Abstract
Objective: To investigate the feasibility and efficacy of sural nerve grafts for repairing sciatic nerve defects using a chitin biological absorbable tube bridge. Methods: A sciatic nerve defect model in the rat was produced, and the sural nerve, cut from the ipsilateral leg, was used as a graft to repair the defect using chitin biological absorbable tube bridges. Then the number and morphology of regenerating myelinated fibers, nerve function index, nerve conduction velocity, and motor end plate and triceps surae muscle morphology were evaluated. Results: The number and morphology of regenerated fibers, nerve function index, nerve conduction velocity, and motor end plate and triceps surae muscle morphology were improved in rats in which the nerve defect was bridged with chitin biological absorbable tubes compared with rats in which the defect was repaired without tubes. Conclusions: The findings suggest that using a sural nerve graft to repair sciatic nerve defects with chitin biological absorbable tubes bridge promotes structural and functional recovery, and improves end plate and muscle fiber morphology as well.
Keywords: Sural nerve, sciatic nerve defect, nerve regeneration, graft, chitin biological absorbable tube
Introduction
Large-segment defects caused by peripheral nerve injury are common in clinical practice. Autologous nerve transplantation is the most common method for repairing nerve defects [1]. Smaller cutaneous nerves or other less important nerves are usually selected as donor nerves. Our previous study demonstrated the feasibility of using small donor nerves to repair large-nerve defects [2]. The donor nerve used in that study was adjacent to the damaged nerve. Therefore, it did not need to be completely cut out; it was sufficient to reposition the donor and suture the proximal end to the damaged nerve. However, when no small donor nerve is available nearby, a nerve segment from more distal donor nerves must be completely excised for transplantation onto the damaged nerve. In these cases, two anastomoses are required. In the proximal anastomosis, the thick damaged nerve is connected to the smaller donor nerve. Consequently, neurites attempting to regenerate from the proximal nerve segment have difficulty squeezing into the smaller donor nerve, thereby reducing the efficiency of peripheral nerve regeneration. Our previous study showed that the use of conduits in nerve transplantation prevents proximal regenerating fibers from escaping to the periphery, and helps them to regrow towards the same type of distal fiber [3]. In the present study, we evaluated the efficacy of sural nerve grafts for repairing sciatic nerve defects using chitin biological absorbable tube bridges by morphological and functional assessment.
Materials and methods
Animals and grouping
Adult male Sprague Dawley rats weighing 200-220 g were obtained from Peking University and were maintained under specific-pathogen-free conditions and a 12 h light/dark cycle, with free access to pellet food and water. The rats were separated into four groups at random (Group A: normal group; Group B: sciatic nerve graft orthotopic transplantation by adventitial suture; Group C: sural nerve graft transplantation by adventitial suture; Group D: sural nerve graft transplantation by bridging with conduit). Efforts were made to minimize possible unnecessary animal sufferings, experimental procedures on animals were performed according to the Guidelines for the Care and Use of Laboratory Animals of Peking University. The study protocol was approved by the Medical Ethics Review Committee of Peking University (2013, Beijing, China).
Materials
The materials used were: chitin biological absorbable tubes (ether-free chitin biological tubes; length, 6 mm; wall thickness, 0.2 mm; inner diameter, 2 mm); a Synergy electrophysiological instrument; A Leica pathological tissue slicer (RM Z135, Leica); a Leica dissecting microscope (MZ75, Leica); a Leica tissue embedding machine (EG 1150H, Leica); and a Leica image collection and analysis system; a footprint measurement box (self-made) and several white papers; Chitin biological absorbable catheters (etherfree chitin biological catheters) were obtained from Peking University People’s Hospital (Patent number: 01136314.2).
Surgical procedures
Preparation of the sural nerve
Surgical procedures were performed in a specific-pathogen-free animal laboratory. Rats in groups C and D were anesthetized with sodium pentobarbital (30 mg/kg, i.p.). Following anesthesia and skin preparation, the skin of the right limb was sterilized, and a longitudinal incision was made along the right tibia, revealing the sural nerve below the shallow muscles. The shallow muscles were cut along the sural nerve, and the sural nerve was carefully separated, avoiding damage to its associated blood vessel. The sural nerve was cut out and then placed in saline for use. The proximal and distal ends were distinguished so that the nerve could be transplanted antegradely. Finally, the muscle and skin were sutured with 4-0 suture. Every effort was made to minimize the incision and reduce damage to animals.
Preparation of the neural transplantation model
In groups B, C and D, the right sciatic nerve was exposed and cut 2 mm and 12 mm above the bifurcation, resulting in a 10 mm defect. In group A, the right sciatic and sural nerves were exposed as described above, and the wound was closed without any operation. In group B, the right sural nerve was exposed, without any operation, and then the wound was closed. A 10 mm sciatic nerve segment was transplanted onto the defect in an antegrade orientation by adventitial suture (Figure 1A). In group C, a 10 mm segment of the sural nerve was cut and transplanted onto the sciatic nerve defect in an antegrade orientation by adventitial suture (Figure 1B). In group D, a 10 mm sural nerve segment was transplanted onto the sciatic nerve defect in an antegrade orientation and bridged with chitin biological absorbable tubes; the gap between the nerve ends was kept at 2 mm (Figure 1C). Every effort was made to minimize the incision and reduce damage to animals. Then, 3 and 6 months after surgery, the animals were used for morphological and functional analyses.
Figure 1.
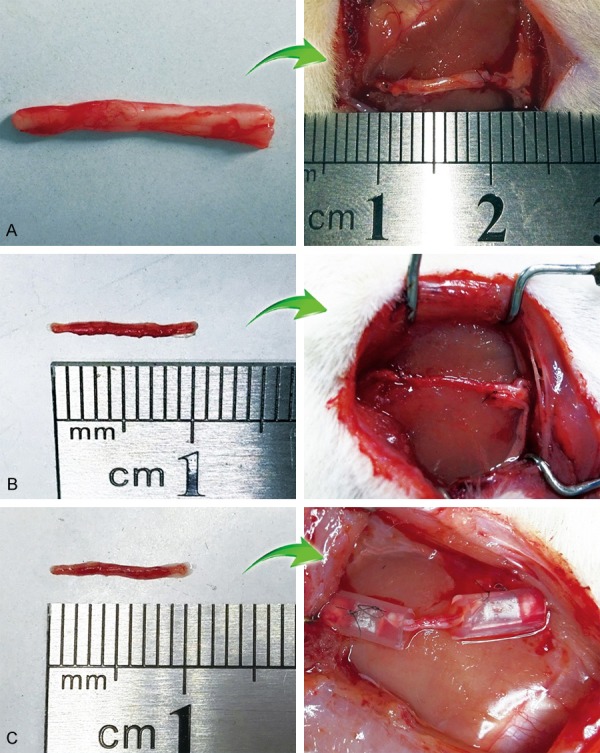
Photographs showing different nerve transplantation models. A: Sciatic nerve graft orthotopic transplantation by adventitial suture, group B. (Left: 10 mm sciatic nerve graft; Right: Sciatic nerve graft was transplanted to the defect by adventitial suture). B: Sural nerve graft transplantation by adventitial suture, group C. (Left: 10 mm sural nerve graft; Right: Sural nerve graft was transplanted to the defect by adventitial suture). C: Sural nerve graft transplantation by bridging with chitin tube, group D. (Left: 10 mm sural nerve graft; Right: Sural nerve graft was transplanted to the defect by bridging with conduit).
Walking track analysis
At 3 and 6 months after the operation, walking track analysis was performed on the animals in each group, as described previously [4]. A confined walking track (10 × 60 cm), darkened at one end, was used. White paper (10 × 60 cm) was placed on the bottom of the track. The hind limbs were dipped into black ink before the animal was placed at the entrance of the walking track. Then, each rat was permitted to walk down the track four or five times, and the bilateral footprints were recorded. Clear and complete prints were chosen for measurement.
Paired footprint parameters for print length (PL; distance from heel to toe), toe spread (TS; distance from first to fifth toe) and intermediary toe spread (IT; distance from second to fourth toe) were recorded for the left normal control foot (NPL, NTS, NIT) and the corresponding right experimental foot (EPL, ETS, EIT) for each rat. Sciatic function index (SFI) and tibial function index (TFI) were calculated according to the Bain-Mackinnon-Hunter formula: SFI = -38.3 ([EPL-NPL]/NPL) + 109.5 ([ETS-NTS]/NTS) + 13.3 ([EIT-NIT]/NIT) - 8.8; TFI = -37.2 ([EPL-NPL]/NPL) + 104.4 ([ETS-NTS]/NTS) + 45.6 ([EIT-NIT]/NIT)-8.8.
Electrophysiological examination
Electrophysiological examination of the sciatic nerve was conducted 3 and 6 months post-surgery, as described previously [2]. The right sciatic nerve was exposed, and stimulating bipolar electrodes were placed proximal and distal to the repaired site. The recording electrode was placed in the triceps surae muscle, while the ground electrode was placed in the subcutaneous tissue between the stimulating and recording electrodes. Rectangular pulses (0.1 ms, 0.9 mA, 10 Hz, six continuous stimuli) were used. The nerve conduction velocity (NCV; m/s) was obtained semi-automatically by dividing the distance between the two stimulating sites by the difference in the conduction time.
Histological analysis of myelinated fibers
The right sciatic nerve was removed from each rat and divided into two parts. One nerve segment was used for optical microscopy. It was fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 h at 4°C. The nerves were then rinsed twice in water for 12 h. A nerve segment 5 mm distal to the distal tube was cut out and stained in 1% osmium tetroxide for 12 h, dehydrated through a graded series of ethanols, immersed in xylene, embedded in paraffin, and sliced into 5-μm cross-sections. Images were acquired under a microscope, from which the number of myelinated axons was evaluated.
The other nerve segment was used for transmission electron microscope (TEM) specimen. It was fixed in 3% glutaraldehyde for 2 h, then post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 2 h, dehydrated in ethanol, immersed in a mixture of epoxy resin and acetone (1:1) for 2.5 h, and embedded in epoxy resin. Transverse ultrathin (thickness: 50.0 nm) sections were prepared. The ultra-thin sections were stained with uranyl acetate and lead citrate and were finally examined under a transmission electron microscope (JEM-100, Tokyo, Japan), from which the morphometrics of the myelinated axons were evaluated.
Histological analysis of neuromuscular junctions
Tissue from the neuromuscular junction of the triceps muscle was removed and stained with gold chloride [5]. The tissue was placed in 15% citric acid aqueous solution until transparent, immersed in 1% aqueous gold chloride solution until golden yellow, immersed in 20% formic acid solution for reduction, and then immersed in glycerol to soften. The tissue was then cut into approximately 8 mm3 pieces, placed on a glass slide with a drop of glycerin gelatin, cover-slipped, and then examined under the light microscope.
Weight and histological analysis of the triceps surae muscle
Triceps surae muscles were freed and cut from the operated and normal limbs of the rats. Nerves, blood vessels and the deep fascia covering the surface of the muscle were stripped off and discarded. To reduce statistical error and ensure objectivity, the triceps surae muscles used for analysis included the tendons. Muscles were then immediately weighed with a microbalance. The recovery rate of muscle wet weight was obtained by dividing the wet weight of the operated muscle by that of the unoperated muscle.
After weighing, the triceps surae muscles were immediately fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 7 days, rinsed in water, dehydrated through a graded series of ethanols, and immersed in xylene and embedded in paraffin. Each muscle segment was sliced into 5-μm-thick cross-sections and then stained in hematoxylin-eosin solution. Images were acquired under a Leica dissecting microscope, from which the cross-sectional area of muscle cells was measured using Image-Proplus software (Media Cybernetics, Inc. Shanghai, China).
Statistical analysis
Statistical analyses were performed with SPSS 19.0 software (IBM, Armonk, NY, USA). One-way analysis of variance (ANOVA) and independent sample t-test were used for comparisons. A value of P less than 0.05 was considered to be statistically significant. All values are presented as the mean ± SD.
Results
Walking track analysis
SFI
As shown in the left part of Figures 2 and 3 months after surgery, the SFIs were significantly lower in groups B, C and D than in group A (P < 0.05), and were significantly lower in groups C and D than in group B (P < 0.05). The SFIs in groups C and D did not statistically differ (P > 0.05). Six months after surgery, the SFIs were significantly lower in groups B, C and D than in group A (P < 0.05), and were significantly lower in groups C and D than in group B (P < 0.05). The SFI was significantly higher in group D than in group C (P < 0.05).
Figure 2.
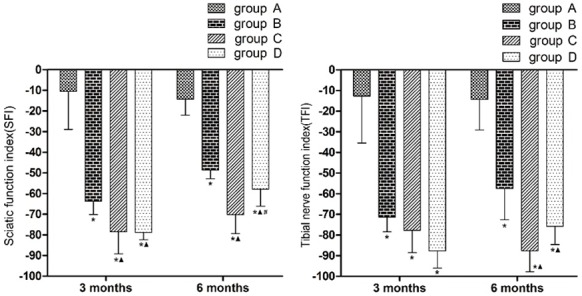
Bar graphs showing motor function in different periods. (Left: SFI in different periods; Right: TFI in different periods; *P < 0.05 versus group A; ▲P < 0.05 versus group B; #P < 0.05 versus group C).
Figure 3.
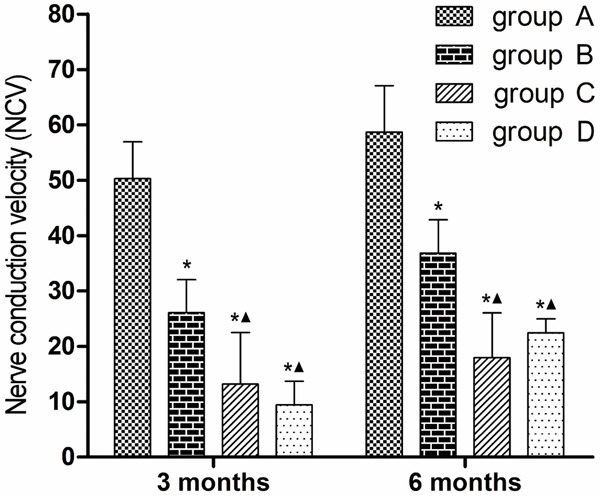
Bar graphs showing electrophysiology of sciatic nerve. (*P < 0.05 versus group A; ▲P < 0.05 versus group B).
TFI
As shown in the right part of Figures 2 and 3 months after surgery, the TFIs were significantly lower in groups B, C and D than in group A (P < 0.05). The TFIs did not differ statistically between any pairwise comparison among groups B, C and D. Six months after surgery, the TFIs were significantly lower in groups B, C and D than in group A (P < 0.05), and were significantly lower in groups C and D than in group B (P < 0.05). The TFIs in groups C and D did not statistically differ.
Electrophysiology of the sciatic nerve
As shown in Figure 3, 3 and 6 months after surgery, the NCVs were significantly lower in groups B, C and D than in group A (P < 0.05), and were significantly lower in groups C and D than in group B (P < 0.05). The NCVs in groups C and D did not statistically differ.
Morphology and morphometrics of regenerated myelin sheaths
Morphology of myelin sheaths
As shown in Figure 4A, 4B, 3 months after surgery, the myelin sheaths in group A were evenly distributed, and appeared uniform and normal. In groups B, C and D, myelin regeneration looked poor, myelin distribution looked uneven, and the myelin sheaths appeared to have a relatively small thickness. The diameter of the myelin sheaths appeared larger in groups B and D than in group C. Six months after surgery, the myelin sheaths in group A looked as good as at 3 months after surgery, while myelin regeneration appeared poorer in groups B and D than in group A, but better than in group C.
Figure 4.
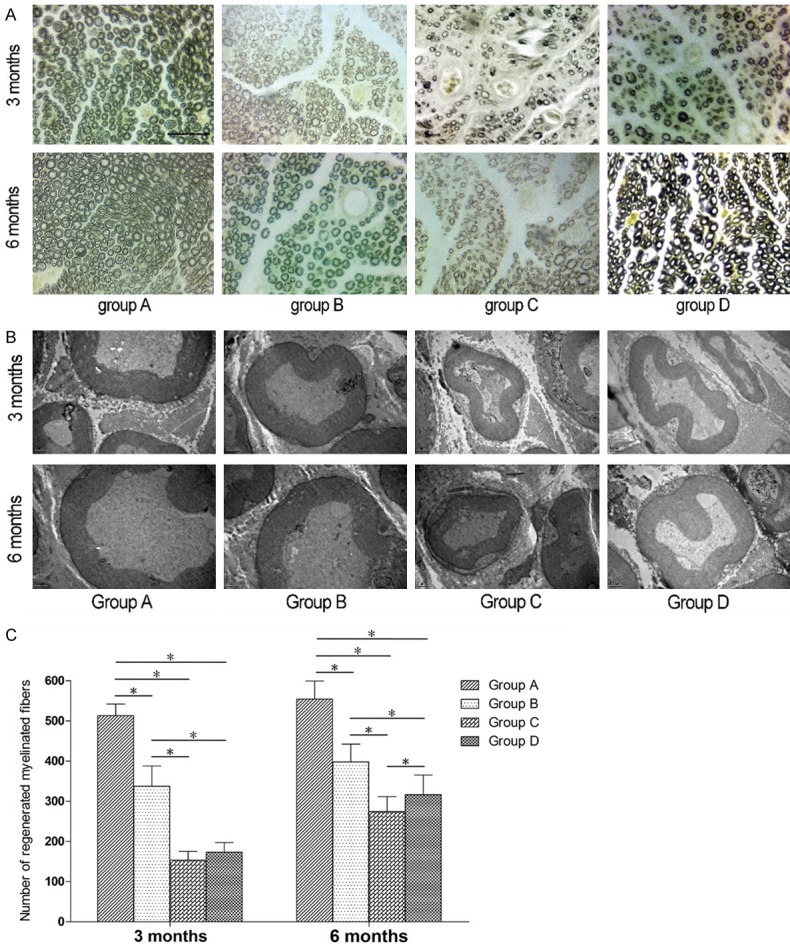
Photomicrographs and bar graphs showing morphology and quantification of myelinated nerve fibers. A: Morphology of myelinated fibers. (Osmium tetroxide staining. Original magnification × 400. Bar = 50 μm). B: Morphology of myelinated fibers. (TEM specimens. Original magnification × 12000. Bar = 1 μm). C: Quantification of myelinated nerve fibers. (The asterisk indicates a statistically significant difference).
Quantification of myelinated nerve fibers
Three months after surgery, the numbers of regenerated myelinated fibers were significantly lower in groups B, C and D than in group A (P < 0.05), and significantly fewer in groups C and D than in group B (P < 0.05). The numbers of regenerated myelinated fibers did not significantly differ between groups C and D. Six months after surgery, the numbers of regenerated myelinated fibers were significant lower in groups B, C and D compared with group A (P < 0.05), and significantly lower in groups C and D than in group B (P < 0.05). The numbers of regenerated myelinated fibers were significantly greater in group D compared with group C (P < 0.05) (Figure 4C).
Morphometrics of myelin sheaths
Morphometric measurements were performed at different postoperative time points. As shown in Figure 5A, 3 months after surgery, axon diameter was significantly larger in group A than in the other groups (P < 0.05), and significantly smaller in groups C and D than in group B (P < 0.05). Axon diameters were not statistically different between groups C and D. Six months after surgery, axon diameters were significantly larger in group A than in the other groups (P < 0.05), and significantly smaller in groups C and D than in group B (P < 0.05). Axon diameter was significantly larger in group D than in group C (P < 0.05).
Figure 5.
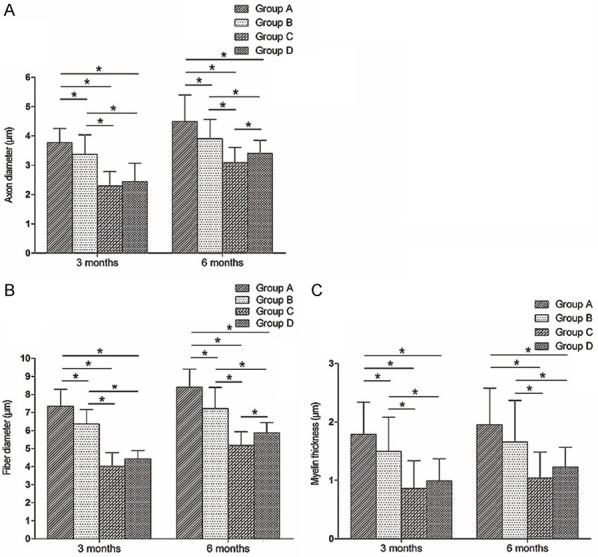
Bar graphs showing morphometrics of myelin sheaths. (A: Axon diameter; B: Fiber diameter; C: Myelin thickness. The asterisk indicates a statistically significant difference).
As shown in Figure 5B, 3 months after surgery, fiber diameters were significantly smaller in groups B, C and D than in group A (P < 0.05), and significantly smaller in groups C and D than in group B (P < 0.05). Fiber diameters were not statistically different between groups C and D. Six months after surgery, fiber diameters were significantly smaller in groups B, C and D compared with group A (P < 0.05), and significantly smaller in groups C and D than in group B (P < 0.05). Fiber diameter was significantly larger in group D than in group C (P < 0.05).
As shown in Figure 5C, 3 months after surgery, myelin thicknesses were significantly smaller in groups B, C and D than in group A (P < 0.05), and significantly smaller in groups C and D compared with group B (P < 0.05). Myelin thicknesses were not statistically different between groups C and D. Six months after surgery myelin thicknesses were significantly smaller in groups C and D than in groups A or B (P < 0.05), but did not differ between groups A and B or between groups C and D.
Neuromuscular junctions
As shown in Figure 6, 3 and 6 months after surgery, in groups A and B there were abundant motor nerve endings and end plates. In groups C and D, motor nerve endings looked sparser, and end plates appeared fewer, compared with groups A or B. The motor nerve endings and end plates in group D appeared more numerous and morphologically more normal than in group C.
Figure 6.
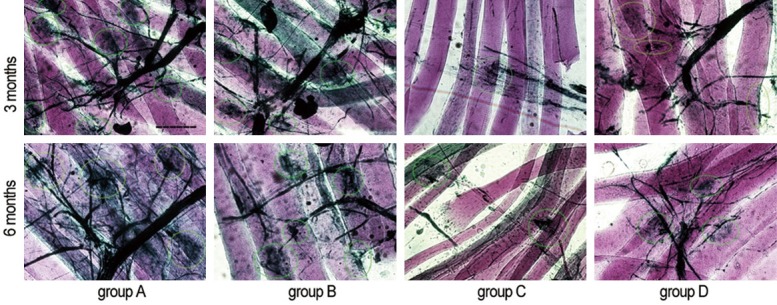
Photomicrograph showing neuromuscular junctions. The light green circles indicate the end plates (Gold chloride staining. Original magnification × 400. Bar = 50 μm).
Recovery of triceps surae muscle mass
At 3 and 6 months after surgery, recovery rates of muscle wet weight were significantly lower in groups B, C and D compared with group A (P < 0.05), and significantly lower in groups C and D than in group B (P < 0.05). The recovery rates were not statistically different between groups C and D (Figure 7).
Figure 7.
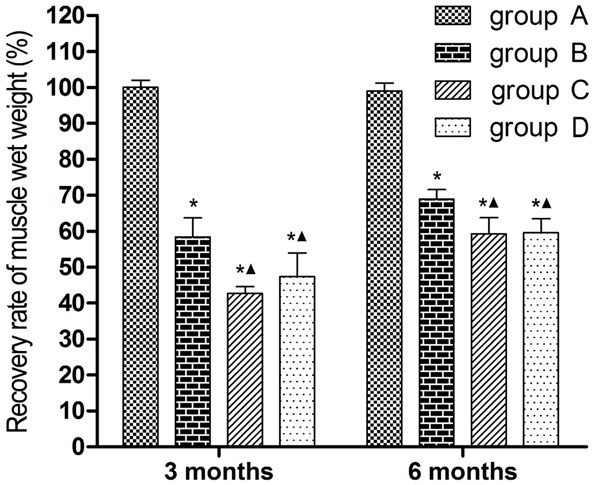
Bar graphs showing recovery of triceps surae muscle mass. (*P < 0.05 versus group A; ▲P < 0.05 versus group B).
Histomorphology of the triceps surae muscle
As shown in Figure 8A, 3 and 6 months after surgery, the muscle fibers in group A were evenly distributed, with a polygonal morphology. The muscle fibers in groups B, C and D exhibited an irregular oval or round shape, and seemed fewer in number than in group A at the same time points.
Figure 8.
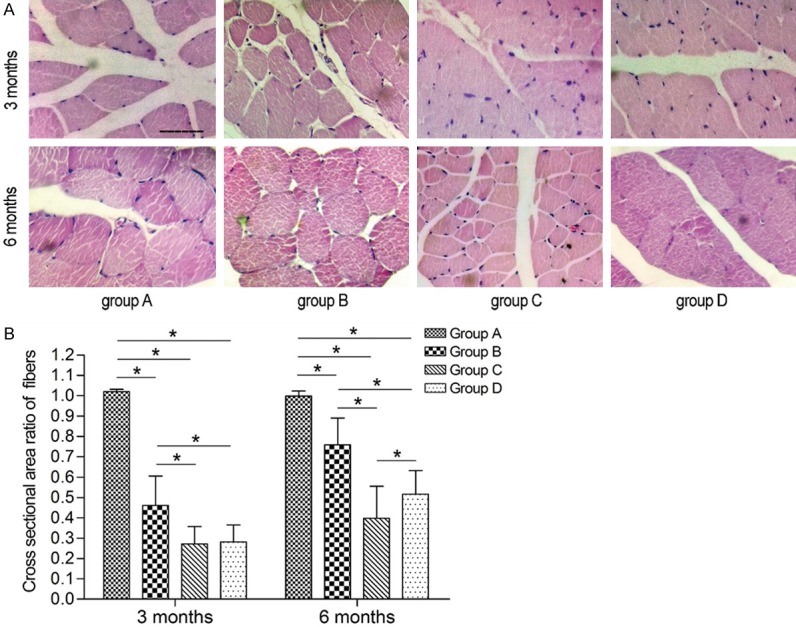
Photomicrograph and Bar graphs showing histomorphology and recovery of the triceps surae muscle. A: Histomorphology of the triceps surae muscle. (Hematoxylin-eosin staining. Original magnification × 400. Bar = 50 μm). B: The recovery of muscle fiber cross-sectional area. (The asterisk indicates a statistically significant difference).
Recovery of muscle fiber cross-sectional area
As shown in Figure 8B, 3 months after surgery, the cross-sectional area ratio of muscle fibers was significantly lower in groups B, C and D than in group A (P < 0.05), and significantly lower in groups C and D than in group B (P < 0.05). The ratios in groups C and D did not statistically differ. Six months after surgery, the ratios were significantly lower in groups B, C and D than in group A (P < 0.05), and significantly lower in groups C and D compared with group B (P < 0.05). The ratio in group D was significantly higher than that in group C (P < 0.05).
Discussion
Transplantation is often required when a large nerve defect is produced by injury. Autologous nerve transplantation is commonly used, as it avoids the immune response [1]. Less important autonomic nerves are often used as donors, such as the sural nerve [6]. When a segment from a more distal nerve is used for transplantation, two anastomoses are required. In the proximal anastomosis, the damaged nerve, which is usually thicker, is connected to the smaller donor nerve. Consequently, neurites extending from the proximal nerve segment face difficulty squeezing into the smaller donor nerve, thereby reducing the efficiency of peripheral nerve regeneration. Furthermore, in both anastomoses, some of the regenerating fibers can escape out of the nerve, as there is no physical constraint. Therefore, in the present study, we evaluated the use of a sural nerve segment to repair a sciatic nerve defect using a chitin biological absorbable tube bridge.
We found that sural nerve transplantation with a chitin biological absorbable tube enhanced nerve regeneration and promoted morphological and functional recovery compared with nerve transplantation without a tube (i.e., by adventitial suture). Six months after surgery, the number, axon diameter and fiber diameter of myelinated nerve fibers were significantly greater in rats given a sural graft using tubes, suggesting that this method promotes nerve regeneration. The motor nerve endings and end plates were also more numerous and appeared more morphologically normal than in rats given a sural transplant without a tube, 3 and 6 months after surgery, suggesting that transplantation with a tube promotes innervation of the effector by regenerating nerve fibers. In addition, 6 months after surgery, the cross-sectional area ratio of muscle fibers was significantly higher in rats given sural nerve transplantation with a tube, suggesting that it enhances target muscle recovery. Recovery of neurological function was also enhanced 6 months after surgery by sural nerve transplantation with a tube, as the SFI was significantly higher than in the group given the sural transplant by adventitial suture.
In the present study, we demonstrate the feasibility of reconstructing a nerve defect with autologous sural nerve bridging, avoiding undesirable immune responses caused by allogeneic nerve transplantation and the need for immunosuppressive treatment [7,8]. Autologous nerve transplantation can guide the proximal axon to grow to the distal endometrial tube, made possible by the presence of Schwann cells and neuroendothelial tubes in the graft.
The regeneration period for large nerve defects is long, and scar tissue or neuromas can form in the anastomosis because of the extrusion and adhesion of adjacent tissue, thereby hindering the directional growth of the nerve fiber [9]. Because of the physical barrier provided by the tube bridge at the two ends, scar tissue and neuromas rarely appear. In addition, the application of tubes prevents the proximal fibers from escaping to the periphery, helping the proximal neurites to grow towards the same type of distant fiber [3].
We also observed that nerve regeneration in the rats given sural transplant with a tube was inferior to that in rats given an orthotopic sciatic nerve graft by adventitial suture. The number, axon diameter, fiber diameter and myelin thickness of myelinated nerve fibers in the latter group were significantly larger than in the former group, 3 and 6 months after surgery. In addition, the motor nerve endings and end plates in rats given orthotopic sciatic transplant appeared more numerous and more normal than in the rats given sural transplant with the tube, 3 and 6 months after surgery. Furthermore, the recovery rate of muscle wet weight and the cross-sectional area ratio of muscle fibers in the rats given the orthotopic sciatic graft were significantly higher than in the rats given sural transplant with a tube, 3 and 6 months after surgery (P < 0.05). The SFI in the former group was also significantly higher than in the latter group, 3 and 6 months after surgery (P < 0.05). Similarly, the TFI in rats given orthotopic sciatic transplant by suture was also significantly higher than in rats given the sural transplant with the tube 6 months after surgery (P < 0.05). These results suggest that the use of a larger graft enhances nerve regeneration. In future studies, double or triple segments of the sural nerve will be combined and used as a graft to repair the defect. We will also use tube bridges to further optimize nerve regeneration.
Neuronal regeneration involves several neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), as well as growth-associated protein 43 (GAP43) [10,11]. After peripheral nerve injury, expression of neurotrophic factors increases at the site of injury. However, this is not sufficient to effectively promote nerve regeneration, and exogenous neurotrophic factors are needed to ameliorate this process. In a future study, local administration of neurotrophic factors will be used after bridging of a sciatic nerve defect with the sural nerve to further enhance nerve repair.
Conclusions
The findings of this study suggest that using a sural nerve graft to repair sciatic nerve defects with a chitin biological tube bridge promotes structural and functional recovery, and improves end plate and muscle fiber morphology. This study gives us a clue for the alternative option in the repairment of nerve defects.
Acknowledgements
This research was continuously funded by Chinese National Key Basic Research 973 Program (2014CB542201); Beijing Science and Technology New Star Cross Subject (2018019); National Natural Science Foundation of China (31571235, 31771322, 51373023, 21171019, 31671248, 31640045, 31571236, 81171146, 31471144, 30971526, 31100860, 31040043, 31371210, 81372044, 81401007); Natural Science Foundation of Beijing Municipality (7162098); Fostering Young Scholars of Peking University Health Science Center (BMU2017PY013).
Disclosure of conflict of interest
None.
References
- 1.Pilanci O, Ozel A, Basaran K, Celikdelen A, Berkoz O, Saydam FA, Kuvat SV. Is there a profit to use the lateral antebrachial cutaneous nerve as a graft source in digital nerve reconstruction? Microsurgery. 2014;34:367–371. doi: 10.1002/micr.22220. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZY, Zhang PX, Han N, Kou YH, Yin XF, Jiang BG. Effect of modified formula radix hedysari on the amplification effect during peripheral nerve regeneration. Evid Based Complement Alternat Med. 2013;2013:647982. doi: 10.1155/2013/647982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Zhang P, Kou Y, Yin X, Han N, Jiang B. Hedysari extract improves regeneration after peripheral nerve injury by enhancing the amplification effect. PLoS One. 2013;8:e67921. doi: 10.1371/journal.pone.0067921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P, Wang Z, Kou Y, Han N, Xu C, Yin X, Wang Y, Feng X. Role of lumbricus extract in the nerve amplification effect during peripheral nerve regeneration. Am J Transl Res. 2014;6:876–885. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Kan S, Zhang B. Pathological changes in neuromuscular junction during ischemia-reperfusion in rat skeletal muscle. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:1103–1108. [PubMed] [Google Scholar]
- 6.Tzou CH, Aszmann OC, Frey M. Bridging peripheral nerve defects using a single-fascicle nerve graft. Plast Reconstr Surg. 2011;128:861–869. doi: 10.1097/PRS.0b013e31821b6369. [DOI] [PubMed] [Google Scholar]
- 7.Arai T, Lundborg G, Dahlin LB. Bioartificial nerve graft for bridging extended nerve defects in rat sciatic nerve based on resorbable guiding filaments. Scand J Plast Reconstr Surg Hand Surg. 2000;34:101–108. doi: 10.1080/02844310050159936. [DOI] [PubMed] [Google Scholar]
- 8.Strasberg SR, Mackinnon SE, Genden EM, Bain JR, Purcell CM, Hunter DA, Hay JB. Long-segment nerve allograft regeneration in the sheep model: experimental study and review of the literature. J Reconstr Microsurg. 1996;12:529–537. doi: 10.1055/s-2007-1006625. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Kou Y, Yin X, Wang Y, Zhang P, Jiang B. The effect of a small gap sleeve suture at the distal anastomosis of a nerve graft on promoting nerve regeneration and functional recovery. Artif Cells Nanomed Biotechnol. 2013;41:282–288. doi: 10.3109/21691401.2012.742097. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Xiao Q, Zhang L, Zhao Y, Yang Y. Nerve growth factor loaded heparin/chitosan scaffolds for accelerating peripheral nerve regeneration. Carbohydr Polym. 2017;171:39–49. doi: 10.1016/j.carbpol.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Sanna MD, Ghelardini C, Galeotti N. HuD-mediated distinct BDNF regulatory pathways promote regeneration after nerve injury. Brain Res. 2017;1659:55–63. doi: 10.1016/j.brainres.2017.01.019. [DOI] [PubMed] [Google Scholar]


