Abstract
Background
Stevia, Stevia rebaudiana (Bertoni), has become an important economic plant for its commercial use as a sweetener. Stevia plays a significant role in the healthcare practice of different cultures and in population. Previous animal and clinical studies demonstrated the efficacy of Stevia against chronic diseases like diabetes and hypertension. This study aimed to investigate the beneficial effect of Stevia in chronic kidney disease (CKD) patients after three (3) months of treatment along with the conventional antihypertensive and anti diabetic medications.
Methods
A prospective, interventional, randomized, single-blind, placebo-controlled trial has been done with 97 participants. Stevia capsule (250 mg) or matching placebo was given to the participants twice daily along with Angiotensin-II Receptor Blocker (ARB) and/or Ca2+ Channel Blocker (CCB). First follow up visits were done after 3 months of the interval. Blood and urine samples were collected for the biochemical tests. A structured questionnaire was used for the baseline assessment. Informed consent was taken from each participant.
Results
Both hypertension and diabetes were found to be associated with CKD. Most of the participants (52.3%) of Stevia group were in CKD Stage II. Significant changes were found in Serum creatinine (p < 0.027), Serum Uric acid (p < 0.009), Fasting blood sugar (p < 0.041) and Postprandial blood sugar (p < 0.013) and Microalbumin (p < 0.041) level in the treatment group.
Conclusion
The initial result demonstrated that Stevia has the potential for a significant improvement of some biochemical parameters in CKD patients. After completion of the nine (9) months clinical trial, the constructive effect of Stevia can be confirmed in this group of patients.
Keywords: Stevia, Chronic kidney disease (CKD), Hypertension, Diabetes mellitus, Angiotensin-II receptor blocker (ARB), Ca2+ channel blocker (CCB)
Abbreviations used: CKD, Chronic Kidney Disease; ARB, Angiotensin-II Receptor Blocker; CCB, Ca2+ Channel Blocker; STV, Stevia; PLC, Placebo; CL, Control (Healthy participant); BMI, Body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FBS, Fasting blood sugar; PBS, Postprandial blood sugar; STP, Serum total protein; S uric acid, Serum uric acid; TCO2, Total CO2; In. phos., Inorganic phosphate; Se. Cr., Serum creatinine; M. albumin, Microalbumin; UTP, Urinary total protein; ACR, Albumin: Creatinine; PCR, Protein: Creatinine; eGFR, estimated glomerular filtration rate
1. Introduction
Stevia, Stevia rebaudiana (Bertoni), a sweet herb native to South America, has long been used in the treatment of diabetes and hypertension [1]. Stevioside (5–10%) and rebaudioside-A (2–4%) are the two most abundant glycosides present in the Stevia leaves of the dry matter, respectively and are responsible for the intensely sweet taste [2]. Commercially, the use of stevioside (as a sweetener) has been established in different countries of the world, such as Australia and Japan. Furthermore, the diverse pharmacological effects of stevioside such as antibacterial effects, anticaries effect, antiedema effects, antifungal effects, antihyperglycemic effects on rabbits, hypotensive effects, and hypoglycemic effects in human were confirmed by some animal and clinical trials [3].
Numerous studies demonstrated that Stevia extract improves glucose tolerance in both diabetic and non-diabetic human. 35% drop in blood glucose was observed in human volunteers after 8 h of consumption of an extract of Stevia [4]. In rats, extract of Stevia given orally for 40–60 days induced diuresis, natriuresis and an indication of vasodilatation on the kidney, increase renal plasma flow and decrease mean arterial pressure [5].
Initial studies showed Stevia also has antioxidant and anti-inflammatory effects which help to reduce cardiovascular damage and metabolic disorders. Since cardiovascular disease and diabetes are now shown to be correlated with oxidative stress and inflammation, it is therefore of great interest to study the protective effect and influence of Stevia rebaudiana on these processes.
Now a day, Chronic Kidney Disease (CKD) becomes a major public health problem worldwide [6]. The prevalence of CKD has reached epidemic status in 10–12% of the populations, and more than 50% of elderly worldwide. Increasing body weight, hypertension and insulin resistance - all contribute to the chance of the increasing the prevalence of CKD with high morbidity and mortality rate [7].
Approximately, more than 450,000 patients in the United States and more than 175,000 patients in Europe are suffering from End-Stage Renal Disease (ESRD) and over one million worldwide currently. But, actually, the overall prevalence of Chronic Kidney Disease (CKD) is expected to be between 30 and 50 fold higher all over the world [8].
The US National Kidney Foundation also strongly recommends that people with diabetes, hypertension or family history of CKD at in higher risk. For the prevention of CKD, screening program and an individualized risk–reduction plan can modify the risk to cause CKD [1]. Again, the NHANES III found diabetes, hypertension, older age, and male sex to be positively associated with increased of elevated Serum creatinine levels to cause CKD [9].
Synthetic antihypertensive medications blocking the renin-angiotensin-aldosterone system (Angiotensin-converting enzyme inhibitors and/or Angiotensin receptor blockers) demonstrated beneficial effects in patients with proteinuria of CKD [10]. Good efficacy and tolerability were found during the long-term use of stevioside in humans. Therefore, the aim of this study was to investigate the effect of Stevia in patients with Chronic Kidney Disease (CKD) stage I to stage III.
2. Materials and methods
2.1. Study design
A total of ninety-seven (97) participants were enrolled in this prospective, interventional, randomized, single-blind study comparing Angiotensin-II Receptor Blocker, Losartan/Valsartan and Ca2+ Channel Blocker, Amlodipine with Stevia or placebo. The stevioside capsules 500 mg (250 mg each) twice a day or matching placebo was prescribed to the participants. Three (3) separate groups were taken to evaluate the drug. In study group-1 (STV), the stevioside capsule was given to 44 patients with conventional antihypertensive treatment along with the treatment of chronic kidney disease. In study group-2 (PLC), matching placebo was given to 43 patients with a similar treatment regimen. In study group-3 (CL), only 10 healthy participants were included as a control. Patients were asked to return for follow up visits every three months during active treatment schedule.
2.2. Study subject
The study involved both male and female participants, ranging in age from 31 to 70 years. CKD patients with stage I to stage III were included in the study. The participants who had a myocardial infarction or had undergone coronary-artery bypass grafting or who had a cerebrovascular accident or have undergone coronary angioplasty or who had a transient ischemic attack or who had any history of heart failure before enrolment or morbid obesity and chronic sepsis were excluded.
2.3. Sample size evaluation
In this prospective study, the sample size was evaluated by following formula:
n = 2[(a+b)2σ2 ]/(μ1-μ2)2, where, n = the sample size in each of the groups, μ1= population mean in treatment group 1, μ2= population mean in treatment group 2, σ2 = population variance (SD), a = conventional multiplier for alpha = 0.05, b = conventional multiplier for power = 0.80. Study power was considered as 80% (0.80) [11].
2.4. Duration of the study
All treatments were performed for nine (9) months. The whole process was divided into four (4) distinct phases. Such as (i) Baseline study (Initial Stage), (ii) First follow up (after 3 months of Baseline), (iii) Second follow up (after 6 months of Baseline) and (iv) Washout Period (after 9 months of Baseline). But in this article, only the data of first three (3) months (from the baseline period to 1st follow up) were considered.
2.5. Study area
The clinical part of this study was conducted in the Kidney Foundation Hospital and Research Institute, Mirpur-2, Dhaka, Bangladesh. It is a tertiary level of Kidney Hospital and non-profit organization of Dhaka City.
2.6. Patient screening
The study was explained to potential participants and they have the opportunity to ask questions about the study and these participants had as much time as required to decide whether they wish to take part. After taking their consent, the blood test had done, urine sample had been taken and either the stevioside capsule or placebo was given to them and proceed to 1st follow up visit.
2.7. Methodology
A pre-structured and validated questionnaire was used for all participants who were reflected in the present study. Full demographic details of the patients were recorded, including age, sex, educational status, monthly income, clinical history, family history, smoking and alcohol intake status, current medication, physical examination (height and weight) and blood pressure (BP) measurement using the validated device. Stage I, stage II, and stage III CKD patients were considered in this study. Most of the CKD patients having either diabetes or systemic hypertension or both and their clinical condition were stable. Anthropometric parameters were assessed using standardized Techniques. Body weight and height were measured. Body mass index (BMI) was calculated on the basis of weight and height [weight (kg)/height (m2).
For Hypertension, the participants who reported current use of antihypertensive medications and those with a Systolic blood pressure (SBP) of 140 mm Hg or greater and Diastolic blood pressure (DBP) of 90 mm Hg or greater was considered [12]. Blood pressure was monitored twice, with an interval of 5 min between each measurement. The mean of these two (2) measurements was recorded. Patients were also encouraged to measure their blood pressure at home in the morning using an automated electronic device.
The blood specimen was obtained for the biochemical investigations, determination of Blood urea, Serum creatinine, Serum electrolytes (Na, K, Cl, TCO2), Ca, Inorganic phosphate (PO4), Serum total protein, Blood sugar (fasting and postprandial), Serum uric acid etc. Urine sample for the calculation of the Microalbumin, Urinary total protein (UTP) (spot), urine for ACR (Albumin: Creatinine), and urine for PCR (Protein: Creatinine), estimated glomerular filtration rate (eGFR) etc. Blood specimens were collected for analysis by the biochemistry laboratory. Remaining samples were collected and stored at −400c.
Serum electrolytes (Na, K, Cl, TCO2), Blood urea, Serum creatinine, Blood sugar (fasting and postprandial) and urine for PCR was measured using Beckman Coulter AU480 Auto Analyzer system. Serum Ca, Serum inorganic phosphate, Serum uric acid, Serum total protein, and urine for ACR were measured using Architect c8000 Biochemistry Auto Analyzer system.
Kidney disease was evaluated by the presence or absence of Microalbuminuria, Serum creatinine level, and eGFR. Microalbuminuria was evaluated and greater than 25 mg/L was considered as positive. Elevated Serum creatinine values were defined as greater than 110.5 mmol/L for male and greater than 98.1 mmol/L for female. The simplified Modification of Diet in Renal Disease (MDRD) equation was used to estimate Glomerular Filtration Rate (GFR) and CKD stages, by following the KDOQI guidelines and the criteria of the US National Kidney Foundation. eGFR = 186.3∗[Serum creatinine−1.154]∗[age−0.203]. Calculated values were multiplied by 0.742 for the woman. CKD Stage I: ACR< 30 mg/g creatinine and eGFR (90 ml/min/1.73 m2 or higher); CKD Stage II: ACR 30–299 mg/g creatinine and eGFR(60–89 ml/min/1.73 m2); CKD Stage III: ACR> 300 mg/g creatinine and eGFR(30–59 ml/min/1.73 m2) [13].
Diabetic condition was evaluated in two (2) ways: (i) Participants who reported a history of diabetes and those with fasting blood sugar values greater than 5.83 mmol/L or postprandial blood sugar level greater than 8 mmol/L were categorized as diabetic, (ii) Participants with non fasting blood sugar levels greater than 8 mmol/L [14].
All the tests were done at the beginning of the treatment and after 3 months of the treatment as well. Approval from the Institutional Review Board (IRB) was collected before starting the research on CKD patients.
2.8. Statistical analysis
Data were checked thoroughly for consistency and completeness after collection and then cleaned, edited and verified to exclude any error or inconsistency. Statistical analysis was performed using the SPSS software for Windows (Version 17.0) (SPSS Inc, Chicago, USA). Descriptive analysis was performed as appropriate. Data of different variables of different (STV, PLC and CL) group of the participants at baseline and after 3 months (1st follow up) of treatment period were analyzed by Paired Sample T-test. Independent Sample T-test was done to do the comparison of different parameters of Group 1 (STV) and Group 2 (PLC) at baseline and 1st follow up study. All the statistical test were considered significant at different levels, (*p < 0.05) represents the significant value, (**p < 0.01) represents the highly significant values and (***p < 0.001) represents the very highly significant value of the parameter. One-way ANOVA followed by Bonferroni's Test were performed to determine the multiple comparisons among the urinary variables of this study. Linear Regression analysis was performed to evaluate the confounding variables.
3. Results
In the Baseline investigation, out of 97 participants, 44 were included in the STV group, 43 were in PLC group and 10 were in the CL group. The Mean (±) age of the participants in STV group was 55 (±11.75) years, in PLC group was 53.6 (±11.27) years and in CL group was 47.20 (±4.87) years, respectively (Table 1).
Table 1.
Status of different variables of the participants at Baseline.
| Different Variables | Group 1- STV |
Group 2- PLC |
Group 3- CL |
|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | |
| Age | 55 (±11.75) | 53.60 (±11.27) | 47.20 (±4.87) |
| Height | 5.31 (±.29) | 5.26 (±.33) | 5.21 (±.30) |
| Weight | 68.82 (±8.23) | 66.52 (±11.38) | 64.20 (±12.89) |
| BMI | 26.34 (±3.46) | 25.79 (±3.31) | 25.45 (±4.11) |
| SBP | 133.86 (±21.37) | 133.84 (±16.83) | 111.00 (±11.005) |
| DBP | 84.77 (±12.66) | 82.67 (±7.74) | 79.00 (±9.944) |
| FBS | 6.94 (±2.27) | 6.28 (±1.45) | 5.53 (±.48) |
| PBS | 9.58 (±3.72) | 9.32 (±4.76) | 6.34 (±1.25) |
| Blood urea | 8.3 (±8.22) | 5.47 (±3.88) | 3.2 (±.91) |
| S. creatinine | 87.2 (±8.22) | 105.3 (±30.7) | 70.9 (±11.04) |
| STP | 71.8 (±11.76) | 74.4 (±5.37) | 71.6 (±3.66) |
| S uric acid | 361.9 (±134.6) | 303.7 (±113.1) | 368 (±108.20) |
| Inorganic phosphate | 1.28 (±.67) | 1.09 (±.20) | 1.14 (±.15) |
| Ca | 2.34 (±.27) | 2.24 (±.16) | 2.30 (±.09) |
| Na | 138.5 (±3.05) | 138.6 (±2.67) | 139.9 (±1.2) |
| K | 3.92 (±.51) | 4.14 (±.55) | 4.24 (±.17) |
| Cl | 102.5 (±4.27) | 103 (±3.748) | 103.7 (±1.16) |
| TCO2 | 27.41 (±1.90) | 26.86 (±1.96) | 26.10 (±.32) |
Values are presented as Mean (±SD). Data were analyzed by Paired sample T-test. Group I- STV = Stevia (n = 44), Group II- PLC=Placebo (n = 43) and Group III- CL=Control (n = 10). Here, BMI = Body mass index, SBP = Systolic blood pressure, DBP = Diastolic blood pressure, FBS= Fasting blood sugar, PBS=Postprandial blood sugar, STP = Serum total protein, S uric acid = Serum uric acid, TCO2 =Total CO2.
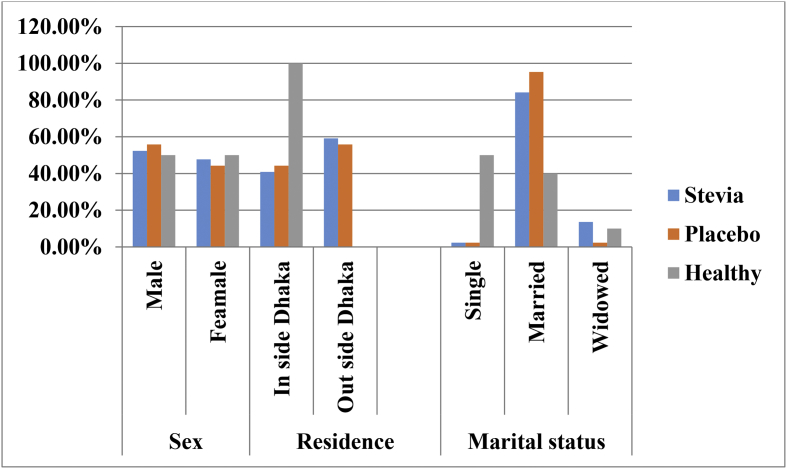
Fig. 1 shows the percentage of the sex, residence and marital status of the participants of the different study group. In this study, most of the participants were male, 53.61% (STV = 52.3%, PLC = 55.8%, CL = 50%). Fifty participants (51.54%) (STV = 59.1%, PLC = 55.8%, CL = 0.00%) were from outside of Dhaka city and the rest of them resided inside. Married participants were 84.5% (STV = 84.1%, PLC = 95.3%, CL = 40%), widowed were 8.2% (STV = 13.6%, PLC = 2.3%, CL = 10%) and single were 7.2% (STV = 2.3%, PLC = 2.3% CL = 50%). In Table 2, from the Stevia group, maximum (29.5%) CKD patients are having one (1) affected family member and in the placebo group 11.6% having more than one (1) affected family member. Maximum overweight participants (47.4%, 46/97) were found from three different study groups. The major participants have CKD stage II (STV = 52.3%, 23/44) and in CKD stage III (PLC = 60.5%, 26/43). Cigarette smokers were found 47.1% in STV, 44.4% in PLC and 8.8% in CL group. Only one (1) participant from STV and two (2) participants from PLC group were taken alcohol occasionally.
Fig. 1.
Distribution (%) of sex, residence and marital status of the Participants.
Table 2.
Characteristics of the study population in three (3) separate groups.
| Different Variables | Group 1- STV N (%) |
Group 2- PLC N (%) |
Group 3- CL N (%) |
Total Participants N (%) |
|
|---|---|---|---|---|---|
| Body Mass Index (BMI) | |||||
| Underweight | 1 (2.3%) | 1 (2.3%) | 0 (0.00%) | 2 (2.1%) | |
| Normal | 16 (36.4%) | 15 (34.9%) | 4 (40%) | 35 (36.1%) | |
| Overweight | 19 (43.2%) | 22 (51.2%) | 5 (50%) | 46 (47.4%) | |
| Obese | 8 (18.2%) | 5 (11.6%) | 1 (10%) | 14 (14.4%) | |
| Stages of CKD (GFR: ml/minutes/1.73 m2)a | |||||
| Stage-I | 5 (11.4%) | 4 (9.3%) | 0 (0%) | 16 (16.5%) | |
| Stage-II | 23(52.3%) | 13 (30.2%) | 0 (0%) | 39 (40.2%) | |
| Stage-III | 16(36.4%) | 26 (60.5%) | 0 (0%) | 42 (43.3%) | |
| No. of Affected family members | |||||
| One (1) | 13 (29.5%) | 5 (11.6%) | 1 (10.0%) | 19 (19.6%) | |
| More than one (1) | 1 (2.3%) | 1 (2.3%) | 0 (0.0%) | 2 (2.1%) | |
| No | 30 (68.2%) | 37 (86.0%) | 9 (90.0%) | 76 (78.4%) | |
| History of Smoking & Alcohol Intake | |||||
| Smoking | YES | 16 (47.1%) | 15 (44.1%) | 3 (8.8%) | 34 (100%) |
| NO | 28 (44.4%) | 28 (44.4%) | 7 (11.1) | 63 (100%) | |
| Alcohol | YES | 1 (33.3) | 2 (66.7%) | 0 (0.0%) | 3 (100%) |
| NO | 43 (45.7%) | 41 (43.6%) | 10 (10.6%) | 94 (100%) | |
Group I- STV= Stevia (n = 44), Group II- PLC= Placebo (n = 43) and Group III- CL= Control (n = 10). Descriptive analysis was performed here. Range of BMI: Underweight = BMI (below 18.5), Normal = BMI (18.5–24.9), Overweight = BMI (25–29.9), Obese = BMI (30+). Different Stages of CKD: Stage I = eGFR 90 ml/min/1.73 m2 or higher, Stage II = eGFR 60–89 ml/min/1.73 m2, Stage III = eGFR 30–59 ml/min/1.73 m2.
National Kidney Foundation (NKF) 2002.
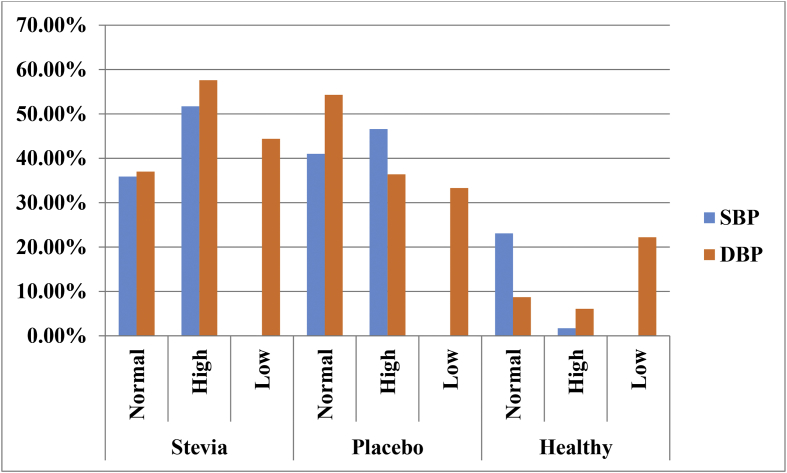
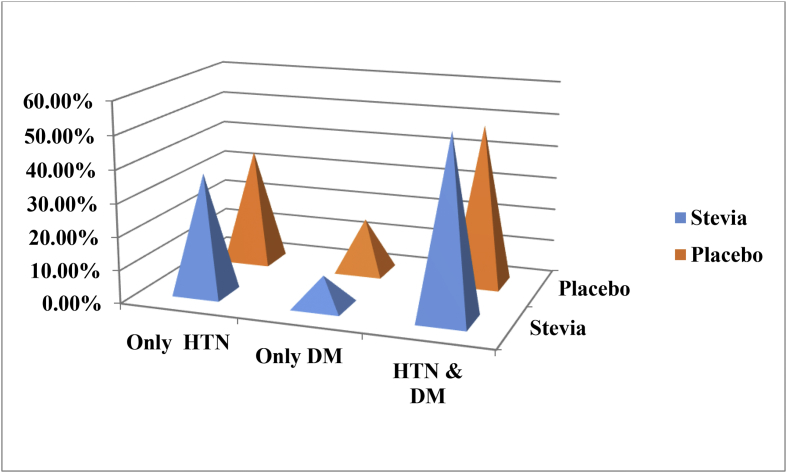
Distribution (%) pattern of the Systolic and Diastolic blood pressure of the participants were presented in Fig. 2. Systolic blood pressure (SBP) was found superior (STV = 51.7% and PLC = 46.6%). Again, high Diastolic blood pressure (DBP) was observed (STV = 57.6%, PLC = 36.4%, CL = 6.1%) and low diastolic blood pressure was observed (STV = 44.4%, PLC = 33.3%, CL = 22.2%). In both study groups, hypertension with diabetes was considered to be the main causes for CKD for these participants (STV = 54.4% and PLC = 48.8%). On the other hand, only hypertension was found in (STV = 36.4% and PLC = 34.9%) and only diabetes was observed for (STV = 9.1% and PLC = 16.3%) participants (Fig. 3).
Fig. 2.
Distribution (%) of the Systolic and Diastolic blood pressure of different groups of participants.
Here, Normal Systolic Blood Pressure (SBP) = >90–120; High Systolic Blood Pressure (SBP) = >120–190; Low Systolic Blood Pressure (SBP) = 70–90; Normal Diastolic Blood Pressure (DBP) = >60–80; High Diastolic Blood Pressure (DBP) = >80–100; Low Diastolic Blood Pressure (DBP) = 40–60.
Fig. 3.
Distribution (%) of the causes of CKD in the participants of STV and PLC groups.
Here, Hypertension = HTN and DM = Diabetes Mellitus.
In Table 3, urinary variables (Microalbumin, UTP, ACR, and PCR) didn't show any significant association among STV, PLC and CL group at the baseline. Again, very highly significant results of eGFR were found between STV and CL (p < 0.000), between PLC and CL (p < 0.000), between CL and STV (p < 0.000), between CL and PLC (p < 0.000) at the baseline.
Table 3.
Multiple comparisons among the urinary variables of different study groups of Baseline.
| Dependent Variable | Different group | Different group | Std. Error | p value | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Microalbumin | STV(n = 44) | PLC | 55.380 | .935 | −191.23 | 78.60 |
| CL | 91.633 | .350 | −78.23 | 368.24 | ||
| PLC(n = 43) | STV | 55.380 | .935 | −78.60 | 191.23 | |
| CL | 92.004 | .093 | −22.82 | 425.46 | ||
| CL(n = 10) | STV | 91.633 | .350 | −368.24 | 78.23 | |
| PLC | 92.004 | .093 | −425.46 | 22.82 | ||
| UTP (Spot) | STV(n = 44) | PLC | 20.685 | 1.000 | −48.15 | 52.64 |
| CL | 34.227 | .336 | −28.47 | 138.29 | ||
| PLC n = 43) | STV | 20.685 | 1.000 | −52.64 | 48.15 | |
| CL | 34.365 | .386 | −31.06 | 136.38 | ||
| CL(n = 10) | STV | 34.227 | .336 | −138.29 | 28.47 | |
| PLC | 34.365 | .386 | −136.38 | 31.06 | ||
| ACR | STV n = 44) | PLC | 83.9729 | 1.000 | −223.435 | 185.716 |
| CL | 138.9435 | .352 | −118.931 | 558.059 | ||
| PLC n = 43) | STV | 83.9729 | 1.000 | −185.716 | 223.435 | |
| CL | 139.5063 | .272 | −101.442 | 578.290 | ||
| CL(n = 10) | STV | 138.9435 | .352 | −558.059 | 118.931 | |
| PLC | 139.5063 | .272 | −578.290 | 101.442 | ||
| PCR | STV (n = 44) | PLC | .5083 | 1.000 | -.812 | 1.665 |
| CL | .8410 | .676 | −1.022 | 3.075 | ||
| PLC (n = 43) | STV | .5083 | 1.000 | −1.665 | .812 | |
| CL | .8444 | 1.000 | −1.457 | 2.657 | ||
| CL(n = 10) | STV | .8410 | .676 | −3.075 | 1.022 | |
| PLC | .8444 | 1.000 | −2.657 | 1.457 | ||
| eGFR | STV (n = 44) | PLC | 4.561 | 1.000 | −7.38 | 14.84 |
| CL | 7.547 | .000*** | −59.75 | −22.98 | ||
| PLC (n = 43) | STV | 4.561 | 1.000 | −14.84 | 7.38 | |
| CL | 7.577 | .000*** | −63.56 | −26.64 | ||
| CL(n = 10) | STV | 7.547 | .000*** | 22.98 | 59.75 | |
| PLC | 7.577 | .000*** | 26.64 | 63.56 | ||
Here, (*p < 0.05) = significant, (**p < 0.01) = highly significant, (***p < 0.001) = very highly significant. The data were analyzed by One-way ANOVA followed by Bonferroni's Test. Here, UTP= Urinary total protein, ACR = Albumin: Creatinine; PCR= Protein: Creatinine; eGFR = estimated glomerular filtration rate.
In Table 4, the comparison of two groups (STV and PLC) was done between Baseline and 1st follow up on data. The Significant difference (p < 0.05) was discovered in Diastolic blood pressure (p < 0.001), Blood urea (p < 0.004), Serum creatinine (p < 0.043), Serum total protein (p < 0.040), Ca (p < 0.008) and Inorganic phosphate (p < 0.043) between STV and PLC group of Baseline data. Whereas, in 1st follow up, a significant difference was found only in Diastolic blood pressure (p < 0.002), urine for PCR (p < 0.050) in both STV and PLC group (Table 4).
Table 4.
Comparison of different parameters of Group 1- STV and Group 2- PLC at Baseline and 1st follow up study.
| Different Variables | Different Study groups | Baseline Data |
1st Follow up Data |
||
|---|---|---|---|---|---|
| Mean=(±SD) | p value | Mean=(±SD) | p value | ||
| Weight | 1 | 68.82(±8.23) | .182 | 68.50(±8.57) | .259 |
| 2 | 66.56(±11.34) | 66.71(±10.90) | |||
| BMI | 1 | 26.341(±3.46) | .540 | 26.156(±3.68) | .094 |
| 2 | 25.798(±3.31) | 25.690(±2.88) | |||
| SBP | 1 | 133.86(±21.37) | .228 | 122.33(±13.42) | .509 |
| 2 | 133.84(±16.82) | 122.00(±14.53) | |||
| DBP | 1 | 84.77(±12.66) | .001*** | 79.30(±5.51) | .002** |
| 2 | 82.67(±7.74) | 78.38(±10.52) | |||
| FBS | 1 | 6.942(±2.26) | .217 | 6.707(±1.96) | .337 |
| 2 | 6.286(±1.42) | 6.985(±2.54) | |||
| PBS | 1 | 9.589(±3.71) | .160 | 9.244(±3.24) | .066 |
| 2 | 9.324(±4.75) | 10.070(±4.59) | |||
| Blood urea | 1 | 8.30(±8.21) | .004** | 5.860(±4.52) | .233 |
| 2 | 5.47(±3.88) | 5.298(±1.44) | |||
| S. creatinine | 1 | 87.226(±42.58) | .043* | 101.072(±23.90) | .054 |
| 2 | 105.258(±30.69) | 110.768(±30.83) | |||
| STP | 1 | 71.84(±11.75) | .040* | 71.930(±10.88) | .102 |
| 2 | 74.40(±5.37) | 74.225(±4.90) | |||
| S. uric acid | 1 | 361.98(±134.66) | .354 | 341.09(±107.63) | .895 |
| 2 | 303.79(±113.11) | 351.25(±105.00) | |||
| Ca | 1 | 3.209(±4.08) | .008** | 2.187(±.14) | .876 |
| 2 | 2.242(±.16) | 2.190(±.13) | |||
| PO4 | 1 | 1.287(±.67) | .043* | 1.172(±.20) | .824 |
| 2 | 1.095(±.20) | 1.120(±.20) | |||
| Na | 1 | 138.57(±3.04) | .323 | 143.95(±30.85) | .114 |
| 2 | 138.63(±2.67) | 140.13(±2.58) | |||
| K | 1 | 3.921(±.51) | .536 | 3.988(±.49) | .692 |
| 2 | 4.149(±.51) | 4.108(±.54) | |||
| Cl | 1 | 102.59(±4.27) | .129 | 103.53(±3.29) | .369 |
| 2 | 103.00(±3.74) | 105.20(±2.83) | |||
| TCO2 | 1 | 27.41(±1.89) | .873 | 27.23(±2.24) | .972 |
| 2 | 26.86(±1.96) | 143.95(±30.85) | |||
| M. albumin | 1 | 133.26(±261.74) | .420 | 172.073(±306.07) | .362 |
| 2 | 187.78(±257.30) | 172.292(±253.98) | |||
| UTP (Spot) | 1 | 49.14(±79.81) | .888 | 71.040(±126.40) | .148 |
| 2 | 56.35(±76.17) | 56.675(±80.60) | |||
| ACR | 1 | 187.341(±412.05) | .761 | 181.404(±345.22) | .348 |
| 2 | 226.819(±351.75) | 168.269(±271.52) | |||
| PCR | 1 | .650(±.92) | .734 | 10.960(±63.65) | .050* |
| 2 | .679(±.86) | .5195(±.65) | |||
| eGFR | 1 | 65.45(±21.93) | .719 | 66.28(±21.95) | .886 |
| 2 | 60.51(±20.26) | 62.58(±20.43) | |||
Values are presented here as Mean (±SD), where, (*p < 0.05) = significant, (**p < 0.01) = highly significant, (***p < 0.001) = very highly significant as compared to the baseline and 1st follow-up data of different study groups. For study group 1- STV (n = 44) and for study group 2- PLC (n = 43). Data were analyzed by the Independent Sample T-test. Here, BMI= Body mass index, SBP= Systolic blood pressure, DBP = Diastolic blood pressure, FBS= Fasting blood sugar, PBS= Postprandial blood sugar, STP= Serum total protein, S. creatinine = Serum creatinine, S. uric acid = Serum uric acid, TCO2= Total CO2, UTP= Urinary total protein, ACR = Albumin: Creatinine; PCR= Protein: Creatinine; eGFR = estimated glomerular filtration rate.
In Table 5, the comparison of Baseline and 1st follow up data was made in two groups (STV and PLC). In PLC group significant difference (p < 0.05) was discovered in Systolic blood pressure (p < 0.000), Diastolic blood pressure (p < 0.010), Serum uric acid (p < 0.002), Sodium (Na) (p < 0.010), Chloride (Cl) (p < 0.001), urine for ACR (p < 0.027) and urine for PCR (p < 0.011). Whereas in STV group significant difference (p < 0.05) were found in Systolic blood pressure (p < 0.042), Diastolic blood pressure (p < 0.008), Fasting blood sugar (p < 0.041), Postprandial blood sugar (p < 0.013), Serum creatinine (p < 0.027), Serum uric acid (p < 0.009), Microalbumin (p < 0.041) between Baseline and 1st follow up on study (Table: 5).
Table 5.
Outcome of participants in (STV and PLC) group after 3 months (1st follow up) of treatment period.
| Different Variables | Group 2- PLC |
Group 1- STV |
||||
|---|---|---|---|---|---|---|
| Baseline Data, Mean (±SD) |
1st follow up Data, Mean (±SD) |
p value | Baseline Data, Mean (±SD) |
1st follow up Data, Mean (±SD) |
p value | |
| Weight | 66.53(±10.94) | 66.17(±10.48) | .295 | 68.82 (±8.23) | 68.94 (±8.97) | .916 |
| BMI | 25.79(±3.16) | 25.64 (±2.90) | .276 | 26.34 (±3.46) | 26.18 (±3.64) | .758 |
| SBP | 133.46 (±16.98) | 122.05 (±14.17) | .000*** | 133.86 (±21.37) | 126.14 (±14.01) | .042* |
| DBP | 82.56 (±7.85) | 78.33 (±10.65) | .010** | 84.77 (±12.66) | 79.32 (±5.45) | .008*** |
| FBS | 6.34 (±1.43) | 7.04 (±2.55) | .092 | 6.94 (±2.26) | 6.19 (±1.49) | .041* |
| PBS | 9.45 (±4.89) | 10.14 (±4.62) | .412 | 9.58 (±3.71) | 8.11 (±2.03) | .013* |
| Blood urea | 5.47 (±4.06) | 5.30 (±1.46) | 0.794 | 8.30 (±8.21) | 5.84 (±4.47) | .092 |
| S creatinine | 104.52 (±29.87) | 110.19 (±31.02) | 0.182 | 87.22 (±42.58) | 101.80 (±24.10) | .027* |
| STP | 74.41 (±5.64) | 74.10 (±4.90) | .783 | 71.84 (±11.75) | 72.09 (±10.80) | .891 |
| S Uric acid | 312.28 (±107.86) | 349.1(±105.52) | .002** | 361.98 (±134.66) | 303.02 (±112.38) | .009*** |
| Ca | 2.23 (±.16) | 2.19 (±.13) | .118 | 3.20 (±4.08) | 2.18 (±.14) | .104 |
| In phos. | 1.10 (±.21) | 1.11 (±.20) | .635 | 1.28 (±.67) | 1.17 (±.20) | .26 |
| Na | 138.77 (±2.46) | 140.18 (±2.59) | .010* | 138.57 (±3.04) | 139.27 (±2.39) | .215 |
| K | 4.18 (±.50) | 4.10 (±.55) | 0.286 | 3.92 (±.51) | 3.99 (±.49) | .419 |
| Cl | 103.28 (±3.30) | 105.28 (±2.82) | .001** | 102.59 (±4.27) | 103.50 (±3.26) | .173 |
| TCO2 | 26.73 (±1.81) | 26.79 (±2.41) | .879 | 27.41 (±1.89) | 27.18 (±2.24) | .490 |
| M albumin | 198.38 (±267.30) | 168.5(±256.16) | .279 | 133.26 (±261.74) | 56.20 (±80.91) | .041* |
| UTP(SPOT) | 59.82 (±79.07) | 56.20 (±81.60) | .767 | 49.14 (±79.81) | 71.13 (±124.92) | .283 |
| ACR | 240.9 (±366) | 160.8 (±270.93) | .027* | 187.34 (±412.05) | 187.64 (±343.68) | .997 |
| PCR | .72(±.89) | .50(±.66) | .011* | .650 (±.92) | 10.73 (±62.92) | .294 |
| e GFR | 62.85 (±18.16) | 62.92 (±20.57) | .964 | 65.45 (±21.93) | 65.84 (±21.82) | .918 |
Values are presented as Mean (±SD), where, (*p < 0.05) = significant, (**p < 0.01) = highly significant, (***p < 0.001) = very highly significant as compared to the baseline and 1st follow up data of different study groups. Data were analyzed by Paired Sample T-test. Here, BMI= Body mass index, SBP= Systolic blood pressure, DBP = Diastolic blood pressure, FBS= Fasting blood sugar, PBS= Postprandial blood sugar, In. phos. = Inorganic phosphate, S. creatinine = Serum creatinine, M. albumin = Microalbumin, STP= Serum total protein, S. uric acid = Serum uric Acid, TCO2 = Total CO2, UTP= Urinary total protein, ACR = Albumin: Creatinine; PCR= Protein: Creatinine; eGFR = estimated glomerular filtration rate.
In Table 6, The multiple linear regressions model showed surprisingly, gender and age were significantly associated with estimated glomerular filtration rate (eGFR) (p < 0.05) in the baseline stage and 1st follow up (Table 6).
Table 6.
Linear Regression analysis of confounding variables considering estimated glomerular filtration rate (eGFR) as dependent variable in the baseline stage and 1st follow up.
| Variables | Beta Coefficient (β) | p-value | 95% confidence interval for β |
|
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Baseline Data | ||||
| (Constant) | 0.000 | 117.699 | 239.844 | |
| Gender | -.223 | 0.026* | −20.221 | −1.291 |
| Age | -.417 | 0.000*** | −1.326 | -.479 |
| BMI | .055 | 0.569 | −0.952 | 1.722 |
| SBP | -.257 | 0.023* | −0.585 | −0.044 |
| DBP | -.070 | 0.514 | −0.653 | 0.329 |
| S. uric acid | -.050 | 0.597 | −0.046 | 0.027 |
| FBS | .010 | 0.913 | −2.338 | 2.612 |
| 1stFollow up | ||||
| (Constant) | 0.000 | 50.294 | 131.088 | |
| Gender | -.252 | 0.010** | −23.880 | −3.389 |
| Age | -.375 | 0.000*** | −1.358 | -.463 |
| BMI | .264 | 0.089 | −0.181 | 2.529 |
| SBP | .151 | 0.438 | −0.230 | 0.526 |
| DBP | .069 | 0.710 | −0.460 | 0.673 |
| S. uric acid | -.092 | 0.354 | −0.065 | 0.024 |
| FBS | -.046 | 0.635 | −2.615 | 1.603 |
Dependent Variable was eGFR in ml/min/1.73m2 in Baseline and 1st Follow up data. Here, (*p < 0.05) = significant, (**p < 0.01) = highly significant, (***p < 0.001) = very highly significant. Linear regression analysis was performed. Here, BMI= Body mass index, SBP= Systolic blood pressure, DBP = Diastolic blood pressure, FBS= Fasting blood sugar, S. uric acid = Serum uric Acid, eGFR = estimated glomerular filtration rate.
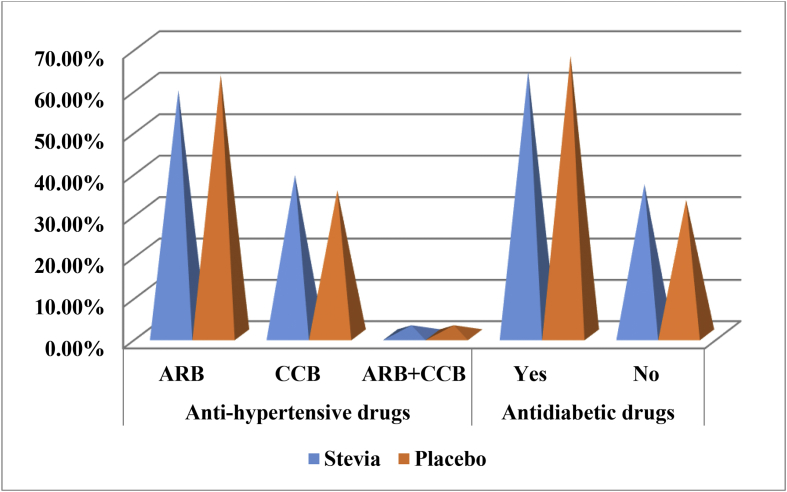
Fig. 4 shows the distribution pattern of Angiotensin-II receptor blocker (ARB), Ca2+ channel blocker (CCB) and Antidiabetic drug among the CKD patients. ARB was taken by (STV = 61.2%, PLC = 65.1%) CKD patients and CCB was taken by (STV = 40.9% and PLC = 37.2%) CKD patients, respectively. Only (STV = 2.3% and PLC = 2.3%) CKD patients were taking combined dose. Antidiabetic drugs were given to (STV = 63.6% and PLC = 67.4%) CKD patients.
Fig. 4.
Distribution (%) of anti-hypertensive and antidiabetic drugs in STV and PLC groups.
Here, ARB = Angiotensin- II Receptor Blocker and CCB= Calcium2+ Channel Blocker.
4. Discussion
The current study demonstrated several insights into the interrelationships between diabetes, hypertension and chronic kidney disease. This study also validated the effect of Stevia in patients with mild to moderate CKD. All the demographic factors like age, gender, educational level, financial condition, affected family members (if); BMI, malnutrition, anemia; causes of the disease like hypertension, diabetes or impaired renal function itself makes overall worsen the condition of the CKD patients [15]. In a different study, it was found that advanced age, female sex, the presence of associated diseases and a low socioeconomic status are the relevant factors to decline the condition of the CKD patients [16]. BMI was part of the most important risk factors for CKD. Overweight and obesity had a graded increase in risk for causing CKD. It was a finding from Singapore and India [17]. In another study, 27% and 64% participants were classified as diabetics and hypertensive respectively. Again, 44% of CKD patients were considered obese (BMI≥ 30.0), including 46% of women and 39% of men [18]. In the current study, 47.4% and 14.4% participants found overweight and obese, respectively. This finding is compatible with the results of other studies.
Previous studies showed that the prevalence of cardiovascular complications among all the patients with CKD. The prevalence of left ventricular hypertrophy is a direct result of the decrease in glomerular filtration rate. Approximately 30% of end-stage renal disease patients demonstrated clinical evidence of ischemic heart disease and heart failure [19]. The present study showed an association between hypertension and diabetes (51.7%) and chronic kidney disease. Another important finding of this study is the improvement of the stage of CKD patients. During 1st follow-up, it was found that out of eighty-three (83) CKD patients, the condition of 72.28% were remaining unchanged and 20.48% patients needed to be improved. However, 7.22% CKD patients were deteriorated. In this regard, treatment with Stevia improved or prevents any further decrease in glomerular filtration rate among CKD patients will be confirmed after finishing the study.
Gislein Elisa et al. found the short duration of action of stevioside. But they didn't find any significant difference in blood glucose levels between placebo and stevioside group (p > 0.05) from 3 months of the treatment period [3]. From another study by Letcia et al. found the significantly decreased (p < 0.05) glucose level during their treatment period for both stevioside and placebo groups [20].
Melis et al. found the significant reduction in blood pressure (p < 0.05) in the active treatment group during the study period. Here, Stevia shows the mechanism of calcium channel antagonism, which is the same as the mechanism of verapamil as antihypertensive agents [21,22]. However, calcium influx in rat smooth muscle cells was inhibited by the action of Stevia is established already from another study [23].
Ana Rebollo-Rubio et al. mentioned that over 77% of the articles in a review process having a larger number of the male population than the female. However, 100% of the studies using sex as a study variable and showed the poor health condition is perceived by women, compared with men [15]. In the present study, the maximum number of randomly selected CKD patients was found male (53.61%).
John Hopkins University reported from a population-based case-control study that severe renal failure clustered within families independent of high blood pressure and diabetes is very common [24]. From another study, it has been observed that a consistent and high baseline rate of familial clustered directly correlated with CKD throughout the world, such as the United States, Western and Eastern Europe, India, South America, The Middle East and Asia [8]. In a different study, the family history is significant risk factors of CKD associated with renal impairment and representing the genetic influence [8,25]. The current study showed, 19.6% participants having one (1) family member and only 2.1% participants had more than one (1) family member affected by CKD. However, 78.4% did not get a family history of CKD. In this regard, no correlation was found between affected family members with CKD.
In a study in India, only 3.3% of subjects were found with low eGFR and the majority of these had eGFR< 30 ml/min/1.73 m2. They also observed an inverse association between renal impairment and history of smoking and alcohol intake [17]. In an Australian Aboriginal Community, a cross-sectional study has been done by McDonald et al. documented the similar observations [26]. Another cross-sectional study also suggested an inverse association between current smoking and low eGFR level in Japan [27]. Wendy et al. mentioned that 45% of participants reported a smoking history and among them, 14% were current smokers [18]. Furthermore, some cross-sectional studies have demonstrated a positive association between smoking, alcohol intake and renal impairment [28]. However, no significant relationship was found between CKD and history of smoking and alcohol intake in the present study.
This study represents CKD patients with Diabetes mellitus was 64.4%. However, the majority (51.8%) of diabetes mellitus was found in stage III CKD patients. New et al. revealed that 30% of diabetic patients had CKD stage III in the UK [29]. Narindar et al. reported that Diabetes has recognized as an emerging epidemic for a long time in India, where every fifth person is hypertensive also and only 20% of hypertensive and 57% of diabetics knew about their disease [17]. In another study, 27% and 64% participants were categorized as diabetics and hypertensive respectively.
Assessment of Kidney disease was inspired by the presence or absence of Microalbuminuria, Serum creatinine level, and eGFR. Wendy et al. mentioned that 29% participants had Microalbuminuria and of them, 34% had diabetes and 14% having an elevated Serum glucose level [18]. Stevia showed a beneficial effect in CKD patients by improving Microalbuminuria in the current study.
Treatment with stevioside reported a significant reduction in Blood urea level and increasing Serum creatinine level. On the other hand, the significant result was noted in sodium (Na) and chloride (Cl) for placebo treatment in this study. Letcia et al. did not find any changes in different parameters of blood analysis, urine analysis, BMI, Serum creatinine, Blood urea, chloride (Cl), potassium (K) and sodium (Na) [20].
The risks of renal failure associated with a wide range of blood pressure levels were determined by a 16 years a large cohort studies. In a separate study, the patients who received the crude stevioside and placebo, decrease the Diastolic blood pressure (p < 0.05). However, several investigations showed that parenteral administration of stevioside decreased blood pressure in rats [20]. But the current study indicated that both the Systolic blood pressure (SBP) and Diastolic blood pressure (DBP) significantly reduced in Stevia and placebo group in human subjects.
Ahmed et al. found the lower prevalence of proteinuria was 2.25% in a cross-sectional study in a rural population in India [30]. While another cross-sectional study from an urban locale estimated a higher prevalence (4.41%) [31]. Kannel et al. suggested the subjects with proteinuria should be worked up adequately because of significant mortality rate [32]. But in this study, placebo group found a significant reduction of Protein: Creatinine (PCR) as well as the Albumin: Creatinine (ACR).
5. Conclusion
In conclusion, our results showed that both diabetes and hypertension are positively associated with the development of CKD. This study revealed the potential benefit of oral Stevia in achieving the reduction in Serum uric acid and Microalbumin. This study highlights the emerging issue of CKD. The rising prevalence of hypertension and diabetes mellitus in CKD patients has the potential to become a future public health problem. Moreover, it is recognized that CKD is an additional cardiac risk factor and this patient is at a higher risk of cardiovascular morbidity. Using oral stevioside with regular drug regimen offers the opportunity for preventing the progression of CKD in human subjects. Benefits and risks of stevioside in CKD patients can be established after completion of the study.
Ethical statement & informed consent
The Chief Clinical Investigator ensures the study was conducted in accordance with the principles of the Declaration of Helsinki and also ensures the study was conducted in full conformity with relevant regulations and with the ICH guidelines for Good Clinical Practice (CPMP/ICH/135/95) July 1996. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki as amended in 1989 and was approved by the Institutional Review Board (IRB) of the Institute of Kidney Foundation Hospital and Research Institute, Mirpur-2, Dhaka. Human Research Ethics Committee Clearance Certificate No. is KFHRI/ECC-001/2016.
Written informed consent was collected from each and every patient or participants and ensures to maintain patient confidentiality.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgment
The authors wish to acknowledge the Kidney Foundation Hospital & Research Institute; Mirpur-2, Dhaka, Bangladesh, for their continued assistance in this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.08.007.
Contributor Information
Farhana Rizwan, Email: farhanarizwan12@gmail.com, frizwan18@yahoo.com, frezwan@ewubd.edu.
Harun Ur Rashid, Email: rashid@bol-online.com.
Saquiba Yesmine, Email: s.yesmine@juniv.edu.
Forhad Monjur, Email: forhadmonjur@gmail.com, monjur_forhad@yahoo.com.
Tapan Kumar Chatterjee, Email: tkchatterjee_81@rediffmail.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39(Suppl 1):S1–S266. ISBN 1-931472-10-6. [PubMed] [Google Scholar]
- 2.Abudula R., Jeppesen P.B., Rolfsen S.E.D., Xiao J., Hermansen K. Rebaudioside A potently stimulates insulin secretion from isolated mouse islets: studies on the dose-, glucose-, and calcium-dependency. Metabolism. 2004;53(10):1378–1381. doi: 10.1016/j.metabol.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 3.da Silva G.E.C., Assef A.H., Albino C.C., Ferri L.A.F., Tasin G., Takahashi M.H., Filho W.E., Bazotte R.B. Investigation of the tolerability of oral stevioside in brazilian hyperlidemic patients. Braz. Arch. Biol. Technol. 2006;49(4):583–587. doi: 10.1590/S1516-89132006000500007. [DOI] [Google Scholar]
- 4.Doughlas Kinghorn A. vol 162. Taylor & Francis; 2002. (Stevia, the Genus Stevia, 8). ISBN 0-203-26058-9 (Adobe eReader Format); ISBN 0-415-26830-3 (Print Edition) [Google Scholar]
- 5.Melis M.S. Chronic administration of aqueous extract of Stevia rebaudiana in rats: renal effects. J. Ethnopharmacol. 1995;47(3):129–134. doi: 10.1016/0378-8741(95)01271-E. [DOI] [PubMed] [Google Scholar]
- 6.Levey A.S., Coresh J., Balk E., Kausz A.T., Levin A., Steffes M.W., Hogg R.J., Perrone R.D., Lau J., Eknoyan G. National Kidney Foundation practice guideline for chronic kidney disease: evaluation, classification, and stratification. Ann. Intern. Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Pestana M. Predicting risk in CKD, port. J. Nephrol. Hypert. 2015;29(3):192–193. http://www.scielo.mec.pt/pdf/nep/v29n3/29n3a02.pdf [Google Scholar]
- 8.Satko S.G., Sedor J.R., Iyengar S.K., Freedman B.I. Familial clustering of chronic kidney disease. Semin. Dial. 2007;20(3):229–236. doi: 10.1111/j.1525-139X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 9.Coresh J., Wei L., McQuillan G. Prevalence of high blood pressure and elevated Serum creatinine level in the United States. Arch. Intern. Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. https://www.ncbi.nlm.nih.gov/pubmed/11343443 [DOI] [PubMed] [Google Scholar]
- 10.Sharma P., Blackburn R.C., Parke C.L. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney diseases. Cochrane Database Syst. Rev. 2011;5(10) doi: 10.1002/14651858.CD007751.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Noordzij M., Tripepi G., Dekker F.W., Zoccali C., Tanck M.W., Jager K.J. Sample size calculations: basic principles and common pitfalls. Nephrol. Dial. Transplant. 2010;25:1388–1393. doi: 10.1093/ndt/gfp732. [DOI] [PubMed] [Google Scholar]
- 12.National High Blood Pressure education program . National Institute of Health, NIH Publication No.; Washington, DC: 1997. The Sixth Report of the Joint National Committee Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; pp. 98–4080.http://www.sld.cu/galerias/pdf/servicios/hta/6to._reporte_del_jnc_usa.pdf [Google Scholar]
- 13.Haneda M. A joint committee on diabetic nephropathy, a new classification of diabetic nephropathy 2014: a report from the joint committee on diabetic nephropathy. J. Diabetes Investig. 2015;6(2):242–246. doi: 10.1111/jdi.12319. Epub 2015 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diagnosis and classification of diabetes mellitus, american diabetes association. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebollo-Rubio A., Morales-Asencio J.M., Pons-Raventos M.E. Review of studies on health-related quality of life in patients with advanced chronic kidney disease in Spain. Nefrologia. 2015;35(1):92–109. doi: 10.3265/Nefrologia.pre2014.Jul.12133. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz M.J., Roman M., Martin G., Alferez M.J., Prieto D. Calidad de vida relacionada con la salud en las diferentes terapias sustitutivas de la insuficiencia renal cronica. Rev. Soc. Esp. Enferm. Nefrol. 2003;6(4):6–16. http://www.revistaseden.org/files/art309_1.pdf [Google Scholar]
- 17.Singh N.P., Ingle G.K., Saini V.K., Jami A., Beniwal P., Lal M., Meena G.S. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: an observational, cross-sectional study. BMC Nephrol. 2009;10:4. doi: 10.1186/1471-2369-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown W.W., Peters R.M., Ohmit R.N.S., Keane W.F., Collins A., Chen S., King K. Early detection of kidney disease in community settings: the kidney early evaluation program (KEEP) Am. J. Kidney Dis. 2003;42(1):22–35. doi: 10.1016/S0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 19.Khan M.K., Rashid H.U., Yesmine S., Mahmood I.H., Habib S.M.A., Hossain A. University Heart Journal; 2013. Assessment of Risk Factors for Cardiovascular Complications in Patients with Chronic Kidney Diseases (CKD) Stage III-V before Dialysis; pp. 25–32.https://www.banglajol.info/index.php/UHJ/article/view/19508/13493 vol. 9 (1) [Google Scholar]
- 20.Ferri L.A.F., Alves-Do-Prado W., Yamada S.S., Gazola S., Batista M.R., Bazotte R.B. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytother Res. 2006;20(9):732–736. doi: 10.1002/ptr.1944. [DOI] [PubMed] [Google Scholar]
- 21.Melis M.S., Sainati A.R. Effect of Calcium and verapamil on renal function of rats during treatment with stevioside. J. Ethnopharmacol. 1991;33(3):257–262. doi: 10.1016/0378-8741(91)90086-s. [DOI] [PubMed] [Google Scholar]
- 22.Evaluation of certain food additives, fifty-first report of the joint FAO/WHO expert committee on food additives, world health. Organ Tech. Report Ser. 2000;891(i-viii):1–168. https://www.ncbi.nlm.nih.gov/pubmed/10876377 [PubMed] [Google Scholar]
- 23.Lee C.N., Wong K.L., Liu J.C. Inhibitory effect of stevioside on calcium influx to produce antihypertension. Planta Med. 2001;67(9):796–799. doi: 10.1055/s-2001-18841. [DOI] [PubMed] [Google Scholar]
- 24.Lei H.H., Perneger T.V., Klag M.J. Familial Aggregation of renal disease in a population-based case-controlled study. J. Am. Soc. Nephrol. 1998;l9(7):1270–1276. doi: 10.1681/ASN.V971270. https://www.ncbi.nlm.nih.gov/pubmed/9644638 [DOI] [PubMed] [Google Scholar]
- 25.Brown W.W., Peters R.M., Ohmit S.E., Keane W.F., Collins A., Chen S.C., King K., Klag M.J., Molony D.A., Flack J.M. Early detection of kidney disease in community settings; the kidney early evaluation program (KEEP) Am. J. Kidney Dis. 2003;42(1):22–35. doi: 10.1016/S0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 26.McDonald S.P., Maguire G.P., Hoy W.E. Renal function and cardiovascular risk markers in a remote australian aboriginal community. Nephrol. Dial. Transplant. 2003;18(8):1555–1561. doi: 10.1093/ndt/gfg199. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaka N., Ishizaka Y., Toda E., Shimomura H., Koike K., Seki G., Nagai R., Yamakado M. Association between cigarette smoking and chronic kidney diseases in Japanese men. Hypertens. Res. 2008;31(3):485–492. doi: 10.1291/hypres.31.485. [DOI] [PubMed] [Google Scholar]
- 28.Briganti E.M., Branley P., Chadban S.J., McNeil J.J., Welborn T.A., Atkins R.C. Smoking is associated with renal impairment and proteinuria in the normal population: the aus diab kidney study. Australian diabetes, obesity and lifestyle study. Am. J. Kidney Dis. 2002;40(4):704–712. doi: 10.1053/ajkd.2002.35677. [DOI] [PubMed] [Google Scholar]
- 29.New J.P., Middleton R.J., Klebe B. Assessing the prevalence, monitoring, and management of chronic Kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet. Med. 2007;24(4):364–369. doi: 10.1111/j.1464-5491.2007.02075.x. http://europepmc.org/abstract/med/6231149 [DOI] [PubMed] [Google Scholar]
- 30.Ahmed I., John G.T., Kirubakaram M.G., Jacob C.K., Muliyil J. Prevalence of Proteinuria in rural adult population in Tamil Naru. Indian J. Med. Res. 2006;124:185–188. https://www.ncbi.nlm.nih.gov/pubmed/17015932 [PubMed] [Google Scholar]
- 31.Agarwal S.K., Dash S.C., Irshad M., Raju S., Singh R., Pandey R.M. Prevalence of Chronic renal failure in adults in Delhi, India. Nephrol. Dial. Transplant. 2005;20:1638–1642. doi: 10.1093/ndt/gfh855. [DOI] [PubMed] [Google Scholar]
- 32.Kannel W.B., Stampfer M.J., Castelli W.P., Verter J. The prognostic significance of proteinuria: the Framingham study. Am. Heart J. 1984;108(5):1347–1352. doi: 10.1016/0002-8703(84)90763-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.