Abstract
Huangqi decoction (HD) is a prescription for the treatment of diabetes in traditional Chinese medicine. The study was aimed to investigate the effect of HD on diabetic nephropathy. Male diabetic db/db mice which develop diabetic nephropathy spontanously and non-diabetic db/m control mice were used in the current study, and received the treatment of HD for 14 consecutive weeks. HD treatment dose-dependently decreased the body weight, urine volume, water intake and food intake, improved glucose tolerance and insulin resistance, and lowered blood glucose, serum glycosylated hemoglobin, insulin and insulin resistance index in db/db mice. The db/db mice also showed low levels of serum creatinine, blood urea nitrogen and urine albumin, and improved renal functions such as glomerular filtration rate after HD treatment. Histological examination showed that HD treatment prevented the deterioration of basement membrane of glomerular capillary, mesangial matrix and renal tubular lumen in the db/db mice. Through examining the cell signaling pathways which might be involved in the pathology of diabetic nephropathy, we found that HD treatment activated phospho-IRY1361, phospho-IRS1Y896, phospho-PI3K, and inhibited phospho-IRS1S636/639, phospho-AKTT308 and phospho-AKTS473. HD treatment abolished the change in the expression of glucose transporters in the diabetic kidney with an increase in GLUT4 but decrease in GLUT1 expression in the kidney in a dose-dependent manner. Our study suggests that HD prevents the development of diabetes and improves renal function in the db/db mice and HD regulation of the IRS1-PI3K-GLUT signaling pathway significantly improves diabetic nephropathy.
Keywords: Astragalus, insulin receptor substrate1, phosphatidylinositol 3 kinase, glucose transporter, db/db mice, diabetic nephropathy
Introduction
Diabetes is a metabolic disease which affects 8.3% of population worldwide and 11.6% of Chinese in population [1]. People with diabetes have a higher risk to develop serious health problems such as heart disease, stroke, renal failure and blindness. Diabetic nephropathy (DN) [2] is one of the most common complications caused by uncontrolled high blood glucose, leading to the life-threatening end-stage renal disease. Therefore, it is very important to prevent and treat diabetic nephropathy in its early stage. Early treatment of diabetic nephropathy mainly focuses on reducing the blood pressure and blood glucose, which makes glucose transport a new therapeutic target [3]. With regulation of glucose transportation, the progression of diabetic nephropathy can be delayed by improving high glucose-induced oxidative stress, inflammation, and lipidoses [4,5].
Insulin resistance, a risk factor for hyperglycemia, diabetes and early index of glucose tolerance, can result in the dysfunction of glucose transportation and consumption [6]. Insulin resistance is also an independent risk factor for microalbuminuria, an early sign of kidney damages [7]. Animal studies in recent years have showed that insulin resistance can lead to kidney damages [8,9]. However, the mechanism of renal injury caused by insulin resistance is still unclear.
Huangqi decoction (HD) is a prescription for the treatment of diabetes in traditional Chinese medicine. A medicinal recipe used for centuries to treat thirsty disease, HD was first recorded by Yang Shiying in the therapeutic guidebook, Renzhai Zhizhi Fanglun in 1264. HD is an extract from 7 herbs such as astragalus, poria, trichosanthes roots, ophiopogon, schisandra, licorice and rehmannia. Modern pharmaceutical techniques have made it possible to unveil the main composition of HD [10]. On the other side, modern pharmacology has proved that herbs composing of HD can be used individually for effective diabetes treatment. For example, Astragaloside IV, the active component of astragalus and astragalus extracts could lower blood glucose level, improve glomerular filtration rate, reduce urine protein, ameliorate the mesangial thickening [11] and delay the progression of the chronic kidney disease in streptozotocin-induced diabetes in rats [12-14]. Poria crude extract and its main chemical component poria acid could be used for the treatment of diabetic db/db mice and streptozotocin-induced diabetic mice [15,16]. Oligosaccharide from ophiopogon could ameliorate the symptoms of type II diabetes in rats [17]. Schisandra extracts could inhibit renal interstitial fibrosis in diabetic nephropathy mice [18], maintain the integrity of podocytes [19], and protect the drug-induced nephrotoxicity [20]. Rehmannia and its extracts could protect renal functions in diabetic animals [21-23]. The beneficial effects of HD might come from the combination of the multiple components from the different herbs. Nowadays HD is widely used in clinical practice in China and shows efficiency on diabetes therapy. However, the mechanism of HD therapy especially how HD protects kidney function is not well understood.
In this study, using db/db mice, a diabetic spontaneous model which develops diabetic nephropathy, we investigated the effect of HD and its protective mechanism on diabetic nephropathy. After 14 consecutive weeks of treatment, we found that HD can protect kidney from diabetic damages, which associates with the regulation of IRS1-PI3K-GLUT signaling pathway.
Materials and methods
Preparation of HD
HD is made from astragalus, poria, trichosanthes roots, ophiopogon, schisandra, licorice and rehmannia. All the herbs used to produce HD in the current study were provided by Shanghai Hua Yu Chinese Herbs Co., LTD (Shanghai, China). Radix astragali 2 kg (H2013031806), wolfiporia extensa 2 kg (H2012082001), fructus trichosanthis 2 kg (H2011011706), radix Ophiopogonis 2 kg (H2012101604), schisandra 1 kg (H2013050303), liquoric root 1 kg (H2013052901), radix rehmanniae 3 kg (H2012091801) were powdered and mixed thoroughly. 52 liter of ddH2O in total were added to extract the powdered herbs 3 times, and the extract was concentrated under vacuum to thick decoction. Ethanol was added to the concentrated decoction to make the final concentration of ethanol in the decoction to be 70%. After an overnight precipitation, the supernatant was collected and dried in an oven at 105°C for 48 h. A total 1730 g of dry decoction was obtained after the drying process and used as HD for animal studies. The production rate was 13.3%. HD was dispensed in 0.5% sodium carboxymethyl cellulose in ddH2O to make a suspension at 0.108, 0.036 and 0.012 g/ml, respectively, for intragastric use in animal study.
Animals
All animal protocols in this study were approved by the Ethics Committee of Putuo Hospital, Shanghai University of Traditional Chinese Medicine. The animal experiments were carried out in accordance with the approved guidelines for the use of experimental animals. Diabetic db/db mice (weight 35 ± 2 g) and non-diabetic db/m (23 ± 2 g) control mice at the age of 5 weeks were purchased from Nanjing Institute of Biomedicine affiliated to Nanjing University (Nanjing, China). Animals were housed in a specific-pathogen-free room at 12:12 h light-dark cycle and were allowed to access to water and chow freely. After one week of adaptive housing and feeding, mice were randomly allocated into different groups for specific treatment. Diabetic db/db mice were divided into 4 groups (n = 10 each) which received vehicle as control, HD at high dose (1.08 g/kg), medium dose (0.36 g/kg) and low dose (0.12 g/kg), respectively. There are two groups (n = 10 each) for non-diabetic db/m control mice which received vehicle and HD at high dose, respectively. HD or vehicle was administered to animals daily in the morning by gavage for 14 consecutive weeks. At the age of 6, 8, 12, 16, 20 weeks, blood glucose, body weight, urine volume, water intake, food intake and blood pressure were measured. Urine, water intake and food intake were measured by placing animals in metabolic cages. Using a non-invasive tail cuff method, the monitor of blood pressure was performed on a blood pressure system (ALC-NIBP) from Shanghai Alcott Biological Technology Co Ltd (Shanghai, China).
At the last day of week 20, animals were sacrificed to harvest tissues in the morning after overnight fasting. The veil blood was collected for blood glucose measurement and serum separation. The left kidney was cut into 2 pieces from the sagittal plane with one half being snap-frozen and the other half submerged into 10% formalin for fixation and the subsequent pathological and immunohistochemical examinations. The cortex of right kidney was isolated and snap-frozen, and then stored at -80°C for protein expression analysis.
Renal function evaluation
Serum creatinine (Scr) was measured by a commercial creatinine colorimetric assay kit from BioVision (Milpitas, San Francisco). Blood urea nitrogen (BUN) was measured by BUN colorimetric detection kit from Arbor Assays (Ann Arbor, Michigan). Urinary albumin and urinary creatinine were measured by albumin mouse enzyme-linked immunosorbent assay (ELISA) kit and creatinine assay kit from Abcam (Cambridge, MA). Glomerular filtration rate (GFR) was calculated based on the endogenous creatinine clearance rate without the calibration of surface area. The formula of GFR (ml/min) = urinary creatinine (μM) × 24 h urinary volume (ml)/serum creatinine (μM).
Histological examination
Paraffin-embedded renal tissues were cut into 3 μm series slices and stained with Periodic Acid-Schiff (PAS). The stained slide was observed under a microscope at 400 × magnification. To determine mesangial hyperplasia, glomerular area and the surrounding mesangial (cell) were measured, and the relative mesangial hyperplasia degree was evaluated as the ratio of the mesangial area/glomerular area, according to the method employed by Zhang et al. [24]. To calculate the average glomerular cross sectional area, twenty glomeruli which randomly shown in the field were measured and averaged. The measurement of areas in slides was performed with Image J V1.50C.
Fasting blood glucose, oral glucose tolerance test (OGTT), insulin tolerance test (IPITT)
Fasting blood glucose was measured at 6, 8, 12, 16, 20 week following dosing after 12 h fasting using a blood glucose meter (OMRON healthcare, Tokyo, Japan). OGTT and IPITT were determined according to the methods used previously in publication [25,26].
Glycosylated hemoglobin, serum insulin and Insulin resistance index
Glycosylated hemoglobin and serum insulin were measured by ELISA kits according to the manuals provided by Millipore (Billerica, MA). Insulin resistance index (IR) was calculated according to the following formula: HOMA-IR = FBG × INS/22.51, in which FBG stands for the level of fasting blood glucose in mmol/L, and INS for the concentration of fasting insulin in μU/ml [27].
Western blot
Renal cortex was homogenized in RIPA lysis buffer (Beyotime, Shanghai, China), and lysates were collected after centrifugation for 10 min at 12,000 g. The concentration of total protein in the tissue lysates was determined using BCA method kit (Pierce, Rockford, IL). A total of 60 μg lysate protein from each animal was subjected to SDS-PAGE (8-12%) for separation and transferred to PVDF membranes (Pierce), and then immunoblotted to primary antibodies against GLUT4, IRS1 (Santa Cruz, Santa Cruz, CA), PI3K, p-PI3K, p-IRS1S636/639 (Cell Signaling, Danvers, MA), GLUT1, p-IRS1Y896, AKT, p-AKTS473, p-AKTT308, p-IRY1361 (Abcam) after blocking in 5% bovine serum albumin in TBST for 1 h. All primary antibodies were incubated at 4°C overnight. All secondary antibodies were obtained from Cell Signaling. Enhanced chemiluminescence (ECL) was performed with ECL reagents from Millipore and a Bio-rad Gel Doc EZ imaging system (BIO-RAD, Hercules, CA) was employed to visualize and capture the blot bands which were quantified by densitometry using the Image J.
Statistical analysis
Experimental results were presented as mean ± standard deviation (x̅ ± sd). Prism 5 (GraphPad software Inc., San Diego, CA) was used for statistical analysis. One-Way ANOVA followed by the Turkey post test was used to determine the statistical significance among groups. A P < 0.05 was considered statistically significant.
Results
HD prevented the development of diabetes in the db/db mice
The db/db mice were obese when they were shipped to the lab as their baseline body weight before HD treatment was much higher than the db/m control mice (db/db: 37.00 ± 1.60 g vs. db/m: 22.61 ± 1.51 g) and the level of blood glucose in the db/db mice was significantly higher than the db/m mice (db/db: 18.13 ± 3.60 vs. db/m: 6.66 ± 1.52). The body weight and blood glucose in the db/db mice continued to grow and be significantly higher than those in the non-diabetic db/m control mice (Figure 1A, 1D). The db/db mice also took more water and food, and the levels of serum insulin and HbA1C than the db/m mice (Figures 1B, 1C, 2A, 2C). All these profiles of db/db mice are consistent with the reports about this diabetic animal model from other groups [28].
Figure 1.
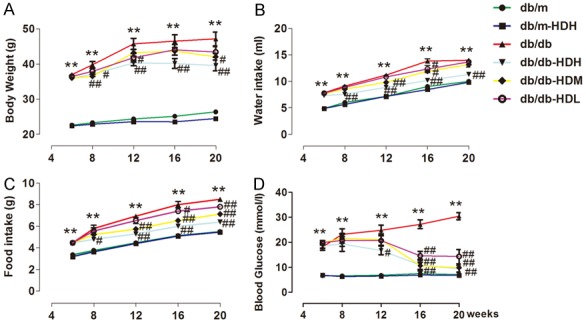
HD prevented the development of diabetes in db/db mice. A-D. Body weight, water intake, food intake and blood glucose, and were higher in the db/db group than in the db/m group during the study, during the 14-week observation period. **P < 0.001, compared with the db/m group; #P < 0.05, ##P < 0.001, compared with the db/db group (n = 10).
Figure 2.
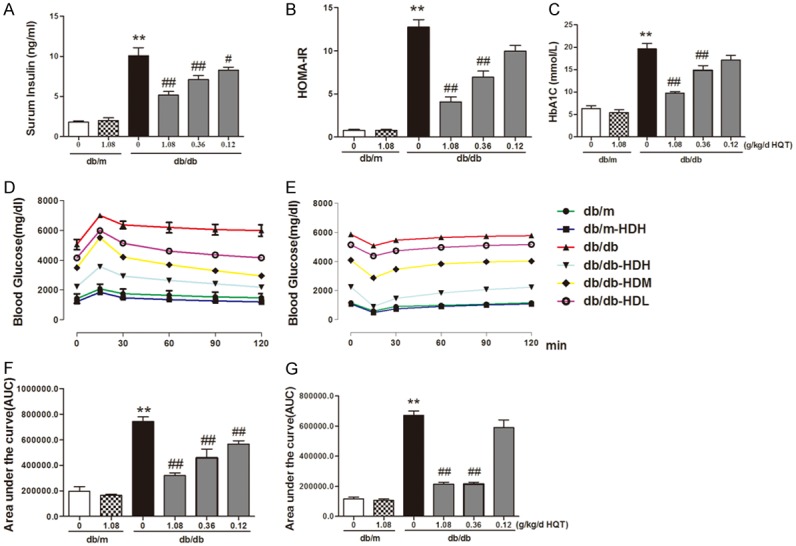
HD treatment inhibited insulin resistance and promoted the ultilization of blood glucose. A. Serum insulin; B. Serum glycosylated hemoglobin; C. Insulin resistance index; D. OGTT (2 g/kg) and fasting blood glucose levels in mice treated with or without HD for 14 weeks; E. IPITT (0.75 U/kg) and fasting blood glucose levels in mice treated with or without HD for 14 weeks. F, G. Area under the curve of OGTT or IPITT. **P < 0.001, compared with db/m group; #P < 0.05, ##P < 0.001, compared with db/db group (n = 10).
HD was administered to both db/db and db/m mice for 14-week treatment. After administration of different dose of HD at 1.08, 0.36, 0.12 g/kg, starting at week 8 we found that high dose of HD treatment (1.08 g/kg) significantly dampened the increase in the body weight, water and food intake and blood glucose over time in the db/db mice (Figure 1A for body weight: db/db-HDH: 39.58 ± 4.84 g vs. db/db: 47.18 ± 5.45 g; Figure 1B for water intake: db/db-HDH: 11.33 ± 0.58 ml vs. db/db: 14.00 ± 0.45 ml; Figure 1C for food intake: db/db-HDH: 6.40 ± 0.17 g vs. db/db: 8.50 ± 0.18 g; and Figure 1D for blood glucose: db/db-HDH: 9.46 ± 3.61 vs. db/db: 30.45 ± 4.08 mM), while low and medium dose groups change these parameters in a dose-dependent manner except water intake which was significantly decreased by high dose of HD. However, HD treatment (1.08 g/kg) had no effect on all the parameters measured in the db/m groups.
HD treatment inhibited insulin resistance and promoted the utilization of blood glucose
db/db mice have significant high level of serum insulin (db/db: 10.11 ± 1.67 vs. db/m: 1.82 ± 0.31 ng/ml), which was dose-dependently decreased by HD treatment (Figure 2A). The HOMA-IR, the insulin resistance indicator calculated based on fasting blood glucose and serum insulin, was higher in the db/db mice, the elevation of which was suppressed by HD treatment in a dose-dependent manner (Figure 2B), indicating an improvement of insulin resistance. The level of HbA1C, measured at week 20 was lowered by HD treatment in a dose-dependent manner (Figure 2C) in the db/db groups compared to db/db vehicle controls, indicated the enhanced glucose consumption and improved insulin resistance by HD.
Oral glucose (2 g/kg) was administered to measure OGTT and IPITT. In the OGTT test, the blood glucose level in the db/db mice significantly increased compared to that in the db/m mice within 2 h after oral glucose (Figure 2D, 2F), indicating that the glucose tolerance was abnormal in the diabetic db/db mice. However, HD treatment (1.08 g/kg) in the db/db mice abolished the elevation in the blood glucose level in OGTT (Figure 2D, 2F), implying the quick clearance of blood glucose and improved glucose tolerance after HD treatment. HD treatment did not change blood glucose in OGTT in the db/m mice. Though as not efficient as high dose of HD, medium and low dose of HD treatment also improved glucose tolerance.
In IPITT test, the blood glucose after insulin injection in the db/db mice was significantly increased at 30, 60, 90 and 120 min (Figure 2E), compared to the db/m mice, indicating that the insulin sensitivity was compromised in the diabetic db/db mice. The db/db mice treated with high dose and medium dose of HD (1.08 g/kg) for 14 weeks have much lower blood glucose at 30, 60, 90 and 120 min in IPITT (Figure 2E, 2G), suggesting HD increase the insulin tolerance in the db/db mice and improve the insulin resistance.
HD treatment protected kidney function in the diabetic mice
By measuring the total urine volume and urine protein, the renal function of the diabetic db/db mice was significantly impaired as early as at the age of 8 weeks and deteriorated as the time went on to week 20 compared to the non-diabetic db/m mice (Figure 3A for total urine volume: db/db: 1.93 ± 0.29 vs. db/m: 1.00 ± 0.12; Figure 3B for urine protein: db/db: 29.96 ± 1.01 vs. db/m 1.19 ± 1.07). The damaged renal function in the diabetic db/db mice was also manifested by the elevation of BUN and serum creatinine (Figure 3C for BUN: db/db: 56.25 ± 2.50 vs. db/m: 19.92 ± 1.28 mg/dl; Figure 3D: db/db: 0.27 ± 0.03 vs. db/m: 0.07 ± 0.03 mg/dl) and the decrease in GFR (Figure 3E, db/db: 0.29 ± 0.06 vs. db/m: 0.52 ± 0.13). At week 12 and the following weeks after HD administration, HD treatment reduced 24 h urine albumin and urine volume in the db/db mice in a dose-dependent manner (Figure 3A, 3B). HD treatment of the db/db mice also dose-dependently prevented the increase of serum creatinine and BUN and rescued GFR (Figure 3C-E), indicating the improved renal function in the db/db mice by HD treatment.
Figure 3.
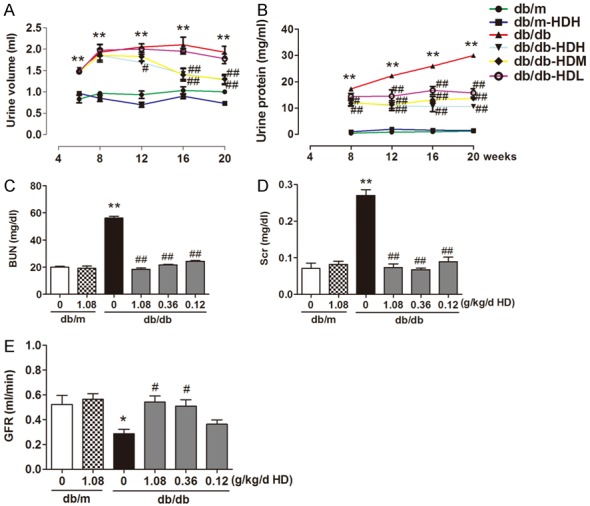
AD treatment protected kidney function in diabetic mice. A. Urine volume; B. Urine albumin; C. blood urea nitrogen; D. Serum creatinine; E. Glomerular filtration rate (GFR); *P < 0.05, **P < 0.001, compared with the db/m group; #P < 0.05, ##P < 0.001, compared with db/db group (n = 10).
HD treatment attenuated the pathological changes in the diabetic kidney
Examing the slides by the PAS staining, we found that the basement membrane of glomerular capillaries in the kidney from the db/db mice became thicken, glycogen and mesangial matrix increased in the mesangial region and the mesangial area became widened (Figure 4). In the kidney slides from HD treated db/db mice (high, medium and low dose groups), less glycogen was deposited in the mesangial region and glycogen disappeared in the glomerular capsule as shown in Figure 4A, implying that HD ameliorated the renal pathological changes from kidney injuries in the diabetic db/db mice.
Figure 4.
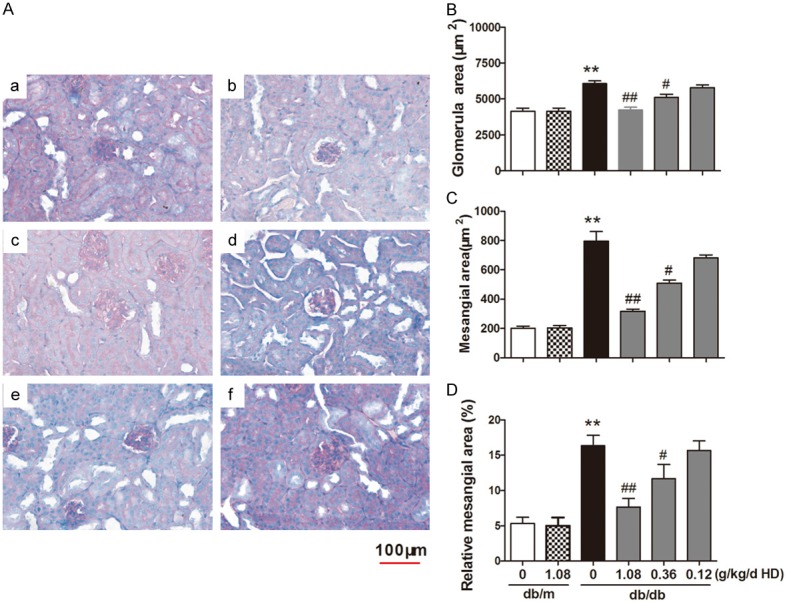
HD treatment attenutates the pathological changes in the diabetic kidney A. PAS stain; B. The average sectional area of glomcrulus; C. Mesangial expansion area; D. Mesangial expansion percentage. a. b. c. d. e. f represented respectively as: db/m group, db/m with high-dose of HD (1.08 g/kg), db/db group, db/db with high-dose of HD (1.08 g/kg); db/db with midium-dose of HD (0.36 g/kg); db/db with low-dose of HD (0.12 g/kg). **P < 0.001, compared with db/m group; ##P < 0.001, #P < 0.05, compared with db/db group (n = 10).
HD regulated IRS1-PI3K/AKT pathway to protect kidney function
To illustrate which cell signals might be involved in the protective effects of HD on diabetic renal pathology, Western blot was performed to detect the expressions of IRS1, IRY, PI3K, AKT in kidney tissue. Compared to the non-diabetic db/m mice, the expression of IRS1, PI3K and AKT did not change in the kidney cortex in the diabetic db/db mice, however, the phosphorylation of these receptors and kinases in the diabetic db/db mice were significantly altered, with an increase in phosphorylation for IRS1S636/639 and AKTT308 and AKTS473 and decrease in phosphorylation for IRS1Y896,7 IRY1361 and PI3K (Figure 5). HD treatment of the diabetic db/db mice did not target the expression of the proteins mentioned above, instead, it regulated the phosphorylation activation of these proteins. High, medium and low doses of HD treatment significantly increased p-IRY1361, phospho-IRS1Y896, phospho-PI3K protein and decreased phospho-IRS1S636/639, phospho-AKTT308, phospho-AKTS473 in a concentration-dependent manner (Figure 5).
Figure 5.
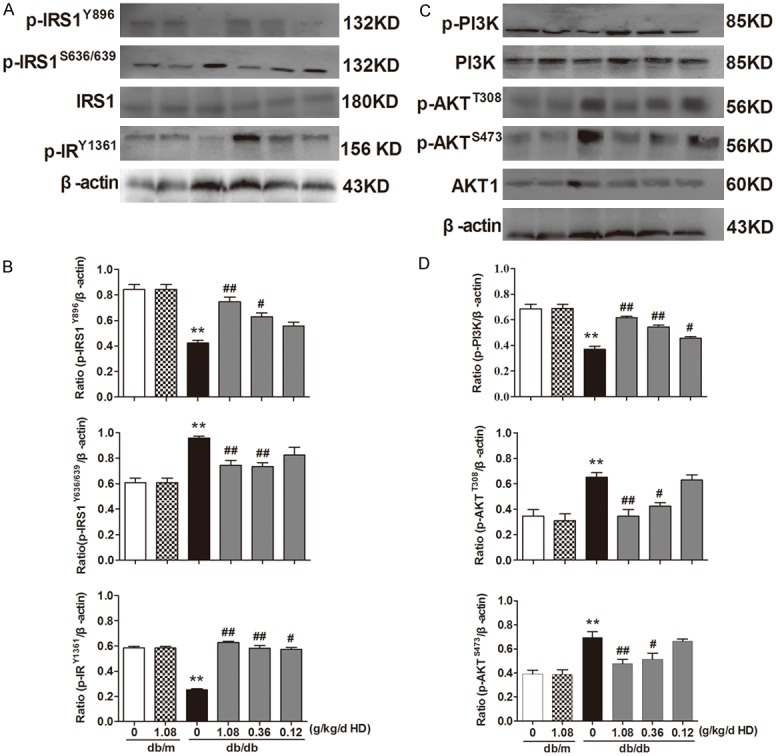
AD regulates IRS1-PI3K/AKT pathway to protect kidney function. Data are representative images for IRS1, phospho-IRS1Y896, phospho-IRY1361, phospho-IRS1S636/639, PI3K, phospho-PI3K, AKT, phospho-AKTT308, phospho-AKTS473. A. Effects of HD on the expression of IRS1, phospho-IRS1307IRS1Y896, phospho--IRY1361, phospho-IRS1S636/639. B. Protein expression is shown relative to β-actin. C. Effect of HD on the expression of PI3K, phospho-PI3K, AKT, phospho-AKTT308, phospho-AKTS473. D. Protein expression is shown relative to β-actin. **P < 0.001, compared with db/m group; ##P < 0.001, #P < 0.05, compared with db/db group (n = 10).
HD abolished the change in the expression of glucose transporters in the diabetic kidney
GLUT1, GLUT4 are two glucose transporters rich in the kidney and function to reabsorb glucose from the filtrate in the tubule lumen. Western blot was performed to detect the expression of GLUT1 and GLUT4 in the kidney and showed that the expression of GLUT1 in the db/db group was significantly higher than that from the db/m group, while GLUT4 expression was significantly lower than that of the db/m group (Figure 6, P < 0.05). High, medium and low doses of HD treatment of the db/db mice dose-dependently decreased the expression of GLUT1 and increased the expression of GLUT4 (Figure 6), indicating an improved glucose reuptake in the kidney.
Figure 6.
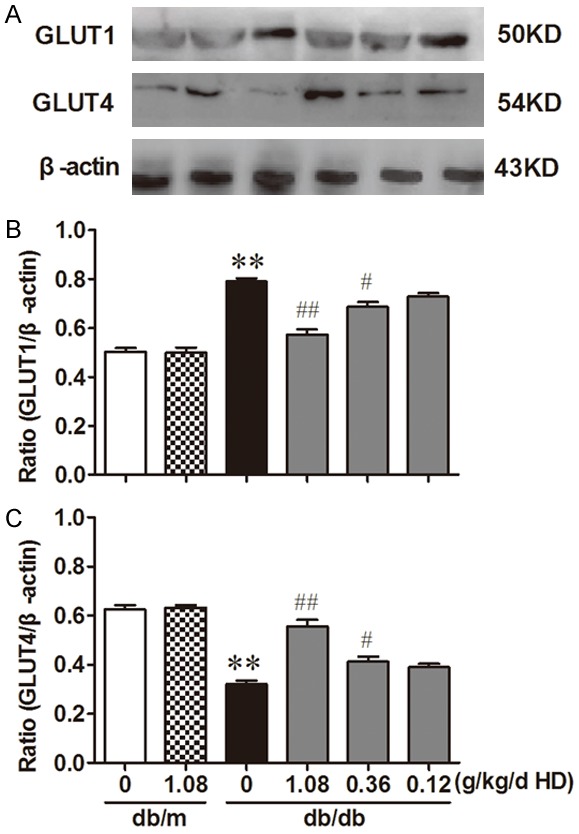
Influence of HD on the expression of GLUT1 and GLUT4 Data are representative images for GLUT1, GLUT4. (A) Influence of HD on the expression of GLUT1 and GLUT4; (B, C) is the statistic analysis of A. Protein expression is shown relative to β-actin. **P < 0.001, compared with db/m group; ##P < 0.001, #P < 0.05, compared with db/db group (n = 10).
Discussion
In the current study, we used db/db mice, a spontaneous type II diabetes model, to investigate the protective mechanism of HD on diabetes and diabetic nephropathy. We found that HD treatment not only prevented the deterioration of diabetes-related symptoms in the db/db mice but also improved renal function and nephropathy, which is associated with the regulation of the IRS1-PI3K-GLUT signaling pathway.
Peripheral insulin resistance and impaired insulin secretion is one of the causes of type II diabetes, and the major clinical symptoms of which are polydipsia, polyuria, polyphagia and obesity [29]. Long-term glucose and lipid metabolic disorders in diabetes can cause kidney pathological changes and renal dysfunction [30]. Our study of HD on the diabetic db/db mice has shown that HD can be used for diabetes treatment as it decreased the body weight, urine volume, water intake and food intake, and lowered the blood glucose and serum glycosylated hemoglobin and improved glucose tolerance and insulin resistance. All these data indicate that HD suppressed the development of diabetes in the db/db mice.
HD treatment dose-dependently reduced 24 h urine albumin and urine volume in the db/db mice and also prevented the increase of serum creatinine and BUN and rescued GFR (Figure 3). In addition, HD treatment attenuated the pathological changes in the diabetic kidney. All of these beneficial effects showed that HD treatment improved renal function and prevented diabetic nephropathy in the db/db mice. One of the pathogenesis of diabetic nephropathy is the disorder of intracellular glucose metabolism. The protection of HD from diabetic damage in kidney may be secondary to the reduced blood glucose from HD treatment. To exclude the effect of lowering blood glucose on the alleviation of DN, previously, we conducted a vitro study that on the condition of co-incubation with 30 mM d-glucose, podocytes was injured and HD rescued podocyte damage. Hence, the reduction of glomerular damage by HD therapy was related with the lowering of blood glucose.
IR/IRS-1/PI3K/GLUT signaling pathway which is activated by insulin activation of insulin receptor (IR) is the most important signal for the regulation of blood glucose [31-34]. To investigate if HD regulates glucose metabolism via this signal pathway in the kidney, we detected signal proteins such as IRS1, IRY, PI3K, AKT in kidney cortex. We found that HD treatment do not change the expression levels of these proteins but regulates their phosphorylation status. HD treatment significantly increased p-IRY1361, phospho-IRS1Y896, phospho-PI3K protein and decreased phospho-IRS1S636/639, phospho-AKTT308, phospho-AKTS473 in a concentration dependent manner (Figure 6), implying that HD regulates signaling pathway associated intracellular glucose metabolism.GLUT1 and GLUT4 are two glucose transporters rich in the kidney and function differently to transport glucose. GLUT1 is the major glucose transporter in the mesangial cells in the kidney. Overexpression of GLUT1 in rat mesangial cells induced an increase in extracellular glucose, and up-regulation of GLUT1 expression lead to less peripheral tissue glucose intake, intracellular glucose metabolism disorders and hyperglycemia [35]. GLUT-4 is the downstream molecule of IR/IRS-1/PI3K/GLUT signaling pathway. In the absence of insulin, GLUT4 is mainly located in intracellular vesicles. The activation of insulin of insulin receptor triggers a series of cascade reactions and causes the translocation of GLUT4 to cell outer membrane, increasing its activity for transporting glucose. GLUT4-mediated glucose transport is the rate-limiting step of glucose utilization in peripheral tissues. Under high extracellular glucose condition, GLUT-4 translocates excess extracellular glucose to cell surface [36,37]. The diabetic db/db mice have higher GLUT1 and lower GLUT4 examined by Western Blot, and HD treat compared to the non-diabetic db/m mice. However, HD treatment of the db/db mice dose-dependently decreased the expression of GLUT1 and increased the expression of GLUT4 (Figure 6), and rescued kidney function. These data indicate that HD regulates the expression of glucose transporter in the kidney and improved glucose reuptake prevents the development of diabetic nephropathy.
Our study of the db/db mouse model showed that the occurrence of insulin resistance symptoms measured appeared at a time the same as the appearance of down-regulation of the p-IRY1361, p-IRS1Y896, p-PI3K and GLUT4 in the kidney, indicating the association of IRS1-PI3K-GLUT signaling with diabetic nephropathy. After the type II diabetic db/db mice were administered HD for 14 weeks, phosphorylation of PI3K in the kidney was increased (Figure 5), implying elevated activity of PI3K by HD treatment. The expression of GLUT4 in the kidney was significantly increased, too, by HD (Figure 6), suggesting an improved glucose transport in the kidney. In addition to ameliorate the renal pathological changes from kidney injuries from diabetes histologically (Figure 4), HD treatment improved insulin signal transduction through promoting the tyrosine phosphorylation of IRS1 in the kidney, which makes HD become a potential potent therapeutic reagent for diabetes and diabetic nephropathy.
In conclusion, HD treatment not only have anti-diabetic effects on the diabetic db/db mice such as reducing blood glucose, promoting glucose utilization and alleviating diabetic symptoms, but also suppressed the development of diabetic nephropathy and improved renal function, which is associated with the regulation of IRS1-PI3K-GLUT signaling pathway. Our study provided solid support for the use of HD for the treatment of diabetic nephropathy in clinical practice.
Acknowledgements
The study was supported by Fund of Shanghai Municipal Health and Planning Commission (No. 20134050, 2016LP016).
Disclosure of conflict of interest
None.
References
- 1.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.Benaissa F, Mohseni-Rad H, Rahimi-Moghaddam P, Mahmoudian M. Berberine reduces the hypoxic-ischemic insult in rat pup brain. Acta Physiol Hung. 2009;96:213–220. doi: 10.1556/APhysiol.96.2009.2.6. [DOI] [PubMed] [Google Scholar]
- 3.Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–894. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 4.Linden KC, DeHaan CL, Zhang Y, Glowacka S, Cox AJ, Kelly DJ, Rogers S. Renal expression and localization of the facilitative glucose transporters GLUT1 and GLUT12 in animal models of hypertension and diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F205–213. doi: 10.1152/ajprenal.00237.2004. [DOI] [PubMed] [Google Scholar]
- 5.Root-Bernstein R, Busik JV, Henry DN. Are diabetic neuropathy, retinopathy and nephropathy caused by hyperglycemic exclusion of dehydroascorbate uptake by glucose transporters? J Theor Biol. 2002;216:345–359. doi: 10.1006/jtbi.2002.2535. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Ghosh R, Maiti S, Khan GA, Sinha AK. The activation by glucose of liver membrane nitric oxide synthase in the synthesis and translocation of glucose transporter-4 in the production of insulin in the mice hepatocytes. PLoS One. 2013;8:e81935. doi: 10.1371/journal.pone.0081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esen B, Atay AE, Gunoz N, Gokmen ES, Sari H, Cakir I, Kayabasi H, Sit D. The relation of mean platelet volume with microalbuminuria and glomerular filtration rate in obese individuals without other metabolic risk factors: the role of platelets on renal functions. Clin Nephrol. 2015;83:322–329. doi: 10.5414/CN108534. [DOI] [PubMed] [Google Scholar]
- 8.Pierine DT, Navarro ME, Minatel IO, Luvizotto RA, Nascimento AF, Ferreira AL, Yeum KJ, Correa CR. Lycopene supplementation reduces TNF-alpha via RAGE in the kidney of obese rats. Nutr Diabetes. 2014;4:e142. doi: 10.1038/nutd.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol. 2007;18:2661–2671. doi: 10.1681/ASN.2006121410. [DOI] [PubMed] [Google Scholar]
- 10.Du JX, Sun MY, Du GL, Li FH, Liu C, Mu YP, Chen GF, Long AH, Bian YQ, Liu J, Liu CH, Hu YY, Xu LM, Liu P. Ingredients of huangqi decoction slow biliary fibrosis progression by inhibiting the activation of the transforming growth factor-beta signaling pathway. BMC Complement Altern Med. 2012;12:33. doi: 10.1186/1472-6882-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehnert S, Baur J, Schmitt A, Neumaier M, Lucke M, Dooley S, Vester H, Wildemann B, Stöckle U, Nussler AK. TGF-beta1 as possible link between loss of bone mineral density and chronic inflammation. PLoS One. 2010;5:e14073. doi: 10.1371/journal.pone.0014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li TH, Hou CC, Chang CL, Yang WC. Anti-Hyperglycemic properties of crude extract and triterpenes from poria cocos. Evid Based Complement Alternat Med. 2011:2011. doi: 10.1155/2011/128402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loniewski I, Wesson DE. Bicarbonate therapy for prevention of chronic kidney disease progression. Kidney Int. 2014;85:529–535. doi: 10.1038/ki.2013.401. [DOI] [PubMed] [Google Scholar]
- 14.Ren S, Zhang H, Mu Y, Sun M, Liu P. Pharmacological effects of astragaloside IV: a literature review. J Tradit Chin Med. 2013;33:413–416. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 15.Huang YC, Chang WL, Huang SF, Lin CY, Lin HC, Chang TC. Pachymic acid stimulates glucose uptake through enhanced GLUT4 expression and translocation. Eur J Pharmacol. 2010;648:39–49. doi: 10.1016/j.ejphar.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Li PB, Lin WL, Wang YG, Peng W, Cai XY, Su WW. Antidiabetic activities of oligosaccharides of Ophiopogonis japonicus in experimental type 2 diabetic rats. Int J Biol Macromol. 2012;51:749–755. doi: 10.1016/j.ijbiomac.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang D, Zhang M. Inhibition mechanism of compound ethanol extracts from wuweizi (fructus schisandrae chinensis) on renal interstitial fibrosis in diabetic nephropathy model mice. J Tradit Chin Med. 2012;32:669–673. doi: 10.1016/s0254-6272(13)60090-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Liu M, Xiong M, Gong J, Tan X. Schisandra chinensis fruit extract attenuates albuminuria and protects podocyte integrity in a mouse model of streptozotocin-induced diabetic nephropathy. J Ethnopharmacol. 2012;141:111–118. doi: 10.1016/j.jep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Wang Y, Chen M, Jin J, Qiu Y, Huang M, Huang Z. Protective effect of schisandrin B against cyclosporine A-induced nephrotoxicity in vitro and in vivo. Am J Chin Med. 2012;40:551–566. doi: 10.1142/S0192415X12500425. [DOI] [PubMed] [Google Scholar]
- 20.Hwang IS, Kim JE, Lee YJ, Kwak MH, Choi YH, Kang BC, Hong JT, Hwang DY. Protective effects of gomisin a isolated from Schisandra chinensis against CCl(4)-induced hepatic and renal injury. Int J Mol Med. 2013;31:888–898. doi: 10.3892/ijmm.2013.1263. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS, Kim ST, Cho DK. Effects of rehmanniae radix water extract on renal function and renin secretion rate in unanesthetized rabbits. Am J Chin Med. 1993;21:179–186. doi: 10.1142/S0192415X93000212. [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Li Y, Chen Y, Lao X, Sheng L, Dai R, Meng W, Deng Y. Hypoglycemic effect of Belamcanda chinensis leaf extract in normal and STZ-induced diabetic rats and its potential active faction. Phytomedicine. 2011;18:292–297. doi: 10.1016/j.phymed.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Yokozawa T, Kim HY, Yamabe N. Amelioration of diabetic nephropathy by dried Rehmanniae Radix (Di Huang) extract. Am J Chin Med. 2004;32:829–839. doi: 10.1142/S0192415X04002442. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Li BY, Li XL, Cheng M, Yu F, Lu WD, Cai Q, Wang JF, Zhou RH, Gao HQ, Shen L. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim Biophys Acta. 2013;1832:805–816. doi: 10.1016/j.bbadis.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 25.He Q, Pu J, Yuan A, Yao T, Ying X, Zhao Y, Xu L, Tong H, He B. Liver X receptor agonist treatment attenuates cardiac dysfunction in type 2 diabetic db/db mice. Cardiovasc Diabetol. 2014;13:149. doi: 10.1186/s12933-014-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Li R, Shi W, Liang X, Liu S, Ye Z, Yu C, Chen Y, Zhang B, Wang W, Lai Y, Ma J, Li Z, Tan X. NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. Br J Pharmacol. 2013;170:426–439. doi: 10.1111/bph.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26:357–366. doi: 10.1016/j.tem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakino S, Hasegawa K, Itoh H. Sirtuin and metabolic kidney disease. Kidney Int. 2015;88:691–8. doi: 10.1038/ki.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 32.Cheng KK, Iglesias MA, Lam KS, Wang Y, Sweeney G, Zhu W, Vanhoutte PM, Kraegen EW, Xu A. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–427. doi: 10.1016/j.cmet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Koren-Gluzer M, Aviram M, Hayek T. Paraoxonase1 (PON1) reduces insulin resistance in mice fed a high-fat diet, and promotes GLUT4 overexpression in myocytes, via the IRS-1/Akt pathway. Atherosclerosis. 2013;229:71–78. doi: 10.1016/j.atherosclerosis.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Prasad J, Maurya CK, Pandey J, Jaiswal N, Madhur G, Srivastava AK, Narender T, Tamrakar AK. Diastereomeric mixture of calophyllic acid and isocalophyllic acid stimulates glucose uptake in skeletal muscle cells: involvement of PI-3-kinase- and ERK1/2-dependent pathways. Mol Cell Endocrinol. 2013;370:11–19. doi: 10.1016/j.mce.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Gnudi L, Viberti G, Raij L, Rodriguez V, Burt D, Cortes P, Hartley B, Thomas S, Maestrini S, Gruden G. GLUT-1 overexpression: Link between hemodynamic and metabolic factors in glomerular injury? Hypertension. 2003;42:19–24. doi: 10.1161/01.HYP.0000075949.19968.EF. [DOI] [PubMed] [Google Scholar]
- 36.Tong KM, Shieh DC, Chen CP, Tzeng CY, Wang SP, Huang KC, Chiu YC, Fong YC, Tang CH. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-kappaB/p300 binding in human synovial fibroblasts. Cell Signal. 2008;20:1478–1488. doi: 10.1016/j.cellsig.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]


