Abstract
Rapid medical countermeasure (MCM) dispensing is an important intervention during a public health emergency. In the United States, MCM planning and exercising efforts have largely focused on dispensing therapeutics, with less emphasis on mass vaccination operations that would require additional specialized staff and infrastructure. Difficulties in distributing vaccines during the 2009 H1N1 influenza pandemic highlighted the need for enhanced planning and exercising of plans for conducting mass vaccination campaigns.
In Taiwan, seasonal influenza mass vaccination campaigns are conducted annually, which both mitigate the effects of seasonal influenza and serve as functional exercises for mass vaccination operations during a pandemic. To identify lessons that can be applied to mass vaccination planning in the United States and elsewhere, we conducted an in-person observation and data review of Taiwan’s annual seasonal influenza mass vaccination efforts in October 2017.
We offer findings and recommendations for enhancing preparedness for seasonal and pandemic influenza and other public health emergencies that would require mass vaccination.
Rapid medical countermeasure (MCM) dispensing—including vaccines, antivirals, and antibiotics—is an important intervention for treating or preventing disease during a public health emergency. In the United States, MCM planning and exercising efforts have largely focused on dispensing therapeutics, with less emphasis on mass vaccination.1 Mass vaccination requires specialized staff and infrastructure (e.g., cold chain) that may not be necessary for dispensing therapeutics.2 In light of these additional challenges, there is a need to develop and exercise plans specifically for conducting mass vaccination campaigns during public health emergencies.
The United States’ 2009 H1N1 influenza pandemic response—which included a nationwide mass vaccination campaign that encompassed the manufacturing, distribution, and administration of pandemic influenza vaccine to more than 80 million individuals3—highlighted a number of challenges associated with mass vaccination. During the response, state and local health departments were responsible for “organizing and implementing population-wide immunization campaigns” at no cost to the individual.4(p323) The mass vaccination campaign commenced in October 2009,5 but by its completion in May 2010, only 32.4% of individuals in the initial priority groups had been vaccinated.3 This was the result of numerous planning and operational challenges, including vaccine shortages, problems tracking vaccine supplies, difficulties in prioritizing high-risk groups, and issues identifying and getting vaccine to individuals in the priority groups.4 The 2009 H1N1 pandemic represented the United States’ first nationwide mass vaccination campaign in more than three decades,6 and because US seasonal influenza immunization is largely conducted by private providers, pharmacies, and workplaces rather than by mass vaccination events, there has been little opportunity for large-scale testing and improvement of mass vaccination planning efforts.
In Taiwan, seasonal influenza mass vaccination campaigns are conducted annually. In addition to mitigating the effects of seasonal influenza, these efforts serve as functional exercises for distributing and dispensing vaccines during larger-scale pandemics. Taiwan gains yearly practice in identifying vaccination sites and providers; maintaining relationships among health officials, other government agencies, and the private sector; and raising public awareness of vaccination activities.
To identify lessons that can be applied to emergency MCM dispensing, we conducted an analysis of seasonal influenza mass vaccination efforts in Taiwan. We conducted this analysis through a literature review, targeted interviews with local- and national-level public health and preparedness officials in Taiwan, and site visits to observe local mass vaccination events. We utilized additional data from the Taiwan Centers for Disease Control (Taiwan CDC) on vaccine purchases and coverage for priority groups to further assess the impact of the mass vaccination program and elucidate lessons that could benefit similar future efforts in the United States and elsewhere.
US MCM DISPENSING APPROACHES
In 2004, the US Centers for Disease Control and Prevention (US CDC) established the Cities Readiness Initiative (CRI) to provide funding for high-risk state and local health departments to “develop, test, and maintain plans to quickly receive medical countermeasures from CDC’s Strategic National Stockpile (SNS) and distribute them to local communities” during a public health emergency.7 The CRI’s stated planning scenario is based on a covert release of Bacillus anthracis, which necessitates the dispensing of antibiotics via points of dispensing (PODs) in less than 48 hours.8 As a result, many jurisdictions focus on dispensing antibiotics, rather than on mass vaccination. One survey of public health disaster planners found that fewer than half had conducted a vaccination clinic exercise during the previous 2 years.9 Mass vaccination efforts do occur in some locations in the United States, but there is no coordinated effort to implement or measure the impact of these programs consistently across most states or the country.
TAIWAN INFLUENZA VACCINATION PROGRAM
Taiwan’s seasonal influenza mass vaccination program, directed and coordinated by Taiwan CDC that is run by the Ministry of Health and Welfare, was initiated in 1998. The first priority groups included individuals aged 65 years or older with specific high-risk comorbidities and residents or staff of long-term care facilities. These priority groups could access the vaccine at little to no cost, as the cost and administration of the vaccine was subsidized by Taiwan CDC. Over the years, increases in funding—which now stands at approximately $40.6 million—has allowed Taiwan CDC to purchase more vaccine doses and subsequently expand the priority populations.
In 1999 and 2000, Taiwan CDC added additional qualifying comorbidities for the priority population aged 65 years or older, finally expanding to include all individuals aged 65 years or older (regardless of comorbidities) in 2001.10 Other priority groups were added starting in 2003, which now include those who are particularly vulnerable to influenza (e.g., individuals aged 50 years or older, preschool-aged children, pregnant women, patients with comorbid conditions), those who work or live with vulnerable populations or in high-risk environments (e.g., health care workers, parents and caregivers of young children, poultry workers), or those who are significant sources of community transmission (e.g., elementary-school and junior and senior high-school students).11,12 In 2007, Taiwan incorporated its seasonal influenza mass vaccination program into the country’s pandemic preparedness plan, recognizing the utility of the campaign in building capacity for larger pandemics.
Taiwan CDC coordinates mass vaccination events in a variety of settings, including nursery schools, elementary schools, and junior and senior high schools; the community; businesses; poultry markets and slaughterhouses; nursing homes; district public health centers; and local clinics, hospitals, and other health care facilities. In addition, local health bureau staff visit elderly and disabled individuals’ homes to ensure vaccine access for those who are homebound. For larger-scale mass vaccination events, local health bureaus coordinate with clinics and hospitals to identify clinicians to perform health screenings (e.g., medical history, physical examination), vaccine administration, adverse event monitoring, and documentation and reporting. Some of these events vaccinate thousands of individuals in a matter of hours.
Public communication and education are a top planning priority. Educational materials—including posters and social media posts as well as the seasonal influenza Web site—are published as early as August. The campaign is a coordinated effort, with multisectoral involvement that includes more than 3500 local clinics and hospitals, 370 district public health centers, 22 local health bureaus, local education departments, and national government agencies such as the Council of Agriculture. The annual campaign prepares health officials and clinicians for the operational challenges inherent to emergency mass vaccination and strengthens relationships between national and local government agencies, the private sector, and the public that are critical to the success of these operations in the midst of an emergency.
ENSURING ADEQUATE PERSONNEL FOR VACCINATION
In the United States and elsewhere, staffing PODs or other dispensing sites with adequate clinical personnel could be a major challenge during a health emergency, particularly for large cities that may require the operation of multiple PODs simultaneously. In the survey discussed previously, only 42.6% of the public health disaster planners “perceived adequate staffing to operate all open PODs.”9 Staffing demands were a particular logistical challenge during the 2009 H1N1 response.4 Most local health departments have limited clinical staff and certainly not enough to conduct vaccination operations for the entire jurisdiction.
With experienced personnel and training plans in place, Taiwan is positioned to respond to health emergencies that require mass vaccination and surge staffing. Taiwan contracts with clinicians—including nurses and physicians—from local hospitals and clinics during annual mass vaccination events, and protocols exist to identify, recruit, and reimburse local clinicians from outside the health bureau for vaccination operations. Annual experience in coordinating and conducting mass vaccination operations with these private-sector clinicians helps to familiarize vaccination personnel with plans and procedures at local PODs.
Influenza pandemics may bring about various disruptions in personnel availability that do not exist during routine seasonal vaccination programs. Taiwan aims to mitigate this issue by routinizing participation of health care providers from across a large number of facilities. During a much more severe pandemic, however, relying on traditional health care professionals could potentially pose problems for personnel availability. Incorporating other health care professionals during annual influenza vaccination programs could help provide the surge capacity needed during a pandemic. In the United States, most states allow pharmacists to administer seasonal influenza vaccinations, which played an integral role during the 2009 H1N1 pandemic as well as in improving annual seasonal vaccination coverage.13–15 Including other volunteers and implementing policies to expand practitioner scopes of practice during pandemics are other ways to help meet surge demand during pandemics.16
ENHANCED ADVERSE EVENT PREVENTION AND SURVEILLANCE
In recent years, increasing public concern about vaccine safety has challenged vaccination efforts in many countries, including the United States. Taiwan faced similar challenges following the death of a physician’s child shortly after receiving the 2009 pandemic H1N1 vaccine, a widely reported event in the Taiwanese media.17,18 Taiwan CDC responded to the public sentiment spurred by this incident by enhancing adverse event reporting and follow-up capabilities, with the dual aim of restoring public trust in the vaccination program and reducing the likelihood that adverse event claims would be reported directly to the media.
Before this incident, Taiwan CDC required local health bureaus to report potential adverse events and conduct follow-up outreach for affected individuals. After this incident, Taiwan CDC re-emphasized the importance of proactive follow-up with additional outreach measures, including educating potentially affected individuals about the vaccine and associated adverse events and, when necessary, assisting them in seeking care and registering for the Vaccine Injury Compensation Program.
Following the occurrence of a number of psychogenic events—including syncopal episodes (i.e., fainting)—among junior and senior high-school students at school-based vaccination events during the 2009 H1N1 pandemic influenza vaccination program, Taiwan CDC updated guidance regarding site layout and patient flow to reduce stress for individuals receiving the vaccine—including queueing children in small groups rather than all at once to reduce both wait times and the number of other children they see receive the vaccine.19 In addition, Taiwan CDC now utilizes social media and other platforms to engage with the public directly, providing guidance aimed at, among other things, reducing vaccination-related stress to mitigate syncopal episodes—including advice to eat before getting vaccinated and to listen to music or talk with friends to reduce stress while waiting in line.
As the United States experiences similar public concerns about the safety of routine vaccinations, it will likely be important that plans for conducting mass vaccination during a pandemic include efforts to collect the information necessary for monitoring for adverse events. This will require determining the legal and epidemiological data requirements for conducting adverse events surveillance and establishing protocols for on-site data collection. Although Taiwan’s adverse event mitigation and monitoring efforts did not appear to have any significant negative impact on POD throughput, it will be important to examine potential effects for US-based mass-vaccination events.
INCREASING VACCINE COVERAGE
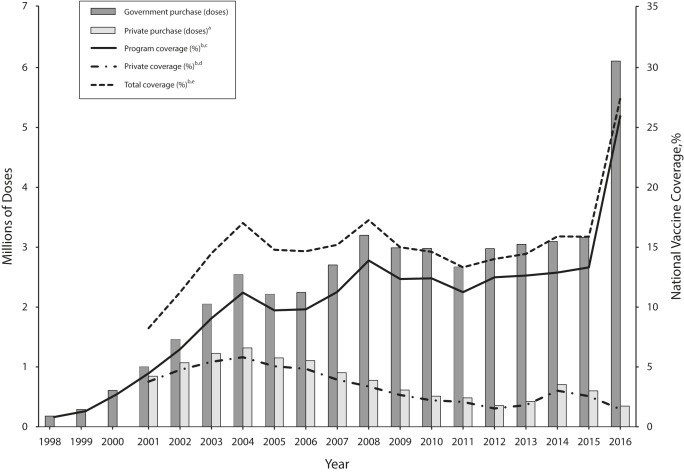
Taiwan appears to be making positive progress toward its goal of boosting vaccine coverage among priority populations, steadily increasing the number of doses purchased for this program from 180 000 in 1998 to 6.1 million in 2016 and 2017 (Figure 1). Taiwan CDC has collected data on vaccine wastage in the seasonal influenza vaccination program since 2007, and fewer than 5% of the doses have failed to be administered each year, with a median of 2.33% over that period. Assuming that essentially all purchased doses were used in 1998, the program would have covered only 0.82% of Taiwan’s entire population. The program now covers an estimated greater than 25% of the population. Seasonal influenza vaccination in Taiwan outside of the mass vaccination program is low and steadily decreasing as more priority groups are added to the program. In fact, only 2% of the Taiwanese population not included in a priority group was vaccinated in 2016 (Figure 1).
FIGURE 1—
Total Vaccine Doses Purchased and Vaccine Coverage Estimates for the Taiwan Annual Seasonal Influenza Mass Vaccination Campaign and the Noneligible Population: 1998–2016
Note. Data on purchases made outside the government-sponsored program are not available for 1998 to 2000. No attempt was made to speculate on the number of doses purchased, private coverage, or total coverage for these years.
aSeasonal influenza vaccine doses purchased outside the government-sponsored vaccination program for individuals not eligible for the program.
bDoes not account for wastage. Vaccine wastage data are only available for 2007 to 2016, but wastage is minimal. The maximum percentage wastage for the government purchases during this period was 4.59% (median = 2.33%).
cEstimated percentage of the total Taiwan population that received the seasonal influenza vaccine using doses purchased by the Taiwan government as part of the annual mass vaccination program.
dEstimated percentage of the total Taiwan population that received the seasonal influenza vaccine using doses purchased outside the government-sponsored vaccination program.
eEstimated percentage of the total Taiwan population that received the seasonal influenza vaccine.
Source. National Development Council.20
Taiwan CDC does not collect vaccine coverage data for priority groups before adding them to the mass vaccination program, but if coverage for these groups was similar to that for the rest of Taiwan, then the program is having a substantial effect on improving coverage for all priority groups. Table 1 shows vaccine coverage for priority groups in Taiwan compared with coverage estimates for the portion of the population not eligible for the vaccination program. All priority groups have markedly better vaccine coverage than what would be expected if they were not part of the mass vaccination program.
TABLE 1—
Taiwan Annual Seasonal Influenza Mass Vaccination Program Priority Group Coverage Estimates by Year: 1998–2016
| Year | Aged ≥ 65 ya and Long-Term Care Residents, % | Health Care Workers, % | Poultry Workers, % | Aged 6 mo–3 y, % | Elementary-School Students,b % | Aged 3–6 y, % | Aged 50–64 y, % | Junior and Senior High-School Students, % | Noneligible Population,c,d % |
| 1998 | 9.99 | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | NA |
| 1999 | 15.78 | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | NA |
| 2000 | 24.48 | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | NA |
| 2001 | 50.70 | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | 3.95 |
| 2002 | 59.90 | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | 5.08 |
| 2003 | 68.40 | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | . . .e | 5.96 |
| 2004 | 60.33 | 88.06 | 64.83 | . . .e | . . .e | . . .e | . . .e | . . .e | 6.54 |
| 2005 | 59.50 | 94.40 | 93.69 | 66.55 | . . .e | . . .e | . . .e | . . .e | 5.60 |
| 2006 | 52.93 | 93.15 | 57.37 | 60.31 | . . .e | . . .e | . . .e | . . .e | 5.37 |
| 2007 | 48.85 | 83.64 | 50.70 | 46.72 | 66.72 | . . .e | . . .e | . . .e | 4.46 |
| 2008 | 52.78 | 87.07 | 66.47 | 48.17 | 78.88 | . . .e | . . .e | . . .e | 3.91 |
| 2009 | 38.14 | 82.31 | 41.48 | 50.62 | 79.19 | 15.21 | . . .e | . . .e | 3.05 |
| 2010 | 36.40 | 85.18 | 44.00 | 30.30 | 68.57 | 19.78 | . . .e | . . .e | 2.54 |
| 2011 | 40.23 | 85.34 | 57.88 | 40.25 | 72.19 | 26.27 | . . .e | . . .e | 2.35 |
| 2012 | 41.62 | 90.19 | 73.12 | 33.09 | 71.11 | 27.63 | . . .e | . . .e | 1.76 |
| 2013 | 41.08 | 94.54 | 81.74 | 31.42 | 71.98 | 29.05 | . . .e | . . .e | 2.08 |
| 2014 | 41.09 | 72.55 | 91.90 | 46.24 | 71.92 | 31.10 | . . .e | . . .e | 3.48 |
| 2015 | 39.43 | 72.64 | 86.87 | 53.31 | 71.91 | 31.19 | . . .e | . . .e | 2.96 |
| 2016 | 49.19 | 78.24 | 97.50 | 55.32 | 74.34 | 42.22 | 20.06 | 75.79 | 1.99 |
Note. NA = not available.
1998–2000: aged ≥ 65 years with specific high-risk comorbidities including cardiopulmonary disease and diabetes; 2001–2016: all individuals aged ≥ 65 years.
2007: aged 7–8 years; 2008–2011: aged 7–10 years; 2012–2016: aged 7–12 years.
Taiwan Centers for Disease Control does not collect vaccine coverage data for priority groups before adding them to the vaccination program. We can assume that the coverage for the period before adding them was approximately equal to that of the population not eligible for the program.
Coverage estimate based on number of vaccine doses purchased outside the government-sponsored program (numerator). Population estimate used for this calculation (denominator) was determined by subtracting the number of government-purchased vaccine doses from the total Taiwan population for that year (data not shown).20
Not designated as a priority population this year.
Although seasonal influenza vaccine coverage in the United States is typically higher than in Taiwan, influenza vaccine uptake in the United States has remained relatively stagnant.21 Annual mass influenza vaccinations already take place in many US cities, but additional efforts at the national level are required to reach established coverage goals. Increasing the number of mass vaccination efforts that take place each year could help boost vaccine coverage, while at the same time enhancing readiness for larger-scale pandemics.
IMPROVING PANDEMIC PREPAREDNESS
The approach to health care delivery in Taiwan—which has nationalized health insurance—makes it unlikely that the United States could fully replicate Taiwan’s seasonal influenza vaccination program, but there are still useful lessons from Taiwan’s experience that can be translated into practice in the United States (and elsewhere) to improve pandemic preparedness. The United States conducts MCM dispensing planning through a variety of federal funding mechanisms, including CRI.7 Similar programs dedicated to mass vaccination could provide the necessary resources to build this capacity across the United States. Many state and local health departments already distribute free or reduced-cost vaccines to the public via vaccination clinics, often with federal support, such as from the Vaccines for Children program.22 Expanding these programs to include mass vaccination events could not only improve vaccination coverage but also provide an opportunity to exercise pandemic response operations similar to the approach in Taiwan. This kind of program could also help establish performance metrics (e.g., POD throughput) and evaluation protocols (e.g., the Technical Assistance Review) similar to those currently used to assess antibiotics dispensing under CRI, thus providing baseline data on current mass vaccination capacities nationwide. It is unlikely that the US government could initiate this type of program at the federal level, but pilot studies in select cities and states could provide evidence-based support for continued funding and nationwide implementation.
CONCLUSION
Existing programs in the United States to improve emergency MCM dispensing capabilities largely focus on anthrax-like scenarios. If one considers the additional challenges required to implement mass vaccination operations, targeted efforts are required to establish and maintain this capacity. Since CRI was established in 2004,23 MCM dispensing capabilities have improved markedly, and the US CDC should consider expanding this program to provide similar assistance and guidance to improve emergency mass vaccination operations. Combining these efforts with the annual implementation of seasonal influenza vaccination programs would provide regular opportunities to exercise the necessary relationships, infrastructure, and operations and to conduct more accurate readiness assessments, including staffing availability and POD throughput, under real-world conditions, with the added benefit of increasing influenza vaccine coverage.
Taiwan’s experience with influenza vaccination campaigns has helped identify challenges inherent to operationalizing mass vaccination programs and has provided unique lessons that may be beneficial to health departments desiring to improve both seasonal vaccination efforts and pandemic readiness. Expanding existing MCM distribution and dispensing preparedness programs in the United States to more explicitly address mass vaccination can support public health officials in developing, exercising, and assessing critical relationships and tools required to implement mass vaccination during an emergency.
ACKNOWLEDGMENTS
This research was conducted by the Johns Hopkins Center for Health Security’s Outbreak Observatory, which is funded by the Open Philanthropy Project.
We would like to acknowledge Michael Snyder and Christopher Hurtado with the Outbreak Observatory team for their help, and our colleagues at the Taiwan Centers for Disease Control for collaborating with the Outbreak Observatory.
HUMAN PARTICIPANT PROTECTION
This research was determined to not be human participant research by the Johns Hopkins Bloomberg School of Public Health institutional review board.
REFERENCES
- 1.Committee on Prepositioned Medical Countermeasures for the Public; Institute of Medicine. Current dispensing strategies for medical countermeasures for anthrax. In: Stroud C, Viswanathan K, Powell T, Bass RR, editors. Prepositioning Antibiotics for Anthrax. Vol. 2011. Washington, DC: National Academies Press; pp. 69–92. Available at: https://www.ncbi.nlm.nih.gov/books/NBK190045. Accessed May 9, 2018. [PubMed] [Google Scholar]
- 2.Zipursky S, Djingarey MH, Lodjo JC, Olodo L, Tiendrebeogo S, Ronveaux O. Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine. 2014;32(13):1431–1435. doi: 10.1016/j.vaccine.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention. Final estimates for 2009–10 seasonal influenza and influenza A (H1N1) 2009 monovalent vaccination coverage—United States, August 2009 through May, 2010. 2011. Available at: https://www.cdc.gov/flu/fluvaxview/coverage_0910estimates.htm. Accessed May 9, 2018.
- 4.Rambhia KJ, Watson M, Sell TK, Waldhorn R, Toner E. Mass vaccinations for the 2009 H1N1 pandemic: approaches, challenges, and recommendations. Biosecur Bioterror. 2010;8(4):321–330. doi: 10.1089/bsp.2010.0043. [DOI] [PubMed] [Google Scholar]
- 5.US Centers for Disease Control and Prevention. The 2009 H1N1 pandemic: summary highlights, April 2009–April 2010. 2010. Available at: https://www.cdc.gov/h1n1flu/cdcresponse.htm. Accessed May 9, 2018.
- 6.Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29–33. doi: 10.3201/eid1201.051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control and Prevention. Medical countermeasure readiness. 2017. Available at: https://www.cdc.gov/phpr/readiness/mcm.html. Accessed July 9, 2018.
- 8.Nelson C, Chan EW, Chandra A Recommended Infrastructure Standards for Mass Antibiotic Dispensing. Arlington, VA: RAND Corporation; 2008. Document no. TR-553-DHHS. Available at: https://www.rand.org/pubs/technical_reports/TR553.html. Accessed May 9, 2018.
- 9.Rebmann T, Loux TM, Swick Z, Dolgin H, Reddick D, Wakefield M. Are US jurisdictions prepared to dispense medical countermeasures through open points of dispensing? Findings from a national study. Health Secur. 2015;13(2):96–105. doi: 10.1089/hs.2014.0080. [DOI] [PubMed] [Google Scholar]
- 10.Tsai YW, Huang WF, Wen YW, Chen PF. The relationship between influenza vaccination and outpatient visits for upper respiratory infection by the elderly in Taiwan. Value Health. 2007;10(2):117–127. doi: 10.1111/j.1524-4733.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- 11.Taiwan Centers for Disease Control Emerging Infectious Disease Incident Team. What is the target of the flu vaccination program this year? 2017. Available at: https://www.cdc.gov.tw/antirumorinfo.aspx?treeid=9B400D6BAFE34857&nowtreeid=1BF9D4691CE7E7DD&tid=B9FC327FD934FBB7. Accessed May 9, 2018.
- 12.Taiwan Centers for Disease Control. As this year’s government-funded influenza vaccination program includes teenage population, Taiwan CDC urges eligible students to receive flu shots for health benefits. November 3, 2016. Available at: http://www.cdc.gov.tw/english/info.aspx?treeid=BC2D4E89B154059B&nowtreeid=EE0A2987CFBA3222&tid=2E61054B1A50C44F. Accessed May 9, 2018.
- 13.Koonin LM, Beauvais DR, Shimabukuro T et al. CDC’s 2009 H1N1 vaccine pharmacy initiative in the United States: implications for future public health and pharmacy collaborations for emergency response. Disaster Med Public Health Prep. 2011;5(4):253–255. doi: 10.1001/dmp.2011.83. [DOI] [PubMed] [Google Scholar]
- 14.Drozd EM, Miller L, Johnsrud M. Impact of pharmacist immunization authority on seasonal influenza immunization rates across states. Clin Ther. 2017;39(8):1563–1580.e17. doi: 10.1016/j.clinthera.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald TJ, Kang Y, Bridges CB et al. Integrating pharmacies into public health program planning for pandemic influenza vaccine response. Vaccine. 2016;34(46):5643–5648. doi: 10.1016/j.vaccine.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtney B, Morhard R, Bouri N, Cicero A. Expanding practitioner scopes of practice during public health emergencies: experiences from the 2009 H1N1 pandemic vaccination efforts. Biosecur Bioterror. 2010;8(3):223–231. doi: 10.1089/bsp.2010.0036. [DOI] [PubMed] [Google Scholar]
- 17.Hsu YC, Chen YL, Wei HN, Yang YW, Chen YH. Risk and outbreak communication: lessons from Taiwan’s experiences in the post-SARS era. Health Secur. 2017;15(2):165–169. doi: 10.1089/hs.2016.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang J. Doctor to sue DOH over vaccinated son’s death. Taipei Times. December 23, 2009. Available at: http://www.taipeitimes.com/News/taiwan/archives/2009/12/23/2003461619. Accessed May 9, 2018.
- 19.Huang WT, Hsu CC, Lee PI, Chuang JH. Mass psychogenic illness in nationwide in-school vaccination for pandemic influenza A(H1N1) 2009, Taiwan, November 2009–January 2010. Euro Surveill. 2010;15(21):19575. doi: 10.2807/ese.15.21.19575-en. [DOI] [PubMed] [Google Scholar]
- 20.National Development Council. Taiwan Statistical Data Book: 2017. Taipei, Taiwan: National Development Council, R.O.C. (Taiwan); 2017. Available at: https://www.ndc.gov.tw/en/News_Content.aspx?n=607ED34345641980&sms=B8A915763E3684AC&s=1897C8025B0899A0. Accessed May 9, 2018.
- 21.US Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016–17 influenza season. 2017. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. Accessed May 9, 2018.
- 22.Hinman AR, Orenstein WA, Rodewald L. Financing immunizations in the United States. Clin Infect Dis. 2004;38(10):1440–1446. doi: 10.1086/420748. [DOI] [PubMed] [Google Scholar]
- 23.Willis HH, Nelson C, Shelton SR Initial Evaluation of the Cities Readiness Initiative. Arlington, VA: RAND Corporation; 2009. Document no. TR-640-CDC. Available at: https://www.rand.org/pubs/technical_reports/TR640.html. Accessed May 9, 2018.