Abstract
Growing evidence supports the notion that serum NAPDH oxidase 2 (NOX2) is an important regulator that contributes to the initiation and progression of various types of diseases. However, so far, it remains elusive about the relationship between levels of serum NOX2 and HBV-related diseases. The overall purpose of the study is to get a better insight into whether or not serum NOX2 is involved in HBV-related disorders. Serum levels of NOX2, from 105 patients with chronic hepatitis B, 58 patients with HBV-related cirrhosis, 48 patients with HBV-related hepatocellular carcinoma and 104 healthy individuals, were measured with sandwich ELISA kits that we developed. In this study, we found that NOX2 values were significantly higher in patients compared to healthy control (P < 0.01) and that the levels of serum NOX2 were significantly correlated with the serum levels of superoxide dismutase (SOD), interleukin-6 (IL-6), interferon-stimulated gene 15 (ISG15), alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT) in chronic hepatitis B, cirrhosis and hepatocellular carcinoma patients. Interestingly, we found that a significant positive correlation between NOX2 and HBV viral load only in patients with chronic hepatitis B and cirrhosis. Therefore, Serum NOX2 levels maybe an important indicator of the pathogenesis of progression of HBV-related disorders.
Keywords: NOX2, ROS, Hepatitis B virus, chronic hepatitis B, cirrhosis, hepatocellular carcinoma
Introduction
Hepatitis B virus (HBV) infection is a major global public health issue, which leads to acute and chronic hepatitis, liver cirrhosis and hepatocellular carcinoma (HCC). Note that (i) approximately 2 billion people have been infected with HBV worldwide [1], (ii) more than 240 million people have been developed chronic HBV infection [2], and (iii) 25% of HBV-infected children will develop HBV-related cirrhosis and HCC as adults [1]. Remarkably, it has been reported that nearly 780,000 people die every year due to HBV-related complications such as liver cirrhosis and HCC [3]. However, despite the widespread distribution of HBV infection and its serious complications related to the persistent infection with the virus, there are limited effective treatment options available for HBV related disorders. Therefore, to ameliorate the disease burden of HBV infection, it may be crucial to early identify patients at risk of development to cirrhosis and HCC, which could allow clinicians to initiate early preventative treatment.
Reactive oxygen species (ROS) are a group of short-lived and highly reactive molecules including hydrogen peroxide (H2O2), superoxide anion (O2 •-) and hydroxyl radicals (•OH), produced as natural byproducts of cellular metabolism [4]. Over the years, it is widely accepted that an imbalance between ROS generation and detoxification can result in disturb the cellular redox homeostasis, which in turn can initiate and promote a variety of diseases such as ischemia reperfusion injury, atherosclerosis, neurodege-neration, cancer, and hypertension [5-7]. However, there is also mounting evidence that ROS may act as important intracellular signaling intermediates in diverse physiological processes, such as cell development, differentiation and survival [6]. For example, it has been demonstrated that reactive molecules H2O2 play an important role in cytokine-, insulin-, growth factor-, and NF-kB-mediated signaling pathways [8,9]. At present, growing evidence indicates that HBV infection and cellular oxidative stress are closely intertwined [10]. This is perhaps best illustrated by the observation that HBV infection related mitochondrial ROS production triggers the up-regulated expression of the transcription factor Snail, which in turn results in the repression of suppressor of cytokine signaling 3 (SOCS3) via DNA methylation with DNMT1 and HDAC1 [11]. Moreover, a recent study by Yu et al. has shown that HBV infection suppresses lipopolysaccharide (LPS)-induced NLRP3 inflammasome activation and IL-1β expression through inhibiting ROS production and the NF-κB signaling pathway [12]. However, the mechanisms underlying how ROS participate in the pathogenesis of HBV-related diseases remain to be established. Therefore, more future research is needed to elucidate the role of ROS in HBV-related pathological processes.
NADPH oxidase 2 (NOX2), encoded by the CYBB gene, is a key protein of the innate immune system via generating superoxide anion [13]. So far, it has been reported that the protein is mainly present in monocytes, leukocytes, platelets and endothelial cells [14]. The significance of NOX2 for human health and development is best illustrated by the existence of severe inherited metabolic diseases (e.g. chronic granulomatous disease) that are caused by a partial or complete dysfunction of the enzyme [15]. Over the past few decades, NOX2 is considered as a critical regulator in mediating the generation of ROS, which plays a pivotal role in development of cardiovascular diseases [16,17]. This hypothesis is mainly based on the observations that (i) the circulating serum levels of NOX2 in hypercholesterolemic patients are significantly higher than those in healthy controls [14], and (ii) a significant correlation between up-regulation of serum NOX2 levels and cardiac Troponin T elevation is observed in patients with myocardial injury [18]. Furthermore, a study by Martinez et al. has shown that NOX2 is a key component of the LC3-associated phagocytosis (LAP) pathway, and that mice and human lacking NOX2 initiate and drive the pathogenesis of systemic lupus erythematosus (SLE) [19].
Recently, it has become increasingly clear that virus infection and NOX2-induced oxidative stress are inextricably linked processes. For example, it has been reported that (i) inhibition of NOX2 oxidase activity suppresses influenza A virus replication in vivo, which in turn ameliorates virus-induced lung inflammation [20]; (ii) NOX2 is a crucial player in Human respiratory syncytial virus (RSV) and Sendai virus-induced NF-κB activation via the phosphorylation of IκBα at Ser32 and of p65 at Ser536 [21]; and (iii) HIV-1 infection can lead to the increased platelet oxidative stress in vivo, which is associated with the activation of NOX2 [22]. However, to date, the interplay between HBV infection and NOX2 remains to be determined. In this context, it is also noteworthy that very little is known about the role of NOX2 in HBV-related diseases.
In this study, we aim to investigate the changes of serum levels of NOX2 in patients with HBV-related disorders and get a better insight into the relationship between serum levels of NOX2 and HBV-related complications.
Material and methods
Human subjects
From February 2015 to April 2017, 105 chronic hepatitis B (CHB) patients, 58 patients diagnosed with HBV-related cirrhosis, 48 HBV-related hepatocellular carcinoma (HCC) patients, 42 patients with alcoholic liver cirrhosis (ALC), 54 patients with alcoholic cirrhosis who abstained from alcohol after contact with the Gastroenterology & Hepatology unit, 39 patients with non-HBV HCC and 104 healthy individuals were enrolled in the study. Written informed consent was obtained from each subject. Patients with CHB, cirrhosis and HCC were diagnosed in accordance with the guidelines on prevention and treatment of chronic hepatitis B (2015 version), issued by the Chinese society of infectious Diseases and Chinese society of Hepatology of Chinese Medical Association. The diagnosis of CHB was based on detectable hepatitis B surface antigen (HBsAg)-positive or HBV-DNA for more than six months. In our study, we also employed imaging techniques including computerized tomography (CT) and magnetic resonance imaging (MRI) to distinguish CHB and other hepatic pathology. The diagnosis of cirrhosis and HCC was confirmed by histological examination of the resected material from patients. The study was carried out according to the principles of the Declaration of Helsinki and was approved by the medical ethics committee of the Second Affiliated Hospital of Chongqing Medical University. The clinical characteristics of study subjects are demonstrated in Table 1.
Table 1.
Clinical characteristics of subjects in this study
| Healthy individuals | CHB patients | Cirrhosis patients | HCC patients | ||
|---|---|---|---|---|---|
| Number of subjects | 104 | 105 | 58 | 48 | |
| Age | (year, ranger) | 25-64 | 17-63 | 29-76 | 33-78 |
| (year, mean) | 39.3 | 37.2 | 49.3 | 51.5 | |
| Sex (male:female) | 58:46 | 64:41 | 40:18 | 36:12 | |
| HBsAg positive | No | Yes | Yes | Yes | |
| NOX 2 (mean ± SD) pg/ml | 930.64 ± 474.10 | 2944.10 ± 1078.74 | 3605.63 ± 857.92 | 4482.48 ± 1086.98 | |
| SOD (mean ± SD) U/ml | 168.1 ± 16.9 | 137.5 ± 31.0 | 135.8 ± 33.6 | 122.7 ± 33.8 | |
| IL-6 (mean ± SD) pg/ml | 1.69 ± 0.54 | 4.06 ± 1.48 | 16.98 ± 4.65 | 22.48 ± 7.19 | |
| ISG15 (mean ± SD) ng/m | 3.21 ± 0.93 | 6.74 ± 2.88 | 15.93 ± 5.30 | 9.21 ± 2.99 | |
| ALP (mean ± SD) U/L | 69.0 ± 17.0 | 94.2 ± 46.7 | 128.8 ± 43.6 | 210.2 ± 119.0 | |
| GGT (mean ± SD) U/L | 23.1 ± 13.3 | 58.5 ± 87.0 | 132.9 ± 71.7 | 188.9 ± 150.4 | |
| ALT (mean ± SD) U/L | 24.0 ± 14.3 | 118.7 ± 244.9 | 248.1 ± 361.9 | 93.5 ± 89.7 | |
| AST (mean ± SD) U/L | 24.1 ± 4.9 | 79.5 ± 117.8 | 240.66 ± 293.4 | 98.1 ± 103.1 | |
| HBV DNA (mean ± SD) copies/ml | - | 10242588 ± 24242785 | 2837049 ± 6971344 | 846385 ± 1833953 | |
Quantification of HBV DNA
The serum levels of HBV DNA were quantified by CFX managerTM software (Bio-Rad, California, USA) using the CFX ConnectTM real time PCR detection system (Bio-Rad, California, USA) according to the manufacturer’s specifications.
Liver function test
Hitachi Modular 7600 chemistry analyzer (Hitachi, Takyo, Japan) with the liver function test kit (Maccura, Sichuan, China) was employed to measure the liver enzymes activity in serum, including alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT).
Serum analysis of SOD
Serum levels of SOD were detected by Hitachi Modular 7600 chemistry analyzer (Hitachi, Takyo, Japan) with the SOD test kit (Medicalsystem Biotechnology, Ningbo, China).
Serum analysis of NOX2, IL-6 and ISG15
Serum soluble NOX2-derived peptide was measured by an ELISA method as previously described by Pignatelli and his colleagues [14]. Here we modified this protocol to evaluate the serum levels of NOX2 in HBV patients. 100 μL of Serum samples or standard (gp91phox peptide from the sequence LFNVEWCVNARVNNSDPYSVALSELGDRQNESYLNFARKRIKNPEG--GLYLAV of gp91phox; Abcam, USA) were added into each capture antibody-coated well (Anti-NOX2/gp91phox antibody; Abcam, USA) and incubated 2 hours at room temperature. After aspirating and washing each well 3 times, 100 μL of diluted detection antibody-conjugated HRP (Rabbit anti-human IgG H&G antibody; Abcam, USA) was added and incubated 1 hour at room temperature while gentle shaking. Repeating the aspiration/wash of each well, 100 μL of substrate solution was added and incubated at room temperature for 20 minutes. Next, adding 50 μL of stop solution (1 mol/L H2SO4) to each well. The absorbance of the colored solution of NOX2 was measured at 450 nm by using a MultiskanTM FC microplate reader (Thermo Scientific, MA, USA).
Serum IL-6 and ISG15 were analyzed utilizing human IL-6 ELISA kit (Multisciences, Hangzhou, China) and human ISG15 ELISA kit (LifeSpan Biosciences, Eching, Germany), according to the manufacturer’s guideline. The absorbance of IL-6 was also read at 450 nm. All samples were run in triplicate and the mean value was used for statistical analysis.
Statistical analysis
Statistics were performed using SPSS software, version 19.0 for windows (SPSS Inc, IL, USA). The biological and clinical characteristics of human subjects were shown as means ± SD (standard deviation). Two groups were compared with a Mann-Whitney test. Spearman’s rank correlation analysis was employed to analyze association between two variables. The significance level was chosen to be P < 0.01.
Results
Serum levels of NOX2 in patients and healthy individuals
As discussed before, it has been reported that NOX2 activity can affect influenza A virus replication in vivo [20]. However, so far, the crosstalk between HBV infection and NOX-derived oxidative stress remains to be established. In a first series of experiments, we examined whether or not the concentrations of serum NOX2 could be elevated in patients with HBV-related disorders. As shown in Figure 1A, we found that the serum levels of NOX2 in CHB patients (mean value: 2944.10 g/ml) were significantly higher than those of healthy individuals (mean value: 930.64 pg/ml, P < 0.01). Here, it is informative to note that (i) the serum concentrations of NOX2 in patients with CHB were considerably lower compared to those with HBV-related cirrhosis (mean value: 3605.63 pg/ml, P < 0.01), and (ii) the NOX2 values in patients with HCC were much higher than any other groups (mean value: 4482.48 pg/ml, P < 0.01, Figure 1A). Next, we analyzed the levels of NOX2 in patients with alcoholic liver cirrhosis. We observed that there was significant difference between patients with HBV-related cirrhosis and patients with alcoholic liver cirrhosis (mean value: 2995.95 pg/ml, P < 0.01, Figure 1B). Remarkably, the serum concentrations of NOX2 were significantly lower in patients with abstinence compared to those without abstinence (mean value: 2029.35 pg/ml, P < 0.01, Figure 1B). In addition, we also found that there was no significant difference between patients with HBV-related HCC and patients with non-HBV-related HCC (mean value: 4611.11 pg/ml, Figure 1C).
Figure 1.

Serum NOX2 concentrations in subjects. A. The scatter dot plot shows the serum levels of NOX2 in healthy individuals and patients with CHB, cirrhosis and HCC patients. B. The scatter dot plot demonstrates the serum levels of NOX2 in patients with HBV-related cirrhosis and alcoholic liver cirrhosis patients. C. The scatter dot plot shows the serum levels of NOX2 in patients with HBV-related HCC and non-HBV-related HCC. The middle horizontal line in the scatter dot plot represents the mean. And the horizontal lines below and above the mean line represent respectively the mean minus and plus one standard deviation. Statistical significance (P) is indicated.
Furthermore, to strengthen our observation that HBV infection can induce NOX2-related oxidative stress, we also investigate whether or not the presence of HBeAg in patients has an impact on the levels of serum NOX2. Our data demonstrated that serum NOX2 expression was clearly affected by the presence of HBeAg in patients with CHB and cirrhosis (Figure 2A, 2B). Intriguingly, no significant difference could be observed between HBeAg (-) and HBeAg (+) patients with HCC in Figure 2C.
Figure 2.

Serum NOX2 concentrations in HBeAg-positive and HBeAg-negative patients. A. The scatter dot plot shows the serum levels of NOX2 in patients with HBeAg-negative CHB and HBeAg-positive CHB. B. The scatter dot plot demonstrates the serum levels of NOX2 in patients with HBeAg-negative cirrhosis and HBeAg-positive cirrhosis. C. The scatter dot plot shows the serum levels of NOX2 in patients with HBeAg-negative HCC and HBeAg-positive HCC. The middle horizontal line in the scatter dot plot represents the mean. And the horizontal lines below and above the mean line represent respectively the mean minus and plus one standard deviation. Statistical significance (P) is indicated.
Correlation between serum levels of NOX2 and HBV viral load
To gain better insight into the link between serum NOX2 levels and HBV-related complications, we assessed whether or not NOX2 is correlated with HBV viral load. In our setting, we found a moderately positive correlation between NOX2 and HBV viral load in patients with HBV-related cirrhosis (r = 0.3009, P < 0.0001). Regarding patients with CHB, our data indicated that there was a strong correlation between these two factors (r = 0.4581, P < 0.0001). Here, it should be noted that there was no correlation between NOX2 and HBV viral load in patients with HCC (r = 0.0141, P = 0.4208) (Figure 3).
Figure 3.

Correlation between NOX2 and HBV viral load in patients. A. Serum NOX2 levels are positively correlated with HBV viral load in patients with CHB. B. Serum NOX2 concentrations are moderately positively correlated with HBV viral load in patients with HBV-related cirrhosis. C. There is no correlation between NOX2 and HBV viral load in patients with HCC. Correlation coefficient (r) and statistical significance (P) are indicated.
Serum levels of SOD in patients and healthy individuals and correlation between serum levels of SOD and NOX2
Numerous clinical and experimental studies have provided clear evidence that there is a good relationship between type and severity of HBV-related diseases and SOD level in the blood [23]. For example, it has been reported that serum SOD levels were significantly reduced in patients with chronic HBV infection [23,24]. To further our understanding of the relationship between NOX2 and HBV-related diseases, we examined whether or not NOX2 is correlated with SOD. In these experiments, we first assessed the levels of serum SOD in patients with HBV-related disorders. As demonstrated in Figure 4A, our data indicated that (i) the levels of SOD in patients with HBV infection were significantly lower than those of healthy subjects, and (ii) serum SOD levels in CHB patients and in cirrhosis patients are comparable, but considerably higher than those in HCC patients. Here, one should keep in mind that our findings were consistent with the results from previous studies in which a slightly different experimental set-up was used [23,24]. In addition, we also found that there was a significant and negative relationship between the NOX2 values and SOD levels in patients with HBV-related liver disorders (Figure 4). In this context, it should be mentioned that there existed a strong correlation between NOX2 and SOD in HCC patients (r = 0.5087, P < 0.0001).
Figure 4.

Correlation between NOX2 and SOD in patients. A. The scatter dot plot shows the serum levels of SOD in CHB, cirrhosis and HCC patients. The middle horizontal line in the scatter dot plot represents the mean. And the horizontal lines below and above the mean line represent respectively the mean minus and plus one standard deviation. B. Serum NOX2 values are negatively correlated with SOD levels in patients with CHB. C. Serum NOX2 concentrations are negatively correlated with SOD levels in cirrhosis patients. D. Serum NOX2 values are negatively correlated with SOD levels in HCC patients. Correlation coefficient (r) and statistical significance (P) are indicated.
Serum levels of IL-6 and ISG15 in patients and healthy individuals and correlation between serum levels of IL-6 and NOX2
Currently, substantial evidence has been collected that the cellular inflammatory cytokines play a crucial role in development and progression of HBV-related disorders [25]. Importantly, given the fact that the levels of pro-inflammatory cytokine IL-6 can be up-regulated in HBV patients, one may also question whether the observed increases of IL-6 in patients with HBV-related complications are associated with NOX2-induced oxidative stress. Our experiments showed that (i) the levels of IL-6 in healthy subjects were significantly lower than those of HBV-infected patients (P < 0.01); (ii) serum IL-6 concentrations were significantly associated with the degree of HBV-related liver diseases, and (iii) a strong and positive correlation between NOX2 and IL-6 existed in CHB patients (r = 0.5445, P < 0.0001), cirrhosis patients (r = 0.4563, P < 0.0001) and HCC patients (r = 0.5991, P < 0.0001) (Figure 5).
Figure 5.

Correlation between NOX2 and IL-6 in patients. A. The scatter dot plot shows the serum levels of IL-6 in CHB, cirrhosis and HCC patients. The middle horizontal line in the scatter dot plot represents the mean. And the horizontal lines below and above the mean line represent respectively the mean minus and plus one standard deviation. B. Serum NOX2 values are positively correlated with IL-6 levels in patients in CHB patients. C. Serum NOX2 values are positively correlated with IL-6 levels in patients in cirrhosis patients. D. Serum NOX2 values are positively correlated with IL-6 levels in patients in HCC patients. Correlation coefficient (r) and statistical significance (P) are indicated.
Furthermore, several studies have indicated that ISG15 plays an important role in the progression of HBV-related liver diseases [26]. In this study, we confirm and extend these findings by showing that serum ISG15 levels in HBV patients were higher than those in healthy individuals and positively associated with HBV-related disorders (Figure 6). Here, it should be noted that the concentrations of serum ISG15 were correlated with serum NOX2 values in HBV patients (Figure 6).
Figure 6.
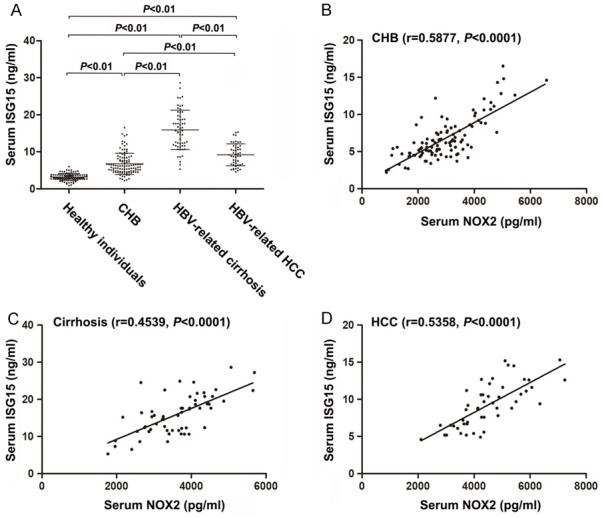
Correlation between NOX2 and ISG15 in patients. A. The scatter dot plot shows the serum levels of SG15 in CHB, cirrhosis and HCC pa-tients. The middle horizontal line in the scatter dot plot represents the mean. And the horizontal lines below and above the mean line represent respectively the mean minus and plus one standard deviation. B. Serum NOX2 values are positively correlated with ISG15 levels in patients in CHB patients. C. Serum NOX2 values are positively correlated with ISG15 levels in patients in cirrhosis patients. D. Serum NOX2 values are positively correlated with IL-6 levels in patients in HCC patients. Correlation coefficient (r) and statistical significance (P) are indicated.
Correlation between serum levels of NOX2 and liver enzyme markers
To gain a more complete picture of how changes in NOX2-induced oxidative insults may affect the levels of liver enzyme marker, including ALP and GGT, we here also investigated the serum levels of ALP and GGT in patients with HBV-related disorders. In a final series of experiments, we found that a strong significant correlation between NOX2 and ALP was observed in patients with CHB (r = 0.3196, P < 0.0001), cirrhosis (r = 0.4145, P < 0.0001), and HCC (r = 0.5823, P < 0.0001) (Figure 7). Interestingly, we also showed the same phenomenon regarding the relationship between NOX2 and GGT. As shown in Figure 8, a significant positive correlation between NOX2 and GGT was observed in CHB patients (r = 0.4318, P < 0.0001), cirrhosis patients (r = 0.4304, P < 0.0001) and HCC patients (r = 0.5119, P < 0.0001).
Figure 7.
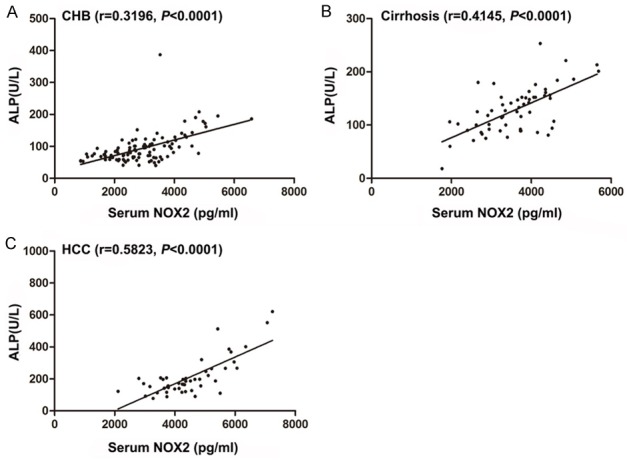
Correlation between NOX2 and ALP in patients. A. Serum NOX2 values are positively correlated with ALP levels in CHB patients. B. Serum NOX2 values are positively correlated with ALP levels in cirrhosis patients. C. Serum NOX2 values are positively correlated with ALP levels in HCC patients. Correlation coefficient (r) and statistical significance (P) are indicated.
Figure 8.

Correlation between NOX2 and GGT in patients. A. Serum NOX2 values are positively correlated with GGT levels in CHB patients. B. Serum NOX2 values are positively correlated with GGT levels in cirrhosis patients. C. Serum NOX2 values are positively correlated with GGT levels in HCC patients. Correlation coefficient (r) and statistical significance (P) are indicated.
Discussion
Recently, strong arguments have been put forward that a dysregulation of ROS may contribute to the etiology and progression of human pathologies related to HBV infection [11]. This postulate is mainly based on the observation that HBV infection-induced mitochondrial ROS accumulation down-regulates SOCS expression via Snail-mediated epigenetic silencing, which results in the sustained activation of IL-6/STAT3 pathway and initiation of hepatocarcinogenesis [11]. So far, the precise role of ROS on HBV infection is not yet clear and has generated much controversy. Mounting evidence has been collected that ROS can create a localized oxidative environment for facilitating HBV infection. This is perhaps best illustrated by the fact that ROS-triggered Hsp90 (heat shock protein 90) conformation change is a crucial for HBV production via promoting HBV capside assembly [27]. However, this model is challenged by a recent research group, who provide evidence that HBV infection inhibits ROS production and the NF-κB signaling pathway which in turn suppress LPS-induced NLRP3 inflammasome activation and IL-1β expression, and evade antiviral immune responses [12]. Regardless of this debate, it is widely assumed that HBV infection and cellular redox state are closely linked [10].
Over the years, it has become evident that NOX2, a major ROS-generating enzyme, can act as crucial players in cellular redox metabolism, and that the NOX2 is involved in a variety of diseases, including chronic granulomatous disease, myocardial infarction, and SLE [15,18,19]. Recently, more attention has focused on the relationship between NOX2 and virus infection. For example, Grandvaux and her colleagues have provided evidence that NOX2-related ROS are crucial to initiate an efficient RIG-I-mediated IRF-3 activation, which triggers the expression of antiviral genes IFNβ and IFIT1 [28]. Additionally, it has already shown that NOX2 plays a pivotal role in influenza a virus, RSV, Sendai virus and HIV infection [20-22]. However, until now, whether or not serum NOX2 is involved in HBV-related liver disorders remains to be determined. In this study, we present strong evidence indicating that the serum NOX2 concentrations are significantly higher in patients with HBV-related complications than those in healthy subjects. In addition, we also have shown that (i) serum NOX2 values are much lower in CHB patients compared to cirrhosis patients, and (ii) the levels of serum NOX2 are more elevated in patients with HCC than in patients with HBV-related cirrhosis. Therefore, our findings support the concepts that the serum NOX2 value may be a useful biomarker of the clinical severity in HBV-related disease, and that NOX2 plays an important role in the progression of HBV infection.
To further our understanding of the changes of serum NOX2 values in other types of liver diseases, we also examined the serum NOX concentration in patient with alcoholic liver cirrhosis and Non-HBV-related HCC. Interestingly, we found that a clear significant difference could be observed between patients with HBV-related cirrhosis and patients with alcoholic liver cirrhosis. Here, it should be mentioned that the serum NOX2 levels are significantly lower in patients with abstinence than those without abstinence. Furthermore, our data indicated that serum NOX2 levels were significantly up-regulated by the presence of HBeAg in CHB patients and cirrhosis patients. Importantly, it is well-known that HBeAg is an important indicator of active viral replication, and that it participates in persistent infection development via regulating adaptive immunity response [29,30]. Thus, these findings strongly support the hypothesis that HBV infection can induce the expression of serum NOX2, which in turn may participate in the progression of HBV-related disorders. However, there is no significant difference in serum NOX2 concentration between HBV-related HCC patients and Non-HBV-related HCC patients. This phenomenon may be explained by the fact that several important risk factors for developing HCC, including HCV, alcoholic liver disease, nonalcoholic fatty liver disease, and intake of aflatoxin contaminated food, are associated with an increase in oxidative stress [31,32]. For example, it has been demonstrated that (i) HCV core protein localized in the outer mitochondrial membrane and endoplasmic reticulum, which in turn enhance oxidative stress [33]; and (ii) alcohol abuse can induce mitochondrial injury, which result in an increase in production and propagation of ROS [34,35]. Note that no difference could be detected between HBeAg negative- and HBeAg positive- patients with HBV-related HCC. This observation stimulates the need to explore in more depth the mechanism underlying this.
Until now, substantial evidence supports the view that oxidative stree may serve as an important player in the pathogenesis of HBV-induced HCC [36]. In order to maintain intracellular and extracellular redox homeostasis, human body contains various antioxidant enzymes, including catalase, SOD, GSTK1 (glutathione S-transferase kappa 1), and peroxiredoxin V (PRDX5) [37]. Recently, growing evidience indicates that SOD plays a pivotal role in onset and progression of HBV-related diseases [23,24]. Chrobot et al. investigated the SOD activity in 100 children with chronic HBV and/or HCV infection, and found that the SOD activity is significantly decreased in children with viral hepatitis compare to the healthy individuals [38]. Additionally, a siginificant decrease of serum SOD activity is observed in patients with acute HBV infection, CHB and HBV-related cirrhosis [23]. Moreover, a recent study by Zhang et al. has demonstrated that serum SOD levels in HCC patients are much lower than those of HBV patients without HCC and healthy subjects [24]. Here, our data also confirm previous observation that the serum SOD levels in patients with CHB, cirrhosis and HCC are much lower than in healthy controls (Figure 4A). Interestingly, regarding serum levels of SOD, there is no difference between CHB patients and cirrhosis patients. Therefore, our findings imply that SOD may be a key biomarker for predicting risk and outcome of HBV-related complications. However, to our knowledge, few studies have assessed the relationship between NOX2 and SOD. In this work, we demonstrate that the serum concentrations NOX2 are significantly negatively correlated with the serum levels of SOD in patients with HBV-related disorders. In this context, it should be mentioned that a stronger correlation is observed between NOX2 and SOD in patients with HCC compared to the two factors in CHB patients (r = 0.5087 vs. 0.4352, respectively). Thus, our data suggest that an increase in serum NOX2 concentrations, combined with serum SOD levels, may become a useful marker for the activity of HBV-related liver diseases.
Currently, it is commonly accepted that the cytokine IL-6 acts as a double-edged sword in HBV-related disorders [39,40]. Several lines of evidence indicate that (i) IL-6 could impact on HBV infection by interference with viral DNA replication and HBV transcription [41,42], and (ii) IL-6 down-regulate the expression of the HBV reporter sodium taurocholate cotransporting polypeptide (NTCP), which results in the suppression of viral entry [43]. On the other hand, as a pro-inflammatory player, IL-6 is involved in initiation and development of HBV-induced cirrhosis and hepatocellular carcinoma [39]. This is nicely illustrated by the observation that IL-6 levels are significantly elevated in severe viral hepatitis, which riggers the activation of natural killer cells and CTLs. These lymphocytes may induce the killing of hepatocytes via necrosis and apoptosis [44]. Furthermore, a recent research work has revealed that IL-6 plays an important role in HBV-associated hepatocarcinogenesis via activation of the STAT3 pathway [11]. Although the effect of IL-6 on HBV infection is controversial, results from various clinical and experimental observations indicate that IL-6 concentrations are significantly higher in patients with HBV-related liver diseases [45-47]. Our findings are also in consistent with these previous reports suggesting that IL-6 is a sensitive indicator for assessing stage or severity of HBV-related complications. Important, we found a strong positive correlation between NOX2 levels and IL-6 concentrations in CHB patients (r = 0.5445, P < 0.0001), cirrhosis patients (r = 0.4563, P < 0.0001) and HCC patients (r = 0.5991, P < 0.0001). Several recent studies have reported that ISG15 acts as a proviral factor in HBV replication, and that this cytokine plays an important role in the progression of HBV-related liver diseases [26]. In our study, we also found that (i) serum ISG15 levels in HBV patients are significantly higher than those of healthy subjects; and (ii) serum ISG15 concentrations are significantly associated with the degree of HBV-related disorders. Importantly, we found that ISG15 levels are significantly positively correlated with the serum NOX2 concentrations in patients with HBV-related disorders. These findings suggest that a combination of NOX2, IL-6 and ISG15 is a suitable prediction tool in the diagnosis and risk assessment of HBV-related liver disorders.
Over the past few decades, it is well known that serum liver enzymes, ALP and GGT, may serve as important markers in HBV-induced liver inflammation and disease progression [48]. Moreover, these authors also showed that ALP and GGT are good parameters for predicting the prognosis of HCC patients [48]. Interestingly, our data also clearly indicate that serum NOX2 values have significant positive correlations with ALP and GGT in patients suffered from HBV-related diseases. Therefore, NOX2 in combination of ALP and GGT may enhance the assessment and prediction of clinical outcomes in HBV infection-related diseases.
In summary, our findings, combined with these previous reports, strongly indicate that serum NOX2 may be one of crucial mediators that contributes to the initiation and development of HBV-related liver diseases. These findings open up the way to potential new candidate for assessing clinical severity in HBV-related diseases. Importantly, further research with a large number of patients also needs to be done to investigate the molecular mechanisms underlying the role of NOX2 in HBV-related pathologies.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81501806), Chongqing Health and Family Planning Commission Foundation (No. 2015MSXM027), Chongqing Science and Technology Commission (No. cstc2017jcyjAx0083), and Chongqing Education Commission (No. KJ1500209).
Disclosure of conflict of interest
None.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging preventionand control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–28. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822:1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Schrader M, Fahimi HD. Growth and division of peroxisomes. Int Rev Cytol. 2006;255:237–290. doi: 10.1016/S0074-7696(06)55005-3. [DOI] [PubMed] [Google Scholar]
- 6.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Titorenko VI, Terlecky SR. Peroxisome metabolism and cellular aging. Traffic. 2011;12:252–259. doi: 10.1111/j.1600-0854.2010.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 9.Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2010;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- 10.Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan K, Lei Y, Chen HN, Chen Y, Zhang T, Li K, Xie N, Wang K, Feng X, Pu Q, Yang W, Wu M, Xiang R, Nice EC, Wei Y, Huang C. HBV-induced ROS accumulation promotes hepatocarcinogenesis through Snail-mediated epigenetic silencing of SOCS3. Cell Death Differ. 2016;23:616–627. doi: 10.1038/cdd.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Lan P, Hou X, Han Q, Lu N, Li T, Jiao C, Zhang J, Zhang C, Tian Z. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κb pathway and ROS production. J Hepatol. 2017;66:693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Ma MW, Wang J, Dhandapani KM, Brann DW. NADPH oxidase 2 regulates NLRP3 inflammasome activation in the brain after traumatic brain injury. Oxid Med Cell Longev. 2017;2017:6057609. doi: 10.1155/2017/6057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pignatelli P, Carnevale R, Cangemi R, Loffredo L, Sanguigni V, Stefanutti C, Basili S, Violi F. Atorvastatin inhibits gp91phox circulating levels in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2010;30:360–367. doi: 10.1161/ATVBAHA.109.198622. [DOI] [PubMed] [Google Scholar]
- 15.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 16.Brandes RP, Weissmann N, Schröder K. NA-DPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cangemi R, Calvieri C, Bucci T, Carnevale R, Casciaro M, Rossi E, Calabrese CM, Taliani G, Grieco S, Falcone M, Palange P, Bertazzoni G, Celestini A, Pignatelli P, Violi F SIXTUS study group. Is NOX2 upregulation implicated in myocardial injury in patients with pneumonia? Antioxid Redox Signal. 2014;20:2949–2954. doi: 10.1089/ars.2013.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, Li QZ, Yan M, Janke L, Guy C, Linkermann A, Virgin HW, Green DR. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533:115–119. doi: 10.1038/nature17950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 2011;7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink K, Duval A, Martel A, Soucy-Faulkner A, Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells. J Immunol. 2008;180:6911–6922. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- 22.Pastori D, Esposito A, Carnevale R, Bartimoccia S, Novo M, Fantauzzi A, Di Campli F, Pignatelli P, Violi F, Mezzaroma I. HIV-1 induces in vivo platelet activation by enhancing platelet NOX2 activity. J Infect. 2015;70:651–658. doi: 10.1016/j.jinf.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Irshad M, Chaudhuri PS, Joshi YK. Superoxide dismutase and total anti-oxidant levels in various forms of liver diseases. Hepatol Res. 2002;23:178–184. doi: 10.1016/s1386-6346(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Lu Y, Rong C, Yang D, Li S, Qin X. Role of superoxide dismutase in hepatitis B virus-related hepatocellular carcinoma. J Res Med Sci. 2016;21:94. doi: 10.4103/1735-1995.192510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Liu J, Xiong Y, Yan Y, Sun B, Zhao Q, Duan L, Li P, Huang Y, Chen W. Soluble E-cadherin as a serum biomarker in patients with HBV-related liver diseases. Clin Biochem. 2016;49:1232–1237. doi: 10.1016/j.clinbiochem.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Hoan NX, Van Tong H, Giang DP, Toan NL, Meyer CG, Bock CT, Kremsner PG, Song LH, Velavan TP. Interferon-stimulated gene 15 in hepatitis B-related liver diseases. Oncotarget. 2016;7:67777–67787. doi: 10.18632/oncotarget.11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YS, Seo HW, Jung G. Reactive oxygen species promote heat shock protein 90-mediated HBV capsid assembly. Biochem Biophys Res Commun. 2015;457:328–333. doi: 10.1016/j.bbrc.2014.12.110. [DOI] [PubMed] [Google Scholar]
- 28.Soucy-Faulkner A, Mukawera E, Fink K, Martel A, Jouan L, Nzengue Y, Lamarre D, Vande Velde C, Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Int. 2009;3:425–433. doi: 10.1007/s12072-009-9140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu B, Zhang B, Wang L, Ma C, Liu X, Zhao Y, Jiao Y. Hepatitis b virus e antigen regulates monocyte function and promotes b lymphocyte activation. Viral Immunol. 2017;30:35–44. doi: 10.1089/vim.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Suppl 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 32.Takaki A, Yamamoto K. Control of oxidative stress in hepatocellular carcinoma: helpful or harmful? World J Hepatol. 2015;7:968–979. doi: 10.4254/wjh.v7.i7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127–2133. doi: 10.1002/hep.22269. [DOI] [PubMed] [Google Scholar]
- 34.Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- 35.Sidharthan S, Kottilil S. Mechanisms of alcohol-induced hepatocellular carcinoma. Hepatol Int. 2014;8:452–457. doi: 10.1007/s12072-013-9494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida N, Kudo M. Oxidative stress and epigenetic instability in human hepatocarcinogenesis. Dig Dis. 2013;31:447–453. doi: 10.1159/000355243. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Van Veldhoven PP, Brees C, Rubio N, Nordgren M, Apanasets O, Kunze M, Baes M, Agostinis P, Fransen M. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radic Biol Med. 2013;65:882–894. doi: 10.1016/j.freeradbiomed.2013.08.173. [DOI] [PubMed] [Google Scholar]
- 38.Chrobot AM, Szaflarska-Szczepanik A, Drewa G. Antioxidant defense in children with chronic viral hepatitis B and C. Med Sci Monit. 2000;6:713–718. [PubMed] [Google Scholar]
- 39.Lan T, Chang L, Wu L, Yuan YF. IL-6 plays a crucial role in HBV infection. J Clin Transl Hepatol. 2015;3:271–276. doi: 10.14218/JCTH.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falasca K, Ucciferri C, Dalessandro M, Zingariello P, Mancino P, Petrarca C, Pizzigallo E, Conti P, Vecchiet J. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36:144–150. [PubMed] [Google Scholar]
- 41.Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S, Kirschning CJ, Langenkamp A, Falk C, Büning H, Rose-John S, Protzer U. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 42.Kuo TM, Hu CP, Chen YL, Hong MH, Jeng KS, Liang CC, Chen ML, Chang C. HBV replication is significantly reduced by IL-6. J Biomed Sci. 2009;16:41. doi: 10.1186/1423-0127-16-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouezzedine F, Fardel O, Gripon P. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology. 2015;481:34–42. doi: 10.1016/j.virol.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Wang JY, Liu P. Abnormal immunity and gene mutation in patients with severe hepatitis-B. World J Gastroenterol. 2003;9:2009–2011. doi: 10.3748/wjg.v9.i9.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ataseven H, Bahcecioglu IH, Kuzu N, Yalniz M, Celebi S, Erensoy A, Ustundag B. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators Inflamm. 2006;2006:78380. doi: 10.1155/MI/2006/78380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan CJ, Wu HL, Kuo SF, Kao JH, Tseng TC, Liu CH, Chen PJ, Liu CJ, Chen DS. Serum interleukin 6 level correlates with outcomes of acute exacerbation of chronic hepatitis B. Hepatol Int. 2012;6:591–597. doi: 10.1007/s12072-011-9299-2. [DOI] [PubMed] [Google Scholar]
- 47.Kao JT, Lai HC, Tsai SM, Lin PC, Chuang PH, Yu CJ, Cheng KS, Su WP, Hsu PN, Peng CY, Wu YY. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naïve hepatitis B infection patients. Liver Int. 2012;32:928–936. doi: 10.1111/j.1478-3231.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 48.Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36:143–151. doi: 10.1016/j.ijsu.2016.10.033. [DOI] [PubMed] [Google Scholar]


