Abstract
Objective: To explore the effects of miRNA-21/phosphatase and tensin homolog (PTEN) on the high glucose-stimulated epithelial-to-mesenchymal transition (EMT) in human peritoneal mesothelial cells (HPMCs). Methods: HPMCs were cultured under control conditions, or with high glucose (HG), HG with miRNA-21 mimic or a miRNA-21 inhibitor. Expression of miRNA-21, α-smooth muscle actin, >fibronectin, E-cadherin and PTEN were measured by real time PCR, Western blotting and immunofluorescence staining. Results: Compared with the control, HG induced the EMT, as shown by upregulation of α-smooth muscle actin and >fibronectin, and downregulation of E-cadherin. We also found that HG upregulated miRNA-21 expression and downregulated PTEN expression; the miRNA-21 inhibitor attenuated the HG-induced EMT in HPMCs by targeting PTEN; the miRNA-21 mimic increased the HG-induced EMT in HPMCs by targeting PTEN. Conclusions: This study demonstrated that miRNA-21 played a vital role in the HG-induced EMT by targeting PTEN in HPMCs.
Keywords: miR-21, PTEN, EMT, HPMCs
Introduction
It is well known that peritoneal dialysis (PD) is a crucial chronic life-sustaining treatment for end stage renal disease (ESRD), and research has shown that the number of patients undergoing PD in both developing and developed countries has increased over the last decade [1]. Due to its simplicity and economic advantages, PD is widely applied to patients who live in remote and rural areas [1]. However, the high glucose-based solutions have disadvantages, such as high lactate, low pH, glucose degradation products and hyperosmolarity [2,3], which can lead to morphological and functional changes of the peritoneum, and eventually cause peritoneal fibrosis and ultrafiltration failure [4].
The epithelial-to mesenchymal transition (EMT) is a crucial and complex phenomenon involved in organ fibrosis, including renal fibrosis, liver fibrosis and peritoneal fibrosis [5-7]. The EMT in HPMCs is considered as an early stage of peritoneal fibrosis [8]. Preventing the EMT in patients undergoing PD could alleviate peritoneal fibrosis, thus protecting the peritoneal mesothelium [9]. The EMT process is accompanied by high expression of mesenchymal markers such as fibronectin (FN) and α-smooth muscle actin (α-SMA), and low expression of epithelial markers such as E-cadherin [10]. In our previous study, we found that high glucose could induce the EMT in HPMCs [11]; however, the underlying molecular mechanisms are not yet fully understood.
microRNAs (miRNAs), which play a key role in regulating targeted genes, have been extensively studied as biomarkers in numerous human diseases [12]. Recent studies have demonstrated that miRNAs are related to the development of the EMT and fibrosis in various organs [13,14]. Among these miRNAs, miRNA-21 has been shown to be upregulated during HG stimulation [15], and it also induced organ fibrosis in liver [16], lungs [17], heart [18] and kidneys [19].
However, the effect of miRNA-21 in the EMT process in HPMCs is not completely understood. In the present study, we aimed to investigate the expression of miRNA-21 and phosphatase and tensin homolog (PTEN) in HPMCs, and investigate their potential roles in the EMT in HPMCs.
Materials and methods
Reagents
RPMI 1640, trypsin-EDTA and fetal bovine serum (FBS) were obtained from Gibco. Bovine serum albumin (BSA) was purchased from Sigma. Rabbit monoclonal α-SMA, rabbit monoclonal E-cadherin, rabbit monoclonal PTEN, mouse monoclonal FN and mouse monoclonal β-actin antibodies were obtained from Abcam. Goat anti-rabbit and goat anti-mouse HRP-conjugated secondary antibodies and FITC-conjugated secondary antibody were purchased from Beyotime biotechnology (Jiangsu, China). The enhanced chemiluminescence (ECL) kit was purchased from Pierce Biotechnology, Inc. miRNA 21 inhibitor/mimic and miRNA 21 inhibitor/mimic negative controls were purchased from Guangzhou RiboBio Co., Ltd (China). Trizol, Lipofectamine 2000 transfection reagent and DAPI were obtained from Invitrogen.
Culture of HPMCs
As mentioned before [11], HPMCs were cultured in RPMI 1640 medium supplemented with 10% FBS, streptomycin and penicillin. Cells were cultured at 37°C with a 5% CO2 atmosphere, the RPMI1640 medium was refreshed every 2-3 days. HPMCs were then digested and passaged with a sub-cultivation ratio of 1:3 to 1:4. HPMCs were cultured with HG of different concentrations (control group, 1.5% HG group, 2.5% HG group, 4.25% HG group) for 24 h, or 2.5% HG for different times (0 h, 6 h, 12 h, 24 h, 48 h). HPMCs were then transfected with miR21 mimic/inhibitor with or without HG treatment.
Transfections
HPMCs were transfected with human miRNA 21 inhibitor/mimic and negative controls using lipofectamine 2000 reagent according to the manufacturer’s instructions. Culture medium was removed after 24 h, and HPMCs were unstimulated or stimulated with HG for another 24 h, cells were then collected for real time PCR, western blotting and immunofluorescence staining.
Western blotting
Western blot analysis was carried out as previously mentioned [11]. The 50 ug proteins from HPMCs were separated by 10% SDS-PAGE at 70 V for 2 h and then transferred to a nitrocellulose membrane at 100 V for 1 h. After blocking with 5% non-fat dry milk, the membranes were incubated at 4°C overnight with dilution of 1:1,000 for primary antibodies and 1:5,000 for secondary antibodies. An ECL kit was used to develop the blots, and images were captured using UVP (G-BOX EF). We applied Image J software to measure the intensity of each band and relative expressions were normalized against β-actin. Experiments were repeated at least three times.
Real-time PCR
Trizol reagent was applied to extract total RNA according to the manufacturer’s protocol. A Reverse Transcription System kit was used to synthesize first-strand cDNA according to the manufacturer’s protocol (Takara). Real-time quantitative PCR was performed using the SYBR Premix Ex Taq II kit (Takara) with ABI 7500 Real-Time PCR System (Applied Biosystems, CA). The relative expression of each gene was measured after normalization with GAPDH. The cDNA sequences were amplified using specific primers, and the sequences were as follows: E-cadherin (sense) 5’-GGGCTGGACCGAGAGAGTTT-3’ and (antisense) 5’-CCTTGTACGTGGTGGGATTGA-3’; α-SMA (sense) 5’-ATCATCACCAACTGGGACGAC-3’ and (antisense) 5’-CTCTTCAGGGGCAACACGAA-3’; FN (sense) 5’-CCAAACCTCAAGCTCCCGTCA-3’ and (antisense) 5’-GAGATTCTGCACATCACGGTCA-3’; PTEN (sense) 5’-CGACGGGAAGACAAGTTCAT-3’ and (antisense) 5’-AGGTTTCCTCTGGTCCTGGT-3’; and GAPDH (sense) 5’-GCACCGTCAAGGCTGAGAAC-3’ and (antisense) 5’-TGGTGAACACGCCAGTGGA-3’.
Immunofluorescence staining with E-cadherin
Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.25% Triton X-100 for 10 min, then incubated with 5% BSA for 30 min at room temperature. HPMCs were then incubated with the primary antibody rabbit anti-E-cadherin (1:200) overnight at 4°C in a humidified chamber. After 3 washes with PBS for 5 min, HPMCs were incubated in FITC-conjugated secondary antibody (1:50) for 1 h at room temperature. The nuclei were stained with DAPI. Images were obtained using a fluorescence microscope (Nikon ECLIPSE Ti).
Statistical analyses
SPSS (version 18) software were used for data analysis. Quantitative data were expressed as means ± SEM. Tukey’s multiple comparison test was used for individual comparisons; standard ANOVA methodology was used for multiple group comparisons (P < 0.05 was considered to be significant).
Results
HG induced the EMT and increased miRNA-21 expression in HPMCs
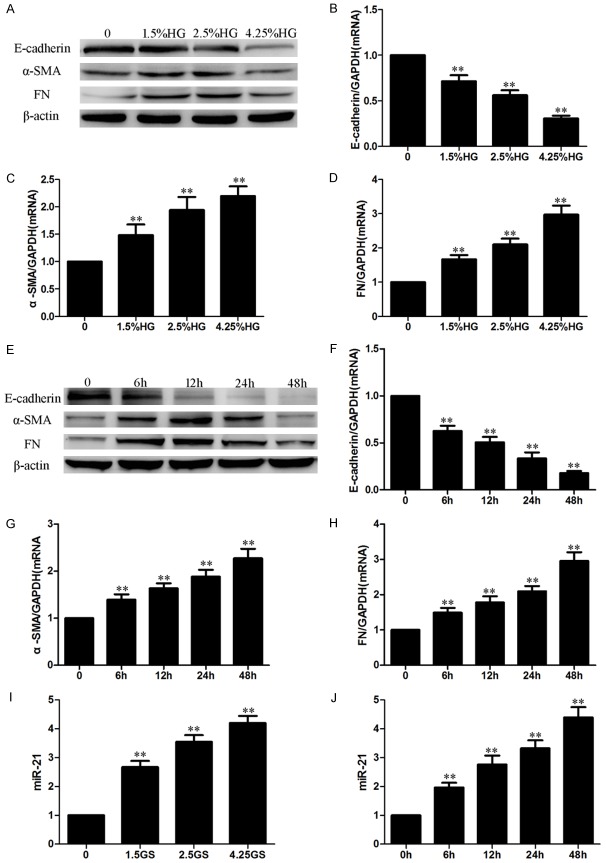
In order to explore the role of the HG-induced EMT in HPMCs, we measured the expression of EMT biomarkers in HG-treated HPMCs. Compared with the control group, HG significantly increased the expression of α-SMA and FN, and decreased the expression of E-cadherin in a dose-dependent (Figure 1A-D) and time-dependent manner (Figure 1E-H) in HPMCs, which means that HG treatment induced the EMT in HPMCs. Figure 1I, 1J showed that HG treatment also remarkably increased miR-21 expression in a dose- and time-dependent manner.
Figure 1.
HG induced the EMT and increased the level of miRNA-21 in HPMCs. HPMCs were cultured with HG of different concentrations (0, 1.5%, 2.5%, 4.25%) for 24 h, or 2.5% HG for different times (0 h, 6 h, 12 h, 24 h, 48 h). (A-D) HG induced the EMT in HPMCs in a dose-dependent manner (A: Western blot; B-D: Real-time PCR). (E-H) HG induced the EMT in HPMCs in a time-dependent manner (E: Western blot; F-H: Real-time PCR). (I, J) HG increased the level of miRNA-21 in a dose-dependent (I) and time-dependent (J) manner (real-time PCR). Each value represents the mean ± SEM (n=6) (**P < 0.01 vs. control).
The miR-21 mimic exacerbated the HG-induced EMT in HPMCs
To confirm the role of miR-21 in the EMT in HPMCs, the miR-21 mimic and negative control were transfected into HPMCs; cells were then cultured with or without HG for another 24 h. Western blotting, real-time PCR and immunofluorescence indicated that the miR-21 mimic significantly increased the levels of α-SMA and FN, and decreased the level of E-cadherin (Figure 2A-D), which suggests that the miR-21 mimic can induce the EMT. Moreover, the miR-21 mimic exacerbated the progress of the HG-induced EMT (Figure 2E-I). These data demonstrated that miR-21 exacerbated the HG-induced EMT in HPMCs.
Figure 2.
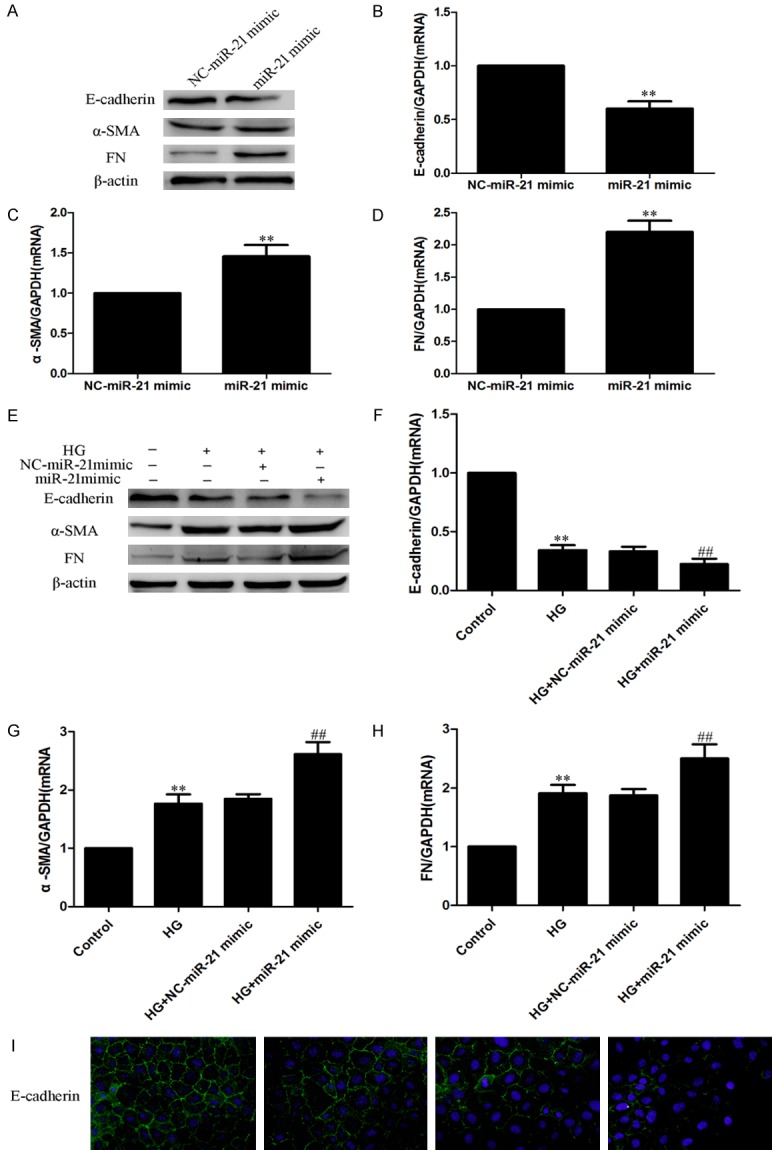
The miR-21 mimic >increased the HG-induced EMT in HPMCs. HPMCs were transfected with miR21 mimic with or without 2.5% HG treatment. (A-D) Overexpression of miR-21 >exacerbated the EMT in HPMCs. (A: Western blot; B-D: Real-time PCR). (E-I) Overexpression of miR-21 >exacerbated the HG-induced EMT in HPMCs. (E: Western blot; F-H: Real-time PCR; I: Immunofluorescence staining of E-cadherin, magnification × 200). Each value represents the mean ± SEM (n=6) (**P < 0.01 vs. control; ##P < 0.01 vs. HG group).
The miR-21 inhibitor alleviated the HG-induced EMT in HPMCs
To further explore the role of miR-21 in the EMT, the miR-21 inhibitor and negative control were transfected into HPMCs; cells were then cultured with or without HG for another 24 h. Western blotting, real-time PCR and immunofluorescence showed that the miR-21 inhibitor significantly alleviated the upregulation of the mesenchymal markers α-SMA and FN and reduced the downregulation of the epithelial marker E-cadherin in HG-treated HPMCs (Figure 3A-E). Therefore, this shows that the miR-21 inhibitor can decrease the HG-induced EMT in HPMCs.
Figure 3.
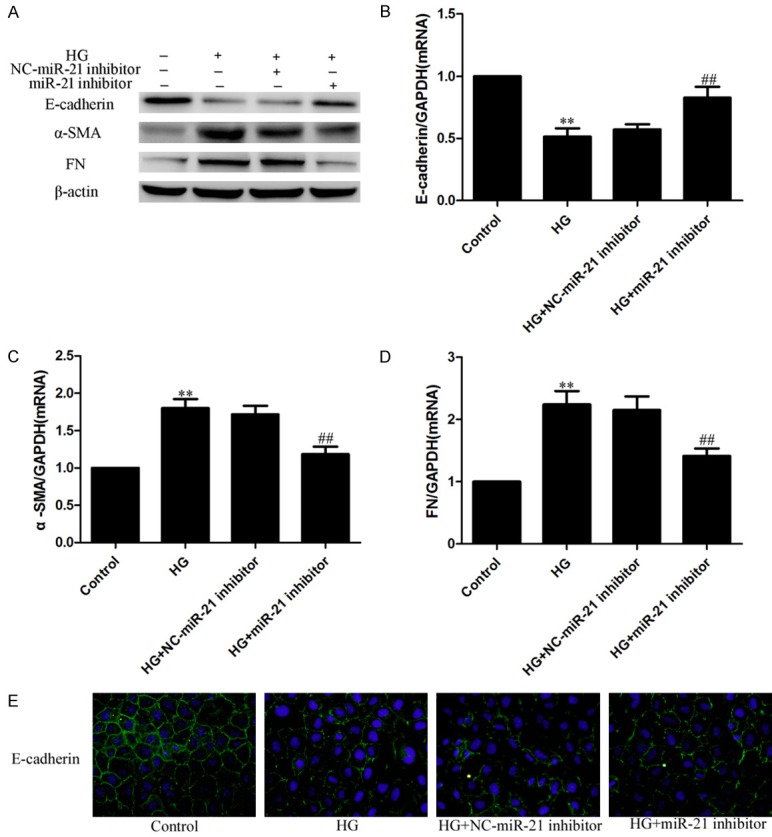
The miR-21 inhibitor alleviated the HG-induced EMT in HPMCs. HPMCs were transfected with miR21 inhibitor followed by 2.5% HG treatment. A: Western blots; B-D: Real-time PCR. Each value represents the mean ± SEM (n=6) (**P < 0.01 vs. control; ##P < 0.01 vs. HG group). E: Immunofluorescence staining of E-cadherin. Magnification × 200.
miR-21 directly targeted PTEN in the HG-induced EMT in HPMCs
To verify whether miR-21 targeted PTEN in the HG-induced EMT, PTEN expression was measured after miR-21 mimic and inhibitor transfection in HPMCs with or without HG treatment. Real-time PCR and Western blotting showed that the miR-21 mimic downregulated PTEN expression (Figure 4A, 4B), and that the miR-21 inhibitor upregulated PTEN expression (Figure 4C, 4D).
Figure 4.
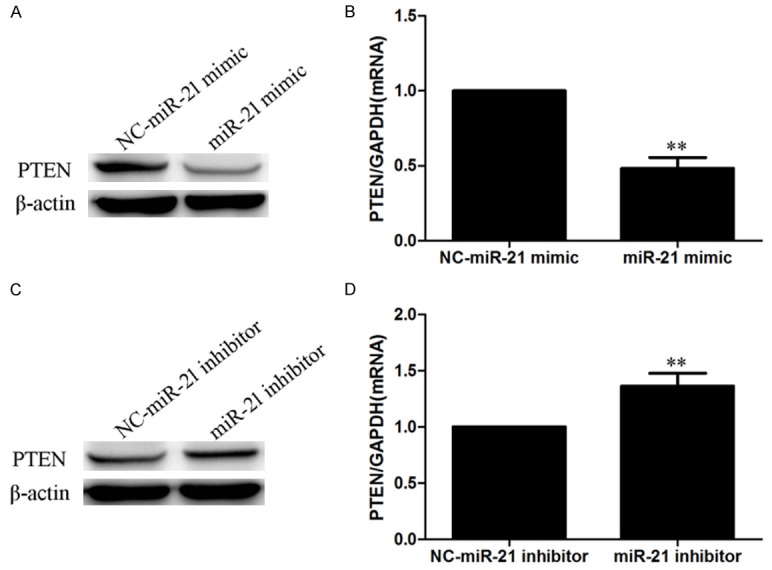
PTEN is a target of miR-21. HPMCs were transfected with miR21 mimic/inhibitor. (A, B) The miR-21 mimic inhibited PTEN expression (A: Western blot; B: Real-time PCR). (C, D) The miR-21 inhibitor upregulated PTEN expression (A: Western blot; B: Real-time PCR). (C: Western blot; D: Real-time PCR). Each value represents the mean ± SEM (n=6) (**P < 0.01 vs. miR-21 negative control).
HG inhibited PTEN expression in a dose- and time-dependent manner (Figure 5A-D). PTEN expression was also measured after miR-21 mimic/inhibitor transfection in HG-treated HPMCs. Compared to the HG group, the miR-21 mimic further inhibited PTEN expression in HG-treated HPMCs (Figure 5E, 5F); the miR-21 inhibitor partly restored the expression of PTEN in HG-treated HPMCs (Figure 5G, 5H). These results demonstrated that miR-21 directly targeted PTEN in HG-treated HPMCs.
Figure 5.
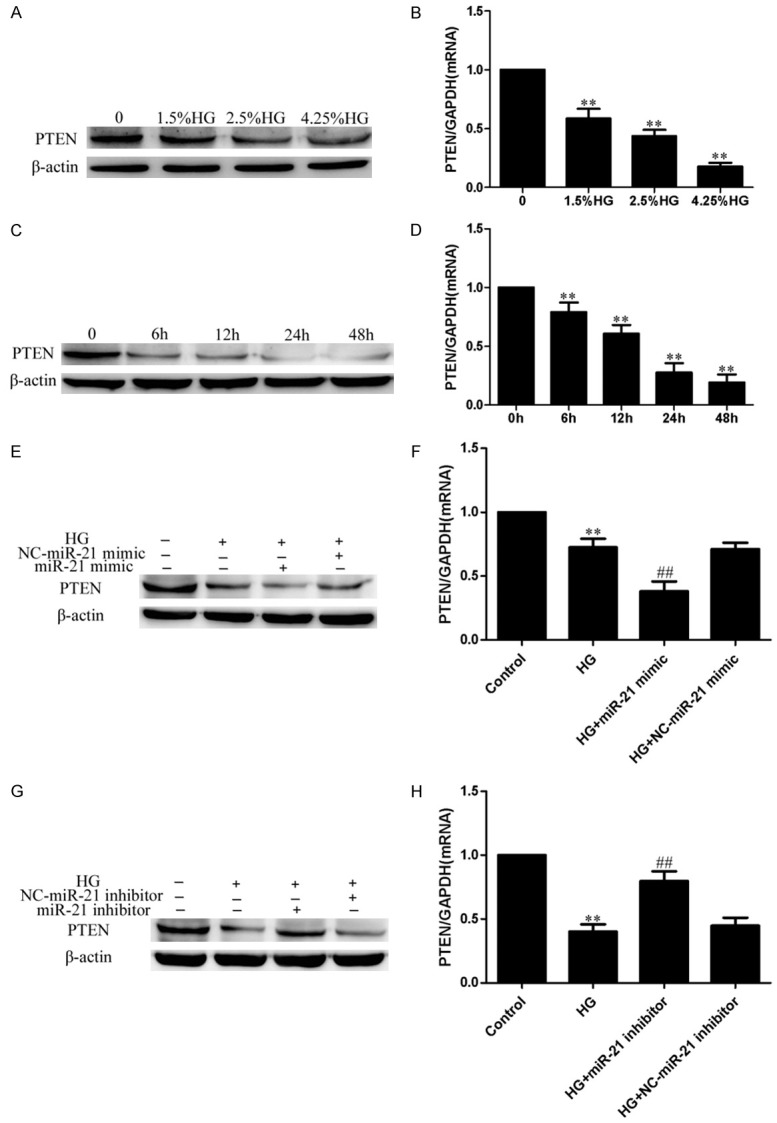
miR-21 directly targeted PTEN in HG-treated HPMCs. HPMCs were cultured with HG of different concentrations (0, 1.5%, 2.5%, 4.25%) for 24 h, or 2.5% HG for different times (0 h, 6 h, 12 h, 24 h, 48 h), then were transfected with miR21 mimic/inhibitor followed by 2.5% HG treatment. (A, B) HG inhibited PTEN expression in a dose-dependent manner (A: Western blot; B: Real-time PCR). (C, D) HG inhibited PTEN expression in a time-dependent manner (C: Western blot; D: Real-time PCR). (E, F) The miR-21 mimic further inhibited PTEN expression in HG-treated HPMCs (E: Western blot; F: Real-time PCR). (G, H) The miR-21 inhibitor partly restored the expression of PTEN in HG-treated HPMCs (G: Western blot; H: Real-time PCR). Each value represents the mean ± SEM (n=6) (**P < 0.01 vs. control; ##P < 0.01 vs. HG group).
Discussion
PD is extensively used for the long-term treatment of ESRD; however, HG solutions can lead to morphological and functional injury in the peritoneum, and may eventually cause peritoneal fibrosis and ultrafiltration failure. The HG-induced EMT in HPMCs plays a crucial role in peritoneal dysfunction and fibrosis [8]. Therefore, preventing the EMT could alleviate peritoneal fibrosis, protecting the peritoneal mesothelium in patients undergoing PD [9].
Increasingly more research have shown that miRNAs play a vital role in the process of EMT [20,21]. miRNA-21 is present in plasma and peritoneal fluid [22-24]. Previous studies have indicated that miRNA21 plays a crucial role in the process of EMT and peritoneal fibrosis in PD [25,26]. However, the molecular mechanisms of miR-21 in the EMT in HPMCs are still poorly understood.
In the current study, we investigated how miRNA-21 could contribute to the EMT in HG-treated HPMCs. We found that HG could induce the EMT in HPMCs, with a concomitant increase in the level of miRNA-21. We also showed that miR-21 could induce the EMT, and the miR-21 mimic increased this HG-induced EMT in HPMCs, while the miR-21 inhibitor attenuated the HG-induced EMT in HPMCs. Overall, we demonstrated that miRNA-21 was involved in the HG-induced EMT in HPMCs.
microRNAs affect organ EMT and fibrosis by regulating the expression of target genes [27]. Studies have shown that PTEN is related to the miR-21-induced EMT and that PTEN is a direct targeted gene of miR-21 [28-30]. Therefore, we explored whether miR-21 was able to regulate the EMT by targeting PTEN. We measured PTEN expression in this context, and found that miR-21 directly targeted PTEN in the HG-induced EMT in HPMCs.
In summary, this investigation has shown that miR-21 plays a vital role in the HG-induced EMT in HPMCs, by targeting PTEN. According to the current investigation, we found that miR-21 could be a promising predictor for diagnosing and treating the effects of HG.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 81300636 and 81370865).
Disclosure of conflict of interest
None.
References
- 1.Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortier S, De Vriese AS, Lameire N. Recent concepts in the molecular biology of the peritoneal membrane-implications for more biocompatible dialysis solutions. Blood Purif. 2013;21:14–23. doi: 10.1159/000067867. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad M, Shah H, Pliakogiannis T, Oreopoulos DG. Prevention of membrane damage in patient on peritoneal dialysis with new peritoneal dialysis solutions. Int Urol Nephrol. 2007;39:299–312. doi: 10.1007/s11255-006-9064-y. [DOI] [PubMed] [Google Scholar]
- 4.Krediet RT, Struijk DG. Peritoneal changes in patients on long-term peritoneal dialysis. Nat Rev Nephrol. 2013;9:419–429. doi: 10.1038/nrneph.2013.99. [DOI] [PubMed] [Google Scholar]
- 5.Ding X, Ma M, Teng J, Shao F, Teng RK, Zhou S, Zhang Y, Wu E, Wang X. Numb induces e-cadherin adhesion dissolution, cytoskeleton reorganization, and migration in tubular epithelial cells contributing to renal fibrosis. Curr Mol Med. 2015;15:368–379. doi: 10.2174/1566524015666150505162015. [DOI] [PubMed] [Google Scholar]
- 6.Zhao S, Zhang Y, Zheng X, Tu X, Li H, Chen J, Zang Y, Zhang J. Loss of microRNA-101 promotes epithelial to mesenchymal transition in hepatocytes. J Cell Physiol. 2015;230:2706–2717. doi: 10.1002/jcp.24995. [DOI] [PubMed] [Google Scholar]
- 7.Strippoli R, Loureiro J, Moreno V, Benedicto I, Lozano ML, Barreiro O, Pellinen T, Minguet S, Foronda M, Osteso MT, Calvo E, Vázquez J, Cabrera ML, Del Pozo MA. Caveolin-1 deficiency induces a MEK-ERK1/2-Snail-1-dependent epithelial-mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Mol Med. 2015;7:357. doi: 10.15252/emmm.201570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V, Castro MA, del Peso G, Cirujeda A, Gamallo C, Sánchez-Madrid F, López-Cabrera M. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–413. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- 9.Yu MA, Shin KS, Kim JH, Kim YI, Chung SS, Park SH, Kim YL, Kang DH. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol. 2009;20:567–581. doi: 10.1681/ASN.2008040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zeng L, Zhao Y, Zhu B, Ren W, Wu C. Selenium suppresses lipopolysaccharide-induced fibrosis in peritoneal mesothelial cells through inhibition of epithelial-to-mesenchymal transition. Biol Trace Elem Res. 2014;161:202–209. doi: 10.1007/s12011-014-0091-8. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Wu L, Zhang X, Hu Y, Fan Y, Ma J. 25(OH)2D3/VDR attenuates high glucose induced epithelial mesenchymal transition in human peritoneal mesothelial cells via the TGFβ/Smad3 pathway. Mol Med Rep. 2017;15:2273–2279. doi: 10.3892/mmr.2017.6276. [DOI] [PubMed] [Google Scholar]
- 12.Liang Z, Xi Y. MicroRNAs mediate therapeutic and preventive effects of natural agents in breast cancer. Chin J Nat Med. 2016;14:881–887. doi: 10.1016/S1875-5364(17)30012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Ye H, Xiang F, Song LJ, Zhou LL, Cai PC, Zhang JC, Yu F, Shi HZ, Su Y, Xin JB, Ma WL. miR-18a-5p inhibits sub-pleural pulmonary fibrosis by targeting TGF-β receptor II. Mol Ther. 2017;25:728–738. doi: 10.1016/j.ymthe.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, Wang G, Zhou H, Cai J, Li P, Zhou M, Lu Y, Jiang X, Huang H, Zhang Y, Gong A. TGF-β1-miR-200a-PTEN induces epithelial-mesenchymal transition and fibrosis of pancreatic stellate cells. Mol Cell Biochem. 2017;431:161–168. doi: 10.1007/s11010-017-2988-y. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Fan Q, Xu L, Li L, Yue Y, Xu Y, Su Y, Zhang D, Wang L. Ursolic acid attenuates diabetic mesangial cell injury through the up-regulation of autophagy via miRNA-21/PTEN/Akt/mTOR suppression. PLoS One. 2015;10:e0117400. doi: 10.1371/journal.pone.0117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noetel A, Kwiecinski M, Elfimova N, Huang J, Odenthal M. MicroRNA are central players in anti- and profibrotic gene regulation during liver fibrosis. Front Physiol. 2012;3:49. doi: 10.3389/fphys.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brønnum H, Andersen DC, Schneider M, Sandberg MB, Eskildsen T, Nielsen SB, Kalluri R, Sheikh SP. MiR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving programmed cell death 4 and sprouty-1. PLoS One. 2013;8:e56280. doi: 10.1371/journal.pone.0056280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice JM, Bouyé S, Hazzan M, Pottier N, Perrais M, Aubert S, Cauffiez C. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One. 2013;8:e58014. doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Li H, Liu X, Xu D, Wang F. MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells by targeting AKT2. Exp Cell Res. 2016;345:115–124. doi: 10.1016/j.yexcr.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 22.Kanaan Z, Rai SN, Eichenberger MR, Roberts H, Keskey B, Pan J, Galandiuk S. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544–551. doi: 10.1097/SLA.0b013e318265bd6f. [DOI] [PubMed] [Google Scholar]
- 23.Cappellesso R, Tinazzi A, Giurici T, Simonato F, Guzzardo V, Ventura L, Crescenzi M, Chiarelli S, Fassina A. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122:685–693. doi: 10.1002/cncy.21442. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H, Kosuga T, Iitaka D, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2011;105:104–111. doi: 10.1038/bjc.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Anton M, Lambie M, Lopez-Cabrera M, Schmitt CP, Ruiz-Carpio V, Bartosova M, Schaefer B, Davies S, Stone T, Jenkins R, Taylor PR, Topley N, Bowen T, Fraser D. miR-21 promotes fibrogenesis in peritoneal dialysis. Am J Pathol. 2017;187:1537–1550. doi: 10.1016/j.ajpath.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Szeto CC, Chow KM, Kwan BC, Cheng PM, Luk CC, Ng JK, Law MC, Leung CB, Li PK. Peritoneal dialysis effluent miR-21 and miR-589 levels correlate with longitudinal change in peritoneal transport characteristics. Clin Chim Acta. 2017;464:106–112. doi: 10.1016/j.cca.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Zou XZ, Liu T, Gong ZC, Hu CP, Zhang Z. MicroRNAs-mediated epithelial-mesenchymal transition in fibrotic diseases. Eur J Pharmacol. 2017;796:190–206. doi: 10.1016/j.ejphar.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L, Wu M, Su C, Chen J. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett. 2013;337:226–236. doi: 10.1016/j.canlet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Xu B. MicroRNA-21 identified as predictor of cancer outcome: a meta-analysis. PLoS One. 2014;9:e103373. doi: 10.1371/journal.pone.0103373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radio resistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]