Abstract
Colorectal cancer (CRC) is highly invasive, and an increasing number of studies report that microRNAs (miRNAs) are involved in CRC cell invasion and metastasis. However, these studies focus on miRNAs with clear functional targets, often overlooking obscure miRNAs. Using TCGA data analysis, we compared differentially expressed miRNAs in colon and rectal adenocarcinoma (stages N0 and N1/N2) to identify miRNAs involved in invasion and metastasis; one identified candidate miRNA was miR-7702. Bioinformatics analysis identified TADA1 as a possible target gene of miR-7702. The relationship between TADA1 and miR-7702 was analyzed using the Oncomine database, CRC tissues at different stages, and CRC cell lines with different metastatic properties. We used a dual luciferase-reporter assay to verify the interaction between miR-7702 and TADA1. We altered miR-7702 and TADA1 expression levels using transfection, and measured cell migration and invasion abilities. The results revealed a negative correlation between miR-7702 and TADA1 in CRC. miR-7702 was significantly downregulated in human CRC cell lines with high invasion capacity. Its overexpression suppressed human CRC cell migration and invasion by directly inhibiting TADA1. This regulatory effect of miR-7702 on TADA1 was observed in human CRC cells but not in mouse cells. These findings indicate that miR-7702 acts as a tumor suppressor in CRC by inhibiting cell migration and invasion, possibly through direct TADA1 inhibition. Our results demonstrate for the first time that miR-7702 and TADA1 are biologically functional in human CRC cells and reveal that the presence and function of miR-7702 may be species-specific.
Keywords: Colorectal cancer, miR-7702, TADA1, migration, invasion, species-specific
Introduction
The primary difficulty with treating colorectal cancer (CRC) is recurrence after surgery and the distant metastasis of cancer cells. CRC tumorigenesis is initiated through the interaction of endogenous and exogenous multi-factor, multi-stage, and multi-molecular events [1]. Exogenous factors are mainly physicochemical and biogenic, and endogenous factors are inherited or acquired through gene instability, microsatellite instability, and chromosomal instability. T molecular events leading to the occurrence and development of CRC can be divided into two categories: the primary genetic event, which is dominated by gene structure, and secondary molecular events, which result in altered gene expression. Dominant oncogenes and invisible tumor-suppressor genes are examples of primary genetic events involved in the development of CRC. Mutations in oncogenes can be initiating factors for tumorigenesis; for example, mutations in Ki-Ras and adenomatous polyposis coli (APC) genes are often associated with CRC [2]. The absence of tumor-suppressor gene expression can allow the original abnormal proliferating cells to acquire malignant biological behavior; for example, the deletion or mutation of the p53 gene can cause colorectal adenoma to develop into CRC. Secondary molecular events play an important role following malignant tumorigenesis. The unbalanced expression or mutation of various genes confer cancer cells with the ability to become invasive. By studying the gene mechanisms involved in molecular events in CRC, we can identify gene expression changes associated with malignant CRC behaviors, such as occurrence, development, invasion, and metastasis, as well as search for early diagnostic and treatment targets.
MicroRNAs (miRNAs) are non-coding small RNAs of about 20 nucleotides in length. miRNA-mediated silencing of specific genes leads to mRNA degradation or inhibition of protein synthesis, regulating gene expression post-transcriptionally. Previous studies confirmed that miRNAs are involved in the regulation of biological growth and development, proliferation, differentiation, and other biological behaviors, and an imbalance in miRNA expression is related to the development of various human diseases, including tumors [3,4]. Cummins et al. confirmed that 18 miRNAs are upregulated in CRC, and 32 miRNAs are decreased or silenced [5]. Monzo et al. found that 28 and 64 miRNAs are differentially expressed in stage I and II CRC, respectively, compared with normal tissues [6]. These studies have shown that miRNAs are closely tied to the occurrence and development of CRC [7].
After tumor cells undergo several cycles of division and proliferation, they tend to exhibit molecular or genetic changes. This heterogeneity leads to differences in tumor growth rate, invasive ability, sensitivity to drugs, and prognosis. Accordingly, miRNA expression varies based on the different stages of CRC [8-10]. Using TCGA database analysis, we found that miR-7702 has noticeable differences in expression in stages N0 and N1/N2 in CRC. Further, our experimental results suggest that miR-7702 may be involved in the regulation of metastasis and invasion in CRC.
Materials and methods
MicroRNA expression data
Level 3 microRNA expression data were retrieved for 455 CRC specimens profiled using the Agilent 4 × 15 k array and miRNA-Seq from the TCGA data portal. Quantification and isoform files of level 3 miRNA data profiled by miRNA-Seq were retrieved from the TCGA data portal along with metafiles annotating each dataset. Permission to access all data was obtained from the Data Access Committee of the National Center for Biotechnology Information Genotypes and Phenotypes Database (dbGAP) at the National Institutes of Health.
Clinical specimens
A total of 28 snap-frozen normal and CRC specimens were collected at the Fuxing Hospital, which is affiliated with the Capital Medical University (Beijing, China). The tissues collected included five normal tissue sections and 20 CRC tissues, including nine N0 CRC and 14 N1/N2 CRC samples. This study was approved by the Fuxing Hospital, Capital Medical University, and written informed consent was obtained from all participants.
Cell culture and transfection
The human CRC cell lines SW620 and SW480 and the mouse CRC cell line CT26, were obtained from the Cell Resource Center, Peking Union Medical College. The cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Invitrogen), 100 U/ml penicillin G, and 100 mg/ml streptomycin sulfate (Sigma-Aldrich) at 37°C with 5% CO2. Cells at 70% confluence were transfected with 20 nM miRNA or vectors using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendations. The plasmid pCMV6-TADA1 was obtained from OriGene (OriGene Technologies, USA). The miRNA mimic and siRNA sequences were as follows: NC mimic: 5’-CUCCGAACGUGUCACGU-3’ (sense), MiR-7702 mimic: 5’-CUUAGACUGCCAGACUCCCUGA-3’ (sense), and TADA1 siRNA: 5’-GAUGAUGACGACUUGAAACdTdT-3’ (sense).
RT-qPCR
Total RNA was isolated using TRIzol Reagent (Invitrogen). The concentrations of RNA samples were quantified using spectrophotometry (GeneQuant, GE Healthcare, Piscataway, USA) at 260 nm. Relative miRNA and mRNA levels were determined using qRT-PCR with an ABI 7500 Real-Time PCR System (Applied Biosystems, Warrington, UK) and SYBR Green. GAPDH was used as the endogenous control for mRNA, and U6 served as the internal control for miRNA. The primers sequences used for RT-qPCR were as follows: TADA1: 5’-GTTCCCACACAATGATGCTT-3’ (forward), 5’-GCTTTCCTTCTTGACACAACTG-3’ (reverse); GAPDH: 5’-ACAACTTTGGTATCGTGGAAGG-3’ (forward), 5’-GCCATCACGCCACAGTTTC-3’ (reverse); MiR-7702: 5’-CAGCTTAGACTGCCAGAC-3’ (forward), 5’-CCAGTTTTTTTTTTTTTTTCAGGGA-3’ (reverse); U6: 5’-CTCGCTTCGGCAGCACA-3’ (forward), 5’-GCGAGCACAGAATTAATACGAC-3’ (reverse).
Western blot analysis
Total cell lysates from CRC cells were prepared using ProteoJET™ Mammalian Cell Lysis Reagent according to the manufacturer’s instructions (Thermo Scientific, Rockford, IL, USA), and protein concentrations were determined using the BCA method. Specific antibodies to TADA1 (ab105712) and GAPDH (ab8245) were purchased from Abcam (Cambridge, UK). The intensities of immunoreactive bands were quantified using the ImageJ software (http://imagej.nih.gov/ij/).
Cell viability assay
Cell viability was assessed indirectly using the CCK-8 assay. Cells were seeded in a 96-well plate at a density of 3000 cells/well. After 24 h, cells were transfected with the indicated miRNAs or vectors and incubated another 48 hours. Then, cells were treated with 100 µl of fresh medium containing 10% CCK-8 reagent (DoJinDo Laboratories, Japan) for 1 h at 37°C. The absorbance values of the wells at 450 nm were detected using an automatic spectrometer (Multimode Reader, PerkinElmer, USA). This procedure was repeated 0, 1, 2, 3, 4 and 5 days after cell seeding.
Migration and invasion assays
Migration and invasion assays of CRC cells were conducted using 24-well Transwell chambers (8-μm pore size polycarbonate membrane; Costar, Corning, NY). For the migration assays, 5 × 103 cells were suspended in 100 μl DMEM and seeded into the upper chamber; 600 μl DMEM containing 10% FBS was added to the outside chamber. After incubating at 37°C under 5% CO2 for 24 hours, the upper chamber was removed and cells that had not migrated were wiped with a cotton swab. After fixing with 4% paraformaldehyde, nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI). Images of different fields were collected, and statistical analyses performed. For invasion assays, Matrigel (80 μg/ml, BD Biosciences) was pre-loaded in the upper chamber, and the remaining steps were performed as outlined for the migration assays.
Statistical analysis
All experiments were performed in triplicate. All data were analyzed using SPSS 19.0 statistics software (IBM). Analysis of variance (ANOVA) was used to evaluate the statistical difference between groups. P-values < 0.05 were considered statistically significant.
Results
Analysis of differentially expressed miRNAs in CRC
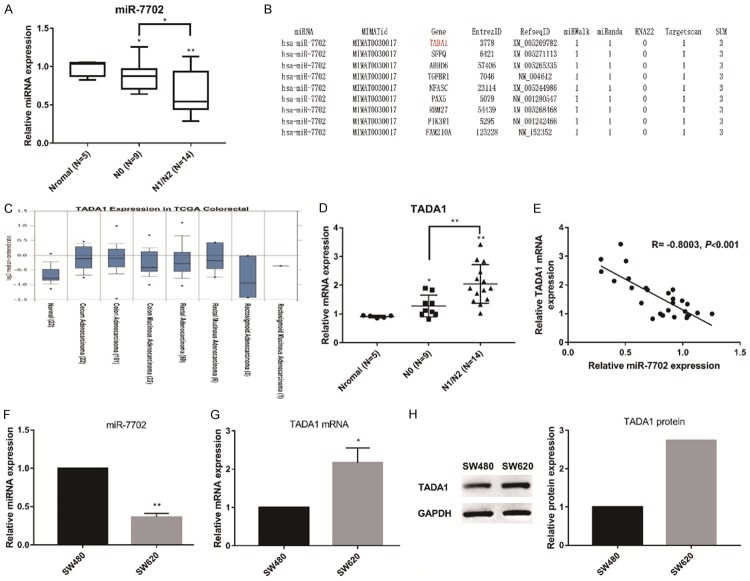
The incidence of CRC was probed based on colon and rectal adenocarcinoma (COAD) keywords from the TCGA database, and a total of 455 miRNA samples were sequenced, including eight cases that included para-carcinoma tissue as negative control. These eight cases were analyzed for differences in miRNA expression, and 100 COAD-related miRNAs were obtained (FDR P < 0.05). Of these eight cases, six cases were stage N0 patients, and two cases were stage N1/N2 patients. The differences in miRNA expression between the two groups were further analyzed, and 83 N0-related miRNAs (second column) and 78 N1/N2-related miRNAs (third column) were obtained (Supplementary Table 1). The three groups of miRNA relationships are shown in Figure 1.
Figure 1.
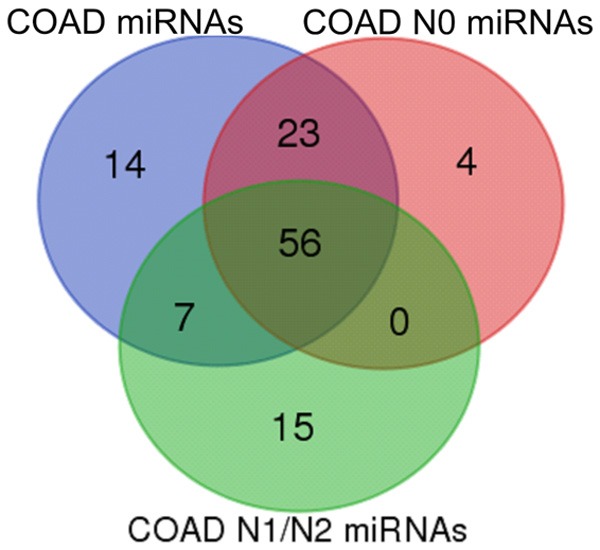
Venn diagram of differentially expressed miRNAs in colorectal cancer. Total colorectal cancer-related miRNAs are shown in blue, stage N0-related miRNAs are in red, and stage N1/N2-related miRNAs are in green.
The following miRNAs were unique to the N1/N2 stage: hsa-miR-369, hsa-miR-193b, hsa-miR-7702, hsa-miR-296, hsa-miR-205, hsa-miR-125a, hsa-miR-3173, hsa-miR-574, hsa-miR-508, hsa-miR-92b, hsa-miR-1296, hsa-miR-1224, hsa-miR-874, hsa-miR-181a-1, and hsa-miR-3150b. Compared with those related to the N0 stage and to common COAD, those miRNAs that exhibit differential expression in N1/N2 cancer tissue could be specific to metastatic foci and may be related to the metastatic phenotype. The following miRNAs were unique to the N0 stage: hsa-miR-192, hsa-miR-301b, hsa-miR-496, and hsa-miR-4662a. The differential expression of these miRNAs may be associated with cancer progression.
Potential regulatory relationship between miR-7702 and TADA1
MiR-7702 is located on chromosome 9, and it was initially reported in 2013. However, to the best of our knowledge, the function of miR-7702 has not been reported. Therefore, we probed whether miR-7702 is related to malignant metastasis in CRC. First, we compared the relative expression levels of miR-7702 in CRC specimens at different stages. The results showed that miR-7702 level was significantly downregulated in N1/N2 CRC specimens compared with that of N0 specimens and normal tissues (Figure 2A). These results indicate that the expression level of miR-7702 might be related to differences in CRC malignancy. We used bioinformatics analysis to identify the regulatory target of miR-7702. Results from three separate analyses-miRWalk, miRanda, and Targetscan-ranked TADA1 as the top predicted target of miR-7702 (Figure 2B). Data analysis using Oncomine (https://www.oncomine.org) revealed that TADA1 mRNA levels were markedly higher in CRC tissues relative to normal tissues (Figure 2C). Subsequently, we examined the relative content of TADA1 mRNA in CRC specimens of different stages. The expression of TADA1 mRNA in N1/N2 CRC specimens was significantly upregulated compared with that in N0 specimens and normal tissues (Figure 2D) and was negatively correlated with the expression of miR-7702 (Figure 2E).
Figure 2.
The negative correlation between mir-7702 and TADA1 in colorectal cancer tissues and cells. A. miR-7702 levels were significantly downregulated in N1/N2 colorectal cancer specimens compared with those in N0 specimens and normal tissues. The miR-7702 expression level was quantified using qRT-PCR in SW480 and SW620 cells. U6 served as an internal control. B. The top nine highest-ranking genes predicted to be candidate targets of miR-7702 by miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2), miRnada (www.microrna.org), RNA22 (https://cm.jefferson.edu/rna22), and Targetscan (www.targetscan.org), among which TADA1 is ranked first. C. Oncomine data mining analysis of mRNA levels from TCGA datasets showed higher TADA1 expression levels in colorectal carcinoma tissues versus normal tissues. D. qRT-PCR assay results showed significant upregulation of TADA1 mRNA in N1/N2 colorectal cancer specimens compared with that in N0 specimens and normal tissues. GAPDH served as an internal reference. E. The expression of miR-7702 negatively correlated with TADA1 (R = -0.8003). F. The expression of miR-7702 in SW620 cells was significantly lower than that in SW480 cells. G. TADA1 mRNA levels in SW620 cells were significantly lower than those in SW480 cells based on qRT-PCR assays. H. TADA1 protein levels in SW620 cells were significantly lower than that in SW480 cells based on western blot analyses. *P < 0.05 and **P < 0.01. Data are representative of three independent experiments (means ± SD.).
Next, we selected two human CRC cell lines, SW620 and SW480, to verify the negative correlation between miR-7702 and TADA1. SW480 is a primary Colon Epithelial Adenocarcinoma tumor line with low metastatic properties, and SW620 is a metastasized variant of SW480 derived from lymph node with high metastatic properties. The level of miR-7702 in SW620 cells was significantly lower than that in SW480 cells (Figure 2F), whereas TADA1 mRNA and protein expression levels showed the opposite pattern (Figure 2G, 2H). In general, the experimental results in both tissues and cells revealed a negative correlation, suggesting a possible regulatory relationship between mir-7702 and TADA1.
MiR-7702 directly targets TADA1 in human CRC cells
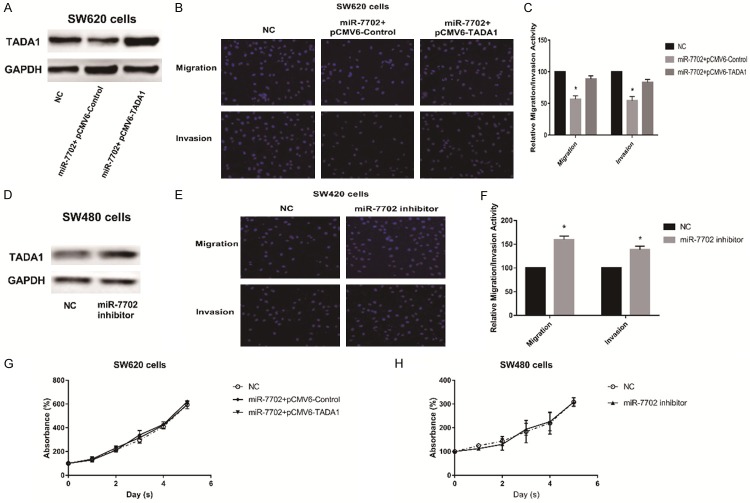
To confirm the direct regulation of TADA1 by miR-7702, luciferase reporter assays were performed using luciferase reporter constructs carrying the TADA1 mRNA 3’ UTR, containing either the wild-type (WT) predicted miRNA-138 target sites (TADA1-wt-3’ UTR) or a mutant (TADA1-mut-3’ UTR) in SW620 cells (Figure 3A). miR-7702 significantly reduced the luciferase activity of the reporter vectors containing WT predicted target sites but had little effect on the mutant (Figure 3B). These results indicate that miR-7702 directly acts on the predicted target sites in the TADA1 3’ UTR. When miR-7702 was overexpressed in SW620 cells, the expression of TADA1 was decreased at both the mRNA and protein levels consistent with the results of the TADA1 siRNA-positive control group (Figure 3C, 3D).
Figure 3.
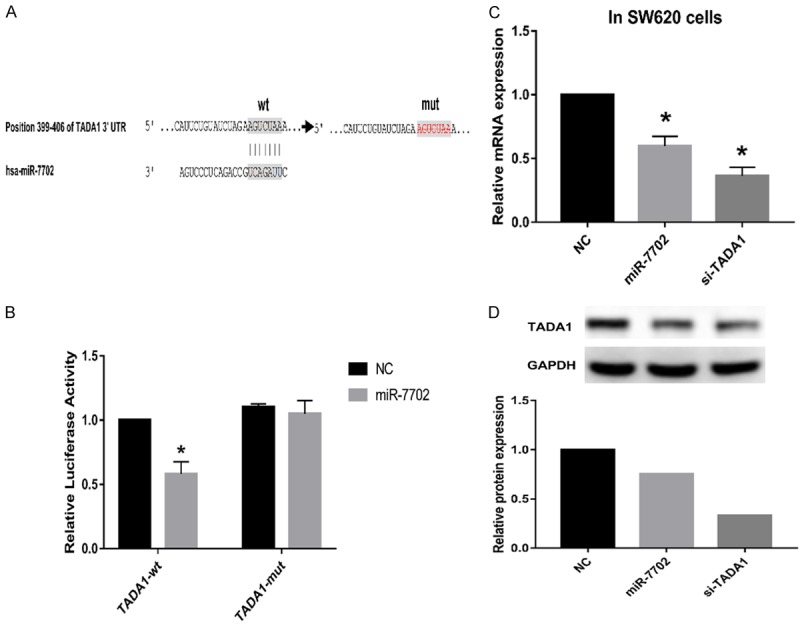
miR-7702 directly targets TADA1 in human colorectal cancer cells. A. A schematic representation of miR-7702 target sites in the 3’ UTR of human TADA1. The artificial mutant 3’ UTR lacks these miR-7702 binding sites. B. Dual luciferase-reporter assays showed that miR-7702 significantly reduced the luciferase activity of reporter vectors containing WT predicted target sites (TADA1-wt-3’ UTR) but had little effect on the mutant group (TADA1-mut-3’ UTR). C. qRT-PCR assays showed that miR-7702 overexpression decreased endogenous TADA1 mRNA levels. GAPDH served as an internal reference. *P < 0.05. Data are representative of three independent experiments (means ± SD.). D. Western blot analyses showed that miR-7702 overexpression reduced endogenous TADA1 protein levels. GAPDH served as an internal control.
Effects of miR-7702 on the migration and invasion of human CRC cells
To investigate the biological significance of the differential expression of miR-7702 in different CRC cells, we performed cell migration and invasion assays. First, given the relatively low expression of miR-7702 in SW620 cells, we overexpressed miR-7702 in SW620 cells to decrease TADA1 expression (Figure 4A). Following transfection with miR-7702, SW620 cells exhibited significantly reduced migration and invasion compared with the control group (Figure 4B, 4C); TADA1 overexpression partially reversed these results. Correspondingly, transfection of a miR-7702 inhibitor into SW480 cells to decreased miR-7702 activity upregulated TADA1 expression (Figure 4D) and increased the migration and invasion (Figure 4E, 4F). Interestingly, miR-7702 overexpression in SW620 cells and miR-7702 inhibition in SW480 cells showed no significant change in cell proliferation (Figure 4G, 4H). Taken together, these results indicate that miR-7702 influences the migration and invasion of CRC cells by modulating TADA1 without affecting cell proliferation.
Figure 4.
miR-7702 inhibits the migration and invasion of human colorectal cancer cells. A. SW620 cells were co-transfected with miR-7702 mimics, pCMV6-control, or pCMV6-TATA1 for 48 h. Western blot analyses were performed to examine the protein expression levels of TADA1. B. miR-7702 overexpression inhibited the migration and invasion of SW620 cells. Migration and invasion was assayed in SW620 cells following co-transfection with miR-7702 minics, pCMV6-control, or pCMV6-TATA1 using the Transwell apparatus. Migrating or invading cells were stained with DAPI and analyzed under a microscope. C. Relative cell migration and invasion were determined based on the number of DAPI-stained SW620 cells. Cell migration and invasion are expressed as a percentage of that observed in control. D. Western blot analyses showed that miR-7702 inhibitor increased endogenous TADA1 protein levels. E. The miR-7702 inhibitor promoted the migration and invasion of SW480 cells. Migration and invasion were assayed in SW480 cells following transfection with the miR-7702 inhibitor. F. Relative cell migration are invasion were determined based on the number of DAPI-stained SW480 cells. G, H. Cell viability assay showed that miR-7702 had no effect on SW620 and SW480 cells proliferation. The line charts show relative CCK-8 absorbance, which indicates cellular viability. Data are the means ± SD. of three independent experiments. *P < 0.05 versus control.
Species specificity of miR-7702 regulation of TADA1
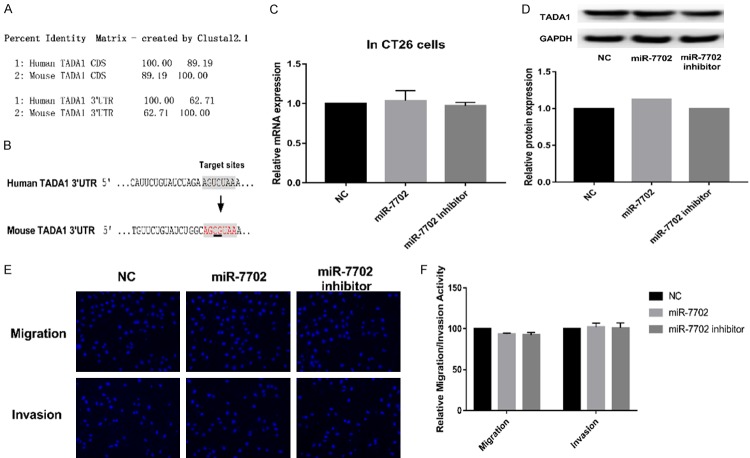
Since miR-7702 has, at present, only been detected in human cells, the presence of miR-7702 is suspected to be genus-specific. In mouse CRC CT26 cells, miR-7702 was not detected using qRT-PCR. The similarity between the mouse TADA1 and human TADA1 mRNA coding regions was 89.19%; however, the sequence similarity of the 3’ UTR is only 62.71% (Figure 5A), and there are no predicted target sites for miR-7702 in the mouse TADA1 3’ UTR (Figure 5B). miR-7702 overexpression in CT26 cells failed to downregulate TADA1 expression (Figure 5C, 5D) and did not inhibit cell migration and invasion (Figure 5E, 5F). These results revealed that the presence of miR-7702 and the regulation of TADA1 are likely species-specific.
Figure 5.
Species specificity of miR-7702 regulation of TADA1. A. Sequence alignment was used to identify the similarity of the TADA1 coding sequence and the TADA1 3’ UTR nucleotide sequences between human and mouse TADA1 using the ClustalW server (http://www.ebi.ac.uk/Tools/msa/clustalw2/). B. A two-base difference between the human and mouse TADA1 mRNA 3’ UTR was observed; thus, mouse TADA1 lacks the miR-7702 targeting sequence. C. Mouse colorectal cancer CT26 cells were transfected with miR-7702 or miR-7702 inhibitor for 48 h. qRT-PCR assays showed that miR-7702 mimic or miR-7702 inhibitor had no effect on endogenous TADA1 gene transcription. GAPDH served as an internal reference. D. Western blot analyses showed that miR-7702 mimic or miR-7702 inhibitor had no effect on endogenous TADA1 protein expression. E. Migration and invasion were assayed in CT26 cells following transfection with the miR-7702 inhibitor. F. Relative cell migration and invasion were determined based on the number of the DAPI-stained SW480 cells.
Discussion
More than 2,500 miRNAs have been found in the human genome, and these non-coding small RNAs are involved in regulating about 30% of the gene expression in the human genome. miRNA can affect cell proliferation, differentiation, apoptosis, insulin secretion, and the development of the heart, brain, and skeletal muscle. Multiple studies have shown that CRC is associated with the mutation or ectopic expression of miRNA, indicating that miRNA can play a tumor-suppressor or oncogene function [11,12]. During tumorigenesis in CRC, studies have found overexpression and silencing of multiple miRNAs. Transcriptional activation and amplification of genes encoding miRNAs lead to the up-regulation of certain miRNAs, which can result in the downregulation and silencing of certain miRNAs due to chromosomal deletions and defects in the generation process [13]. miRNAs can affect the development of CRC by regulating proteins that affect the tumorigenesis pathway in CRC. These proteins include Wnt-catenin [14,15], PI3K-AKT-mTOR-pathway-related proteins [16-18], and K-RAS [19,20]. miRNAs can also influence tumor invasion and metastasis by affecting extracellular matrix degradation, the epithelial-mesenchymal transition, and the PI3K-AKT-mTOR and TGF-β signaling pathways [21,22]. Studying these miRNAs will provide a better understanding of tumorigenesis in CRC and facilitate the development of drugs that target these miRNAs to treat CRC.
The CRC-associated miRNAs reported here, including miR-143, miR-18a, miR-135, miR-18, miR-20, and miR-21, play similar roles in other tumors [23-26]. However, tumors are heterogeneous, and some miRNAs that cannot target “star” molecules are often overlooked. We hypothesized that miR-7702 belonged to this category. TCGA database analysis revealed that miR-7702 has significantly different expression levels between stages N0 and N1/N2 in CRC. Bioinformatics analysis showed that the expression of TADA1, a potential target of miR-7702, in CRC is inversely related to that of miR-7702. Furthermore, our cell-based experiments demonstrated that miR-7702 inhibits the expression of TADA1 and thereby participates in the regulation of CRC cell invasion and migration. The purpose of miR-7702 downregulation in malignant CRC, and the mechanism by which TADA1 affects the invasion and migration of CRC cells, are both topics worthy of further investigation.
Although miR-7702 was identified in 2013 [27], no reports about its function have been published, and it appears to exist only in human cells. We have experimentally demonstrated that miR-7702 is indeed absent in mouse CRC cells. Overexpression of miR-7702 did not inhibit the expression of TADA1 in mice, and it was not involved in the regulation of mouse CRC cell migration and invasion. It is also interesting to note that no precise function of TADA1 related to tumorigenesis and development has been identified. In 2014, Shalem et al., using the genome-scale CRISPR-Cas9 knockout technique to screen for genes related to the viability of melanoma cells, identified TADA1 as a candidate gene [28]. However, there are no apparent follow-up reports. Our studies have preliminarily identified the role of TADA1 in CRC. Overall, our study showed that miR-7702 acts as a tumor suppressor in CRC by inhibiting cell migration and invasion, which may be associated with its direct inhibition on TADA1. Our results demonstrate for the first time that miR-7702 and TADA1 are biologically functional in human CRC cells, and revealed that the presence and function of miR-7702 is likely species-specific.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Broderick P, Dobbins SE, Chubb D, Kinnersley B, Dunlop MG, Tomlinson I, Houlston RS. Validation of recently proposed colorectal cancer susceptibility gene variants in an analysis of families and patients-a systematic review. Gastroenterology. 2017;152:75–77. e4. doi: 10.1053/j.gastro.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218–229. doi: 10.1038/nrgastro.2017.1. [DOI] [PubMed] [Google Scholar]
- 3.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719–732. doi: 10.1038/nrg.2016.134. [DOI] [PubMed] [Google Scholar]
- 4.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 5.Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, Raymond CK, Roberts BS, Juhl H, Kinzler KW, Vogelstein B, Velculescu VE. The colorectal microRNAome. Proc Natl Acad Sci U S A. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monzo M, Navarro A, Bandres E, Artells R, Moreno I, Gel B, Ibeas R, Moreno J, Martinez F, Diaz T, Martinez A, Balagué O, Garcia-Foncillas J. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell Res. 2008;18:823–833. doi: 10.1038/cr.2008.81. [DOI] [PubMed] [Google Scholar]
- 7.Strubberg AM, Madison BB. MicroRNAs in the etiology of colorectal cancer: pathways and clinical implications. Dis Model Mech. 2017;10:197–214. doi: 10.1242/dmm.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slattery ML, Herrick JS, Pellatt DF, Stevens JR, Mullany LE, Wolff E, Hoffman MD, Samowitz WS, Wolff RK. MicroRNA profiles in colorectal carcinomas, adenomas and normal colonic mucosa: variations in miRNA expression and disease progression. Carcinogenesis. 2016;37:245–261. doi: 10.1093/carcin/bgv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas J, Ohtsuka M, Pichler M, Ling H. MicroRNAs: clinical relevance in colorectal cancer. Int J Mol Sci. 2015;16:28063–28076. doi: 10.3390/ijms161226080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valeri N, Croce CM, Fabbri M. Pathogenetic and clinical relevance of microRNAs in colorectal cancer. Cancer Genomics Proteomics. 2009;6:195–204. [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S, Kopetz S, Davuluri R, Hamilton SR, Calin GA. MicroRNAs, ultraconserved genes and colorectal cancers. Int J Biochem Cell Biol. 2010;42:1291–1297. doi: 10.1016/j.biocel.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Yamada N, Noguchi S, Mori T, Naoe T, Maruo K, Akao Y. Tumor-suppressive microRNA-145 targets catenin δ-1 to regulate Wnt/β-catenin signaling in human colon cancer cells. Cancer Lett. 2013;335:332–342. doi: 10.1016/j.canlet.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 15.Strillacci A, Valerii MC, Sansone P, Caggiano C, Sgromo A, Vittori L, Fiorentino M, Poggioli G, Rizzello F, Campieri M, Spisni E. Loss of miR-101 expression promotes Wnt/β-catenin signalling pathway activation and malignancy in colon cancer cells. J Pathol. 2013;229:379–389. doi: 10.1002/path.4097. [DOI] [PubMed] [Google Scholar]
- 16.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke TW, Wei PL, Yeh KT, Chen WT, Cheng YW. MiR-92a promotes cell metastasis of colorectal cancer through PTEN-Mediated PI3K/AKT pathway. Ann Surg Oncol. 2015;22:2649–2655. doi: 10.1245/s10434-014-4305-2. [DOI] [PubMed] [Google Scholar]
- 18.Wu W, Yang J, Feng X, Wang H, Ye S, Yang P, Tan W, Wei G, Zhou Y. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30. doi: 10.1186/1476-4598-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang WP, Kwok TT. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30:953–959. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, Cai X, Wang K, Wang G, Ba Y, Zhu L, Wang J, Yang R, Zhang Y, Ren Z, Zen K, Zhang J, Zhang CY. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 21.Bu P, Wang L, Chen KY, Rakhilin N, Sun J, Closa A, Tung KL, King S, Kristine VA, Xu Y, Huan CJ, Zessin AS, Shealy J, Cummings B, Hsu D, Lipkin SM, Moreno V, Gümüş ZH, Shen X. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-β. Nat Commun. 2015;6:6879. doi: 10.1038/ncomms7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling H, Pickard K, Ivan C, Isella C, Ikuo M, Mitter R, Spizzo R, Bullock M, Braicu C, Pileczki V, Vincent K, Pichler M, Stiegelbauer V, Hoefler G, Almeida MI, Hsiao A, Zhang X, Primrose J, Packham G, Liu K, Bojja K, Gafà R, Xiao L, Rossi S, Song JH, Vannini I, Fanini F, Kopetz S, Zweidler-McKay P, Wang X, Ionescu C, Irimie A, Fabbri M, Lanza G, Hamilton SR, Berindan-Neagoe I, Medico E, Mirnezami A, Calin GA, Nicoloso MS. The clinical and biological significance of MIR-224 expression in colorectal cancer metastasis. Gut. 2016;65:977–989. doi: 10.1136/gutjnl-2015-309372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kent OA, McCall MN, Cornish TC, Halushka MK. Lessons from miR-143/145: the importance of cell-type localization of miRNAs. Nucleic Acids Res. 2014;42:7528–7538. doi: 10.1093/nar/gku461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu S, Ichikawa D, Takeshita H, Morimura R, Hirajima S, Tsujiura M, Kawaguchi T, Miyamae M, Nagata H, Konishi H, Shiozaki A, Otsuji E. Circulating miR-18a: a sensitive cancer screening biomarker in human cancer. In Vivo. 2014;28:293–297. [PubMed] [Google Scholar]
- 25.Chandra V, Kim JJ, Mittal B, Rai R. MicroRNA aberrations: an emerging field for gallbladder cancer management. World J Gastroenterol. 2016;22:1787–1799. doi: 10.3748/wjg.v22.i5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer SR, Yang CH, Pfeffer LM. The role of miR-21 in cancer. Drug Dev Res. 2015;76:270–277. doi: 10.1002/ddr.21257. [DOI] [PubMed] [Google Scholar]
- 27.Swaminathan S, Hu X, Zheng X, Kriga Y, Shetty J, Zhao Y, Stephens R, Tran B, Baseler MW, Yang J, Lempicki RA, Huang D, Lane HC, Imamichi T. Interleukin-27 treated human macrophages induce the expression of novel microRNAs which may mediate anti-viral properties. Biochem Biophys Res Commun. 2013;434:228–234. doi: 10.1016/j.bbrc.2013.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.