Abstract
Portulacae Oleracea L. (POL) is a traditional Chinese medicine and also an edible vegetable used to treat diarrhea in china for thousands years. Though the therapeutic effect has been proved in clinical trials, the concrete effective component and mechanisms remained elusive. Polysaccharide from POL has been extracted previously and the experiment suggested that POLP could diminish the weight loss and improve the health conditions of mice with DSS induced colitis. Hematoxylin & eosin staining revealed that POLP could improve the histopathological structure of the colon tissue. For the notably variation curve of TNF-α in control, colitis and treatment group, NF-κB was enrolled to investigate the molecular mechanisms of the protective effect of POLP. The protein expression level of NF-κBp65 in cytoplasm increased after POLP treatment of the induced colitis. However, the protein level of NF-κBp65 in the nucleus decreased after administration of POLP. The expression levels of IκBα and NF-κB related proteins Bcl-2 and survivin were also detected and the results suggested that POLP could inhibit the degradation of IκBα and decrease the protein levels of Bcl-2 and Survivin in colitis. It was concluded that POLP could improve the health condition of mice with DSS induced colitis and the mechanisms were closely related with NF-κB via inhibiting the degradation of IκBα.
Keywords: Colitis, Portulacae Oleracea L., polysaccharide, NF-κB, DSS
Introduction
Inflammatory bowel disease (IBD) is characterized by chronic, unregulated and relapsing intestinal inflammation, which can affect any part of the gastrointestinal tract [1]. While the pathophysiology of IBD remains unclear, it has been considered that a complex interaction of genetic, immunoregulatory and environmental factors contributes to the occurrence and the perpetuation of this disease [2,3]. The main symptoms of this disease are diarrhea, weight loss, abdominal pain, rectal bleeding and vomiting and the incidence in humans appears to be increased [4]. Though the etiology and pathogenesis of IBD have not been elucidated, it is confirmed that inflammatory cytokines play an essential role in the occurrence and progression of IBD [5]. Moreover, accumulating experimental indicates that these inflammatory cytokines are connected with some signaling pathways to modulate the inflammation [6,7].
Nuclear factor-kappa B (NF-κB) is a key transcription factor that has been most extensively studied for its universal existence and functional diversity. It can induce the expression of a large number of inflammatory mediators [8,9]. Guo and co-workers have been reported that NF-κB played a vital role in the pathogenesis of IBD, and the degree of activated NF-κB was significantly correlated with the severity of inflammation of the intestine and the colon [10]. The NF-κB family consists of several proteins, p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1) and p100/52 (NF-κB2). These proteins can form homo- and heterodimeric complexes by associating each other with transcriptionally regulate target genes [11,12]. The signaling of NF-κB is normally suppressed by a family of inhibitory molecules termed IκB proteins, such as IκBα which binds to NF-κB to prevent its activation in the cytoplasm [13].
Current pharmaceutical treatment of IBD including anti-inflammatory agents and immunomodulators are available, but the limitations in efficacy and safety remains unsolved [2]. Portulaca oleracea L. (POL) is a traditional Chinese medicine and used to treated IBD in clinical [14]. It is a warm-climate annual plant and has a cosmopolitan distribution [15]. The plant is a rich source of antioxidants, omega-3 fatty acids, polysaccharide, alkaloids and various kinds of flavonoids [16]. In many countries in the world, it is an edible vegetable and a wide range of pharmacological effects, such as anti-inflammatory, antibacterial, analgesic and wound-healing activities have been reported [17,18]. Due to its multiple medicinal usages, POL is listed by the World Health Organization as one of the most used medicinal plants [19].
In our previous work, Polysaccharide from POL (POLP) was extracted by traditional process combined with membrane separation technology [20]. And it was found to be effective in protecting mice against experimentally induced colitis by dextran sulfate sodium (DSS) and the inflammatory mediators TNF-α was found to have changed in the group with POLP treatment. Furthermore, TNF-α is closely related to NF-κB. After combining this evidence, we hypothesized that the protective effect of POLP against DSS induced colitis was compactly correlated with NF-κB.
Materials and methods
Preparation of polysaccharide from Portulacae Oleracea L.
The polysaccharide was extracted from Portulacae Oleracea L. via traditional process combining with membrane separation technology reported in our previous work.
DSS mouse models
The experiment animals enrolled in our work were all male Kung ming (KM) mice weighting 20±2 g and purchased from Laboratory Animal Center of Xi’an Jiao Tong University Health Science Center. Animals were maintained under standard laboratory conditions and were given autoclaved ultra-filtered water and animal feed ad libitum. Animal handling and scoring of colitis were performed in a consequently blinded experimental design.
DSS (40,000 Da) was purchased from MP biomedicals (USA) and dissolved in distilled water. Colitis was induced by providing drinking water containing 5% DSS for 5 to 7 days. Control mice received distilled water. Positive control mice were provided sulfasalazine with concentration of 9 mg/mL through intragastrical administration for 7 days [5]. The other three groups were given 0.5 mL POLP twice a day with concentrations of 0.75 g/mL, 0.5 g/mL and 0.25 g/mL for 7 days.
Clinical activity score
A clinical activity score involving animals’ body weight loss, stool consistency and rectal bleeding was recorded daily during the experiment as described previously [21]. The total clinical score was ranged from 0.0 (healthy) to 4.0 (maximal activity of colitis).
Cytokine assays
The experimental animals were sacrificed after 14 days and eyeballs were excised to take blood. Subsequently, the blood was centrifuged to test the levels of IL-8, IL-10, IL-1β and TNF-α using Elisa kits according to the manufacturer’s instructions. The Elisa kit of TNF-α was obtained from Neobioscience (China; Batch Code, 201701). The Elisa kit of IL-8 was purchased from Nanjing Jiancheng Bioengineering Institute (China; Batch Code, 20160323). The Elisa kits of IL-1β and IL-10 were purchased from Suzhou Calvin Biological Science and Technology Co. (China; Batch Code, 201611).
Histopathological evaluation
The colon was spread onto a plastic sheet, fixed with 10% neutral-buffered formalin for 48 h, and embedded in paraffin block. Sections of paraffin-embedded tissues were subjected to hematoxylin & eosin (H & E) staining for measuring the severity of inflammation. Histologic scoring was performed by a pathologist. For infiltration of inflammatory cells, rare inflammatory cells in the lamina propria were counted as 0; increased numbers of inflammatory cells in the lamina propria as 1; confluence of inflammatory cells, extending into the submucosa as 2; and a score of 3 was given for transmural extension of the infiltrate. For tissue damage, no mucosal damage was counted as 0, discrete lymphoepithelial lesions were counted as 1, surface mucosal erosion was counted as 2, and a score of 3 was given for extensive mucosal damage and extension through deeper structures of the bowel wall. The combined histologic score ranged from 0 (no changes) to 6 (extensive cell infiltration and tissue damage).
Immunohistochemistry
Tissues sections for immunohistochemistry were prepared from formalin fixed, paraffin-embedded colon tissue. Stains against NF-κBp65 (CST, #8242S) was performed according to the kit protocol (KGOS60, KeyGEN, Nanjing, China). The slides were briefly deparaffinized. Antigen unmasking was carried out by incubation in 100°C water bath in 10 mM sodium citrate buffer with 0.1% Tween 20 for 20 min. Slides were incubated with primary antibodies. HRP Polymer were added and incubated at room temperature for 30 min. And the sections were stained with DAB substrate and counterstained with hematoxylin after 40 min. Image Pro Plus 6.0 software (Media Cybernetics, Silver Spring, USA) was used for automatic counting of labeled inflammatory cytokines to quantify the experimental data.
RT-qPCR
Total RNAs from colon tissues were extracted using RNA-isolation kit (Takara, Japan). The purity of RNA obtained was evaluated by the calculated A260/A280 (1.9-2.0). GAPDH was used as internal loading controls for mRNA, 5’-TCAATGGCTACTCAGGACCA-3’ and 5’-CGGCAGTCCTTTCCTACAAG-3’ for NF-κBp65, 5’-GCACCGTCAAGCTGAGAAC-3’ and 5’-TGGTGAAGACGCCAGTGGA-3’ for GAPDH. qPCR reactions were run in triplicate and relative expression levels of miRNA or mRNA were analyzed using the Bio-Rad C1000 Thermal Cycler (Bio-Rad, CA, USA).
Western blot analysis
Tissues sections were grinded to homogenate in homogenizer and washed with ice-cold phosphate-buffered saline (PBS) and lysed using RIPA buffer supplemented with protease and phosphatase inhibitor mixtures (Heart Biological Technology Co., Xi’an, China) on ice. Lysates were separated by centrifugation at 4°C and 14,000 g for 10 min. Protein concentration was determined by BCA assay (Pierce, CO, USA). 50 mg total protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (Millipore, Bedford, MA, USA). After blocking with 5% non-fat milk, the membranes were incubated with rabbit mAbs specific for NF-κBp65 (CST, #8242S), IκBα (CST, #4812S), Bcl-2 (CST, #D17C4) and Survivin (71G4B7) overnight at 4°C, next day the membranes were incubated with horseradish peroxidase conjugated secondary antibodies (1:3000) 37°C for 1 h. Then immunoreactive proteins were visualized using ECL Western blotting detection reagent (Millipore, Billerica, MA, USA) and detected using Multi Image Light Cabinet Filter Positions (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Student’s t-test was performed to compare the values of the test and control samples. All experiments were repeated at least three times. The data are presented as the mean ± standard deviation (SD). SPSS 19.0 software was used for all statistical analysis. *P<0.05 was considered statistically significant.
Results
General observations on the colitis mice
To evaluate the health conditions of the experimental animals, Clinical activity score (CAS) was employed in our work. In addition, the CAS reflected the animals’ body weight loss, stool consistency and rectal bleeding. The results of Figure 1 showed the activity scores of each experiment group during 14 days. The line chart of the colitis group indicated that the colitis model was successful comparing with the CAS of control group. After treatment for 7 days, both the sulfasalazine (positive control) group and the three POLP groups had the CAS sharply decreased, indicating therapeutic effects of sulfasalazine and POLP. In summary, the results of CAS suggested that POLP could diminish the weight loss and improve the health conditions of the experimentally diseased mice. And the therapeutic effect of 0.75 g/ml of POLP was almost equal to that of the sulfasalazine control group.
Figure 1.
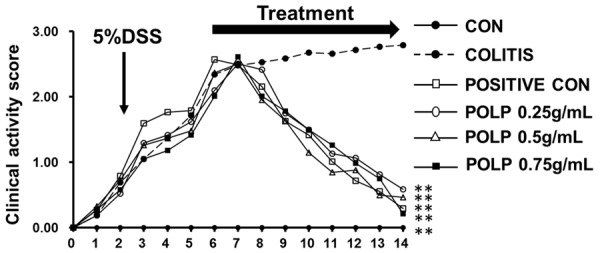
Clinical activity score (CAS) of mice. After colitis induction, the animals were treated for 7 days with distilled water, DSS, sulfasalazine (“positive con”) and three concentrations of POLP. The untreated showed no significant improvement after 14 days, while treatment with sulfasalazine and three concentrations of POLP has significantly improved the CAS of colitis mice. n=6, **P<0.01 compared with colitis.
POLP decreased the levels of IL-6 and TNF-α
Inflammatory cytokines always exert significant roles in keeping humans healthy. And the imbalances of pro-inflammatory and anti-inflammatory cytokines are closely related with the occurrence and progression of colitis. In our experiment, four inflammatory cytokines, including three pro-inflammatory cytokines IL-8, IL-1β and TNF-α and one anti-inflammatory cytokine IL-10 were investigated. (Figure 2) And the results exhibited that the levels of pro-inflammatory cytokines IL-1β, IL-8 and TNF-α were all increased in colitis comparing with the negative control group, indicating a close relationship with their occurrence and colitis. Contrarily, the level of anti-inflammatory cytokine IL-10 decreased (compared to the control group) in the colitis group. However, after treatment with sulfasalazine and three POLP, the level of TNF-α decreased greatly comparing with the colitis group, while the levels of IL-1β, IL-8 and IL-10 changed insignificantly. Therefore, TNF-α were chosen to elucidate the molecular mechanism of the therapeutic role of POLP.
Figure 2.
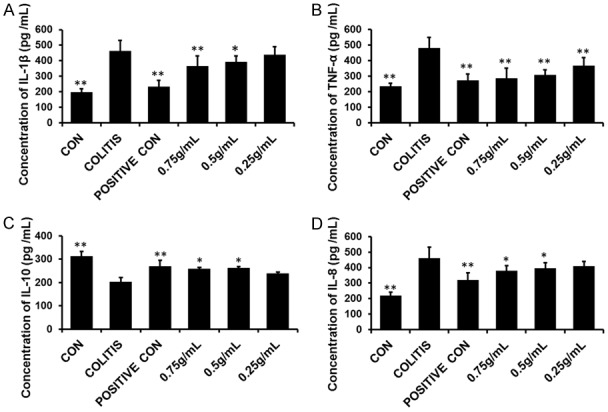
Levels of pro-inflammatory cytokines IL-1β, IL-8 and TNF-α and anti-inflammatory cytokines IL-10 in each group. A. The concentration of IL-1β in each group; B. The concentration of TNF-α in each group. After treatment with sulfasalazine and three concentration of POLP, the levels of TNF-α decreased sharply. C. The concentration of anti-inflammatory cytokines IL-10 in each group; D. The concentration of IL-8 in each group. Error bars represent means ± SEM of n=3, *P<0.05 and **P<0.01 compared with colitis.
POLP improves the morphological structure of colitis
The experiment of H & E staining was enrolled to confirm the colon damage of DSS in colitis and the therapeutic effects of POLP on the pathological structure of the colon. The results in Figure 3 exhibited the histologic characteristics of each group in our experiment. The intact colon structure in control group numbered Figure 3A, including mucosa, submucosa, inner circular muscle and outer longitudinal muscle, was clearly visible, with the crypts and goblet cells regularly arranged. The infiltration of inflammatory cells was observed in the colitis group. The crypts and goblet cells almost disappeared, revealing multiple erosive lesions and inflammatory cell infiltration composed mainly of macrophages with fewer lymphocytes. The inflammatory process was limited to the inner circular muscle. The results of Figure 3C showed the therapeutic effects of sulfasalazine. Though the structure of colon was intact, inflammatory cells were also observed. The treatment effect of POLP with concentration of 0.75 g/mL was showed in Figure 3D. This result indicated that 0.75 g/mL POLP could ameliorate the colitis condition induced by DSS comparing with colitis group. And the structure of colon was intact and the inflammatory cells were almost disappeared, indicating the satisfied therapeutic effect of POLP with concentration of 0.75 g/mL. The H & E staining result of Figure 3E illustrates the treatment effects of POLP with concentration of 0.5 g/mL. Though the colon structure was intact, the damage to colon tissues caused by inflammatory cell infiltration was not completely annihilated. The results of Figure 3F exhibited the effect of 0.25 g/mL POLP. Comparing with the results of positive control and 0.75 g/mL POLP, a lot of inflammatory cells were still observed in mucosa. The crypts and goblet cells almost disappeared. The columns in Figure 3G showed the histopathological scores of each group. The column heights of sulfasalazine control and three POLP were considerably lower than that of colitis group, indicating the good therapeutic effect.
Figure 3.
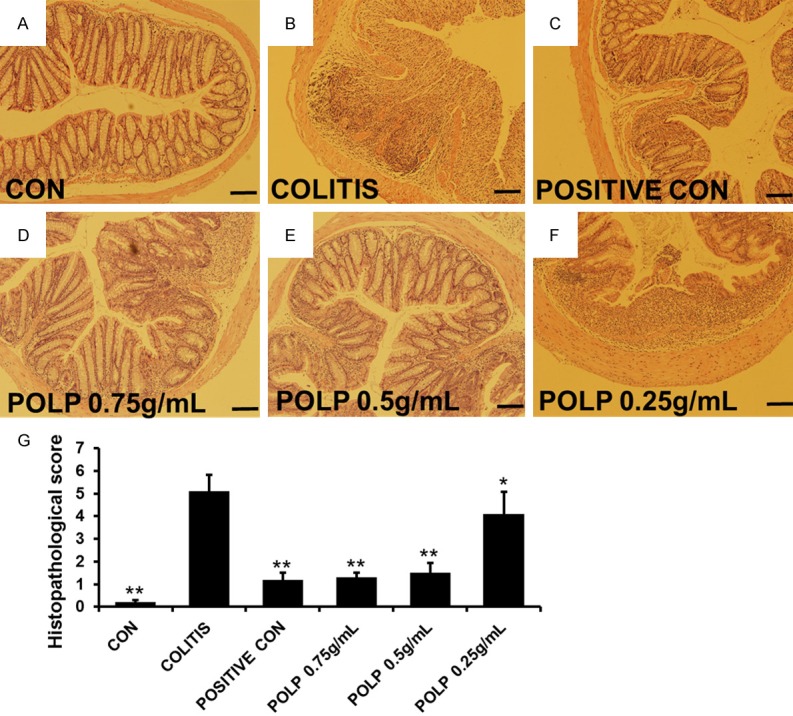
Histologic characteristics of each group, magnification ×100. A. Normal colonic mucosa with regularly formed colonic folds covered by intact mucosa. B. The histologic characteristics of colitis group. Inflammatory cell completely infiltrated to mucosa and submucosa. C. The histologic characteristics of sulfasalazine control group. D. The histologic characteristics of POLP group with a concentration of 0.75 g/mL. E. The histologic characteristics of POLP group with a concentration of 0.5 g/mL. F. The histologic characteristics of POLP group with a concentration of 0.25 g/mL. G. The sum of histological score obtained from blinded histopathological analysis. Error bars represented means ± SEM of n=3, *P<0.05 and **P<0.01 compared with colitis.
POLP inhibited the protein expression level of NF-κBp65
NF-κB is a key signaling protein that could induce the expression of a large number of inflammatory mediators and is recognized as a good target to treat some diseases with inflammation. In normal times, NF-κB is sequestered in cytoplasm. Upon activation, NF-κB translocate into nucleus and initiates transcription of genes associated with inflammation and immunity [22]. Therefore, the expression level of NF-κBp65 was detected by immunohistochemistry. The results of Figure 4 showed the protein expression level and mRNA level of NF-κBp65 were all increased considerably in the colitis group induced by DSS compared with control group, indicating the close relationship with colitis. The protein expression levels of NF-κBp65 in Figure 4C, 4D were all decreased. Furthermore, the column in Figure 4E exhibited that the height in of POLP with concentration of 0.5 g/mL was lower than that of the sulfasalazine control. To investigate the protective mechanism of POLP on colitis deeply, the mRNA levels of NF-κBp65 in each group were detected. The results in Figure 4F gave the similar changed cure compared with that in Figure 4E, indicating the close relationship of NF-κBp65 and the protective mechanism of POLP.
Figure 4.
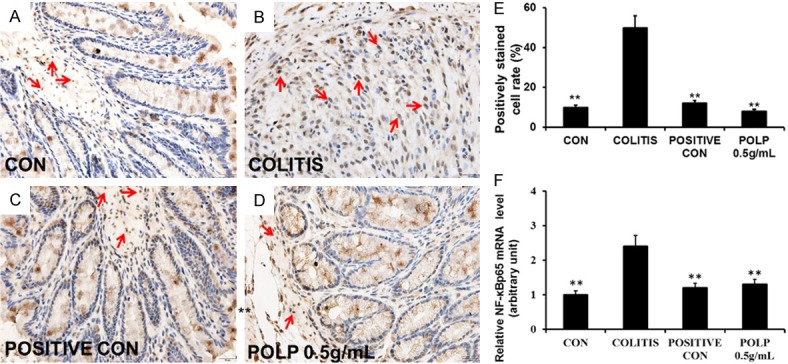
POLP inhibited the expression of NF-κBp65, magnification ×400. A. Immunohistochemistry result of control group with expressing NF-κBp65 barely. B. The expression level of NF-κBp65 increased sharply with crypts and goblet cells disappeared. C. Immunohistochemistry result of sulfasalazine control. D. Immunohistochemistry result of POLP with a concentration of 0.5 g/mL. E. The column heights represented the positively stained cell rates. F. The results of RT-qPCR. The column heights represented the mRNA level of NF-κBp65. Error bars represented means ± SEM of n=3, *P<0.05 and **P<0.01 compared with colitis.
POLP prevented NF-κBp65 transferring to nucleus and attenuated the NF-κBp65 related proteins in mice
NF-κBp65 is normally located in cytoplasm, but once NF-κBp65 becomes activated by signaling transduction, it transfers to the nucleus to execute its functions. And NF-κB is normally suppressed by IκB proteins, such as IκBα which binds to NF-κB to prevent its activation in the cytoplasm [23]. Therefore, the expression levels of NF-κBp65 in cytoplasm and nucleus always reflected the state of NF-κBp65. Additionally, the protein expression levels of NF-κBp65 and IκBα were investigated by Western blot. The results showed that the protein level of NF-κBp65 in cytoplasm was decreased comparing with the control group. After administration of sulfasalazine and POLP, the protein levels were all increased. In addition, the protein level in POLP group was higher than that of the sulfasalazine control (Supplementary Figure 1). The variation curve of NF-κBp65 concentration in the nucleus was contrary to that in the cytoplasm (Supplementary Figure 2). These results indicated that NF-κBp65 was closely related to the occurrence of colitis and POLP could regulate the protein level of NF-κBp65 to exert its therapeutic function.
The protein level of IκBα was also tested in our experiment and the results showed that the protein level of IκBα to be sharply decreased in colitis group compared to control group, indicating the activation of NF-κBp65. After treatment with sulfasalazine and POLP, the protein levels of IκBα were all much increased. And the column height of POLP group was higher than that of positive control (Supplementary Figure 3). Altogether, these results do indicate that NF-κBp65 is a key protein related to the occurrence and progression of colitis and POLP could inhibit its transfer to the nucleus via suppressing the degradation of IκBα (Figure 5).
Figure 5.
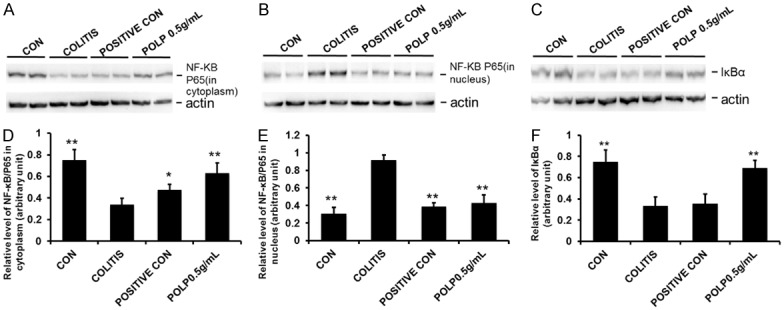
Effects of POLP on the protein levels of NF-κBp65 and IκBα. A. Results of POLP with on the protein level of NF-κBp65 in cytoplasm; B. The protein levels of NF-κBp65 in nucleus; C. Effects of POLP on the protein levels of IκBα; D. The column charts represented the relative protein level of NF-κBp65 in cytoplasm; E. The column charts represented the relative protein level of NF-κBp65 in nucleus; F. The column charts represented the relative protein level of IκBα. Error bars represented means ± SEM of n=3, *P<0.05 and **P<0.01 compared with colitis.
It is well known NF-κB is coordinated with mediators of pro-survival and proliferative signals during the chronic inflammation in colon [24]. As shown in Figure 6, it was observed that DSS increased the expression levels of NF-κB related proteins, that was, survivin and Bcl-2 family proteins whereas POLP reduced these increases in colonic tissue of DSS-induced mice (Supplementary Figures 4, 5). Altogether, these results indicated POLP inhibited NF-κB activation and that this was followed by down-regulations in the expressions of NF-κB related proteins.
Figure 6.
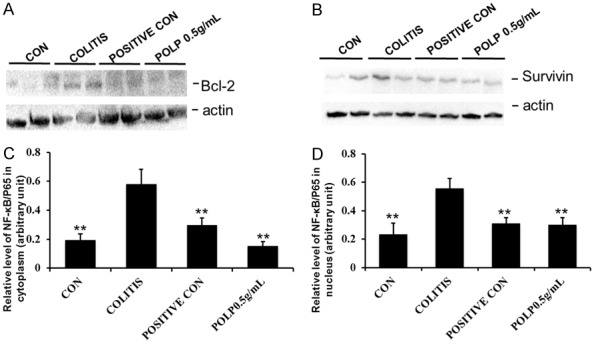
Effects of POLP on the protein levels of Bcl-2 and Survivin. A. Results of POLP with on the protein level of Bcl-2; B. The protein levels of Survivin; C. The column charts represented the relative protein level of Bcl-2; D. The column charts represented the relative protein level of Survivin. Error bars represented means ± SEM of n=3, *P<0.05 and **P<0.01 compared with colitis.
Discussion
IBD is a chronic debilitating condition that includes ulcerative colitis and Crohn’s disease, and that may eventually results in hyperplasia and neoplastic growth. The hall mark of this disease is uncontrolled inflammation which can affect any part of the gastrointestinal tract [25]. Though the pathophysiology of IBD remains unclear, it has been widely considered that the imbalance of pro-inflammatory cytokines and anti-inflammatory cytokines exert key roles in the occurrence and perpetuation of this disease. POL is a traditional Chinese medicine to treat diarrhea. And it is also an edible vegetable wildly spread in many countries. In our previous work, polysaccharide from POL was extracted and the protective effect against colitis induced by DSS was observed. However, the concrete mechanisms of this protective were elusive.
In our experiment, the pro-inflammatory cytokines IL-8, IL-1β and TNF-α and one anti-inflammatory cytokine IL-10 were detected and the results showed that the expression levels of these four cytokines were all changed, indicating the close relationship with the occurrence of colitis. In addition, after administration of POLP, the protein level of TNF-α was sharply decreased. The results of H & E staining showed that 0.75 g/ml POLP could improve the morphological structure of colitis and the inflammatory cells were almost disappeared, indicating the satisfied therapeutic effect. The protein expression level of NF-κBp65 was evaluated by immunochemistry and Western blot analysis. The results showed that the protein level of NF-κBp65 in cytoplasm was decreased in the colitis group and the level increased after administration of POLP. The protein level of NF-κBp65 in the nucleus showed reverse relation to the level in the cytoplasm. The results of RT-qPCR gave the same variation curve compared with the results of immunochemistry. These results indicated that NF-κBp65 was closely related to the occurrence of colitis and POLP could regulate the protein level of NF-κBp65 to exert its therapeutic function. Finally, the expression level of IκBα was also detected and the results showed the protein level of IκBα to be sharply decreased in colitis group compared to control group, indicating the activation of NF-κBp65. The protein level of IκBα was increased after POLP treatment, implying that POLP could inhibit the degradation of IκBα to regulate the state of NF-κBp65.
Taken together, our experiment exhibited that POLP could ameliorate the health condition of colitic mice and improve the morphological structure of colon. The concrete mechanisms were related to the activation of NF-κB and may be via inhibiting the degradation of IκBα.
Acknowledgements
The present work was supported by the Program for the National Natural Science Foundation of China (NO 81703924), Youth Talent Lifts Plan of Shaanxi Association for Science and Technology (NO 20160229).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lee C, Kim BG, Kim JH, Chun J, Im JP, Kim JS. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int Immunopharmacol. 2017;51:47–56. doi: 10.1016/j.intimp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Xi M, Wang X, Ge J, Yin D. N’-[(3-[benzyloxy] benzylidene]-3,4,5-trihydroxybenzohydrazide (1) protects mice against colitis induced by dextran sulfate sodium through inhibiting NFkappaB/IL-6/STAT3 pathway. Biochem Biophys Res Commun. 2016;477:290–296. doi: 10.1016/j.bbrc.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T, Higashitsuji H, Kashida H, Watanabe T, Komeda Y, Nagai T, Hagiwara S, Kitano M, Nishida N, Abe T, Kiyonari H, Itoh K, Fujita J, Kudo M. The oncoprotein gankyrin promotes the development of colitis-associated cancer through activation of STAT3. Oncotarget. 2017;8:24762–24776. doi: 10.18632/oncotarget.14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JH, Chung KS, Jin BR, Cheon SY, Nugroho A, Roh SS, An HJ. Anti-inflammatory effects of an ethanol extract of aster glehni via inhibition of NF-kappaB activation in mice with DSS-induced colitis. Food Funct. 2017;8:2611–2620. doi: 10.1039/c7fo00369b. [DOI] [PubMed] [Google Scholar]
- 5.Oz HS, Chen T, de Villiers WJ. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front Immunol. 2013;4:132. doi: 10.3389/fimmu.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–3503. doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YY, Ma ZB, Xu HY, Shi LJ, Li DY, Sun LY, Yin XH, Sang GY, Xu D, Tang YH, Wang X, Li P, Wu F, Zhou J. IL-6/STAT3/SOCS3 signaling pathway playing a regulatory role in ulcerative colitis carcinogenesis. Int J Clin Exp Med. 2015;8:12009–12017. [PMC free article] [PubMed] [Google Scholar]
- 8.El-Salhy M, Umezawa K. Effects of AP1 and NFkappaB inhibitors on colonic endocrine cells in rats with TNBS induced colitis. Mol Med Rep. 2016;14:1515–1522. doi: 10.3892/mmr.2016.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandurangan AK, Mohebali N, Hasanpourghadi M, Looi CY, Mustafa MR, Mohd Esa N. Boldine suppresses dextran sulfate sodium-induced mouse experimental colitis: NF-kappaB and IL-6/STAT3 as potential targets. Biofactors. 2016;42:247–258. doi: 10.1002/biof.1267. [DOI] [PubMed] [Google Scholar]
- 10.Guo T, Lin Q, Li X, Nie Y, Wang L, Shi L, Xu W, Hu T, Guo T, Luo F. Octacosanol attenuates inflammation in both RAW264.7 macrophages and a mouse model of colitis. J Agric Food Chem. 2017;65:3647–3658. doi: 10.1021/acs.jafc.6b05465. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Li Y, Chen C, Ma J, Sun W, Tian Z, Li J, Xu J, Liu CS, Zhang D, Huang C, Huang H. NF-kappaB p65 overexpression promotes bladder cancer cell migration via FBW7-mediated degradation of RhoGDIalpha protein. Neoplasia. 2017;19:672–683. doi: 10.1016/j.neo.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu GR, Mu TC, Gao ZX, Wang J, Sy MS, Li CY. Prion protein is required for tumor necrosis factor alpha (TNFalpha)-triggered nuclear factor kappa B (NF-kappaB) signaling and cytokine production. J Biol Chem. 2017 doi: 10.1074/jbc.M117.787283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Yang H, Tang C, Yao G, Kong L, He H, Zhou Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-kappaB signal in type 2 diabetic rats. Int Immunopharmacol. 2015;28:744–750. doi: 10.1016/j.intimp.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Yan Y, Li J, Tang Z, Sun J, Zhang H, Hao S, Wen A, Liu L. Protective effects of ethanol extract from Portulaca oleracea L on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am J Transl Res. 2016;8:2138–2148. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou YX, Xin HL, Rahman K, Wang SJ, Peng C, Zhang H. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. Biomed Res Int. 2015;2015:925631. doi: 10.1155/2015/925631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo M, Conte E, Naviglio D. Analysis and comparison of the antioxidant component of portulaca oleracea leaves obtained by different solid-liquid extraction techniques. Antioxidants (Basel) 2017:6. doi: 10.3390/antiox6030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Liu X, Tang G, Liu H, Zhang Y, Zhang B, Zhao X, Wang W. Ethanol extract of Portulaca Oleracea L. reduced the carbon tetrachloride induced liver injury in mice involving enhancement of NF-kappaB activity. Am J Transl Res. 2014;6:746–755. [PMC free article] [PubMed] [Google Scholar]
- 18.Jin H, Chen L, Wang S, Chao D. Portulaca oleracea extract can inhibit nodule formation of colon cancer stem cells by regulating gene expression of the Notch signal transduction pathway. Tumour Biol. 2017;39:1010428317708699. doi: 10.1177/1010428317708699. [DOI] [PubMed] [Google Scholar]
- 19.Kaveh M, Eidi A, Nemati A, Boskabady MH. Modulation of lung inflammation and immune markers in asthmatic rats treated by portulaca oleracea. Avicenna J Phytomed. 2017;7:409–416. [PMC free article] [PubMed] [Google Scholar]
- 20.Li Wang ZW, Jiao Z, Tang ZS, Sun XC, Song ZX, Huang WJ, Liu L. Extraction and isolation of polysaccharide from portulaca oleracea by traditionalprocess combined with membrane separation technology and evaluation of itsanti-oxidant activity. Chinese Traditional and Herbal Drugs. 2016;47:6. [Google Scholar]
- 21.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 22.Mu P, Akashi T, Lu F, Kishida S, Kadomatsu K. A novel nuclear complex of DRR1, F-actin and COMMD1 involved in NF-kappaB degradation and cell growth suppression in neuroblastoma. Oncogene. 2017;36:5745–5756. doi: 10.1038/onc.2017.181. [DOI] [PubMed] [Google Scholar]
- 23.Wen Y, Yu Y, Fu X. LncRNA Gm4419 contributes to OGD/R injury of cerebral microglial cells via IkappaB phosphorylation and NF-kappaB activation. Biochem Biophys Res Commun. 2017;487:923–929. doi: 10.1016/j.bbrc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami H, Tomita M, Matsuda T, Ohta T, Tanaka Y, Fujii M, Hatano M, Tokuhisa T, Mori N. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. Int J Cancer. 2005;115:967–974. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- 25.Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-inflammatory action of angiotensin 1-7 in experimental colitis. PLoS One. 2016;11:e0150861. doi: 10.1371/journal.pone.0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


