Abstract
Anesthesia and surgery (A + S) are risk factors for patients to develop postoperative delirium (POD). However, the pathogenesis of POD remains largely to be determined. We employed battery of behavioral tests including open-filed test (OFT), elevated plus maze test (EPMT) and buried food test (BFT) to investigate the role of oxidative stress in the development of POD and to explore the therapeutic target for POD in mice after A + S (simple laparotomy under 1.4% isoflurane anesthesia). We initially found that 6 hours after A + S, mice failed to alter the behavioral changes in OFT and the adenosine triphosphate (ATP) level in hippocampus. After hierarchical cluster analysis, however, there was a significant change in the behavior tests between POD unsusceptible (non-POD) and susceptible (POD-like) mice. Interestingly, cyclosporine A, an inhibitor of mitochondrial permeability transition pore (mPTP) opening, exerted pharmacologically beneficial effects on symptoms, decreased reactive oxygen species (ROS) and ATP, and increased superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) levels in the hippocampus of POD-like mice. These findings suggest that abnormally activated oxidative stress might be involved in the underlying mechanisms of POD. Novel therapeutic agents targeting inhibition of oxidative stress would provide an available strategy for POD treatment.
Keywords: Postoperative delirium, oxidative stress, cyclosporine A, anesthesia, surgery
Introduction
Delirium is an acute and fluctuating alteration of mental state of reduced awareness and disturbance of attention [1-3]. Postoperative delirium (POD) refers to the delirium that occurs after surgery, and it mainly starts in the recovery room and occurs up to 5 days after surgery [3,4]. It is currently well known that POD prolongs hospitalization, increases medical expense, and has potential to induce postoperative short-term or long-term complications, such as postoperative cognitive dysfunction (POCD) [5]. However, the vast majority of POD patients fail to gain enough attentions and appropriate effective treatments from anesthesiologists, and that the pathogeneses and molecular mechanisms underlying POD, to date, have not yet been fully determined.
A review including 25 clinical trials showed that the incidence of POD is ranged from 5.1% to 52.2% [6]. It seems that differential enrollment of individuals, evaluation scales and medical treatments might be of great relevance to the variations in the incidence of POD [7]. Although studies on the pathophysiologic and therapeutic mechanisms of delirium are mainly via animal models, to the best of our knowledge [8,9], there is currently no animal model better simulating POD. It could be easily mimicked by the anesthesia and/or surgery [10,11]. However, POD does not occur in every subject after anesthesia and/or surgery. It is therefore likely that, in current preclinical studies, false positives would be mixed in the POD group, probably resulting in a possibility that our results fail to support our conclusions.
It is well recognized that several similarities in the pathogeneses and clinical symptoms between POD and POCD, whereas there are also many differences [5,12,13]. Previous studies reported that POD mainly occurs within postoperatively 3 days, the onset of POCD, however, starts from 1 week to 2 or 3 years after surgeries [5]. Although the treatment strategies for POD and POCD are still controversial [5,14], clinical guidelines recommend that anti-schizophrenia drug haloperidol could be prescribed for POD alleviation [15,16], and that anti-Alzheimer drugs may exert pharmacologically beneficial effects on POCD symptoms [17,18]. It is therefore of particular importance to perform studies on building the POD animal models, which might provide available alternatives to investigate the underlying molecular mechanisms and therapeutic approaches of POD.
In the present study, we aimed to perform hierarchical cluster analysis of behavior tests, e.g., open-filed test (OFT), elevated plus maze test (EPMT) and buried food test (BFT), to classify the mice undergoing anesthesia and surgery (A + S) into POD susceptible (POD-like) and unsusceptible (non-POD). This was designed to effectively exclude mice that are unsusceptible to POD, and thus to avoid the impacts of false-positive factors on the results. Furthermore, increasing evidence demonstrated that abnormality in oxidative stress potentially plays a pivotal role in the pathogenesis and molecular mechanisms of neurological diseases or disorders [19,20]. cyclosporine A (CsA), an immunosuppressive agent, has been commonly reported as an inhibitor of mitochondrial permeability transition pore (mPTP) opening to suppress oxidative stress [21,22]. We therefore applied to investigate its facilitating role in the alleviation of POD, and to measure its effects on hippocampal levels of reactive oxygen species (ROS), adenosine triphosphate (ATP), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) which are highly related to the oxidative stress.
Material and methods
Animals
C57BL/6J mice (8 weeks old, male) weighing approximately 25 g with food and water ad libitum were purchased from the Experimental Animal Center of Tongji Medical College (Wuhan, China). All experimental protocols were performed in accordance with the National Institute of Health guidelines and regulations. The experimental protocols were approved by the Committee of Experimental Animals of Tongji Medical College (Wuhan, China).
Drugs and reagents
CsA (Cat. NO.: HY-B0579, MedChemExpress, Monmouth Junction, NJ, US) was dissolved in 1% DMSO. For the interventional studies, CsA (10 mg/kg) or isovolumetric saline were intraperitoneally injected to mice 30 min before the anesthesia and surgery. Compound lidocaine cream (Cat. NO.: H20063466, Ziguang pharmaceutical Co. Ltd., Beijing, China) was given to the wound for the incision pain. Isoflurane (Cat. NO.: 217170701, RWD, Shenzhen, China) was used for anesthesia in the present study.
Anesthesia and surgery
All mice were randomly assigned to either anesthesia + surgery group (A + S group) or control group. A + S group mice received 1.4% isoflurane and 100% oxygen in a transparent acrylic chamber for 15 min as our previous study [22,23]. Then the heads of mice were put into a mask to maintain 1.4% isoflurane with 100% oxygen. The concentration of isoflurane was monitored by an infrared probe (OhmedaS/5 Compact, Datex-Ohmeda, Louisville, CO). After that, simple laparotomy was performed. We used longitudinal midline incision from the xiphoid to the 0.5 centimeter proximal pubic symphysis on the skin, abdominal muscles and finally peritoneum and sutured the wound layer by layer using 5-0 vicryl thread. After the surgery, compound lidocaine cream was given to the wound for the incision pain until all experiments finished. The mice were then put back to the chamber for up to 2 hours anesthesia in total. After that, the mice were returned to their own cage with food and water ad libitum. The mice in control group were placed in a similar transparent acrylic chamber with 100% oxygen for 2 hours. The rectal temperature of all mice was maintained at 37±0.5°C use heating blanket.
Behavior tests
All mice were singly raised in controlled temperature (22±2°C), relative humidity (55±10%), 12 hours light/dark cycle for new environment one week before our study. One day before anesthesia and surgery, we measured the basic behavior of all mice, including OFT, EPMT and finally BFT. Then we performed all above behavior tests at 6, 9 and 24 hour after the anesthesia and surgery respectively. Before the behavior tests, all mice were put into the test environment for 1 hour. After every test, the mice returned to their own cage respectively. All apparatus were cleaned with 70% ethanol after removal of each mouse and we changed new gloves for the next mouse.
OFT: According to our previous study [23], each mouse was gently placed into the center of an open field chamber (40×40×40 cm) constructed of Plexiglas alone for 5 min. The movement parameters of all mice were monitored and analyzed using the EthoVision tracking system (Noldus Information Technology, Wageningen, the Netherlands) including the total distance moved (meters), times of crossing the center, the time (seconds) spent in the center of the open field and times of crossing each zone.
EPMT: The maze included four arms (every arm length: 50 cm; width: 10 cm, center region: 10*10 cm, and height from floor: 50 cm) with a cross-shape. Two arms had 3-sides opaque Perspex which was 9 cm high, while the other two were open. When the test started, mice were placed in center region and faced one open arm. All mice were evaluated in a 5 min session for the entering times of open arm. The entry was defined as 2 paws crossing the dividing line between the open arm and the center region [24].
BFT: Forty-eight hours before buried food test, each mouse was received 1 piece of butter bread. When the elevated plus maze test finished, mice were put into a clean cage with padding of 3 cm high in which we buried 1 pellet of butter bread below the padding. Its location was freely chosen and kept out of sight. The test started in the center of the cage. The latency was defined from when mice were placed in the cage to mice found the food and caught it with forepaws or teeth. When mice found the pellet within 5 min, they were permitted to eat up the pellet and then would be taken back to their own cage. But if they couldn’t find the pellet, they would be taken back when up to 5 min and the latency was recorded as 300 s [23].
Hippocampal levels of oxidative cytokines
Mice were killed with CO2 asphyxiation. Next we removed the brains immediately and harvested the hippocampus at 4°C. Then the hippocampus was homogenized using RIPA lysis buffer (20 mM Tris, pH 8.0, 137 mM NaCl, 0.5 mM EDTA, 1% Nonidet P-40, 10% glycerol, 10 mM Na2P2O7, 10 mM NaF, 1 g/ml aprotinin, 10 ng/ml leupeptin, 1 mM vanadate, and 1 mM PMSF) at 4°C for 30 min. The lysates were collected and centrifuged at 12,000 rpm for 5 min at 4°C. The supernatant was quantified for total proteins by a BCA assay kit (Boster, Wuhan, China). Mouse ROS (MDL biotech, Beijing, China), ATP (Unionhonest, Shanghai, China), SOD (Xinfan, Nanjing, China), GSH-Px (Jiancheng, Nanjing, China) and CAT (MDL biotech, Beijing, China) enzyme linked immunosorbent assay (ELISA) kits were used to measure their levels in hippocampus of mice following the protocols provided by the manufacturers.
Statistical analysis
The data show as the mean ± standard error of the mean (SEM). Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). Comparisons among groups were performed using the one-way analysis of variance (ANOVA) or two-way ANOVA, followed by post-hoc Tukey test. In Hierarchical cluster analysis, the data were firstly standardized by z scores. Then, hierarchical cluster analysis of OFT, EPMT and BFT was performed using Ward’s method and applying squared Euclidean distance as the distance measure, and mice were classified as POD susceptible, unsusceptible or undetermined clusters. The P-values of less than 0.05 were considered statistically significant.
Results
Effects of A + S on center cross, center duration, zone crossing and total distance of mice in the OFT
OFT is a classical behavior test to show the fear of novel surroundings in rodents [23]. Currently, it has been used to indirectly evaluate the severity of POD [22]. We here showed that center cross, center duration and total distance were not significantly changed after A + S. Interestingly, 6 and 9 hours after intervention, A + S mice showed a significant change in zone crossing (Figure 1).
Figure 1.
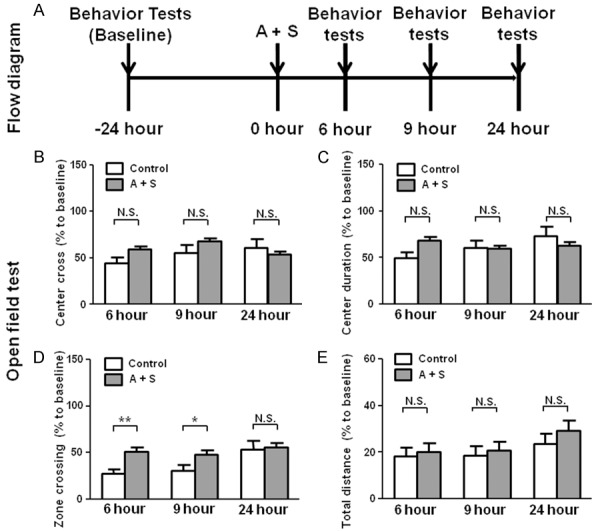
The flow diagram and OFT results in the control and A + S groups. A: The flow diagram. Behavior tests, including open filed test, evaluated plus maze test and buried food test were performed 24 hours (baseline) before and 6, 9 and 24 hours after A + S, respectively. B: Center cross (Two-way ANOVA. Time: F2,44 = 10.87, P < 0.01; Group: F1,44 = 1.043, P = 0.3126; Interaction: F2,44 = 15.27, P < 0.01). C: Center duration (Time: F2,44 = 13.64, P < 0.05; Group: F1,44 = 0.1048, P = 0.7477; Interaction: F2,44 = 29.21, P < 0.001). D: Zone crossing (Time: F2,44 = 24.95, P < 0.001; Group: F1,44 = 3.792, P = 0.0479; Interaction: F2,44 = 9.685, P < 0.01). E: Total distance: (Time: F2,44 = 7.057, P < 0.01; Group: F1,44 = 0.2497, P = 0.6198; Interaction: F2,44 = 0.4298, P = 0.652). ANOVA: analysis of variance; A + S: anesthesia and surgery; CsA: cyclosporine A; N.S.: not significant; OFT: open field test. Data are shown as mean ± S.E.M. (n = 14 or 46). *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of A + S on open-arm entry and total distance of mice in the EPMT
In the EPMT, we also found no significant changes in total distance between control and A + S groups at three time points after intervention. Furthermore, compared with control group, mice in A + S group significantly changed the open-arm entry 6 and 9 hours after intervention. However, the effects of A + S on open-arm entry disappeared after 24 hours (Figure 2).
Figure 2.
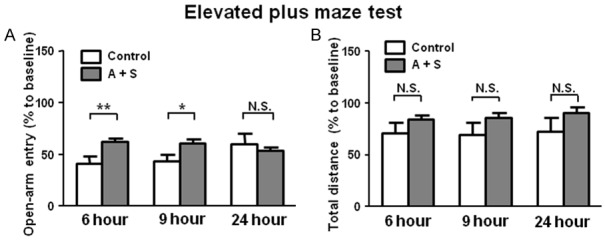
EPMT results in the control and A + S groups. A: Open-arm entry (Two-way ANOVA. Time: F2,44 = 1.254, P = 0.293; Group: F1,44 = 5.366, P < 0.05; Interaction: F2,44 = 8.647, P < 0.001). B: Total distance (Time: F2,44 = 5.31, P < 0.01; Group: F2,44 = 2.479, P = 0.1226; Interaction: F2,44 = 1.408, P = 0.25). ANOVA: analysis of variance; A + S: anesthesia and surgery; EPMT: elevated plus maze test; N.S.: not significant. Data are shown as mean ± S.E.M. (n = 14 or 46). *P < 0.05, ***P < 0.001.
Latency to eat food in the BFT after A + S intervention
In this study, we detected the latency to eat food in BFT and found a significant increase in A + S group than those in control group. The effects of A + S on latency to eat food in the BFT disappeared after 24 hours (Figure 3).
Figure 3.
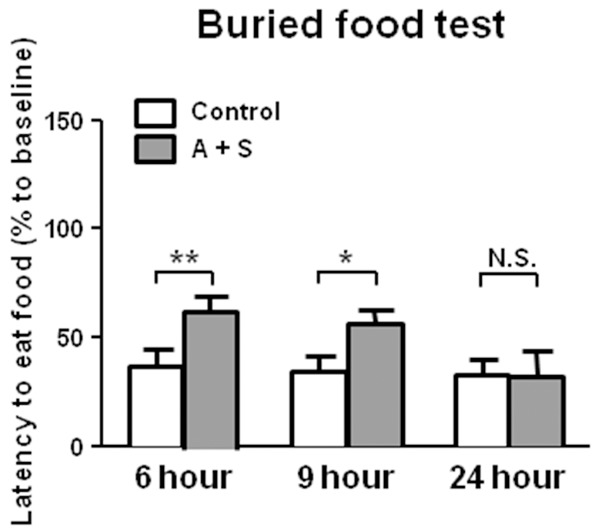
BFT results in the control and A + S groups. Latency to eat food (Two-way ANOVA. Time: F2,44 = 1.334, P = 0.2686; Group: F1,44 = 11.14, P < 0.001; Interaction: F2,44 = 1.805, P = 0.1706). ANOVA: analysis of variance; A + S: anesthesia and surgery; BFT: buried food test; N.S.: not significant. Data are shown as mean ± S.E.M. (n = 14 or 46). *P < 0.05, **P < 0.01.
Hippocampal levels of ROS, ATP, SOD, GSH-Px and CAT after A + S intervention
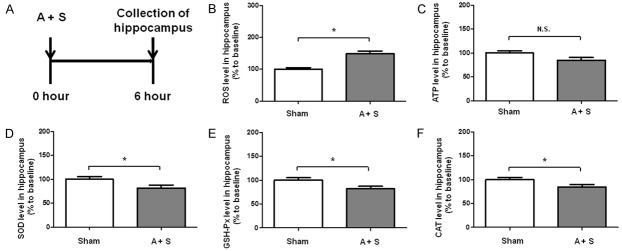
A + S mice significantly increased the level of ROS in the hippocampus. As to the hippocampal level of ATP, mice in the A + S group, however, failed to induce a significant change as compared with that of control mice. SOD, GSH-Px and CAT levels were significantly decreased in the hippocampus of A + S mice than those in control group (Figure 4).
Figure 4.
Hippocampal levels of ROS, ATP, SOD, GSH-Px and CAT in the control and A + S groups. A: The flow diagram. B: ROS level in hippocampus (t = 2.59, P < 0.05); C: ATP level in hippocampus (t = 1.242, P = 0.2194); D: SOD level in hippocampus (t = 2.163, P = 0.0371); E: GSH-Px level in hippocampus (t = 2.489, P = 0.0173); F: CAT level in hippocampus (t = 2.125, P = 0.0379). A + S: anesthesia and surgery; ATP: adenosine triphosphate; CAT: catalase; GSH-Px: glutathione peroxidase; N.S.: not significant; ROS: reactive oxygen species; SOD: superoxide dismutase. Data are shown as mean ± S.E.M. (n = 14 or 46). *P < 0.05.
Comparisons of behavior tests in control, POD unsusceptible and susceptible groups
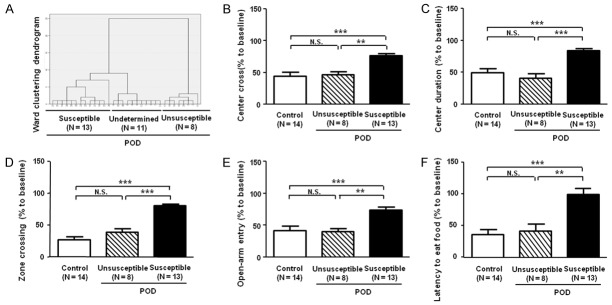
Most of previous studies simply mimicked POD or POCD animal model after anesthesia and/or surgery, instead of classification of susceptible or unsusceptible phenotype to POD or POCD [25,26]. Here we applied hierarchical cluster analysis (Ward’s method) to classify the A + S mice into three clusters: POD susceptible, unsusceptible and undetermined mice. In order to distinguish between the POD susceptible and unsusceptible, the results in the OFT, EPMT and BFT were standardized by z scores and then analyzed by two-way ANOVA. Then, 13 of 32 (approximately 40%) mice showing POD-like phenotypes were regarded as “POD susceptible” and 11 mice displaying non-POD-like phenotypes were regarded as “POD unsusceptible”. However, the others (8 mice) were regarded as “POD undetermined”.
Next, we investigated the differential results of behavior tests in control, POD unsusceptible and susceptible groups. We found that there are no significant differences in center cross, center duration, zone crossing, open-arm entry and latency to eat food between POD unsusceptible and control mice. Moreover, our results revealed that center cross, center duration, zone crossing and open-arm entry were significantly changed in POD susceptible mice than those in control or unsusceptible group. Similarly, latency to eat food showed a significant increase in POD susceptible mice as compared with that of control or POD unsusceptible group (Figure 5).
Figure 5.
Comparisons of behavior tests in the control, POD unsusceptible and susceptible groups. A: Dendrogram of hierarchical clustering analysis. A total of 32 mice after A + S treatment were divided into POD susceptible, unsusceptible and undetermined groups by the results of behavior tests of hierarchical clustering analysis. B: Center cross (One-way ANOVA. F2,31 = 12.74, P < 0.001). C: Center duration (F2,31 = 16.22, P < 0.001). D: Zone crossing (F2,31 = 47.42, P < 0.001). E: Open-arm entry (F2,31 = 11.78, P < 0.001). F: Lantebcy to eat food (F2,31 = 11.17, P < 0.001). ANOVA: analysis of variance; N.S.: not significant; POD: postoperative delirium. Data are shown as mean ± S.E.M. (n = 8~14). **P < 0.01, ***P < 0.001.
Effects of CsA on behavior tests in A + S mice
CsA, an immunosuppressant agent, has been commonly reported to be effective in inhibiting oxidative stress by the improving impaired mitochondrion function [21,22,27]. In this study, we investigated the behavior tests consisting of OFT, EPMT and BFT, and found that CsA exerts substantially improving actions on these abnormal behaviors in A + S mice. These findings suggest that mechanisms underlying POD are probably associated with abnormally activated oxidative stress. On the contrary, therapeutic strategy inhibiting oxidative stress may produce beneficial effects on POD treatment. This implies that inhibition of oxidative stress would to be a promising therapeutic target for POD (Figure 6).
Figure 6.
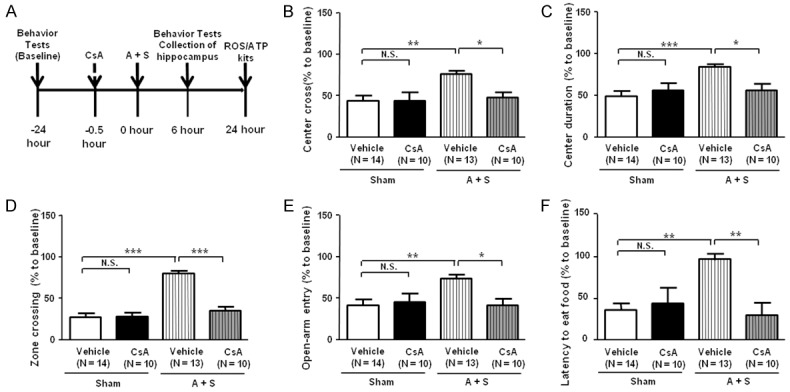
Effects of CsA on behavior tests in A + S mice. A: The flow diagram. Behavior tests, including open filed test, evaluated plus maze test and buried food test. B: Center corss (Two-way ANOVA. Drug: F1,43 = 4.689, P < 0.05; Group: F1,43 = 7.604, P < 0.01; Interaction: F1,43 = 4.789, P < 0.05). C: Center duration (Drug: F1,43 = 4.226, P = 0.0459; Group: F1,43 = 9.925, P < 0.01; Interaction: F1,43 = 6.471, P < 0.05). D: Zone crossing (Drug: F1,43 = 26.03, P < 0.001; Group: F1,43 = 48.11, P < 0.01; Interaction: F1,43 = 29.2, P < 0.01). E: Open-arm entry (Drug: F1,43 = 4.51, P < 0.01; Group: F1,43 = 4.5, P < 0.01; Interaction: F1,43 = 5.551, P < 0.01). F: Latency to eat food (Drug: F1,43 = 9.215, P < 0.01; Group: F1,43 = 7.151, P < 0.05; Interaction: F1,43 = 5.024, P < 0.05). ANOVA: analysis of variance; A + S: anesthesia and surgery; CsA: cyclosporine A; N.S.: not significant. Data are shown as mean ± S.E.M. (n = 8~14). **P < 0.01, ***P < 0.001.
Effects of CsA on hippocampal levels of ROS, ATP SOD, GSH-Px and CAT in A + S mice
ROS is a series of reactive oxygen species produced by aerobic cells during the metabolic process [28,29]. Similarly, ATP is made up of adenine, ribose and 3 phosphoric acid perssads, releasing energy when hydrolyzed, which is the also most direct source of energy in the organism [30]. We found in the present study that there were no changes in the hippocampal levels of ROS and ATP between vehicle and CsA administered in control mice. Additionally, A + S mice markedly up-regulated the ROS level, while significantly down-regulated the ATP level in the hippocampus. In contrast, CsA significantly attenuated the increased ROS level and the decreased ATP level in the hippocampus of A + S mice.
Anti-oxidative stress biomarkers, including SOD, GSH-Px and CAT, were significantly decreased in the hippocampus of vehicle-treated A + S mice, while CsA administration significantly attenuated the decreased levels of SOD, GSH-Px and CAT (Figure 7).
Figure 7.
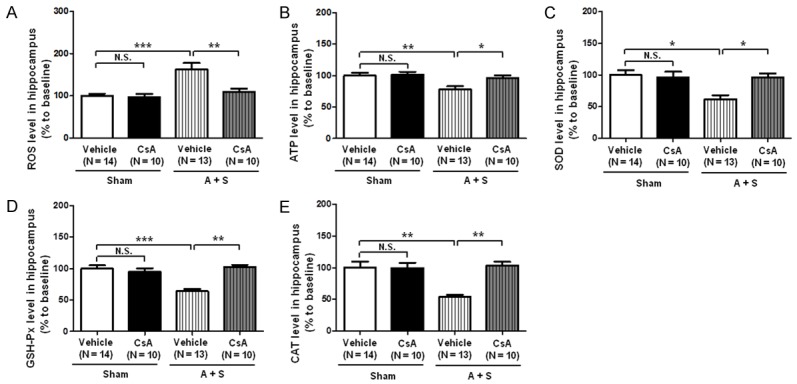
Effects of CsA on levels of ROS and ATP in the hippocampus of A + S mice. A: Hippocampal ROS level (Two-way ANOVA. Drug: F1,33 = 7.773, P < 0.01; Group: F1,33 = 14.38, P < 0.01; Interaction: F1,33 = 6.386, P < 0.05); B: Hippocampal ATP level (Drug: F1,43 = 4.662, P < 0.05; Group: F1,43 = 8.78, P < 0.01; Interaction: F1,43 = 3.119, P = 0.086). C: Hippocampal SOD level (Drug: F1,43 = 4.721, P < 0.01; Group: F1,43 = 7.258, P < 0.01; Interaction: F1,43 = 7.207, P < 0.01); D: Hippocampal GSH-Px level (Drug: F1,43 = 9.672, P < 0.01; Group: F1,43 = 13.43, P < 0.001; Interaction: F1,43 = 22.32, P < 0.0001); E: Hippocampal CAT level (Drug: F1,43 = 8.488, P < 0.01; Group: F1,43 = 11.84, P < 0.01; Interaction: F1,43 = 12.23, P < 0.01). ANOVA: analysis of variance; A + S: anesthesia and surgery; CAT: catalase; CsA: cyclosporine A; GSH-Px: glutathione peroxidase; N.S.: not significant; SOD: superoxide dismutase. Data are shown as mean ± S.E.M. (n = 8~14). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The major findings of the present study are as follows: Firstly, not all the subjects undergoing anesthesia and surgery suffer from POD. Secondly, oxidative stress is of greatly importance to the pathogenesis and therapeutic mechanisms of POD. These findings suggest that false-positive subjects exist in the intervention group that would greatly cause errors in the experiment results and adversely impact the conclusions, and that oxidative stress-lowing agents could alleviate POD symptoms.
To date, the study of POD mainly depends on behavioral results [9,22], and we found that behavioral results are not stable and fluctuate greatly. A large number of studies and our experience in clinical practice suggested that the incidence of POD fluctuates greatly, as mentioned previously, approximately 5.1% to 52.2% [6,31,32]. In this study, we observed that in the OFT although the mice experienced a slighter increase in the center duration after anesthesia and surgery, there was no significant difference. Interestingly, after hierarchical cluster analysis, we found a significant difference in OFT between POD susceptible and unsusceptible mice. However, most previous studies did not distinguish POD susceptible and unsusceptible subjects. To the best of our knowledge, this is the first report that the false positive factors were excluded from the POD group by hierarchical cluster analysis.
Abnormal alterations in oxidative stress system play an important role in neurological diseases [19,20]. A clinical study by Karlidag et al. [33] reported that there are no significant changes in plasma levels of oxidative stress biomarkers including superoxide dismutase (SOD) and malondialdehyde (MDA) between delirium and non-delirium individuals preoperatively. Post-operative plasma levels of SOD and MDA, however, were up-regulated in delirium group, but not in non-delirium group, as compared with those in the preoperative period. Collectively, these findings suggest that excessively activated oxidative stress is likely to be involved in the onset of POD. In this study, the serum oxidative stress biomarker ROS was detected, the results in the subjects undergoing anesthesia and surgery showed a significant increase. Interestingly, we further divided the mice in anesthesia and surgery group into POD susceptible (POD-like), unsusceptible (non-POD) and undetermined phenotype via hierarchical cluster analysis, and that ROS showed a significant increase in POD-like phenotype mice than that of non-POD. As to the serum level of ATP, we failed to observe a significant change between the two groups (Control and A + S groups). While these data showed significant differences after hierarchical clustering analysis. Additionally, SOD, GSH-Px and CAT were significantly decreased in the hippocampus of A + S mice. These findings suggest that oxidative and anti-oxidative responses imbalance in hippocampus is probably involved in the underlying mechanisms of delirium-like behaviors in mice after A + S.
It is commonly recognized that CsA plays a significant role in the organ transplantation since it is clinically used as an immunosuppressant [21,22,27]. Recently, studies suggested that CsA has pharmacologically therapeutic effects to treat neurodegenerative diseases [34,35]. Kumar et al. [35] demonstrated that cyclosporine A (2.5, 5 and 10 mg/kg, p.o.) significantly attenuated 3-nitropropionic acid (3-NA)-induced cognitive dysfunctions by restoration of glutathione level and acetylcholinesterase activity, indicating that the inhibition of oxidative stress would be implicated in the improving effects of CsA on 3-NA induced neurodegenerative diseases. We found in this study that CsA alleviated behavioral abnormalities in mice after A + S. More importantly, CsA markedly restored up-regulated ROS and downregulated ATP, SOD, GSH-Px and CAT levels in the hippocampus of A + S mice. Altogether, our findings indicate that inhibiting oxidative stress might be associated with the therapeutic effects of CsA on POD. Further detailed studies on the favorable actions of CsA on POD and the role of oxidative stress in the pathogenesis and molecular mechanisms of POD are extremely required.
POD, as described previously, has a higher incidence in the elderly patients [36-38]. It was reported that the incidence of POD in the elderly patients undergoing large surgeries, e.g., abdominal surgery and cardiac surgery, is up to approximately 50% [3,38]. In this study, we did not adopt geriatric mice because our preliminary study found that the survival rate was slightly reduced after anesthesia and surgery in the elderly mice with an age of 18 months. Additionally, although the fact that aging is a key inducing factor for POD, we suggested using the non-elderly rodents to study POD mechanisms since this protocol only focused on the effects of anesthesia and surgery on POD, and most importantly, avoided aging-related confounding factors, at least partially, that has already existed before experiments. Furthermore, although mounting studies suggested that POD is different from POCD [5,12,13], there was less evidence on the differences in molecular mechanisms. We will explore the topic further by using hierarchical cluster analysis.
In conclusion, hierarchical cluster analysis could be effectively applied to distinguish POD-like from non-POD phenotype mice, thus it would be better to investigate the pathogenic and therapeutic mechanisms of POD. Moreover, oxidative stress might underlie the molecular mechanisms of POD and that CsA has pharmacologically beneficial effects on POD symptoms probably by inhibiting oxidative stress. Subsequent large-scale studies on the underlying mechanisms of POD are greatly needed.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No.: 81500931, 81571047, 81771159 and 81703482) and Foundation of Hubei province (No. 2017CFB171).
Disclosure of conflict of interest
None.
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456–1466. doi: 10.1056/NEJMcp1605501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldecoa C, Bettelli G, Bilotta F. European society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214. doi: 10.1097/EJA.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 4.Kassie GM, Nguyen TA, Kalisch Ellett LM, Pratt NL, Roughead EE. Preoperative medication use and postoperative delirium: a systematic review. BMC Geriatr. 2017;17:298. doi: 10.1186/s12877-017-0695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. doi: 10.3389/fnagi.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54:1578–1589. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 7.Bilotta F, Lauretta MP, Borozdina A, Mizikov VM, Rosa G. Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol. 2013;79:1066–1076. [PubMed] [Google Scholar]
- 8.Long LS, Wolpaw JT, Leung JM. Sensitivity and specificity of the animal fluency test for predicting postoperative delirium. Can J Anaesth. 2015;62:603–608. doi: 10.1007/s12630-014-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner LA. Postoperative delirium. Part 1: pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28:628–636. doi: 10.1097/EJA.0b013e328349b7f5. [DOI] [PubMed] [Google Scholar]
- 10.Acharya NK, Goldwaser EL, Forsberg MM, Godsey GA, Johnson CA, Sarkar A, DeMarshall C, Kosciuk MC, Dash JM, Hale CP, Leonard DM, Appelt DM, Nagele RG. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res. 2015;1620:29–41. doi: 10.1016/j.brainres.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 11.Steinmetz J, Rasmussen LS. Peri-operative cognitive dysfunction and protection. Anaes-thesia. 2016;71:58–63. doi: 10.1111/anae.13308. [DOI] [PubMed] [Google Scholar]
- 12.Krenk L, Rasmussen LS. Postoperative delirium and postoperative cognitive dysfunction in the elderly-what are the differences? Minerva Anestesiol. 2011;77:742–749. [PubMed] [Google Scholar]
- 13.Jildenstal PK, Rawal N, Hallen JL, Berggren L, Jakobsson JG. Perioperative management in order to minimise postoperative delirium and postoperative cognitive dysfunction: results from a Swedish web-based survey. Ann Med Surg (Lond) 2014;3:100–107. doi: 10.1016/j.amsu.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17:376–381. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirota T, Kishi T. Prophylactic antipsychotic use for postoperative delirium: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74:e1136–1144. doi: 10.4088/JCP.13r08512. [DOI] [PubMed] [Google Scholar]
- 16.Javedan H, Tulebaev S. Management of common postoperative complications: delirium. Clin Geriatr Med. 2014;30:271–278. doi: 10.1016/j.cger.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Jungwirth B, Zieglgansberger W, Kochs E, Rammes G. Anesthesia and postoperative cognitive dysfunction (POCD) Mini Rev Med Chem. 2009;9:1568–1579. doi: 10.2174/138955709791012229. [DOI] [PubMed] [Google Scholar]
- 18.Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111:119–125. doi: 10.3238/arztebl.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci. 2016;37:768–778. doi: 10.1016/j.tips.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Yu S, Zheng Y, Yang H, Zhang J. Oxidative modification and its implications for theneurodegeneration of parkinson’s disease. Mol Neurobiol. 2017;54:1404–1418. doi: 10.1007/s12035-016-9743-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Dong Y, Xu Z, Xie Z. Propofol and magnesium attenuate isoflurane-induced caspase-3 activation via inhibiting mitochondrial permeability transition pore. Med Gas Res. 2012;2:20. doi: 10.1186/2045-9912-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng M, Zhang C, Dong Y, Zhang Y, Nakazawa H, Kaneki M, Zheng H, Shen Y, Marcantonio ER, Xie Z. Battery of behavioral tests in mice to study postoperative delirium. Sci Rep. 2016;6:29874. doi: 10.1038/srep29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Q, Peng M, Dong Y, Zhang Y, Chen M, Yin N, Marcantonio ER, Xie Z. Surgery plus anesthesia induces loss of attention in mice. Front Cell Neurosci. 2015;9:346. doi: 10.3389/fncel.2015.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochoa-Sanchez R, Rainer Q, Comai S, Spadoni G, Bedini A, Rivara S, Fraschini F, Mor M, Tarzia G, Gobbi G. Anxiolytic effects of the melatonin MT(2) receptor partial agonist UCM765: comparison with melatonin and diazepam. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:318–325. doi: 10.1016/j.pnpbp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Chen D, Huang X, Huang L, Tang L, Jiang J, Chen L, Li S. Neuroprotective effects of HTR1A antagonist WAY-100635 on scopolamine-induced delirium in rats and underlying molecular mechanisms. BMC Neurosci. 2016;17:66. doi: 10.1186/s12868-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi H, Kawano T, Tamura T, Iwata H, Takahashi Y, Eguchi S, Yamazaki F, Kumagai N, Yoko-yama M. Postoperative pain impairs subsequent performance on a spatial memory task via effects on N-methyl-D-aspartate receptor in aged rats. Life Sci. 2013;93:986–993. doi: 10.1016/j.lfs.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai N, Zhou W, Ye LL, Chen J, Liang QN, Chang G, Chen JJ. The STAT3 inhibitor pimozide impedes cell proliferation and induces ROS generation in human osteosarcoma by suppressing catalase expression. Am J Transl Res. 2017;9:3853–3866. [PMC free article] [PubMed] [Google Scholar]
- 29.Tabassum S, Haider S, Ahmad S, Madiha S, Parveen T. Chronic choline supplementation improves cognitive and motor performance via modulating oxidative and neurochemical status in rats. Pharmacol Biochem Behav. 2017;159:90–99. doi: 10.1016/j.pbb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Engl E, Attwell D. Non-signalling energy use in the brain. J Physiol. 2015;593:3417–3429. doi: 10.1113/jphysiol.2014.282517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldroyd C, Scholz AFM, Hinchliffe RJ, McCarthy K, Hewitt J, Quinn TJ. A systematic review and meta-analysis of factors for delirium in vascular surgical patients. J Vasc Surg. 2017;66:1269–1279. doi: 10.1016/j.jvs.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 32.Liao Y, Flaherty JH, Yue J, Wang Y, Deng C, Chen L. The incidence of delirium after cardiac surgery in the elderly: protocol for a systematic review and meta-analysis. BMJ Open. 2017;7:e014726. doi: 10.1136/bmjopen-2016-014726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlidag R, Unal S, Sezer OH, Bay Karabulut A, Battaloğlu B, But A, Ozcan C. The role of oxidative stress in postoperative delirium. Gen Hosp Psychiatry. 2006;28:418–423. doi: 10.1016/j.genhosppsych.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Zhang S, Xu C, Barron A, Galiano F, Patel D, Lee YJ, Caldwell GA, Caldwell KA, Witt SN. Chemical compensation of mitochondrial phospholipid depletion in yeast and animal models of Parkinson’s disease. PLoS One. 2016;11:e0164465. doi: 10.1371/journal.pone.0164465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P, Kalonia H, Kumar A. Cyclosporine A attenuates 3-nitropropionic acid-induced Huntington-like symptoms in rats: possible nitric oxide mechanism. Int J Toxicol. 2010;29:318–325. doi: 10.1177/1091581810365568. [DOI] [PubMed] [Google Scholar]
- 36.Raats JW, van Eijsden WA, Crolla RM, Steyerberg EW, van der Laan L. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One. 2015;10:e0136071. doi: 10.1371/journal.pone.0136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouchoux C, Rippert P, Duclos A, Fassier T, Bonnefoy M, Comte B, Heitz D, Colin C, Krolak-Salmon P. Impact of a multifaceted program to prevent postoperative delirium in the elderly: the CONFUCIUS stepped wedge protocol. BMC Geriatr. 2011;11:25. doi: 10.1186/1471-2318-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]