Abstract
Prompt treatment of ill persons with influenza antivirals will be an important part of a future pandemic influenza response.
This essay reviews key lessons learned from the 2009 H1N1 pandemic and the changing landscape of antiviral drug availability, and identifies and describes the multiple components needed to ensure the timely administration of antiviral drugs during a future pandemic.
Fortunately, many of these planning efforts can take place before a pandemic strikes to improve outcomes during a future public health emergency.
Over the past decade, pandemic influenza planning efforts have been a major priority of the global health community. Preparedness planning has focused on a multifaceted approach to mitigate widespread morbidity and mortality from a novel, highly transmissible influenza A virus. Medical countermeasures such as vaccine and antiviral drugs play a critical role in a pandemic response. A vaccine that is effective against a circulating virus would have the greatest overall public health impact by preventing persons from becoming ill. However, antiviral drugs are also critically important for use in treating persons who are sick, and can thus mitigate the effects of emerging pandemics before effective vaccines become available. When used appropriately, antiviral drugs can reduce the severity of influenza symptoms and shorten the time of illness by approximately 1 or 2 days. Research also suggests that in past pandemics, hospitalizations were likely averted through use of antiviral drugs.1
Since the 2009 influenza A H1N1 pandemic, the antiviral drug landscape—from drug availability to usage—has evolved significantly. In addition, the 2009 H1N1 response demonstrated many factors crucial to the well-timed distribution and dispensing of antivirals during a response that need to be considered. Influenza antivirals are used to treat seasonal flu, and the high demand for these medications during the 2017–2018 season illustrates the importance of additional planning to ensure access to antivirals when needed. We examine these factors and identify areas in which preparedness efforts can improve the timely administration of antiviral drugs during a pandemic influenza response.
NEED FOR TIMELY ADMINISTRATION OF ANTIVIRAL DRUGS
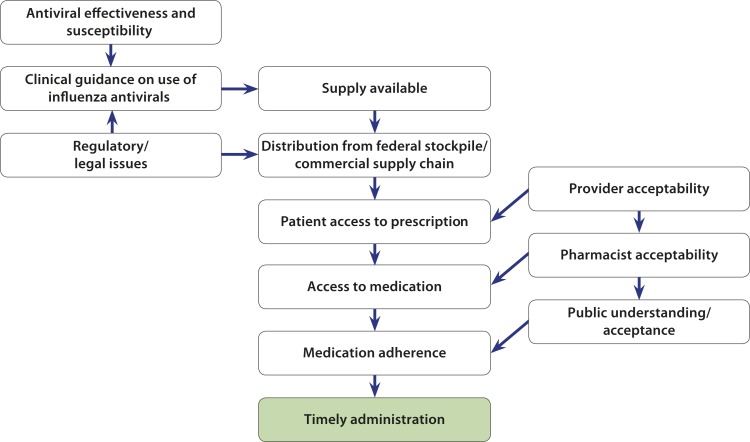
Antiviral drugs are currently the only pharmacological option available for treating patients with influenza. During a pandemic influenza response, timely administration of antivirals will be a critical factor in mitigating the effects of the outbreak through reducing the impact of illness. Influenza antiviral drugs work best when started as early as possible after symptoms arise—preferably within 48 hours after a person becomes ill—but have also shown effectiveness when used after this time period.2 There are numerous, complex factors that enable timely administration of antivirals during a pandemic, including the following: availability of expert clinical guidance on the use of antivirals based on the epidemiology and virulence of the pandemic virus; viral susceptibility to the available drugs; regulatory and legal considerations; a sufficient drug supply; acceptance of the drug by health care providers, pharmacists, and the public; adequate drug distribution systems; patient access to prescriptions and medication; and patient adherence to the drug regimen (Figure 1).
FIGURE 1—
Timely Antiviral Treatment During an Influenza Pandemic Depends Upon Success at Every Step
Antiviral Effectiveness and Susceptibility
The first step in timely administration of antiviral drugs is having medications that are effective and for which the novel virus is susceptible. To treat influenza, the Centers for Disease Control and Prevention (CDC) currently recommends 3 antiviral drugs approved by the Food and Drug Administration (FDA): oseltamivir (capsule and suspension formulation), zanamivir (inhaled formulation), and peramivir (intravenous formulation). Oseltamivir and zanamivir are also recommended for chemoprophylaxis in certain situations.3 Although the effectiveness of influenza antiviral drugs has been questioned by some researchers,4 current evidence shows that antiviral drugs can reduce the severity of influenza symptoms and shorten the time of illness by approximately 1 or 2 days, avert hospitalizations,1 reduce time spent in the hospital and admissions to intensive care units, decrease the development of pneumonia, and, in high-risk populations, reduce the chance of death for those hospitalized.4
In addition, novel influenza viruses may have genomic configurations or develop genetic changes during the outbreak that confer resistance to the antiviral drugs currently available.5 Older drugs such as amantadine and rimantadine are approved by the FDA for treatment and prevention of influenza A, but many strains of influenza are now resistant to these 2 antivirals.5 Ongoing efforts to monitor and rapidly detect antiviral resistance are a key component of seasonal influenza surveillance activities.6 Additionally, multiple modeling studies have explored the timing and use of antivirals to minimize risk of resistance and have described strategies for the use of other antivirals or a combination of antivirals if resistance emerges.7,8 Current efforts by drug manufacturers and the Department of Health and Human Services are focused on developing new therapeutics for influenza with improved spectrums of activity or better pharmacological profiles relative to current treatments; these therapeutics, which include next-generation monoclonal antibody treatments and novel endonuclease inhibitors, could be used against an emerging pandemic virus.9,10 In consultation with the FDA, drugs and biologics in development may also be considered for use during an emergency on the basis of various factors, including assessment of the risk–benefit ratio and sufficiency of data to support investigational product use.11
Clinical Guidance
Recommendations and clinical guidance will be needed on the use of available therapeutic options for managing sick and exposed persons. Clinical guidance on the use of antiviral agents for the prevention and treatment of seasonal and novel influenza is routinely developed by the CDC12,13 and its Advisory Committee on Immunization Practices.14 During a pandemic influenza event, the CDC will issue to health care providers guidance specific to the circulating virus regarding diagnosing, testing, and chemoprophylaxis and treatment of persons with antivirals.15
Regulatory and Legal Considerations
During an emergency, distribution, access, and utilization of antiviral drugs may be dependent on implementation of certain legal and regulatory authorities. The FDA, which is responsible for making sure that safe and efficacious drugs are on the market, is the regulatory authority for prescription medications. Under the Food, Drug and Cosmetics Act, as amended by the Project Bioshield Act of 2004 and the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), if requested, the FDA commissioner has the authority to make available promising new drugs that are not yet FDA approved, or to allow unapproved use of an approved medication during a public health emergency response through issuing an Emergency Use Authorization.11,16 During the 2009 H1N1 response, upon CDC request, the FDA issued its first Emergency Use Authorization for an investigational antiviral drug, peramivir. Under this authorization, and in accordance with the CDC’s clinical guidance, peramivir was allowed to be used in certain patients hospitalized with known or suspected 2009 H1N1 influenza.17 Additionally, PAHPRA provides key legal authorities to support public health emergencies by allowing the FDA to extend the expiration dating of certain stockpiled products such as antiviral drugs held in state and federal stockpiles.18 PAHPRA also authorized the CDC to issue special Emergency Use Instructions for the use of certain FDA-approved medical countermeasures during an emergency without the FDA needing to issue an Emergency Use Authorization. This includes possible use of Emergency Use Instructions to extend the use of antiviral drugs beyond the labeled time frame of use within 48 hours of illness onset, or for use in unapproved populations. The Public Readiness and Emergency Preparedness Act is also critical to a pandemic response as it provides necessary liability protections for individuals involved in the development, manufacturing, testing, distribution, dispensing, and use of federally stockpiled antivirals, which are covered countermeasures through this act.19
Supply
To meet patient demand during a pandemic influenza response, it will be important to have an adequate supply of appropriate influenza antiviral medications. In the United States, prescription drugs, including antivirals, are provided through the commercial supply chain; manufacturers sell these drugs to distributors, who then provide products downstream to pharmacies, hospitals, clinics, nursing homes, and others to support seasonal influenza demand. Typically each year (excluding the 2017–2018 influenza season), approximately 1.4 million to 8.7 million antiviral drug courses are prescribed to treat seasonal influenza.20 However, the amount of antiviral drugs needed for even a mild to moderate pandemic is projected to vastly exceed the amount of product normally available in the United States through manufacturers.21 Therefore, in 200422 the US government began stockpiling influenza antiviral drugs in the CDC’s Strategic National Stockpile (SNS) for use during a pandemic influenza public health emergency, when local supplies are depleted. In addition to stockpiling, strategies to support domestic surge production of influenza antivirals are also critical; these include Department of Health and Human Services and CDC collaboration with pharmaceutical manufacturers to understand current manufacturing capacity for brand and generic drug products, to identify the rate-limiting factors in antiviral drug production (e.g., supply of raw materials, production time, packaging time), and to use this information to develop solutions to secure an adequate pandemic drug supply for the United States. With multiple FDA-approved generic alternatives for oseltamivir entering the market, manufacturing surge capabilities may also change and should be reevaluated.
Antiviral Drug Distribution
Since 2001, the CDC has provided technical assistance to state and local health departments about methods to receive, stage, store, and further distribute medications provided by the SNS during a public health emergency. Public health dispensing plans were first developed and targeted to support intentional biological attacks (e.g., anthrax response). Planning efforts emphasized mass dispensing strategies by public health aimed at getting “pills into people” as quickly as possible. This entailed public health authorities and departments establishing plans to distribute medical countermeasures received from the SNS to specific locations within each jurisdiction designated as points of dispensing; these were envisioned as short-term response efforts.23 By contrast, a pandemic influenza response requires rapid targeted antiviral treatment strategies (which require an individual prescription) rather than mass dispensing (which does not require a prescription for each person receiving medication). In addition, the duration of a pandemic (multiple waves likely occurring in each community, with the national response lasting more than 12 months) would mean that distribution and dispensing efforts need to be sustained over a long period of time. It is also critical that plans for the distribution and dispensing of public health supplies of influenza antiviral drugs match demand, are scalable to respond to a nationwide event, and allow for sufficient tracking and accountability of the product.
The current federal plan for the distribution and dispensing of SNS antivirals during an influenza pandemic relies on sending influenza antivirals to state health departments after a pandemic emerges and the need for these drugs arises. Federal planning envisions that state and local health departments would serve as primary distributors of antivirals to other facilities in their jurisdiction and to the public during a pandemic. This plan was developed in 2006 and executed during the 2009 H1N1 pandemic response. A portion of the antivirals from the SNS stockpile was distributed early in this response to state health departments, with amounts of medication released based on the size of a jurisdiction’s population. State health departments, often in collaboration with relevant local health departments, used various ways to further distribute and dispense antiviral drugs for the duration of the year-long response. However, numerous issues arose at the state and local level with these efforts, including stresses caused by the increased logistics burden of distributing and dispensing large quantities of antivirals over a 1-year period—specifically, issues with scalability, long-term inventory storage and management, and product allocation and tracking.24 Many states created partnerships with pharmacies to assist in helping them dispense antiviral medications.24 Pharmacies were well-suited partners for dispensing these medications because of their existing capacities, including trained expert staff, multiple locations, and familiarity within the community.
Experience during the 2009 H1N1 pandemic, as well as reductions in the public health workforce since then, have prompted CDC’s exploration of new antiviral stockpile distribution plans that include not only direct distribution and dispensing by public health agencies but also private sector distribution partners such as pharmaceutical distributors and pharmacies.25 Most Americans (91%) live within 5 miles of a pharmacy.26 The revised concept would shift some of the responsibility for antiviral distribution and dispensing to distributors and pharmacy partners that routinely handle such distribution, dispensing, and tracking of pharmaceuticals on a daily basis. Health departments could then focus on distributing and dispensing to populations that may be hard to reach or underserved and use public health clinics and outreach methods to serve those populations. Exploration of the feasibility and acceptability of establishing partnerships with these private sector partners has been completed. If executed, such partnerships may improve access to public health–provided antiviral drugs during a pandemic.27 In addition, there has been an increase in the use of mail-order pharmacies and home delivery of prescription medications, and several private sector entities now routinely provide rapid (within 24 hours) home delivery of consumer goods. The feasibility of these novel approaches for the distribution and dispensing of stockpiled antivirals during an emergency should be examined.
Patient Access to Prescription
Currently, influenza antivirals are only available by prescription from a licensed health care provider. No change to this policy is envisioned during a future pandemic; therefore, ill persons will need to contact a licensed provider to receive a prescription. The requirement of a prescription for this medication conforms to the FDA’s determination of safety requirements and prevents inappropriate use of influenza antivirals and possible hoarding of a (potentially) scarce resource. However, the requirement for a prescription could be a bottleneck and create delays to treatment if there is a surge at health care facilities during a pandemic with high severity, or when a large number of sick people are simultaneously seeking medical care.
To help decrease demand on health care facilities, federal efforts are exploring the potential for rapid care and treatment by telemedicine approaches without requiring an in-person visit to a health care provider to receive a prescription.28 The CDC and its public health partners have developed—and are testing—a telephone triage capability called Flu on Call that, when warranted, can be activated during a pandemic to improve access to care and prescriptions for antivirals.28,29 Other strategies to improve access to prescriptions may include authorizing the use of collaborative practice agreements between physicians and pharmacists to enable ill patients who meet certain criteria to obtain antivirals in pharmacies without an additional provider visit.30
Furthermore, as noted, during a severe pandemic demand for antivirals might exceed the available supply. When antiviral supplies are limited, the CDC’s recommendations for antiviral treatment and chemoprophylaxis might differ according to disease incidence, severity of illness, and likelihood for influenza-related complications. Therefore, health care providers may be advised by the CDC and other public health authorities to prioritize the use of antivirals for those at higher risk for complications or severe illness (e.g., infants and young children, pregnant women). Public health and providers will need to clearly explain to the public that this medication is in short supply and therefore prescriptions are being reserved to treat those at the highest risk for complications.
Health Care Provider Acceptability
Clinicians and pharmacists must be aware of the utility of antivirals during a pandemic, the need for rapid treatment, and the importance of prompt treatment for those at higher risk of influenza complications.3 Providers play an important role in communicating with and educating patients about antivirals, particularly during a pandemic emergency. Although clinical benefit is greatest when antiviral treatment is administered as early as possible after illness onset (within 48 hours), and recommendations for prompt treatment of those at higher risk for complications of seasonal influenza have been published and disseminated, 1 study showed that fewer than a fifth of those who were ill and at higher risk for complications were offered antivirals after the diagnosis of influenza was confirmed by laboratory tests.31 Positive influenza test findings have been associated with appropriate antiviral prescribing.32 Newer rapid influenza diagnostic tests with improved accuracy have been developed and are now on the market, which may improve antiviral prescribing. However, additional communication and educational efforts and tools for providers are needed.33 To emphasize the importance of early treatment—especially for high-risk populations—the CDC continues to focus activities on health care provider outreach (updated antiviral guidance and information on the CDC Web site, clinician outreach calls, Health Alert Network messages, pharmacist outreach, and webinars). In addition, pharmacists must be aware of the efficacy and safety profiles of antiviral drugs as patients will likely have questions and need accurate information about these medications. A recent poll of over 1000 community pharmacists found that they were amenable to a new role as distributors and dispensers of stockpiled antivirals during a pandemic, and expected that their own pharmacy would participate in such an effort.27
Public Understanding and Acceptance
Because antivirals must be administered promptly, the public must be informed that it is important to seek care soon after symptoms arise, that there are medications that can be used to treat influenza, and that these medications are only available by contacting a health care provider. Providers play an important role in communicating with patients about seeking care early after influenza symptoms arise and the appropriate use of antivirals. Researchers conducting a study of 5 recent seasons of seasonal flu found that fewer than half (40%) of high-risk outpatients with influenza sought medical care early in the onset of their disease.34 During the 2009 H1N1 pandemic, only 40% of ill persons sought medical care within 3 days of becoming ill.35 During a future pandemic, it will be critical that the public know that sick persons should access care in a timely fashion to reduce morbidity and mortality. Public health and provider communication strategies targeted to the public will be vital to increase awareness of influenza symptoms, provide guidance on when and where to seek care, and emphasize the importance of early treatment with antivirals.
Patient Access to Medication
During a severe pandemic, the supply of antiviral drugs may fluctuate and not be consistently available at all pharmacies, clinics, or public health points of dispensing. Supplies may be available through commercial inventories or public health supplies. Knowing where to go to get prescriptions filled will be critical for patients, health care providers, and public health. Currently, the standard of care is for prescriptions to be electronically generated and sent directly to a pharmacy from the point of care (“e-prescribing”). Therefore, providers need to be able to send prescriptions to pharmacies or other dispensing sites that have confirmed available supplies of antiviral medications. Patients who receive a paper prescription will need to know where to access available supplies of these drugs. A Web site called MedFinder, that can display available supplies of antivirals (by zip code) during a pandemic is being developed by CDC on the basis of currently available similar services (e.g., Health Map Vaccine Finder, https://vaccinefinder.org).
In addition, once a patient identifies the location that may have an antiviral supply, it is also critical that the cost of the drug or fees associated with medication dispensing do not create a barrier to access. Although the price of antivirals varies somewhat, the average cost of a regimen of currently approved antiviral drugs (absent third-party coverage) ranges from approximately $70 (zanamivir)36 to $115 (oseltamivir capsules)36 to $950 (intravenous peramivir).36 Although antiviral drugs provided through public health stockpiles are free to the public, pharmacies dispensing these medications may charge a dispensing fee. Solutions are needed, in collaboration with the commercial supply chain (manufacturers, pharmacies, pharmacy benefits managers, payers) and public health, to ensure that costs are minimized and are not a barrier to access for those needing these critical medications during a public health emergency. Strategies could include providing discount codes, waiving fees, or other solutions.
Adherence With Prescribed Medication
Once patients have received their medications, it will be imperative to ensure that they receive proper treatment of the illness, that they start taking the medicine as soon as possible, and that they take every dose in the regimen in order for the medication to be most effective. Technology tools could help increase such medication compliance. For example, reminders sent by mobile texting have been shown to improve drug adherence, especially those that are personalized, nonrepetitive, and tailored to patients’ needs.37
Side effects that result from taking medication can influence adherence to antiviral therapy. Nausea and vomiting have been reported as side effects in adults (19%) and children (25%) receiving oseltamivir for treatment in clinical studies.38 However, these may be minimized by taking the medication with food, a message that will need to be effectively communicated to patients. Additionally, risk perception related to the severity of the pandemic may influence adherence to antiviral therapy.39
CONCLUSIONS
Ensuring availability and accessibility of antiviral medication to sick persons will be a key factor in mitigating a novel influenza pandemic. There are multiple, complex, and interrelated components that factor into ensuring such timely access to these medications. We have described these components and offered ideas for improving each part that will be critical during an influenza pandemic. Fortunately, many of these planning efforts can take place before a pandemic strikes to improve outcomes during a future public health emergency.
HUMAN PARTICIPANT PROTECTION
No human participation protection was necessary because data were obtained from secondary sources for this essay.
REFERENCES
- 1.Atkins CY, Patel A, Taylor TH, Jr et al. Estimating effect of antiviral drug use during pandemic (H1N1) 2009 outbreak, United States. Emerg Infect Dis. 2011;17(9):1591–1598. doi: 10.3201/eid1709.110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fry AM, Goswami D, Nahar K et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14(2):109–118. doi: 10.1016/S1473-3099(13)70267-6. [DOI] [PubMed] [Google Scholar]
- 3.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- 4.Muthuri SG, Myles PR, Venkatesan S, Leonardi-Bee J, Nguyen-Van-Tam JS. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207(4):553–563. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayden FG, de Jong MD. Emerging influenza antiviral resistance threats. J Infect Dis. 2011;203(1):6–10. doi: 10.1093/infdis/jiq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Influenza antiviral drug resistance. 2018. Available at: https://www.cdc.gov/flu/about/qa/antiviralresistance.htm. Accessed August 13, 2018.
- 7.Althouse BM, Patterson-Lomba O, Goerg GM, Hebert-Dufresne L. The timing and targeting of treatment in influenza pandemics influences the emergence of resistance in structured populations. PLOS Comput Biol. 2013;9(2):e1002912. doi: 10.1371/journal.pcbi.1002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipsitch M, Cohen T, Murray M, Levin BR. Antiviral resistance and the control of pandemic influenza. PLoS Med. 2007;4(1):e15. doi: 10.1371/journal.pmed.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dept of Health and Human Services. Janssen Research & Development join forces on innovative influenza products. Available at: https://www.phe.gov/Preparedness/news/Pages/janssen-flu.aspx. Accessed August 13, 2018.
- 10.A flu drug may be poised to upend treatment in US. Available at: https://www.scientificamerican.com/article/a-flu-drug-may-be-poised-to-upend-treatment-in-u-s. Accessed August 13, 2018.
- 11.Food and Drug Administration. Emergency use authorization of medical products and related authorities. Guidance for industry and other stakeholders. 2017. Available at: https://www.fda.gov/RegulatoryInformation/Guidances/ucm125127.htm. Accessed August 13, 2018.
- 12.Centers for Disease Control and Prevention. Seasonal influenza A(H3N2) activity and antiviral treatment of patients with influenza. 2017. Available at: https://emergency.cdc.gov/han/han00409.asp. Accessed August 13, 2018.
- 13.Centers for Disease Control and Prevention. Interim guidance on the use of antiviral medications for treatment of human infections with novel influenza A viruses associated with severe human disease. 2016. Available at: https://www.cdc.gov/flu/avianflu/novel-av-treatment-guidance.htm. Accessed August 13, 2018.
- 14.Smith JC. The structure, role, and procedures of the US Advisory Committee on Immunization Practices (ACIP) Vaccine. 2010;28(suppl 1):A68–A75. doi: 10.1016/j.vaccine.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. 2009. Available at: https://www.cdc.gov/h1n1flu/recommendations.htm. Accessed August 13, 2018.
- 16.Food and Drug Administration. MCM-related counterterrorism legislation. 2018. Available at: https://www.fda.gov/emergencypreparedness/counterterrorism/medicalcountermeasures/mcmlegalregulatoryandpolicyframework/ucm2007271.htm. Accessed August 13, 2018.
- 17.Yu Y, Garg S, Yu PA et al. Peramivir use for treatment of hospitalized patients with influenza A(H1N1)pdm09 under emergency use authorization, October 2009–June 2010. Clin Infect Dis. 2012;55(1):8–15. doi: 10.1093/cid/cis352. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Summary of PAHPRA’s MCM provisions. 2017. Available at: https://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/MCMLegalRegulatoryandPolicyFramework/ucm346195.htm. Accessed August 13, 2018.
- 19.Dept of Health and Human Services. Public Readiness and Emergency Preparedness Act. Available at: https://www.phe.gov/Preparedness/legal/prepact/Pages/default.aspx. Accessed August 13, 2018.
- 20.Suda KJ, Hunkler RJ, Matusiak LM, Schumock GT. Influenza antiviral expenditures and outpatient prescriptions in the United States, 2003–2012. Pharmacotherapy. 2015;35(11):991–997. doi: 10.1002/phar.1656. [DOI] [PubMed] [Google Scholar]
- 21.O’Hagan JJ, Wong KK, Campbell AP et al. Estimating the United States demand for influenza antivirals and the effect on severe influenza disease during a potential pandemic. Clin Infect Dis. 2015;60(suppl 1):S30–S41. doi: 10.1093/cid/civ084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel A, Gorman SE. Stockpiling antiviral drugs for the next influenza pandemic. Clin Pharmacol Ther. 2009;86(3):241–243. doi: 10.1038/clpt.2009.142. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Point of dispensing (POD) standards. 2008. Available at: https://www.cdc.gov/phpr/documents/coopagreement-archive/fy2008/dispensingstandards.pdf. Accessed August 13, 2018.
- 24.Association of State and Territorial Health Officials. National Association of County & City Health Officials. Managing antiviral medication during the 2009 H1N1 influenza pandemic: state and local public health department responsibilities. 2013. Available at: http://www.astho.org/Programs/Infectious-Disease/Antiviral-Distribution/Managing-Antiviral-Medication-during-the-2009-H1N1-Influenza-Pandemic. Accessed August 13, 2018.
- 25.Koonin LM, Patel A. New strategies for pandemic antiviral distribution and dispensing: a workshop for state and local public health. Paper presented at: Public Health Preparedness Summit; March 12–15, 2013; Atlanta, GA.
- 26.National Association of Chain Drug Stores. Face-to-face with community pharmacies. Available at: https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf. Accessed August 13, 2018.
- 27.SteelFisher GK, Benson JM, Caporello H et al. Pharmacist views on alternative methods for antiviral distribution and dispensing during an influenza pandemic. Health Secur. 2018;16(2):108–118. doi: 10.1089/hs.2017.0068. [DOI] [PubMed] [Google Scholar]
- 28.Dept of Health and Human Services. Pandemic Influenza Plan 2017 UPDATE. Available at: https://www.cdc.gov/flu/pandemic-resources/pdf/pan-flu-report-2017v2.pdf. Accessed August 13, 2018.
- 29.Koonin LM, Hanfling D. Broadening access to medical care during a severe influenza pandemic: the CDC nurse triage line project. Biosecur Bioterror. 2013;11(1):75–80. doi: 10.1089/bsp.2013.0012. [DOI] [PubMed] [Google Scholar]
- 30.Northwest Center for Public Health Practice. University of Washington. Collaborative Drug Therapy Agreement for Influenza Antivirals in Washington State. Available at: http://www.nwcphp.org/training/opportunities/toolkits-guides/collaborative-drug-therapy-agreement-for-influenza-antivirals-in-washington-state. Accessed August 13, 2018. [PubMed]
- 31.Havers F, Thaker S, Clippard JR et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012–2013 influenza season. Clin Infect Dis. 2014;59(6):774–782. doi: 10.1093/cid/ciu422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller MR, Smith PJ, Baumbach JP et al. Influenza testing and antiviral prescribing practices among emergency department clinicians in 9 states during the 2006 to 2007 influenza season. Ann Emerg Med. 2010;55(1):32–39. doi: 10.1016/j.annemergmed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Ison MG. Editorial commentary: failing our patients by suboptimally treating influenza infections. Clin Infect Dis. 2014;59(6):783–786. doi: 10.1093/cid/ciu425. [DOI] [PubMed] [Google Scholar]
- 34.Stewart RJ, Flannery B, Chung JR et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during five influenza seasons—United States, 2011–2016. Clin Infect Dis. 2018;66(7):1035–1041. doi: 10.1093/cid/cix922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biggerstaff M, Jhung M, Reed C, Fry AM, Balluz L, Finelli L. Influenza-like illness, the time to seek healthcare, and influenza antiviral receipt during the 2010–2011 influenza season-United States. J Infect Dis. 2014;210(4):535–544. doi: 10.1093/infdis/jiu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GoodRx. Flu medications. 2018. Available at: https://www.goodrx.com/flu/drugs. Accessed August 13, 2018.
- 37.Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health. 2015;36(1):393–415. doi: 10.1146/annurev-publhealth-031914-122855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche Laboratories Inc. Tamiflu (oseltamivir phosphate) capsules and oral suspension. 2009. Available at: https://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/ucm147992.pdf. Accessed August 13, 2018.
- 39.Smith LE, D’Antoni D, Jain V, Pearce JM, Weinman J, Rubin GJ. A systematic review of factors affecting intended and actual adherence with antiviral medication as treatment or prophylaxis in seasonal and pandemic flu. Influenza Other Respir Viruses. 2016;10(6):462–478. doi: 10.1111/irv.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]