Abstract
Inflammation is a major pathological event following ischemic stroke that contributes to secondary brain tissue damage leading to poor functional recovery. Following the initial ischemic insult, post-stroke inflammatory damage is driven by initiation of a central and peripheral innate immune response and disruption of the blood-brain barrier (BBB), both of which are triggered by the release of pro-inflammatory cytokines and infiltration of circulating immune cells. Stroke therapies are limited to early cerebral blood flow reperfusion, and whilst current strategies aim at targeting neurodegeneration and/or neuroinflammation, innovative research in the field of regenerative medicine aims at developing effective treatments that target both the acute and chronic phase of inflammation. Anti-inflammatory regenerative strategies include the use of nanoparticles and hydrogels, proposed as therapeutic agents and as a delivery vehicle for encapsulated therapeutic biological factors, anti-inflammatory drugs, stem cells, and gene therapies. Biomaterial strategies—through nanoparticles and hydrogels—enable the administration of treatments that can more effectively cross the BBB when injected systemically, can be injected directly into the brain, and can be 3D-bioprinted to create bespoke implants within the site of ischemic injury. In this review, these emerging regenerative and anti-inflammatory approaches will be discussed in relation to ischemic stroke, with a perspective on the future of stroke therapies.
Keywords: stroke, neuroinflammation, stem cells, hydrogels, nanoparticles
Introduction
Stroke is the second leading cause of death worldwide, causing 6.2 million deaths each year accounting for 12 percent of all deaths, with stroke-related illness, disability and early death set to double by 2035 (1–3). A stroke occurs due to the disruption of blood flow to the brain by a bleed (hemorrhagic stroke) (4) or a blockage (ischemic stroke), accounting for 15 and 85% of all strokes respectively. While brain tissue ischemia occurs in ischemic stroke, it remains unclear whether cerebral ischemia plays an important role during hemorrhagic stroke. In both cases however, acute insult to the brain leads to the formation of a cavity, or necrotic infarct and a cavity (5). Current therapy for ischemic stroke is limited to thrombolysis by intravenous (i.v.) administration of recombinant tissue plasminogen activator (rt-PA) given within 4.5 h of symptom onset, but is associated with unwanted effects (6), or endovascular thrombectomy to physically remove the blood clot (7). An endovascular thrombectomy can be performed as a complement to rt-PA, but like thrombolysis, it has to be carried out within hours of stroke onset and can be given to only a limited number of patients (7). Ultimately, long-term rehabilitation therapy is available to most stroke patients receiving daily sessions of motor functions, cognitive, and speech language therapies, which has proven beneficial to regain functional recovery to some extent (8).
The past decades has seen a large number of promising therapeutic approaches in pre-clinical settings, however most have failed to translate into clinical application. The reasons for these failures remain largely unknown, and the Stroke Therapy Academic Industry Roundtable (STAIR) (9) followed by STAIR meetings (10) formulated several recommendations with the hope that ongoing preclinical strategies could translate into successful therapies. One main hypothesis behind the failure of clinical trials in stroke is that current animal models are inadequate and simply do not replicate the human pathology. As a result, current therapies remain exclusively limited to thrombolysis and thrombectomy, and with an aging population and access of developing countries to western lifestyle, the clinical and socioeconomic impact of stroke and stroke-related complications is on the rise, which is further potentiated by decreased post-stroke mortality rate and patient care costs due to better rehabilitation and clinical management procedures.
Despite the aforementioned interventions, no effective treatment to promote brain tissue repair and restore brain functions after stroke exist. Regenerative medicine is an emerging paradigm in the field of stroke therapy that offers the potential to promote recovery and regeneration of damaged neurovascular tissue at previously unattainable levels. This builds on previous research into neuroinflammation intertwined with the multidisciplinary research field of regenerative medicine, utilizing biomaterials science and mechanical engineering, as well as cell and gene therapies. This review focusses on the use and limitations of anti-inflammatory regenerative medicine therapies for stroke, with specific focus on the use of nanoparticles (NPs), hydrogels, stem cells and gene-editing technologies to repair the damaged brain tissue after stroke. The use of NPs and hydrogels in particular has the potential to improve the administration of drug and cell-based therapies through a controlled release of therapeutics at appropriate doses, and therefore may enable the repurposing or revised investigation of previously ineffective therapeutics.
Inflammation in stroke
Shortly after vessel occlusion, post-ischemic inflammation begins in the vascular compartment, peaking during the first days after stroke onset (11). Post-stroke inflammation response is characterized by blood-brain barrier (BBB) disruption, infiltration of peripheral leukocytes, activation of glial cells and the release of molecules known as damage-associated molecular patterns (DAMPs) by injured and dying cells (Figure 1). Activated immune cells, triggered by DAMPs, produce inflammatory cytokines, chemokines, and other cytotoxic mediators, leading to exacerbation of cerebral ischemic injury (12). During the sub-acute phase of stroke (weeks to months after stroke onset) chronic inflammation and tissue remodeling (neurogenesis and angiogenesis) take place, although ultimately repair is limited and a fluid filled cavity develops, preventing full functional recovery (13, 14).
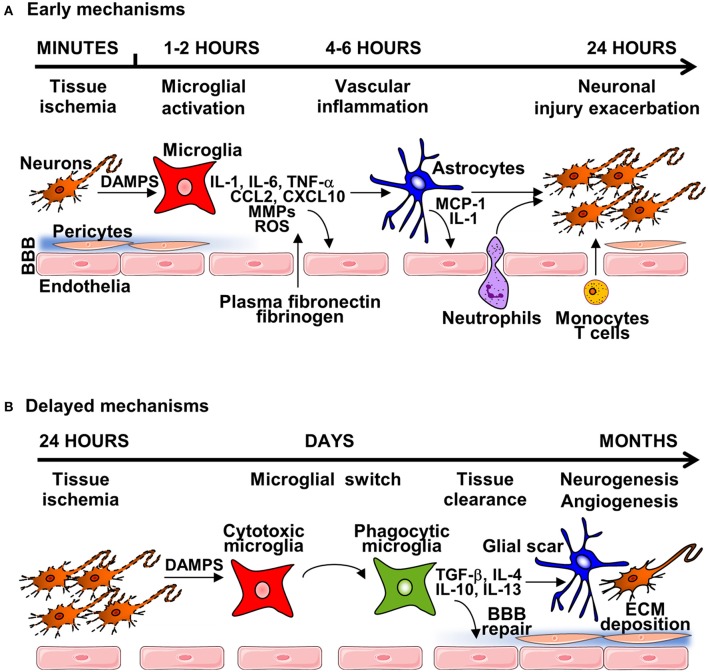
Figure 1.
The general mechanisms of neuroinflammation post-stroke. (A) Early mechanisms of neuroinflammation are initiated by acute neuronal injury producing DAMPS, leading to microglial and endothelial cell activation and disruption of the BBB, through release of pro-inflammatory cytokines, chemokines, reactive oxygen species (ROS) and matrix-metalloproteinases (MMPs). Degradation of the extracellular matrix (ECM)—in both the parenchyma and basement membrane induces astrocyte endfeet and pericytes lifting from the endothelium. Damage of the BBB enables infiltration of circulatory cells with transmigration of neutrophils and immune cells. This damage can lead to brain oedema and hemorrhage, causing further neuroinflammation and tissue damage. (B) During the subacute phase of injury, microglial switch from cytotoxic to phagocytic phenotype occurs, leading to tissue clearance, and expression of anti-inflammatory mediators and neurotrophic factors that leads to the formation of the glial scar, and initiation of brain repair mechanisms, including neurogenesis, angiogenesis and BBB repair.
Early mechanisms of neuroinflammation
Under normal conditions, microglia, the main resident immune cells in the brain, are primarily involved in monitoring (surveying) the brain parenchyma, and are known to play an important homeostatic role (15). In response to cerebral ischemia, microglia are rapidly activated, switching from a resting state to an activated state (16). The inflammatory phenotype of early activated microglia is characterized by the production of a variety of pro-inflammatory cytokines including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, chemokines CCL2, and CXCL10, reactive oxygen species (ROS), nitric oxide (NO), and proteolytic enzymes such as matrix metalloproteinase (MMP)-9 and MMP-3 (17). The release of cytokines and chemokines by activated microglia/macrophage promotes recruitment of circulating immune cells to damaged brain tissue that plays a critical role in pathophysiological events following very acute stroke onset (18). Such events are associated with BBB disruption and degradation of the associated extracellular matrix (ECM), alongside activation of perivascular astrocytes. After the onset of stroke, the BBB is rapidly disrupted allowing uncontrolled entry of circulating molecules into the brain parenchyma, and this disruption persists for days through the acute and early subacute phases of stroke (19). Clinically, BBB disruption leads to the development of hemorrhagic transformation that is associated with worse stroke outcome (20), and MMPs have been identified to play a key role in this process, degrading all components of the ECM including laminin, collagen and fibronectin, and the endothelial junction proteins claudin-5, occluding, and zona occludens (ZO)-1 (21). Opening of the BBB allows penetration of plasma-derived factors (plasma fibronectin, fibrinogen) and inflammatory cells into the brain tissue, causing edema and cell death (22). Astrocytic death is a critical contributing step of BBB dysfunction in stroke by decreasing expression of tight junction proteins (23). Perivascular astrocytes express the passive water channel aquaporin 4 (AQP4) at astrocytic end-feet localized adjacent to the brain endothelium that contributes to post-stroke edema (24). In addition, astrocytes secrete chemokines such as monocyte chemoattractant protein-1 (MCP-1), a critical mediator involved in opening of the BBB after stroke (25). Astrocytes also synthesize a large array of cytokines (i.e., IL-1α, IL-1β, TNF-α) (26) that can directly trigger endothelial cell activation that contributes directly to BBB disruption (27).
Delayed mechanisms of neuroinflammation and brain repair
Cerebral ischemia also activates important delayed endogenous repair processes such as BBB repair, neurogenesis, and angiogenesis that are important for functional recovery and patient rehabilitation in clinical settings, and evidence suggests that the sub-acute phase of inflammation plays a key role in this process. The anti-inflammatory phenotype of microglia exhibits neuroprotective and anti-inflammatory effects during the delayed phase of post-stroke inflammation, producing anti- inflammatory cytokines such as IL-10, transforming growth factor (TGF)-β, IL-4, and IL-13, as well as scavenge receptors, contributing to inhibiting inflammation and promoting tissue repair mechanisms (28). Of those, neurogenesis—known to take place in the sub granular zone of the dentate gyrus of the hippocampus and in the sub ventricular zone adjacent to the third ventricle (29)—is increased following experimental stroke (30), and is regulated by inflammatory mediators expressed during the acute phase of stroke (31). However, neurogenesis in the adult mammalian brain has been debated, with Sorrells and colleagues, reporting that human hippocampal neurogenesis declines rapidly during early childhood and is rarely detected in adult humans (32), and the role of neurogenesis on functional recovery in human remains unclear. In parallel, reactive astrocytes form a glial scar around the ischemic infarct by 14 days (33). Astrocytes initially proliferate and then migrate toward the site of ischemic injury that becomes surrounded by multiple layers of reactive astrocytes interspersed with activated microglia and a dense network of ECM proteins such as laminin, fibronectin, and chondroitin sulfate proteoglycans, resulting in the formation of a very tight glial scar (34). Angiogenesis is also a mechanism of recovery induced by inflammation after an ischemic stroke (35) that is essential for the reoxygenation of post-ischemic brain tissue, and is also an essential step for BBB repair, neurogenesis, and neuronal synaptic plasticity (11).
Although there is clinical evidence to support that inflammation plays a key role in stroke (36–39), the failure of anti-inflammatory strategies have raised hypotheses that inflammation might not play a significant role in stroke pathophysiology. This alterative hypothesis should be investigated more extensively, and future anti-inflammatory therapies may prove to be successful in the treatment of human stroke, providing more convincing evidence for the role of inflammation in stroke pathophysiology.
Anti-inflammatory strategies in regenerative medicine
Several strategies to prevent neuroinflammation and modulate the immune response post-stroke have been studied in experimental models and explored in clinical trials (Table 1). For instance, minocycline is a semi-synthetic tetracycline derivative (51). In animal models of cerebral ischemia, minocycline administration correlates with the reduction of several pro-inflammatory cytokines, as well as ROS and NO (52). A recent comprehensive systematic review and meta-analysis by Malhotra and colleagues showed that minocycline is safe in ischemic stroke patients and demonstrated efficacy and a neuroprotective role, particularly in the acute ischemic stroke (53). Furthermore, several approaches aimed at preventing neutrophil infiltration, trafficking and/or activation have been explored; experimental models using pharmacological agents to block leukocyte adhesion and migration into the ischemic brain have shown promising results (54, 55). In particular, Fingolimod (FTY720) a sphingosine 1-phosphate receptor (S1PR) modulator that prevents the egress of lymphocytes from lymph nodes, has shown promise in preclinical models of stroke (56). This is evident in a systematic review and meta-analysis which reports that fingolimod reduced brain injury in eight out of nine studies (57). There is an ongoing Phase 2 randomized, open-label trial of patients receiving fingolimod within 72 h of ischemic stroke or spontaneous intracerebral hemorrhage. Main outcome measures include NIHSS, BI, mRS, GCS at d7, d14, d30, d90, brain MRI, and immune markers (58). In patients with small- to moderate-sized deep primary supratentorial ICH, administration of fingolimod reduced perihematomal edema, attenuated neurologic deficits, and promoted recovery. The results for ischemic stroke patients have not yet been reported. However, clinical trials testing antibodies against adhesion molecules such as intercellular cell adhesion molecule (ICAM)-1 or by administering neutrophil inhibiting factor have been inconclusive (59, 60). A recent phase 2 clinical trial, involving subcutaneous administration of interleukin-1 receptor antagonist (IL-1Ra) in ischemic stroke has shown promising results (61). IL-1Ra is known to block actions of the pro-inflammatory cytokine IL-1, which has a deleterious role in cerebral ischemia. IL-1Ra reduced plasma inflammatory markers, which are known to be associated with worse clinical outcome in ischemic stroke. Previous to this phase 2 clinical trial, recombinant human IL-1Ra (anakinra) was administered as an i.v. formulation, although it is no longer manufactured in this way (58). Anakinra was evaluated in a UK Phase 2 randomized controlled trial (RCT) in patients presenting within 6 h of acute stroke onset (39). The drug was administered intravenously and there were no significant safety concerns. Patients that received anakinra showed better clinical outcome overall and had reduced neutrophil leukocytosis, plasma C-reactive protein and plasma IL-6 levels during the 72 h infusion. Statins inhibit the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase, lowering the level of low-density lipoprotein (LDL) cholesterol in the blood (38). In a Phase 2 RCT, patients were treated with simvastatin (40 mg/day) within 24 h after the onset of acute ischemic stroke (62). Serum TNF-α levels were marginally lower at day 3 in the simvastatin-treated group, however no clinical outcomes were reported. The safety and efficacy of simvastatin, in combination with rt-PA, is currently being evaluated in the STARS07 trial (58). Edaravone (MCI-186) is an antioxidant and free radical scavenger evaluated in a Phase 2 clinical trial for the treatment of patients with acute ischemic stroke within 24 h from the onset of symptoms (63). MCI-186 was shown to be well-tolerated and safe. However, there were no differences in clinical outcome measures after 1 year. The calcineurin inhibitor cyclosporin A has shown efficacy in preclinical stroke models. Studies reported a reduction of infarct size and inflammation as a result of the drugs suppression of cytokines, T cell activation, and ROS production (51, 64). A Phase 2 clinical study investigating the effect of cyclosporin A (single i.v. dose of cyclosporin A after i.v. thrombolysis within 4.5 h of stroke onset) on MR infarct volume at day 30 has been completed, and the results of this study has yet to be published (65).
Table 1.
Regenerative medicine therapies for ischemic stroke.
| Therapeutic | Preclinical or clinical | Outcome | References |
|---|---|---|---|
| Polyethylene glycol-melanin nanoparticles (PEG-MeNPs) | Preclinical; rat model with middle cerebral artery occlusion (MCAO) | Pre-injection of PEG-MeNPs significantly reduced infarct volume and decreased superoxide levels in brain tissues; in vitro, NPs decreased expression of pro-inflammatory cytokines | (40) |
| Carbon NPs (hydrophilic carbon clusters conjugated to PEG) termed PEG-HCCs | Preclinical; transient MCAO in acutely hyperglycemic rats | Reduction in infarct size, hemisphere swelling, hemorrhage score, and improvement in Bederson score | (41) |
| Perflutren lipid microspheres trademarked as Definity (Lantheus Medical Imaging) | Clinical; FDA approved (2001) ultrasound contrast agent | Safety/efficacy study for use as an ultrasound enhancer for acute ischemic stroke | (42) |
| Microporous Annealing Particle (MAP) hydrogels | Preclinical; mouse model with MCAO | Injection of MAP hydrogels in the stroke cavity reduces gliosis and inflammation and promotes neural progenitor cell migration to the lesion | (43) |
| Hyaluronic acid hydrogel mixed with poly(lactic-co-glycolic acid) microspheres (HA–PLGA) containing vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang1) | Preclinical; mouse model with MCAO | Inhibition of brain inflammation and gliosis after implantation in brain, behavior improvement recorded by cylinder testing and enhanced angiogenesis | (44) |
| HA gel + heparin nanoparticles (nH) with VEGF binding | Preclinical; mouse model with distal MCAO | HA gel + nH injection into the stroke cavity reduced inflammation (activated microglia and reactive astrocytes) and significantly increased vascularization within the stroke cavity and the peri-infarct area | (45) |
| Hydrophobic (HP) carbon nanotubes (CNTs) impregnated with subventricular zone neural progenitor cells (SVZ NPCs) | Preclinical; rat model of transient MCAO | HP CNT-SVZ NPC transplants reduced infarct cyst volume and infarct cyst area. Improved rat behavior and stem cell differentiation. Reduced inflammation (activated microglia) | (46) |
| Amine-modified single-walled carbon nanotubes (a-SWNTs) | Preclinical; rat model of MCAO | Injection of the right lateral ventricles 1 week before induction of ischemic stroke reduced stroke infarct volume, apoptotic, angiogenic and inflammation markers. Behavioral recovery evaluated by the Rota-Rod treadmill test | (47) |
| Multipotent adult progenitor cells (MAPCs) trademarked as MultiStem | Clinical: MultiStem phase II clinical trial; treatment of patients with acute ischemic stroke | Intravenous MultiStem treatment was safe and well tolerated. Lower rates of life-threatening adverse events or death and of secondary infections. Reduced biomarkers of post-stroke inflammation | (48) |
| CTX stem cell therapy (neural stem cell line) | Clinical; phase II clinical trial (PISCES II) for patients with motor disability as a result of ischemic stroke | Treatment was well tolerated and patients showed clinically relevant improvements in the Action Research Arm Test (ARAT) scores, Modified Rankin Scale and Barthel Index | (49) |
| Gene therapy; Interleukin-1 receptor antagonist (IL-1Ra)-producing bone marrow (BM) cells | Preclinical; mouse model of permanent or transient MCAO | Therapeutic injection of IL-1Ra-producing BM cells post-stroke amplified microglial production of IL-1Ra and reduced brain levels of IL-1β, collectively leading to smaller infarcts and improved functional outcome | (50) |
Past and current anti-inflammatory therapies have not translated into a successful clinical treatment for ischemic stroke. Regenerative medicine therapies for stroke may alleviate some of these challenges by providing a structural support, localizing therapy to the site of action, and/or modulating endogenous regenerative cues to brain cells. The multidisciplinary nature of the regenerative medicine approach improves the likelihood of the development of an effective therapy for ischemic stroke. When considering the aforementioned therapies, cyclosporin A, edaravone, and IL-1Ra are the best candidate drugs for combination with NP delivery as they are administered intravenously, thus encapsulation into NPs would potentially improve blood circulation half-life and allow for a more targeted and controlled drug delivery. NPs could be used for the targeted therapeutic delivery of rt-PA, and Tadayon; colleagues have studied the potential of silica-coated magnetic NPs as nanocarriers for rtPA, showing promising results (66). The CTX stem cell therapy developed by ReNeuron could be encapsulated into a hydrogel, for injection into the ischemic brain, enabling controlled delivery over time and better cell survival.
Demonstrating the efficacy of emerging regenerative medicine therapies for ischemic stroke is important and challenging. Magnetic resonance imaging (MRI) can be used to visualize and quantify the infarct volume at multiple time points after stroke (67). This method can be applied to both animal models and stroke patients, and is arguably far more accurate than the determination of infarct volume by immunohistostaining such as NeuN immunostaining or cresyl violet staining (68, 69), which cannot be achieved in humans. Stroke cavity size can also be determined in order to evaluate whether a therapy is promoting repair after stroke injury. For example, Wang and colleagues used cresyl violet staining to quantify the cavity size in a mouse model of stroke (70). As demonstrated by Zhang and colleagues, the effect of scaffold implantation on the integrity of brain shape can be simply shown by haematoxylin and eosin staining of rat brain sections or by extracting and visually observing the whole brain (71). Regenerative medicine therapies may increase post-stroke neurogenesis (72), which can be assessed by doublecortin and NeuN/BrdU immunohistochemistry (73). Induction of post-stroke angiogenesis is considered to be beneficial and can be imaged by laminin immunohistochemistry (44). Immunohistochemical staining for reactive astrocytes and activated microglia is commonly used to determine whether a regenerative medicine therapy is attenuating the inflammatory response after experimental stroke (72). The translocator 18 kDa protein (TSPO) has been used in PET imaging studies to image glial activation and neuroinflammation (74). Recently, improved radioligands for this protein have been developed and approved for human imaging including (11C)PBR28, (18F)DPA-714, and (18F)FEPPA (75). Ultra-small superparamagnetic particles of iron oxide (USPIO) can be used for human imaging of monocyte/macrophage tracking, and have been used successfully to study neuroinflammation in stroke patients (76). The PET ligand 11C-flumazenil (FMZ), which targets GABA-A receptors, has been used for imaging neuronal integrity in human stroke, with patients showing reduced FMZ binding potential in ischemic brain regions (77). Tracking of transplanted stem cells is essential to monitor safety and efficiency of cell-based therapies. Citrate-coated superparamagnetic iron oxide NPs have been used for in vivo stem cell tracking by MRI (78). Zhu and colleagues reported a case of labeling human neural stem cells (NSC) with superparamagnetic iron oxide NPs and tracking their survival, migration, and distribution in a patient with brain trauma by MRI (79). Additional promising imaging modalities for tracking stem cells include nuclear imaging [Positron emission tomography (PET) and Single-photon emission computed tomography (SPECT)] and optical imaging (80). To evaluate changes in neurological function, animals can be subjected to a variety of somatosensory, motor, learning, and memory tests before and after surgery. For example, the rotarod test is widely used for evaluating motor function and balance in rats and mice (81). In addition, the pole test and wire hanging test can be used to assess motor dysfunction after stroke (82). Cognitive deficits including memory problems occur in human stroke survivors thus memory tests have been developed for use in animals such as water maze and passive avoidance task (83). Tests that assess anxiety-like behavior in rodent models have been developed, in order to address post-stroke anxiety that affects up to 40% of stroke survivors (84). Popular tests for this include dark-light box, Vogel conflict test, Geller- Seifter conflict test, elevated plus maze, and open field (81).
Anti-inflammatory properties of nanoparticles (NPs)
Nanoparticles (NPs) are colloidal carriers that can be of natural or synthetic origin and can vary in size from 1 to 1,000 nm (85). Natural NPs are primarily composed of molecules such as proteins (albumin), polysaccharides, or chitosan for instance (86). Synthetic NPs are made from common polymers such as poly(lactic-co-glycolic acid) (PLGA), poly(ethylenimine) (PEI), polyesters poly(lactic acid) (PLA), or from inorganic agents such as gold, silica or alumina (87). NPs can be spherical, cubic and rod-like in shape, and they can have negative, zwitterionic, or positive charge, affecting interactions with biological substrates and the BBB (85). NPs can be coated and functionalized with different types of ligands; some are capable of mediating protein adsorption, others are able to interact directly with the BBB, increase hydrophobicity, or are able to improve blood circulation (88). NPs are versatile drug delivery systems that can be used for the targeted delivery of therapeutic agents into normally inaccessible organs like the brain, and can also be used for the delivery of lyophobic drugs (89).
Recently, it has been suggested that NPs could exert potent anti-inflammatory effects by acting on ROS production, a key process in stroke pathogenesis, since oxidative stress contributes to the initiation of the post-ischemic inflammatory response (90). Recent work from Liu et al. (40) has shown that polyethylene glycol-melanin NPs (PEG-MeNPs) exhibit broad anti-oxidative properties against multiple toxic reactive oxygen and nitrogen species (RONS) including superoxide ions (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), peroxinitrite (ONOO–), and NO, highlighting their potential as a robust RONS scavenger (40). Using a rat model of ischemic stroke, they showed that pre-injection of PEG-MeNPs can significantly decrease ischemic brain injury. In vitro, the NPs were shown to be anti-inflammatory, decreasing the expression of cyclo-oxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), TNF-α, and IL-1β in lipopolysaccharides(LPS)-stimulated macrophages. Biocompatibility was assessed in vitro and in vivo, with NPs demonstrating no obvious toxicity. Another type of NPs, retinoic acid-loaded polymeric NPs (RA-NP) have been developed to modulate microglial response toward an anti-inflammatory and somehow neuroprotective phenotype (28). RA-NP were internalized by murine N9 microglial cell line and inhibited LPS-induced iNOS expression and NO release, whilst promoting arginase-1 and IL-4 production. Additionally, RA-NP effects on microglial phenotype, promoted tissue viability and neuronal survival in organotypic hippocampal slice cultures exposed to an inflammatory stimulus. A new class of antioxidant NPs composed with hydrophilic carbon clusters conjugated to poly(ethylene glycol), named PEG-HCCs, have been recently developed (41). They are effective at scavenging hydroxyl radical and have been found to reduce infarct size when administered during the reperfusion period after experimentally-induced stroke in rat (41).
Over the last decade, a variety of NPs (metal-based, carbon-based, polymer-based, biological-based, and lipid-based) have been investigated for their use in biomedical imaging (91). In particular, the potential uses of iron oxide NPs as MRI contrast agents has been an area of intense interest, and several types of these particles, such as ferumoxytol, have been approved by the Food and Drug Administration (USA) for their use in clinical diagnosis (92). Europium-doped very small iron oxide NPs have been used to visualize neuroinflammation with MRI combined with fluorescence microscopy (93). In addition, there have been recent developments in molecular imaging techniques using organic NPs and quantum dot applications for visualizing in vivo molecular pathways (94, 95). Clinically approved NPs are currently limited to SPECT imaging of peripheral organs such as gastrointestinal tract, liver, and spleen (96). Although nanotechnology has relieved many problems in biomedical imaging, the clinical translation of many types of NPs is impeded by fundamental limitations of human physiology (i.e., vessel pore size, renal, and hepatic clearance), potential toxicity, and/or interference with other medical tests. Hence, a refined NP design and extensive toxicity studies will help facilitate the clinical translation of new NPs that have unique advantages over conventional imaging agents.
Anti-inflammatory properties of hydrogels
Hydrogels are acellular polymeric networks that replicate the intrinsic properties of the native ECM of the neurovascular unit (NVU) (97, 98), and are therefore used commonly for in vitro cell culture and as an in vivo therapeutic tool. The polymeric constituents of hydrogels are termed as biopolymers, which are of natural or synthetic origin (97–99). Natural hydrogels are formed of protein and polysaccharide biopolymers that are either native constituents of the ECM, i.e., collagen, laminin or hyaluronic acid, or can be structurally similar to the native ECM, like alginate and gellan gum. Synthetic hydrogels are chemically synthesized biopolymers—commonly peptide based—that can be designed to assemble into an ECM-like conformation (also known as self-assembling peptides). Hydrogels provide a supportive 3D microenvironment that is similar to the native ECM. This enables the encapsulation of cells, drugs or growth factors for injection or implantation into the brain.
The primary aim of using hydrogels in stroke recovery is to provide an exogenous ECM-based network that allows structural support within the cerebrospinal fluid-filled cavity and promotes endogenous brain tissue repair around the ischemic lesion. To this end, hydrogels must have appropriate properties for the brain tissue, with the neurovascular environment having different ECM properties compared to other peripheral organs. Furthermore, hydrogels must be biocompatible, without activating an immune response from the native tissue, whilst promoting anti-inflammatory activity and recovery. Key physical parameters for hydrogel biocompatibility include porosity, stiffness, and preferentially the physico-chemical presence of cell adhesion peptide (CAP) domains (72, 97, 100, 101). Hydrogel porosity enables the diffusion of nutrients throughout the 3D structure. If the pore size of a hydrogel is too low then nutrients and oxygen within media may not efficiently permeate through the entire structure, or could cause a concentration gradient; potentially leading to necrotic regions (102, 103). The stiffness of a hydrogel regulates the phenotype of cells, with mechanical interactions between cells of the NVU and the ECM through hydrostatic pressures (104) and CAP binding (105, 106), even directing the differentiation of stem cells (105–108). Crosslinking is the mechanism by which a pre-gelation hydrogel becomes solid, with the initiation of inter-molecular physical or chemical bonds maintaining a 3D structure. Therefore, crosslinking dictates the administration technique used for delivering hydrogel to the site of injury, with injectable hydrogels requiring crosslinking (gelation) to occur under physiological conditions [Figure 2; (109, 110)], whereas implanted hydrogels can be crosslinked in a controlled in vitro situation. Hydrogel injection has been achieved with both synthetic (43, 111, 112) and natural biopolymer hydrogels (109, 113–115) for stroke and other CNS applications.
Figure 2.
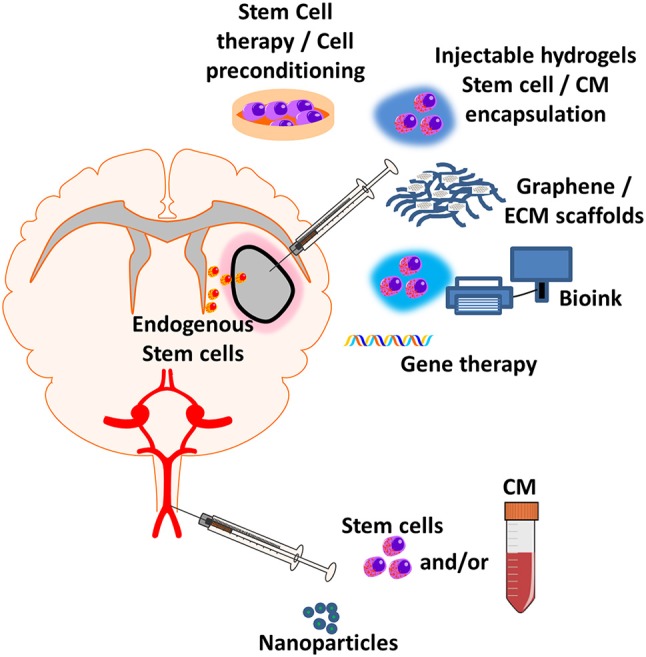
Regenerative medicine applications for treating post-stroke inflammation. New emerging regenerative medicine approaches include central injection of cell therapy and/or encapsulated factor loaded hydrogels, graphene and ECM scaffolds, 3D-bioprinting of cell therapy and/or encapsulated factor loaded bioink, gene therapy which can be implanted directly into the site of injury. Systemic injection of nanoparticles with encapsulated anti-inflammatory factor, nanoparticles or cell therapy can either elicit an effect at the blood-brain barrier (BBB) or enter the parenchyma to elicit an effect at the site of injury. Exogenous administration of new regenerative medicine therapies could lead to the recruitment and infiltration of endogenous stem cells to stroke site.
The interactions between implanted hydrogel and endogenous brain cells have the potential to induce many different reparative and anti-inflammatory cellular pathways, through binding of CAPs (including RGD, IKVAV, and YISGR motifs) to specific cell surface receptors (101). Anti-inflammatory targets of CAPs include; cell adhesion molecules (CAMs), which are involved in the recruitment and trafficking of leukocytes (51, 116); integrin receptors, which in addition to having anti-inflammatory effects can have proangiogenic properties (115, 117, 118), reduce reactive gliosis (43, 119) and promote the infiltration of neural progenitor cells to the site of injury (43, 111); and growth factor receptors that can initiate similar anti-inflammatory effects (120). CAPs can also be used to mimic growth factors and initiate preferential cellular pathways. For example a peptide (QK) which binds to the vascular endothelial growth factor (VEGF) receptor has been incorporated into a hydrogel to promote angiogenesis and may also have an anti-inflammatory effect similar to that observed after recombinant VEGF administration (120). Hydrogels like this VEGF-mimetic structure could aid recovery and promote anti-inflammatory processes following stroke, and the technique used here could be implemented for a number of growth factor mimetic peptides to promote the desired anti-inflammatory actions.
Tissues can be decellularised to isolate the native ECM, which has been used to create hydrogels with anti-inflammatory effects, whilst also aiding clearance of necrotic debris and providing a platform for regeneration through infiltration of endogenous cells to the stroke site (100, 121). Isolation of single ECM components for hydrogels enables the determination of the positive or negative effects of different biopolymers on brain tissue, with some native ECM biopolymers inducing an anti-inflammatory response on their own; Hyaluronic acid (HA) hydrogels in particular have been used frequently in stroke studies (43, 115, 122–126), owing to their anti-inflammatory effects through CAPs binding to CD44, which inhibits inflammation (127) as well as leukocyte rolling and extravasation through the BBB to the brain parenchyma (128). Similarly, gelatin has been shown to exhibit native anti-inflammatory effects in the brain following injury through repairing the BBB, reducing circulatory molecules and cells from entering the brain parenchyma and shifting the microglial response from neurotoxic to a neuroreparative phenotype (129).
Implantation of hydrogels into the brain would require invasive surgery and therefore is a higher risk regenerative strategy than hydrogel injection and other therapeutic techniques, but does offer certain advantages. Through use of 3D-bioprinting, a printable hydrogel (bioink) and brain scans, an implantable structure can be created with patient specific dimensions (Figure 2). Anti-inflammatory and patient specific bioinks can be created with use of a patient platelet-rich plasma (PRP)—a platelet rich fraction of blood that contains a number of growth factors—allowing for printed 3D-anti-inflammatory structures to be implanted (130). Certain hydrogels and bioinks require potentially toxic reagents or ultraviolet-light to initiate crosslinking through the creation of ROS, potentially damaging the cellular contents or initiating downstream pro-inflammatory pathways (131). This further highlights the need for appropriate selection of hydrogel to ensure that anti-inflammatory effects are not negated by the production procedure.
Hydrogels for delivery of anti-inflammatory therapeutic agents
Hydrogels can also be used as a vehicle for the delivery of drugs, growth factors, stem cells, and NPs, to control delivery of therapeutics over time in conjunction with the rate of hydrogel degradation. Hydrogel degradation and gradual release of therapeutics can be tuned to the release profile desired by modifying the physical properties of the biopolymer, or by simply selecting a hydrogel with the appropriate physical profile. Whilst there has been a level of success with anti-inflammatory drugs for post-stroke recovery, the therapeutic window and dosing strategies of these drugs could be enhanced by encapsulation and controlled release from a hydrogel or NP structure (Figure 2). The half-life of drugs injected without a controlled release system is limited, whereas when administered within a hydrogel or NP, the drug can be present at the site of stroke damage for days or even weeks (70, 72, 125, 132). This is a concept which can be applied to various neurodegenerative diseases and to repairing the nervous system, justifying the re-investigation of previously promising drugs and drug targets in a hydrogel- or NP-based administration system (133, 134). Controlled release has also been used in regenerative cardiology, where the use of a hydrogel-based oxygen release system provided a sustained release of oxygen to cardiac tissue in a model of heart failure for up to 4 weeks, significantly reducing inflammation, ROS production and promoting functional recovery of the damaged tissue (135). This system could be applied to treating ischemic regions following stroke and could allow sustained release of oxygen to promote tissue recovery and regeneration. Hydrogels also allow for the controlled release of NPs into the surrounding stroked tissue, for the controlled release of anti-inflammatory NPs (45) delivering encapsulated anti-inflammatory drugs to the site of injury (44, 125).
Conditioned media are commonly produced in in vitro research, with astrocyte-derived conditioned media known to improve the survival and function of other cells of the NVU (136–138). The potential benefit of using cell-derived conditioned media (without cells) is to implant cell secretomes without inducing an immunogenic response from the host tissue. This also presents the opportunity to prime cells to secrete beneficial factors that can reduce inflammation and promote neurorepair in the post-stroke brain (139). Recent research has shown alterations in the secretome of mesenchymal (stromal) stem cells (MSCs) following priming with IL-1, which promotes the secretion of anti-inflammatory and proangiogenic growth factors that could aid recovery (140, 141). Similarly, encapsulation of pro-angiogenic fibroblast growth factor (FGF)-2 within a collagen-alginate hydrogel controlled release system has been shown to be beneficial to ischemic tissues in zebrafish models (142). By using hydrogel controlled release systems, it is possible to therapeutically release anti-inflammatory secretomes to aid regeneration of damaged brain tissue.
Carbon based substrates
The integration of carbon-based substrates to the brain and in hydrogels has been investigated previously for neural tissue engineering. Two of the most commonly investigated carbon substrates for potential stroke therapy are carbon nanotubes (CNTs) and graphene, which have conductive properties that promote neurons and NSC activity and survival. CNTs have been used previously for neural tissue engineering due to their strong conductive properties, which can promote the differentiation and function of neurons (143, 144). CNTs, used as a substrate within hydrogels, have been used to promote both the expression of neural phenotypes and to secrete neurotrophic factors that could reduce inflammation (145). The transplantation of CNTs directly into the post-stroke brain has been shown to reduce microglial activation in the weeks following stroke, as well as promoting neural progenitor cell differentiation to functioning neurons (46). The administration of CNTs before stroke also exhibited enhanced recovery following stroke, with a reduced level of inflammatory markers (47).
Graphene is a biomaterial consisting of carbon in a 2D plane, like the 3D structure of graphite, but with only a single-atom thickness. For biomedical applications, graphene is commonly oxidized (graphene oxide, GO) to make the material hydrophilic and to improve biocompatibility (146, 147). The structural advantage of using GO over the 3D counterpart (graphite oxide for instance) is the enhanced surface area and hydrophilicity that is gained from having atom-thick layers (146). GO can be integrated into hydrogels—as a substrate or graphene foams—for implantation after stroke, due to its mechanical, physical, and electrical properties (148–150). GO has been shown to have ROS scavenging and immune modulating properties when conjugated with a synthetic hydrogel and injected into the post-myocardial infarction heart (151), as well as reducing neuroinflammation in a poly-ε-caprolactone scaffold through inhibition of reactive gliosis and subsequent reduction in glial scarring (148). This positive immunomodulatory response shows promise for the use of GO in hydrogel systems for stroke. More research in graphene derivatives is needed to determine toxicity and immunogenic responses when introduced into living systems—especially for prolonged periods of time—before translation to humans can be considered (152).
Stem cell therapies
Stem cell therapy is a promising therapeutic approach in stroke and is a research priority (153). Stem cells can differentiate into many cell types including neuronal and endothelial lineage, and it has been widely assumed that once implanted they may promote recovery by repopulating the necrotic cavity present within the area of ischemic damage (154). Indeed, several studies have tested the effect of embryonic-derived NSC, induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), MSCs, and bone marrow stem cells (BMSCs) in pre-clinical stroke models (155). Further, the world's first fully-regulated open-label clinical trial of neural stem cell (NSC) therapy in stroke (Pilot Investigation of Stem Cells in Stroke, PISCES I, ReNeuron, UK), followed by the current Phase II trial (PISCES-II) appeared safe with suggestion of functional improvement (49), whilst autologous transplantations of MSCs in stroke patients appear safe and are associated with clinical improvement (156). Although it has been long assumed that cell replacement is the primary mechanism of action of implanted stem cells, a new paradigm of stem cell actions has recently focused on their paracrine actions. It is known that MSCs for instance exert unique therapeutic effects by secreting anti-inflammatory and trophic factors that can transform the local inflammatory environment when implanted locally (157), and the anti-inflammatory theory has been established for other types of stem cell (158). To induce anti-inflammatory mechanisms, stem cells can be manipulated or genetically edited to express certain proteins that are neuroprotective and anti-inflammatory.
A type of anti-inflammatory cell therapy is the transplantation of stem cells that activate downstream cellular pathways and promote infiltration of endogenous NSC to the site of stroke injury (Figure 2). This involves the transplantation of stem cells which have either been differentiated from iPSCs, ESCs, MSCs, or BMSCs to a neural progenitor state, or are un-differentiated. The delivery of neural progenitor cells to the site of injury triggers recovery through reducing inflammation and reactive gliosis as well as promoting angiogenesis (159). The transplantation of un-differentiated pluripotent stem cells (iPSCs and ESCs) has a heightened risk of teratomas and is therefore investigated to a lesser extent (160, 161). In contrast, BMSCs and MSCs have been shown to have beneficial anti-inflammatory effects through inhibition of microglia activation without the heightened risk of tumorigenesis (162). Further research is needed to try and optimize the transplantation of pluripotent stem cells to avoid tumorigenic complications, with the transplantation of cells within a hydrogel of growth factors to direct differentiation potentially offering a better therapeutic approach.
Cell therapies are commonly administered through i.v. injection, requiring cells to cross the BBB. The selective permeability of the brain endothelium restricts cell infiltration resulting in much larger doses of cell therapy being needed to have a therapeutic effect (163, 164). To circumvent this limitation, dual therapies including stem cells administered with biomaterial, astrocyte-derived conditioned medium or drugs that transiently open the BBB have been considered (163, 165). Alternatively, therapies based on administration of T-cell (Treg), known not to cross the BBB, are able to dampen the immune response in the brain and subsequently exert anti-inflammatory actions after stroke (166–169). The anti-inflammatory and neuroprotective effect of Tregs occurs through C-C Chemokine Receptor Type 5 (CCR5) interaction with the endothelial vessel wall, which allows the Tregs to interact with circulatory macrophages and neutrophils (167). This information suggests CCR5 as a potential therapeutic target for enhancing the therapeutic effect of Tregs as well as a sole target without Treg therapy.
Studies have reported modest recovery and highlighted the need to develop new strategies to improve the safety and efficacy of stem cell therapies in stroke. In vitro pre-treatment of stem cells by specific culture conditions and/or biological agents (also known as “preconditioning” or “priming”) can improve the survival, engraftment, immunosuppressive and paracrine properties of stem cells, therefore enhancing their regenerative capacity. For MSCs, preconditioning strategies have been explored in order to enhance the anti-inflammatory properties of MSCs, including exposure to hypoxia/growth factors (170) and inflammatory cytokines (171), whilst the only preconditioning strategy in human stroke patients (STARTING-2) tested the transplantation of autologous MSCs exposed to autologous serum obtained at stroke onset (172). Further, the encapsulation of cells within a hydrogel can create a pre-made tissue to help promote brain repair following stroke. This approach also improves the rate of stem cell survival from implantation, as the cells have a support matrix to aid their integration in the host tissue. This has been shown through using a HA based hydrogel with growth factors, cell adhesion domains (RGD, IKVAV, and YISGR) and neural stem cells, which enhanced stem cell survival following injection in stroked mice (122). A similar HA has been used to inject NSC and subsequently differentiate to a neuronal lineage (123).
Gene therapies
The mass advancements of gene-editing technologies has enhanced the capabilities of both cell and gene therapies, with beneficial genes being introduced to cells in vitro or in vivo to promote the expression of neuroprotective or anti-inflammatory factors. These advancements also raise ethical considerations as editing of the germ line coding sequences results in permanent and hereditary genetic changes, as opposed to editing non-germ line genes. In addition to ethical considerations it is important to ensure that editing a certain gene does not have off-target effects that could cause adverse events in patients.
The use of gene therapies offers the potential to alter cellular and molecular processes that are important to recovery from ischemic stroke, reducing the inflammatory response and initiating regeneration of damaged tissue. This approach has been used to deliver anti-inflammatory gene therapies that promote production of VEGF (116, 173), anti-inflammatory neural cell adhesion molecule (NCAM) (116), or IL-1Ra (50). These therapies were administered in rodents by intrathecal injection, but could be improved through encapsulation within a hydrogel for injection or implantation as this would control the release of gene-edited cells over time to increase the therapeutic effect.
Hydrogel- and NPs-based delivery systems enable the optimization of cell and gene therapy delivery to the site of stroke injury. Systemically injected NPs can optimize BBB permeability through precise surface chemistry and can be designed for controlled release of encapsulated cells. While hydrogels must be injected directly to the brain, they provide ECM mimetic support for both the encapsulated cells and the surrounding host tissue. Like with NPs, the degradation profile of the hydrogel biomaterial can enable the controlled release of cells to the brain; both prolonging the application of anti-inflammatory factors over time rather than having a short therapeutic effect.
Targeting genes that affect neuroinflammation has the potential to be used as an effective therapy for multiple different neurological diseases, with many of these diseases having an inflammatory pathophysiology implicated in either disease onset or progression (174, 175). As an example, pre-clinical Alzheimer's disease research has identified anti-inflammatory mediators that could be targeted using gene therapies to modulate disease pathology (176–178). In a mouse model of Alzheimer's disease, viral vectors have been used to increase gene expression of anti-inflammatory cytokines IL-2 (178) and IL-10 (177) which had a positive effect on pathology and cognitive function in mice. Similar approaches to inhibit neuroinflammation have been applied to other neurological conditions, with a multiple sclerosis gene therapy showing neuroprotective and even disease reversing clinical outcomes in a mouse model (179). The principles of gene-editing that have been developed in these neurological disease models has the potential to influence stroke gene therapy progression, with shared inflammatory pathways in stroke allowing for similar treatments to aid tissue regeneration in the post-stroke brain.
Concluding remarks
Regenerative medicine is an emerging field of interdisciplinary research, providing potential future solutions for the treatment of stroke and other neuroinflammatory conditions. The efficacy of the regenerative approaches discussed in this review has been explored mainly in pre-clinical models showing reductions in inflammatory responses and improved recovery of brain tissue. These pre-clinical studies form the basis of scientific evidence to progress the translation of regenerative therapies toward clinical applications. In particular, the use of biomaterials as anti-inflammatory agents—or as vehicles for controlled release of anti-inflammatory agents—in the form of NPs or hydrogels present as attractive candidates for improving the efficacy of stroke therapies. The development and administration of biomaterials with appropriate physical properties to treat post-stroke inflammation is crucial; with additional complexities and potential advantages being acquired from the bioprinting of implantable tissues. Overall the vast array of NPs, hydrogels, and cell and gene therapies being investigated for the treatment of stroke is very promising and may lead to the licensing of a regenerative medicine inspired treatment in the years to come.
Author contributions
OR and GP formulated original idea. EP contributed to the design of the review. OR, GP, and EP wrote, reviewed and approved the manuscript. GP designed the figures.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by the Centre for Doctoral Training (CDT) in Regenerative Medicine (EP/L014904/1) (University of Manchester), funded by the Medical Research Council (MRC, UK), and Engineering and Physical Sciences Research Council (EPSRC, UK).
References
- 1.Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. (2004) 13:171–7. 10.1016/j.jstrokecerebrovasdis.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet (2014) 383:245–54. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroke Association State of the Nation: Stroke Statistics (2018). Available online at: https://www.stroke.org.uk/system/files/sotn_2018.pdf (Accessed August 19, 2018).
- 4.Musuka TD, Wilton SB, Traboulsi M, Hill MD. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ (2015) 187:887–93. 10.1503/cmaj.140355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkho BZ, Zhao X. Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther. (2011) 6:327–38. 10.2174/157488811797904362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth JM. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proc (Bayl Univ Med Cent) (2011) 24:257–59. 10.1080/08998280.2011.11928729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BCV, Donnan GA, Mitchell PJ, Davis SM. Endovascular thrombectomy for stroke: current best practice and future goals. BMJ (2016) 1:16–22. 10.1136/svn-2015-000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enderby P, Pandyan A, Bowen A, Hearnden D, Ashburn A, Conroy P, et al. Accessing rehabilitation after stroke–a guessing game? Disabil Rehabil. (2017) 39:709–13. 10.3109/09638288.2016.1160448 [DOI] [PubMed] [Google Scholar]
- 9.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M, STAIR VI consortium. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke (2009) 40:2594–600. 10.1161/STROKEAHA.109.552554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saver JL, Jovin TG, Smith WS, Albers GW, Baron JC, Boltze J, et al. Stroke treatment academic industry roundtable: research priorities in the assessment of neurothrombectomy devices. Stroke (2013) 44:3596–601. 10.1161/STROKEAHA.113.002769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaphingst KA, Persky S, McCall C, Lachance C, Beall AC, Blascovich J. Testing communication strategies to convey genomic concepts using virtual reality technology. J Health Commun. (2009) 14:384–99. 10.1080/10810730902873927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics (2016) 13:661–70. 10.1007/s13311-016-0483-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. (2014) 24:7053–62. 10.1002/adfm.201401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rewell SSJ, Churilov L, Sidon TK, Aleksoska E, Cox SF, Macleod MR, et al. Evolution of ischemic damage and behavioural deficit over 6 months after MCAo in the rat: Selecting the optimal outcomes and statistical power for multi-centre preclinical trials. PLoS ONE (2017) 12:e0171688. 10.1371/journal.pone.0171688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. (2014) 15:300–12. 10.1038/nrn3722 [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. (2018) 18:225–42. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- 17.Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol. (2013) 5:73–90. [PMC free article] [PubMed] [Google Scholar]
- 18.Tam WY, Ma CHE. Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci Rep. (2014) 4:7279. 10.1038/srep07279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haley MJ, Lawrence CB. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab. (2016) 37:456–70. 10.1177/0271678X16629976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassner A, Merali Z. Assessment of blood-brain barrier disruption in stroke. Stroke (2015) 46:3310–5. 10.1161/STROKEAHA.115.008861 [DOI] [PubMed] [Google Scholar]
- 21.Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. (2016) 10:56. 10.3389/fncel.2016.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dejonckheere E, Vandenbroucke RE, Libert C. Matrix metalloproteinases as drug targets in ischemia/reperfusion injury. Drug Discov Today (2011) 16:762–78. 10.1016/j.drudis.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 23.Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des. (2012) 18:3624–44. 10.2174/138161212802002625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, et al. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. (1994) 107:1347–57. [DOI] [PubMed] [Google Scholar]
- 25.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. (2006) 26:797–810. 10.1038/sj.jcbfm.9600229 [DOI] [PubMed] [Google Scholar]
- 26.Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. (2011) 11:164–73. 10.2174/187152411796011303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. (2017) 60:1–12. 10.1016/j.bbi.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 28.Machado-Pereira M, Santos T, Ferreira L, Bernardino L, Ferreira R. Anti-inflammatory strategy for M2 microglial polarization using retinoic acid-loaded nanoparticles. Mediat Inflamm. (2017) 2017:6742427. 10.1155/2017/6742427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Manaenko A, Hu Q. Targeting adult neurogenesis for poststroke therapy. Stem Cells Int. (2017) 2017:5868632. 10.1155/2017/5868632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. (2017) 8:3–13. 10.1007/s12975-016-0460-z [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, et al. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation (2015) 12:15. 10.1186/s12974-014-0227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature (2018) 555:377–81. 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cekanaviciute E, Buckwalter MS. Astrocytes: integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics (2016) 13:685–701. 10.1007/s13311-016-0477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zbesko JC, Nguyen TV, Yang T, Frye JB, Hussain O, Hayes M, et al. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis. (2018) 112:63–78. 10.1016/j.nbd.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes Migr. (2009) 3:216–23. 10.4161/cam.3.2.8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke (2003) 34:2518–32. 10.1161/01.STR.0000089015.51603.CC [DOI] [PubMed] [Google Scholar]
- 37.McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience (2009) 158:1049–61. 10.1016/j.neuroscience.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 38.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ (2003) 326:1423. 10.1136/bmj.326.7404.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley HCA, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry (2005) 76:1366–72. 10.1136/jnnp.2004.054882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Ai K, Ji X, Askhatova D, Du R, Lu L, et al. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J Am Chem Soc. (2017) 139:856–62. 10.1021/Jacs.6b11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabian RH, Derry PJ, Rea HC, Dalmeida WV, Nilewski LG, Sikkema WKA, et al. Efficacy of novel carbon nanoparticle antioxidant therapy in a severe model of reversible middle cerebral artery stroke in acutely hyperglycemic rats. Front Neurol. (2018) 9:199. 10.3389/fneur.2018.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke (2008) 39:1464–9. 10.1161/STROKEAHA.107.505727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nih LR, Sideris E, Carmichael ST, Segura T. Injection of Microporous Annealing Particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv Mater. (2017) 29:1606471. 10.1002/adma.201606471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju R, Wen Y, Gou R, Wang Y, Xu Q. The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant. (2014) 23(Suppl. 1):S83–95. 10.3727/096368914X684998 [DOI] [PubMed] [Google Scholar]
- 45.Nih LR, Gojgini S, Carmichael ST, Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat Mater. (2018)17:642–51. 10.1038/s41563-018-0083-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon SU, Kim J, Bokara KK, Kim JY, Khang D, Webster TJ, et al. Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int J Nanomed. (2012) 7:2751–65. 10.2147/IJN.S30273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HJ, Park J, Yoon OJ, Kim HW, Lee DY, Kim DH, et al. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol. (2011) 6:121–5. 10.1038/nnano.2010.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. (2017) 16:360–8. 10.1016/S1474-4422(17)30046-7 [DOI] [PubMed] [Google Scholar]
- 49.Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet (2016) 388:787–96. 10.1016/S0140-6736(16)30513-X [DOI] [PubMed] [Google Scholar]
- 50.Clausen BH, Lambertsen KL, Dagnæs-Hansen F, Babcock AA, von Linstow CU, Meldgaard M, et al. Cell therapy centered on IL-1Ra is neuroprotective in experimental stroke. Acta Neuropathol. (2016) 131:775–91. 10.1007/s00401-016-1541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol. (2017) 8:467. 10.3389/fneur.2017.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation (2015) 12:26. 10.1186/s12974-015-0245-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malhotra K, Chang JJ, Khunger A, Blacker D, Switzer JA, Goyal N, et al. Minocycline for acute stroke treatment : a systematic review and meta- analysis of randomized clinical trials. J Neurol. (2018) 265:1871–9. 10.1007/s00415-018-8935-3 [DOI] [PubMed] [Google Scholar]
- 54.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. (1996) 2:788–94. 10.1038/nm0796-788 [DOI] [PubMed] [Google Scholar]
- 55.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, et al. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. (1998) 153:223–3. 10.1006/exnr.1998.6876 [DOI] [PubMed] [Google Scholar]
- 56.Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann Neurol. (2011) 69:759–77. 10.1002/ana.22426 [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of Sphingosine-1- phosphate (S1P) receptor agonist FTY720 (Fingolimod) in animal models of stroke. Int J Neurosci. (2013) 123:163–9. 10.3109/00207454.2012.749255 [DOI] [PubMed] [Google Scholar]
- 58.Smith CJ, Denes A, Tyrrell PJ, Di Napoli M. Phase II anti-inflammatory and immune-modulating drugs for acute ischaemic stroke. Expert Opin Investig Drugs (2015) 24:623–43. 10.1517/13543784.2015.1020110 [DOI] [PubMed] [Google Scholar]
- 59.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. (2002) 18:s18–22. 10.1185/030079902125000688 [DOI] [PubMed] [Google Scholar]
- 60.Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke (2003) 34:2543–8. 10.1161/01.STR.0000092527.33910.89 [DOI] [PubMed] [Google Scholar]
- 61.Smith CJ, Hulme S, Vail A, Heal C, Parry-Jones AR, Scarth S, et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke). Stroke (2018) 49:1210–6. 10.1161/STROKEAHA.118.020750 [DOI] [PubMed] [Google Scholar]
- 62.Szczepanska-Szerej A, Kurzepa J, Wojczal J, Stelmasiak Z. Simvastatin-induced prevention of the increase in TNF- alpha level in the acute phase of ischemic stroke. Pharmacol Rep. (2007) 59:94–7. [PubMed] [Google Scholar]
- 63.Kaste M, Murayama S, Ford GA, Dippel DWJ, Walters MR, Tatlisumak T. Safety, tolerability and pharmacokinetics of mci-186 in patients with acute ischemic stroke: new formulation and dosing regimen. Cerebrovasc Dis. (2013) 36:196–204. 10.1159/000353680 [DOI] [PubMed] [Google Scholar]
- 64.Yuen CM, Sun CK, Lin YC, Chang LT, Kao YH, Yen CH, et al. Combination of cyclosporine and erythropoietin improves brain infarct size and neurological function in rats after ischemic stroke. J Transl Med. (2011) 9:114. 10.1186/1479-5876-9-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawakami M. Molecular dissection of cyclosporin A's neuroprotective effect reveals potential therapeutics for ischemic brain injury. Brain Sci. (2013) 3:1325–56. 10.3390/brainsci3031325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tadayon A, Jamshidi R, Esmaeili A. Delivery of tissue plasminogen activator and streptokinase magnetic nanoparticles to target vascular diseases. Int J Pharm. (2015) 495:428–38. 10.1016/j.ijpharm.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 67.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. (2002) 8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 68.Leithner C, Füchtemeier M, Jorks D, Mueller S, Dirnagl U, Royl G. Infarct volume prediction by early magnetic resonance imaging in a murine stroke model depends on ischemia duration and time of imaging. Stroke (2015) 46:3249–59. 10.1161/STROKEAHA.114.007832 [DOI] [PubMed] [Google Scholar]
- 69.Pialat JB, Wiart M, Nighoghossian N, Adeleine P, Derex L, Hermier M, et al. Evolution of lesion volume in acute stroke treated by intravenous t-PA. J Magn Reson Imaging (2005) 22:23–8. 10.1002/jmri.20363 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Cooke MJ, Morshead CM, Shoichet MS. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials (2012) 33:2681–92. 10.1016/j.biomaterials.2011.12.031 [DOI] [PubMed] [Google Scholar]
- 71.Zhang HZ, Hayashi T, Tsuru K, Deguchi K, Nagahara M, Hayakawa S, et al. Vascular endothelial growth factor promotes brain tissue regeneration with a novel biomaterial polydimethylsiloxane-tetraethoxysilane. Brain Res. (2007) 1132:29–35. 10.1016/j.brainres.2006.09.117 [DOI] [PubMed] [Google Scholar]
- 72.Tuladhar A, Payne SL, Shoichet MS. Harnessing the potential of biomaterials for brain repair after stroke. Front Mater (2018) 5:14 10.3389/fmats.2018.00014 [DOI] [Google Scholar]
- 73.Cook DJ, Nguyen C, Chun HN, L Llorente I, Chiu AS, Machnicki M, et al. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. (2017) 37:1030–45. 10.1177/0271678X16649964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janssen B, Vugts DJ, Windhorst AD, Mach RH. PET imaging of microglial activation - Beyond targeting TSPO. Molecules (2018) 23:E607. 10.3390/molecules23030607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrera-Rivero M, Heneka MT, Papadopoulos V. Translocator protein and new targets for neuroinflammation. Clin Transl Imaging (2015) 3:391–402. 10.1007/s40336-015-0151-x [DOI] [Google Scholar]
- 76.Nighoghossian N, Wiart M, Cakmak S, Berthezène Y, Derex L, Cho TH, et al. Inflammatory response after ischemic stroke: a USPIO-enhanced MRI study in patients. Stroke (2007) 38:303–7. 10.1161/01.STR.0000254548.30258.f2 [DOI] [PubMed] [Google Scholar]
- 77.Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab. (2014) 34:2–18. 10.1038/jcbfm.2013.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, et al. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials (2012) 33:4515–25. 10.1016/j.biomaterials.2012.02.064 [DOI] [PubMed] [Google Scholar]
- 79.Hospital MG. Tracking neural stem cells in patients with brain trauma. N Engl J Med. (2006) 355:2376–8. 10.1056/NEJMc055304 [DOI] [PubMed] [Google Scholar]
- 80.Zheng Y, Huang J, Zhu T, Li R, Wang Z, Ma F, et al. Stem cell tracking technologies for neurological regenerative medicine purposes. Stem Cells Int. (2017) 2017:2934149. 10.1155/2017/2934149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balkaya M, Kröber JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. (2013) 33:330–8. 10.1038/jcbfm.2012.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balkaya M, Kröber J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Methods (2013) 213:179–87. 10.1016/j.jneumeth.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 83.Doeppner TR, Kaltwasser B, Bãhr M, Hermann DM. Effects of neural progenitor cells on post-stroke neurological impairment—a detailed and comprehensive analysis of behavioral tests. Front Cell Neurosci (2014) 8: 338. 10.3389/fncel.2014.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell Burton CA, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int J Stroke (2013) 8:545–59. 10.1111/j.1747-4949.2012.00906.x [DOI] [PubMed] [Google Scholar]
- 85.Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release (2016) 235:34–47. 10.1016/j.jconrel.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 86.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. (2002) 28:1–13. 10.1081/DDC-120001481 [DOI] [PubMed] [Google Scholar]
- 87.Cupaioli FA, Zucca FA, Boraschi D, Zecca L. Engineered nanoparticles. How brain friendly is this new guest? Prog Neurobiol. (2014) 119–20:20–38. 10.1016/j.pneurobio.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 88.Betzer O, Shilo M, Opochinsky R, Barnoy E, Motiei M, Okun E, et al. The effect of nanoparticle size on the ability to cross the blood–brain barrier: an in vivo study. Nanomedicine (2017) 12:1533–46. 10.2217/nnm-2017-0022 [DOI] [PubMed] [Google Scholar]
- 89.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B (2016) 6:268–86. 10.1016/J.APSB.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. (2009) 7:97. 10.1186/1479-5876-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging (2010) 9:291–310. 10.2310/7290.2010.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ajetunmobi A, Prina-Mello A, Volkov Y, Corvin A, Tropea D. Nanotechnologies for the study of the central nervous system. Prog Neurobiol. (2014) 123:18–36. 10.1016/J.PNEUROBIO.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 93.Millward JM, Ariza de Schellenberger A, Berndt D, Hanke-Vela L, Schellenberger E, Waiczies S, et al. Application of europium-doped very small iron oxide nanoparticles to visualize neuroinflammation with MRI and fluorescence microscopy. Neuroscience (2018). 10.1016/j.neuroscience.2017.12.014 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 94.Hill RT, Lyon JL, Allen R, Stevenson KJ, Shear JB. Microfabrication of three-dimensional bioelectronic architectures. J Am Chem Soc. (2005) 127:10707–11. 10.1021/ja052211f [DOI] [PubMed] [Google Scholar]
- 95.Albertazzi L, Gherardini L, Brondi M, Sulis Sato S, Bifone A, Pizzorusso T, et al. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm. (2013) 10:249–60. 10.1021/mp300391v [DOI] [PubMed] [Google Scholar]
- 96.Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically approved nanoparticle imaging agents. J Nucl Med. (2016) 57:1833–7. 10.2967/jnumed.116.181362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Potjewyd G, Moxon S, Wang T, Domingos M, Hooper NM. Tissue engineering 3D neurovascular units: a biomaterials and bioprinting perspective. Trends Biotechnol. (2018) 10.1016/j.tibtech.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 98.Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods (2016) 13:405–14. 10.1038/nmeth.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gyles DA, Castro LD, Silva JOC, Ribeiro-Costa RM. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur Polym J. (2017) 88:373–92. 10.1016/j.eurpolymj.2017.01.027 [DOI] [Google Scholar]
- 100.Ghuman H, Massensini AR, Donnelly J, Kim SM, Medberry CJ, Badylak SF, et al. ECM hydrogel for the treatment of stroke: characterization of the host cell infiltrate. Biomat. (2016) 91:166–81. 10.1016/j.biomaterials.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huettner N, Dargaville TR, Forget A. Discovering cell-adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. (2018) 36:372–83. 10.1016/j.tibtech.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 102.Fu J, Wiraja C, Muhammad HB, Xu C, Wang DA. Improvement of endothelial progenitor outgrowth cell (EPOC)-mediated vascularization in gelatin-based hydrogels through pore size manipulation. Acta Biomater. (2017) 58:225–37. 10.1016/j.actbio.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 103.Murphy AR, Laslett A, O'Brien CM, Cameron NR. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. (2017) 54:1–20. 10.1016/j.actbio.2017.02.046 [DOI] [PubMed] [Google Scholar]
- 104.Ahearne M. Introduction to cell – hydrogel mechanosensing. Interface Focus (2014) 4:20130038. 10.1098/rsfs.2013.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang ZN, Freitas BC, Qian H, Lux J, Acab A, Trujillo CA, et al. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc Natl Acad Sci USA. (2016) 113:3185–90. 10.1073/pnas.1521255113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stukel JM, Willits RK. The interplay of peptide affinity and scaffold stiffness on neuronal differentiation of neural stem cells. Biomed Mater. (2017) 13:024102. 10.1088/1748-605X/aa9a4b [DOI] [PubMed] [Google Scholar]
- 107.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell (2006) 126:677–89. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 108.Her GJ, Wu HC, Chen MH, Chen MY, Chang SC, Wang TW. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomater. (2013) 9:5170–80. 10.1016/j.actbio.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 109.Arulmoli J, Wright HJ, Phan DTT, Sheth U, Que RA, Botten GA, et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. (2016) 43:122–38. 10.1016/j.actbio.2016.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nih LR, Carmichael ST, Segura T. Hydrogels for brain repair after stroke: an emerging treatment option. Curr Opin Biotechnol. (2016) 40:155–63. 10.1016/j.copbio.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang TW, Chang KC, Chen LH, Liao SY, Yeh CW, Chuang YJ. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale (2017) 9:16281–92. 10.1039/C7NR06528K [DOI] [PubMed] [Google Scholar]
- 112.Cheng TY, Chen MH, Chang WH, Huang MY, Wang TW. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials (2013) 34:2005–16. 10.1016/j.biomaterials.2012.11.043 [DOI] [PubMed] [Google Scholar]
- 113.Coutinho DF, Sant SV, Shin H, Oliveira JT, Gomes ME, Neves NM, et al. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials (2010) 31:7494–502. 10.1016/j.biomaterials.2010.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adil MM, Vazin T, Ananthanarayanan B, Rodrigues GMC, Rao AT, Kulkarni RU, et al. Engineered hyaluronic acid hydrogels increase the post-transplantation survival of encapsulated hPSC-derived midbrain dopaminergic neurons. Biomaterials (2016) 136:1–11. 10.1016/j.biomaterials.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 115.Li S, Nih LR, Bachman H, Fei P, Li Y, Nam E, et al. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater. (2017) 16:953–61. 10.1038/nmat4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sokolov ME, Bashirov FV, Markosyan VA, Povysheva TV, Fadeev FO, Izmailov AA, et al. Triple-gene therapy for stroke: a proof-of-concept in vivo study in rats. Front Pharmacol (2018) 9:111. 10.3389/fphar.2018.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee H, Jin YC, Kim SW, Kim ID, Lee HK, Lee JK. Proangiogenic functions of an RGD-SLAY-containing osteopontin icosamer peptide in HUVECs and in the postischemic brain. Exp Mol Med. (2018) 50:e430. 10.1038/emm.2017.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park EJ, Yuki Y, Kiyono H, Shimaoka M. Structural basis of blocking integrin activation and deactivation for anti-inflammation. J Biomed Sci. (2015) 22:51. 10.1186/s12929-015-0159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Srivastava T, Sherman LS, Back SA, Srivastava T, Diba P, Dean JM, et al. A TLR/AKT/FoxO3 immune tolerance – like pathway disrupts the repair capacity of oligodendrocyte progenitors Find the latest version : a TLR/AKT/FoxO3 immune tolerance – like pathway disrupts the repair capacity of oligodendrocyte progenitors. J Clin Invest. (2018) 128:2025–41. 10.1172/JCI94158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cai L, Dinh CB, Heilshorn SC. One-pot synthesis of elastin-like polypeptide hydrogels with grafted VEGF-mimetic peptides. Biomater Sci. (2014) 2:757–65. 10.1039/C3BM60293A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ghuman H, Gerwig M, Nicholls FJ, Liu JR, Donnelly J, Badylak SF, et al. Long-term retention of ECM hydrogel after implantation into a sub-acute stroke cavity reduces lesion volume. Acta Biomater. (2017) 63:50–63. 10.1016/j.actbio.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials (2016) 105:145–55. 10.1016/j.biomaterials.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. (2015) 24:7053–62. 10.1002/adfm.201401483.Delivery [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nih LR, Moshayedi P, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, et al. Engineered HA hydrogel for stem cell transplantation in the brain: Biocompatibility data using a design of experiment approach. Data Br. (2017) 10:202–9. 10.1016/j.dib.2016.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caicco MJ, Cooke MJ, Wang Y, Tuladhar A, Morshead CM, Shoichet MS. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. J Control Release (2013) 166:197–202. 10.1016/j.jconrel.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 126.Moshayedi P, Carmichael ST. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter (2013) 3:e23863. 10.4161/biom.23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cooper CA, Brown KK, Meletis CD, Zabriskie N. Inflammation and Hyaluronic Acid. Altern Complement Ther. (2008) 14:78–84. 10.1089/act.2008.14201 [DOI] [Google Scholar]
- 128.Forrester JV, Wilkinson PC. Inhibition of leukocyte locomotion by hyaluronic acid. J Cell Sci. (1981) 48:315–31. [DOI] [PubMed] [Google Scholar]
- 129.Kumosa LS, Zetterberg V, Schouenborg J. Gelatin promotes rapid restoration of the blood brain barrier after acute brain injury. Acta Biomater. (2017) 65:137–49. 10.1016/j.actbio.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 130.Faramarzi N, Yazdi IK, Nabavinia M, Gemma A, Fanelli A, Caizzone A, et al. Patient-specific bioinks for 3D bioprinting of tissue engineering scaffolds. (2018) 7:e1701347. 10.1002/adhm.201701347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mironi-Harpaz I, Wang DY, Venkatraman S, Seliktar D. Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. (2012) 8:1838–48. 10.1016/j.actbio.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 132.Tuladhar A, Morshead CM, Shoichet MS. Circumventing the blood-brain barrier: Local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J Control Release (2015) 215:1–11. 10.1016/j.jconrel.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 133.Sánchez-López E, Ettcheto M, Egea MA, Espina M, Cano A, Calpena AC, et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer's disease: in vitro and in vivo characterization. J Nanobiotechnol. (2018) 16:32. 10.1186/s12951-018-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khaing ZZ, Thomas RC, Geissler SA, Schmidt CE. Advanced biomaterials for repairing the nervous system: What can hydrogels do for the brain? Mater Today (2014) 17:332–40. 10.1016/j.mattod.2014.05.011 [DOI] [Google Scholar]
- 135.Fan Z, Xu Z, Niu H, Gao N, Guan Y, Li C, et al. An injectable oxygen release system to augment cell survival and promote cardiac repair following myocardial infarction. Sci Rep. (2018) 8:1371. 10.1038/s41598-018-19906-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. (2007) 1147:39–50. 10.1016/j.brainres.2007.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Dev Brain Res. (1987) 36:155–9. 10.1016/0165-3806(87)90075-7 [DOI] [PubMed] [Google Scholar]
- 138.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. (2016) 35:239–57. 10.15252/embj.201592705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cunningham CJ, Redondo-castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. (2018) 38:1276–92. 10.1177/0271678X18776802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Redondo-Castro E, Cunningham CJ, Miller J, Brown H, Allan SM, Pinteaux E. Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res Ther. (2018) 9:11. 10.1186/s13287-017-0753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Salmeron K, Aihara T, Redondo-Castro E, Pinteaux E, Bix G. IL-1alpha induces angiogenesis in brain endothelial cells in vitro: implications for brain angiogenesis after acute injury. J Neurochem. (2016) 136:573–80. 10.1111/jnc.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ali Z, Islam A, Sherrell P, Le-Moine M, Lolas G, Syrigos K, et al. Adjustable delivery of pro-angiogenic FGF-2 by collagen-alginate microspheres. Biol Open (2018) 7:bio027060. 10.1242/bio.027060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, et al. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. (2005) 5:1107–10. 10.1021/nl050637m [DOI] [PubMed] [Google Scholar]
- 144.Shin J, Choi EJ, Cho JH, Cho AN, Jin Y, Yang K, et al. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules (2017) 18:3060–72. 10.1021/acs.biomac.7b00568 [DOI] [PubMed] [Google Scholar]
- 145.Lee JH, Lee JY, Yang SH, Lee EJ, Kim HW. Carbon nanotube-collagen three-dimensional culture of mesenchymal stem cells promotes expression of neural phenotypes and secretion of neurotrophic factors. Acta Biomater. (2014) 10:4425–36. 10.1016/j.actbio.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 146.Chung C, Kim YK, Shin D, Ryoo SR, Hong BH, Min DH. Biomedical applications of graphene and graphene oxide. Acc Chem Res. (2013) 46:2211–24. 10.1021/ar300159f [DOI] [PubMed] [Google Scholar]
- 147.Lee SK, Kim H, Shim BS. Graphene: an emerging material for biological tissue engineering. Carbon Lett. (2013) 14:63–75. 10.5714/CL.2013.14.2.063 [DOI] [Google Scholar]
- 148.Zhou K, Motamed S, Thouas GA, Bernard CC, Li D, Parkington HC, et al. Graphene functionalized scaffolds reduce the inflammatory response and supports endogenous neuroblast migration when implanted in the adult brain. PLoS ONE (2016) 11:e0151589. 10.1371/journal.pone.0151589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li N, Zhang Q, Gao S, Song Q, Huang R, Wang L, et al. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci Rep. (2013) 3:1604. 10.1038/srep01604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng HM. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater. (2011) 10:424–8. 10.1038/nmat3001 [DOI] [PubMed] [Google Scholar]
- 151.Han J, Kim YS, Lim MY, Kim HY, Kong S, Kang M, et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano (2018) 12:1959–77. 10.1021/acsnano.7b09107 [DOI] [PubMed] [Google Scholar]
- 152.Mattei TA. How graphene is expected to impact neurotherapeutics in the near future. Expert Rev Neurother. (2014) 14:845–7. 10.1586/14737175.2014.925804 [DOI] [PubMed] [Google Scholar]
- 153.Bang OY, Kim EH, Cha JM, Moon GJ. Adult stem cell therapy for stroke: challenges and progress. J Stroke (2016) 18:256–66. 10.5853/jos.2016.01263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stroemer P, Hope A, Patel S, Pollock K, Sinden J. Development of a human neural stem cell line for use in recovery from disability after stroke. Front Biosci. (2008) 13:2290–2. 10.2741/2842 [DOI] [PubMed] [Google Scholar]
- 155.Savitz SI. Are stem cells the next generation of stroke therapeutics? Stroke (2018) 49:1056–7. 10.1161/STROKEAHA.118.019561 [DOI] [PubMed] [Google Scholar]
- 156.Dulamea AO. The potential use of mesenchymal stem cells in stroke therapy—From bench to bedside. J Neurol Sci. (2015) 352:1–11. 10.1016/J.JNS.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 157.Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in “innate tolerance”? Immunology (2012) 137:206–13. 10.1111/j.1365-2567.2012.03621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ulrich H, do Nascimento IC, Bocsi J, Tárnok A. Immunomodulation in stem cell differentiation into neurons and brain repair. Stem Cell Rev Rep. (2015) 11:474–86. 10.1007/s12015-014-9556-6 [DOI] [PubMed] [Google Scholar]
- 159.Lee IH, Huang SS, Chuang CY, Liao KH, Chang LH, Chuang CC, et al. Delayed epidural transplantation of human induced pluripotent stem cell-derived neural progenitors enhances functional recovery after stroke. Sci Rep. (2017) 7:1943. 10.1038/s41598-017-02137-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. (2010) 30:1487–93. 10.1038/jcbfm.2010.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. (2011) 20:883–91. 10.3727/096368910X539092 [DOI] [PubMed] [Google Scholar]
- 162.Zhu S, Szeto V, Bao M, Sun H, Feng Z. Pharmacological approaches promoting stem cell- based therapy following ischemic stroke insults. Acta Pharmacol Sin. (2018) 39:695–712. 10.1038/aps.2018.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tajiri N, Lee JY, Acosta S, Sanberg PR, Borlongan CV. Breaking the blood–brain barrier with mannitol to aid stem cell therapeutics in the chronic stroke brain. Cell Transplant. (2016) 25:1453–60. 10.3727/096368916X690971 [DOI] [PubMed] [Google Scholar]
- 164.Ahn JH, Chen BH, Park JH, Shin BN, Lee TK, Cho JH, et al. Early IV-injected human dermis-derived mesenchymal stem cells after transient global cerebral ischemia do not pass through damaged blood-brain barrier. J Tissue Eng Regen Med. (2018) 12:1646–57. 10.1002/term.2692 [DOI] [PubMed] [Google Scholar]
- 165.Borlongan CV, Glover LE, Sanberg PR, Hess DC. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Curr Pharm Des. (2012) 18:3670–6. 10.2174/138161212802002841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. (2013) 74:458–71. 10.1002/ana.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li P, Wang L, Zhou Y, Gan Y, Zhu W, Xia Y, et al. C-C chemokine receptor type 5 (CCR5)-mediated docking of transferred Tregs protects against early blood-brain barrier disruption after stroke. J Am Heart Assoc. (2017) 6:e006387. 10.1161/JAHA.117.006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xia Y, Cai W, Thomson AW, Hu X. Regulatory T cell therapy for ischemic stroke: how far from clinical translation? Transl Stroke Res. (2016) 7:415–9. 10.1007/s12975-016-0476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liesz A, Kleinschnitz C. Regulatory T Cells in post-stroke immune homeostasis. Transl Stroke Res. (2016) 7:313–21. 10.1007/s12975-016-0465-7 [DOI] [PubMed] [Google Scholar]
- 170.Schäfer R, Spohn G, Baer PC. Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus Med Hemother. (2016) 43:256–67. 10.1159/000447458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. (2017) 8:79. 10.1186/s13287-017-0531-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim S, Moon G, Chang W, Kim YH, Bang O. Intravenous transplantation of mesenchymal stem cells preconditioned with early phase stroke serum: current evidence and study protocol for a randomized trial. Trials (2013) 14:317. 10.1186/1745-6215-14-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells (2011) 29:274–85. 10.1002/stem.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Skaper SD, Facci L, Zusso M, Giusti P. An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci. (2018) 12:72. 10.3389/fncel.2018.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Amor S, Puentes F, Baker D, Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology (2010) 129:154–69. 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiang T, Tan L, Zhu XC, Zhang QQ, Cao L, Tan MS, et al. Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer's disease. Neuropsychopharmacology (2014) 39:2949–62. 10.1038/npp.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APPPS1 mice. Gene Ther. (2012) 19:724–33. 10.1038/gt.2011.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alves S, Churlaud G, Audrain M, Michaelsen-Preusse K, Fol R, Souchet B, et al. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer's disease mice. Brain (2017) 140:826–42. 10.1093/brain/aww330 [DOI] [PubMed] [Google Scholar]
- 179.Keeler GD, Kumar S, Palaschak B, Silverberg EL, Markusic DM, Jones NT, et al. Gene therapy-induced antigen-specific tregs inhibit neuro-inflammation and reverse disease in a mouse model of multiple sclerosis. Mol Ther. (2018) 26:173–83. 10.1016/j.ymthe.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]