Abstract
Transplantation research has focused on cytotoxic T-cell and plasma cell/B-cell-targeted strategies, but little attention has been paid to the role of T helper 17 (Th17) cells in allograft dysfunction. However, accumulating evidence suggests that Th17 cells contribute to the development of acute and chronic allograft injury after transplantation of various organs, including the kidney. This review summarizes recent reports on the role of Th17 cells in kidney transplantation. Means of improving allograft outcomes by targeting the Th17 pathway are also suggested.
Keywords: Allograft rejection, Chronic allograft dysfunction, Kidney transplantation, Mammalian target of rapamycin, Th17 cells
INTRODUCTION
Kidney transplantation (KT) is the best available treatment option for end-stage renal disease. However, the long-term success rate of KT is limited by acute or chronic allograft rejection due to progressive destruction caused by recognition of donor alloantigens by the recipient’s immune system [1]. Allograft rejection after KT is mediated mainly by helper T-cells. The Th1–Th2 balance is regarded as the major mechanism of rejection [2]. However, certain immunologic events occurring after KT cannot be explained solely by the Th1–Th2 balance. Therefore, the mechanism of rejection has been expanded to include T helper 17 (Th17) cells, which secrete the proinflammatory cytokine interleukin 17 (IL-17) [3].
Th17 cells were identified as a subset distinct from T helper type 1 and 2 cells in 2005 [3-5]. Th17 cells were first found to be clinically important in autoimmune disorders, and are now of interest in transplantation [6-8]. Indeed, Th17 cells and their associated cytokines play an important role in the development of acute and chronic allograft injury after organ transplantation [1,8-12]. For example, IL-17 messenger RNA (mRNA) and protein levels are elevated in animal models of acute rejection [13]. In KT, the IL-17 mRNA level is elevated in proximal tubular epithelial cells from allograft tissue with subclinical rejection [14]. Moreover, the IL‑17 protein level is elevated, and IL-17 mRNA is detectable, in kidney allografts with subclinical rejection [7]. Here, we review the findings on the role of Th17 cells in the development of acute or chronic rejection in KT, and discuss strategies to control the Th17 pathway.
OVERVIEW OF TH17 CELLS
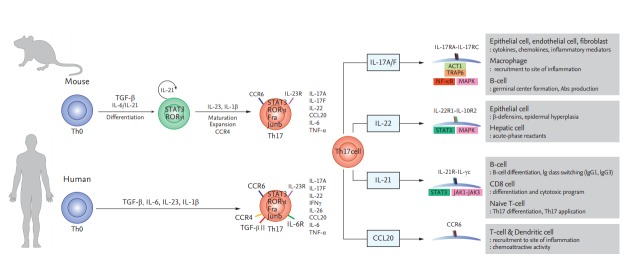
Th17 cells secrete IL-17, which recruits monocytes and neutrophils and acts in synergy with other proinflammatory cytokines [3]. Transforming growth factor β, IL-6, and IL-1β mediate the induction of immature Th17 cells [15,16]. However, they have a different nuclear transcript factor profile compared to classical Th1 cells; i.e., RORγt (RAR-related orphan receptor γt) and signal transducer and activator of transcription 3 (STAT3) [3,17]. Finally, differentiation into effector Th17 cells is mediated by the IL-23–IL-23R interaction [18]. Mature Th17 cells produce IL-22, express C-C motif chemokine receptor (CCR)-6, -4, and -10, and do not produce IL-10 (Fig. 1) [19-21].
Figure 1.
Differentiation of T helper 17 (Th17) cells in mice and humans and the functions of Th17 cytokines and chemokines. Th17 cells secrete interleukin 17A/F (IL-17A/F), interleukin 22 (IL-22), interleukin 21 (IL-21), and C-C motif chemokine ligand 20 (CCL20), which modulate inflammation and immune cell recruitment. TGF-β, Transforming growth factor β; STAT3, signal transducer and activator of transcription 3; RORγt, RAR-related orphan receptor γt; CCR, C-C motif chemokine receptor; ACT1, actin 1; TRAP6, thrombin receptor-activating peptide-6; NF-κB, nuclear factor κB; MAPK, mitogen-activated protein kinase; IgG, immunoglobulin G.
TH17 CELLS IN KIDNEY ALLOGRAFT TISSUE WITH ACUTE ALLOGRAFT REJECTION
Acute allograft rejection is initiated by alloreactive T-cells primed in secondary lymphoid organs and recruited to the graft. The production of various proinflammatory cytokines by infiltrating cells is increased during acute kidney allograft rejection [22-24]. Therefore, diagnosis and staging of allograft rejection are based on the severity or pattern of immune cell infiltration and evidence of local immune system activation in allograft tissue [25-28]. Increased expression of IL-17 in local tissue is associated with allograft rejection in vivo [7,14,29]. Also, greater infiltration of Th17 cells compared to FOXP3+ (forkhead box P3+) regulatory T-cells (Tregs) is associated with both the severity of T-cell–mediated rejection (TCMR) and the subsequent clinical prognosis. A lower Treg/Th17 infiltration ratio is significantly associated with reduced allograft function and more severe interstitial and tubular injury [26]. Also, greater infiltration of Th17 cells is significantly associated with steroid-resistant rejection, incomplete recovery, recurrent TCMR, and a lower allograft survival rate after rejection. Several mechanisms have been suggested to underlie the above. First, renal epithelial cells exposed to IL-17 produce inflammatory mediators that stimulate early alloimmune responses [13]. Second, IL-17 induces neutrophil recruitment during severe rejection, as seen in biopsies [30]. Third, Th17 cells drive alloimmune responses by promoting lymphoid neogenesis [12]. Thus, Th17 cells induce stronger and more sustained alloimmune responses, which can result in severe injury to allograft tissue.
ROLE OF TH17 CELLS IN CHRONIC KIDNEY ALLOGRAFT DYSFUNCTION
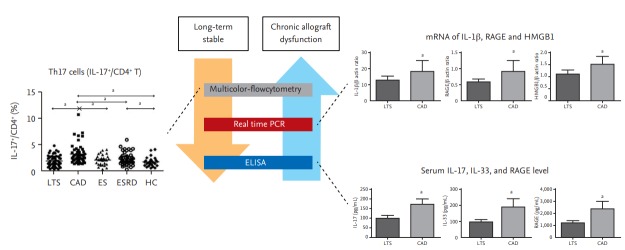
Th17 cells may be associated with severe allograft rejection. Therefore, we investigated whether Th17 cells are involved in chronic allograft dysfunction (CAD), which is the main cause of allograft failure. In human proximal renal tubular epithelial cells (HPRTEpiCs), IL-17 not only increases the production of markers of acute inflammation (IL-6 and IL-8) but also modulates the expression of profibrotic markers; e.g., CTGF (connective tissue growth factor) and ACTA-2 (α-actin 2) [31-33]. The proportion of peripheral blood Th17 cells from KT recipients with CAD was significantly increased in comparison to those of KT recipients with stable allograft function, irrespective of follow-up duration. Interestingly, the proportion of Th17 cells was higher in CAD patients than in transplant-naïve chronic kidney disease (CKD) patients, although renal function was lower in the CKD patients. This is important because uremia itself can increase the proportion of IL-17–producing effector T-cells [34]. Our results suggest that the immune response rather than renal dysfunction is responsible for the increased proportion of Th17 cells in patients with CAD (Fig. 2) [33].
Figure 2.
Activation of the T helper 17 (Th17) pathway in kidney transplantation recipients with chronic allograft dysfunction (CAD). The proportion of Th17 cells (interleukin 17+ [IL-17+]/cluster of differentiation 4+ [CD4+]); the IL-1β, receptor for advanced glycation end products (RAGE), and high mobility group box 1 (HMGB-1) messenger RNA (mRNA) levels; and the IL17, interleukin 33 (IL-33), and RAGE levels in peripheral blood are higher in patients with CAD. Modified from Chung et al. [41]. LTS, long-term stable; ES, early stable; ESRD, end stage renal disease; HC, healthy control; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay. ap < 0.05 for each comparison.
EFFECTS OF IMMUNOSUPPRESSIVE AGENTS ON TH17 CELLS
The incidence of early allograft loss due to acute rejection can be significantly reduced by use of immune suppressants [35]. T-cells are suppressed by treatment with a combination of tacrolimus (Tac), mycophenolate mofetil, and steroid. In addition, induction therapy using basiliximab, an anti-CD25 monoclonal antibody, suppresses the proliferation of T-cells [1]. Although maintenance immune suppression can improve the allograft survival rate in the first year after KT, there has been little improvement in the long-term outcomes [36], suggesting that the currently used immune-suppressant regimen has limitations. Tac, the major immune-suppressant used in KT recipients, blocks Th1- and Th2-associated alloimmune responses [37-39]. In contrast, few animal studies have investigated the effect of calcineurin inhibitors (CNIs) on Th17 responses. In an animal model of heart transplantation, CNI did not suppress the Th17-associated response [40]. Both Th1 and Th2 cytokines are required to reduce the IL-17 level, while administration of an anti-Th1 or -Th2 cytokine antibody increases the IL-17 level [3]. Therefore, administration of Tac may actually enhance the Th17 response. Interestingly, the percentage of Th17 cells and production of IL‑17 by effector memory T-cells (TEM) are significantly (p < 0.05) increased at 3 months after KT compared to baseline, whereas the proportions of Th1/Th2 cells and TEM cells are decreased in the early post-transplantation period [41]. In addition, Tac suppresses Th1 and Th2 cells in a concentration-dependent manner, but even a high concentration has no effect on Th17 cells in vitro [41]. This suggests that Tac-based immunosuppression may be inadequate to suppress Th17 cells in KT recipients.
STRATEGIES TARGETING TH17 CELLS
mTOR inhibitors
Activation of Th17 cells is related to more severe allograft rejection and subsequent adverse outcomes [26,42]. In addition, an increased proportion of Th17 cells in peripheral blood is associated with CAD [33]. Therefore, modulation of the Th17 response may improve allograft outcomes in KT. Mammalian target of rapamycin (mTOR) is an important regulator of helper T-cell differentiation [43-47]. Cluster of differentiation 4+ (CD4+) T-cells lacking or deficient in mTOR fail to differentiate into effector cells or Treg cells under appropriate conditions [48,49]. Also, mTOR inhibition abrogates the reprogramming of Treg cells into pathogenic Th1/Th17 effector cells [50]. Sirolimus (SRL), an mTOR inhibitor, suppresses Th17 cells in KT recipients [50,51]. Moreover, conversion from Tac to SRL inhibits the proliferation of allogeneic CD4+ T-cells and Th17 cells in vitro and ex vivo [52].
Regulation of Th17 cells by 1α,25-dihydroxyvitamin D3
Another strategy to regulate Th17 cells is to compensate Tac instead of conversion to mTOR inhibitor. Metabolic regulators, such as vitamin D, have therapeutic effects on immunologic disorders that involve the Th17 response [53-55]. Indeed, a low serum 25‑hydroxyvitamin D (25-[OH]D) level is associated with high Th17 activity in patients with autoimmune diseases or graft-versus-host disease after hematopoietic stem-cell transplantation. Furthermore, treatment with 1α,25-dihydroxyvitamin D3 (1,25[OH]2D3) ameliorates these disorders by modulating the Th17 response [53,56-58] through suppression of the mTOR/STAT3 pathway [57,59,60]. Indeed, addition of 1,25(OH)2D3 to Tac significantly inhibits Th17 cells in vitro and reduces the IL-17 and IL-22 mRNA levels in peripheral blood mononuclear cells [61]. In Jurkat cells, the mTOR/STAT3 pathway is downregulated by the addition of 1,25(OH)2D3 to Tac [61]. In an ex vivo study, treatment with 1,25(OH)2D3 for 6 months significantly decreased the Th17 level compared to baseline in 42 KT recipients [61]. Furthermore, resveratrol regulates the Th17 pathway by activating AMPK and suppressing mTOR (unpublished data). mTOR-targeted therapy suppresses Th17-related immune responses in KT recipients (Fig. 3).
Figure 3.
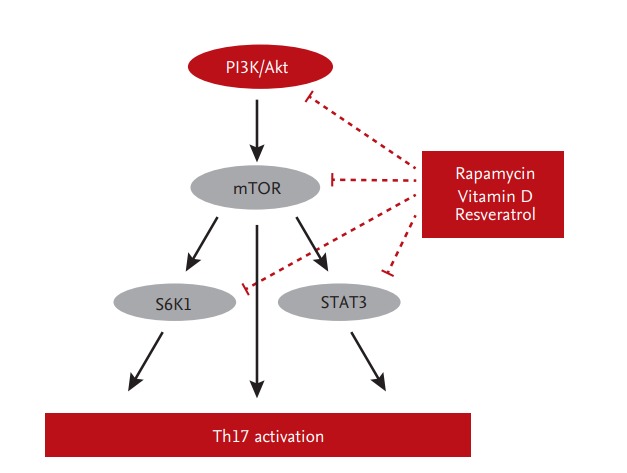
Effec inhibitors on the mTOR/signal transducer and activator of transcription 3 (STAT3) signaling pathway in T helper 17 (Th17) cells. PI3K, phosphoinositide 3-kinase; S6K1, ribosomal protein S6 kinase beta-1.
CONCLUSIONS
This review summarizes the role of Th17 cells in KT. The Th17 pathway plays an important role in various types of allograft injury. Tac-based immunosuppression has a limited impact on Th17-cell-induced allograft injury. Several recent studies, including ours, suggest that mTOR-targeted strategies could suppress the Th17 pathway, but the clinical relevance is unclear. Therefore, development of strategies targeting the Th17 pathway is required to improve allograft outcomes.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Fan H, Jiang S. CD4(+) T-cell subsets in transplantation. Immunol Rev. 2013;252:183–191. doi: 10.1111/imr.12038. [DOI] [PubMed] [Google Scholar]
- 3.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 6.Loong CC, Lin CY, Lui WY. Expression of interleukin-17 as a predictive parameter in acute renal allograft rejection. Transplant Proc. 2000;32:1773. doi: 10.1016/s0041-1345(00)01382-8. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14:287–298. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14:326–331. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 9.Calvo-Turrubiartes M, Romano-Moreno S, Garcia-Hernandez M, et al. Quantitative analysis of regulatory T cells in kidney graft recipients: a relationship with calcineurin inhibitor level. Transpl Immunol. 2009;21:43–49. doi: 10.1016/j.trim.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Warrens AN. Pharmacological control of the immune response in renal transplantation. BJU Int. 2002;90:784–791. doi: 10.1046/j.1464-410x.2002.03006.x. [DOI] [PubMed] [Google Scholar]
- 11.Crispim JC, Grespan R, Martelli-Palomino G, et al. Interleukin-17 and kidney allograft outcome. Transplant Proc. 2009;41:1562–1564. doi: 10.1016/j.transproceed.2009.01.092. [DOI] [PubMed] [Google Scholar]
- 12.Deteix C, Attuil-Audenis V, Duthey A, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 13.Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197:322–332. doi: 10.1002/path.1117. [DOI] [PubMed] [Google Scholar]
- 14.Van Kooten C, Boonstra JG, Paape ME, et al. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9:1526–1534. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 15.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Kuchroo VK, Awasthi A. Emerging new roles of Th17 cells. Eur J Immunol. 2012;42:2211–2214. doi: 10.1002/eji.201242872. [DOI] [PubMed] [Google Scholar]
- 19.Van Voorhis M, Fechner JH, Zhang X, Mezrich JD. The aryl hydrocarbon receptor: a novel target for immunomodulation in organ transplantation. Transplantation. 2013;95:983–990. doi: 10.1097/TP.0b013e31827a3d1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 21.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 22.Merville P, Pouteil-Noble C, Wijdenes J, Potaux L, Touraine JL, Banchereau J. Cells infiltrating rejected human kidney allografts secrete IFN-gamma, IL-6, and IL-10, and are modulated by IL-2 and IL-4. Transplant Proc. 1993;25(1 Pt 1):111–113. [PubMed] [Google Scholar]
- 23.Merville P, Pouteil-Noble C, Wijdenes J, Potaux L, Touraine JL, Banchereau J. Detection of single cells secreting IFN-gamma, IL-6, and IL-10 in irreversibly rejected human kidney allografts, and their modulation by IL-2 and IL-4. Transplantation. 1993;55:639–646. doi: 10.1097/00007890-199303000-00032. [DOI] [PubMed] [Google Scholar]
- 24.Pavlakis M, Strehlau J, Lipman M, Shapiro M, Maslinski W, Strom TB. Intragraft IL-15 transcripts are increased in human renal allograft rejection. Transplantation. 1996;62:543–545. doi: 10.1097/00007890-199608270-00020. [DOI] [PubMed] [Google Scholar]
- 25.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 26.Chung BH, Oh HJ, Piao SG, et al. Higher infiltration by Th17 cells compared with regulatory T cells is associated with severe acute T-cell-mediated graft rejection. Exp Mol Med. 2011;43:630–637. doi: 10.3858/emm.2011.43.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8:670–678. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 28.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574–582. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 29.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 30.Healy DG, Watson RW, O'Keane C, et al. Neutrophil transendothelial migration potential predicts rejection severity in human cardiac transplantation. Eur J Cardiothorac Surg. 2006;29:760–766. doi: 10.1016/j.ejcts.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 31.Gore-Hyer E, Shegogue D, Markiewicz M, et al. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol Renal Physiol. 2002;283:F707–F716. doi: 10.1152/ajprenal.00007.2002. [DOI] [PubMed] [Google Scholar]
- 32.LeBleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung BH, Kim KW, Kim BM, Doh KC, Cho ML, Yang CW. Increase of Th17 cell phenotype in kidney transplant recipients with chronic allograft dysfunction. PLoS One. 2015;10:e0145258. doi: 10.1371/journal.pone.0145258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung BH, Kim KW, Sun IO, et al. Increased interleukin-17 producing effector memory T cells in the end-stage renal disease patients. Immunol Lett. 2012;141:181–189. doi: 10.1016/j.imlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 36.Guerra G, Srinivas TR, Meier-Kriesche HU. Calcineurin inhibitor-free immunosuppression in kidney transplantation. Transpl Int. 2007;20:813–827. doi: 10.1111/j.1432-2277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 37.Rentenaar RJ, van Diepen FN, Meijer RT, et al. Immune responsiveness in renal transplant recipients: mycophenolic acid severely depresses humoral immunity in vivo. Kidney Int. 2002;62:319–328. doi: 10.1046/j.1523-1755.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 38.Takatsuki M, Uemoto S, Inomata Y, et al. Analysis of alloreactivity and intragraft cytokine profiles in living donor liver transplant recipients with graft acceptance. Transpl Immunol. 2001;8:279–286. doi: 10.1016/s0966-3274(01)00027-2. [DOI] [PubMed] [Google Scholar]
- 39.Weimer R, Melk A, Daniel V, Friemann S, Padberg W, Opelz G. Switch from cyclosporine A to tacrolimus in renal transplant recipients: impact on Th1, Th2, and monokine responses. Hum Immunol. 2000;61:884–897. doi: 10.1016/s0198-8859(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 40.Syrjala SO, Keranen MA, Tuuminen R, et al. Increased Th17 rather than Th1 alloimmune response is associated with cardiac allograft vasculopathy after hypothermic preservation in the rat. J Heart Lung Transplant. 2010;29:1047–1057. doi: 10.1016/j.healun.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Chung BH, Kim KW, Kim BM, et al. Dysregulation of Th17 cells during the early post-transplant period in patients under calcineurin inhibitor based immunosuppression. PLoS One. 2012;7:e42011. doi: 10.1371/journal.pone.0042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung BH, Oh HJ, Piao SG, et al. Clinical significance of the ratio between FOXP3 positive regulatory T cell and interleukin-17 secreting cell in renal allograft biopsies with acute T-cell-mediated rejection. Immunology. 2012;136:344–351. doi: 10.1111/j.1365-2567.2012.03588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flechner SM, Kurian SM, Solez K, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant. 2004;4:1776–1785. doi: 10.1111/j.1600-6143.2004.00627.x. [DOI] [PubMed] [Google Scholar]
- 46.Larson TS, Dean PG, Stegall MD, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant. 2006;6:514–522. doi: 10.1111/j.1600-6143.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 47.Hackstein H. Rapamycin and dendritic cells: keep on movin'. Transplantation. 2006;82:739–740. doi: 10.1097/01.tp.0000235438.11132.8f. [DOI] [PubMed] [Google Scholar]
- 48.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yurchenko E, Shio MT, Huang TC, et al. Inflammation-driven reprogramming of CD4+ Foxp3+ regulatory T cells into pathogenic Th1/Th17 T effectors is abrogated by mTOR inhibition in vivo. PLoS One. 2012;7:e35572. doi: 10.1371/journal.pone.0035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Shi Y, Huang Z, et al. CNI induced Th17/Treg imbalance and susceptibility to renal dysfunction in renal transplantation. Int Immunopharmacol. 2011;11:2033–2038. doi: 10.1016/j.intimp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Kim KW, Chung BH, Kim BM, Cho ML, Yang CW. The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients. Immunology. 2015;144:68–78. doi: 10.1111/imm.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev. 2011;10:733–743. doi: 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Smolders J, Menheere P, Thewissen M, et al. Regulatory T cell function correlates with serum 25-hydroxyvitamin D, but not with 1,25-dihydroxyvitamin D, parathyroid hormone and calcium levels in patients with relapsing remitting multiple sclerosis. J Steroid Biochem Mol Biol. 2010;121:243–246. doi: 10.1016/j.jsbmb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Datta Mitra A, Raychaudhuri SP, Abria CJ, et al. 1α,25-Dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR signaling cascades: a therapeutic agent for psoriasis. J Invest Dermatol. 2013;133:1556–1564. doi: 10.1038/jid.2013.3. [DOI] [PubMed] [Google Scholar]
- 58.Ranganathan P, Khalatbari S, Yalavarthi S, Marder W, Brook R, Kaplan MJ. Vitamin D deficiency, interleukin 17, and vascular function in rheumatoid arthritis. J Rheumatol. 2013;40:1529–1534. doi: 10.3899/jrheum.130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011;10:1888–1889. doi: 10.4161/cc.10.12.15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lisse TS, Liu T, Irmler M, et al. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J. 2011;25:937–947. doi: 10.1096/fj.10-172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung BH, Kim BM, Doh KC, et al. Suppressive effect of 1α,25-dihydroxyvitamin D3 on Th17-immune responses in kidney transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2017;101:1711–1719. doi: 10.1097/TP.0000000000001516. [DOI] [PubMed] [Google Scholar]