enhanced accumulation of CRT on the cell surface of NDV-infected cells, indicating the translocation of CRT to the cell membrane upon NDV infection. We further demonstrated that NDV infection induced the release of secreted HMGB1 and HSP70/90 by examining the concentrated supernatants of NDV-infected cells. Furthermore, pre-treatment with either the pan-caspase inhibitor z-VAD-FMK or the necrosis inhibitor Necrostain-1, had no impact on NDV-induced release of ICD determinants in lung cancer cells. Rather, depletion of autophagy-related genes in lung cancer cells significantly inhibited the induction of ICD determinants by NDV. Of translational importance, in a lung cancer xenograft model, treatment of mice with supernatants from NDV-infected cells significantly inhibited tumour growth. Together, these results indicate that oncolytic NDV is a potent ICD-inducer and that autophagy contributes to NDV-mediated induction of ICD in lung cancer cells.
Keywords: Newcastle disease virus, immunogenic cell death, apoptosis, autophagy, lung cancer
Introduction
Oncolytic viruses (OVs) have been emerging as novel anti-cancer immunotherapeutic agents as they induce immunogenic cell death (ICD) in addition to direct tumor cell lysis [1-4]. In general, tumor cells undergoing OV-induced ICD release tumor-associated antigens (TAAs) accompanied by diverse danger-associated molecular patterns (DAMPs) and inflammatory cytokines to restore the immunosuppressive tumor microenvironment. In addition, they can trigger TAA-specific antitumor immunity [1,2]. The three major ICD determinants include the release of ATP, high-mobility group box B1 protein (HMGB1) and the pre-apoptotic cell surface exposure of calreticulin (CRT). Heat shock proteins (HSP) HSP70/90, which are exposed on the membrane of cells undergoing severe stress, act as danger signals thereby contributing to the stimulation of antigen-presenting cells. In preclinical studies, several OVs such as adenoviruses, herpes simplex virus, measles virus, parvovirus and vesicular stomatitis virus have been shown to trigger ICD-like features in a variety of cancers [5-12], activating dendritic cell (DC) and increasing levels of inflammatory cytokines, natural killer (NK) cells and CD8+ T-cell responses [3]. These observations would suggest a potential role for OVs as anti-cancer immunotherapeutic modalities that may be used in combination with pharmacologic or other immunotherapeutic agents to further enhance anti-tumor immunity and overall therapeutic efficacy [4].
Oncolytic Newcastle disease virus (NDV) has displayed potent anti-tumor activities both in preclinical investigations and in clinical trials [13-18]. In addition to inducing apoptosis, autophagy-related cell death and necroptosis in a range of cancers, a recent study demonstrated that NDV induced ICD in gliomas [19]. In line with these findings in NDV-infected gliomas, our previous study showed that oncolytic NDV triggered the release of HMGB1 in drug-resistant lung cancer cells [20], suggesting that NDV may induce ICD-like features in different cancer cell types. However, the mechanism(s) by which NDV induces ICD in infected cancer cells remains largely unknown.
The aim of the current study was to establish whether (i) oncolytic NDV triggers the release of CRT, ATP, HMGB1 and HSP70/90 in human lung cancer cells, thereby inducing ICD; (ii) if apoptosis and autophagy play a critical role in this NDV-induced ICD.
Materials and methods
Cell culture and viral infection
Human lung cancer cell lines A549 (ATCC® Number: CRM-CCL-185TM), H1650 (ATCC® Number: CRL-5883TM) and H460 (ATCC® Number: HTB-177TM) were purchased from the American Type Culture Collection (ATCC). A549 cells was cultured in DMEM and H1650 and H460 cell were cultured in Roswell Park Memorial Institute (RPMI-1640) medium, supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. Oncolytic NDV strain NDV/FMW, which has been previously shown to induce cytotoxic effects in lung cancer cells [21,22], was used throughout the study. The propagation and titration of NDV/FMW were performed as previously described [21,22]. Lung cancer cells were infected with NDV/FMW at a multiplicity of infection (MOI) of 1 and recovered at several time-points post-infection. To inactivate NDV/FMW, the virus was ultraviolet-irradiated for 60 min at intensity of 0.15 mW cm-2.
Antibodies and reagents
The following antibodies from Cell Signaling Technology were used: cleaved caspase-3 (#9662S), HMGB1 (#3935), HSP70 (#4876), poly (ADP-ribose) polymerase (PARP) (#9532S). Anti-microtubule-associated protein 1 light chain 3 (LC3) (#L7543) and anti-a-tubulin (#T6074) were purchased from Sigma. Anti-calreticulin (CRT) (#ab2907) and anti-E-cadherin (#ab40772) antibodies were purchased from Abcam. Anti-hemagglutinin-neuraminidase protein (HN) (#sc-53562) and anti-HSP90 (#sc-69703) antibodies were purchased from Santa cruz. Anti-β-actin (#66009-1-Ig) and goat anti-rabbit antibody (#SA00001-2) were purchased from Proteintech. Goat anti-mouse antibody (#bs12478) was obtained from Bioworld. Alexa 488 (#A11070) and Alexa 568 (#A11031) were obtained from Invitrogen. Anti-ATG5 antibody (#NB110-53818) was purchased from NOVUS. DAPI (#C1002) was purchased from Beyotime. Mitoxantrone (MTX) (#S1889), Necrostain-1 (Nec-1) (#S8037) and Z-VAD-FMK (#S7083) were purchased from Selleckchem. Drugs were dissolved in dimethyl sulfoxide (DMSO) as stock solutions and stored at -20°C. Mem-PERTMPlus Membrane Protein Extraction Kit (#89842) and Pierce®Protein Concentrator PES (#88517) were obtained from Thermo Scientific. ENLITEN®ATP Assay System Bioluminescence Detection Kit for ATP Measurement (#FF2000) was obtained from Promega. HMGB1 ELISA Kit II (#L534) was purchased from SHINO-TEST CORPORATION.
Lentiviral constructs and stable cell lines
A549 cell lines stably depleted of ATG5 or LC3 were established, as described in our previous studies [23]. The following lentiviral constructs were purchased from Santa Cruz: ATG5 shRNA (#sc-41445-V), MAP LC3β shRNA (#sc-43390-V) and noncoding shRNA (#sc-108080).
Membrane protein extraction and preparation of concentrated supernatants
Cell lysates of virus-infected cells were collected and membrane proteins were extracted using the Mem-PERTMPlus Membrane Protein Extraction Kit, according to the manufacturer’s instructions. The supernatants (5 ml) of infected cells were collected and concentrated to 100 μl using Pierce®Protein Concentrator 2-6 ml/10K filters, according to the manufacturer’s instructions.
Confocal microscopy
For the detection of CRT on the cell surface, virus-infected cells were subjected to confocal laser microscopy analysis as previously described [23]. Briefly, cells were fixed and incubated with a rabbit monoclonal antibody against CRT, following incubation with Alexa Fluor 488 goat anti-rabbit IgG. Nuclei were stained with 5 μg/mL DAPI (Sigma) in PBS. Images were obtained using a confocal laser microscope (Leica TCS SP5) with a ×60 oil objective. Data were analyzed using the open source ImageJ (64 Bit for Windows) imaging platform.
Apoptosis
Cell death was assessed by double staining with fluorescein isothiocyanate (FITC)-coupled annexin-V (AnnexinV-FITC, BD Bioscience, #C34554) and propidium iodide (PI) staining as previously described [21]. The cell population in the lower right quadrant (PI-negative, Annexin V-positive) corresponding to apoptotic cells were quantified.
Flow cytometric analysis
Ecto-CRT detection was performed as previously documented by Khou et al. [24]. Briefly, cells were incubated with an anti-CRT antibody, followed by incubation with AlexaFluor 488-conjugates. PI was added to the final concentration of 1 μg/ml, and samples were analyzed by flow cytometry analysis. CRT-positive cells were gated on live (PI-negative) cells.
ATP and enzyme-linked immunosorbent assays
The cell-free supernatants of NDV-infected lung cancer cells and mock-infected control cells were collected. Secreted extracellular ATP in the supernatants was measured using the ENLITEN ATP assay (Promega, Madison, WI, USA) using a multifunctional enzyme labeling instrument (Enspire2300, Perkin Elmer, USA). Supernatants were also used to detect HMGB1 using the HMGB1 ELISA Kit II (Shino-Test, Kanagawa, Japan). Enzyme-linked immunosorbent assays (ELISA) were performed using a luminometer.
Immunoblot analysis
Immunoblot (IB) analysis was carried out as previously described [25]. For detection of CRT on the cell surface, cell membrane fractions of NDV/FMW-infected lung cancer cells were isolated and subjected to SDS-PAGE and analyzed by immunoblotting. E-cadherin was used as a positive control for membrane protein expression. To examine secreted HMGB1 and HSP70/90, the concentrated cell-free supernatants of virus-treated cells were subjected to IB analysis.
Animal experiments
BALB/c nude mice (female, 6 weeks old) were purchased from the Experimental Animal Center of Dalian Medical University. Briefly, cultured A549 and H460 cells were injected subcutaneously into the right flank of mice. Mice were divided into four groups and ten mice were included in each treatment group. (a) PBS control, (b) concentrated cell-free supernatants from NDV/FMW-infected cells (the supernatants were ultraviolet-irradiated for 60 min at intensity of 0.15 mW cm-2), (c) NDV/FMW (1×107 TCID50 per dose), (d) inactivated NDV/FMW. When tumors reached 200 mm3, tumors received an intratumoral injection every three days. Tumor-bearing mice were also treated with either NDV/FMW or inactivated NDV/FMW for control. Tumor growth and survival was monitored every 5 days by digital calipers. After 40 days, mice were sacrificed under anesthesia. Euthanasia: Treat mice in an inhalation anesthesia machine with 5% isoflurane; 2.4 L/min N2O; 1.2 L/min O2. Observe the mice (The depth of anesthetization is sufficient when the following vital criteria are reached: regular spontaneous breathing. No reflex after setting of pain stimuli between toes, and no response to pain). Carotid after anesthesia mice was killed off. The animals were tested in a biosafety cabinet of the SPF laboratory animal center of the Dalian Medical University (Dalian, China), all procedures involving animals and their care complied with the China National Institutes of Healthy Guidelines for the Care and Use of Laboratory Animals. Ethical approval for the study was granted by the Ethics Committee of Dalian Medical University. Mouse experiments were carried out as described previously [21,26].
Statistical analysis
Statistical analyses were performed using the Student’s t-test with Microsoft Excel (Microsoft, Redmond, WA, USA). Results were expressed as the Mean ± SD of at least three independent experiments. To assess the in vivo oncolytic effects, statistical significance between groups was calculated using LSD test and SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). Differences with a p value of P<0.05 were considered statistically significant.
Results
Oncolytic NDV induces apoptosis in lung cancer cells
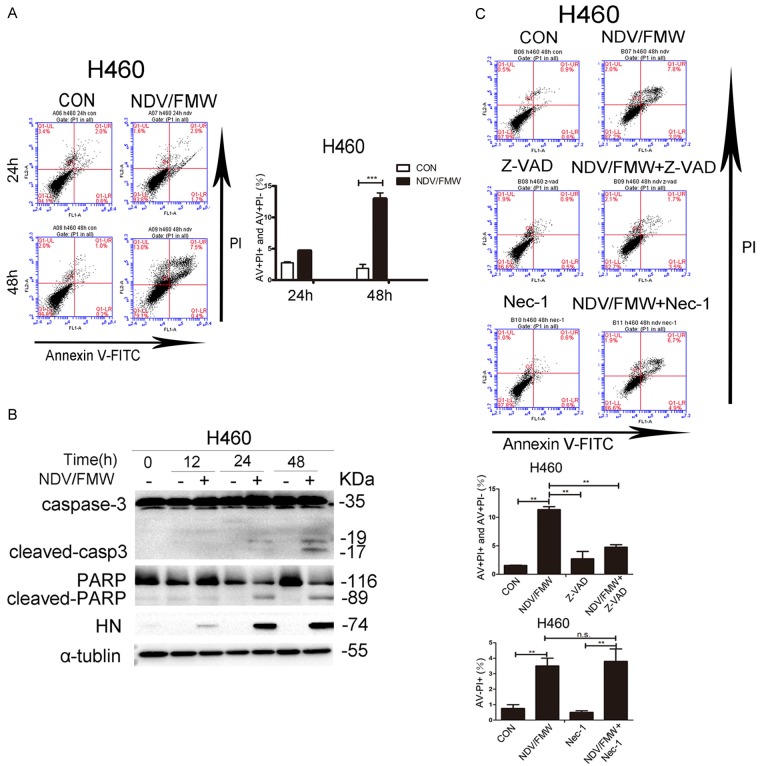
Our previous work showed that oncolytic NDV, strain FMW (NDV/FMW), induced apoptosis in human lung cancer A549 cells [21,27,28]. We determined the apoptotic effects of NDV/FMW on lung H460 cells. NDV/FMW was inoculated at an MOI of 1 for different times and apoptosis was analyzed by flow cytometry with FITC-conjugated Annexin V and PI double staining. Relative to controls, NDV/FMW infection triggered a significant increase in the percentage of apoptotic cells in H460 cells at 48 h post-infection (hpi) (Figure 1A). Moreover, we observed a profound cleavage of caspase-3 and poly (ADP-ribose) polymerase (PARP), two classical markers of apoptosis, in NDV/FMW-infected H460 cells at 24 and 48 hpi as assessed by immunoblot analysis (Figure 1B). These data indicate that NDV/FMW induces apoptosis in H460 cells. To further examine the apoptotic effect of NDV/FMW on H460 lung cancer cells, cells were pre-treated with either the broad specificity caspase inhibitor, Z-VAD-FMK, the necrosis inhibitor, Necrostain-1, or mock-treated. Pre-treatment with Z-VAD-FMK (but not Necrostain-1) significantly decreased the number of apoptotic cells in NDV/FMW-infected H460 cells (Figure 1C), further confirming the induction of apoptosis in NDV/FMW-treated H460 cells. In addition, marked expression of NDV HN protein in H460 cells was detected at 12, 24 and 48 hpi (Figure 1B), indicating viral replication. These findings are in agreement with our previous observations [21,27] whereby NDV/FMW infection triggered apoptosis and expression of HN protein in A549 cells (data not shown).
Figure 1.
Induction of apoptosis by oncolytic NDV/FMW in lung cancer cells. A. H460 cells were infected with or without (mock-infected) NDV/FMW (MOI = 1) for the indicated time-points. Cells at 24 and 48 h post-infection (hpi) were double-stained with Annexin V and propidium iodide (PI) and analyzed by flow cytometry. The cell population in the right lower quadrant (PI-negative, Annexin V-positive) and the right upper quadrant (Annexin V/PI positive) are represented. Data shown are representative of three independent experiments (***P<0.001). B. Cells at 12, 24 and 48 hpi were lysed and activation of caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) was examined by immunoblot analysis (n = 2). Replication of NDV/FMW was detected by examination of the expression of hemagglutinin-neuraminidase protein (HN) protein. To control for loading, a-tubulin was also used. Immunoblots shown are representative of two independent experiments. C. Cells were pre-treated with either Z-VAD-FMK (Z-VAD, 100 μM) or Necrostain-1 (Nec-1, 20 μM) or mock-treated, following infection of NDV/FMW for 48 h. Apoptosis was analyzed by flow cytometry. Data are representative of three independent experiments (**P<0.01, n.s = not significant).
NDV induces CRT exposure in lung cancer cells
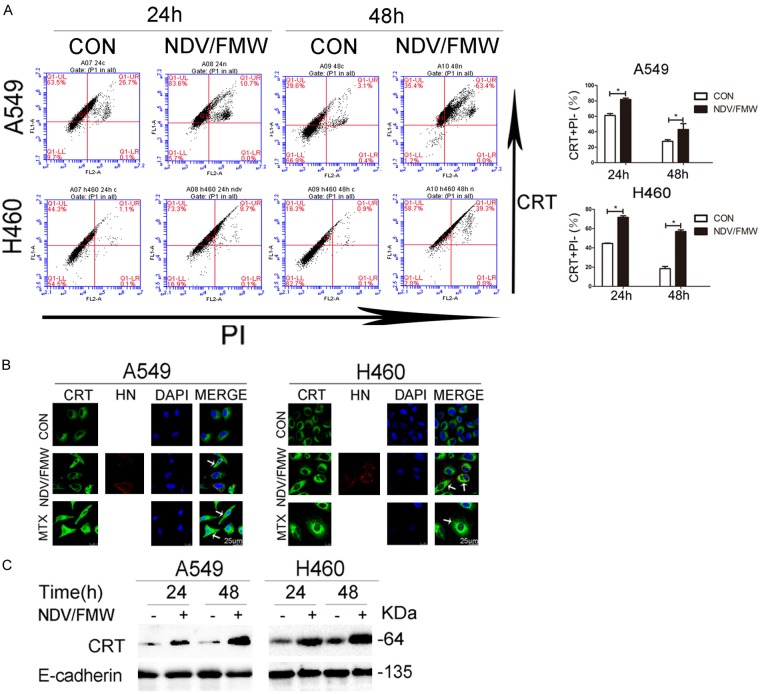
Oncolytic NDV was shown to induce ICD in gliomas and to trigger the release of HMGB1 in drug-resistant lung cancer cells [19,20]. We hypothesized that NDV/FMW induces ICD in lung cancer cells. Ecto-CRT is the most important determinant of ICD [1-3]. Following the triggering of immunogenic apoptosis, CRT translocates from the lumen of the endoplasmic reticulum to the surface of dying cells where it functions as an ‘eat-me’ signal for professional phagocytes [29,30]. FACS analysis following co-staining of NDV/FMW-infected A549 and H460 cells with anti-CRT antibody and PI, showed a significant increase in the number of PI-negative cells at 24 and 48 hpi (Figure 2A), suggesting that NDV infection may trigger CRT translocation to the cell surface. To examine whether NDV/FMW induced ecto-CRT, NDV/FMW-infected lung cancer cells were stained with an anti-CRT antibody and analyzed by confocal imaging. Mitoxantrine (MTX) [31], a bona fide ICD inducer, was used as a positive control. Increased accumulation of CRT (Green) on the cell surface was observed in NDV/FMW-infected A549 and H460 cells at 48 hpi compared to mock-infected cells by immunofluorescence (Figure 2B). As expected, MTX treatment of A549 and H460 cells induced greater exposure of CRT (Green) (Figure 2B). The NDV envelope protein, HN (Red), was evident in NDV-infected cells but not in mock-infected or MTX-treated cells by immunostaining with anti-HN antibody (Figure 2B). To further corroborate the above findings, we analyzed the expression levels of CRT on the cell plasma membrane by immunoblotting. NDV/FMW infection indeed triggered an increase in membrane-fractioned CRT levels in both A549 and H460 cells compared to mock-infected cells (Figure 2C), indicating that NDV/FMW induces ecto-CRT in lung cancer cells.
Figure 2.
NDV induces calreticulin (CRT) exposure in response to NDV. A. A549 and H460 cells were infected or mock-infected with NDV/FMW (MOI = 1) for 24 and 48 h. Cells were subjected to surface immunofluorescence staining to detect CRT in viable, PI-negative cells. Representative dot plots (left panel) and quantification data (right panel) are shown. Data are shown as Mean ± S.D. for three independent replicates (*P<0.05). B. Translocation of calreticulin (CRT) was assessed by immunofluorescence staining. The ICD inducer, mitoxantrine (MTX), was used as a positive control. DAPI was used for nuclear staining. NDV was detected based on the expression of hemagglutinin-neuraminidase protein (HN). Images were obtained using confocal microscopy (scale bar = 25 μm), arrowheads indicate positive area. Images are representative of three independent experiments. C. Cell membranes was isolated and subjected to immunoblot (IB) analysis while E-cadherin was used as a membrane marker. Immunoblots shown are representative of two independent experiments.
NDV infection of lung cancer cells triggers release of HMGB1and HSP70/90
We next investigated the release of secreted DAMPs such as ATP, HMGB1 and HSP70/90 in NDV/FMW-infected lung cancer cells. Immunoblot analysis of the concentrated supernatants of NDV-infected A549 and H460 cells demonstrated that extracellular HMGB1 levels were markedly increased after 48 h incubation compared to mock-infected cells (Figure 3A and 3B). Surprisingly, marked induction of HMGB1 was detected in the whole cell lysates of NDV-infected H460 cells but not in A549 cells (Figure 3B). Consistently, a significant increase in HMGB1 levels was observed in the supernatants of NDV/FMW-infected lung cancer cells compared to mock-infected cells as detected by ELISA (Figure 3C). Furthermore, extracellular HSP70/90 levels were found in concentrated supernatants of A549 cells from 12 h to 48 h following NDV/FMW infection (Figure 3A), but were only detectable in H460 cells at 48 hpi (Figure 3B). In addition, no extracellularly secreted ATP was found in NDV/FMW-infected lung cancer cells at 12, 24 and 48 hpi (data not shown).
Figure 3.
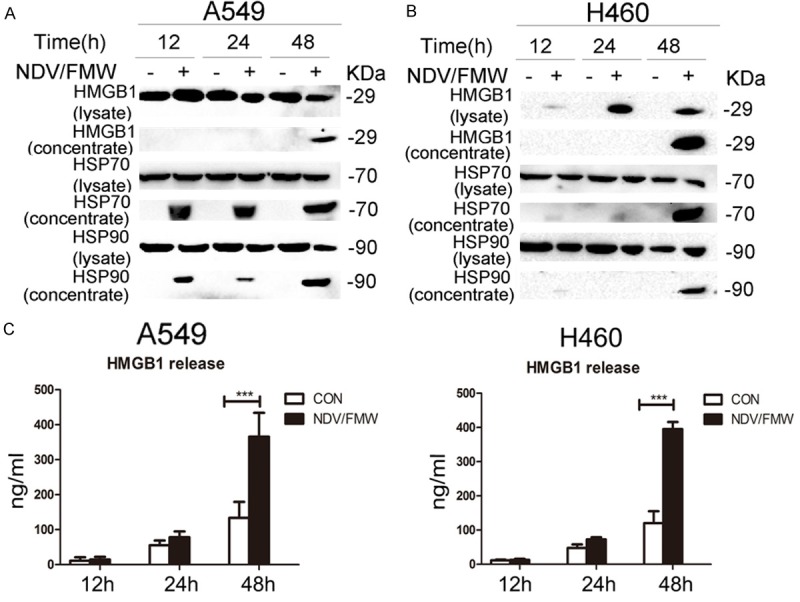
Release of immunologic signals upon NDV infection. A, B. Cell-free supernatants (concentrated) and whole cell lysates were collected from A549 and H460 cells infected with NDV/FMW (MOI = 10) for distinct periods of 12, 24 and 48 h. Immunoblot (IB) analysis of HMGB1 and HSP70/90 in either whole cell lysates or concentrated supernatants of A549 and H460 cells. Data shown are representative of two independent experiments. C. Enzyme-linked immunosorbent (ELISA) detection of HMGB1 release in NDV/FMW cell supernatants compared to uninfected control cells (***P<0.001, n = 4).
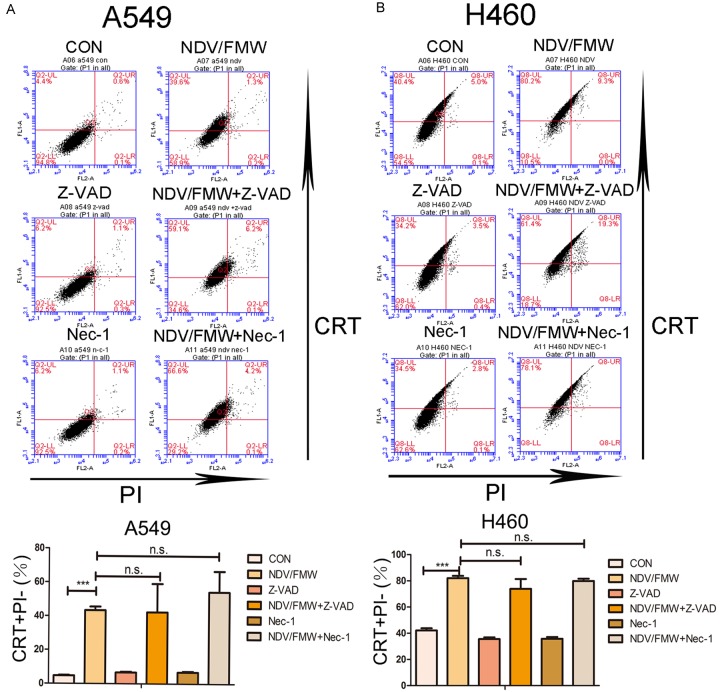
Inhibition of apoptosis does not affect NDV-induced ICD in lung cancer cells
We sought to test whether apoptosis plays a role in NDV/FMW-induced ICD in lung cancer cells. Pretreatment of A549 (Figure 4A) and H460 (Figure 4B) cells with either Z-VAD-FMK or necrostain-1 had no effect in decreasing ecto-CRT on the cell surface in NDV/FMW-infected cells at 24 hpi compared to virus-only-treated cells. In addition, Z-VAD-FMK and necrostain-1 showed no inhibitory effect on the release of HMGB1 or HSP70/90 in NDV/FMW-infected A549 and H460 cells (data not shown).
Figure 4.
Effects of pharmacological modulation of apoptosis and necroptosis on NDV-induced CRT exposure. A549 (A) and H460 (B) lung cancer cells were pre-treated with either Z-VAD-FMK (100 μM) or Necrostain-1 (Nec-1, 20 μM), and subsequently infected or mock-infected with NDV/FMW (MOI = 1) for 24 h. Cells were stained for the detection of CRT in viable, PI-negative cells (Figure 2A). Representative dot plots (top panel) and quantification data (lower panel) are shown for three independent experimental replicates (***P<0.001, n.s = not significant).
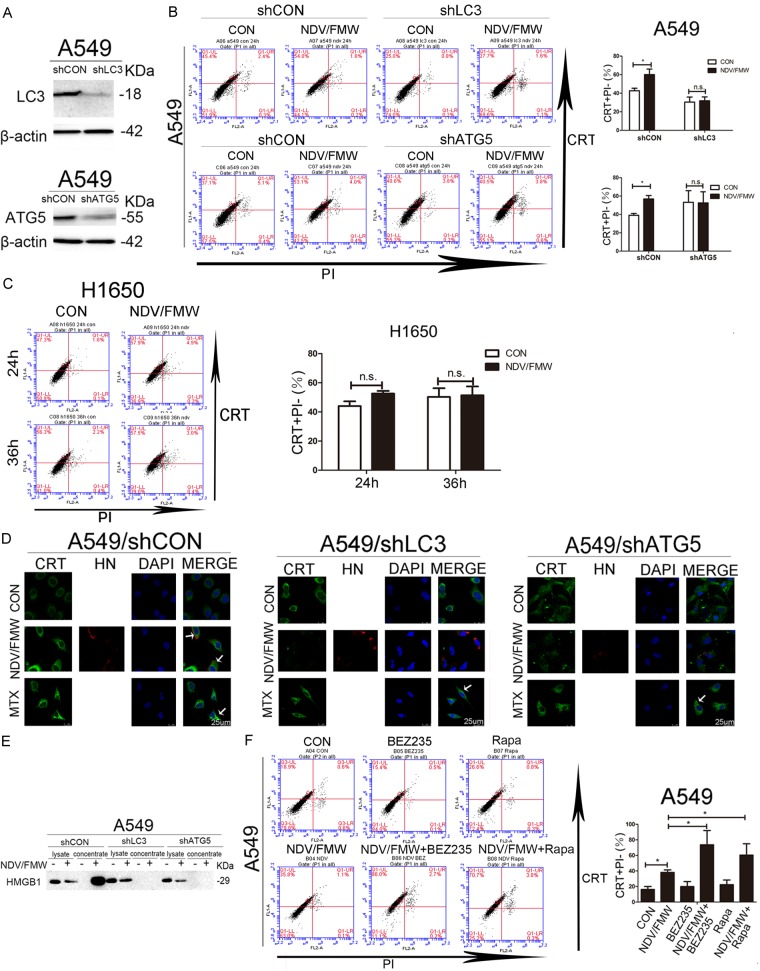
Depletion of key autophagy gene decreases the induction of ICD in lung cancer cells upon NDV infection
We and others previously reported that oncolytic NDV/FMW triggers autophagy in infected cells [19,20,32-35]. Given that autophagy may contribute to the induction of ICD [36,37], we examined whether depletion of the autophagy-related gene (Atg) would impact on NDV/FMW-mediated ICD in lung cancer cells. To test this, lentiviruses expressing different short hairpin RNAs (shRNAs) against either ATG5 or microtubule associated protein 1 light chain 3 (LC3), two key autophagy genes in autophagosome formation, were used to stably deplete ATG5 and LC3 in A549 cells. Lentiviruses expressing the scrambled shRNAs were used as controls. Depletion of ATG5 and LC3 was confirmed by immunoblot analysis (Figure 5A). FACS analysis indicated that NDV/FMW-induced CRT exposure, which was significantly suppressed in ATG5- and LC3-depleted A549 cells at 24 hpi compared to A549-scrambled controls (Figure 5B). It was reported that the human lung adenocarcinoma H1650 cell line, which has lost Atg7 expression, is deficient in Atg7-dependent autophagy [38]. Interestingly, we did not observe any CRT exposure in H1650 cells upon NDV/FMW infection at 24 and 36 hpi (Figure 5C). Consistently, confocal analysis of NDV/FMW-infected cells at 36 hpi showed that depletion of either ATG5 or LC3 markedly abolished the induction of CRT exposure compared to scrambled control cells (Figure 5D). Moreover, immunoblot analysis of concentrated supernatants from NDV/FMW-infected A549 cells at 48 hpi demonstrated that depletion of either ATG5 or LC3 profoundly decreased the release of HMGB1 (Figure 5E). In addition, we did not observe the release of HMGB1 in NDV-infected H1650 cells (data not shown). We further examined whether pharmacological modulation of autophagy could affect NDV-mediated induction of ICD in lung cancer cells. As shown in Figure 5F, pretreatment with two well known autophagy inducers, Rapamycin and BEZ235, significantly increased NDV/FMW-induced CRT exposure in lung cancer cells compared with NDV/FMW alone.
Figure 5.
Effects of depletion of autophagy-related genes on NDV-triggered ICD determinants. (A) Confirmation of the depletion efficiency in A549 cells stably depleted of ATG5 (shATG5) or LC3 (shLC3) by immunoblot analysis. β-actin was used as a loading control. ATG5- or LC3-depleted A549 cells (B) and H1650 cells (C) were infected or mock-infected with NDV/FMW (MOI = 1) for 24 in A549 cells and 24 and 36 h in H1650 cells. Flow-cytometric analysis of CRT exposure was performed (Figure 2A). Data are shown as Mean ± S.D. for three independent experiments (*P<0.05, n.s = not significant). (D) ATG5- or LC3-depleted A549 cells and control cells were infected or mock-infected with NDV/FMW (MOI = 1) for 36 h. Confocal analysis of CRT exposure was carried out (Figure 2B). MTX was used as a positive control, arrowheads indicate positive area. Representative images are shown for three independent experiments. (E) IB analysis of released HMGB1 in whole cell lysates and concentrated supernatants of NDV/FMW-infected A549, ATG5- and LC3-depleted A549 cells. Blots shown are representative of two independent experiments. (F) A549 cells were treated with vehicle, Rapamycin (1 μM) or BEZ235 (1 μM), then they were infected with vehicle or NDV/FMW (1 MOI) for 24 h. Flow-cytometric analysis for CRT exposure. Representative images are shown for three independent experiments.
Supernatants derived from NDV-infected cells reduce tumor growth in mice
We explored the potential of supernatants from NDV/FMW-infected lung cancer cells to suppress tumor growth in vivo. Mice with A549 or H460-derived tumors were injected with concentrated supernatants from NDV/FMW-infected A549 and H460 cells every three days. The supernatants were irradiated with UV to inactivate the infectious virus. The growth of supernatant-injected tumors was significantly reduced compared to PBS-injected tumors (Figure 6A). As expected, injection with NDV/FMW, but not UV-inactivated virus, significantly decreased tumor growth (Figure 6A). In addition, the CRT exposure in mice tumor samples in NDV/FMW-treated group and comparative control group were assessed by Immunohistochemistry. Figure 6B shows that the CRT exposure was evidently observed in tumor samples of mice treated with NDV/FMW and with the supernatants.
Figure 6.
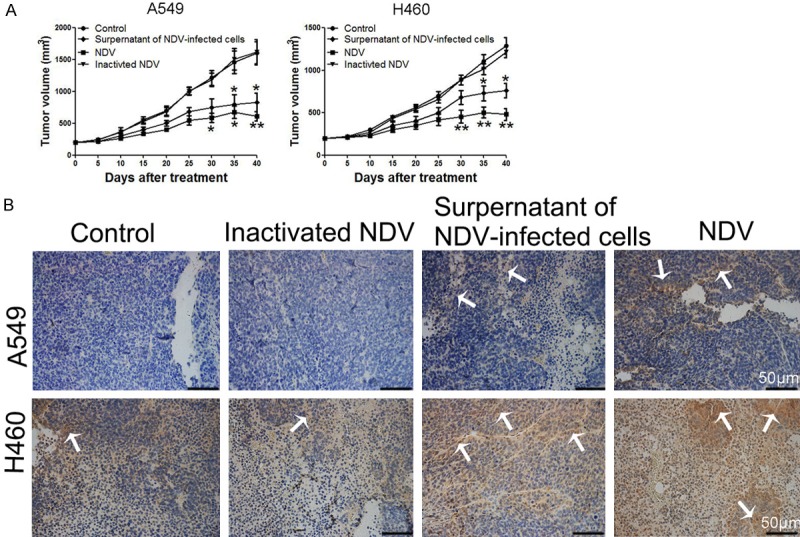
Tumor growth in response to supernatants from NDV-infected lung cancer cells. A. A549 and H460 cells were injected subcutaneously into the right flanks of mice to establish tumors. When tumors reached approximately 200 mm3, mice received an intratumoral injection of either PBS, the concentrated cell-free supernatants of NDV/FMW (50 μl), NDV or inactivated NDV every three days. Tumor volumes were measured at 5-day intervals for 40 days after injections and expressed as the Mean ± SD (n = 10) and represented as tumor volume-time curves to show any differences in tumor regression (*P<0.05, **P<0.01). B. Immunohistochemistry assay for expressions of CRT protein in four groups of mice model, arrowheads indicate positive area. Scale bar = 50 μm.
Discussion
In this study, we show that oncolytic NDV/FMW triggers CRT exposure and HMGB1 and HSP70/90 release in human lung cancer cells in vitro, while supernatants from NDV-infected cells reduced tumor formation in a xenograft model indicating that NDV-induced lung cancer cell death is immunogenic and associated with the release of the major ICD determinants. Notably, we demonstrate that autophagy plays a role in NDV-induced ICD in lung cancer cells. Thus, to our knowledge, this is the first study describing the induction of ICD by NDV in lung cancer cells and a potential mechanism by which NDV induces ICD in this cancer type.
A growing number of oncolytic viruses such as coxsackie B3 virus, herpes simplex virus type 1, measles virus, parvovirus, vaccinia virus have been shown to induce ICD in a variety of cancer cells [8,11,39-42]. Recently Koks et al. reported that NDV induced ICD accompanying the release and surface exposure of DAMPs in glioma cells [19]. However, whether oncolytic NDV triggers ICD in other cancer types has yet to be reported. In the present study, we identified the ICD markers HMGB1, HSP70/90 and ATP in cell culture supernatants following viral infection and examined cell surface of infected lung cancer cells for CRT expression. We found that NDV infection of lung cancer cells triggered the release or expression of several DAMPs, including ecto-CRT, HMGB1and HSP70/90, consistent with the observation by Koks et al. in glioma GL261 cells [19]. Thus, our data indicate that NDV induces ICD in lung cancer cells. It has been shown that ATP is an important ICD determinant [43]. Interestingly, similar to the study in NDV-infected glioma cells [19], no change was observed in the levels of secreted ATP in NDV-infected lung cancer cells. Nevertheless, this study and work by others strongly suggest that induction of ICD in cancer cells may be a common feature for NDV during infection.
Recent studies have highlighted the role of the immune response, initiated by NDV, to mediate antitumor effects in addition to direct oncolysis. Zamarin et al. reported that localized therapy with oncolytic NDV induces inflammatory immune infiltrates in distant melanoma [17]. Studies by Schwaiger et al. showed that NDV mediates pancreatic tumor rejection via NK cell activation and prevents cancer relapse by prompting an adaptive immune response [44]. Thus, the induction of ICD by NDV in cancer cells may induce antitumor immune response in vivo. Remarkably, a reduction in tumor formation in xenografts injected with concentrated supernatants of NDV-infected lung cancer cells was observed, suggesting that non-infectious molecules including the DAMPs in the supernatants of NDV-infected cells inhibited the growth of tumors via tumor immunity. However, whether NDV-triggered ICD could exert direct effects on the activation and maturation of immune cells such as DCs and cytotoxic T lymphocytes (CTLs), as well as the release of inflammatory cytokines in lung cancers, has not been determined in the current study. However, previous reports have shown that NDV-induced ICD can prime adaptive antitumor immunity in an orthotopic, syngeneic murine glioma model [19]. Of interest, immune checkpoint blockade of PD-1/PD-L1 in conjunction with oncolytic NDV-mediated therapy have been recently investigated [17,45]. Therefore, it will be interesting to explore whether and how the induction of ICD by NDV affects the antitumor response using these combinatorial approaches in pre-clinical studies and subsequent clinical trials.
Several forms of cell death such as apoptosis, autophagic cell death, necroptosis and endoplasmic reticulum stress, play a role in the induction of ICD in response to different stimuli [2,43]. Given the importance of ICD in NDV-mediated antitumor immune response, we investigated whether NDV-induced apoptosis and/or autophagy contribute to the induction of ICD in infected lung cancer cells. Our data demonstrate that pharmacological inhibition of either apoptosis or necroptosis does not display any inhibitory effect on NDV-triggered release or expression of ecto-CRT, HMGB1 and HSP70/90 in lung cancer cells, indicating that, in addition to apoptosis and necroptosis, other cell death pathways may be involved in NDV-mediated induction of ICD. This finding is, at least in part, consistent with that observed in NDV-infected gliomas, indicating that NDV-induced ICD is blocked by inhibition of necroptosis and is independently of caspase signaling [19]. However, we demonstrate that depletion of Atg genes in lung cancer cells suppressed the induction of NDV-triggered release of DAMPs, revealing the contribution of autophagy to NDV-induced ICD. Together, our data indicate that the induction of ICD by NDV in lung cancer cells, to some extent, relies on autophagy. In this regard, strategies targeting autophagy may modulate NDV-triggered ICD and thereby affect the NDV-mediated immune response in lung cancer.
In conclusion, our data show that oncolytic NDV infection of lung cancer cells triggers the release of ICD determinants including ecto-CRT, HMGB1 and HSP70/90. Furthermore, we demonstrate in this study that autophagy contributes to NDV-mediated induction of ICD. Further studies are however warranted in vivo to validate these data, which may have potential implications as NDV-based virotherapy in combination with conventional cancer treatments in the clinical setting.
Acknowledgements
This work was supported by the National Science Foundation of China (81502674 to Ke Jiang, 81372471, 81572707 and 81772973 to Songshu Meng, 31530074 to Chan Ding) and the National Science Foundation of Liaoning Province (2015020655 to Guirong Zhang). Guiding Funds for the Development of Local Science and Technology by the Central Government (2017106014 to Haozhe Piao) and Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-B04-2015 to Haozhe Piao). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21:39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aurelian L. Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco Targets Ther. 2016;9:2627–2637. doi: 10.2147/OTT.S63049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo ZS, Liu Z, Kowalsky S, Feist M, Kalinski P, Lu B, Storkus WJ, Bartlett DL. Oncolytic immunotherapy: conceptual evolution, current strategies, and future perspectives. Front Immunol. 2017;8:555. doi: 10.3389/fimmu.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridle BW, Boudreau JE, Lichty BD, Brunelliere J, Stephenson K, Koshy S, Bramson JL, Wan Y. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, Bramante S, Parviainen S, Kanerva A, Loskog AS, Eliopoulos AG, Pesonen S, Hemminki A. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 7.Grekova SP, Raykov Z, Zawatzky R, Rommelaere J, Koch U. Activation of a glioma-specific immune response by oncolytic parvovirus minute virus of mice infection. Cancer Gene Ther. 2012;19:468–475. doi: 10.1038/cgt.2012.20. [DOI] [PubMed] [Google Scholar]
- 8.Sieben M, Schafer P, Dinsart C, Galle PR, Moehler M. Activation of the human immune system via toll-like receptors by the oncolytic parvovirus H-1. Int J Cancer. 2013;132:2548–2556. doi: 10.1002/ijc.27938. [DOI] [PubMed] [Google Scholar]
- 9.Workenhe ST, Simmons G, Pol JG, Lichty BD, Halford WP, Mossman KL. Immunogenic HSV-mediated oncolysis shapes the antitumor immune response and contributes to therapeutic efficacy. Mol Ther. 2014;22:123–131. doi: 10.1038/mt.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liikanen I, Koski A, Merisalo-Soikkeli M, Hemminki O, Oksanen M, Kairemo K, Joensuu T, Kanerva A, Hemminki A. Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology. 2015;4:e989771. doi: 10.4161/2162402X.2014.989771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly OG, Errington-Mais F, Steele L, Hadac E, Jennings V, Scott K, Peach H, Phillips RM, Bond J, Pandha H, Harrington K, Vile R, Russell S, Selby P, Melcher AA. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20:7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollino D, Colunga A, Li B, Aurelian L. DeltaPK oncolytic activity includes modulation of the tumour cell milieu. J Gen Virol. 2016;97:496–508. doi: 10.1099/jgv.0.000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schirrmacher V. Fifty years of clinical application of newcastle disease virus: time to celebrate! Biomedicines. 2016;4 doi: 10.3390/biomedicines4030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schirrmacher V. Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance. Expert Opin Biol Ther. 2015;15:1757–1771. doi: 10.1517/14712598.2015.1088000. [DOI] [PubMed] [Google Scholar]
- 16.Cuadrado-Castano S, Sanchez-Aparicio MT, Garcia-Sastre A, Villar E. The therapeutic effect of death: Newcastle disease virus and its antitumor potential. Virus Res. 2015;209:56–66. doi: 10.1016/j.virusres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra232. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tayeb S, Zakay-Rones Z, Panet A. Therapeutic potential of oncolytic Newcastle disease virus: a critical review. Oncolytic Virother. 2015;4:49–62. doi: 10.2147/OV.S78600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, Boon L, De Vleeschouwer S, Agostinis P, Graf N, Van Gool SW. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer. 2015;136:E313–325. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 20.Jiang K, Li Y, Zhu Q, Xu J, Wang Y, Deng W, Liu Q, Zhang G, Meng S. Pharmacological modulation of autophagy enhances Newcastle disease virus-mediated oncolysis in drug-resistant lung cancer cells. BMC Cancer. 2014;14:551. doi: 10.1186/1471-2407-14-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng S, Zhou Z, Chen F, Kong X, Liu H, Jiang K, Liu W, Hu M, Zhang X, Ding C, Wu Y. Newcastle disease virus induces apoptosis in cisplatin-resistant human lung adenocarcinoma A549 cells in vitro and in vivo. Cancer Lett. 2012;317:56–64. doi: 10.1016/j.canlet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Bian J, Wang K, Kong X, Liu H, Chen F, Hu M, Zhang X, Jiao X, Ge B, Wu Y, Meng S. Caspase-and p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells by Newcastle disease virus. Arch Virol. 2011;156:1335–1344. doi: 10.1007/s00705-011-0987-y. [DOI] [PubMed] [Google Scholar]
- 23.Jiang K, Liu M, Lin G, Mao B, Cheng W, Liu H, Gal J, Zhu H, Yuan Z, Deng W, Liu Q, Gong P, Bi X, Meng S. Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death. Oncotarget. 2016;7:25652–25667. doi: 10.18632/oncotarget.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Forveille S, Sauvat A, Yamazaki T, Senovilla L, Ma Y, Liu P, Yang H, Bezu L, Muller K, Zitvogel L, Rekdal O, Kepp O, Kroemer G. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016;7:e2134. doi: 10.1038/cddis.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng S, Gui Q, Xu Q, Lu K, Jiao X, Fan J, Ge B, Ke Y, Zhang S, Wu J, Wang C. Association of Shp2 with phosphorylated IL-22R1 is required for interleukin-22-induced MAP kinase activation. J Mol Cell Biol. 2010;2:223–230. doi: 10.1093/jmcb/mjq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Zhang X, Wang X, Wang L, Hu S, Liu X, Meng S. Apoptin enhances the oncolytic properties of Newcastle disease virus. Intervirology. 2012;55:276–286. doi: 10.1159/000328325. [DOI] [PubMed] [Google Scholar]
- 27.Wei D, Sun N, Nan G, Wang Y, Liu HQ, Peeters B, Chen ZN, Bian H. Construction of recombinant Newcastle disease virus Italien strain for oncolytic virotherapy of tumors. Hum Gene Ther. 2012;23:700–710. doi: 10.1089/hum.2011.207. [DOI] [PubMed] [Google Scholar]
- 28.Hu L, Sun S, Wang T, Li Y, Jiang K, Lin G, Ma Y, Barr MP, Song F, Zhang G, Meng S. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am J Cancer Res. 2015;5:3612–3623. [PMC free article] [PubMed] [Google Scholar]
- 29.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 30.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, Kroemer G. Screening of novel immunogenic cell death inducers within the NCI Mechanistic Diversity Set. Oncoimmunology. 2014;3:e28473. doi: 10.4161/onci.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng C, Zhou Z, Jiang K, Yu S, Jia L, Wu Y, Liu Y, Meng S, Ding C. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch Virol. 2012;157:1011–1018. doi: 10.1007/s00705-012-1270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Yu S, Ding N, Meng C, Meng S, Zhang S, Zhan Y, Qiu X, Tan L, Chen H, Song C, Ding C. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J Virol. 2014;88:525–537. doi: 10.1128/JVI.01849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng G, Xia M, Wang D, Chen A, Wang Y, Wang H, Yu D, Wei J. Mitophagy promotes replication of oncolytic Newcastle disease virus by blocking intrinsic apoptosis in lung cancer cells. Oncotarget. 2014;5:6365–6374. doi: 10.18632/oncotarget.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang Y, Yuan R, Xiang B, Zhao X, Gao P, Dai X, Liao M, Ren T. Newcastle disease virus-induced autophagy mediates antiapoptotic signaling responses in vitro and in vivo. Oncotarget. 2017;8:73981–73993. doi: 10.18632/oncotarget.18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Martins I, Ma Y, Kepp O, Galluzzi L, Kroemer G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy. 2013;9:1624–1625. doi: 10.4161/auto.25873. [DOI] [PubMed] [Google Scholar]
- 37.Garg AD, Dudek AM, Agostinis P. Autophagy-dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. Oncoimmunology. 2013;2:e26260. doi: 10.4161/onci.26260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandelbaum J, Rollins N, Shah P, Bowman D, Lee JY, Tayber O, Bernard H, LeRoy P, Li P, Koenig E, Brownell JE, D’Amore N. Identification of a lung cancer cell line deficient in atg7-dependent autophagy. Autophagy. 2015 doi: 10.1080/15548627.2015.1056966. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto S, Inoue H, Nakamura T, Yamada M, Sakamoto C, Urata Y, Okazaki T, Marumoto T, Takahashi A, Takayama K, Nakanishi Y, Shimizu H, Tani K. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 2012;72:2609–2621. doi: 10.1158/0008-5472.CAN-11-3185. [DOI] [PubMed] [Google Scholar]
- 40.Takasu A, Masui A, Hamada M, Imai T, Iwai S, Yura Y. Immunogenic cell death by oncolytic herpes simplex virus type 1 in squamous cell carcinoma cells. Cancer Gene Ther. 2016;23:107–113. doi: 10.1038/cgt.2016.8. [DOI] [PubMed] [Google Scholar]
- 41.Angelova AL, Grekova SP, Heller A, Kuhlmann O, Soyka E, Giese T, Aprahamian M, Bour G, Ruffer S, Cziepluch C, Daeffler L, Rommelaere J, Werner J, Raykov Z, Giese NA. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J Virol. 2014;88:5263–5276. doi: 10.1128/JVI.03688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinrich B, Klein J, Delic M, Goepfert K, Engel V, Geberzahn L, Lusky M, Erbs P, Preville X, Moehler M. Immunogenicity of oncolytic vaccinia viruses JX-GFP and TG6002 in a human melanoma in vitro model: studying immunogenic cell death, dendritic cell maturation and interaction with cytotoxic T lymphocytes. Onco Targets Ther. 2017;10:2389–2401. doi: 10.2147/OTT.S126320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, Bracci L, Breckpot K, Brough D, Buque A, Castro MG, Cirone M, Colombo MI, Cremer I, Demaria S, Dini L, Eliopoulos AG, Faggioni A, Formenti SC, Fucikova J, Gabriele L, Gaipl US, Galon J, Garg A, Ghiringhelli F, Giese NA, Guo ZS, Hemminki A, Herrmann M, Hodge JW, Holdenrieder S, Honeychurch J, Hu HM, Huang X, Illidge TM, Kono K, Korbelik M, Krysko DV, Loi S, Lowenstein PR, Lugli E, Ma Y, Madeo F, Manfredi AA, Martins I, Mavilio D, Menger L, Merendino N, Michaud M, Mignot G, Mossman KL, Multhoff G, Oehler R, Palombo F, Panaretakis T, Pol J, Proietti E, Ricci JE, Riganti C, Rovere-Querini P, Rubartelli A, Sistigu A, Smyth MJ, Sonnemann J, Spisek R, Stagg J, Sukkurwala AQ, Tartour E, Thorburn A, Thorne SH, Vandenabeele P, Velotti F, Workenhe ST, Yang H, Zong WX, Zitvogel L, Kroemer G, Galluzzi L. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwaiger T, Knittler MR, Grund C, Roemer-Oberdoerfer A, Kapp JF, Lerch MM, Mettenleiter TC, Mayerle J, Blohm U. Newcastle disease virus mediates pancreatic tumor rejection via NK cell activation and prevents cancer relapse by prompting adaptive immunity. Int J Cancer. 2017;141:2505–2516. doi: 10.1002/ijc.31026. [DOI] [PubMed] [Google Scholar]
- 45.Zamarin D, Ricca JM, Sadekova S, Oseledchyk A, Yu Y, Blumenschein WM, Wong J, Gigoux M, Merghoub T, Wolchok JD. PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy. J Clin Invest. 2018;128:1413–1428. doi: 10.1172/JCI98047. [DOI] [PMC free article] [PubMed] [Google Scholar]