Abstract
Immunotherapy is one of the methods that can change the survival rate of patients with malignant tumors, in addition to surgery therapy, radiotherapy, chemotherapy and targeted therapy. Among various immunotherapy methods, immunoprecipitation inhibitors have been the most effective medications developed in recent years. At present, more in-depth studies have been conducted for two immune checkpoint inhibitor pathways, programmed cell death protein 1 (PD-1)/Programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), and a variety of medications for those above mentioned. The present study briefly reviews the results of clinical trials for relevant immune checkpoint inhibitors in lung cancer.
Keywords: PD-1/PD-L1, CTLA-4, NSCLC, immunotherapy, biomarkers
Introduction
Lung cancer is one of the most fatal malignant tumors, and has been ranked as the first killer among male patients. The five-year survival rate of patients with advanced stage non-small cell lung cancer (NSCLC) remains low despite recent advances in surgery, irradiation, chemotherapy and targeted therapy, and the prognosis of advanced NSCLC remains unsatisfying [1]. Recent breakthroughs in the understanding of tumor immune biology and the development of newer generations of cancer immunotherapies have opened a brand new chapter in the war against cancer [2]. Immune checkpoint blockade (ICB) has emerged as a novel treatment modality that reshapes the immune system of cancer patients to kill tumor cells, and this has achieved inspired success in solid tumors. In the present study, focus was mainly given on the advance of lung cancer immunotherapy [3].
The immune system has the ability to recognize tumor-associated antigens and regulate the body’s ability to attack tumor cells. The immunotherapy of tumors is a therapy that works against tumors by repairing and enhancing the body’s immune system, controlling and killing tumor cells. In 1893, Dr. Coley et al. discovered that Streptococcus pyogenes could reduce tumors, and this allowed the academy to recognize an immune phenomenon for the first time. In 1991, Weissman et al. first reported the anti-tumor efficacy evaluation data of CIK cells and pushed immunotherapy to the spotlight. Since then, tumor immunotherapy research has opened up a new chapter, and cancer immunotherapy has come of age [4].
At present, the most widely studied immunotherapy checkpoints include inhibitors of CTLA-4 and PD-1 and its ligand programmed cell death ligand 1 (PD-L1). An immunological checkpoint inhibitor acts as an immune blockade that prevents the release of tumors to the microenvironment, and induces the re-activation of T cells for immune response to the tumor effect, thereby achieving an anti-tumor role, making it a new weapon against tumors (Figure 1).
Figure 1.
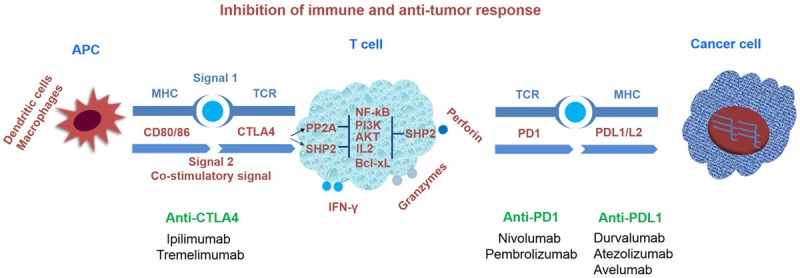
Inhibition of immune and anti-tumor response.
The anti-PD-1/PD-L1 pathway
PD-1 is a member of the extended CD28/CTLA-4 family of T cell regulators and proteins that are expressed after T cells are induced (mature T cells) [5]. Its ligands include PD-L1 and PD-L2, and tumors mainly express PD-L1 [6]. The combination of PD-1 and PD-L1 inhibits the proliferation and viability of CD4+ T and CD8+ T cells, which have been shown in normal individuals, in order to reduce the damage of the immune response to surrounding tissues and prevent the development of autoimmune diseases [6,7]. In addition, for patients with tumors, it can reduce T-cell immunity killing in the tumor local microenvironment, leading to tumor immune escape and the promotion of tumor growth [8]. A number of clinical studies [9-14] have demonstrated that PD-1/PD-L1 inhibitors have excellent efficacy in advanced NSCLC. Furthermore, it was found that PD-L1 expression is closely correlated to its efficacy, and is a direct predictor of its efficacy. Therefore, it is important to understand the proportion of PD-L1 expression in advanced NSCLC, and determine whether it is correlated to the type of tissue.
Nivolumab
Nivolumab is a full human IgG4 monoclonal antibody against PD-1 [15,16], and is also the first listed drug approved by the Food and Drug Administration (FDA) for advanced NSCLC patients. A Phase I, Dose-Hill study enrolled 129 advanced NSCLC patients. Among these patients, the treatment ratio of three lines and above was 54.3%, the median overall survival (OS) was 9.9 months, the best effect was at 3 mg/kg, and the median OS was 14.9 months, when compared with 9.2 months at 1 or 10 mg/kg. CheckMate-063 was also a single-arm, phase II clinical trial for evaluating monotherapy [12]. Patients in the study were limited to advanced resistant squamous cell carcinoma, and most of these patients were in the third-line and later treatment. The results revealed that in the single-agent treatment of nivolumab, the objective response rate (ORR) was 14.5%, median OS was 8.2 months, and 1-year survival rate reached 40.8%. This survival data far exceeds previous findings on advanced resistant squamous cell carcinoma. Thus, nivolumab has become the first immune checkpoint inhibitor approved by the US FDA for advanced squamous cell carcinoma. Two subsequent Phase III-randomized controlled clinical studies conducted by CheckMate-017 [13] and CheckMate-057 [17] explored the efficacy of nivolumab, with focus on advanced NSCLC patients with first-line platinum-based regimen. In the phase III clinical trials, among the 272 squamous NSCLC patients in the CheckMate-017 [13] study, the median OS was 9.2 and 6.0 months in the nivolumab and docetaxel groups, respectively, with a 41% reduction on the risk of death in the nivolumab group (HR = 0.59, P = 0.001). Furthermore, in these two groups, the ORR was 20% and 9%, respectively (P = 0.008), and the median progression-free survival (PFS) was 3.5 and 2.8 months, respectively (HR = 0.62, P < 0.001). A total of 582 patients were enrolled in the CheckMate-057 [17] study. In these two groups, the median OS was 12.2 and 9.4 months, respectively (HR = 0.73, P = 0.002), and the ORR was 19% and 12%, respectively (P = 0.02). However, there was no significant difference in median PFS. Unlike the CheckMate-017 study, the present study found that as the PD-L1 expression level increased, the efficacy of nivolumab also improved. However, no specific values were provided.
Pembrolizumab (MK-3475)
Pembrolizumab is a human monoclonal antibody against PD-1 and its ligand PD-L1, which can release T cells and achieve the effect of eliminating tumor cells. KEYNOTE-001 [9] was the first phase I clinical trial conducted. This study revealed that the ORR and duration of response (DOR) of pembrolizumab monotherapy was 19.4% and 12.5 months, respectively, while median PFS and OS were 3.7 and 12 months, respectively. The subgroup analysis revealed that patients who were initially treated had better efficacy than those who were subsequently treated, and the ORR was 6.8% (24.8% and 18%, respectively). In addition, PD-L1 overexpression (TPS ≥ 50%) had better efficacy, with a PFS of more than one year (12.5 months). Therefore, the FDA approved pembrolizumab was used to treat the disease progression of metastatic NSCLC patients with PD-L1 ≥ 50% during or after platinum-containing chemotherapy on October 2, 2015.
The KEYNOTE-010 [10] study was the first randomized controlled study that compared pembrolizumab with chemotherapy in patients with advanced NSCLC, who required first-line post-treatment following progression and were PD-L1 positive (L1 p). It was also prospective to use the expression PD-L1 to predict pembrolizumab efficacy, and it was ultimately confirmed that PD-L1 expression levels can be used as a biomarker for pembrolizumab efficacy. Moreover, it was also determined that the optimal dose was 2 mg/kg. The KEYNOTE-024 [11] is another clinical trial that compared pembrolizumab with standard platinum-based chemotherapy in patients with high PD-L1 expression (TPS pat). The results revealed that PFS was prolonged by 4.3 months (10.3 months vs. 6.0 months, HR = 0.50, P < 0.001) in the pembrolizumab group. Furthermore, pembrolizumab was also superior to chemotherapy in terms of ORR (44.8% vs. 27.8%), and had a longer DOR. The KEYNOTE-021 study [18] set a goal to compare the efficacy and safety of pembrolizumab + chemotherapy and chemotherapy as a first-line treatment for advanced NSCLC. The results revealed that tissue type was limited to adenocarcinoma, ORR was 55% vs. 29%, and PFS was 13 and 8.9 months (HR = 0.53, P = 0.01) in the two groups, respectively. In pre-treatment, for PD-L1-expressing SCLC patients, the KEYNOTE-028 [19] study revealed that pembrolizumab also had an ORR of 33% (95% CI: 16%-55%), demonstrating its promising antitumor activity.
Atezolizumab (MPDL3280A)
Atezolizumab is a fully humanized, engineered monoclonal antibody of the IgG1 isotype against PD-L1. Early non-randomized controlled clinical studies have confirmed that atezolizumab has an exact effect and low incidence of adverse reactions for the treatment of advanced NSCLC [20]. The POPLAR [21] study is a randomized phase II clinical trial designed to evaluate the efficacy and safety of atezolizumab for second-line lung cancer therapy, and compare with docetaxel. The results revealed that the primary end-point OS was 12.6 and 9.7 months in the atezolizumab and docetaxel groups, respectively (HR = 0.73, P = 0.04), but PFS had no significant difference (2.7 and 3.0 months, respectively; HR = 0.94). Furthermore, DOR was significantly better in the atezolizumab group than in the docetaxel group (14.3 and 7.2 months, respectively). The clinical trial OAK [14] showcased that atezolizumab prolonged OS, compared with chemotherapy (13.8 months vs. 9.6 months, HR = 0.74, P = 0.0004) in second-line or third-line IIIB/IV stage NSCLC after first line therapy failure. Another study was the BIRCH [22] study, which revealed that atezolizumab had a higher disease control rate (DCR) rate (27%), and was better than chemotherapy. It is noteworthy that the detection method for PD-L1 expression induced by atezolizumab was different from that induced by nivolumab or pembrolizumab, and this method mainly focused on PD-L1 expression in tumor cells and immune cells.
Durvalumab (MEDI4736)
Durvalumab is an FDA-approved immunotherapy for cancer, which was developed by Medimmune/AstraZeneca. It is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of PD-L1 with PD-1 and CD80 (B7.1), and this exhibited a high degree of safety and clinical activity in previous treatment-naive [23] patients with advanced non-small-cell lung cancer. ARCTIC [24] (NCT02352948) is a global, phase III, randomized, open-label multicenter study that included patients with advanced NSCLC. This study assessed the safety and clinical activity of durvalumab vs. standard care (SoC; erlotinib, gemcitabine, or vinorelbine) in patients with PD-L1(+) tumors (slbine) stained using ventana PD-L1 [SP263] CDx Assay (Sub-study A) and the combination of durvalumab + tremelimumab, or either agent as monotherapy vs. SoC in patients with PD-L1(-) tumors (Sub-study B). The recruitment started on January 2015, and is presently ongoing. Indeed, a recent phase 3 study of durvalumab demonstrated improved PFS for patients with surgically unresectable, locally advanced, stage III NSCLC. The median PFS was substantially improved in the durvalumab arm, with a hazard ratio of 0.52 (16.8 vs. 5.6 months) [25].
Avelumab
Avelumab (Bavencio) is another human Ig-G1 monoclonal antibody that targets PD-L1, and this was approved by the US FDA. In the dose-expansion cohort of this multicenter, open-label, phase 1 study [26,27], 92 (50%) of 184 patients achieved disease control (they had a confirmed response or stable disease as their best overall response). According to the latest study by PACIFIC, Durvalumab can improve the PFS of patients with locally advanced, unresectable stage III lung cancer. The median PFS in the Durvalumab group was 16.8 vs. 5.6 (months), and the hazard ratio (HR) was 0.52, when compared with the placebo [28]. This revealed an acceptable safety profile and antitumor activity in patients with progressive or treatment-resistant NSCLC, providing a rationale for further studies of avelumab in this disease setting.
Summary
A summary of the design of phase II/III studies leading to IO registrations in NSCLC is presented in Table 1.
Table 1.
Summary of the design of phase II/III studies leading to IO registrations in NSCLC
| Study | Phase | Agent | Control arm | Line of therapy | PD-L1 selection | Primary endpoint | ||
|---|---|---|---|---|---|---|---|---|
| CM026 | III | Nivo. | Plat. Cx | 1st | PD-L1 ≥ 1% | ≥ 5% PD-L1+PFS (m): Nivo vs. Chemo: 4.2 vs. 5.9 | ||
| KN024 | III | Pembro. | Plat. Cx | 1st | PD-L1 ≥ 50% | ≥ 50% PD-L1+PFS (m): Pembro. vs. Chemo: 10.3 vs. 6.0 | ||
| KN021G | II | Pembro./Cx | Plat. Cx | 1st | None | ORR: Pembro./Cx vs. Chemo: 56.7% vs. 30.2% | ||
| BIRCH | II | Atezo. | None | 1st | PD-L1 positive | Arm A ORR: ITT: 25% | ||
| KN001 | Ib | Pembro. | None | 1st & ≥ 2nd | None | ORR: | 1st | ≥ 2nd |
| PD-L1 < 1% | 10% | 9.9% | ||||||
| PD-L1 ≥ 1% | 17.4% | 12.9% | ||||||
| PD-L1 ≥ 50% | 58.3% | 38.3% | ||||||
| KN010 | I/II | Pembro.2 mg/10 mg | Docetaxel | 2nd | PD-L1 ≥ 1% | ORR: 30%/29% vs. 8% | ||
| CM017 | III | Nivo. | Docetaxel | 2nd | None | mOS (m): 9.2 vs. 6.0 | ||
| CM057 | III | Nivo. | Docetaxel | 2nd | None | mOS (m): 12.2 vs. 9.5 | ||
| POPLAR | II | Atezo | Docetaxel | 2nd | None | mOS (m): ITT group 12.6 vs. 9.7 | ||
| OAK | III | Atezo | Docetaxel | 2nd | None | mOS (m): ITT group 13.8 vs. 9.6 | ||
Note: NSCLC, non-small cell lung cancer; PD-L1, programmed death ligand 1.
The anti-CTLA-4 pathway
CTLA4 is a member of the immunoglobulin superfamily, is expressed by activated T cells, and transmits an inhibitory signal to T cells [29]. CTLA4 is homologous to the T-cell co-stimulatory protein, CD28, and both molecules bind to CD80 and CD86, which are also called B7-1 and B7-2, respectively, on antigen-presenting cells. CTLA-4 binds CD80 and CD86 with greater affinity and avidity, when compared to CD28, thereby enabling it to outcompete CD28 for its ligands. CTLA4 transmits an inhibitory signal to T cells, whereas CD28 transmits a stimulatory signal. CTLA4 was also found in regulatory T cells, which contributed to its inhibitory function. T cell activation through the T cell receptor and CD28 led to the increased expression of CTLA-4 [30].
The mechanism by which CTLA-4 acts in T cells remains somewhat controversial [31,32]. Biochemical evidence suggests that CTLA-4 recruits a phosphatase to the T cell receptor (TCR), thereby attenuating the signal. This work remains unconfirmed in literature since its first publication. A more recent work has suggested that CTLA-4 may function in vivo by capturing and removing B7-1 and B7-2 from the membranes of antigen-presenting cells, thereby making these unavailable for triggering CD28.
At present, the main CTLA-4 inhibitors are ipilimumab and tremelimumab, among which ipilimumab was the earliest inhibitor approved by the FDA, and the earliest inhibitor used as a clinical immunological checkpoint inhibitor.
Ipilimumab
Ipilimumab is a fully humanized IgG1 monoclonal antibody capable of effectively blocking CTLA-4 from binding to its ligand. Early clinical trials have shown that phased ipilimumab plus paclitaxel and carboplatin had improved irPFS and PFS in lung cancer patients, which support the additional investigation of ipilimumab in NSCLC [33]. However, another phase III trial of ipilimumab combined with paclitaxel and carboplatin did not prolong OS, when compared with chemotherapy alone, in patients with advanced squamous NSCLC. The safety profile of chemotherapy plus ipilimumab was consistent with that observed in previous lung and melanoma studies [34]. Ongoing studies are evaluating ipilimumab in combination with nivolumab in this population. The same conclusion was also obtained for newly diagnosed extensive-stage disease SCLCs, and the addition of ipilimumab to chemotherapy did not prolong OS, when compared with chemotherapy alone [35].
Tremelimumab
DETERMINE [36] was a double-blind, placebo-controlled, phase 2b trial performed on 105 study centers across 19 countries in patients with unresectable pleural or peritoneal malignant mesothelioma, who progressed after one or two previous systemic treatments for advanced disease. Tremelimumab did not significantly prolong overall survival, when compared with placebo in patients with previously treated malignant mesothelioma. The safety profile of tremelimumab was consistent with the known safety profile of CTLA-4 inhibitors. Investigations on whether immunotherapy combination regimens can provide greater efficacy, when compared with monotherapies, in lung cancer (NCT02179671, NCT02000947, etc.) are ongoing.
Novel immunological checkpoint treatment target
Lymphocyte activation gene-3 (LAG3)
Despite the impressive impact of CTLA4 and PD1-PDL1-targeted cancer immunotherapy, a large proportion of patients with many tumor types have failed to respond. LAG3 is the third inhibitory receptor targeted in clinic, which has consequently garnered considerable interest and scrutiny [37,38]. This is an immunoassay molecule expressed in activated T cells, NK cells, B cells and plasma cell-like dendritic cells. Studies have shown that LAG-3 selectively upregulates CD4 on the surface of Treg. Thus, LAG-3 antibodies can reduce Treg activity in vivo. The inhibition or knockout of LAG-3 would release the inhibitory effect of Treg on T cells. In addition, in the absence of CD4+ T cells, LAG-3 antibody can increase the function of CD8+ T cells. PD-1/LAG-3 double knockout mice exhibited anti-tumor activity when implanted with tumor cells, while PD-1 knockout mice exhibited tumor growth delay. To date, clinical trials that use the LAG-3 inhibitor or combines with the PD-1 inhibitor are ongoing for advanced lung cancer (NCT02966548 and NCT01968109).
TIM-3
TIM-3 is an immune checkpoint receptor that is constitutively expressed by CD4+ T helper 1 (Th1), CD8+ T cytotoxic 1 (Tc1) and Th17 cells [39,40]. The interaction between TIM-3 and its ligand galectin-9 inhibits Th1 and Th17 response, which induces peripheral tolerance. Other ligands of TIM-3, such as phosphatidyl serine (PtdSer), high mobility group protein B1 (HMGB1) and Ceacam-1, have been identified to have a primary role in innate immune response. Unlike other immunological checkpoint molecules, TIM-3 was not upregulated after all T cells were activated, and merely CD4+ Th1 and CD8+ cytotoxic T cells were involved in its synergistic inhibition. When activated by its ligand galectin-9, TIM-3 inhibits the activity of T cells and causes peripheral tolerance. TIM-3 plays a key role in the loss of T cells in tumors. Furthermore, TIM-3 is highly expressed in the T cells of animals resistant to treatment with anti-PD-1. In an independent experiment, the combination of anti-TIM-3 antibody and anti-PD-1 drug can inhibit the anti-PD-1 treatment of drug resistance [41]. In addition, TIM-3 expression was upregulated in CD4+ and CD8+ tumor-infiltrating lymphocytes in patients with lung cancer, and TIM-3 expression in CD4+ T cells was correlated with lymph node metastasis and lung cancer staging [42]. To date, the anti-TIM3 monoclonal antibody (TSR-022, TESARO and MBG453, Novartis) is being tested in two clinical trials for advanced lung cancer (NCT02817633 and NCT02608268) as a monotherapy or in combination with an anti-PD1 antibody.
KIRs
KIRs are regulatory glycoproteins that belong to the superfamily of immunoglobulins [43]. These are principally expressed on the surface of both NK cells and T CD8+ cells, which modulate the cytotoxic activity of both cell types. New drugs that target NK cells are in development, and the most advanced compound is the anti-KIR monoclonal antibody (lirilumab), which recognizes KIR-2DL-1, -2 and -3, and has the ability to block the interaction with HLA-I ligands. The Phase I study of lirilumab combined with ipilimumab (NCT01750580) for advanced solid tumors, which included NSCLC, has been completed, and the data revealed no additional safety concerns in the combination group. Another Phase I/II study that assessed the safety, tolerability and anti-tumor activity of lirilumab in combination with nivolumab (NCT01714739) is presently recruiting.
Influence factor of immune checkpoint blockade (ICB) therapy
Although these immunological checkpoint inhibitors exhibited an inspiring effect on melanoma, NSCLC, renal cell carcinoma, Hodgkin’s lymphoma, bladder cancer and other cancers, the clinical outcome of ICB therapy remains challenged by a lot of influential factors. Based on the mechanism and principle of PD-1/PD-L1 inhibitors, among the many factors presently used to predict the response of PD-1/PD-L1 inhibitors, the expression level of PD-L1 was the most attractive detection hot spot. CheckMate-012, KEYNOTE-001, OAK and other clinical trial data revealed that the ORRs of PD-1/PD-L1 inhibitors were positively correlated with PD-L1 expression level in tumor tissues. Furthermore, a higher response rate to pembrolizumab has been shown with a tumor cell PD-L1 expression of ≥ 50% in the KEYNOTE-010 [10] and KEYNOTE-024 [6] studies, while the research data in Checkmate-057 [8] revealed that the expression of PD-L1 in tumor tissue was not associated with the prognosis or disease control rate of patients. Furthermore, a recent meta-analysis [44] revealed that ORR was 29.6% in PD-L1 positive patients and 13.5% in negative patients, and the difference was statistically significant (P < 0.001). Therefore, the reliability of PD-L1 as a clinical biomarker requires a large number of clinical trials to validate. There are also some other factors that influence ICB therapies below, and these are summarized in Figure 2.
Figure 2.
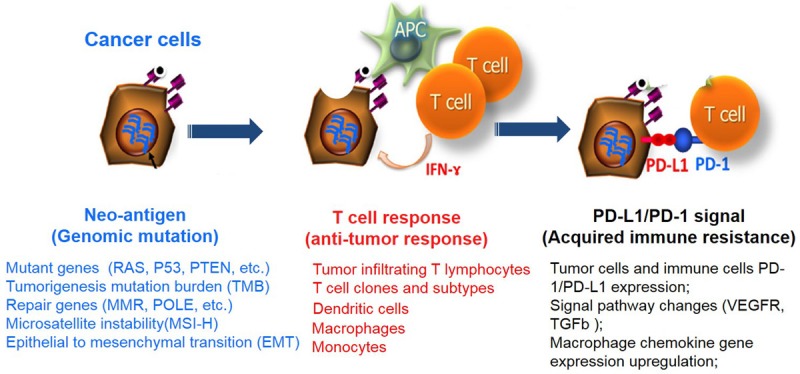
Summary of immunotherapy biomarkers.
Tumor mutation burden (TMB)
Microsatellite instability (MSI)
MSI is the condition of genetic hypermutability (predisposition to mutation) that results from impaired DNA mismatch repair (MMR) [45,46]. The presence of MSI represents phenotypic evidence that MMR is not functioning normally. Furthermore, it has a higher mutation load and percentage of proliferative tumor-lymphocytes, as well as multiple immunological checkpoints, including PD-1, PDL1, CTLA-4, LAG-3 and IDO, in MSI-high patients, when compared to MSI-low patients. Hence, MSI-high patients usually have a better prognosis with a lower recurrence rate.
Polymerase epsilon (POLE)
POLE gene mutation appears to present an enhanced immune microenvironment and a high mutation burden [47]. It has a higher likelihood of gene mutation in the process of tumor cell proliferation and mitosis with POLE-mutated patients, because the DNA repair mechanism is destroyed.
TP53
It has been reported that TP53 mutation significantly increased the expression of immune checkpoints, and activated T-effector and interferon-γ signatures [48]. More importantly, the TP53/KRAS comutated subgroup manifested an exclusive increased expression of PD-L1 and the highest proportion of PD-L1+/CD8A+. Meanwhile, TP53- or KRAS-mutated tumors exhibited prominently elevated mutation burdens, and were specifically enriched in the transversion-high (TH) cohort. This shows that TP53 and KRAS mutation in lung adenocarcinoma may serve as a pair of potential predictive factors in guiding anti-PD-1/PD-L1 immunotherapy.
KRAS
In contrast to MSI, KRAS and NRAS mutations are associated with relatively few immune cell infiltrations and a relatively low expression of inhibitory molecules. Therefore, when KRAS [49] mutations in colorectal cancer (CRC) is in a relatively static immune state in the tumor microenvironment, ICB treatment may be poor. On the contrary, chemotherapy would be relatively suitable.
BRAF
The BRAF mutation [49] in lung cancer usually means poor prognosis, while using BRAF inhibitors combined with ICB can further enhance immune activation, and may provide a new idea for treatment program selection.
Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK)
EGFR and ALK tyrosine kinase inhibitors (TKIs) have significantly improved clinical outcomes, when compared with chemotherapy, in NSCLC patients with sensitizing EGFR mutation or ALK amplification. However, almost all patients treated with TKIs eventually develop acquired resistance. Combination approaches [50-53], including TKIs therapy, on the basis of PD-1/PD-L1 inhibitors, are presently being designed to re-energize the immune system with complementary/synergetic mechanisms, and these can achieve durable antineoplastic effects in NSCLC.
Tumor immune microenvironment
The concept of an immune microenvironment has become increasingly clear since the “seed-soil” theory of Stephen Paget’s tumor-specific tumor metastasis in 1889. The tumor microenvironment [54,55] comprises of endothelial cells, as well as its precursor cells, pericytes, myeloid-derived suppressor cells (MDSC), tumor-associated fibroblast (CAF), tumor-associated macrophages (TAM), T cells and B Cells, NK cells, DC cells, etc. There are factors that affect the activation and function of T cells in the tumor microenvironment. The microenvironment of the tumor affects the activation and metabolism of T cells through various mechanisms, promotes the uptake of T cell surface inhibitory molecules, and induces T cells to differentiate to the terminal state, causing T cells to be depleted and incompetent. Tumor-infiltrating T lymphocyte density: Tumor-infiltrating T lymphocytes have an immune surveillance effect that inhibits the binding to PD-1 and PD-L1, which is positively correlated with resistance, and is resistant to tumorigenesis and development. These are used to predict the occurrence of tumors. Co-stimulatory receptor: Tumor infiltrating T cells can be activated by co-stimulatory receptors (e.g. tumor necrosis factor receptor superfamily members OX40, CD40, 41BB and B7-CD28, and immunoglobulin superfamily member ICOS, etc.) and a costimulatory receptor in combination with its ligand by enhancing the function of Th1 cells or inhibiting the function of Treg cells, killing tumor cells. Comeric stimulating agent agonists can enhance the activation of T cells. Its combination with ICB can enhance its anti-tumor efficiency. MDSC can directly act on T cells to inhibit its activation, and also generates active nitrogen-induced CCL2 into N-CCL2, thereby inhibiting T cell infiltration, and helping tumor cells achieve immune escape. Treg cells can promote the production of vascular endothelial growth factor (VEGF) in tumor cells and CAF, and reduce IFN-γ and granzyme produced by T cells to inhibit immune killing. In tumor patients, Treg cells inhibit specific T cell responses and express high levels of glucocorticoid-induced tumor necrosis factor receptor-associated proteins (GITR) and CTLA-4.
The expression or uptake of a particular signaling pathway
The MAPK pathway [56] leads to the production of VEGF and IL-8, thereby inhibiting T cell recruitment and function. In a variety of tumors, the absent expression of tumor suppressor gene PTEN and the enhanced PI3K pathway are highly correlated with the decrease in IFNγ, granzyme B gene expression and the number of tumor-infiltrating CD8+ T cells.
The sustained expression of the WNT/β-catenin [57] signaling pathway causes the WNT signaling pathway to remain active by stabilizing beta-catenin, thereby removing T cells out of the tumor, and inhibiting immunotherapy.
IFNγ [58] produced by tumor-specific T cells is capable of recognizing the corresponding receptors on tumor cells or antigen presenting cells, thereby exhibiting an effective anti-tumor immune response. IFNγ enhances the expression of MHC molecules, thereby enhancing tumor antigen presentation. Furthermore, IFNγ can also recruit other immune cells, or directly inhibit the proliferation of tumor cells, and promote its apoptosis. Therefore, the mutations and deletions of IFNγ pathway-related proteins, such as IFNγ1 and IFNGR2, and JAK1 and JAK2, STATs, and IRF1, in IFNγ receptor chains on tumor cells lead to resistance to immunosuppressive inhibitors.
Conclusion
Immunotherapy is the most revolutionary advance in tumor research. This new treatment approach not only prolongs the survival of patients, but also indicates the direction for future tumor research [59]. Immunological checkpoint inhibitors, such as PD-1/PD-L1 and CTLA-4, show an inspiring effect in melanoma, NSCLC, renal cell carcinoma, Hodgkin’s lymphoma, bladder cancer and other cancers in a number of multi-center clinical trials. However, to date, its benefit remains limited to a minority of patients with certain cancer types. In addition, as a result of more successful immunotherapy treatments, we now have a significant subset of patients who initially respond, but eventually relapse. Cancer immunotherapy continues to face some challenges: (1) Determining how to scientifically evaluate the efficacy of immunotherapy; (2) Determining the best strategy for tumor immunotherapy combined with other treatments; (3) Finding suitable predictors for screening the effect of ICB therapies; (4) Reducing the incidence of adverse reactions and mortality of ICB.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–668. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 10.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall survival and long-term safety of nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2017;35:3832–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 22.Wakelee H, Patel JD, Heist R, Balmanoukian A, Besse B, Felip E, Carcereny Costa E, Chow LQ, Koczywas M, Garassino MC, Christoph D, Toh CK, Johnson ML, Chaft J, Kurata T, Qiu J, Kowanetz M, Coleman S, Mocci S, Sandler A, Gettinger SN, Peters S. ORAL01.04: phase II trial of atezolizumab for patients with PD-L1-selected advanced NSCLC (BIRCH): updated efficacy and exploratory biomarker results. J Thorac Oncol. 2016;11:S251–S252. [Google Scholar]
- 23.Antonia S, Rizvi N, Brahmer J, Ou SH, Khleif SN, Hwu WJ, Gutierrez M, Schoffski P, Hamid O, Weiss J, Lutzky J, Maio M, Nemunaitis J, Jaeger D, Balmanoukian A, Rebelatto MC, Steele KE, Jin X, Robbins PB, Blake-Haskins JA, Segal NH. Safety and clinical activity of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC) [abstract] Cancer Immunol Res. 2016;4(Suppl):A047. [Google Scholar]
- 24.Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, Shi K, Soria JC. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17:232–236. doi: 10.1016/j.cllc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 26.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, Heydebreck AV, Chin K, Cuillerot JM, Kelly K. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18:599–610. doi: 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerusalem G, Chen F, Spigel D, Iannotti N, Mcclay E, Redfern C, Bennouna J, Taylor M, Kaufman H, Kelly K, Chand V, Heydebreck AV, Verschraegen C. JAVELIN solid tumor: safety and clinical activity of avelumab (anti-PD-L1) as first-line treatment in patients with advanced NSCLC [abstract] . J Thorac Oncol. 2017;12:S252. [Google Scholar]
- 28.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 29.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 34.Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V, Fadeeva N, Lee KH, Kurata T, Zhang L, Tamura T, Postmus PE, Jassem J, O’Byrne K, Kopit J, Li M, Tschaika M, Reck M. Phase III trial of lpilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J. Clin. Oncol. 2017;35:3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 35.Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, Felip E, Gold K, Horn L, Aerts J, Nakagawa K, Lorigan P, Pieters A, Kong Sanchez T, Fairchild J, Spigel D. Phase III randomized trial of lipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 2016;34:3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 36.Maio M, Scherpereel A, Calabrò L, Aerts J, Perez SC, Bearz A, Nackaerts K, Fennell DA, Kowalski D, Tsao AS, Taylor P, Grosso F, Antonia SJ, Nowak AK, Taboada M, Puglisi M, Stockman PK, Kindler HL. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18:1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 37.Okamura T, Fujio K, Sumitomo S, Yamamoto K. Roles of LAG3 and EGR2 in regulatory T cells. Ann Rheum Dis. 2012;71(Suppl 2):i96–i100. doi: 10.1136/annrheumdis-2011-200588. [DOI] [PubMed] [Google Scholar]
- 38.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, Zhang Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huard B, Karlsson L. KIR expression on self-reactive CD8+ T cells is controlled by Tcell receptor engagement. Nature. 2000;403:325–328. doi: 10.1038/35002105. [DOI] [PubMed] [Google Scholar]
- 45.Zhu L, Jing S, Wang B, Wu K, Shenglin MA, Zhang S. Anti-PD-1/PD-L1 therapy as a promising option for non-small cell lung cancer: a single arm meta-analysis. Pathol Oncol Res. 2016;22:331–339. doi: 10.1007/s12253-015-0011-z. [DOI] [PubMed] [Google Scholar]
- 46.Maruvka YE, Mouw KW, Karlic R, Parasuraman P, Kamburov A, Polak P, Haradhvala NJ, Hess JM, Rheinbay E, Brody Y, Koren A, Braunstein LZ, D’Andrea A, Lawrence MS, Bass A, Bernards A, Michor F, Getz G. Analysis of somatic microsatellite indels identifies driver events in human tumors. Nat Biotechnol. 2017;35:951–959. doi: 10.1038/nbt.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson AL, Chen R, Loeb LA. Induction of microsatellite instability by oxidative DNA damage. Proc Natl Acad Sci U S A. 1998;95:12468–12473. doi: 10.1073/pnas.95.21.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrianova MA, Bazykin GA, Nikolaev SI, Seplyarskiy VB. Human mismatch repair system balances mutation rates between strands by removing more mismatches from the lagging strand. Genome Res. 2017;27:1336–1343. doi: 10.1101/gr.219915.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, Yang JJ, Yang XN, Lin JX, Yan HH, Zhai HR, Yan LX, Liao RQ, Wu SP, Wu YL. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23:3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 50.Soo RA, Kim HR, Asuncion BR, Fazreen Z, Omar MFM, Herrera MC, Yun Lim JS, Sia G, Soong R, Cho BC. Significance of immune checkpoint proteins in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017;105:17–22. doi: 10.1016/j.lungcan.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Gibbons DL, Chow LQ, Kim DW, Kim SW, Yeh T, Song X, Jiang H, Taylor R, Karakunnel J, Creelan B. Efficacy, safety and tolerability of MEDI4736 (durvalumab [D] ), a human IgG1 antiprogrammed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11:S79. [Google Scholar]
- 53.Su C, Zhou F, Shen J, Zhao J, O’Brien M. Treatment of elderly patients or patients who are performance status 2 (PS2) with advanced non-small cell lung cancer without epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations-Still a daily challenge. Eur J Cancer. 2017;83:266–278. doi: 10.1016/j.ejca.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Turcotte S, Rosenberg SA. Immunotherapy of metastatic solid cancers. Adv Surg. 2011;45:341–360. doi: 10.1016/j.yasu.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Jia Y, Zhao S, Zhang X, Wang X, Han X, Wang Y, Ma M, Shi J, Liu L. BIN1 reverses PD-L1-mediated immune escape by inactivating the c-MYC and EGFR/MAPK signaling pathways in non-small cell lung cancer. Oncogene. 2017;36:6235–6243. doi: 10.1038/onc.2017.217. [DOI] [PubMed] [Google Scholar]
- 57.Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10:101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nirschl CJ, Suárez-Fariñas M, Izar B, Prakadan S, Dannenfelser R, Tirosh I, Liu Y, Zhu Q, Devi KSP, Carroll SL, Chau D, Rezaee M, Kim TG, Huang R, Fuentes-Duculan J, Song-Zhao GX, Gulati N, Lowes MA, King SL, Quintana FJ, Lee YS, Krueger JG, Sarin KY, Yoon CH, Garraway L, Regev A, Shalek AK, Troyanskaya O, Anandasabapathy N. IFNγ-dependent tissue-immune homeostasis is co-opted in the tumor microenvironment. Cell. 2017;170:127–141. e15. doi: 10.1016/j.cell.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colli LM, Machiela MJ, Zhang H, Myers TA, Jessop L, Delattre O, Yu K, Chanock SJ. Landscape of combination immunotherapy and targeted therapy to improve cancer management. Cancer Res. 2017;77:3666–3671. doi: 10.1158/0008-5472.CAN-16-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]


