Abstract
The aim of the present research was to investigate the association between the BRAF mutation and papillary thyroid carcinoma (PTC), and further explore the relationship between the systemic inflammation response index (SIRI) and BRAF mutation in patients with PTC. The clinicopathological data were extracted from the patients’ medical records from June 2012 to June 2014 in our hospital. We enrolled 95 patients with PTC that have received the total or near-total thyroidectomy and pretracheal and paratracheal lymph node dissection. The blood samples were obtained before surgery. According to the BRAF mutation analysis, the patients were divided into two groups: BRAF mutation positive group and BRAF mutation negative group. The receiver operating characteristic curve (ROC) for the presence of BRAF mutation was used to evaluate the optimal cutoff value of SIRI. The ratio closest to the point with maximum sensitivity and specificity was defined as the optimal cutoff value. Univariate and multivariate logistic regression model were used to confirm the independent factors and compare observed and predicted outcomes. The BRAF mutation rates were 62.1% (59/95). The results indicated that BRAF mutation was significantly correlated with pathological TNM stage, monocyte, SIRI and Galectin-3. The pathological TNM stage, monocyte, SIRI and Galectin-3 were the significant risk factors associated with the presence of BRAF mutation. Moreover, we found that patients with low SIRI had higher BRAF mutation percentage than those with high SIRI, and patients with low monocyte had higher percentage than those with high monocyte. BRAF mutation is associated with the pathological TNM stage, monocyte, SIRI and Galectin-3, and SIRI was the significant risk factor with the BRAF mutation and patients with low SIRI have higher BRAF mutation.
Keywords: Papillary thyroid carcinoma (PTC), BRAF, systemic inflammation response index (SIRI), mutation, endocrine malignancy
Introduction
Thyroid cancer is one of the common endocrine malignancy all over the world, influencing the health and quality of life of people, which has become the eighth most common malignant cancer in women in China [1]. Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer. With the increasing prevalence of PTC, the worldwide incidence of thyroid cancer has been remarkably raising in the lately several decades [2]. Meanwhile, with the advance of ultrasonographic (US) and fine needle aspiration biopsy (FNAB), more thyroid cancer has been detected at early stage, even the papillary thyroid microcarcinoma. Although the incidence of thyroid cancer is raising rapidly, the cancer-related mortality rate is still relatively stable and the PTC has a better prognosis with an average 10-year survival rate of around 90% [3,4]. However, above 50% of PTC usually has performed lymph node metastasis and less than 10% of PTC has showed distant metastasis with lung and bone [5]. PTC treatment, included surgical resection, radio-iodine (131I) ablation, levothyroxine suppression therapy and so forth, has treated successfully and shown a better prognosis. Many researches have indicated that some clinicopathologic factors, such as age, gender, histological variants, infiltrative growth pattern, tumor size and genetic aberrations, has been proved to be related to the prognostic of PTC and might reflected the recurrence and metastasis [6-8]. However, these factors were not accurate as the individual patient’s likelihood of recurrence and metastasis. Therefore, it is essential to study the molecular mechanisms and identify the markers to guide the appropriate clinical strategy and management of thyroid cancer.
Nowadays, genetic aberrations have been proved to the influence factors to the development, progression and metastasis of PTC [9,10]. The relationship between BRAF and PTC has been a hot research aspect. BRAF gene mutation was usually the T (thymine) to A (adenine) substitution at nucleotide position 1799 point, resulted in a valine-to-glutamic acid at residue 600 (V600E) within exon 15 of the kinase domain [11,12]. This genetic change would influence and activate the mitogen-activated protein kinase (MAPK) signaling pathway, and lead to influence the cell proliferation, cell differentiation, apoptosis and development and so forth [13]. Some research have indicated that BRAF mutation was high frequency in PTC, which has been found to be conformed to more aggressiveness, with higher risk of recurrence and mortality, and worse prognosis [9,14]. Thus, BRAF should be further study the molecular mechanism of tumor and cancer metastasis and provide the new direction for the therapy of PTC.
However, the relationship between BRAF and cancer-related inflammation was rarely investigated, which would show whether or not to the recurrence and metastasis of PTC. Lots of studies have indicated the he inflammatory cells, such as neutrophil, lymphocyte, monocyte, platelet, may play an important role in tumor development and progression [15-17]. A novel systemic inflammation response index named SIRI (SIRI = N × M/L), which based on neutrophil (N), monocyte (M) and lymphocyte (L) counts, has been reported to be associated with survival time and clinical outcome in some tumors. The present study was aimed to investigate the association between the BRAF mutation and papillary thyroid carcinoma (PTC), and further study the relationship between the systemic inflammation response index (SIRI) and BRAF mutation in patients with PTC.
Materials and methods
This retrospective research was conducted under the understanding and written consent of individual patient. The present study was approved by the ethics committee of our hospital and in accordance with the ethical standards of the Declaration of Helsinki and its later ethical standards. The written informed consent was obtained from all patients. All experiments were performed in accordance with relevant guidelines and regulations.
Patients selection
The present research was retrospective and all medical records data of patients were kept anonymous. We enrolled 95 patients with PTC, who had received the total or near-total thyroidectomy and pretracheal and paratracheal lymph node dissection in our hospital from June 2012 to June 2014. All patients with PTC were confirmed in accordance with pathological evidence. Inclusion criteria included: (1) patients with PTC were confirmed in accordance with endoscopic evaluation with histological pathology; (2) no previous treatment, such as radiation exposure and suppressive therapy with thyroid hormones. Exclusion criteria included: (1) patients with medullary, analplastic and follicular carcinoma; (2) with any other malignancy or autoimmune disease; (3) patients with serious complications, such as infections.
BRAF mutation detection
BRAF mutation analysis was performed at the pathology department of our hospital. Before BRAF mutation analysis, we marked the specimens that were enriched tumor cell by hematoxylin and eosin staining. DNA was extracted from the postoperative formalin fixed paraffin embedded (FFPE) tissue, and 5 to 10 paraffin sections with a thickness of 6-10 um that contained the tumor tissue. According to the manufacturer’s protocol, we used the QIAamp DNA FFPE Tissue kit (Qiagen, Hilden, Germany) to conduct the DNA of PTC. The NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to evaluate the absorbance and quality of the extracted DNA. We tested the BRAF exon 15 by the human BRAF V600E ARMS-PCR kit (Amoy Diagnostics Co. Ltd, Xiamen, China) and followed the manufacturer’s protocol. The polymerase chain reaction (PCR) was performed as follows: 95°C for 5 min, followed by 35 cycles of 30 sec at 95°C, 64°C for 20 sec, 72°C for 30 sec, then 10 min extension at 72°C. The PCR products were using 2% gel-purified by the QIAgen gel extraction kit (Qiagen). The BRAF mutation was determined by comparing the SSCP banding pattern of the case with positive and negative controls.
Blood sample
The peripheral venous blood samples were collected from the PTC patients before surgery. These samples were collected into a sterile ethylenediaminetetraacetic acid (EDTA) tube. The systemic inflammation response index (SIRI) was defined as N × M/L, where N, M, and L are the pretreatment peripheral neutrophil, monocyte, and lymphocyte counts, respectively.
Statistical analysis
Statistical Package for Social Sciences (SPSS) 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (Inc, La Jolla, CA) were used to perform all statistical analyses. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of SIRI. The ratio closest to the point with maximum sensitivity and specificity was defined as the optimal cutoff value. The categorical variables were presented as frequencies and percentages (%), and the Chi-square test or Fisher exact test was used to analyze the categorical variables as appropriate. The continuous variables were provided with mean ± standard deviation (SD), and compared by Student’s t test. Univariate and multivariate logistic regression model were used to confirm the independent factors and compare observed and predicted outcomes. A two-tailed P < 0.05 was considered to indicate statistical significance.
Results
BRAF mutation analysis
All of the enrolled patients were divided into two groups basing on the presence or absence of BRAF mutation. There were 59 patients (62.1%) with BRAF mutation, 36 patients (37.9%) without BRAF mutation. All cases were the BRAFV600E mutation subtype. Pathological examination confirmed the diagnosis of papillary thyroid carcinoma by haematoxylin-eosin stain (Figure 1). An immunohistochemistry method was applied to detect the expression of BRAF in PTC tissues and matched normal PTC tissues (Figure 2A, 2B). The BRAF exon 15 was tested by the human BRAF V600E ARMS-PCR kit. The DNA sequence was indicated the results of the PTC tissues with mutations (Figure 3A) and without mutations (Figure 3B). The amplification plot of BRAF showed BRAF mutation type and BRAF wild type (Figure 4A, 4B).
Figure 1.
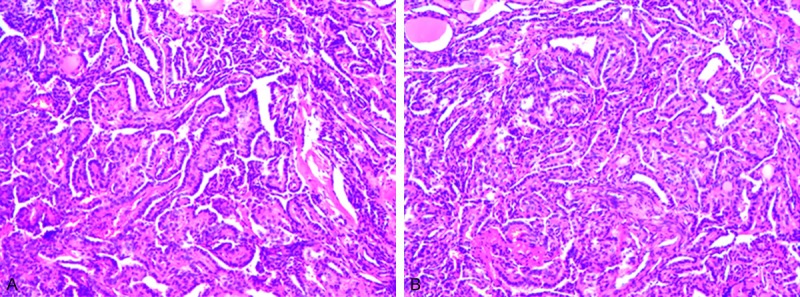
Hematoxylin and eosin staining of papillary thyroid carcinoma (PTC) (magnification, × 100) Micropapillary structures with thin fibrovascular stroma are shown. The epithelial cells are mostly small with an increased nuclear to cytoplasmic ratio.
Figure 2.
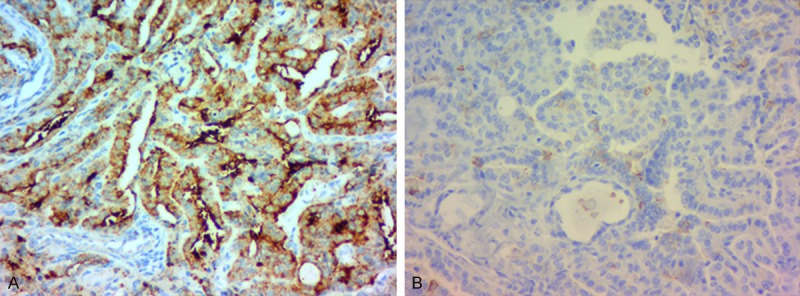
Immunohistochemical staining in papillary thyroid carcinoma (PTC). A. The expression of BRAF in PTC tissues × 400; B. The expression of BRAF in matched normal PTC tissues × 400.
Figure 3.
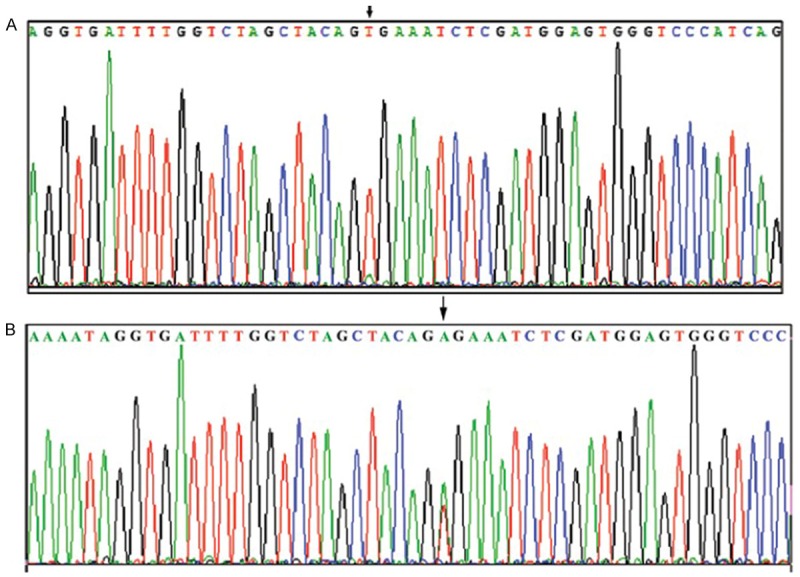
Representative sequencing of exon 15 of BRAF. A. BRAF mutation type sequence; B. BRAF wild type sequence. *Arrow indicates mutated base in BRAF V600E mutation. BRAF, B-Raf proto oncogene serine/threonine kinase.
Figure 4.
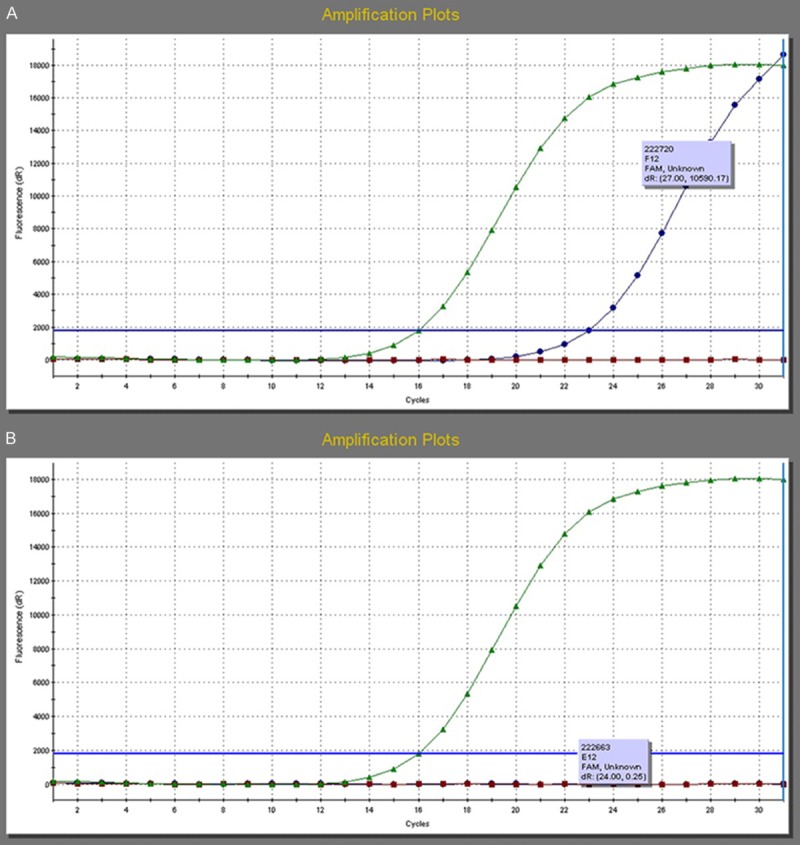
The amplification plot of BRAF. A. The amplification plot of BRAF showed BRAF mutation type; B. The amplification plot of BRAF showed BRAF wild type.
Clinicopathological characteristics of patients with PTC
We enrolled 95 patients with PTC in this study. 59 patients were with BRAF mutation and 36 patients without BRAF mutation. There were 29 males and 66 females, respectively. The median age was 49 years, range 11-71 years. The mean tumor size was 11.03 ± 1.03 mm, and median size was 8.00 mm. Thus, the patients were stratified into two groups: BRAF mutation positive group and BRAF mutation negative group. There were differences of demographic and clinicopathological features between the two groups (Table 1). The BRAF mutation positive group was significantly correlated with pathological TNM stage (χ2 = 4.252, P = 0.039), monocyte (χ2 = 5.298, P = 0.021), SIRI (χ2 = 4.004, P = 0.045) and Galectin-3 (χ2 = 6.283, P = 0.012).
Table 1.
BRAF mutation and clinicopathological characteristics of papillary thyroid cancer (PTC)
| Parameters | Number (%) | BRAF mutation (+) | BRAF mutation (-) | χ2 | P value |
|---|---|---|---|---|---|
| Cases (n) | 59 | 36 | |||
| Age (years) | 0.043 | 0.835 | |||
| < 45 | 37 (38.9%) | 23 (39.0%) | 14 (38.9%) | ||
| ≥ 45 | 58 (61.1%) | 36 (61.0%) | 22 (61.1%) | ||
| Gender | 3.356 | 0.067 | |||
| Male | 29 (30.5%) | 22 (37.3%) | 7 (19.4%) | ||
| Female | 66 (69.5%) | 37 (62.7%) | 29 (80.6%) | ||
| Type of surgery | 1.141 | 0.565 | |||
| Right resection | 29 (30.5%) | 20 (33.9%) | 9 (25.0%) | ||
| Left resection | 34 (35.8%) | 19 (32.2%) | 15 (41.7%) | ||
| Total resection | 32 (33.7%) | 20 (33.9%) | 12 (33.3%) | ||
| Tumor size (mm) | 0.431 | 0.512 | |||
| < 10 | 54 (56.8%) | 32 (54.2%) | 22 (61.1%) | ||
| ≥ 10 | 41 (43.2%) | 27 (45.8%) | 14 (38.9%) | ||
| Tumor focus | 0.206 | 0.650 | |||
| Unifocal | 66 (69.5%) | 40 (67.8%) | 26 (72.2%) | ||
| Multifocal | 29 (30.5%) | 19 (32.2%) | 10 (27.8%) | ||
| Lymph node metastasis | 2.154 | 0.142 | |||
| Yes | 38 (40.0%) | 27 (45.8%) | 11 (30.6%) | ||
| No | 57 (60.0%) | 32 (54.2%) | 25 (69.4%) | ||
| Pathological TNM classification | |||||
| T stage | 0.127 | 0.721# | |||
| T1 | 87 (91.6%) | 54 (91.5%) | 33 (91.7%) | ||
| T2 | 6 (6.3%) | 3 (5.1%) | 3 (8.3%) | ||
| T3 | 2 (2.1%) | 2 (3.4%) | 0 (0.0%) | ||
| N stage | 2.263 | 0.322# | |||
| N0 | 61 (64.2%) | 35 (59.3%) | 26 (72.2%) | ||
| N1a | 20 (21.1%) | 13 (22.0%) | 7 (19.5%) | ||
| N1b | 14 (14.7%) | 11 (18.7%) | 3 (8.3%) | ||
| TNM stage | 4.252 | 0.039# | |||
| I | 77 (81.1%) | 44 (74.5%) | 33 (91.7%) | ||
| II | 1 (1.1%) | 1 (1.7%) | 0 (0.0%) | ||
| III | 10 (10.5%) | 7 (11.9%) | 3 (8.3%) | ||
| IVa | 7 (7.3%) | 7 (11.9%) | 0 (0.0%) | ||
| White blood cell (W) | 0.118 | 0.732 | |||
| < 5.82 | 47 (49.5%) | 30 (50.9%) | 17 (47.2%) | ||
| ≥ 5.82 | 48 (50.5%) | 29 (49.1%) | 19 (52.8%) | ||
| Hemoglobin (Hb) | 2.795 | 0.095 | |||
| < 137 | 45 (47.4%) | 24 (40.7%) | 21 (58.3%) | ||
| ≥ 137 | 50 (52.6%) | 35 (59.3%) | 15 (41.7%) | ||
| Neutrophils (N) | 0.199 | 0.656 | |||
| < 3.30 | 45 (47.4%) | 29 (49.1%) | 16 (44.4%) | ||
| ≥ 3.30 | 50 (52.6%) | 30 (50.9%) | 20 (55.6%) | ||
| Monocyte (M) | 5.298 | 0.021 | |||
| < 0.30 | 26 (27.4%) | 21 (35.6%) | 5 (13.9%) | ||
| ≥ 0.30 | 69 (72.6%) | 38 (64.4%) | 31 (86.1%) | ||
| Platelet (P) | 2.598 | 0.107 | |||
| < 221 | 47 (49.5%) | 33 (55.9%) | 14 (38.9%) | ||
| ≥ 221 | 48 (50.5%) | 26 (44.1%) | 22 (61.1%) | ||
| Lymphocyte (L) | 1.286 | 0.257 | |||
| < 1.90 | 44 (46.3%) | 30 (50.9%) | 14 (38.9%) | ||
| ≥ 1.90 | 51 (53.7%) | 29 (49.1%) | 22 (61.1%) | ||
| SIRI | 4.004 | 0.045 | |||
| < 0.43 | 33 (34.7%) | 25 (42.4%) | 8 (22.2%) | ||
| ≥ 0.43 | 62 (65.3%) | 34 (57.6%) | 28 (77.8%) | ||
| Ki-67 | 0.621 | 0.431 | |||
| Positive | 64 (67.4%) | 38 (64.4%) | 26 (72.2%) | ||
| Negative | 31 (32.6%) | 21 (35.6%) | 10 (27.8%) | ||
| P53 | 0.104 | 0.745 | |||
| Positive | 35 (36.8%) | 21 (35.6%) | 14 (38.9%) | ||
| Negative | 60 (63.2%) | 38 (64.4%) | 22 (61.1%) | ||
| CK19 | 0.696 | 0.404 | |||
| Positive | 85 (89.5%) | 54 (91.5%) | 31 (86.1%) | ||
| Negative | 10 (10.5%) | 5 (8.5%) | 5 (13.9%) | ||
| Galectin-3 | 6.283 | 0.012# | |||
| Positive | 82 (86.3%) | 55 (93.2%) | 27 (75.0%) | ||
| Negative | 13 (13.7%) | 4 (6.8%) | 9 (25.0%) | ||
| CD56 | 0.402 | 0.526 | |||
| Positive | 23 (24.2%) | 13 (22.0%) | 10 (27.8%) | ||
| Negative | 72 (75.8%) | 46 (78.0%) | 26 (72.2%) |
Fisher exact test.
Univariate and multivariate logistic regression model analyses with BRAF mutation
Univariate analysis showed that pathological TNM stage (OR = 0.533, 95% CI: 0.292-0.972; P = 0.040), monocyte (OR = 3.426, 95% CI: 1.158-10.135; P = 0.026), SIRI (OR = 2.206, 95% CI: 0.884-5.503; P = 0.033) and Galectin-3 (OR = 4.583, 95% CI: 1.294-16.233; P = 0.018) were the four significant risk factors associated with the presence of BRAF mutation. The results of multivariate logistic regression model analyses of the factors associated with BRAF mutation were pathological TNM stage (OR = 0.503, 95% CI: 0.266-0.951; P = 0.035), monocyte (OR = 4.056, 95% CI: 1.268-12.973; P = 0.018), SIRI (OR = 2.574, 95% CI: 1.005-6.590; P = 0.049) and Galectin-3 (OR = 7.116, 95% CI: 1.598-31.699; P = 0.010) (Table 2).
Table 2.
Univariate and multivariate logistic regression model analyses with BRAF mutation
| Parameters | Univerlate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age (years) | ||||
| < 45 vs ≥ 45 | 5.458 (0.989-30.125) | 0.052 | ||
| Gender | ||||
| Male vs Female | 1.242 (0.328-4.710) | 0.75 | ||
| Type of surgery | ||||
| Right resection vs Left and total resection | 1.669 (0.638-4.365) | 0.296 | ||
| Tumor size (mm) | ||||
| < 10 vs ≥ 10 | 0.970 (0.269-3.504) | 0.963 | ||
| Tumor focus | ||||
| Unifocal vs Multifocal | 0.506 (0.096-2.658) | 0.421 | ||
| Lymph node metastasis | ||||
| Yes vs No | 0.343 (0.041-2.868) | 0.323 | ||
| Pathological TNM classification | ||||
| T stage | ||||
| T1 vs T2+T3 | 1.271 (0.211-7.660) | 0.793 | ||
| N stage | ||||
| N0 vs N1a+N1b | 0.548 (0.117-2.572) | 0.446 | ||
| TNM stage | ||||
| I+II vs III+VIa | 0.533 (0.292-0.972) | 0.04 | 0.503 (0.266-0.951) | 0.035 |
| White blood cell (W) | ||||
| < 5.82 vs ≥ 5.82 | 0.155 (0.022-1.087) | 0.061 | ||
| Hemoglobin (Hb) | ||||
| < 137 vs ≥ 137 | 0.979 (0.247-3.885) | 0.976 | ||
| Neutrophils (N) | ||||
| < 3.30 vs ≥ 3.30 | 6.646 (0.790-55.880) | 0.081 | ||
| Monocyte (M) | ||||
| < 0.30 vs ≥ 0.30 | 3.426 (1.158-10.135) | 0.026 | 4.056 (1.268-12.973) | 0.018 |
| Platelet (P) | ||||
| < 221 vs ≥ 221 | 2.028 (0.559-7.354) | 0.282 | ||
| Lymphocyte (L) | ||||
| < 1.90 vs ≥ 1.90 | 1.991 (0.482-8.226) | 0.341 | ||
| SIRI | ||||
| < 0.43 vs ≥ 0.43 | 2.206 (0.884-5.503) | 0.033 | 2.574 (1.005-6.590) | 0.049 |
| Ki-67 | ||||
| Positive vs Negative | 0.588 (0.143-2.425) | 0.463 | ||
| P53 | ||||
| Positive vs Negative | 0.598 (0.182-1.967) | 0.398 | ||
| CK19 | ||||
| Positive vs Negative | 1.742 (0.467-6.494) | 0.408 | ||
| Galectin-3 | ||||
| Positive vs Negative | 4.583 (1.294-16.233) | 0.018 | 7.116 (1.598-31.699) | 0.010 |
| CD56 | ||||
| Positive vs Negative | 0.624 (0.156-2.497) | 0.505 | ||
Correlation between BRAF mutation and SIRI or monocyte
The peripheral venous blood samples were collected from the PTC patients before surgery. We used receiver operating characteristic curve (ROC) for the presence of BRAF mutation with respect to preoperative SIRI. The optimal cutoff value of SIRI was 0.43, with the highest sensitivity and specificity. The patients were stratified into two groups by the optimal cutoff value of SIRI: a low SIRI group (SIRI < 0.43 × 109/L) and a high SIRI group (SIRI ≥ 0.43 × 109/L). In univariate and multivariate logistic regression analysis, we found that SIRI was the significant risk factor with the BRAF mutation. In order to further study the significant risk factor of BRAF mutation, the BRAF mutation was analyzed by the SIRI. Patients with low SIRI by BRAF mutation were 33, and there were 25 patients (75.8%) with BRAF mutation, 8 patients (24.2%) without BRAF mutation. Patients with high SIRI by BRAF mutation were 62, and there were 34 patients (54.8%) with BRAF mutation, 28 patients (45.2%) without BRAF mutation (Figure 5A). We found that patients with low SIRI had higher BRAF mutation percentage than those with high SIRI. Moreover, with the same method, the optimal cutoff value of monocyte was 0.30. Patients with low monocyte by BRAF mutation were 26, 21 patients (80.8%) with BRAF mutation, 5 patients (19.2%) without BRAF mutation. Patients with high monocyte by BRAF mutation were 69, and there were 38 patients (55.1%) with BRAF mutation, 31 patients (44.9%) without BRAF mutation (Figure 5B). We found that patients with low monocyte had higher BRAF mutation percentage than those with high monocyte.
Figure 5.
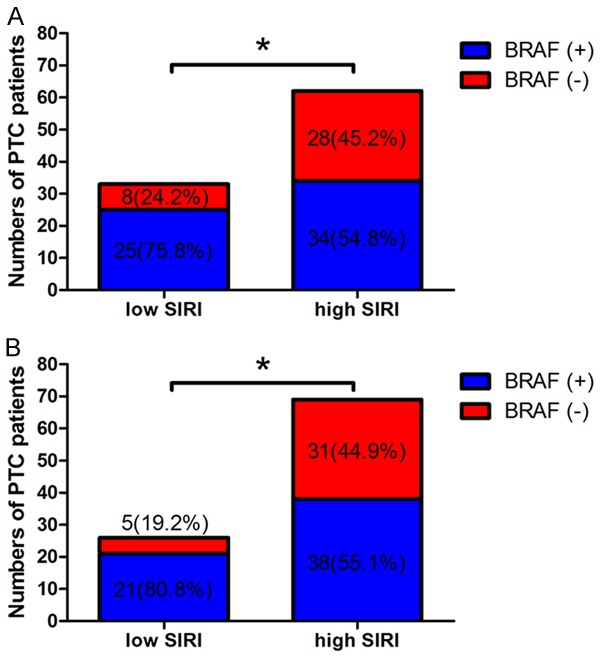
BRAF mutation for papillary thyroid carcinoma (PTC). A. BRAF mutation for papillary thyroid carcinoma (PTC) of two SIRI groups; B. BRAF mutation for papillary thyroid carcinoma (PTC) of two SIRI groups. *P < 0.05.
Discussion
Papillary thyroid carcinoma (PTC), which accounts for the majority of thyroid cancers, is not a lethal carcinoma and with long-term survival rate [18,19]. This subtype thyroid cancer is accounting for 1.5% of all malignant cancers in American, and up to Arab countries [20,21]. Although PTC has excellent prognosis, half of them will have lymph node metastasis and about 10% of them show distant metastasis and recurrence [22]. Nowadays, the incidence of this subtype thyroid cancer has been raising quickly among the females, and the second fastest-raising incidence among males [23]. The current standard treatments, such as surgical excision, 131I ablation and oral levothyroxine suppression, has improved the differentiated thyroid cancer quality of life, however, failed to the aggressive or incurable disease. Therefore, new therapeutic methods should be researched and discovered, and especially for the aggressive thyroid cancers.
The genetic techniques have been successfully applied and identified to the diagnosis and treatment of many malignant tumors. In the latest several years, some genes have been found and further studied, and proved that are associated to the occurrence, development, and metastasis of thyroid cancer [24]. BRAF, named as murine sarcoma viral carcinogenic homology B1, was identified as an oncogene [25]. It can induce the cell proliferation, such as avian primary cells and NIH3T3 cells, and act as specific serine/threonine kinase to activate the mitogen-activated protein kinase (MAPK) signaling pathway [26,27]. Various studies have proved that the V600E mutation is located in the activation region of BRAF (CR3), and can lead to the aberrant activation of BRAF, and enhance to activate downstream kinases compared with not mutation [28]. With continuous activation, the RAS/RAF/MEK/MAPK signaling pathway will promote the well-differentiated papillary carcinoma cells to become the poorly differentiated and undifferentiated tumor cells [29].
Nowadays, as far as we known, inflammation is a key component of tumor microenvironment and plays a critical role in cancer cell proliferation, carcinogenesis and invasion [17]. The proinflammatory mediators are influenced by the cancer cells and stimulate the production of systemic inflammatory response. Furthermore, tumor-inflammation interaction may represent a possible therapeutic target for thyroid cancer. The cellular components, such as neutrophils, monocytes, platelets and lymphocytes, can reflect the clinical outcome for thyroid cancer. SIRI has been studied and reported that it is related to the clinical outcome and provide critical information for the cancer treatment. However, the SIRI has been researched rarely.
In the present study, we studied the BRAF mutation in patients with PTC, and analyze the relationship between BRAF mutation and clinicopathological characteristics. We found that BRAF mutation was significantly correlated with pathological TNM stage, monocyte, SIRI and Galectin-3. Meanwhile, we used the univariate and multivariate logistic regression model to further study the risk factors associated with BRAF mutation. The results indicated that pathological TNM stage, monocyte, SIRI and Galectin-3 were the significant risk factors associated with the presence of BRAF mutation. Moreover, we further studied the correlation between BRAF mutation and SIRI. We found that patients with low SIRI had higher percentage than those with high SIRI, and patients with low monocyte had higher percentage than those with high monocyte.
In this study, the BRAF mutation rates were 62.1% (59/95), and the rates had been reported from 28% to 83%, which is primarily related to epidemiological factors and ethnic backgrounds [30,31]. With the development and advance of ultrasonographic (US), fine needle aspiration biopsy (FNAB) technology and color doppler ultrasound instruments with high resolution, the incidence of thyroid tumors is increasingly. Some studies indicated that the extra-thyroidal extension, higher clinical stage, and older age were the significant risk factors, and associated with higher rates of thyroid cancer recurrence and related mortality [32,33]. In our study, the pathological TNM stage was associated to the BRAF mutation, and the pathological I stage accounted for the majority. The latest research had indicated that neutrophil-to-lymphocyte ratio (NLR) is associated to extrathyroidal invasion, multifocality, bilaterality, and lymph node metastasis of PTC [34]. However, the relationship between inflammation and PTC with BRAF mutation remains to be elucidated. Whether the SIRI respresents the inflammatory microenvironment results the PTC development or BRAF mutation remains to be further to research and study.
In conclusion, the present research has confirmed that the BRAF mutation is associated with the pathological TNM stage, monocyte, SIRI and Galectin-3, and patients with low SIRI has higher BRAF mutation. However, we can’t conclude that BRAF mutation accurate the poor prognosis in PTC, and we need further prospective and well-designed randomized controlled trials investigation.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.La VC, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136:2187. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Suh S, Yun HK, Goh TS, Jin L, Jeong DC, Oh SO, Hong JC, Kim SJ, Kim IJ, Pak K. Outcome prediction with the revised American joint committee on cancer staging system and American thyroid association guidelines for thyroid cancer. Endocrine. 2017;58:1–8. doi: 10.1007/s12020-017-1449-4. [DOI] [PubMed] [Google Scholar]
- 5.Livolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(Suppl 2):S1–9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 6.Lacouture ME, Ciccolini K, Kloos RT, Agulnik M. Overview and management of dermatologic events associated with targeted therapies for medullary thyroid cancer. Thyroid. 2014;24:1329–1340. doi: 10.1089/thy.2013.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers MB, Mckim KL, Parsons BL. A subset of papillary thyroid carcinomas contain KRAS mutant subpopulations at levels above normal thyroid. Mol Carcinog. 2014;53:159–67. doi: 10.1002/mc.21953. [DOI] [PubMed] [Google Scholar]
- 8.Chung SY, Lee JS, Lee H, Park SH, Kim SJ, Han SR. Cytomorphological factors and braf mutation predicting risk of lymph node metastasis in preoperative liquid-based fine needle aspirations of papillary thyroid carcinoma. Acta Cytol. 2013;57:252–258. doi: 10.1159/000343617. [DOI] [PubMed] [Google Scholar]
- 9.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Chen T, Liu Z. Associations between BRAF V600E and prognostic factors and poor outcomes in papillary thyroid carcinoma: a meta-analysis. World J Surg Oncol. 2016;14:241. doi: 10.1186/s12957-016-0979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carta C, Moretti S, Passeri L, Barbi F, Avenia N, Cavaliere A, Monacelli M, Macchiarulo A, Santeusanio F, Tartaglia M. Genotyping of an Italian papillary thyroid carcinoma cohort revealed high prevalence of BRAF mutations, absence of RAS mutations and allowed the detection of a new mutation of BRAF oncoprotein (BRAF(V599lns)) Clin Endocrinol (Oxf) 2006;64:105–9. doi: 10.1111/j.1365-2265.2005.02401.x. [DOI] [PubMed] [Google Scholar]
- 12.Moretti S, Macchiarulo A, De FV, Avenia N, Barbi F, Carta C, Cavaliere A, Melillo RM, Passeri L, Santeusanio F. Biochemical and molecular characterization of the novel BRAF(V599Ins) mutation detected in a classic papillary thyroid carcinoma. Oncogene. 2006;25:4235–4240. doi: 10.1038/sj.onc.1209448. [DOI] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab. 2012;97:4559–4570. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diakos CI, Charles KA, Mcmillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 16.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis--tracing the accessory. Oncogene. 2014;33:3217–24. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinstein WS. Endocrine cancer predisposition syndromes: hereditary paraganglioma, multiple endocrine neoplasia type 1, multiple endocrine neoplasia type 2, and hereditary thyroid cancer. Hematol Oncol Clin North Am. 2010;24:907–937. doi: 10.1016/j.hoc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Kushchayeva YS, Kushchayev SV, Wexler JA, Carroll NM, Preul MC, Teytelboym OM, Sonntag VK, Van ND, Burman KD, Boyle LM. Current treatment modalities for spinal metastases secondary to thyroid carcinoma. Thyroid. 2014;24:1443–55. doi: 10.1089/thy.2013.0634. [DOI] [PubMed] [Google Scholar]
- 20.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 21.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Ca Cancer J Clin. 2015;54:8–29. [Google Scholar]
- 22.Wang F, Yu X, Shen X, Zhu G, Huang Y, Liu R, Viola D, Elisei R, Puxeddu E, Fugazzola L. The prognostic value of tumor multifocality in clinical outcomes of papillary thyroid cancer. J Clin Endocrinol Metab. 2017;102:3241–3250. doi: 10.1210/jc.2017-00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carhill AA, Litofsky DR, Ross DS, Jonklaas J, Cooper DS, Brierley JD, Ladenson PW, Ain KB, Fein HG, Haugen BR. Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987-2012. J Clin Endocrinol Metab. 2015;100:3270–3279. doi: 10.1210/JC.2015-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukushima T, Suzuki S, Mashiko M, Ohtake T, Endo Y, Takebayashi Y, Sekikawa K, Hagiwara K, Takenoshita S. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22:6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 25.Lee MY, Bo MK, Kim HS, Ji YL, Lim SH, Sun JM, Lee SH, Park K, Oh YL, Hong M. Genetic alterations and their clinical implications in high-recurrence risk papillary thyroid cancer. Cancer Res Treat. 2016;49:906–914. doi: 10.4143/crt.2016.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004;45:818–21. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 27.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 28.Poyrazoğlu Ş, Bundak R, Baş F, Yeğen G, Şanlı Y, Darendeliler F. Clinicopathological characteristics of papillary thyroid cancer in children with emphasis on pubertal status and association with brafv600e mutation. J Clin Res Pediatr Endocrinol. 2017;9:185–193. doi: 10.4274/jcrpe.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikiforova MN, Ciampi R, Salvatore G, Santoro M, Gandhi M, Knauf JA, Thomas GA, Jeremiah S, Bogdanova TI, Tronko MD. Low prevalence of BRAF mutations in radiation-induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett. 2004;209:1–6. doi: 10.1016/j.canlet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Jung CK, Im SY, Kang YJ, Lee H, Jung ES, Kang CS, Bae JS, Choi YJ. Mutational patterns and novel mutations of the BRAF gene in a large cohort of Korean patients with papillary thyroid carcinoma. Thyroid. 2012;22:791–7. doi: 10.1089/thy.2011.0123. [DOI] [PubMed] [Google Scholar]
- 31.Can N, Celik M, Sezer YA, Ozyilmaz F, Ayturk S, Tastekin E, Sut N, Gurkan H, Ustun F, Bulbul BY. Follicular morphological characteristics may be associated with invasion in follicular thyroid neoplasms with papillary-like nuclear features. Bosn J Basic Med Sci. 2017;17:211–220. doi: 10.17305/bjbms.2017.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fugazzola L, Puxeddu E, Avenia N, Romei C, Cirello V, Cavaliere A, Faviana P, Mannavola D, Moretti S, Rossi S. Correlation between BRAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13:455–464. doi: 10.1677/erc.1.01086. [DOI] [PubMed] [Google Scholar]
- 33.Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, Mazzon E. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 34.Manatakis DK, Tselenibalafouta S, Balalis D, Soulou VN, Korkolis DP, Sakorafas GH, Plataniotis G, Gontikakis E. Association of baseline neutrophil-to-lymphocyte ratio with clinicopathological characteristics of papillary thyroid carcinoma. Int J Endocrinol. 2017;2017:8471235. doi: 10.1155/2017/8471235. [DOI] [PMC free article] [PubMed] [Google Scholar]


