Abstract
Hyperoxia induces activation of the renin-angiotensin system (RAS) in newborn rat lungs. This study investigated the therapeutic effects of human mesenchymal stem cells (MSCs) on lung development and RAS expression in neonatal rats exposed to hyperoxia. Sprague-Dawley rat pups were exposed to either room air (RA) or oxygen-enriched atmosphere (O2) treatment from postnatal days 1 to 14. Human MSCs (1 × 105 cells) in 0.03 mL of normal saline (NS) were administered intratracheally on postnatal day 5, and four study groups were obtained: RA + NS, RA + MSCs, O2 + NS, and O2 + MSCs. The lungs were excised for cytokine, expression of RAS components, and histological analyses on postnatal day 14. Body and lung weights were significantly lower in rats reared in hyperoxia than in those reared in RA. The rats reared in hyperoxia and treated with NS exhibited significantly higher tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels, mean linear intercept (MLI), and expression of angiotensin II, angiotensin II type 1 receptor, and angiotensin-converting enzyme than those reared in RA and treated with NS or MSCs did. Administering MSC to hyperoxia-exposed rats reduced TNF-α and IL-6 levels, improved MLI, and decreased expression of angiotensin II, angiotensin II type 1 receptor, and angiotensin-converting enzyme to normoxic levels. Thus, human MSCs attenuated hyperoxia-induced lung injury through inhibition of the RAS in newborn rats.
Keywords: Angiotensin, angiotensin-converting enzyme, bronchopulmonary dysplasia, cytokine, mean linear intercept
Introduction
Oxygen is often required to treat newborns with respiratory disorders. However, supplemental oxygen administered to newborn infants with respiratory disorders causes lung injury [1]. In neonatal rats, prolonged exposure to hyperoxia induces inflammation, reduces alveolar septation, and increases terminal air space size [2-4]. Therapeutic strategies for hyperoxia-induced lung injury have been a focus in neonatal medicine; however, effective therapies have not been confirmed.
The renin-angiotensin (Ang) system (RAS) is a key regulator of blood pressure and fluid home-ostasis [5]. Local RASs have been found in several tissues, including lung, kidney, adrenal gland, heart, vasculature, and nervous system, and have exhibited a variety of functions, including local cardiovascular regulation in association with or independently of the systemic RAS, as well as noncardiovascular functions [6]. Consequently, a compartmentalized RAS may work within individual organ systems with some degree of autonomy to influence regional response. High Ang II concentrations and angiotensinogen and Ang II type 1 receptors (AT1R) have been expressed in rat lung tissue [7,8]. Rat somatic Ang-converting enzyme (ACE) is first detectible at 18 days of gestation and increases afterwards until postnatal weeks 2-6 in rat lungs [9]. Ang II produced from proteolytic processing of angiotensinogen and generated locally in the lung tissue may have autocrine and paracrine actions at the cellular level [10].
Mesenchymal stem cells (MSCs) are multipotent stromal cells that self-renew and differentiate into various cell types including bone, cartilage, adipose tissue, muscle, and tendon cells [11]. MSCs have immunomodulatory, anti-inflammatory, and regenerative effects. Preclinical studies have provided evidence for the therapeutic benefits of MSCs modified by Ang-converting enzyme 2 (ACE2) in bleomycin- or lipopolysaccharide-induced lung injury [12,13]. We demonstrated activation of the lung RAS in rat pups exposed to 1 week of >95% O2 and a further 2 weeks of 60% O2; administration of AT1R antagonist attenuated lung injury in hyperoxia-exposed newborn rats [14,15]. However, the therapeutic effects of MSCs on the RAS in hyperoxia-induced lung injury are largely unknown. We hypothesized that the MSCs would inhibit RAS and reduce hyperoxia-induced lung injury. To test this hypothesis, neonatal rats were exposed to hyperoxia, and the expression of the RAS components and lung development was examined.
Materials and methods
Isolation of human MSCs
Human MSCs were isolated as described in a previous report [16]. Briefly, the stem cells were isolated from placental-derived tissues and characterized by analyzing the expression of CD markers (CD44, CD73, CD90, and CD105) and the cell surface receptor HLA-DR using flow cytometry (BD Stemflow hMSC Analysis Kit; BD Biosciences, Franklin Lakes, NJ, USA). The capability of trilineage differentiation (chondrocyte, adipocyte, and osteocyte) and the karyotyping results were examined, demonstrating positive results.
Animal model
This study was approved by the Animal Care and Use Committee at Taipei Medical University. Time-dated pregnant Sprague-Dawley rats were housed in individual cages with 12 h light-dark cycles. Laboratory food and water were available ad libitum. The rat dams were allowed to deliver vaginally at term. Within 12 h of birth, litters were pooled and randomly redistributed to the newly delivered mothers; the pups were then randomly assigned to room air (RA) or oxygen-enriched atmosphere (O2) treatment. The pups in O2 treatment subgroups were reared in an atmosphere containing 85% O2 from postnatal days 1 to 14. The pups in the RA control subgroups were reared in normal RA for 14 days. To avoid oxygen toxicity in the nursing mothers, they were rotated between the O2 treatment and RA control litters every 24 h. An oxygen-rich atmosphere was maintained in a transparent 40 × 50 × 60 cm plexiglass chamber receiving O2 continuously at 4 L/min. Oxygen levels were monitored using a ProOx P110 monitor (Bio-Spherix; Redfield, NY, USA).
Transplantation of human MSCs
Human MSCs (1 × 105 cells) in 0.03 mL of NS were administered intratracheally on postnatal day 5. The cell numbers used in this experiment were based on a previous study that investigated surfactant effects on the viability and function of human MSCs in a similar animal model [17]. For intratracheal transplantation, the rats were anesthetized with 1% isoflurane (Halocarbon Laboratories; River Edge, NJ, USA) and restrained on a board at a fixed angle. MSCs were injected into the trachea through a syringe with a 30-gauge needle. After the procedure, the animals were allowed to recover from anesthesia and were returned to their mothers. We obtained four study groups as follows: RA + NS, RA + MSC (1 × 105 cells), O2 + NS, and O2 + MSC (1 × 105 cells). Pups from each group were deeply anesthetized with an overdose of isoflurane on postnatal day 14, and body and lung weights were recorded. Immediately after death, the left lung was ligated and the right lung was fixed by tracheal instillation of 10% buffered formalin at a pressure of 25 cmH2O for 10 min.
Cytokine levels
Lung tissue was homogenized in 1 mL of ice-cold lysis buffer containing 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.01 M deoxycholic acid, and a complete protease cocktail inhibitor. Cell extracts were centrifuged and the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in supernatants were measured using an enzyme-linked immunosorbent assay kit (Cloud-Clone Corp., Houston, TX, USA).
Western blot analysis
Lung tissues (0.06 g) were homogenized in 0.6 mL of ice-cold lysis buffer containing 1% Nonidet P-40, 0.1% SDS, 0.01 M deoxycholic acid, and a complete protease cocktail inhibitor (s8830, Sigma-Aldrich, St Louis, MO, USA). The samples were then centrifuged at 13,000 rpm for 20 min at 4°C and the supernatants were aliquoted and stored at -20°C. Proteins (30 µg) were resolved in 8-10% SDS-PAGE in reduced conditions and electroblotted to an Immobilon-P polyvinylidene difluoride membrane (IPVH-00010; Millipore Corporation, Bedford, MA, USA). After they had been blocked with 5% non-fat milk, the membranes were incubated with anti-Ang II (GTX37789, GeneTex, San Antonio, Texas, USA), anti-ACE (2E2, Santa Cruz Biotechnology, Dallas, Texas, USA), and anti-ACE2 (E-11, Santa Cruz Biotechnology) at 4°C overnight. Mouse anti-β-actin mAb (C4, 1:1000; Santa Cruz Biotechnology) was used as an internal control. The densitometry unit of the protein expression in room air-exposed lungs was assigned as 1 after it had been normalized to ß-actin.
Histology and morphological analysis
The lung lobes were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The tissue was then dehydrated in alcohol, cleared in xylene, and embedded in paraffin. Five-micrometer-thick sections were cut for further processing.
After deparaffinization and rehydration, the lung sections were stained with hematoxylin and eosin for the general morphological observation and for morphometric analysis. Mean linear intercept (MLI), an indicator of mean alveolar diameter, was assessed in 10 nonoverlapping fields. In brief, 10 nonoverlapping fields of every section were randomly selected and each alveolar wall was counted as two crossings, which was counted first in the horizontal direction and then in the vertical direction by turning the ocular 90 degrees. To obtain the MLI values, an average of two numbers per field were used in the equation: Lm = (0.57/average intercepts) × 1000 (μm).
Immunohistochemistry
Immunostaining was performed on 5-µm-thick paraffin sections, followed by immunoperoxidase visualization. After endogenous peroxidase activity and nonspecific binding of antibodies had been blocked, the sections were preincubated for 1 h at 37°C in 0.1 M phosphate-buffered saline containing 10% normal goat serum and 0.3% H2O2, and then incubated for 20 h at 4°C with rabbit polyclonal anti-Ang II (1:50; GeneTax Inc., Irvine, CA, USA), anti-AT1R (N-10; 1:50; Santa Cruz Biotechnology Inc., Dallas, TX, USA), and mouse monoclonal anti-ACE and anti-ACE2 (1:50; Santa Cruz Biotechnology Inc.) primary antibodies. Subsequently, the sections were treated for 1 h at 37°C with biotinylated goat anti-rabbit IgG (1:200; Jackson ImmunoResearch Laboratories Inc., PA, USA) or rabbit anti-mouse IgG (1:200; Sigma-Aldrich Inc., St. Louis, MO, USA), followed by a reaction with the reagents from an avidin-biotin complex kit (Vector, CA, USA); the brown reaction products were visualized using a diaminobenzidine substrate kit (Vector) as per the manufacturer’s recommendations.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analyses were performed using one-way analysis of variance with a Bonferroni post hoc test for multiple group comparisons. The survival rate was evaluated using the Kaplan-Meier method, and the log-rank test was used for intergroup comparisons. Differences were considered statistically significant when P < 0.05.
Results
Six dams gave birth to a total of 68 pups; 34 pups each were randomly distributed to RA and hyperoxia groups. Next, 17 and 17 pups and 15 and 19 pups were treated with NS and human MSCs (1 × 105 cells) in the RA and hyperoxia groups, respectively.
Survival
The rats reared in RA and treated with NS or MSCs all survived (Figure 1). The rats reared in hyperoxia and receiving NS or MSCs exhibited a lower survival rate after postnatal day 7. On postnatal day 14, the survival rates between rats treated with NS or MSCs were comparable.
Figure 1.
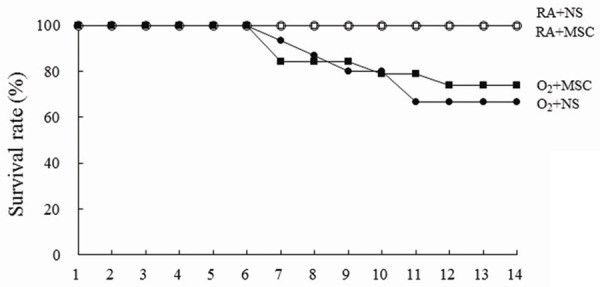
Effects of human MSCs on the survival rate on postnatal day 14. The rats reared in RA and treated with NS or MSCs all survived. The rats reared in hyperoxia and receiving NS or MSCs exhibited a lower survival rate after postnatal day 7. RA = room air; NS = normal saline; MSC = mesenchymal stem cell.
Body weight, lung weight, and lung to body weight ratios
The rats reared in hyperoxia and treated with NS or MSCs exhibited significantly lower body and lung weights on postnatal day 14 than those reared in RA and treated with NS or MSCs (Table 1). Treatment with MSCs did not significantly influence body or lung weights. Lung to body weight ratios were comparable among rats treated with NS or MSCs.
Table 1.
Body weights, lung weights, and lung to body weight ratios in rat pups on postnatal day 14
| Treatment | n | Body weight (g) | Lung weight (g) | Lung to body weight (%) |
|---|---|---|---|---|
| RA + NS | 17 | 23.37 ± 2.15 | 0.34 ± 0.03 | 1.47 ± 0.15 |
| RA + MSCs | 17 | 24.28 ± 2.18 | 0.36 ± 0.03 | 1.47 ± 0.11 |
| O2 + NS | 10 | 18.85 ± 3.64*** | 0.30 ± 0.06* | 1.61 ± 0.22 |
| O2 + MSCs | 14 | 18.03 ± 2.89*** | 0.27 ± 0.06*** | 1.47 ± 0.15 |
Values are mean ± SD. RA = room air; NS = normal saline; MSCs = mesenchymal stem cells.
P < 0.05 vs. RA + MSCs.
P < 0.001 vs. RA + NS and RA + MSCs.
Cytokine levels
The rats reared in hyperoxia and treated with NS exhibited significantly higher TNF-α and IL-6 levels than those reared in RA and treated with NS or MSCs (Figure 2A, 2B). Furthermore, the rats reared in hyperoxia and treated with MSCs exhibited a significantly lower TNF-α and IL-6 levels than those treated with NS did.
Figure 2.
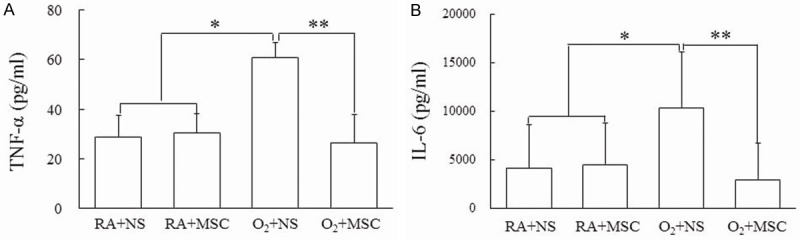
(A) Tumor necrosis factor (TNF)-α and (B) interleukin (IL)-6 levels in lung tissues of 14-day-old rats exposed to postnatal RA or hyperoxia and treated with NS or MSCs on postnatal day 5. Cytokine analysis for TNF-α and IL-6 were performed using ELISA. The rats reared in hyperoxia and treated with NS exhibited significantly higher TNF-α and IL-6 levels than those reared in RA and treated with NS or MSCs. Treatment with MSCs significantly decreased the hyperoxia-induced increase in cytokines. Data represents mean ± SD. *P < 0.05, **P < 0.01. RA = room air; NS = normal saline; MSC = mesenchymal stem cell.
Histology results
Representative lung sections stained with hematoxylin and eosin on postnatal day 14 are shown in Figure 3A. The lungs of rats reared in hyperoxia and treated with NS contained large thin-walled air spaces and exhibited a significantly higher MLI than those reared in RA and treated with NS or MSCs did (Figure 3B). Alveolar macrophages were detected in the hyperoxia groups. Treatment with MSCs significantly decreased the hyperoxia-induced increase in the MLI and restored the thickness of alveolar wall.
Figure 3.
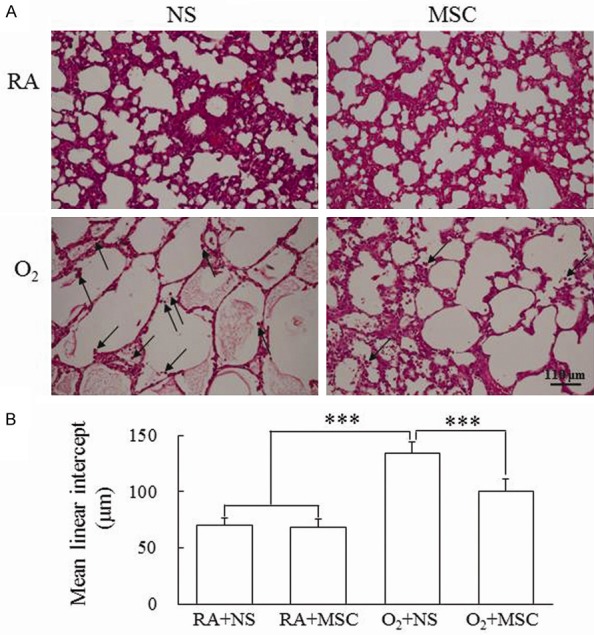
(A) Representative histology and (B) mean linear intercept in 14-day-old rats exposed to postnatal RA or hyperoxia and treated with NS or MSCs on postnatal day 5. Mean linear intercept, an indicator of mean alveolar diameter, was assessed in 10 nonoverlapping fields. The alveolar macrophages (arrow) were detected in the hyperoxia groups. The rats reared in hyperoxia and treated with NS exhibited a significantly higher MLI than those reared in RA and treated with NS or MSCs did. Treatment with MSCs significantly decreased the hyperoxia-induced increase in the MLI. Data represents mean ± SD. ***P < 0.001. RA = room air; NS = normal saline; MSC = mesenchymal stem cell.
Protein expression of RAS components
The rats reared in hyperoxia and treated with NS exhibited significantly higher Ang II and ACE expression than those reared in RA and treated with NS or MSCs did (Figure 4A, 4B). Treatment with MSCs significantly decreased the hyperoxia-induced increase in the Ang II and ACE expression. The rats reared in hyperoxia and treated with NS exhibited lower ACE2 expression than those reared in RA and treated with NS or MSCs did. Treatment with MSCs intensified the hyperoxia-induced reduction in ACE2 expression. The differences were not statistically significant.
Figure 4.
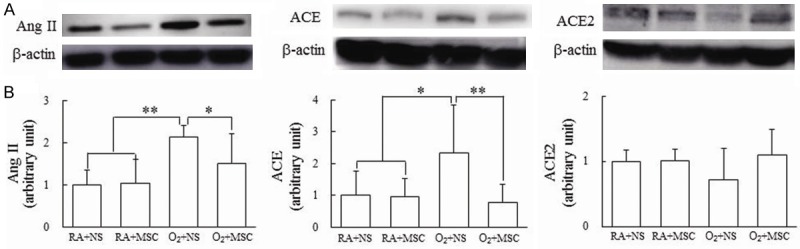
(A) Representative Western blots and (B) protein expression of the renin-angiotensin system components in lung tissues on postnatal day 14. The rats reared in hyperoxia and treated with NS exhibited significantly higher angiotensin (Ang) II and angiotensin-converting enzyme (ACE) expression than those reared in RA and treated with NS or MSCs did. Treatment with MSCs significantly decreased the hyperoxia-induced increase in Ang II and ACE expression and increased the hyperoxia-induced decrease in the ACE2 expression. Data represents mean ± SD. *P < 0.05, **P < 0.01. RA = room air; NS = normal saline; MSC = mesenchymal stem cell.
Immunohistochemistry of RAS components
The immunoreactivity levels of Ang II, AT1R, ACE, and ACE2 were mainly detected in the endothelium of small-to-medium pulmonary vessels and type I and type II alveolar cells (Figure 5A-D). Ang II immunoreactivity was also demonstrated in the epithelium of bronchus. The rats reared in hyperoxia and treated with NS displayed relatively prominent immunoreactivity of Ang II, AT1R, and ACE, whereas the rats reared in RA displayed relatively high ACE2 immunoreactivity. Treatment with MSCs weakened the hyperoxia-induced increase in immunoreactivity of Ang II, AT1R, and ACE, and slightly increased the immunoreactivity of ACE2.
Figure 5.
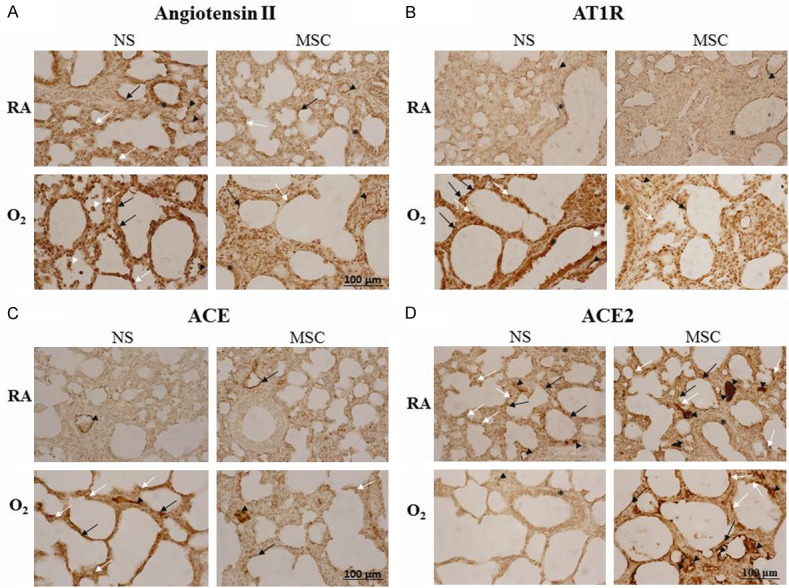
Representative immunohistochemical staining for (A) angiotensin (Ang) II, (B) Ang II type-1 receptor (AT1R), (C) angiotensin-converting enzyme (ACE), and (D) angiotensin-converting enzyme 2 (ACE2) expression in 14-day-old rats exposed to postnatal RA or hyperoxia and treated with NS or MSCs on postnatal day 5. The immunoreactivity of the renin-angiotensin system components was mainly detected in the endothelium of small-to-medium pulmonary vessels (black arrowhead), type I (black arrow) and type II (white arrow) alveolar cells, and epithelium of bronchus (asterisk). Ang II and AT1R were also demonstrated in alveolar macrophages (white arrowhead). The rats reared in hyperoxia and treated with NS displayed prominent immunoreactivity of Ang II, AT1R, and ACE, whereas the rats reared in RA and treated with NS SCs displayed higher ACE2 immunoreactivity. Treatment with MSCs decreased the hyperoxia-induced increase in immunoreactivity of Ang II, AT1R, and ACE and slightly increased immunoreactivity of ACE2. RA = room air; NS = normal saline; MSC = mesenchymal stem cell.
Discussion
Our in vivo model revealed that neonatal hyperoxia exposure increased cytokine levels and arrested alveolarization in the lungs of newborn rats on postnatal day 14. Hyperoxia-induced lung injury was associated with increased Ang II, AT1R, and ACE expression. Intratracheal administration of MSCs on postnatal day 5 decreased cytokine levels, improved alveolarization, and decreased lung Ang II, AT1R, and ACE protein expression. The major finding is that the intratracheal administration of human MSCs attenuated hyperoxia-induced lung injury by inhibiting RAS in an animal model of bronchopulmonary dysplasia (BPD). These results were compatible with previous findings in that AT1R antagonist attenuated hyperoxia-induced lung injury in newborn rats, suggesting that human MSCs attenuated hyperoxia-induced lung injury, probably by inhibiting the RAS.
Our study demonstrated that rats reared in hyperoxia and treated with NS or MSCs exhibited significantly lower body and lung weights on postnatal day 14 than those reared in RA and treated with NS or MSCs did. Lung to body weight ratios were comparable among the rats reared in RA or hyperoxia and treated with NS or MSCs. These results suggested that body and lung weights were mainly influenced by hyperoxia; MSC treatment did not increase body weight and lung growth in hyperoxia-exposed newborn rats.
Supplemental oxygen administered to newborn infants with respiratory distress increases oxidative stress and causes cytokine production. Human and animal studies have shown that increased cytokine levels and inflammatory cells are associated with the development of BPD [18,19]. In this study, we found increased cytokine levels in the rats reared in hyperoxia. The administration of MSCs reduced the postnatal hyperoxia-induced increase in TNF-α and IL-6 levels. These reduction effects of human MSCs on cytokines are consistent with relevant studies [20-22]. These results suggested that the therapeutic effects of MSCs on developing lungs are partially mediated through the inhibition of proinflammatory cytokine production.
In this study, although the survival rate was not significantly improved, administering human MSCs to rats exposed to postnatal hyperoxia significantly improved lung development in the surviving animals. The rats reared in hyperoxia exhibited a lower survival rate after postnatal day 6. Treatment with MSCs improved the survival rate from postnatal days 11 to 14. The differences in the survival rates between hyperoxia-exposed rats treated with NS and MSCs were not significant on postnatal day 14. These results suggested that a higher dose or an additional dose of MSCs is required to maintain the survival rate.
The circulating RAS has played a well-described role in cardiovascular homeostasis. Local tissue-based RASs have also been described to play key roles in hyperoxia-induced lung injury [14,15,23,24]. The expression of RAS components and elevation of ACE suggest the existence of a pulmonary RAS as well as that Ang II could mediate the response to hyperoxia-induced lung injury. The main function of ACE2 is to convert the octapeptide Ang II to a heptapeptide Ang-(1-7), thereby decreasing Ang II accumulation. Hyperoxia was reported to downregulate ACE2 expression in human fetal lung fibroblasts [25]. Administration of purified recombinant human ACE2 attenuated bleomycin-induced lung collagen accumulation in mice [26]. MSCs carrying ACE2 were more effective than MSCs alone in alleviating lipopolysaccharide-induced acute lung injury in mice [12]. The effects of MSCs on RAS in hyperoxia-induced lung injury are unknown. In this study, we found that intratracheal administration of MSCs improved alveolarization and decreased lung Ang II, AT1R, and ACE protein expression. Treatment of MSCs did not significantly increase ACE2 protein expression. These results support that MSCs must carry ACE2 to exert their beneficial effects and suggest that human MSCs attenuated hyperoxia-induced lung injury, probably through the inhibition of the RAS.
Conclusion
We have demonstrated that intratracheal administration of human MSCs decreased cytokine levels and improved alveolarization in hyperoxia-exposed newborn rat lungs. These effects are associated with the inhibition of the RAS. These results can be used to construct a rational procedure that may characterize an RAS inhibitory pathway motivated by MSCs.
Disclosure of conflict of interest
None.
References
- 1.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manji JS, O’Kelly CJ, Leung WI, Olson DM. Timing of hyperoxic exposure during alveolarization influences damage mediated by leukotrienes. Am J Physiol Lung Cell Mol Physiol. 2001;281:L799–L806. doi: 10.1152/ajplung.2001.281.4.L799. [DOI] [PubMed] [Google Scholar]
- 3.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen CM, Wang LF, Chou HC, Lan YD, Yi-Ping Lai. Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res. 2007;62:128–133. doi: 10.1203/PDR.0b013e3180987202. [DOI] [PubMed] [Google Scholar]
- 5.Coble JP, Grobe JL, Johnson AK, Sigmund CD. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: importance of the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2015;308:R238–R249. doi: 10.1152/ajpregu.00486.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 7.Tryka AF, Witschi H, Gosslee DG, McArthur AH, Clapp NK. Patterns of cell proliferation during recovery from oxygen injury. Species differences. Am Rev Respir Dis. 1986;133:1055–1059. doi: 10.1164/arrd.1986.133.6.1055. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Kladis A, Valentijn AJ. Effects of losartan on angiotensin and bradykinin peptides and angiotensin converting enzyme. J Cardiovasc Pharmacol. 1995;26:233–240. doi: 10.1097/00005344-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Sim MK, Seng KM. Development of angiotensin converting enzyme in fetal lung and placenta of the rat and human. Clin Exp Pharmacol Physiol. 1984;11:497–501. doi: 10.1111/j.1440-1681.1984.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 10.Filippatos G, Tilak M, Pinillos H, Uhal BD. Regulation of apoptosis by angiotensin II in the heart and lungs. Int J Mol Med. 2001;7:273–280. doi: 10.3892/ijmm.7.3.273. [DOI] [PubMed] [Google Scholar]
- 11.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 12.He H, Liu L, Chen Q, Liu A, Cai S, Yang Y, Lu X, Qiu H. Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant. 2015;24:1699–1715. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 13.Min F, Gao F, Li Q, Liu Z. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep. 2015;11:2387–2396. doi: 10.3892/mmr.2014.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou HC, Lang YD, Wang LF, Wu TY, Hsieh YF, Chen CM. Angiotensin II type 1 receptor antagonist attenuates lung fibrosis in hyperoxia-exposed newborn rats. J Pharmacol Exp Ther. 2012;340:169–175. doi: 10.1124/jpet.111.186288. [DOI] [PubMed] [Google Scholar]
- 15.Jiang JS, Lang YD, Chou HC, Shih CM, Wu MY, Chen CM, Wang LF. Activation of the renin-angiotensin system in hyperoxia-induced lung fibrosis in neonatal rats. Neonatology. 2012;101:47–54. doi: 10.1159/000329451. [DOI] [PubMed] [Google Scholar]
- 16.Chou HC, Li YT, Chen CM. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am J Transl Res. 2016;8:342–353. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CM, Chou HC, Lin W, Tseng C. Surfactant effects on the viability and function of human mesenchymal stem cells: in vitro and in vivo assessment. Stem Cell Res Ther. 2017;8:180. doi: 10.1186/s13287-017-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res. 2014;76:294–301. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Fang J, Su H, Yang M, Lai W, Mai Y, Wu Y. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 2012;16:589–598. doi: 10.1111/j.1399-3046.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36:589–594. doi: 10.1042/CBI20110447. [DOI] [PubMed] [Google Scholar]
- 22.Chang YS, Ahn SY, Jeon HB, Sung DK, Kim ES, Sung SI, Yoo HS, Choi SJ, Oh WI, Park WS. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;51:391–399. doi: 10.1165/rcmb.2013-0385OC. [DOI] [PubMed] [Google Scholar]
- 23.Wagenaar GT, Laghmani el H, Fidder M, Sengers RM, de Visser YP, de Vries L, Rink R, Roks AJ, Folkerts G, Walther FJ. Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L341–L351. doi: 10.1152/ajplung.00360.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagenaar GT, Sengers RM, Laghmani el H, Chen X, Lindeboom MP, Roks AJ, Folkerts G, Walther FJ. Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. Am J Physiol Lung Cell Mol Physiol. 2014;307:L261–L272. doi: 10.1152/ajplung.00345.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oarhe CI, Dang V, Dang M, Nguyen H, Gopallawa I, Gewolb IH, Uhal BD. Hyperoxia downregulates angiotensin-converting enzyme-2 in human fetal lung fibroblasts. Pediatr Res. 2015;77:656–662. doi: 10.1038/pr.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


