Abstract
Different types of cancers exhibit disparate spectra of genomic alterations (germline and/or somatic). These alterations can include single nucleotide variants (SNVs), copy number alterations (CNAs) or structural changes (e.g. gene fusions and chromosomal rearrangements). Identification of those genomic alterations has provided the opportune element to derive new strategies for molecular-based precision medicine of adult and pediatric cancers including risk assessment, non-invasive detection, molecular diagnosis and personalized therapy. Moreover, it is now becoming clear that the spectra of genomic-based alterations and mechanisms in pediatric malignancies are different from those predominantly occurring in adult cancer. Adult cancers on average exhibit substantially higher mutational burdens compared with the vast majority of childhood tumors. Accumulating evidence also suggests that the type of genomic alterations frequently encountered in adult cancers is different from those observed in pediatric malignancies. In this review, we discuss the state of knowledge on adult and pediatric cancer genomes (or “mutatomes”), specifically focusing on solid tumors. We present an overview of mutational signatures and processes in cancer as well as comprehensively compare and contrast the diverse spectra of genomic alterations (somatic and familial) among major adult and pediatric solid tumors. The review also discusses the role of genomics in molecular-based precision medicine of adult and pediatric solid malignancies as well as comprehending resistance mechanisms to various targeted therapies. In addition, we present a perspective that discusses upon emerging concepts in cancer genomics including intratumoral heterogeneity, the precancer (premalignant) genome as well as the interface between the host immune response and tumor genome - immunogenomics - as they relate to adult and pediatric tumors.
Keywords: Cancer genomics, driver mutations, mutation signatures, mutation spectra, precision medicine, genomic medicine
Introduction
With the advent of massively parallel sequencing technology (or next-generation sequencing/NGS), we now know that different types of cancers exhibit disparate spectra of genomic alterations (germline and/or somatic) and, thus, mutational profiles [1]. These alterations can include single nucleotide variants (SNVs), copy number alterations (CNAs) or structural changes (e.g. gene fusions and chromosomal rearrangements) [2]. These genetic/genomic changes, whether clonal or subclonal, are thought to confer a selective advantage to malignant cells - analogous to natural selection phenomena posited earlier in Darwinian evolution [3]. Specific cancers, particularly those of the skin and lung, are driven by carcinogenic exposures (e.g., ultraviolet radiation and cigarette smoke) and display elevated mutational burdens and unique spectra of base substitutions [4]. Also, different malignancies, or even subtypes of particular cancers, exhibit an overabundance of alterations in disparate genes that are thought to contribute to cancer initiation, termed drivers [5]. Understanding the spectra of these genomic changes, particularly genomic drivers, is crucial to discern the pathogenesis of cancer. Importantly, identification of genomic alterations has provided the opportune element to derive new targeted strategies for the clinical management of adult and pediatric cancers - referred to as genomic (or precision) medicine - including risk assessment, non-invasive detection, molecular diagnosis and personalized therapy [6].
It has been suggested that pediatric malignancies often manifest in precursor cells of non-self-renewing tissues as compared to cells-of-origin of adult tumors such as those of the gastrointestinal tract and skin, thus arising in a precursor cell that has accumulated a lower number of mutations [5]. In this context, it is thought that the spectra of genomic-based alterations and mechanisms in pediatric malignancies are different from those predominantly occurring in adult cancer [7]. Epithelial cancers, which primarily manifest in adults, are hypothesized to arise after accumulation of multiple sequential mutations directly linked to environmental exposures, and arise within differentiated adult tissues [5]. Mesenchymal tumors - such as sarcomas - occur in both pediatric and adult age groups, but specific histologic subtypes and clinical behavior are also age-dependent, suggesting differential pathogenetic and underlying molecular mechanisms for tumor initiation and progression in the different age groups [8]. The paucity of epithelial cancers in childhood underscores the importance of accumulating environmental insults in epithelial tumor initiation, and suggests an alternative etiologic mechanism for childhood tumors. Indeed, the peak incidence rates of many types of childhood solid tumors have been noted to correspond to the maturation/differentiation stage of the underlying organ of origin, favoring a developmental model for childhood cancer initiation [9-11]. As such, immature cells that undergo substantial expansion during early organ formation, growth and maturation, acquire a deleterious mutation in genes that are both important for cell cycle arrest as well as organ differentiation at a particular developmental stage [9,12-14]. These premises suggest that pathogenomic mechanisms among solid adult and pediatric malignancies are likely very different.
In this review, we overall compare and contrast genomes (or “mutatomes”) of adult and pediatric malignancies with a focus on solid tumors. We discuss mutational signatures and processes in cancer as well as comprehensively compare and contrast the diverse spectra of genomic alterations (somatic and familial) among major adult and pediatric solid tumors. The review also discusses the role of genomics in molecular-based precision medicine of adult and pediatric solid malignancies including risk assessment, diagnosis, personalized therapy, as well as comprehending resistance mechanisms to various therapies. The review’s perspective touches upon emerging concepts in cancer genomics including intratumoral heterogeneity and its impact on precision medicine, non-invasive genomics-based diagnostics, the genome of premalignant conditions (premalignant genome) as well as the interface between the host immune response and genome - immunogenomics.
Cancer genomics of adult and pediatric solid tumors
Genomic profiles of tumors, for example genome-wide mutation analyses, shed light on their molecular pathogenesis and, thus, have the potential to enhance our capacity to demarcate the origins of different types or even subtypes of malignancies. Recent studies have discerned the genomic spectra of major adult and pediatric solid tumors including specific driver alterations (SNVs, CNAs and structural variation), and work has begun on the application of genomics in targeted and precision medicine.
Mutation signatures
Studies have shown that genomic landscapes, mutational loads (or mutation burdens) and mutational signatures inform of the pathobiology of different malignancies [5]. Perhaps the most striking finding across genomic studies has been the lack of a high mutation burden in the vast majority of childhood tumors, with very few exceptions [15]. Studies have quantified mutation burden in many pediatric cancers to be in the range of 5-10 protein-coding variants per tumor across multiple tumor types [5,15]. Osteosarcoma is an exception, with an average of 25 protein-affecting mutations per genome, which is significantly higher than the majority of other childhood cancers [16,17]. However, even that remains markedly less than the number of protein-affecting mutations seen in most adult cancers, including melanoma, lung, and colon cancer. For example, in adult cancers the average number of mutations ranges between 33-66 by tumor type (such as in colon, brain, breast and pancreatic cancers), increases up to 200 in mutagen-caused adult tumors (such as melanoma and lung cancer), and even to the 1000s for tumors with mismatch-repair defects [5,15].
Using Pan-cancer analysis to examine commonalities and differences among various cancer types, a recent report by Gröbner et al. showed that 47% of pediatric tumors harbor at least one significantly mutated gene, with most tumors having only one. In contrast, 93% of adult tumors harbor at least one mutation in an adult cancer-related significantly mutated gene and 76% show recurrent mutation in multiple genes. Additionally, 30% of pediatric most commonly recurrent genes overlapped with adult most frequently mutated genes. TP53 is predicted to be the most common somatically mutated gene in both pediatric and adult cancers, albeit more frequently in the latter [18].
Total mutational burdens (most notably point mutations including SNVs and indels) observed in adult cancers are the outcome of multiple mutagenic processes (e.g. smoking exposure or ultraviolet radiation exposure) that have been occurring over a long course, sometime over the lifetime of an individual [4,19]. Each process is thought to result in distinctive mutational signatures in the cancer genome [4,19]. These different genome-wide mutational signatures are demarcated by different types of base substitutions (e.g. C > A transversions and C > T transitions) that are often influenced by the type of DNA damage and DNA repair processes [4,19]. A noteworthy study by Alexandrov et al. analyzed 4,938,362 mutations from 7,042 cancers extracting more than 20 distinct mutational signatures [4]. Two signatures named 1A and 1B exhibited strong positive correlations with age in the majority of cancer types of childhood and adulthood malignancies. Both are characterized by prominence of C > T substitutions at NpCpG trinucleotides. Signature 1A/B was reasoned to be probably linked to elevated rates of spontaneous deamination of 5-methyl-cytosine resulting in C > T transitions that predominantly occur at NpCpG trinucleotides. Furthermore, analyses of mutated genes in lung and skin tumors have shown that the type of point mutations found in these genes are corroborative with the overall mutational spectra induced by tobacco carcinogens and ultraviolet (UV) radiation respectively, the major known exogenous carcinogenic stimuli that are causally linked to these two highly mutagenized cancer types [4,20,21]. Notably, C:G > A:T transversions predominate in smoking-associated lung cancer, whereas C:G > T:A transitions that occur mainly at dipyrimidines and CC:GG > TT:AA double nucleotide substitutions are more common in UV-exposed skin tumors [4]. Endogenous and intracellular mechanisms and processes (e.g. cellular metabolism and lipid peroxidation, apolipoprotein B mRNA editing enzyme catalytic polypeptide-like (APOBEC) family of deaminases) have also been implicated in the somatic acquisition of mutations in cancer [4]. It cannot be neglected that these intracellular mechanisms and exogenous carcinogenic insults (e.g., smoking) are not entirely mutually exclusive but rather related or causally linked; thus, often resulting in exacerbated mutational spectra with complex heterogeneity in adult malignancies [4,19].
Single nucleotide variants (SNVs) and small insertions/deletions (indels)
Various comprehensive genomic surveys have shown that somatically acquired genomic events particularly in adult cancers are characterized by accumulation of SNVs and indels in driver genes [22-32]. Specific malignancies, particularly those of the skin and lung driven by chronic environmental (e.g., ultraviolet radiation) and carcinogen (e.g., cigarette smoke) exposures, respectively, display elevated mutational burdens and unique spectra of base substitutions [4,19]. Melanoma comprising the highest total mutational burden among all malignancies (16.8 mutations/Mb), harbor recurrent point mutations in driver genes such as BRAF, NRAS, CDKN2A, TP53, PTEN, RAC1, MAP2K1, PPP6C, and ARID2 [33]. Moreover, point mutations in RB1, KIAA1211, COL22A1, RGS7 and FPR1 were reported in small-cell lung carcinoma (SCLC) with smokers comprising the overwhelming majority of this malignancy [29]. Of note, SCLC genomes exhibited extremely high mutation rates of 8.62 non-synonomous mutations per million base pairs (Mb) [29]. Additionally, urothelial carcinoma of the bladder harbors a large number of DNA alterations, slightly fewer than those observed in melanoma and lung cancer. Recurrent SNVs and indels were reported in TP53, TSC1, RB1, KDM6A, FGFR3, and PIK3CA among others. Of note, FGFR3 mutation is a common feature of low-grade non-invasive papillary urothelial bladder cancer [34]. Furthermore, APC, KRAS, TP53, FBXW7, PIK3CA, BRAF, SMAD4 and TCF7L2 were among the recurrently mutated genes driving the “adenoma-to-carcinoma” progression model of colorectal cancer mediated by the sequential accumulation of molecular alterations in specific genes [5,22,25,35]. As for glioblastoma, one of the most challenging cancers to treat, recurrent driver substitutions and indels were reported in SPTA1, GABRA6, KEL, CDH18, SEMA3C, COL1A2, ABCC9, NLRP5, DRD5, TCHH and SCN9A [36]. Additionally, recurrent point mutations were observed in breast cancer in driver genes such as TBX3, RUNX1, PIK3CA, MYC and FOXA1. Notably, stark differences in the frequency of those point mutations were observed between the two breast cancer subtypes (ductile adenocarcinoma and lobular carcinoma) suggesting divergent pathways in the pathogenesis of the different subtypes of breast tumors [37]. Despite having an overall simple mutational spectrum, ovarian carcinoma was shown to harbor recurrent mutations in NF1, BRCA1, BRCA2, RB1 and CDK12, in addition to TP53 mutations which is present in almost all tumors (96%) [38]. It is noteworthy that somatic point mutations are less common in prostate cancer relative to most other solid tumors [39].
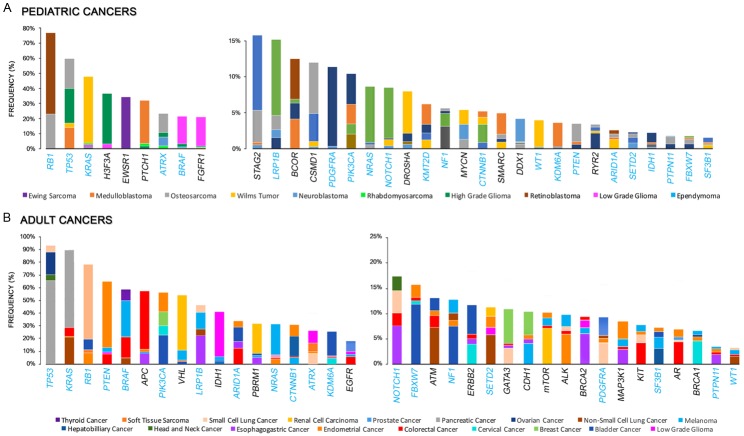
In sharp contrast, SNVs and indels are far less common in childhood malignancies. For instance, osteosarcoma exhibits a relatively high number of mutations as compared to other childhood tumors, but this rate is still much lower than numbers observed in adult tumors [16,17]. Osteosarcomas commonly harbor genetic aberrations in the p53 and RB1 genes, as well as recurrent mutations in ATRX, DLG2, and PTEN [16,17]. Furthermore, the BRAF V600E variant was recurrently noted in low-grade gliomas, such as ganglioglioma and pleomorphic xanthoastrocytoma, and may be associated with a worse outcome [40-45]. Other activating mutated genes driving low-grade glioma include variants of PTPN11, NTRK2, and FGFR1 [46-48]. Pediatric high-grade gliomas (HGG) are characterized by a higher preponderance of mutations in histones such as histone 3.3 and histone 3.1, as well as alterations in PDGF and PDGFR. Unlike adult-onset HGG, there is a low onset of PTEN or EGFR mutations in pediatric HGGs. IDH1 mutations have been described in HGG that occur in older adolescents, but not in younger children [49-53]. SMARCB1 (and less commonly SMARCA4) mutations characterize the aggressive childhood tumor atypical teratoid rhabdoid tumors (AT/RT), with paucity of any other concurrent recurring genomic abnormalities [54,55]. As for medulloblastoma, genomic studies have resulted in a novel WHO molecular classification, with 4 identified subtypes: WNT-activated, SHH-activated & TP53-mutant, SHH-activated & TP53-wildtype, and non-WNT/nonSHH subtypes [56,57]. WNT medulloblastoma is characterized by CTNNB1 mutations in the vast majority, with a minority having APC mutation instead [58]. SHH-medulloblastoma is characterized by mutations in PTCH1, PTCH2, SMO, SUFU or GLI2 and TERT promoter mutations are also common in adult-onset tumors [59]. CTNNB1 mutations are also a common driver in hepatoblastoma [60]. Novel mutations have been identified in Ewing sarcoma, such as STAG2 mutations that occur in 15-20% of cases, and are associated with metastatic tumors [61,62]. Rhabdomyosarcoma, especially the embryonal (which is the most common) subtype, is characterized by recurrent mutations in genes of the RAS pathway, as well as mutations in FGFR4, PIK3CA, BCOR, CTNNB1, and FBXW7 [16,63], whereas the vast majority of spindle cell/sclerosing rhabdomyosarcoma have a specific MYOD1 mutation p.L122R [64]. As for neuroblastoma, the most common mutations are in the ALK gene, occurring in approximately 10% of cases [65]. In addition, ATRX mutations are found in 10-20% of high-risk neuroblastoma, and are most commonly found in older children [66]. As has been long identified, both heritable and sporadic retinoblastoma are caused by mutation or deletion of RB1, with a few other rare recurring mutations recently identified [67,68]. Wilms tumor, a pediatric embryonal tumor of the kidney, has long been recognized to be associated in around 30% of cases with mutations in WT1, WTX, or CTNNB1 [69]. Recently, mutations in genes involved in miRNA processing, including DICER, DROSHA, and others, have been identified as possible drivers of a subset of Wilms tumor [70]. Other recurrent mutations have been identified, with primary function in early renal development and differentiation, such as SIX1, SIX2, among others [71], with p53 mutations occurring in anaplastic tumors [72]. Figure 1 summarizes the reported frequency of the identified drivers of different (a) pediatric and (b) adult solid tumors.
Figure 1.
Reported spectrum of significantly mutated driver genes in pediatric and adult cancers. A. Most common recurrently mutated genes in adult cancers. B. Most common recurrently mutated genes in pediatric cancers. Different types of cancers are denoted by different colors, and the frequency (%) of the mutated gene in each cancer is represented by the upper margin of the respective cancer. For each bar/mutated gene the top four tumors with recurrence of the mutated gene are depicted. Mutated genes in blue represent common recurrently mutated genes in both pediatric and adult cancers. Data for the significantly mutated genes in adult and pediatric cancer were retrieved from cBioPortal for Cancer Genomics [249,250] and St. Jude Cloud PeCan, respectively.
Copy number alterations (CNAs)
CNAs are common in both adult and pediatric cancers. In some cases, focal CNAs have led to the identification of cancer-causing genes and suggested specific therapeutic approaches [73-76]. Amplifications of ERBB2 and IGF2 have been reported to be recurrent in colorectal cancer [25]. Prostate cancers also display varying degrees of CNAs, with more aggressive primary and metastatic tumors exhibiting more extensive burdens compared with indolent and low-Gleason tumors [77-79]. Commonly amplified loci include MYC on 8q24.21 and NCOA2 amplification on 8q13.3 [39,80]. Additionally, somatic CNAs in lung adenocarcinomas include significant amplifications in NKX2-1, TERT, MDM2, MYC, KRAS, EGFR, MET, CCNE1, CCND1, TITF-1, TERC, MECOM, CDK4 and ERBB2 [31,81]. The most significant focal regions of deletions included tumor-suppressor genes such as CDKN2A and PTEN [31,81]. Furthermore, oncogene amplification (e.g., SOX2, PDGFRA and/or KIT, EGFR and FGFR1) and tumor suppressor loss (CDKN2A, FBXW7, SMARCA4, NF1 and TSC1) were reported in lung squamous cell carcinoma [82]. Copy number gain/amplification of MYC and CCND1 have been described in pancreatic ductile adenocarcinoma [83]. Of note, amplification of the 8q24 locus comprising MYC was significantly associated with poor clinical outcome [83]. Amplifications of PPARG, E2F3, EGFR, CCND1 and MDM2, as well as loss of CDKN2A and RB1 were reported in urothelial carcinoma of the bladder [34].
Similarly, recurrent CNAs including those associated with different clinical outcomes, were reported in childhood malignancies. A distinct group of pediatric high grade glioma exhibits amplifications in common adult-type glioblastoma genes such as PDGFRA, EGFR, Cyclin D/CDKs and MYC [84,85]. Moreover, amplifications in MYC have been shown to define a clinically relevant molecular subgroup of medulloblastomas, the non-WNT/non-Shh subtype or Group 3 subtype, especially in younger children, that exhibit relatively poor prognosis compared with tumors associated with NMYC and CDK6 amplifications [57,86,87]. Amplification of NMYC and OTX2 were also reported in pediatric retinoblastoma [67,68]. Of note, NMYC amplification on chromosome 17q has been long identified as the most important genomic feature influencing neuroblastoma outcome and as a marker of high-risk tumors [88,89]. Novel detection of a recurrent amplification of the C19MC region on chromosome 19, has resulted in re-classification of embryonal brain tumors, with the entity ‘embryonal tumor with multilayered rosettes, C19MC-altered’, replacing prior generic classification of primitive neuroectodermal tumors [90-92]. Rhabdomyosarcoma of embryonal histology, one of the most common soft tissue tumors in children, is characterized by LOH at 11p15 and gains on chromosome 8 [93]. LOH at 11p15 is a common finding in Wilms tumor, and is responsible for WT2 inactivation as a driver genetic lesion [94,95]. Also, gain of chromosome 1q occurs in approximately a third of Wilms tumors, and heralds a worse outcome [96,97]. Other recurrent areas of LOH in Wilms tumor include chromosomes 16q and 1p, and also correlate with patient outcome [98]. Additionally, MYCN amplification has been reported in Wilms tumor, with a higher frequency in anaplastic tumors [99].
Structural changes (gene fusions and chromosomal rearrangements)
A major characteristic of childhood cancer pathogenesis is the relatively high prevalence of specific structural variations, and the specificity of their association with histologic tumor subtypes [16,100,101]. Specific translocations leading to oncogenic fusion proteins seem to play a driver role in tumor initiation. For instance, BRAF-KIAA1549 fusion gene was identified as an important diagnostic biomarker in pilocytic astrocytoma and other low-grade gliomas, such as ganglioglioma and pleomorphic xanthoastrocytoma [102-104]. C11orf95-RELA translocations were reported in most of supratentorial ependymoma cases, characterized by low rates of coding mutations, and a relatively unfavorable outcome [105]. As a result the WHO classification currently lists RELA-positive supratentorial ependymoma as a distinct diagnostic category [90]. Many specific recurrent fusion genes are characteristic and diagnostic of pediatric sarcomas, such as EWS-associated fusion genes (EWS-FLI1, EWS-ERG, and multiple other lower-frequency partners) in Ewing sarcoma [106]. More recently, novel recurrent fusion genes were identified in tumors histopathologically diagnosed as Ewing sarcoma, but which may represent genomically-distinct entitities, including BCOR-CCNB3, CIC-DUX4, and CIC-FOX4 positive tumors [107,108]. Other fusion genes characteristic of pediatric sarcomas include PAX3-FOXO1 and PAX7-FOXO1 in alveolar rhabdomyosarcoma, NCOA2 fusion genes in spindle cell/sclerosing rhabdomyosarcoma, ETV6-NTRK3 in infantile fibrosarcoma, and YWHAE-NUTM2 in clear cell sarcoma of the kidney [101,108-110]. Osteosarcoma is an exception, which is characterized by a high rate of chromothripsis but without a characteristic specific fusion oncoprotein. Interestingly, though, non-coding translocations in osteosarcoma were found to act by interrupting the first intron of the tumor suppressor TP53 [16]. In medulloblastoma, non-coding recurrent translocations were also found to act by promoting enhancer hijacking [100]. Another exception is neuroblastoma, where chromothripsis is also identified in a subset of tumors, and there seems to be a lack of unifying driver lesions, but rather different drivers in different tumor subtypes [66,111-113]. In contrast, structural variations are less commonly identified in adult solid tumors. Structural variations in adult cancer include NAV2-TCF7L1 fusion in colorectal cancer [25], TMPRSS2-ERG fusion in prostate cancer [114], EML4-ALK, KIF5B-RET [115], ROS-SCL34a2 [116] fusion genes in non-small cell lung cancers, and FGFR3-TACC3 fusion in urothelial carcinoma of the bladder [34] among others. These structural variations in adult cancers, although not frequent, are considered major drivers and pliable therapeutic targets [117-120].
The epigenome and epigenetic modifiers
Genes involved in epigenetic regulation are driving events in a significant proportion of childhood tumors. For example, atypical teratoid rhabdoid tumor (ATRT) is an aggressive tumor characterized by biallelic loss of the SMARCB1 gene, involved in chromatin remodeling [55,121]. Interestingly, three distinctive subsets of ATRT were identified through the use of DNA methylation arrays [122]. Posterior fossa ependymoma has also been sub-classified based on methylation profile, with distinct biologic subgroups of CpG island methylator phenotype (CIMP) positive and CIMP-negative subtypes, with distinct clinical behavior, specifically with CIMP-positive tumors associated with an inferior outcome [123-125]. Chromatin remodeling is also a likely driver in other brain tumors, though by different mechanisms. Specifically, mutations in histone 3 are common in high-grade and midline gliomas [126], and are thought to drive tumorigenesis by altering the chromatin landscape and inhibiting cellular differentiation [127]. In medulloblastoma, almost half of recurrent gene mutations are in epigenetic modifiers [128,129]. Retinoblastoma is characterized with very few genetic mutations but an altered epigenome [130]. Fusion oncoproteins in alveolar rhabdomyosarcoma, Ewing sarcoma, and synovial sarcoma have also been shown to have effector functions on the epigenome [101].
On the other hand, oncogenic activating mutations in adult cancers are also now known to occur in a number of epigenetic modifiers (e.g. IDH1/2, EZH2, DNMT3A, MLL2, SMARCA4, SETD2, ARID1A, U2AF1) pinpointing epigenetic pathways that are involved in tumorigenesis [39,82,131-138]. Similarly, investigations into the role of inactivating mutations in chromatin modifiers (e.g. KDM6A, CREBBP/EP300, SMARCB1) implicate many of these genes as tumor suppressors [29,132,135,138-141]. Intriguingly, a number of neoplasms are defined by a plethora of mutations in epigenetic regulators, including renal, bladder, lung and adenoid cystic carcinomas [82,138,142-146]. Nonetheless, the fundamental processes underlying the development of tumors, particularly those arising from genetic/epigenetic interaction with environmental exposures, seem to operate across all ages covering both pediatric and adult tumors [147].
Genomics of familial cancers
Advances in cancer genetics and sequencing have helped characterize mutated cancer predisposition genes that account for 5-10% of cancers, and that manifest with a Mendelian pattern of inheritance [148]; the majority being transmitted in an autosomal dominant manner with incomplete penetrance [149]. Familial cancers are relatively rare compared with sporadic malignancies [150] and include heritable mutations. Major tumor suppressor genes implicated in common sporadic tumors are often different from those involved in familial forms [151]. The vast majority of familial cancers harbor mutations in tumor suppressor genes, including what are known as caretaker genes (genes involved in the maintenance of the genome stability and DNA repair) and gatekeeper genes (genes that inhibit cell growth or induce apoptosis). Mutations in caretaker genes were reported in hereditary breast ovarian cancer syndrome, the most common form of inherited breast cancer, caused by germline mutations in BRCA1 on 17q11 and BRCA2 on 13q12-q13 [152,153]. Whole-exome sequencing studies have revealed additional genes with variable penetrance in familial breast cancer, such as P53, PTEN, STK11, PALB2 or ATM together accounting for approximately 35% of familial cases. The majority of non-BRCA1/BRCA2 breast cancer families might be explained by the action of those moderate and/or low penetrance susceptibility alleles [154]. Additionally, about 45% to 70% of hereditary nonpolyposis colorectal cancer (HNPCC) families harbor germline mutations in one of five DNA mismatch repair genes MLH1, MSH2, MSH6, PMS1 and PMS2 [155-159]. Familial adenomatous polyposis (FAP) is a familial disease that accounts for approximately 1% of hereditary colorectal cancer. FAP is an autosomal dominant condition caused by germline mutations in the gatekeeper gene adenomatous polyposis coli (APC) and is characterized by the development of hundreds to thousands of adenomatous polyps throughout the colon and rectum, with an extremely high lifetime risk of colon cancer [160,161]. Probably the oldest recognized tumor predisposition syndrome secondary to specific germline mutations in a gatekeeper gene was reported in Li-Fraumeni syndrome (LFS), which is associated in most cases with mutations in the TP53 tumor suppressor gene [162]. LFS shows phenotypic heterogeneity exemplified by various cancers including sarcomas, breast cancer, brain tumors, adrenocortical carcinoma and acute leukemias, occurring at a young age and frequently in childhood [163-166]. Another tumor predisposition syndrome that has long been characterized is heritable retinoblastoma. In children with retinoblastoma, approximately 25% have familial predisposition that manifests as bilateral multifocal retinal tumors, with 90% of such patients diagnosed before the age of 5 years. Approximately 85% of these patients carry an identifiable germline mutation in RB1 and are at high risk of developing second malignancies, especially sarcomas, embryonal brain tumors, and melanoma. They also have a higher incidence of common epithelial cancers in adulthood such as breast and colon cancer [167].
More recent insights have occurred in other types of familial cancers due to genome-wide analyses. For example, complete sequencing of protein-coding genes in patients with familial pancreatic cancer has identified PALB2 as a susceptibility gene [168]. In malignant melanoma, approximately 3-15% of all cases are familial [169]. Melanoma kindreds demonstrated genetic linkage to chromosome 1p as well as 9p12-22, [170-172] suggesting genetic heterogeneity. Candidate gene surveys identified the gatekeeper gene CDKN2A, which encodes the cell cycle and cyclin-dependent kinase inhibitor p16, as a likely candidate for the melanoma predisposing gene in most 9p21-linked families [172]. Exome (168 samples) and whole-genome (16 samples) sequencing of familial melanoma cases by Robles-Espinoza et al. suggested that loss-of-function variants in the Protection of Telomeres 1 gene (POT1) predispose to melanoma formation via a direct effect on telomeres [173]. Additionally, pleuropulmonary blastoma, a rare pediatric lung cancer of embryonal origin, has been associated with germline DICER1 mutations [174].
Importantly, the recent genomic analyses on childhood tumors have identified a prevalence of predisposition to cancer in approximately 10-15% of all pediatric cancer cases [175-177]. The characterization of these cases has led to new recommendations regarding genetic screening of individuals at risk, and is expected to continue to evolve as new findings are uncovered by continued genomic analyses of rarer tumor subtypes and more patient numbers [178,179]. In fact, strategies for cancer surveillance and prevention have recently started to be developed and are currently being incorporated into oncologic practice [180-184].
Precsion medicine of pediatric and adult cancer
It is increasingly being ascertained that each tumor comprises its own set of mutational changes - both genetic and somatic. Understanding these changes provides opportune windows for more effective and personalized treatments tailored to mutational profiles of each cancer patient. The Precision Medicine Initiative defines precision medicine as an emerging approach for disease prevention and treatment that takes into account individuals’ variability in environment, lifestyle and genes [185,186]. In this section of the review, we summarize the application of genomics in precision medicine of adult and pediatric malignancies.
Genomics in risk stratification and therapy selection
Elucidating the disparate spectra of genomic alterations in cancers has eased the identification of specific genomic subsets of particular tumors, which allowed the association of powerful prognostic features that are being translated into therapy reduction (for good-risk patients) or intensification (for high-risk patients). This was possibly the highest area of direct impact of recent genomic studies in childhood cancers. Examples are in medulloblastoma, where the WNT pathway subtype has been identified to have a particularly good prognosis, and therefore these patients will receive less intensive therapy in ongoing prospective clinical trials, thus potentially diminishing short- and long-term side effects of treatment [187-189]. Other risk stratification criteria based on the genomic alterations discussed in the above sections for multiple tumor types including ependymoma, glioma, among others, are expected to be refined and incorporated into future clinical trials.
Similarly, colorectal cancer subtypes in adults, defined by proposed etiologic pathways (MSI, CIMP, BRAF-mutation, and KRAS-mutation status), were shown to be associated with marked differences in survival. For instance, patients with MSI-high subtypes of disease had the most favorable survival, whereas those with MSS/MSI-low, CIMP-positive, BRAF-mutated, KRAS-mutation-negative had the highest mortality [190]. Furthermore, four clinically relevant molecular subtypes linked to distinct patterns of molecular alterations, disease progression and prognosis, were identified for gastric cancer. The worst prognosis was associated with the mesenchymal-like subtype which has the highest recurrence frequency (63%) among the four subtypes. Microsatellite-unstable tumors were shown to have the best overall prognosis and the lowest frequency of recurrence (22%) compared with the tumor protein 53 (TP53)-active and TP53-inactive types which include patients with intermediate prognosis and recurrence rates, with the TP53-active group showing better prognosis.
In this context, a system named PRECOG (Prediction of Clinical Outcomes from Genomic Profiles) has been established for querying associations between genomic profiles and cancer outcomes. PRECOG encompasses 166 cancer expression data sets, including overall survival data for ~18,000 patients diagnosed with 39 distinct malignancies. Using this resource, Gentles, A. J., et al. has identified a forkhead box MI (FOXM1) regulatory network as a major predictor of adverse outcomes in many tumors. In contrast, the expression of KLRB1 (encoding CD161), reflects favorable prognosis [191]. More advances are expected in this area as more population genomes are being sequenced and as data sharing among scientists becomes more common and organized.
Personalized therapy
Targeted therapy
Pediatric applications in genomic precision medicine are best demonstrated by the multiple ongoing genomically-driven clinical trials in childhood tumors [176,192-201]. In addition to providing important insight into tumor characterization and biology, these studies have to date demonstrated that approximately 30-40% of cases have ‘actionable’ mutations. Importantly, in a small subset of pediatric solid tumors, genetic findings have resulted in direct therapeutic applications by offering a target for treatment, with resulting improvements in patient outcome. Examples are targeting ALK, which has shown success in treatment of childhood inflammatory myofibroblastic tumors [202] and anaplastic large cell lymphoma (ALCL), but less so in neuroblastoma due to mutation-specific structural variants in the ALK kinase domain that confer resistance to currently available ALK inhibitors [203]. Other promising targets for which pharmacologic agents are already available and which are currently in clinical trials include BRAF in a subset of gliomas [204], hedgehog pathway alteration in a subset of medulloblastoma [205], and recently TRK inhibition in infantile fibrosarcoma, a tumor characterized by a fusion protein that activates NTRK [206].
In contrast, targeted therapy has been more frequently applied in clinical management of adult solid cancers. Imatinib, which was the first selective tyrosine-kinase inhibitor to be approved for the treatment of leukemia, is used as neoadjuvant (preoperative) and adjuvant (postoperative) therapy for patients with gastrointestinal stromal tumors harboring mutations in the KIT proto-oncogene [207]. Crizotinib, a tyrosine kinase inhibitor, is approved for treatment of most anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancers (NSCLCs) which proved to be highly responsive to treatment. Treatment with tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) (e.g., erlotinib, gefitinib) is now the standard-of-care for lung adenocarcinomas with activating mutations (e.g. L858R) in EGFR, particularly non-smoker patients or those of East Asian ethnicity [208]. Also, trastuzumab (Herceptin) is approved as first-line treatment of a significant fraction (~25-30%) of breast cancers, those with amplification of the ERBB2 oncogene [209]. Moreover, CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) are recommended for hormone-positive breast cancer [210]. Vemurafenib, a BRAF enzyme inhibitor, is employed for treatment of BRAF mutant late-stage melanomas [211]. Table 1 summarizes notable targeted therapy strategies along with their respective targets that are currently approved for the treatment of adult cancers.
Table 1.
Summary of currently approved targeted therapy for adult cancers and their respective targets
| Target Gene | Drug | Type of cancer |
|---|---|---|
| C-Kit | Imatinib | Gastrointestinal stromal cancers |
| EGFR | Erlotinib | Non-small cell lung carcinoma (NSCLC) |
| Gefitinib | Non-small cell lung carcinoma (NSCLC) | |
| Panitumumab/cetuximab | Colorectal cancer (CRC) | |
| EML4-ALK | Crizotinib | Non-small cell lung carcinoma (NSCLC) |
| BRAF | Vemurafenib | Melanoma |
| Sorafenib | Renal cell carcinoma | |
| Hepatocellular carcinoma | ||
| Thyroid carcinoma | ||
| MEK | Trametinib | Melanoma |
| Non-small cell lung carcinoma (NSCLC) | ||
| HER2 | Trastuzumab | Breast cancer |
| Gastric cancer | ||
| Esophageal cancer | ||
| CDK4/6 | Palbociclib | Breast cancer |
Resistance and relapse
Cancer relapse and drug resistance continue to be a major impediment in medical oncology. Recurrent tumors are often phenotypically very different from their primary counterparts, representing the end product of in vivo selection that is often associated with acquired genomic alterations [212-214]. Whole-genome sequencing of relapsed breast cancer showed that relapsed tumors acquired driver mutations not seen in primary tumors. For instance, samples from relapses harbored a higher number of driver point mutations on average than those in the primary tumor, these include a number of clinically actionable alterations and mutations inactivating SWI-SNF, such as ARID1A, ARID1B and ARID2. Of note, those genes were also acquired in metastatic endometrial cancer [215], and hepatocellular carcinoma [216]. Additionally, JAK2 and STAT3 were also shown as significantly mutated in the relapse tumors. Many of those newly acquired mutations are driven by selective pressure exerted by therapeutic interventions such as ARID1A and ARID2 after taxane chemotherapy, and amplifications in MDM4, FGFR1, and CCND1 after endocrine therapy [217]. Similarly, acquired BRAF V600E mutation was speculated as a potential mechanism of resistance after Osimertinib treatment in non-small-cell lung carcinomas [218]. Studies in limited numbers of the childhood tumor neuroblastoma have shown occurrence of mutations and structural alterations activating the RAS/MAPK pathway in the majority of tumors at relapse, suggesting a central role for this pathway in disease reactivation. Other mutations identified included those affecting ALK and CHD5 genes [219]. However, studying the genomic alterations in pediatric solid tumors at relapse has been relatively slow because of the paucity of biopsies and the relative rarity of individual tumor subtypes [220]. Recent efforts within pediatric oncology have been focusing on prospective studies that incorporate tumor biopsy at relapse, to better understand tumor biology and enable further progress in understanding tumor progression and therapeutic targeting [221].
Perspective
Studies have shown that various cancers when diagnosed at earlier stages (e.g. stages I or II) exhibit significantly improved outcomes (e.g. following definitive treatment or surgery) compared to when the disease is diagnosed at more advanced (e.g. metastatic) stages [222,223]. These data suggest that early detection and intervention are likely to be effective means for reducing morbidity and mortality of cancer. It is conceivable that development of non-invasive molecular methods or assays (liquid biopsies) can impact early detection (particularly for early-stage disease) and possibly clinical outcome [224]. Circulating tumor cells (CTC) and circulating tumor DNA spread in blood and/or lymphatic vessels from solid tumors. These CTCs can remain loose in circulation, cluster together as they travel, or lodge themselves in new tissues [225]. Numerous studies have shown that CTCs and ctDNA may be used as a marker to predict disease progression and survival in metastatic [226,227] and possibly even in early-stage cancer patients [228]. However, there has been very little work performed to date on studying circulating tumor cells and their markers for understanding tumor biology in childhood cancers.
Immune-based cancer therapies have come to the forefront of targeted therapeutic strategies for the clinical management of cancer, mostly adult malignancies [229]. Immune-based cancer therapies are already revolutionizing the management of several types of intractable cancers [230]. Two main immunotherapeutic approaches (i.e., checkpoint inhibition and cellular therapy with autologous (‘self’) chimeric antigen receptor T cells (CAR T cells)), currently show undeniable evidence of efficacy in several cancer types, and promise yet more rapid progress as they are refined and combined with existing conventional therapies and with each other [230,231]. The remarkable success of immune checkpoint blockade, including in non-small cell lung cancer [232], advanced melanoma [233] and renal cancer [234], pinpoints that many cancers are actually spontaneously immunogenic (i.e. can be detected by the immune system), but the immune response is inhibited by factors in the tumor microenvironment [235,236]. In fact, it has been demonstrated that patients’ sensitivity to immune checkpoint inhibitors is influenced by mutations that lead to “neoantigens” [237]. In a landmark study, Rizvi and colleagues demonstrated that increased somatic mutational burden in non-small cell lung cancer is associated with elevated sensitivity to PD-1 checkpoint blockade [238]. Additionally, McGranahan and colleagues found that a high burden of clonal tumor neoantigens (shared by all tumor cells) is indicative of better patient survival, increased infiltration by lymphocytes, and a more durable response to immunotherapy [239]. In contrast, patients with a high subclonal neoantigen fraction (present in only a fraction of cells) showed little response to PD-1 blockade [239]. Moreover, it has been shown that tumor immunogenicity differs greatly between different types of cancer and cancers of the same type in different individuals [236]. Consequently, it will be crucial to design therapeutic interventions with T-cell reactivity selectively enhanced against tumor-specific clonal neoantigens [240,241]. In contrast, studies probing neoantigen landscapes of pediatric tumors are scarce compared with their adult counterparts. One study characterized the neo-antigen repertoire of 23 types of pediatric cancers (540 childhood cancer genomes) by whole-genome sequencing [242]. Owing to the low mutation rate in pediatric cancers compared to adult tumors, it is conceivable that the number of predicted neoantigens in pediatric malignancies is lower than that reported in adult cancers [242]. Notwithstanding, the neoantigen repertoire is ideal for the development of novel immunotherapeutic modalities for the treatment of pediatric tumors, including tumor vaccines and adoptively transferred tumor-reactive T cells. Alternatively, such neoantigens will help in defining the subset of children who will mostly benefit from immune checkpoint inhibitors.
Molecular-based personalized therapeutic strategies, be it targeted anti-cancer drugs or immune-based cancer therapy, must take into account the heterogeneity observed within a tumor. In addition to the extensive heterogeneity between individual tumors demonstrated by large-scale sequencing analyses of solid cancers [243], genomic intra-tumor heterogeneity (ITH) has also been shown to be a common attribute of several adult malignancies [244]. Furthermore, studies comparing mutational profiles of primary tumors and their corresponding metastatic lesions [214,245] or local recurrences [213,217] displayed ITH at the nucleotide level. Moreover, ITH associated with heterogeneous protein function, may promote tumor adaptation and therapeutic failure through Darwinian selection [244]. Thus, intra-tumor heterogeneity may have important consequences for personalized-medicine approaches that commonly portray mutational landscapes of the tumor by relying on single tumor-biopsy [243]. It is noteworthy that therapy itself may be a source of acquired ITH. For example, temozolomide treatment has been shown to leave a signature in the cancer genome manifested as an elevated rate of C > T transitions, mainly at CpC and CpT sites [4]. Furthermore, therapy-induced mutations may enhance total neoantigen burden, but without eliciting an effective anti-cancer response to immunotherapy, probably because of the subclonal nature of these neoantigens [239]. Thus, the identification of cytotoxic tumor-infiltrating T cells that recognize specifically clonal mutations, shared by all tumor cells, might hold promise for adoptive therapy strategies to address the challenges of ITH. It is also largely unknown whether the high degree of genomic ITH observed in major adult cancers is also present in tumors from infants and children, given the short period of time during which pediatric tumors develop. Remarkably, one study has identified intratumoral diversity in all pediatric patients analyzed after chemotherapy including those with neuroblastoma, nephroblastoma and rhabdoid tumors [246]. Thus, this present study of clonal evolution under chemotherapy does not support the notion of childhood cancers being overall genetically stable [246]. Yet, more studies are still needed to further elucidate ITH in pediatric cancers.
With all the complexity and heterogeneity seen in cancer, it has been suggested that prevention remains the best “cure” [5]. Yet, this is only possible when we acquire a deep understanding of premalignant biology. Unlike the extensive efforts put to profile advanced stage tumors, studies profiling genomic alterations in precancerous tissues are very rare. Histologic changes preceding the development of invasive carcinoma characterize premalignant lesions [247]. These lesions are often identified in biopsies from patients with a suspected tumor, from samples during preventive screening, or from neighboring regions of an invasive cancer [248]. Identifying the molecular alterations in precancerous tissues and elucidating the associated changes in the microenvironment would facilitate the development of biomarkers for the early detection of cancer. In addition, such studies would hasten the development of preventive measures to delay or reverse the development of tumors [248]. Joshua et al. have made the call for a new collaborative initiative analogous to “The Cancer Genome Atlas”, entitled the “Pre-Cancer Genome Atlas (PCGA)”. This initiative aims to comprehensively profile the genomics of premalignant lesions and their associated field of injury, combined with clinical data including histology and outcome (progression/regression) [248]. It is believed that such an initiative would help elucidate the sequence of genomic events characterizing the progression of precancerous lesions to malignant ones [248]. Hence, our ability to predict which lesions are at higher risk of progression to invasive tumors would be greatly improved, allowing for the development of novel targeted early interventional and therapeutic strategies [248]. However, questions are raised on the feasibility of such a “Pre-Cancer Genome Atlas” for pediatric tumors due to the paucity of epithelial cancers (hypothesized to arise from accumulating environmental insults), and the different etiologic mechanism for childhood tumors.
Acknowledgements
Supported in part by American University of Beirut Medical physician practice (MPP) grants to HK and RS.
Disclosure of conflict of interest
None.
References
- 1.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11:685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 2.Ding L, Wendl MC, Koboldt DC, Mardis ER. Analysis of next-generation genomic data in cancer: accomplishments and challenges. Hum Mol Genet. 2010;19:R188–196. doi: 10.1093/hmg/ddq391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernards RA, Weinberg RA. Metastasis genes: a progression puzzle. Nature. 2002;418:823–823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børesen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van ‘t Veer L, Vincent-Salomon A, Waddell N, Yates LR Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain. Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. . Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa H, Fujita M. Whole genome sequencing analysis for cancer genomics and precision medicine. Cancer Sci. 2018;109:513–522. doi: 10.1111/cas.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downing JR, Wilson RK, Zhang J, Mardis ER, Pui CH, Ding L, Ley TJ, Evans WE. The pediatric cancer genome project. Nature Genetics. 2012;44:619. doi: 10.1038/ng.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain S, Xu R, Prieto VG, Lee P. Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol. 2010;3:416–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Pappo A, Dyer MA. Pediatric solid tumor genomics and developmental pliancy. Oncogene. 2015;34:5207–5215. doi: 10.1038/onc.2014.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018;2018:9059382. doi: 10.1155/2018/9059382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker SJ, Ellison DW, Gutmann DH. Pediatric gliomas as neurodevelopmental disorders. Glia. 2016;64:879–895. doi: 10.1002/glia.22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puisieux A, Pommier RM, Morel AP, Lavial F. Cellular pliancy and the multistep process of tumorigenesis. Cancer Cell. 2018;33:164–172. doi: 10.1016/j.ccell.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Sanjuan-Pla A, Bueno C, Prieto C, Acha P, Stam RW, Marschalek R, Menédez P. Revisiting the biology of infant t(4;11)/MLL-AF4(+) B-cell acute lymphoblastic leukemia. Blood. 2015;126:2676–2685. doi: 10.1182/blood-2015-09-667378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan P, Leavey PJ, Galindo RL. PAX genes in childhood oncogenesis: developmental biology gone awry? Oncogene. 2014;34:2681. doi: 10.1038/onc.2014.209. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, Parker M, Rusch M, Nagahawatte P, Wu J, Mao S, Boggs K, Mulder H, Yergeau D, Lu C, Ding L, Edmonson M, Qu C, Wang J, Li Y, Navid F, Daw NC, Mardis ER, Wilson RK, Downing JR, Zhang J, Dyer MA. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7:104–112. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS, Helman E, Taylor-Weiner A, McKenna A, DeLuca DS, Lawrence MS, Ambrogio L, Sougnez C, Sivachenko A, Walensky LD, Wagle N, Mora J, de Torres C, Lavarino C, Dos Santos Aguiar S, Yunes JA, Brandalise SR, Mercado-Celis GE, Melendez-Zajgla J, Cardenas-Cardos R, Velasco-Hidalgo L, Roberts CW, Garraway LA, Rodriguez-Galindo C, Gabriel SB, Lander ES, Golub TR, Orkin SH, Getz G, Janeway KA. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A. 2014;111:E5564–5573. doi: 10.1073/pnas.1419260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gröbner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, Johann PD, Balasubramanian GP, Segura-Wang M, Brabetz S, Bender S, Hutter B, Sturm D, Pfaff E, Hübschmann D, Zipprich G, Heinold M, Eils J, Lawerenz C, Erkek S, Lambo S, Waszak S, Blattmann C, Borkhardt A, Kuhlen M, Eggert A, Fulda S, Gessler M, Wegert J, Kappler R, Baumhoer D, Burdach S, Kirschner-Schwabe R, Kontny U, Kulozik AE, Lohmann D, Hettmer S, Eckert C, Bielack S, Nathrath M, Niemeyer C, Richter GH, Schulte J, Siebert R, Westermann F, Molenaar JJ, Vassal G, Witt H ICGC PedBrain-Seq Project; ICGC MMML-Seq Project. Burkhardt B, Kratz CP, Witt O, van Tilburg CM, Kramm CM, Fleischhack G, Dirksen U, Rutkowski S, Frühwald M, von Hoff K, Wolf S, Klingebiel T, Koscielniak E, Landgraf P, Koster J, Resnick AC, Zhang J, Liu Y, Zhou X, Waanders AJ, Zwijnenburg DA, Raman P, Brors B, Weber UD, Northcott PA, Pajtler KW, Kool M, Piro RM, Korbel JO, Schlesner M, Eils R, Jones DTW, Lichter P, Chavez L, Zapatka M, Pfister SM. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321. doi: 10.1038/nature25480. [DOI] [PubMed] [Google Scholar]
- 19.Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014;15:585. doi: 10.1038/nrg3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainaut P, Pfeifer GP. Patterns of p53 G-- > T transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis. 2001;22:367–374. doi: 10.1093/carcin/22.3.367. [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 22.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 23.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brannon AR, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, Kania K, Viale A, Oschwald DM, Vacic V, Emde AK, Cercek A, Yaeger R, Kemeny NE, Saltz LB, Shia J, D’Angelica MI, Weiser MR, Solit DB, Berger MF. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014;15:454. doi: 10.1186/s13059-014-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, Pugh M, Jones L, Russell R, Sammut SJ, Tsui DW, Liu B, Dawson SJ, Abraham J, Northen H, Peden JF, Mukherjee A, Turashvili G, Green AR, McKinney S, Oloumi A, Shah S, Rosenfeld N, Murphy L, Bentley DR, Ellis IO, Purushotham A, Pinder SE, Borresen-Dale AL, Earl HM, Pharoah PD, Ross MT, Aparicio S, Caldas C. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, Leenders F, Lu X, Fernandez-Cuesta L, Bosco G, Muller C, Dahmen I, Jahchan NS, Park KS, Yang D, Karnezis AN, Vaka D, Torres A, Wang MS, Korbel JO, Menon R, Chun SM, Kim D, Wilkerson M, Hayes N, Engelmann D, Putzer B, Bos M, Michels S, Vlasic I, Seidel D, Pinther B, Schaub P, Becker C, Altmuller J, Yokota J, Kohno T, Iwakawa R, Tsuta K, Noguchi M, Muley T, Hoffmann H, Schnabel PA, Petersen I, Chen Y, Soltermann A, Tischler V, Choi CM, Kim YH, Massion PP, Zou Y, Jovanovic D, Kontic M, Wright GM, Russell PA, Solomon B, Koch I, Lindner M, Muscarella LA, la Torre A, Field JK, Jakopovic M, Knezevic J, Castanos-Velez E, Roz L, Pastorino U, Brustugun OT, Lund-Iversen M, Thunnissen E, Kohler J, Schuler M, Botling J, Sandelin M, Sanchez-Cespedes M, Salvesen HB, Achter V, Lang U, Bogus M, Schneider PM, Zander T, Ansen S, Hallek M, Wolf J, Vingron M, Yatabe Y, Travis WD, Nurnberg P, Reinhardt C, Perner S, Heukamp L, Buttner R, Haas SA, Brambilla E, Peifer M, Sage J, Thomas RK. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, Imielinski M, Hu X, Ling S, Akbani R, Rosenberg M, Cibulskis C, Ramachandran A, Collisson EA, Kwiatkowski DJ, Lawrence MS, Weinstein JN, Verhaak RG, Wu CJ, Hammerman PS, Cherniack AD, Getz G, Artyomov MN, Schreiber R, Govindan R, Meyerson M. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, Chang MT, Ni A, Kundra R, Jonsson P, Jayakumaran G, Gao SP, Johnsen HC, Hanrahan AJ, Zehir A, Rekhtman N, Ginsberg MS, Li BT, Yu HA, Paik PK, Drilon A, Hellmann MD, Reales DN, Benayed R, Rusch VW, Kris MG, Chaft JE, Baselga J, Taylor BS, Schultz N, Rudin CM, Hyman DM, Berger MF, Solit DB, Ladanyi M, Riely GJ. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7:596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, Shiah YJ, Yousif F, Lin X, Masella AP, Fox NS, Xie M, Prokopec SD, Berlin A, Lalonde E, Ahmed M, Trudel D, Luo X, Beck TA, Meng A, Zhang J, D’Costa A, Denroche RE, Kong H, Espiritu SM, Chua ML, Wong A, Chong T, Sam M, Johns J, Timms L, Buchner NB, Orain M, Picard V, Hovington H, Murison A, Kron K, Harding NJ, P’ng C, Houlahan KE, Chu KC, Lo B, Nguyen F, Li CH, Sun RX, de Borja R, Cooper CI, Hopkins JF, Govind SK, Fung C, Waggott D, Green J, Haider S, Chan-Seng-Yue MA, Jung E, Wang Z, Bergeron A, Dal Pra A, Lacombe L, Collins CC, Sahinalp C, Lupien M, Fleshner NE, He HH, Fradet Y, Tetu B, van der Kwast T, McPherson JD, Bristow RG, Boutros PC. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. 2017;541:359–364. doi: 10.1038/nature20788. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA, Perou CM. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, Storm PB, Biegel JA. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12:621–630. doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR, Batchelor TT, Ligon KL, Iafrate AJ, Ligon AH, Louis DN, Santagata S. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6:e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 43.Dahiya S, Emnett RJ, Haydon DH, Leonard JR, Phillips JJ, Perry A, Gutmann DH. BRAF-V600E mutation in pediatric and adult glioblastoma. Neuro Oncol. 2014;16:318–319. doi: 10.1093/neuonc/not146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho CY, Mobley BC, Gordish-Dressman H, VandenBussche CJ, Mason GE, Bornhorst M, Esbenshade AJ, Tehrani M, Orr BA, LaFrance DR, Devaney JM, Meltzer BW, Hofherr SE, Burger PC, Packer RJ, Rodriguez FJ. A clinicopathologic study of diencephalic pediatric low-grade gliomas with BRAF V600 mutation. Acta Neuropathol. 2015;130:575–585. doi: 10.1007/s00401-015-1467-3. [DOI] [PubMed] [Google Scholar]
- 45.Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, Stavropoulos J, Alon N, Pole JD, Ray PN, Navickiene V, Mangerel J, Remke M, Buczkowicz P, Ramaswamy V, Guerreiro Stucklin A, Li M, Young EJ, Zhang C, Castelo-Branco P, Bakry D, Laughlin S, Shlien A, Chan J, Ligon KL, Rutka JT, Dirks PB, Taylor MD, Greenberg M, Malkin D, Huang A, Bouffet E, Hawkins CE, Tabori U. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J. Clin. Oncol. 2015;33:1015–1022. doi: 10.1200/JCO.2014.58.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones DT, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA, Fontebasso AM, Stutz AM, Hutter S, Zuckermann M, Sturm D, Gronych J, Lasitschka B, Schmidt S, Seker-Cin H, Witt H, Sultan M, Ralser M, Northcott PA, Hovestadt V, Bender S, Pfaff E, Stark S, Faury D, Schwartzentruber J, Majewski J, Weber UD, Zapatka M, Raeder B, Schlesner M, Worth CL, Bartholomae CC, von Kalle C, Imbusch CD, Radomski S, Lawerenz C, van Sluis P, Koster J, Volckmann R, Versteeg R, Lehrach H, Monoranu C, Winkler B, Unterberg A, Herold-Mende C, Milde T, Kulozik AE, Ebinger M, Schuhmann MU, Cho YJ, Pomeroy SL, von Deimling A, Witt O, Taylor MD, Wolf S, Karajannis MA, Eberhart CG, Scheurlen W, Hasselblatt M, Ligon KL, Kieran MW, Korbel JO, Yaspo ML, Brors B, Felsberg J, Reifenberger G, Collins VP, Jabado N, Eils R, Lichter P, Pfister SM. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J, Mulder H, Boggs K, Shao Y, Rusch M, Becksfort J, Gupta P, Wang S, Lee RP, Brat D, Peter Collins V, Dahiya S, George D, Konomos W, Kurian KM, McFadden K, Serafini LN, Nickols H, Perry A, Shurtleff S, Gajjar A, Boop FA, Klimo PD Jr, Mardis ER, Wilson RK, Baker SJ, Zhang J, Wu G, Downing JR, Tatevossian RG, Ellison DW. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131:833–845. doi: 10.1007/s00401-016-1539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollack IF, Hamilton RL, Sobol RW, Nikiforova MN, Lyons-Weiler MA, LaFramboise WA, Burger PC, Brat DJ, Rosenblum MK, Holmes EJ, Zhou T, Jakacki RI. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children’s oncology group. Childs Nerv Syst. 2011;27:87–94. doi: 10.1007/s00381-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 51.Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Zhang J, Baker SJ. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Fruhwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Durken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, Meyer J, Schrimpf D, Kool M, Northcott PA, Zheludkova O, Milde T, Witt O, Kulozik AE, Reifenberger G, Jabado N, Perry A, Lichter P, von Deimling A, Pfister SM, Jones DT. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129:669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 54.Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–3467. [PubMed] [Google Scholar]
- 55.Lee RS, Stewart C, Carter SL, Ambrogio L, Cibulskis K, Sougnez C, Lawrence MS, Auclair D, Mora J, Golub TR, Biegel JA, Getz G, Roberts CW. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122:2983–2988. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, Gröbner S, Segura-Wang M, Zichner T, Rudneva VA, Warnatz HJ, Sidiropoulos N, Phillips AH, Schumacher S, Kleinheinz K, Waszak SM, Erkek S, Jones DTW, Worst BC, Kool M, Zapatka M, Jäger N, Chavez L, Hutter B, Bieg M, Paramasivam N, Heinold M, Gu Z, Ishaque N, Jäger-Schmidt C, Imbusch CD, Jugold A, Hübschmann D, Risch T, Amstislavskiy V, Gonzalez FGR, Weber UD, Wolf S, Robinson GW, Zhou X, Wu G, Finkelstein D, Liu Y, Cavalli FMG, Luu B, Ramaswamy V, Wu X, Koster J, Ryzhova M, Cho YJ, Pomeroy SL, Herold-Mende C, Schuhmann M, Ebinger M, Liau LM, Mora J, McLendon RE, Jabado N, Kumabe T, Chuah E, Ma Y, Moore RA, Mungall AJ, Mungall KL, Thiessen N, Tse K, Wong T, Jones SJM, Witt O, Milde T, Von Deimling A, Capper D, Korshunov A, Yaspo ML, Kriwacki R, Gajjar A, Zhang J, Beroukhim R, Fraenkel E, Korbel JO, Brors B, Schlesner M, Eils R, Marra MA, Pfister SM, Taylor MD, Lichter P. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547:311. doi: 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, Garzia L, Torchia J, Nor C, Morrissy AS, Agnihotri S, Thompson YY, Kuzan-Fischer CM, Farooq H, Isaev K, Daniels C, Cho BK, Kim SK, Wang KC, Lee JY, Grajkowska WA, Perek-Polnik M, Vasiljevic A, Faure-Conter C, Jouvet A, Giannini C, Nageswara Rao AA, Li KKW, Ng HK, Eberhart CG, Pollack IF, Hamilton RL, Gillespie GY, Olson JM, Leary S, Weiss WA, Lach B, Chambless LB, Thompson RC, Cooper MK, Vibhakar R, Hauser P, van Veelen MC, Kros JM, French PJ, Ra YS, Kumabe T, Lopez-Aguilar E, Zitterbart K, Sterba J, Finocchiaro G, Massimino M, Van Meir EG, Osuka S, Shofuda T, Klekner A, Zollo M, Leonard JR, Rubin JB, Jabado N, Albrecht S, Mora J, Van Meter TE, Jung S, Moore AS, Hallahan AR, Chan JA, Tirapelli DPC, Carlotti CG, Fouladi M, Pimentel J, Faria CC, Saad AG, Massimi L, Liau LM, Wheeler H, Nakamura H, Elbabaa SK, Perezpena-Diazconti M, Chico Ponce de Leon F, Robinson S, Zapotocky M, Lassaletta A, Huang A, Hawkins CE, Tabori U, Bouffet E, Bartels U, Dirks PB, Rutka JT, Bader GD, Reimand J, Goldenberg A, Ramaswamy V, Taylor MD. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31:737–754. e736. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crippa S, Ancey PB, Vazquez J, Angelino P, Rougemont AL, Guettier C, Zoete V, Delorenzi M, Michielin O, Meylan E. Mutant CTNNB1 and histological heterogeneity define metabolic subtypes of hepatoblastoma. EMBO Mol Med. 2017;9:1589–1604. doi: 10.15252/emmm.201707814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tirode F, Surdez D, Ma X, Parker M, Le Deley MC, Bahrami A, Zhang Z, Lapouble E, Grossetete-Lalami S, Rusch M, Reynaud S, Rio-Frio T, Hedlund E, Wu G, Chen X, Pierron G, Oberlin O, Zaidi S, Lemmon G, Gupta P, Vadodaria B, Easton J, Gut M, Ding L, Mardis ER, Wilson RK, Shurtleff S, Laurence V, Michon J, Marec-Berard P, Gut I, Downing J, Dyer M, Zhang J, Delattre O. Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discov. 2014;4:1342–1353. doi: 10.1158/2159-8290.CD-14-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crompton BD, Stewart C, Taylor-Weiner A, Alexe G, Kurek KC, Calicchio ML, Kiezun A, Carter SL, Shukla SA, Mehta SS, Thorner AR, de Torres C, Lavarino C, Sunol M, McKenna A, Sivachenko A, Cibulskis K, Lawrence MS, Stojanov P, Rosenberg M, Ambrogio L, Auclair D, Seepo S, Blumenstiel B, DeFelice M, Imaz-Rosshandler I, Schwarz-Cruz Y Celis A, Rivera MN, Rodriguez-Galindo C, Fleming MD, Golub TR, Getz G, Mora J, Stegmaier K. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4:1326–1341. doi: 10.1158/2159-8290.CD-13-1037. [DOI] [PubMed] [Google Scholar]
- 63.Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, Ambrogio L, Auclair D, Wang J, Song YK, Tolman C, Hurd L, Liao H, Zhang S, Bogen D, Brohl AS, Sindiri S, Catchpoole D, Badgett T, Getz G, Mora J, Anderson JR, Skapek SX, Barr FG, Meyerson M, Hawkins DS, Khan J. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohsaka S, Shukla N, Ameur N, Ito T, Ng CK, Wang L, Lim D, Marchetti A, Viale A, Pirun M, Socci ND, Qin LX, Sciot R, Bridge J, Singer S, Meyers P, Wexler LH, Barr FG, Dogan S, Fletcher JA, Reis-Filho JS, Ladanyi M. A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nat Genet. 2014;46:595–600. doi: 10.1038/ng.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska K, Ryles H, Laudenslager M, Rappaport EF, Wood AC, McGrady PW, Hogarty MD, London WB, Radhakrishnan R, Lemmon MA, Mosse YP. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014;26:682–694. doi: 10.1016/j.ccell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung NK, Zhang J, Lu C, Parker M, Bahrami A, Tickoo SK, Heguy A, Pappo AS, Federico S, Dalton J, Cheung IY, Ding L, Fulton R, Wang J, Chen X, Becksfort J, Wu J, Billups CA, Ellison D, Mardis ER, Wilson RK, Downing JR, Dyer MA. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307:1062–1071. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X, Ulyanov A, Wu G, Wilson M, Wang J, Brennan R, Rusch M, Manning AL, Ma J, Easton J, Shurtleff S, Mullighan C, Pounds S, Mukatira S, Gupta P, Neale G, Zhao D, Lu C, Fulton RS, Fulton LL, Hong X, Dooling DJ, Ochoa K, Naeve C, Dyson NJ, Mardis ER, Bahrami A, Ellison D, Wilson RK, Downing JR, Dyer MA. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481:329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Theriault BL, Prigoda-Lee NL, Spencer C, Dimaras H, Corson TW, Pang R, Massey C, Godbout R, Jiang Z, Zacksenhaus E, Paton K, Moll AC, Houdayer C, Raizis A, Halliday W, Lam WL, Boutros PC, Lohmann D, Dorsman JC, Gallie BL. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. 2013;14:327–334. doi: 10.1016/S1470-2045(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 69.Gadd S, Huff V, Walz AL, Ooms AHAG, Armstrong AE, Gerhard DS, Smith MA, Auvil JMG, Meerzaman D, Chen QR, Hsu CH, Yan C, Nguyen C, Hu Y, Hermida LC, Davidsen T, Gesuwan P, Ma Y, Zong Z, Mungall AJ, Moore RA, Marra MA, Dome JS, Mullighan CG, Ma J, Wheeler DA, Hampton OA, Ross N, Gastier-Foster JM, Arold ST, Perlman EJ. A Children’s oncology group and TARGET initiative exploring the genetic landscape of Wilms tumor. Nat Genet. 2017;49:1487–1494. doi: 10.1038/ng.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walz AL, Ooms A, Gadd S, Gerhard DS, Smith MA, Guidry Auvil JM, Meerzaman D, Chen QR, Hsu CH, Yan C, Nguyen C, Hu Y, Bowlby R, Brooks D, Ma Y, Mungall AJ, Moore RA, Schein J, Marra MA, Huff V, Dome JS, Chi YY, Mullighan CG, Ma J, Wheeler DA, Hampton OA, Jafari N, Ross N, Gastier-Foster JM, Perlman EJ. Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer Cell. 2015;27:286–297. doi: 10.1016/j.ccell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wegert J, Ishaque N, Vardapour R, Georg C, Gu Z, Bieg M, Ziegler B, Bausenwein S, Nourkami N, Ludwig N, Keller A, Grimm C, Kneitz S, Williams RD, Chagtai T, Pritchard-Jones K, van Sluis P, Volckmann R, Koster J, Versteeg R, Acha T, O’Sullivan MJ, Bode PK, Niggli F, Tytgat GA, van Tinteren H, van den Heuvel-Eibrink MM, Meese E, Vokuhl C, Leuschner I, Graf N, Eils R, Pfister SM, Kool M, Gessler M. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell. 2015;27:298–311. doi: 10.1016/j.ccell.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Bardeesy N, Falkoff D, Petruzzi MJ, Nowak N, Zabel B, Adam M, Aguiar MC, Grundy P, Shows T, Pelletier J. Anaplastic Wilms’ tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet. 1994;7:91–97. doi: 10.1038/ng0594-91. [DOI] [PubMed] [Google Scholar]
- 73.Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, Shah K, Sato M, Thomas RK, Barletta JA, Borecki IB, Broderick S, Chang AC, Chiang DY, Chirieac LR, Cho J, Fujii Y, Gazdar AF, Giordano T, Greulich H, Hanna M, Johnson BE, Kris MG, Lash A, Lin L, Lindeman N, Mardis ER, McPherson JD, Minna JD, Morgan MB, Nadel M, Orringer MB, Osborne JR, Ozenberger B, Ramos AH, Robinson J, Roth JA, Rusch V, Sasaki H, Shepherd F, Sougnez C, Spitz MR, Tsao MS, Twomey D, Verhaak RG, Weinstock GM, Wheeler DA, Winckler W, Yoshizawa A, Yu S, Zakowski MF, Zhang Q, Beer DG, Wistuba II, Watson MA, Garraway LA, Ladanyi M, Travis WD, Pao W, Rubin MA, Gabriel SB, Gibbs RA, Varmus HE, Wilson RK, Lander ES, Meyerson M. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, Kango-Singh M, Lu KH, Warneke CL, Atkinson EN, Bedrosian I, Keyomarsi K, Kuo WL, Gray JW, Yin JC, Liu J, Halder G, Mills GB. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, Houlston RS. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hieronymus H, Schultz N, Gopalan A, Carver BS, Chang MT, Xiao Y, Heguy A, Huberman K, Bernstein M, Assel M, Murali R, Vickers A, Scardino PT, Sander C, Reuter V, Taylor BS, Sawyers CL. Copy number alteration burden predicts prostate cancer relapse. Proc Natl Acad Sci U S A. 2014;111:11139–11144. doi: 10.1073/pnas.1411446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lalonde E, Ishkanian AS, Sykes J, Fraser M, Ross-Adams H, Erho N, Dunning MJ, Halim S, Lamb AD, Moon NC, Zafarana G, Warren AY, Meng X, Thoms J, Grzadkowski MR, Berlin A, Have CL, Ramnarine VR, Yao CQ, Malloff CA, Lam LL, Xie H, Harding NJ, Mak DY, Chu KC, Chong LC, Sendorek DH, P’ng C, Collins CC, Squire JA, Jurisica I, Cooper C, Eeles R, Pintilie M, Dal Pra A, Davicioni E, Lam WL, Milosevic M, Neal DE, van der Kwast T, Boutros PC, Bristow RG. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol. 2014;15:1521–1532. doi: 10.1016/S1470-2045(14)71021-6. [DOI] [PubMed] [Google Scholar]
- 80.Troutman SM, Price DK, Figg WD. Prostate cancer genomic signature offers prognostic value. Cancer Biol Ther. 2010;10:1079–1080. doi: 10.4161/cbt.10.11.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M, Sivachenko A, Sougnez C, Auclair D, Lawrence MS, Stojanov P, Cibulskis K, Choi K, de Waal L, Sharifnia T, Brooks A, Greulich H, Banerji S, Zander T, Seidel D, Leenders F, Ansen S, Ludwig C, Engel-Riedel W, Stoelben E, Wolf J, Goparju C, Thompson K, Winckler W, Kwiatkowski D, Johnson BE, Janne PA, Miller VA, Pao W, Travis WD, Pass HI, Gabriel SB, Lander ES, Thomas RK, Garraway LA, Getz G, Meyerson M. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, Choti MA, Yeo CJ, McCue P, White MA, Knudsen ES. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paugh BS, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, Bax DA, Coyle B, Barrow J, Hargrave D, Lowe J, Gajjar A, Zhao W, Broniscer A, Ellison DW, Grundy RG, Baker SJ. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bax DA, Mackay A, Little SE, Carvalho D, Viana-Pereira M, Tamber N, Grigoriadis AE, Ashworth A, Reis RM, Ellison DW, Al-Sarraj S, Hargrave D, Jones C. A distinct spectrum of copy number aberrations in pediatric high-grade gliomas. Clin Cancer Res. 2010;16:3368–3377. doi: 10.1158/1078-0432.CCR-10-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J. Clin. Oncol. 2015;33:2986–2998. doi: 10.1200/JCO.2014.59.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011;29:1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J. Clin. Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schleiermacher G, Mosseri V, London WB, Maris JM, Brodeur GM, Attiyeh E, Haber M, Khan J, Nakagawara A, Speleman F, Noguera R, Tonini GP, Fischer M, Ambros I, Monclair T, Matthay KK, Ambros P, Cohn SL, Pearson AD. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012;107:1418. doi: 10.1038/bjc.2012.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 91.Korshunov A, Sturm D, Ryzhova M, Hovestadt V, Gessi M, Jones DT, Remke M, Northcott P, Perry A, Picard D, Rosenblum M, Antonelli M, Aronica E, Schuller U, Hasselblatt M, Woehrer A, Zheludkova O, Kumirova E, Puget S, Taylor MD, Giangaspero F, Peter Collins V, von Deimling A, Lichter P, Huang A, Pietsch T, Pfister SM, Kool M. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol. 2014;128:279–289. doi: 10.1007/s00401-013-1228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kleinman CL, Gerges N, Papillon-Cavanagh S, Sin-Chan P, Pramatarova A, Quang DA, Adoue V, Busche S, Caron M, Djambazian H, Bemmo A, Fontebasso AM, Spence T, Schwartzentruber J, Albrecht S, Hauser P, Garami M, Klekner A, Bognar L, Montes JL, Staffa A, Montpetit A, Berube P, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel PM, Duchaine T, Perotti C, Fleming A, Faury D, Remke M, Gallo M, Dirks P, Taylor MD, Sladek R, Pastinen T, Chan JA, Huang A, Majewski J, Jabado N. Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet. 2014;46:39–44. doi: 10.1038/ng.2849. [DOI] [PubMed] [Google Scholar]
- 93.Merlino G, Helman LJ. Rhabdomyosarcoma--working out the pathways. Oncogene. 1999;18:5340–5348. doi: 10.1038/sj.onc.1203038. [DOI] [PubMed] [Google Scholar]
- 94.Scott RH, Murray A, Baskcomb L, Turnbull C, Loveday C, Al-Saadi R, Williams R, Breatnach F, Gerrard M, Hale J, Kohler J, Lapunzina P, Levitt GA, Picton S, Pizer B, Ronghe MD, Traunecker H, Williams D, Kelsey A, Vujanic GM, Sebire NJ, Grundy P, Stiller CA, Pritchard-Jones K, Douglas J, Rahman N. Stratification of Wilms tumor by genetic and epigenetic analysis. Oncotarget. 2012;3:327–335. doi: 10.18632/oncotarget.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gadd S, Huff V, Huang CC, Ruteshouser EC, Dome JS, Grundy PE, Breslow N, Jennings L, Green DM, Beckwith JB, Perlman EJ. Clinically relevant subsets identified by gene expression patterns support a revised ontogenic model of wilms tumor: a children’s oncology group study. Neoplasia (New York, N.Y.) 2012;14:742–756. doi: 10.1593/neo.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gratias EJ, Dome JS, Jennings LJ, Chi YY, Tian J, Anderson J, Grundy P, Mullen EA, Geller JI, Fernandez CV, Perlman EJ. Association of chromosome 1q gain with inferior survival in favorable-histology wilms tumor: a report from the children’s oncology group. J. Clin. Oncol. 2016;34:3189–3194. doi: 10.1200/JCO.2015.66.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chagtai T, Zill C, Dainese L, Wegert J, Savola S, Popov S, Mifsud W, Vujanić G, Sebire N, Le Bouc Y, Ambros PF, Kager L, O’Sullivan MJ, Blaise A, Bergeron C, Mengelbier LH, Gisselsson D, Kool M, Tytgat GA, van den Heuvel-Eibrink MM, Graf N, van Tinteren H, Coulomb A, Gessler M, Williams RD, Pritchard-Jones K. Gain of 1q as a prognostic biomarker in wilms tumors (WTs) treated with preoperative chemotherapy in the international society of paediatric oncology (SIOP) WT 2001 trial: a SIOP renal tumours biology consortium study. J. Clin. Oncol. 2016;34:3195–3203. doi: 10.1200/JCO.2015.66.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grundy PE, Breslow NE, Li S, Perlman E, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, D’Angio GJ, Donaldson M, Coppes MJ, Malogolowkin M, Shearer P, Thomas PR, Macklis R, Tomlinson G, Huff V, Green DM. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology wilms tumor: a report from the national wilms tumor study group. J. Clin. Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 99.Williams RD, Chagtai T, Alcaide-German M, Apps J, Wegert J, Popov S, Vujanic G, van Tinteren H, van den Heuvel-Eibrink MM, Kool M, de Kraker J, Gisselsson D, Graf N, Gessler M, Pritchard-Jones K. Multiple mechanisms of MYCN dysregulation in Wilms tumour. Oncotarget. 2015;6:7232–7243. doi: 10.18632/oncotarget.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Northcott PA, Lee C, Zichner T, Stutz AM, Erkek S, Kawauchi D, Shih DJ, Hovestadt V, Zapatka M, Sturm D, Jones DT, Kool M, Remke M, Cavalli FM, Zuyderduyn S, Bader GD, VandenBerg S, Esparza LA, Ryzhova M, Wang W, Wittmann A, Stark S, Sieber L, Seker-Cin H, Linke L, Kratochwil F, Jager N, Buchhalter I, Imbusch CD, Zipprich G, Raeder B, Schmidt S, Diessl N, Wolf S, Wiemann S, Brors B, Lawerenz C, Eils J, Warnatz HJ, Risch T, Yaspo ML, Weber UD, Bartholomae CC, von Kalle C, Turanyi E, Hauser P, Sanden E, Darabi A, Siesjo P, Sterba J, Zitterbart K, Sumerauer D, van Sluis P, Versteeg R, Volckmann R, Koster J, Schuhmann MU, Ebinger M, Grimes HL, Robinson GW, Gajjar A, Mynarek M, von Hoff K, Rutkowski S, Pietsch T, Scheurlen W, Felsberg J, Reifenberger G, Kulozik AE, von Deimling A, Witt O, Eils R, Gilbertson RJ, Korshunov A, Taylor MD, Lichter P, Korbel JO, Wechsler-Reya RJ, Pfister SM. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature. 2014;511:428–434. doi: 10.1038/nature13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson JL, Denny CT, Tap WD, Federman N. Pediatric sarcomas: translating molecular pathogenesis of disease to novel therapeutic possibilities. Pediatr Res. 2012;72:112–121. doi: 10.1038/pr.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korshunov A, Meyer J, Capper D, Christians A, Remke M, Witt H, Pfister S, von Deimling A, Hartmann C. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 104.Lin A, Rodriguez FJ, Karajannis MA, Williams SC, Legault G, Zagzag D, Burger PC, Allen JC, Eberhart CG, Bar EE. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, White E, Tang B, Orisme W, Gupta K, Rusch M, Chen X, Li Y, Nagahawhatte P, Hedlund E, Finkelstein D, Wu G, Shurtleff S, Easton J, Boggs K, Yergeau D, Vadodaria B, Mulder HL, Becksfort J, Gupta P, Huether R, Ma J, Song G, Gajjar A, Merchant T, Boop F, Smith AA, Ding L, Lu C, Ochoa K, Zhao D, Fulton RS, Fulton LL, Mardis ER, Wilson RK, Downing JR, Green DR, Zhang J, Ellison DW, Gilbertson RJ. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506:451–455. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ, et al. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 107.Sturm D, Orr BA, Toprak UH, Hovestadt V, Jones DTW, Capper D, Sill M, Buchhalter I, Northcott PA, Leis I, Ryzhova M, Koelsche C, Pfaff E, Allen SJ, Balasubramanian G, Worst BC, Pajtler KW, Brabetz S, Johann PD, Sahm F, Reimand J, Mackay A, Carvalho DM, Remke M, Phillips JJ, Perry A, Cowdrey C, Drissi R, Fouladi M, Giangaspero F, Lastowska M, Grajkowska W, Scheurlen W, Pietsch T, Hagel C, Gojo J, Lotsch D, Berger W, Slavc I, Haberler C, Jouvet A, Holm S, Hofer S, Prinz M, Keohane C, Fried I, Mawrin C, Scheie D, Mobley BC, Schniederjan MJ, Santi M, Buccoliero AM, Dahiya S, Kramm CM, von Bueren AO, von Hoff K, Rutkowski S, Herold-Mende C, Fruhwald MC, Milde T, Hasselblatt M, Wesseling P, Rossler J, Schuller U, Ebinger M, Schittenhelm J, Frank S, Grobholz R, Vajtai I, Hans V, Schneppenheim R, Zitterbart K, Collins VP, Aronica E, Varlet P, Puget S, Dufour C, Grill J, Figarella-Branger D, Wolter M, Schuhmann MU, Shalaby T, Grotzer M, van Meter T, Monoranu CM, Felsberg J, Reifenberger G, Snuderl M, Forrester LA, Koster J, Versteeg R, Volckmann R, van Sluis P, Wolf S, Mikkelsen T, Gajjar A, Aldape K, Moore AS, Taylor MD, Jones C, Jabado N, Karajannis MA, Eils R, Schlesner M, Lichter P, von Deimling A, Pfister SM, Ellison DW, Korshunov A, Kool M. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell. 2016;164:1060–1072. doi: 10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pierron G, Tirode F, Lucchesi C, Reynaud S, Ballet S, Cohen-Gogo S, Perrin V, Coindre JM, Delattre O. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44:461–466. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]
- 109.Sugita S, Arai Y, Tonooka A, Hama N, Totoki Y, Fujii T, Aoyama T, Asanuma H, Tsukahara T, Kaya M, Shibata T, Hasegawa T. A novel CIC-FOXO4 gene fusion in undifferentiated small round cell sarcoma: a genetically distinct variant of Ewing-like sarcoma. Am J Surg Pathol. 2014;38:1571–1576. doi: 10.1097/PAS.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 110.O’Meara E, Stack D, Lee CH, Garvin AJ, Morris T, Argani P, Han JS, Karlsson J, Gisselson D, Leuschner I, Gessler M, Graf N, Fletcher JA, O’Sullivan MJ. Characterization of the chromosomal translocation t(10;17)(q22;p13) in clear cell sarcoma of kidney. J Pathol. 2012;227:72–80. doi: 10.1002/path.3985. [DOI] [PubMed] [Google Scholar]
- 111.Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, Hamdi M, van Nes J, Westerman BA, van Arkel J, Ebus ME, Haneveld F, Lakeman A, Schild L, Molenaar P, Stroeken P, van Noesel MM, Øra I, Santo EE, Caron HN, Westerhout EM, Versteeg R. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 112.Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, Carter SL, Cibulskis K, Hanna M, Kiezun A, Kim J, Lawrence MS, Lichenstein L, McKenna A, Pedamallu CS, Ramos AH, Shefler E, Sivachenko A, Sougnez C, Stewart C, Ally A, Birol I, Chiu R, Corbett RD, Hirst M, Jackman SD, Kamoh B, Khodabakshi AH, Krzywinski M, Lo A, Moore RA, Mungall KL, Qian J, Tam A, Thiessen N, Zhao Y, Cole KA, Diamond M, Diskin SJ, Mosse YP, Wood AC, Ji L, Sposto R, Badgett T, London WB, Moyer Y, Gastier-Foster JM, Smith MA, Auvil JMG, Gerhard DS, Hogarty MD, Jones SJ, Lander ES, Gabriel SB, Getz G, Seeger RC, Khan J, Marra MA, Meyerson M, Maris JM. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45:279. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sausen M, Leary RJ, Jones S, Wu J, Reynolds CP, Liu X, Blackford A, Parmigiani G, Diaz LA, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE, Hogarty MD. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z, Wang Y, Zhang J, Hu Q, Zhi F, Zhang S, Mao D, Zhang Y, Liang H. Significance of the TMPRSS2: ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450–5458. doi: 10.3892/mmr.2017.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L, Wallis J, Chen K, Walker J, McDonald S, Bose R, Ornitz D, Xiong D, You M, Dooling DJ, Watson M, Mardis ER, Wilson RK. Genomic landscape of non-small cell lung cancer in smokers and never smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, Terracciano LM, Incarbone M, Roncalli M, Cappuzzo F, Camidge DR, Varella-Garcia M, Doebele RC. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Graff RE, Pettersson A, Lis RT, DuPre N, Jordahl KM, Nuttall E, Rider JR, Fiorentino M, Sesso HD, Kenfield SA, Loda M, Giovannucci EL, Rosner B, Nguyen PL, Sweeney CJ, Mucci LA. The TMPRSS2: ERG fusion and response to androgen deprivation therapy for prostate cancer. Prostate. 2015;75:897–906. doi: 10.1002/pros.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Mello RA, Liu DJ, Aguiar PN, Tadokoro H. EGFR and EML4-ALK updated therapies in non-small cell lung cancer. Recent Pat Anticancer Drug Discov. 2016;11:393–400. doi: 10.2174/1574892811666160803090944. [DOI] [PubMed] [Google Scholar]
- 119.Sarfaty M, Moore A, Neiman V, Dudnik E, Ilouze M, Gottfried M, Katznelson R, Nechushtan H, Sorotsky HG, Paz K, Katz A, Saute M, Wolner M, Moskovitz M, Miller V, Elvin J, Lipson D, Ali S, Gutman LS, Dvir A, Gordon N, Peled N. RET fusion lung carcinoma: response to therapy and clinical features in a case series of 14 patients. Clin Lung Cancer. 2017;18:e223–e232. doi: 10.1016/j.cllc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Roskoski R Jr. ROS1 protein-tyrosine kinase inhibitors in the treatment of ROS1 fusion protein-driven non-small cell lung cancers. Pharmacol Res. 2017;121:202–212. doi: 10.1016/j.phrs.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 121.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 122.Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V, Jones DT, Sturm D, Hermann C, Segura Wang M, Korshunov A, Rhyzova M, Grobner S, Brabetz S, Chavez L, Bens S, Groschel S, Kratochwil F, Wittmann A, Sieber L, Georg C, Wolf S, Beck K, Oyen F, Capper D, van Sluis P, Volckmann R, Koster J, Versteeg R, von Deimling A, Milde T, Witt O, Kulozik AE, Ebinger M, Shalaby T, Grotzer M, Sumerauer D, Zamecnik J, Mora J, Jabado N, Taylor MD, Huang A, Aronica E, Bertoni A, Radlwimmer B, Pietsch T, Schuller U, Schneppenheim R, Northcott PA, Korbel JO, Siebert R, Fruhwald MC, Lichter P, Eils R, Gajjar A, Hasselblatt M, Pfister SM, Kool M. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016;29:379–393. doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, Reimand J, Warnatz HJ, Ryzhova M, Mack S, Ramaswamy V, Capper D, Schweizer L, Sieber L, Wittmann A, Huang Z, van Sluis P, Volckmann R, Koster J, Versteeg R, Fults D, Toledano H, Avigad S, Hoffman LM, Donson AM, Foreman N, Hewer E, Zitterbart K, Gilbert M, Armstrong TS, Gupta N, Allen JC, Karajannis MA, Zagzag D, Hasselblatt M, Kulozik AE, Witt O, Collins VP, von Hoff K, Rutkowski S, Pietsch T, Bader G, Yaspo ML, von Deimling A, Lichter P, Taylor MD, Gilbertson R, Ellison DW, Aldape K, Korshunov A, Kool M, Pfister SM. Molecular classification of ependymal tumors across All CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27:728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, Benner A, Hielscher T, Milde T, Remke M, Jones DT, Northcott PA, Garzia L, Bertrand KC, Wittmann A, Yao Y, Roberts SS, Massimi L, Van Meter T, Weiss WA, Gupta N, Grajkowska W, Lach B, Cho YJ, von Deimling A, Kulozik AE, Witt O, Bader GD, Hawkins CE, Tabori U, Guha A, Rutka JT, Lichter P, Korshunov A, Taylor MD, Pfister SM. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20:143–157. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mack SC, Witt H, Piro RM, Gu L, Zuyderduyn S, Stütz AM, Wang X, Gallo M, Garzia L, Zayne K, Zhang X, Ramaswamy V, Jäger N, Jones DTW, Sill M, Pugh TJ, Ryzhova M, Wani KM, Shih DJH, Head R, Remke M, Bailey SD, Zichner T, Faria CC, Barszczyk M, Stark S, Seker-Cin H, Hutter S, Johann P, Bender S, Hovestadt V, Tzaridis T, Dubuc AM, Northcott PA, Peacock J, Bertrand KC, Agnihotri S, Cavalli FM, Clarke I, Nethery-Brokx K, Creasy CL, Verma SK, Koster J, Wu X, Yao Y, Milde T, Sin-Chan P, Zuccaro J, Lau L, Pereira S, Castelo-Branco P, Hirst M, Marra MA, Roberts SS, Fults D, Massimi L, Cho YJ, Van Meter T, Grajkowska W, Lach B, Kulozik AE, von Deimling A, Witt O, Scherer SW, Fan X, Muraszko KM, Kool M, Pomeroy SL, Gupta N, Phillips J, Huang A, Tabori U, Hawkins C, Malkin D, Kongkham PN, Weiss WA, Jabado N, Rutka JT, Bouffet E, Korbel JO, Lupien M, Aldape KD, Bader GD, Eils R, Lichter P, Dirks PB, Pfister SM, Korshunov A, Taylor MD. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diaz AK, Baker SJ. The genetic signatures of pediatric high-grade glioma: no longer a one-act play. Semin Radiat Oncol. 2014;24:240–247. doi: 10.1016/j.semradonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weinberg DN, Allis CD, Lu C. Oncogenic mechanisms of histone H3 mutations. Cold Spring Harb Perspect Med. 2017:7. doi: 10.1101/cshperspect.a026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Batora NV, Sturm D, Jones DT, Kool M, Pfister SM, Northcott PA. Transitioning from genotypes to epigenotypes: why the time has come for medulloblastoma epigenomics. Neuroscience. 2014;264:171–185. doi: 10.1016/j.neuroscience.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 129.Fontebasso AM, Jabado N. Pediatric brain tumors: genomics and epigenomics pave the way. Crit Rev Oncog. 2015;20:271–299. doi: 10.1615/critrevoncog.2015013565. [DOI] [PubMed] [Google Scholar]
- 130.Benavente CA, Dyer MA. Genetics and epigenetics of human retinoblastoma. Annu Rev Pathol. 2015;10:547–562. doi: 10.1146/annurev-pathol-012414-040259. [DOI] [PubMed] [Google Scholar]
- 131.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M, Jackman S, Krzywinski M, Scott DW, Trinh DL, Tamura-Wells J, Li S, Firme MR, Rogic S, Griffith M, Chan S, Yakovenko O, Meyer IM, Zhao EY, Smailus D, Moksa M, Chittaranjan S, Rimsza L, Brooks-Wilson A, Spinelli JJ, Ben-Neriah S, Meissner B, Woolcock B, Boyle M, McDonald H, Tam A, Zhao Y, Delaney A, Zeng T, Tse K, Butterfield Y, Birol I, Holt R, Schein J, Horsman DE, Moore R, Jones SJ, Connors JM, Hirst M, Gascoyne RD, Marra MA. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ Jr, Laird PW, Baty JD, Fulton LL, Fulton R, Heath SE, Kalicki-Veizer J, Kandoth C, Klco JM, Koboldt DC, Kanchi KL, Kulkarni S, Lamprecht TL, Larson DE, Lin L, Lu C, McLellan MD, McMichael JF, Payton J, Schmidt H, Spencer DH, Tomasson MH, Wallis JW, Wartman LD, Watson MA, Welch J, Wendl MC, Ally A, Balasundaram M, Birol I, Butterfield Y, Chiu R, Chu A, Chuah E, Chun HJ, Corbett R, Dhalla N, Guin R, He A, Hirst C, Hirst M, Holt RA, Jones S, Karsan A, Lee D, Li HI, Marra MA, Mayo M, Moore RA, Mungall K, Parker J, Pleasance E, Plettner P, Schein J, Stoll D, Swanson L, Tam A, Thiessen N, Varhol R, Wye N, Zhao Y, Gabriel S, Getz G, Sougnez C, Zou L, Leiserson MD, Vandin F, Wu HT, Applebaum F, Baylin SB, Akbani R, Broom BM, Chen K, Motter TC, Nguyen K, Weinstein JN, Zhang N, Ferguson ML, Adams C, Black A, Bowen J, Gastier-Foster J, Grossman T, Lichtenberg T, Wise L, Davidsen T, Demchok JA, Shaw KR, Sheth M, Sofia HJ, Yang L, Downing JR, Eley G. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, Kasper LH, Lerach S, Tang H, Ma J, Rossi D, Chadburn A, Murty VV, Mullighan CG, Gaidano G, Rabadan R, Brindle PK, Dalla-Favera R. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sivakumar S, Lucas FAS, McDowell TL, Lang W, Xu L, Fujimoto J, Zhang J, Futreal PA, Fukuoka J, Yatabe Y, Dubinett SM, Spira AE, Fowler J, Hawk ET, Wistuba II, Scheet P, Kadara H. Genomic landscape of atypical adenomatous hyperplasia reveals divergent modes to lung adenocarcinoma. Cancer Res. 2017;77:6119–6130. doi: 10.1158/0008-5472.CAN-17-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Puente XS, Bea S, Valdes-Mas R, Villamor N, Gutierrez-Abril J, Martin-Subero JI, Munar M, Rubio-Perez C, Jares P, Aymerich M, Baumann T, Beekman R, Belver L, Carrio A, Castellano G, Clot G, Colado E, Colomer D, Costa D, Delgado J, Enjuanes A, Estivill X, Ferrando AA, Gelpi JL, Gonzalez B, Gonzalez S, Gonzalez M, Gut M, Hernandez-Rivas JM, Lopez-Guerra M, Martin-Garcia D, Navarro A, Nicolas P, Orozco M, Payer AR, Pinyol M, Pisano DG, Puente DA, Queiros AC, Quesada V, Romeo-Casabona CM, Royo C, Royo R, Rozman M, Russinol N, Salaverria I, Stamatopoulos K, Stunnenberg HG, Tamborero D, Terol MJ, Valencia A, Lopez-Bigas N, Torrents D, Gut I, Lopez-Guillermo A, Lopez-Otin C, Campo E. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature. 2015;526:519–524. doi: 10.1038/nature14666. [DOI] [PubMed] [Google Scholar]
- 138.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grutzmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 140.Okosun J, Bödör C, Wang J, Araf S, Yang CY, Pan C, Boller S, Cittaro D, Bozek M, Iqbal S, Matthews J, Wrench D, Marzec J, Tawana K, Popov N, O’Riain C, O’Shea D, Carlotti E, Davies A, Lawrie CH, Matolcsy A, Calaminici M, Norton A, Byers RJ, Mein C, Stupka E, Lister TA, Lenz G, Montoto S, Gribben JG, Fan Y, Grosschedl R, Chelala C, Fitzgibbon J. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nature Genetics. 2013;46:176. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T, Menon R, Koker M, Dahmen I, Müller C, Di Cerbo V, Schildhaus HU, Altmüller J, Baessmann I, Becker C, de Wilde B, Vandesompele J, Böhm D, Ansén S, Gabler F, Wilkening I, Heynck S, Heuckmann JM, Lu X, Carter SL, Cibulskis K, Banerji S, Getz G, Park KS, Rauh D, Grütter C, Fischer M, Pasqualucci L, Wright G, Wainer Z, Russell P, Petersen I, Chen Y, Stoelben E, Ludwig C, Schnabel P, Hoffmann H, Muley T, Brockmann M, Engel-Riedel W, Muscarella LA, Fazio VM, Groen H, Timens W, Sietsma H, Thunnissen E, Smit E, Heideman DA, Snijders PJ, Cappuzzo F, Ligorio C, Damiani S, Field J, Solberg S, Brustugun OT, Lund-Iversen M, Sänger J, Clement JH, Soltermann A, Moch H, Weder W, Solomon B, Soria JC, Validire P, Besse B, Brambilla E, Brambilla C, Lantuejoul S, Lorimier P, Schneider PM, Hallek M, Pao W, Meyerson M, Sage J, Shendure J, Schneider R, Büttner R, Wolf J, Nürnberg P, Perner S, Heukamp LC, Brindle PK, Haas S, Thomas RK. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nature Genetics. 2012;44:1104. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roy DM, Walsh LA, Chan TA. Driver mutations of cancer epigenomes. Protein Cell. 2014;5:265–296. doi: 10.1007/s13238-014-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT, Zhang J, Dolgalev I, Huberman K, Heguy A, Viale A, Drobnjak M, Leversha MA, Rice CE, Singh B, Iyer NG, Leemans CR, Bloemena E, Ferris RL, Seethala RR, Gross BE, Liang Y, Sinha R, Peng L, Raphael BJ, Turcan S, Gong Y, Schultz N, Kim S, Chiosea S, Shah JP, Sander C, Lee W, Chan TA. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45:791–798. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, Wu R, Chen C, Li X, Zhou L, He M, Li Z, Sun X, Jia W, Chen J, Yang S, Zhou F, Zhao X, Wan S, Ye R, Liang C, Liu Z, Huang P, Liu C, Jiang H, Wang Y, Zheng H, Sun L, Liu X, Jiang Z, Feng D, Chen J, Wu S, Zou J, Zhang Z, Yang R, Zhao J, Xu C, Yin W, Guan Z, Ye J, Zhang H, Li J, Kristiansen K, Nickerson ML, Theodorescu D, Li Y, Zhang X, Li S, Wang J, Yang H, Wang J, Cai Z. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, Teague J, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Forbes S, Jia M, Jones D, Knott H, Kok CY, Lau KW, Leroy C, Lin ML, McBride DJ, Maddison M, Maguire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, O’Meara S, Pleasance E, Rajasingham A, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turrell K, Dykema KJ, Khoo SK, Petillo D, Wondergem B, Anema J, Kahnoski RJ, Teh BT, Stratton MR, Futreal PA. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Behrens C, Solis LM, Lin H, Yuan P, Tang X, Kadara H, Riquelme E, Galindo H, Moran CA, Kalhor N, Swisher SG, Simon GR, Stewart DJ, Lee JJ, Wistuba II. EZH2 protein expression associates with the early pathogenesis, tumor progression and prognosis of non-small cell lung carcinoma. Clin Cancer Res. 2013;19:6556–6565. doi: 10.1158/1078-0432.CCR-12-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murphy MFG, Bithell JF, Stiller CA, Kendall GM, O’Neill KA. Childhood and adult cancers: Contrasts and commonalities. Maturitas. 2013;76:95–98. doi: 10.1016/j.maturitas.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 148.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J. Clin. Oncol. 2005;23:276–292. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 149.Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004;23:6445–6470. doi: 10.1038/sj.onc.1207714. [DOI] [PubMed] [Google Scholar]
- 150.Hodgson S. Mechanisms of inherited cancer susceptibility. J Zhejiang Univ Sci B. 2008;9:1–4. doi: 10.1631/jzus.B073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Strachan T, Goodship J, Chinnery P. Genetics and genomics in medicine. Taylor Francis. 2014 [Google Scholar]
- 152.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 153.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 154.Gracia-Aznarez FJ, Fernandez V, Pita G, Peterlongo P, Dominguez O, de la Hoya M, Duran M, Osorio A, Moreno L, Gonzalez-Neira A, Rosa-Rosa JM, Sinilnikova O, Mazoyer S, Hopper J, Lazaro C, Southey M, Odefrey F, Manoukian S, Catucci I, Caldes T, Lynch HT, Hilbers FS, van Asperen CJ, Vasen HF, Goldgar D, Radice P, Devilee P, Benitez J. Whole exome sequencing suggests much of non-BRCA1/BRCA2 familial breast cancer is due to moderate and low penetrance susceptibility alleles. PLoS One. 2013;8:e55681. doi: 10.1371/journal.pone.0055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomaki P, Sistonen P, Aaltonen LA, Nyström-Lahti M, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 156.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 157.Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomaki P, de la Chapelle A, Hamilton SR, Vogelstein B, Kinzler KW. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169–174. doi: 10.1038/nm0296-169. [DOI] [PubMed] [Google Scholar]
- 158.Wijnen JT, Vasen HF, Khan PM, Zwinderman AH, van der Klift H, Mulder A, Tops C, Moller P, Fodde R. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med. 1998;339:511–518. doi: 10.1056/NEJM199808203390804. [DOI] [PubMed] [Google Scholar]
- 159.Liu T, Yan H, Kuismanen S, Percesepe A, Bisgaard ML, Pedroni M, Benatti P, Kinzler KW, Vogelstein B, Ponz de Leon M, Peltomaki P, Lindblom A. The role of hPMS1 and hPMS2 in predisposing to colorectal cancer. Cancer Res. 2001;61:7798–7802. [PubMed] [Google Scholar]
- 160.Burn J, Chapman PD, Eastham EJ. Familial adenomatous polyposis. Arch Dis Child. 1994;71:103–105. doi: 10.1136/adc.71.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jarvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut. 1992;33:357–360. doi: 10.1136/gut.33.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nichols KE, Malkin D, Garber JE, Fraumeni JF Jr, Li FP. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev. 2001;10:83–87. [PubMed] [Google Scholar]
- 163.Tomlinson MJ, Bullimore JA. Adrenal-cell carcinoma and rhabdomyosarcoma occurring in father and daughter: “SBLA” syndrome? Br J Radiol. 1987;60:89–90. doi: 10.1259/0007-1285-60-709-89. [DOI] [PubMed] [Google Scholar]
- 164.Li FP, Fraumeni JF Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, Miller RW. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48:5358–5362. [PubMed] [Google Scholar]
- 165.Malkin D. Adrenocortical carcinoma. Springer; 2009. Li-Fraumeni syndrome; pp. 173–191. [Google Scholar]
- 166.Hisada M, Garber JE, Li FP, Fung CY, Fraumeni JF Jr. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst. 1998;90:606–11. doi: 10.1093/jnci/90.8.606. [DOI] [PubMed] [Google Scholar]
- 167.Rodríguez-Martín C, Cidre F, Fernández-Teijeiro A, Gómez-Mariano G, de la Vega L, Ramos P, Zaballos Á, Monzón S, Alonso J. Familial retinoblastoma due to intronic LINE-1 insertion causes aberrant and noncanonical mRNA splicing of the RB1 gene. J Hum Genet. 2016;61:463. doi: 10.1038/jhg.2015.173. [DOI] [PubMed] [Google Scholar]
- 168.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Cheng J, Lin H, Palmisano E, Brune K, Jaffee EM, Iacobuzio CA, Maitra A, Parmigini G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217–217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jarvinen HJ. Hereditary cancer: guidelines in clinical practice. Colorectal cancer genetics. Ann Oncol. 2004;15(Suppl 4):iv127–131. doi: 10.1093/annonc/mdh916. [DOI] [PubMed] [Google Scholar]
- 170.Bale SJ, Dracopoli NC, Tucker MA, Clark WHJ, Fraser MC, Stanger BZ, Green P, Donis-Keller H, Housman DE, Greene MH. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome Lp. N Engl J Med. 1989;320:1367–1372. doi: 10.1056/NEJM198905253202102. [DOI] [PubMed] [Google Scholar]
- 171.Goldstein AM, Dracopoli NC, Engelstein M, Fraser MC, Clark WH, Tucker MA. Linkage of cutaneous malignant melanoma/dysplastic nevi to chromosome 9p, and evidence for genetic heterogeneity. Am J Hum Genet. 1994;54:489–496. [PMC free article] [PubMed] [Google Scholar]
- 172.Cannon-Albright LA, Goldgar DE, Meyer LJ, Lewis CM, Anderson DE, Fountain JW, Hegi ME, Wiseman RW, Petty EM, Bale AE, et al. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992;258:1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- 173.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, Pooley KA, Pritchard AL, Tiffen JC, Petljak M, Palmer JM, Symmons J, Johansson P, Stark MS, Gartside MG, Snowden H, Montgomery GW, Martin NG, Liu JZ, Choi J, Makowski M, Brown KM, Dunning AM, Keane TM, Lopez-Otin C, Gruis NA, Hayward NK, Bishop DT, Newton-Bishop JA, Adams DJ. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schultz KA, Yang J, Doros L, Williams GM, Harris A, Stewart DR, Messinger Y, Field A, Dehner LP, Hill DA. DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome: a unique constellation of neoplastic conditions. Pathol Case Rev. 2014;19:90–100. doi: 10.1097/PCR.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parsons DW, Roy A, Yang Y, Wang T, Scollon S, Bergstrom K, Kerstein RA, Gutierrez S, Petersen AK, Bavle A, Lin FY, Lopez-Terrada DH, Monzon FA, Hicks MJ, Eldin KW, Quintanilla NM, Adesina AM, Mohila CA, Whitehead W, Jea A, Vasudevan SA, Nuchtern JG, Ramamurthy U, McGuire AL, Hilsenbeck SG, Reid JG, Muzny DM, Wheeler DA, Berg SL, Chintagumpala MM, Eng CM, Gibbs RA, Plon SE. Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumors. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5699. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang W, Brohl AS, Patidar R, Sindiri S, Shern JF, Wei JS, Song YK, Yohe ME, Gryder B, Zhang S, Calzone KA, Shivaprasad N, Wen X, Badgett TC, Miettinen M, Hartman KR, League-Pascual JC, Trahair TN, Widemann BC, Merchant MS, Kaplan RN, Lin JC, Khan J. MultiDimensional ClinOmics for precision therapy of children and adolescent young adults with relapsed and refractory cancer: a report from the center for cancer research. Clin Cancer Res. 2016;22:3810–3820. doi: 10.1158/1078-0432.CCR-15-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, Wilkinson MR, Vadodaria B, Chen X, McGee RB, Hines-Dowell S, Nuccio R, Quinn E, Shurtleff SA, Rusch M, Patel A, Becksfort JB, Wang S, Weaver MS, Ding L, Mardis ER, Wilson RK, Gajjar A, Ellison DW, Pappo AS, Pui CH, Nichols KE, Downing JR. Germline mutations in predisposition genes in pediatric cancer. New Engl J Med. 2015;373:2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ripperger T, Bielack SS, Borkhardt A, Brecht IB, Burkhardt B, Calaminus G, Debatin KM, Deubzer H, Dirksen U, Eckert C, Eggert A, Erlacher M, Fleischhack G, Fruhwald MC, Gnekow A, Goehring G, Graf N, Hanenberg H, Hauer J, Hero B, Hettmer S, von Hoff K, Horstmann M, Hoyer J, Illig T, Kaatsch P, Kappler R, Kerl K, Klingebiel T, Kontny U, Kordes U, Korholz D, Koscielniak E, Kramm CM, Kuhlen M, Kulozik AE, Lamottke B, Leuschner I, Lohmann DR, Meinhardt A, Metzler M, Meyer LH, Moser O, Nathrath M, Niemeyer CM, Nustede R, Pajtler KW, Paret C, Rasche M, Reinhardt D, Riess O, Russo A, Rutkowski S, Schlegelberger B, Schneider D, Schneppenheim R, Schrappe M, Schroeder C, von Schweinitz D, Simon T, Sparber-Sauer M, Spix C, Stanulla M, Steinemann D, Strahm B, Temming P, Thomay K, von Bueren AO, Vorwerk P, Witt O, Wlodarski M, Wossmann W, Zenker M, Zimmermann S, Pfister SM, Kratz CP. Childhood cancer predisposition syndromes-A concise review and recommendations by the cancer predisposition working group of the society for pediatric oncology and hematology. Am J Med Genet A. 2017;173:1017–1037. doi: 10.1002/ajmg.a.38142. [DOI] [PubMed] [Google Scholar]
- 179.Scollon S, Anglin AK, Thomas M, Turner JT, Wolfe Schneider K. A comprehensive review of pediatric tumors and associated cancer predisposition syndromes. J Genet Couns. 2017;26:387–434. doi: 10.1007/s10897-017-0077-8. [DOI] [PubMed] [Google Scholar]
- 180.Stadler ZK, Thom P, Robson ME, Weitzel JN, Kauff ND, Hurley KE, Devlin V, Gold B, Klein RJ, Offit K. Genome-wide association studies of cancer. J. Clin. Oncol. 2010;28:4255–4267. doi: 10.1200/JCO.2009.25.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim HL, Puymon MR, Qin M, Guru K, Mohler JL. NCCN clinical practice guidelines in oncology™. 2009 [Google Scholar]
- 182.Robson M, Offit K. Management of an inherited predisposition to breast cancer. N Engl J Med. 2007;357:154–162. doi: 10.1056/NEJMcp071286. [DOI] [PubMed] [Google Scholar]
- 183.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, Isaacs C, Evans DG, Lynch H, Eeles RA, Neuhausen SL, Daly MB, Matloff E, Blum JL, Sabbatini P, Barakat RR, Hudis C, Norton L, Offit K, Rebbeck TR. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J. Clin. Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, Lynch P, Burke W, Press N. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 185.Porche DJ. Precision medicine initiative. Am J Mens Health. 2015;9:177. doi: 10.1177/1557988315574512. [DOI] [PubMed] [Google Scholar]
- 186.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Müller K, Goschzik T, zur Mühlen A, Gerber N, Warmuth-Metz M, Soerensen N, Deinlein F, Benesch M, Zwiener I, Kwiecien R, Faldum A, Bode U, Fleischhack G, Hovestadt V, Kool M, Jones D, Northcott P, Kuehl J, Pfister S, Pietsch T, Rutkowski S. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J. Clin. Oncol. 2016;34:4151–4160. doi: 10.1200/JCO.2016.67.2428. [DOI] [PubMed] [Google Scholar]
- 188.Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M, Dufour C, Vassal G, Milde T, Witt O, von Hoff K, Pietsch T, Northcott PA, Gajjar A, Robinson GW, Padovani L, André N, Massimino M, Pizer B, Packer R, Rutkowski S, Pfister SM, Taylor MD, Pomeroy SL. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathologica. 2016;131:821–831. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Clifford SC, Lannering B, Schwalbe EC, Hicks D, O’Toole K, Nicholson SL, Goschzik T, Zur Muhlen A, Figarella-Branger D, Doz F, Rutkowski S, Gustafsson G, Pietsch T. Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: results from the multi-center HIT-SIOP-PNET4 clinical trial. Oncotarget. 2015;6:38827–38839. doi: 10.18632/oncotarget.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, Sinicrope FA, Rosty C, Buchanan DD, Potter JD, Newcomb PA. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87. e72. doi: 10.1053/j.gastro.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shlien A, Malkin D, Tabori U. Translational childhood cancer genomics: the future is now. JAMA Oncol. 2016;2:384–385. doi: 10.1001/jamaoncol.2015.5076. [DOI] [PubMed] [Google Scholar]
- 193.Mody RJ, Wu YM, Lonigro RJ, Cao X, Roychowdhury S, Vats P, Frank KM, Prensner JR, Asangani I, Palanisamy N, Dillman JR, Rabah RM, Kunju LP, Everett J, Raymond VM, Ning Y, Su F, Wang R, Stoffel EM, Innis JW, Roberts JS, Robertson PL, Yanik G, Chamdin A, Connelly JA, Choi S, Harris AC, Kitko C, Rao RJ, Levine JE, Castle VP, Hutchinson RJ, Talpaz M, Robinson DR, Chinnaiyan AM. Integrative clinical sequencing in the management of refractory or relapsed cancer in youth. JAMA. 2015;314:913–925. doi: 10.1001/jama.2015.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Allen CE, Laetsch TW, Mody R, Irwin MS, Lim MS, Adamson PC, Seibel NL, Parsons DW, Cho YJ, Janeway K. Target and agent prioritization for the Children’s oncology group-national cancer institute pediatric MATCH trial. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saulnier Sholler GL, Bond JP, Bergendahl G, Dutta A, Dragon J, Neville K, Ferguson W, Roberts W, Eslin D, Kraveka J, Kaplan J, Mitchell D, Parikh N, Merchant M, Ashikaga T, Hanna G, Lescault PJ, Siniard A, Corneveaux J, Huentelman M, Trent J. Feasibility of implementing molecular-guided therapy for the treatment of patients with relapsed or refractory neuroblastoma. Cancer Med. 2015;4:871–886. doi: 10.1002/cam4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pincez T, Clément N, Lapouble E, Pierron G, Kamal M, Bieche I, Bernard V, Fréneaux P, Michon J, Orbach D, Aerts I, Pacquement H, Bourdeaut F, Jiménez I, Thébaud E, Oudot C, Vérité C, Taque S, Owens C, Doz F, Tourneau C, Delattre O, Schleiermacher G. Feasibility and clinical integration of molecular profiling for target identification in pediatric solid tumors. Pediatric Blood Cancer. 2017;64:e26365. doi: 10.1002/pbc.26365. [DOI] [PubMed] [Google Scholar]
- 197.Kline CN, Joseph NM, Grenert JP, van Ziffle J, Talevich E, Onodera C, Aboian M, Cha S, Raleigh DR, Braunstein S, Torkildson J, Samuel D, Bloomer M, Campomanes AGA, Banerjee A, Butowski N, Raffel C, Tihan T, Bollen AW, Phillips JJ, Korn WM, Yeh I, Bastian BC, Gupta N, Mueller S, Perry A, Nicolaides T, Solomon DA. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol. 2017;19:699–709. doi: 10.1093/neuonc/now254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Harris MH, DuBois SG, Glade Bender JL, Kim A, Crompton BD, Parker E, Dumont IP, Hong AL, Guo D, Church A, Stegmaier K, Roberts CW, Shusterman S, London WB, MacConaill LE, Lindeman NI, Diller L, Rodriguez-Galindo C, Janeway KA. Multicenter feasibility study of tumor molecular profiling to inform therapeutic decisions in advanced pediatric solid tumors: the individualized cancer therapy (iCat) study. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5689. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 199.Harttrampf AC, Lacroix L, Deloger M, Deschamps F, Puget S, Auger N, Vielh P, Varlet P, Balogh Z, Abbou S, Allorant A, Valteau-Couanet D, Sarnacki S, Gamiche-Rolland L, Meurice G, Minard-Colin V, Grill J, Brugieres L, Dufour C, Gaspar N, Michiels S, Vassal G, Soria JC, Geoerger B. Molecular screening for cancer treatment optimization (MOSCATO-01) in pediatric patients: a single-institutional prospective molecular stratification trial. Clin Cancer Res. 2017;23:6101. doi: 10.1158/1078-0432.CCR-17-0381. [DOI] [PubMed] [Google Scholar]
- 200.Oberg JA, Glade Bender JL, Sulis ML, Pendrick D, Sireci AN, Hsiao SJ, Turk AT, Dela Cruz FS, Hibshoosh H, Remotti H, Zylber RJ, Pang J, Diolaiti D, Koval C, Andrews SJ, Garvin JH, Yamashiro DJ, Chung WK, Emerson SG, Nagy PL, Mansukhani MM, Kung AL. Implementation of next generation sequencing into pediatric hematology-oncology practice: moving beyond actionable alterations. Genome Medicine. 2016;8:133. doi: 10.1186/s13073-016-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marks LJ, Oberg JA, Pendrick D, Sireci AN, Glasser C, Coval C, Zylber RJ, Chung WK, Pang J, Turk AT, Hsiao SJ, Mansukhani MM, Glade Bender JL, Kung AL, Sulis ML. Precision medicine in children and young adults with hematologic malignancies and blood disorders: the columbia university experience. Front Pediatr. 2017;5:265. doi: 10.3389/fped.2017.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Caporalini C, Moscardi S, Tamburini A, Pierossi N, Di Maurizio M, Buccoliero AM. Inflammatory myofibroblastic tumor of the tongue. Report of a pediatric case and review of the literature. Fetal Pediatr Pathol. 2018;37:117–125. doi: 10.1080/15513815.2017.1385667. [DOI] [PubMed] [Google Scholar]
- 203.Tucker ER, Danielson LS, Innocenti P, Chesler L. Tackling crizotinib resistance: the pathway from drug discovery to the pediatric clinic. Cancer Res. 2015;75:2770. doi: 10.1158/0008-5472.CAN-14-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Penman CL, Faulkner C, Lowis SP, Kurian KM. Current understanding of BRAF alterations in diagnosis, prognosis, and therapeutic targeting in pediatric low-grade gliomas. Front Oncol. 2015;5:54. doi: 10.3389/fonc.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, Chintagumpala M, Takebe N, Kaste SC, Rusch M, Allen SJ, Onar-Thomas A, Stewart CF, Fouladi M, Boyett JM, Gilbertson RJ, Curran T, Ellison DW, Gajjar A. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015;33:2646–2654. doi: 10.1200/JCO.2014.60.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Balachandran VP, DeMatteo RP. GIST tumors: who should get imatinib and for how long? Adv Surg. 2014;48:165–183. doi: 10.1016/j.yasu.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y, Schmid-Bindert G, Zhou C. Erlotinib in the treatment of advanced non-small cell lung cancer: an update for clinicians. Ther Adv Med Oncol. 2012;4:19–29. doi: 10.1177/1758834011427927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dean L. Trastuzumab (Herceptin) Therapy and ERBB2 (HER2) Genotype. In: Pratt V, McLeod H, Dean L, Malheiro A, Rubinstein W, editors. Medical Genetics Summaries. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [Google Scholar]
- 210.Polk A, Kolmos IL, Kümler I, Nielsen DL. Specific CDK4/6 inhibition in breast cancer: a systematic review of current clinical evidence. ESMO Open. 2017;1:e000093. doi: 10.1136/esmoopen-2016-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chapman PB, Robert C, Larkin J, Haanen JB, Ribas A, Hogg D, Hamid O, Ascierto PA, Testori A, Lorigan PC, Dummer R, Sosman JA, Flaherty KT, Chang I, Coleman S, Caro I, Hauschild A, McArthur GA. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol. 2017;28:2581–2587. doi: 10.1093/annonc/mdx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ding L, Bailey MH, Porta-Pardo E, Thorsson V, Colaprico A, Bertrand D, Gibbs DL, Weerasinghe A, Huang KL, Tokheim C, Cortes-Ciriano I, Jayasinghe R, Chen F, Yu L, Sun S, Olsen C, Kim J, Taylor AM, Cherniack AD, Akbani R, Suphavilai C, Nagarajan N, Stuart JM, Mills GB, Wyczalkowski MA, Vincent BG, Hutter CM, Zenklusen JC, Hoadley KA, Wendl MC, Shmulevich L, Lazar AJ, Wheeler DA, Getz G. Perspective on oncogenic processes at the end of the beginning of cancer genomics. Cell. 2018;173:305–320. e310. doi: 10.1016/j.cell.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Meric-Bernstam F, Frampton GM, Ferrer-Lozano J, Yelensky R, Pérez-Fidalgo JA, Wang Y, Palmer GA, Ross JS, Miller VA, Su X, Eroles P, Barrera JA, Burgues O, Lluch AM, Zheng X, Sahin A, Stephens PJ, Mills GB, Cronin MT, Gonzalez-Angulo AM. Concordance of genomic alterations between primary and recurrent breast cancer. Mol Cancer Ther. 2014;13:1382–1389. doi: 10.1158/1535-7163.MCT-13-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bertucci F, Finetti P, Guille A, Adélaïde J, Garnier S, Carbuccia N, Monneur A, Charafe-Jauffret E, Goncalves A, Viens P, Birnbaum D, Chaffanet M. Comparative genomic analysis of primary tumors and metastases in breast cancer. Oncotarget. 2016;7:27208–27219. doi: 10.18632/oncotarget.8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gibson WJ, Hoivik EA, Halle MK, Taylor-Weiner A, Cherniack AD, Berg A, Holst F, Zack TI, Werner HM, Staby KM, Rosenberg M, Stefansson IM, Kusonmano K, Chevalier A, Mauland KK, Trovik J, Krakstad C, Giannakis M, Hodis E, Woie K, Bjorge L, Vintermyr OK, Wala JA, Lawrence MS, Getz G, Carter SL, Beroukhim R, Salvesen HB. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat Genet. 2016;48:848–855. doi: 10.1038/ng.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.He F, Li J, Xu J, Zhang S, Xu Y, Zhao W, Yin Z, Wang X. Decreased expression of ARID1A associates with poor prognosis and promotes metastases of hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:47. doi: 10.1186/s13046-015-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yates LR, Knappskog S, Wedge D, Farmery JHR, Gonzalez S, Martincorena I, Alexandrov LB, Van Loo P, Haugland HK, Lilleng PK, Gundem G, Gerstung M, Pappaemmanuil E, Gazinska P, Bhosle SG, Jones D, Raine K, Mudie L, Latimer C, Sawyer E, Desmedt C, Sotiriou C, Stratton MR, Sieuwerts AM, Lynch AG, Martens JW, Richardson AL, Tutt A, Lønning PE, Campbell PJ. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell. 2017;32:169–184. e167. doi: 10.1016/j.ccell.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lin CC, Shih JY, Yu CJ, Ho CC, Liao WY, Lee JH, Tsai TH, Su KY, Hsieh MS, Chang YL, Bai YY, Huang DD, Thress KS, Yang JC. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir Med. 2018;6:107–116. doi: 10.1016/S2213-2600(17)30480-0. [DOI] [PubMed] [Google Scholar]
- 219.Eleveld TF, Oldridge DA, Bernard V, Koster J, Daage LC, Diskin SJ, Schild L, Bentahar NB, Bellini A, Chicard M, Lapouble E, Combaret V, Legoix-Ne P, Michon J, Pugh TJ, Hart LS, Rader J, Attiyeh EF, Wei JS, Zhang S, Naranjo A, Gastier-Foster JM, Hogarty MD, Asgharzadeh S, Smith MA, Guidry Auvil JM, Watkins TB, Zwijnenburg DA, Ebus ME, van Sluis P, Hakkert A, van Wezel E, van der Schoot CE, Westerhout EM, Schulte JH, Tytgat GA, Dolman ME, Janoueix-Lerosey I, Gerhard DS, Caron HN, Delattre O, Khan J, Versteeg R, Schleiermacher G, Molenaar JJ, Maris JM. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47:864–871. doi: 10.1038/ng.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 221.Hingorani P, Janeway K, Crompton BD, Kadoch C, Mackall CL, Khan J, Shern JF, Schiffman J, Mirabello L, Savage SA, Ladanyi M, Meltzer P, Bult CJ, Adamson PC, Lupo PJ, Mody R, DuBois SG, Parsons DW, Khanna C, Lau C, Hawkins DS, Randall RL, Smith M, Sorensen PH, Plon SE, Skapek SX, Lessnick S, Gorlick R, Reed DR. Current state of pediatric sarcoma biology and opportunities for future discovery: a report from the sarcoma translational research workshop. Cancer Genet. 2016;209:182–194. doi: 10.1016/j.cancergen.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat Rev Cancer. 2003;3:243. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 223.Miller AB. Early detection and mass screening for cancer. Canadian Family Physician. 1972;18:76–79. [PMC free article] [PubMed] [Google Scholar]
- 224.Heitzer E, Perakis S, Geigl JB, Speicher MR. The potential of liquid biopsies for the early detection of cancer. NPJ Precis Oncol. 2017;1:36. doi: 10.1038/s41698-017-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Williams SC. Circulating tumor cells. Proc Natl Acad Sci U S A. 2013;110:4861. doi: 10.1073/pnas.1304186110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Calabuig-Fariñas S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer-which one will win? Transl Lung Cancer Res. 2016;5:466–482. doi: 10.21037/tlcr.2016.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhou L, Dicker DT, Matthew E, El-Deiry WS, Alpaugh RK. Circulating tumor cells: silent predictors of metastasis. F1000Res. 2017:6. doi: 10.12688/f1000research.11313.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ried K, Eng P, Sali A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: an observational study. Asian Pac J Cancer Prev. 2017;18:2275–2285. doi: 10.22034/APJCP.2017.18.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Neves H, Kwok HF. Recent advances in the field of anti-cancer immunotherapy. BBA Clinical. 2015;3:280–288. doi: 10.1016/j.bbacli.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Trapani JA, Darcy PK. Immunotherapy of cancer. Aust Fam Physician. 2017;46:194–199. [PubMed] [Google Scholar]
- 231.Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. Immunotherapeutic approaches for cancer therapy: an updated review. Artif Cells Nanomed Biotechnol. 2016;44:769–779. doi: 10.3109/21691401.2015.1019669. [DOI] [PubMed] [Google Scholar]
- 232.Johnson DB, Rioth MJ, Horn L. Immune checkpoint inhibitors in NSCLC. Curr Treat Options Oncol. 2014;15:658–669. doi: 10.1007/s11864-014-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Investig Dermatol. 2017;10:325–339. doi: 10.2147/CCID.S120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Atkins MB, Clark JI, Quinn DI. Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Ann Oncol. 2017;28:1484–1494. doi: 10.1093/annonc/mdx151. [DOI] [PubMed] [Google Scholar]
- 235.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12:307. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28:411–419. doi: 10.1093/intimm/dxw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28:22–27. doi: 10.1016/j.smim.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wirth TC, Kühnel F. Neoantigen targeting-dawn of a new era in cancer immunotherapy? Front Immunol. 2017;8:1848. doi: 10.3389/fimmu.2017.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chang TC, Carter RA, Li Y, Li Y, Wang H, Edmonson MN, Chen X, Arnold P, Geiger TL, Wu G, Peng J, Dyer M, Downing JR, Green DR, Thomas PG, Zhang J. The neoepitope landscape in pediatric cancers. Genome Medicine. 2017;9:78. doi: 10.1186/s13073-017-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Swanton C. Intratumour heterogeneity: evolution through space and time. Cancer Res. 2012;72:4875–4882. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vakiani E, Janakiraman M, Shen R, Sinha R, Zeng Z, Shia J, Cercek A, Kemeny N, D’Angelica M, Viale A, Heguy A, Paty P, Chan TA, Saltz LB, Weiser M, Solit DB. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J. Clin. Oncol. 2012;30:2956–2962. doi: 10.1200/JCO.2011.38.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mengelbier LH, Karlsson J, Lindgren D, Valind A, Lilljebjörn H, Jansson C, Bexell D, Braekeveldt N, Ameur A, Jonson T, Kultima HG, Isaksson A, Asmundsson J, Versteeg R, Rissler M, Fioretos T, Sandstedt B, Börjesson A, Backman T, Pal N, Øra I, Mayrhofer M, Gisselsson D. Intratumoral genome diversity parallels progression and predicts outcome in pediatric cancer. Nat Commun. 2015;6:6125. doi: 10.1038/ncomms7125. [DOI] [PubMed] [Google Scholar]
- 247.Wacholder S. Precursors in cancer epidemiology: aligning definition and function. Cancer Epidemiol Biomarkers Prev. 2013;22:521. doi: 10.1158/1055-9965.EPI-13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Campbell JD, Mazzilli SA, Reid ME, Dhillon SS, Platero S, Beane J, Spira AE. The case for a pre-cancer genome atlas (PCGA) Cancer Prev Res (Phila) 2016;9:119–24. doi: 10.1158/1940-6207.CAPR-16-0024. [DOI] [PubMed] [Google Scholar]
- 249.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]