Abstract
Multiple myeloma (MM) is an extremely malignant plasma cell disease, which is still incurable due to its drug resistance. Lithium chloride (LiCl) functions in many pathological processes, including bipolar disorder, acute brain injuries, and chronic neurodegenerative diseases, but its antagonistic role in MM progression has not been reported thus far. In this study, we found that LiCl inhibited MM cell proliferation and induced MM cell cycle G2/M phase arrest in a dose-dependent manner. Moreover, LiCl overcomes bortezomib (BTZ)-mediated resistance in MM cells and induces apoptosis in BTZ-resistant cells. Our data preliminarily indicate that LiCl induces MM cell apoptosis via activating the Wnt/β-catenin signaling pathway. Overall, our results define LiCl as an inducer of MM cell apoptosis and unveil a crosstalk between BTZ and LiCl in facilitating cell apoptosis.
Keywords: Lithium chloride, apoptosis, multiple myeloma, β-catenin
Introduction
Multiple myeloma (MM), a malignant disease of plasma cells, is one of the most frequent types of hematological tumors [1]. Due to its high complexity and heterogeneity, MM can induce various severe complications, such as infection, renal failure, fractures, and spinal cord compression [2]. In recent years, many novel drugs, such as thalidomide, bortezomib (BTZ), lenalidomide, and elotuzumab, have been applied as treatment for MM, but the disease continues to be incurable [3]. Because patients with MM continue to experience easy relapse and drug resistance, it is urgent to develop new molecular targeted therapies that can be used to treat MM.
Numerous research studies have delved into the mechanisms of MM pathogenesis, and many pathways, including Wnt/β-catenin, NF-κB, and Janus kinase/signal transducers and activators of transcription (JAK/STAT) have been reported to be involved in MM survival [4-6]. Among them, the canonical Wnt/β-catenin signaling pathway is associated with MM progression [7]. Normally, the protein level of β-catenin in the cytoplasm is mainly regulated via phosphorylation and ubiquitin-dependent degradation. However, under certain physiological or pathological conditions, β-catenin is not phosphorylated by glycogen synthase kinase-3 (GSK-3β), which subsequently inhibits β-catenin ubiquitination and degradation. Then, the stabilized β-catenin translocates into the nucleus and regulates the expression of downstream genes along with the T-cell factor 4/lymphoid enhancer-binding factor (TCF4/LEF) family [8,9].
Lithium chloride (LiCl) plays a vital function in the treatment of bipolar disorder, acute brain injuries, and chronic neurodegenerative diseases [10-12]. Recently, LiCl has been reported to promote human acute promyelocytic leukemia cell apoptosis [13]. A major antagonistic effect of lithium is to inhibit the kinase activity of GSK-3β, which is a crucial mediator of the Wnt/β-catenin signaling pathway [14]. Nevertheless, whether and how LiCl is involved in MM progression is currently unknown.
In our present study, we found that LiCl inhibits MM cell line viability in a concentration- and time-dependent manner. We also present evidence that LiCl overcomes BTZ resistance for MM cells. Moreover, the data from western blot (WB) and luciferase reporter assay indicated that LiCl triggered cell cycle arrest and apoptosis in MM cells via the inhibition of GSK-3β and subsequent activation of the Wnt/β-catenin signaling pathway. These data preliminarily provide an insight into the mechanism of LiCl-mediated cell death in MM and also contribute to developing LiCl as a novel therapeutic intervention agent for relapsed/refractory MM.
Materials and methods
Cell culture
Human MM cell lines (RPMI-8226 and U266) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The BTZ-resistant RPMI-8226 cell line (RPMI8226/BTZ100) was kindly provided by Dr. Jacqueline Cloos (VU University Medical Center, The Netherlands) [15], and 100 nM of these cells was cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 incubator. Human embryonic kidney 293T (HEK293T) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) medium with 10% FBS.
Cell viability assay
The indicated MM cells were seeded at 1 × 104 cells per well in 96-well plates and dose-dependently treated with LiCl for 48 h or with 40 mM LiCl for different times. Then, the cell viability was detected using a Cell Counting Kit-8 (CCK-8) kit (Beyotime, China). Additionally, RPMI-8226 cells treated with 40 mM LiCl for 48 h were measured using a BrdU cell proliferation Detection Kit based on FITC-labeled flow cytometry analysis (KeyGEN, China).
Western blot
The WB was performed as previously described [16]. Antibodies were used against β-actin, H3, cyclin D1, cyclin B1, PARP1, GSK-3β, and β-catenin (Proteintech, Chicago, IL, USA), caspase-9 and caspase-3 (Cell Signaling Technology, Beverley, MA, USA), and p-GSK-3β and p-β-catenin (Affinity Biosciences, Cincinnati, OH, USA).
Cell cycle and apoptosis assay
For the cell cycle assay, the indicated cells were dispensed into a 6-well plate and treated with different concentrations of LiCl for 48 h. Then, the cells were incubated with 50 μg/ml propidium iodide (PI) (KeyGEN, China), and analysis was performed using a FACSCaliburTM (BD Biosciences). Similarly, MM cells stimulated by LiCl were treated according to the Annexin V-fluorescein isothiocyanate/propidium iodide (FITC/PI) cell apoptosis detection kit (KeyGEN, China). The cell apoptosis rate was measured by flow cytometry.
Luciferase reporter assay
The luciferase assay was performed according to a previously reported procedure [16]. TOP Flash or FOP Flash reporter gene plasmids were purchased from Upstate Biotechnology, Lake Placid, NY, USA. The luciferase activities were measured according to a standard protocol (Promega).
Statistical analysis
The data from the above experiments are presented as the mean ± SD. Student’s t-test was used to assess the difference between two groups. P < 0.05 was considered to be statistically significant. All analyses were conducted using GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA).
Results
The cytotoxic effect of LiCl on MM cell survival
To illuminate the relevance between LiCl and MM cell proliferation, we first treated RPMI-8226 cells with different doses of LiCl (0, 5 mM, 10 mM, 20 mM, and 40 mM) for 48 h. The results showed that LiCl inhibited RPMI-8226 cell viability in a dose-dependent manner (Figure 1A). Similarly, U266 cells treated with LiCl also showed decreased cell survival in a concentration-dependent manner (Figure 1B). Additionally, we observed that LiCl clearly reduced the MM cell viability in a time-dependent manner (Figure 1C, 1D). We also detected the cell proliferation rate by FITC-labeled BrdU assay, and the flow cytometry analysis demonstrated that LiCl clearly decreased RPMI-8226 cell survival (Figure 1E). Therefore, we speculated that LiCl was responsible for cell cytotoxicity and growth inhibition.
Figure 1.
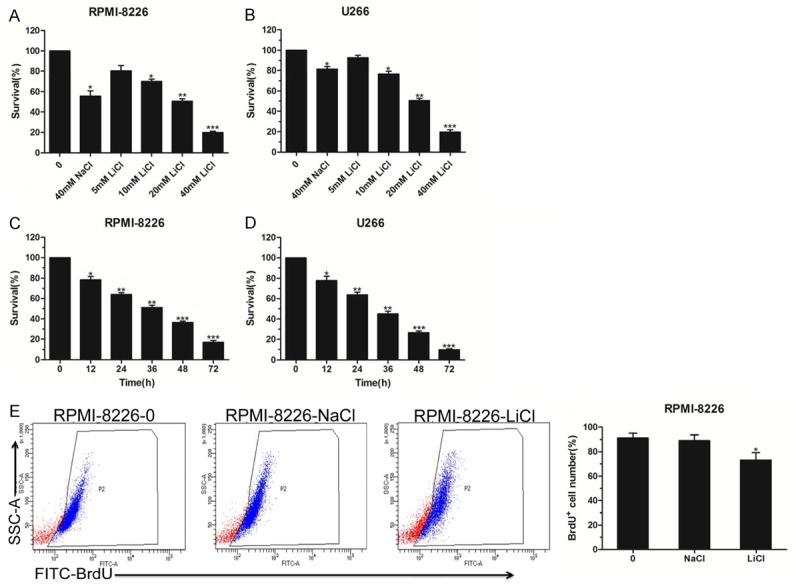
The cytotoxic effect of LiCl on MM cell survival. A, B. Indicated cells were seeded at 1 × 104 cells per well in 96-well plates and cell proliferation detection of RPMI-8226 and U266 cells treated with LiCl in different concentrations (0, 5 mM, 10 mM, 20 mM, and 40 mM) for 48 h, with 40 mM NaCl used as a negative control. C, D. Indicated cells were seeded at 1 × 104 cells per well in 96-well plates and CCK-8 analysis of RPMI-8226 and U266 cells exposed to 40 mM LiCl at different times (12, 24, 36, 48 and 72 h). E. BrdU incorporation assay, using RPMI-8226 cells that were treated with 40 mM LiCl for 48 h and labeled with FITC-BrdU; cell proliferation was examined by flow cytometry. The data represent three independent experiments. Error bars, mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
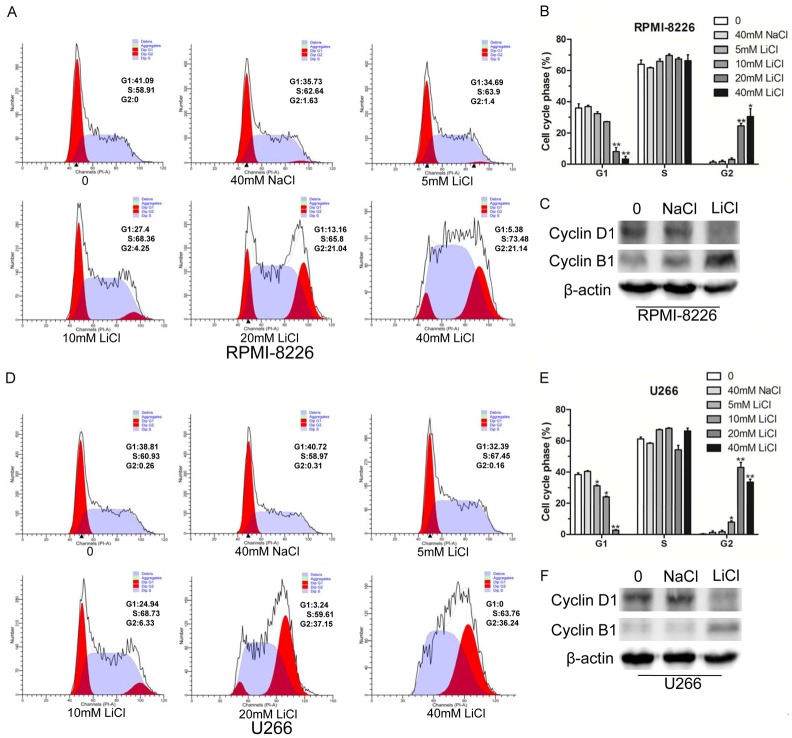
LiCl induced cell cycle arrest
To demonstrate the impact of LiCl on the cell cycle, we determined the MM cell cycle distribution by flow cytometry. As shown in Figure 2A, 2B, LiCl treatment induced a percentage of RPMI-8226 cells to enter G2 phase in a dose-dependent manner, corresponding to the decrease in G1 phase. As expected, cyclin D1, a regulator of G1-S phase transition, exhibited a lower level expression in RPMI-8226 cells treated with 40 mM LiCl. Likewise, cyclin B1, which is highly expressed in G2/M phase, exhibited a high level of expression in the treated group (Figure 2C). Subsequently, we also evaluated the effect of LiCl on the U266 cell cycle, and the results showed that LiCl treatment led to cell cycle arrest at G2/M phase (Figure 2D, 2E). The WB results indicated that the expression of cyclin D1 was decreased along with elevated expression of cyclin B1 in U266 cells treated by LiCl (Figure 2F). The above results showed that LiCl induced MM cell cycle arrest at G2/M phase.
Figure 2.
LiCl induced cell cycle G2/M phase arrest. A, B. RPMI-8226 cells were treated with different concentrations (5 mM, 10 mM, 20 mM, and 40 mM) of LiCl for 48 h, with 40 mM NaCl used as a negative control. Then the cell cycle was detected by flow cytometry. C. Western blot analyses of cell cycle-associated proteins cyclin D1 and cyclin B1 in RPMI-8226 cells treated with 40 mM LiCl, β-actin was used as internal reference. D, E. Cell cycle analysis of U266 cells treated with different doses (5 mM, 10 mM, 20 mM, and 40 mM) of LiCl for 48 h, and the distribution of the subpopulation was analyzed based on three independent experiments. F. Cell cycle-associated proteins cyclin D1 and cyclin B1 were detected by Western blot in U266 cells treated with 40 mM LiCl for 48 h. All experiments were conducted at least three times. Error bars, mean ± SD. *, P < 0.05; **, P < 0.01.
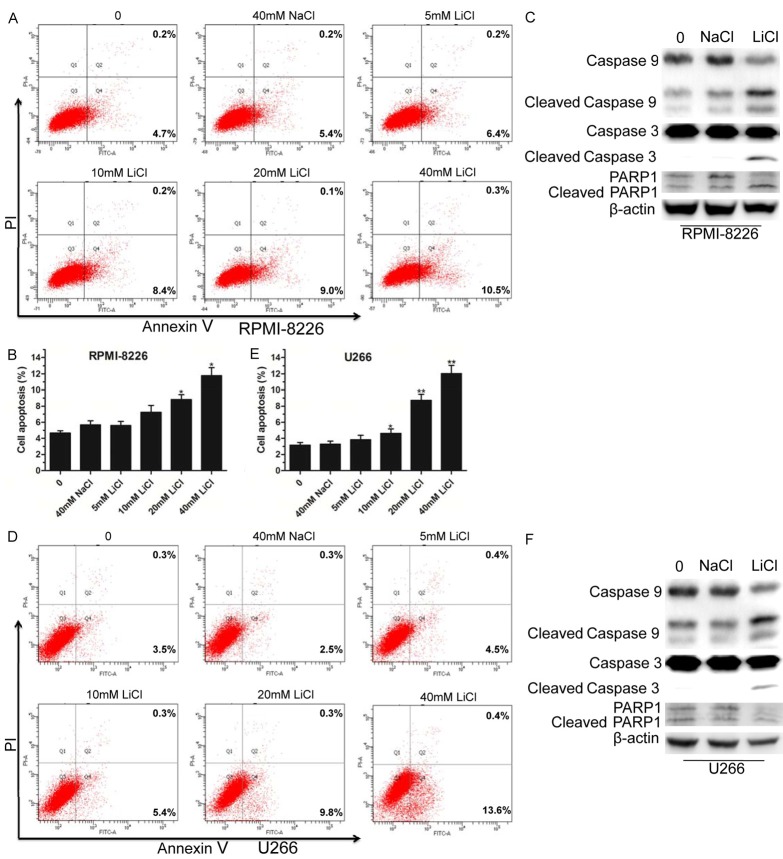
LiCl triggered MM cell apoptosis
Due to its effect on cell proliferation and the cell cycle, we explored whether LiCl promoted MM cell apoptosis. We first performed a cell apoptosis assay by flow cytometry using the RPMI-8226 cell line. The results showed that LiCl increased cell apoptosis in a concentration-dependent manner (Figure 3A, 3B). Then, we detected the expression of apoptosis-associated proteins. As shown in Figure 3C, caspase-9, caspase-3, and poly (ADP-ribose) polymerase 1 (PARP1) proteins were significantly activated when RPMI-8226 cells were treated with 40 mM LiCl compared with 40 mM NaCl. Furthermore, we also found that LiCl treatment dose-dependently increased the U266 cell apoptosis rate (Figure 3D, 3E). Surprisingly, the WB results showed elevated activated caspase-9 and caspase-3, but not PARP1 in U266 cell lines (Figure 3F). These results suggested that LiCl promoted MM cell apoptosis.
Figure 3.
LiCl induced MM cell apoptosis. A, B. After treatment with LiCl for 48 h, RPMI-8226 cells were analyzed using an Annexin V-FITC/PI double staining detection kit. C. Western blot analyses of apoptosis-related proteins caspase-9, caspase-3, PARP1, and corresponding cleaved forms, β-actin was used as internal reference. D, E. Flow cytometry analyses of U266 cell apoptosis following treatment with different concentrations (5 mM, 10 mM, 20 mM, and 40 mM) of LiCl for 48 h, with 40 mM NaCl used as a negative control. F. Assessment of caspase-9, caspase-3, PARP1, and corresponding cleaved forms in U266 cells treated by 40 mM LiCl for 48 h. All experiments were repeated three times. Error bars, mean ± SD. *, P < 0.05; **, P < 0.01.
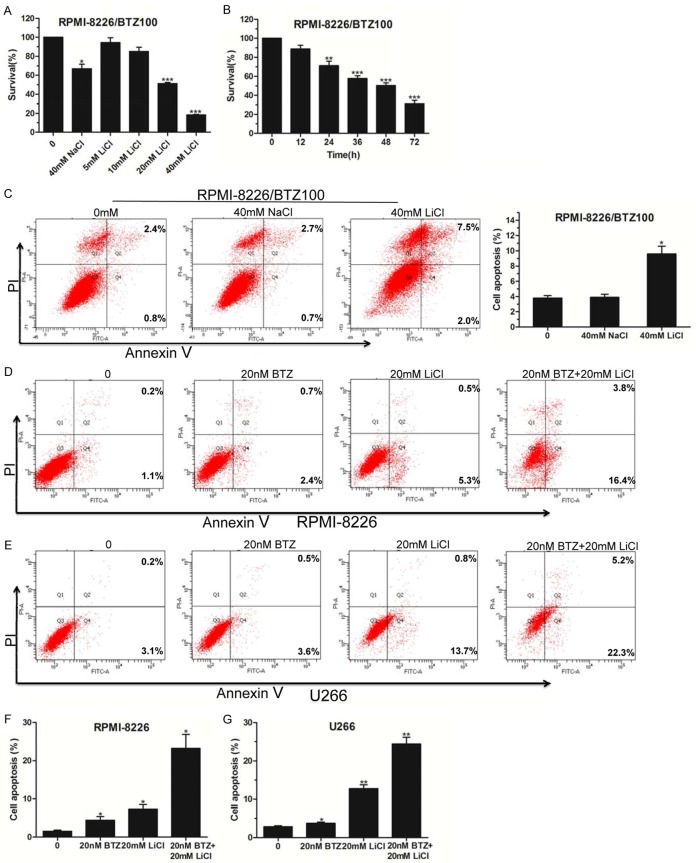
LiCl overcame BTZ-mediated resistance for MM cells
As a first-in-class proteasome inhibitor, bortezomib (BTZ) has been successfully used for MM treatment for many years. However, because of the resistance to it often encountered in relapsed/refractory MM, its efficiency has been greatly restricted [17]. Herein, we performed tests to determine whether LiCl had a cytotoxic effect on BTZ-resistant cell survival. As shown in Figure 4A, 4B, LiCl inhibited 100 nM BTZ-resistant RPMI-8226 (RPMI-8226/BTZ100) cell survival in a dose- and time-dependent manner. The proportion of apoptotic RPMI-8226/BTZ100 cells was evidently elevated by 40 mM LiCl treatment compared with the NaCl control group (Figure 4C). Subsequently, we conducted Annexin V/PI staining and flow cytometry analysis by combining LiCl with bortezomib treatment. The results showed that multiple drugs combined with BTZ and LiCl treatment further enhanced MM cell apoptosis compared with the BTZ- or LiCl-treated group (Figure 4D-G). Taken together, our data suggested that LiCl overcame the BTZ-mediated resistance of MM cells and inhibited BTZ-resistant cell apoptosis. Also, our data unveiled a crosstalk between BTZ and LiCl that facilitated MM cell apoptosis.
Figure 4.
LiCl overcame the drug resistance of bortezomib. A. Cell viabilities were detected by CCK-8 assay in RPMI-8226/BTZ100 cells treated with different doses (5 mM, 10 mM, 20 mM, and 40 mM) of LiCl for 48 h, with 40 mM NaCl used as a negative control. B. The survival rate of RPMI-8226/BTZ100 cells exposed to 40 mM LiCl for 12, 24, 36, 48 and 72 h was measured by CCK-8 assay. C. Flow cytometry analysis of apoptosis of RPMI-8226/BTZ100 cells treated with 40 mM LiCl for 48 h. D-G. The apoptosis of RPMI-8226 and U266 cells treated with 20 nM BTZ and/or 20 mM LiCl for 48 h was detected by flow cytometry. All experiments were conducted three times. Error bars, mean ± SD. *, P < 0.05; **, P < 0.01.
LiCl triggered cell apoptosis via activating the Wnt/β-catenin signaling pathway
Emerging evidence shows that LiCl inhibits the activity of glycogen synthase kinase 3 (GSK-3β) [18,19]. To explore the molecular mechanism of LiCl-induced MM cell apoptosis, we first detected the expression level of GSK-3β and p-GSK-3β in the RPMI-8226 and U266 cell lines treated by LiCl. The results showed that LiCl treatment did not change the protein level of GSK-3β, but clearly increased the level of p-GSK-3β, implying the loss of activity of GSK-3β (Figure 5A, 5B). Additionally, we extracted the nucleus proteins from RPMI-8226 and U266 cells via treatment with 40 mM LiCl. As shown in Figure 5C, 5D, LiCl treatment led to increased protein levels of β-catenin corresponding to decreased phosphorylation levels of β-catenin. Normally, once β-catenin translocates into the nucleus, it will activate the expression of downstream target genes. Therefore, we conducted the TOP/FOP Flash luciferase reporter gene assay and found that LiCl enhanced the activities of the Wnt reporter gene in the HEK293T and RPMI-8226 cell lines (Figure 5E, 5F). Collectively, our study preliminarily shows that LiCl induced apoptosis of MM cells via activation of the Wnt/β-catenin signaling pathway.
Figure 5.
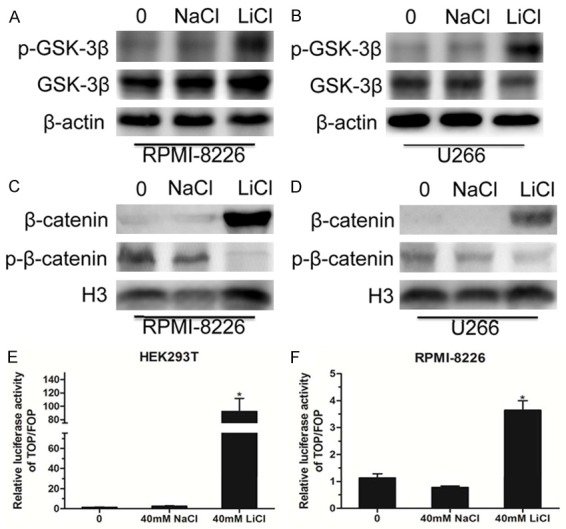
The Wnt/β-catenin signaling pathway was activated in MM cells. A, B. Western blot analyses of the GSK-3β and p-GSK-3β protein levels in RPMI-8226 and U266 cells treated with 40 mM NaCl or 40 mM LiCl for 48 h, and using β-actin as an internal reference. C, D. RPMI-8226 and U266 cells were treated with 40 mM NaCl or 40 mM LiCl, the nuclear proteins were extracted, and then Western blot analyses of β-catenin and p-β-catenin protein levels were performed, using histone H3 as an internal reference. E, F. TOP/FOP Flash luciferase reporter assay indicating the activity of the downstream transcriptional factor of the Wnt pathway in HEK293T and RPMI-8226 cells following 40 mM LiCl treatment. Error bars, mean ± SD. *, P < 0.05.
Discussion
In the current study, we carried out CCK-8 and BrdU assays to measure the proliferation of MM cells treated with different doses of LiCl. The results showed that LiCl inhibited MM cell survival in a dose-dependent manner (at concentrations of 5 mM, 10 mM, 20 mM, and 40 mM) and reached its peak at 40 mM (Figure 1). Interestingly, LiCl promoted metanephric mesenchyme cell proliferation at 40 mM and inhibited it at 50 mM [20]. Another study also indicated that LiCl increased the proliferation of hippocampal neural stem/progenitor cells at low concentration [21]. The main reason for this phenomenon is likely to be the variant background of different sources of cells. These studies also indicated that LiCl, as the main drug used for bipolar disorder, is closely associated with cell proliferation.
In addition, we found that LiCl induced MM cell cycle arrest at G2/M phase as well as cell apoptosis in a concentration-dependent manner (Figures 2 and 3). Generally, the Wnt signaling pathway plays an important role in tumor cell cycle distribution and cell apoptosis [22-25]. Nevertheless, it has been reported that LiCl inhibited GSK-3β activity [19,26]. In this study, we confirmed that LiCl treatment increased the level of p-GSK-3β and further inhibited the activity of GSK-3β (Figure 5A, 5B). Then, GSK-3β did not phosphorylate β-catenin, and the unphosphorylated form of β-catenin translocated into the nucleus, which was also confirmed by our data showing that LiCl treatment led to an increased level of β-catenin protein in the nucleus (Figure 5C, 5D). The TOP/FOP Flash luciferase reporter gene assay further indicated that LiCl activated the canonical Wnt signaling pathway to induce MM cell apoptosis (Figure 5E, 5F).
Despite having been successfully used to treat MM, bortezomib is not an optimal treatment because of the drug resistance that is often encountered with its use [27]. Here, we found that LiCl further reduced the viability of 100 nM BTZ-resistant cells in a dose- and time-dependent manner (Figure 4A, 4B). LiCl also induced apoptosis in BTZ-resistant cells (Figure 4C). Surprisingly, both RPMI-8226 and U266 cells were insensitive to low concentrations of bortezomib (5 nM and 10 nM), and IM9 cells were sensitive to 10 nM bortezomib (data not shown). Therefore, our results showed that multiple drugs combined with 20 nM BTZ and 20 mM LiCl treatment further enhanced MM cell apoptosis compared with the BTZ- or LiCl-treated groups (Figure 4D, 4E). The above results indicate that LiCl was able to overcome bortezomib-mediated resistance of MM cells.
In conclusion, our data revealed that LiCl inhibited MM cell proliferation and triggered cell cycle arrest in a dose-dependent manner. We also found that LiCl repressed the viability in BTZ-resistant cells and overcame the drug resistance to BTZ in MM cells. Finally, we preliminarily confirmed that LiCl induced cell death via transcriptional activation of the Wnt/β-catenin signaling pathway. These results contribute to a better understanding of the impact of LiCl on MM progression.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81600173); Natural Science Foundation of Jiangsu Province (BK20160230); Postdoctoral Science Foundation of China (2016M601895) and Postdoctoral Science Foundation of Jiangsu Province (1601092B); Science and Technology Special Project in Clinical Medicine of Jiangsu Province (BL2013010); Jiangsu Provincial Key Research and Development Program (BE2017638); Science and Technology Project of Xuzhou City (KC16SY149).
Disclosure of conflict of interest
None.
References
- 1.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 2.Dispenzieri A, Kyle RA. Multiple myeloma: clinical features and indications for therapy. Best Pract Res Clin Haematol. 2005;18:553–568. doi: 10.1016/j.beha.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol. 2015;43:732–741. doi: 10.1016/j.exphem.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolosenko I, Grander D, Tamm KP. IL-6 activated JAK/STAT3 pathway and sensitivity to Hsp90 inhibitors in multiple myeloma. Curr Med Chem. 2014;21:3042–3047. doi: 10.2174/0929867321666140414100831. [DOI] [PubMed] [Google Scholar]
- 7.Schmeel LC, Schmeel FC, Kim Y, Endo T, Lu D, Schmidt-Wolf IG. Targeting the Wnt/beta-catenin pathway in multiple myeloma. Anticancer Res. 2013;33:4719–4726. [PubMed] [Google Scholar]
- 8.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell. 2007;131:1204. doi: 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Ketter TA, Frye MA. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J Affect Disord. 2002;69:1–14. doi: 10.1016/s0165-0327(00)00361-x. [DOI] [PubMed] [Google Scholar]
- 11.Gurvich N, Klein PS. Lithium, valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol Ther. 2002;96:45–66. doi: 10.1016/s0163-7258(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 12.Forlenza OV, De-Paula VJ, Diniz BS. Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci. 2014;5:443–450. doi: 10.1021/cn5000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Song H, Zhong L, Yang R, Yang XQ, Jiang KL, Liu BZ. Lithium chloride promotes apoptosis in human leukemia nb4 cells by inhibiting glycogen synthase Kinase-3 beta. Int J Med Sci. 2015;12:805–810. doi: 10.7150/ijms.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Schulze TG, Nagarajan V, Akula N, Corona W, Jiang XY, Hunter N, McMahon FJ, Detera-Wadleigh SD. Interaction networks of lithium and valproate molecular targets reveal a striking enrichment of apoptosis functional clusters and neurotrophin signaling. Pharmacogenomics J. 2012;12:328–341. doi: 10.1038/tpj.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke NE, Niewerth D, Assaraf YG, van Meerloo J, Vojtekova K, van Zantwijk CH, Zweegman S, Chan ET, Kirk CJ, Geerke DP, Schimmer AD, Kaspers GJ, Jansen G, Cloos J. Impaired bortezomib binding to mutant beta5 subunit of the proteasome is the underlying basis for bortezomib resistance in leukemia cells. Leukemia. 2012;26:757–768. doi: 10.1038/leu.2011.256. [DOI] [PubMed] [Google Scholar]
- 16.Yao R, Jiang H, Ma Y, Wang L, Wang L, Du J, Hou P, Gao Y, Zhao L, Wang G, Zhang Y, Liu DX, Huang B, Lu J. PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Res. 2014;74:5656–5667. doi: 10.1158/0008-5472.CAN-14-0800. [DOI] [PubMed] [Google Scholar]
- 17.Murray MY, Auger MJ, Bowles KM. Overcoming bortezomib resistance in multiple myeloma. Biochem Soc Trans. 2014;42:804–808. doi: 10.1042/BST20140126. [DOI] [PubMed] [Google Scholar]
- 18.Galli C, Piemontese M, Lumetti S, Manfredi E, Macaluso GM, Passeri G. GSK3b-inhibitor lithium chloride enhances activation of Wnt canonical signaling and osteoblast differentiation on hydrophilic titanium surfaces. Clin Oral Implants Res. 2013;24:921–927. doi: 10.1111/j.1600-0501.2012.02488.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Ju P, Zhou Y, Zhao Y, Xie Y, Long Y, Gu Y, Ni D, Lyv Z, Mao Z, Hao J, Li Y, Wan Q, Kanyomse Q, Liu Y, Xiang Y, Wang R, Chen X, Zhang J, Liu X, Zhao H, Zhou Q, Li G. Six2 is a coordinator of LiCl-Induced cell proliferation and apoptosis. Int J Mol Sci. 2016;17:1504. doi: 10.3390/ijms17091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanni G, Di Martino E, Omelyanenko A, Andang M, Delle U, Elmroth K, Blomgren K. Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro. Oncotarget. 2015;6:37083–37097. doi: 10.18632/oncotarget.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 2010;20:453–460. doi: 10.1016/j.tcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Orte E, Saenz-Narciso B, Moreno S, Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 2013;29:545–553. doi: 10.1016/j.tig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Pecina-Slaus N. Wnt signal transduction pathway and apoptosis: a review. Cancer Cell Int. 2010;10:22. doi: 10.1186/1475-2867-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodine PV. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–253. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- 26.Zaragosi LE, Wdziekonski B, Fontaine C, Villageois P, Peraldi P, Dani C. Effects of GSK3 inhibitors on in vitro expansion and differentiation of human adipose-derived stem cells into adipocytes. BMC Cell Biol. 2008;9:11. doi: 10.1186/1471-2121-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlogie B, Shaughnessy J, Tricot G, Jacobson J, Zangari M, Anaissie E, Walker R, Crowley J. Treatment of multiple myeloma. Blood. 2004;103:20–32. doi: 10.1182/blood-2003-04-1045. [DOI] [PubMed] [Google Scholar]