Abstract
This article contains data on the proteins expressed in the ovaries of Anopheles stephensi, a major vector of malaria in India. Data acquisition was performed using a high-resolution Orbitrap-Velos mass spectrometer. The acquired MS/MS data was searched against An. stephensi protein database comprising of 11,789 sequences. Overall, 4407 proteins were identified, functional analysis was performed for the identified proteins and a protein-protein interaction map predicted. The data provided here is also related to a published article - “Integrating transcriptomics and proteomics data for accurate assembly and annotation of genomes” (Prasad et al., 2017) [1].
Specifications Table
| Subject area | Biology |
| More specific subject area | Vector biology |
| Type of data | Excel files, figures |
| How data was acquired | LTQ-Orbitrap Velos ETD mass spectrometer (Thermo Scientific, Bremen, Germany) |
| Proteome Discoverer 2.1 and MASCOT search engine (Matrix Science, London, UK; version 2.2) | |
| Protein database Anopheles stephensi Liston (Indian strain) (www.VectorBase.org, release February 25, 2014) | |
| Data format | Analyzed |
| Experimental factors | Ovaries were dissected from sugar fed mosquitoes and proteins extracted. |
| Experimental features | Proteome profiling of Anopheles stephensi ovaries |
| Data source location | Goa and Bangalore, India |
| Data accessibility | Raw mass spectrometric data is available from a web application (ProteomeXchange) Consortium |
| (http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD001128). | |
| Analyzed data is provided along with this article as excel sheets. | |
| Related research article | Prasad et al. [1]. |
Value of the data
-
•
The data tabulates proteins identified in the adult An. stephensi ovaries, which, plays an essential role in determining the fecundity of this important malaria vector.
-
•
The data provides better understanding of the underlying physiology of An. stephensi females that can be manipulated to prevent the spread of malaria.
-
•
The data provides potential protein-protein interactions among the proteins identified in the ovaries. Further study of these proteins can provide deeper insights in to the underlying physiological processes in female An. stephensi.
-
•
The data provides a baseline platform for further studies on vector-pathogen interactions in female An. stephensi mosquitoes.
1. Data
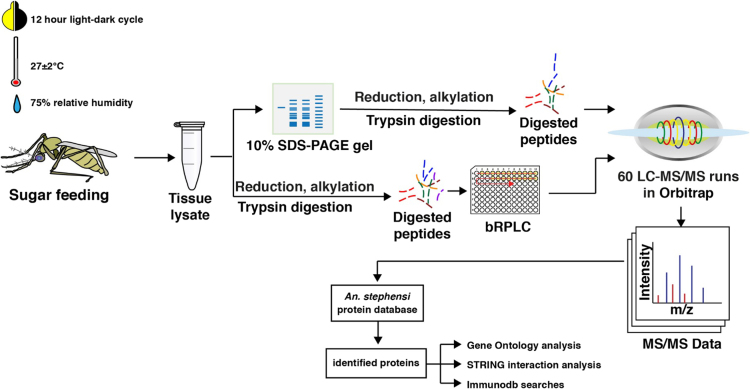
To identify proteins expressed in the mosquito ovaries, we carried out high-resolution mass spectrometry-based proteomic analysis of ovaries dissected from sugar fed female An. stephensi Liston (Fig. 1). Extracted proteins were digested using trypsin followed by fractionation at protein level using SDS-PAGE. In addition, we also performed basic reverse phase liquid chromatograhy (bRPLC) fractionation at the peptide level. Each of these fractions were then analyzed on a high-resolution LTQ-Orbitrap Velos mass spectrometer and the acquired data searched against An. stephensi protein database. A total of 4407 were identified of which, 4284 proteins were identified with multiple PSMs. The complete list of proteins identified, and their corresponding peptides are provided in Supplementary Table S1 and S2.
Fig. 1.
Pictorial representation of mosquito insectary conditions (light-dark cycle, temperature and humidity), sample processing steps, fractionation method used, and workflow of data analysis undertaken in the study.
2. Experimental design, materials and methods
2.1. Maintenance of mosquito colony
Continuous cyclic colony of An. stephensi mosquitoes were obtained from the insectary at ICMR-National Institute of Malaria Research, Field Station Goa. Colonies were maintained in a cycle of 12 hours in light and 12 hours in darkness, at 27±2 °C, 75% relative humidity. Adult mosquitoes were fed on 10% glucose solution.
2.2. Protein extraction
Altogether, 3,500 pairs of ovaries were dissected in 0.65% normal saline under dissecting microscope and homogenized in 200 µl of 4% SDS solution using a probe sonicator. Supernatant was collected after centrifugation of tissue lysate at 14,000 rpm for 10 minutes at 4 °C. Quantification of the extracted proteins were carried out according to Lowry׳s method (Bio-Rad DC Protein assay).
2.3. Fractionation
Proteins extracted from ovaries were fractionated as discussed in our previous studies [1], [2], [3]. For fractionation on gel, 300 µg of protein was resolved on a 10% SDS-PAGE, and 24 bands were excised. Excised bands were destained, reduced and alkylated followed by overnight digestion at 37°C using sequencing grade trypsin (Promega). Digested peptides were extracted and stored at -20 °C, until further use.
For fractionation at peptide level, 500 µg of protein were reduced and alkylated prior to trypsin digestion at 37 °C for 16 hours. Digested peptides were cleaned using Sepak C18 column and lyophilized prior to bRPLC fractionation. The lyophilized samples were reconstituted in bRPLC solvent A (10 mM triethyl ammonium bicarbonate (TEABC) in water at, pH 9.5), loaded on XBridge C18, 5 µm 250×4.6 mm column (Waters, Milford, MA) connected to Agilent 1100 series HPLC system. The peptide digest was resolved using a gradient of 5% to 100% solvent B (10 mM TEABC in Acetonitrile, pH 9.5) in 70 minutes. The total fractionation time was 60 minutes. Eluting peptides were collected in a 96 well plate and then concatenated into 36 fractions. Fractions were dried and reconstituted in 20 μl of 0.1% formic acid before mass spectrometric analysis [4].
2.4. Mass spectrometry analysis
The fractionated and cleaned peptides were analyzed on LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific, Bremen, Germany) interfaced with Easy-nLCII (Thermo Scienific, Bremen, Germany). Peptides were enriched on a pre-column (2 cm, 5μ–100Ǻ), followed by separation on an analytical column (11 cm, 3μ–100Ǻ) packed in-house with magic AQ C18 material (Michrom Bioresources, Inc, Auburn, CA). The solvents used contained 0.1% aqueous formic acid as solvent A and 95% acetonitrile, 0.1% formic acid as solvent B. Solvent A was used to load peptides on to the trap column, followed by resolution on the analytical column using a gradient of 10–35% solvent B for 75 min at a constant flow rate of 0.25 μl/min. The spray voltage and heated capillary temperature were set to 2.0 kV and 220 °C. Data acquisition was carried out in a data dependent manner with a full scan in the range of m/z 350 to 2000. Orbitrap mass analyzer was used at both MS and MS/MS scans. Full MS scans were measured at a resolution 30,000 at m/z 400 and fifteen most intense precursors were selected for MS/MS fragmentation. Fragmentation was carried using higher-energy collisional dissociation (HCD) method and detected at a mass resolution of 15,000 at m/z 400. For full FTMS automatic gain control (AGC) was set to 1 million ions while FT MS/MS was set to 0.1 million ions with maximum accumulation time of 100 ms and 200 ms, respectively.
2.5. Functional categorization and prediction of interaction map
Categorization of the Gene Ontology terms for the identified proteins was performed by fetching information provided in the Panther database [5]. As Panther database has identifiers only for An. gambiae, which, is an ortholog of An. stephensi, we fetched the An. gambiae orthologs for the identified An. stephensi proteins using Biomart (version 0.7) [6] tool provided through VectorBase [7] (Supplementary Table S3). These An. gambiae identifiers were then used to fetch the Gene Ontology information. A predicted protein-protein interaction map of the identified proteins was then generated using STRING (Search Tool for the Retrieval Interacting Genes/Proteins) online tool (version 10.5) [8].
2.6. Data analysis
The mass spectrometry-derived data was searched against a database containing An. stephensi proteins along with known contaminants using Proteome Discoverer software, version 2.1 (Thermo Fischer Scientific, Bremen, Germany). The workflow consisted of Spectrum selector and SEQUEST and MASCOT search nodes. Trypsin was used as an enzyme allowing a single missed cleavage and 6 amino acids as the minimum peptide length. Search parameters also included carbamidomethylation of cysteine as static modifications and oxidation of methionine as a variable modification. Results were retrieved using a 1% false discovery rate (FDR) at the peptide level.
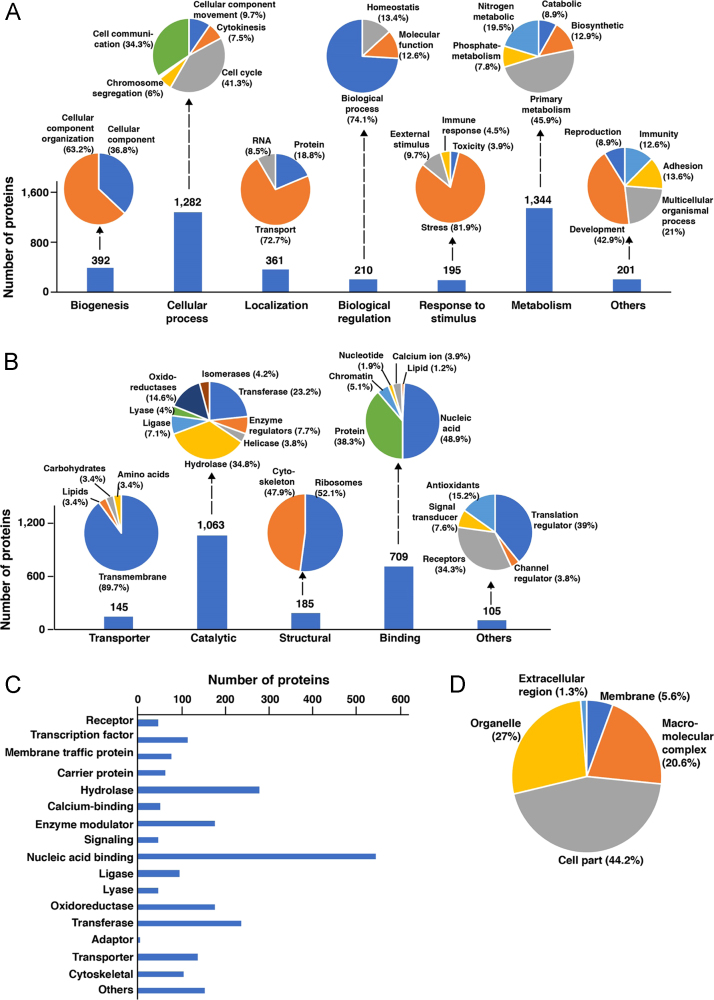
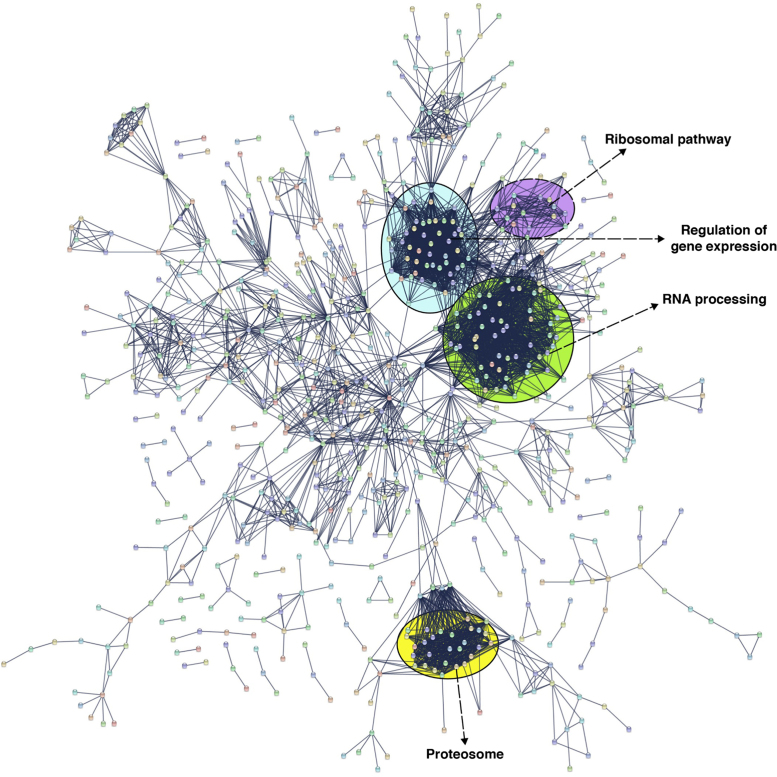
The proteins identified were annotated for their role in biological processes, molecular functions and their localization using Gene Ontology. Most of the identified proteins belonged to the protein class of nucleic acid binding proteins (23.1%), hydrolases (11.8%), transferases (10%) and oxidoreductases (7.5%). Functional annotations categorized the identified proteins to be associated with biological processes such as metabolism (33.6%), cellular processes (32%) and biogenesis (9.8%) (Fig. 2). We also generated a predictive protein-protein interaction map for the proteins identified in ovaries (Fig. 3, Supplementary Table S4). The interaction map showed distinctive clusters associated with ribosomal pathway, regulation of gene expression, RNA processing and proteasome pathways.
Fig. 2.
Functional analysis of the identified protein in An. stephensi ovaries. Gene Ontology information was fetched from PANTHER database. A) Biological processes, B) Molecular function, are represented in bar graph and major process and functions are further categorized into their sub-classes and represented as pie charts; C) Bar graph representation of Protein Class; D) Pie chart of Sub-cellular localization of the identified proteins.
Fig. 3.
Predicted protein-protein interaction map of proteins identified in the ovary. Few of the identified proteins were found to cluster together into distinct clusters with associated roles in ribosomal pathway, regulation of gene expression, RNA processing and proteasomal pathway.
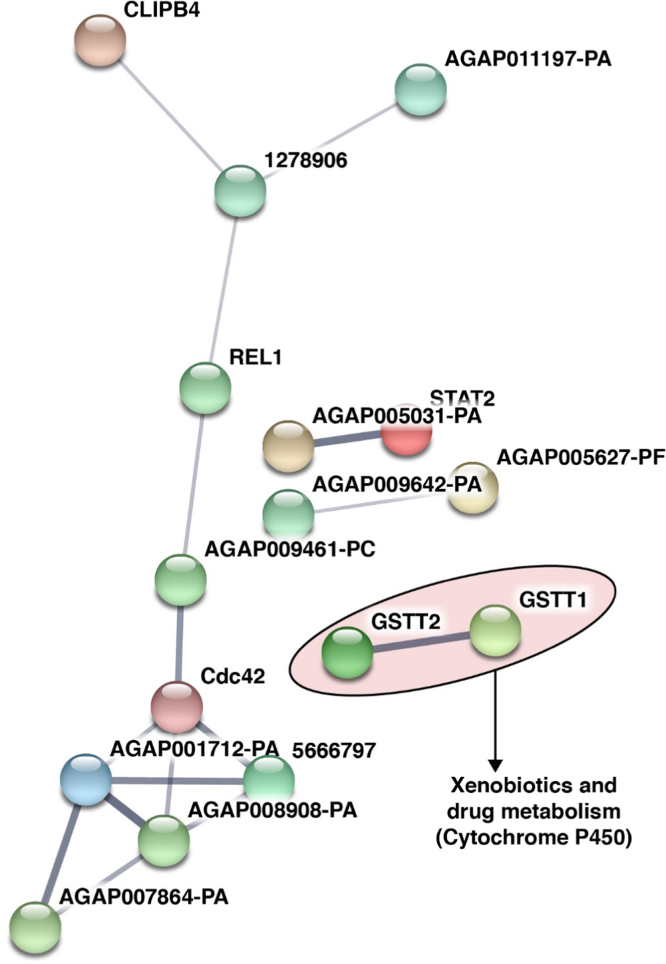
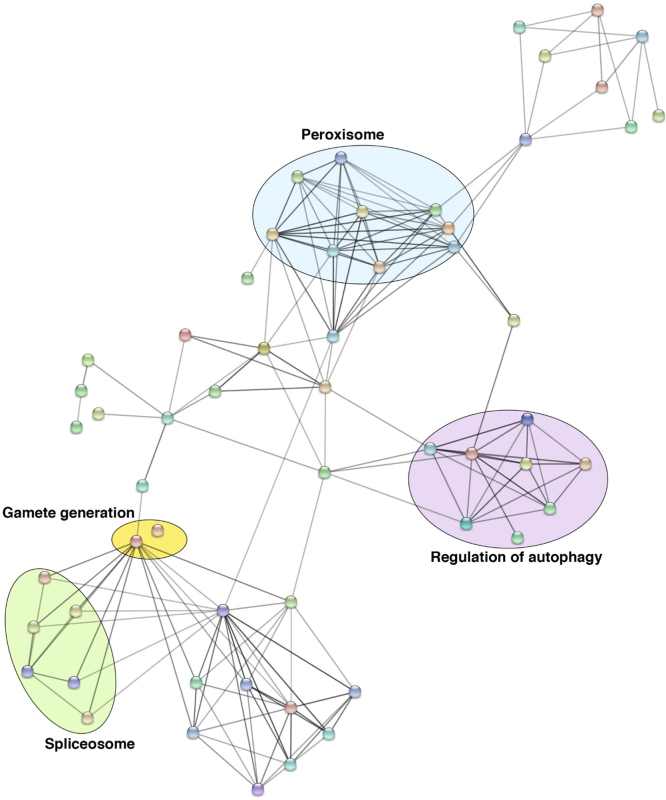
The potential role of certain genes in mosquitoes as agonist or antagonist to the development of Plasmodium parasite in the mosquito midgut and salivary glands has been studied through gene knockdown experiments [9]. We observed 30 of such proteins to be expressed in the An. stephensi ovaries with potential role in xenobiotics and drug metabolism that involves Cytochrome P450 (Fig. 4, Supplementary Table S5). However, these proteins were not restricted only to the ovaries and were also observed to be expressed in midgut, fatbody and salivary glands [1], [10], [11], [12]. The complete list of proteins identified in ovaries that mapped to the list of vector proteins with experimentally proven roles in parasite development is provided in (Supplementary Table S6). To further evaluate for related functions of the proteins identified in the ovaries, we mapped our data against Immunodb, which is a resource that provides a list of immune-related gene families. Seventy of the identified proteins were observed to have a potential role in mosquito vector immune responses. The list of proteins mapping to Immunodb is provided in (Supplementary Table S7). To evaluate the interaction network of the proteins mapping to Immunodb data, we generated a putative interacting map of the 70 proteins using STRING online tool (Fig. 5, Supplementary Table S8). Most of these proteins were found to be associated with peroxisome, regulation of autophagy, gamete generation and spliceosome.
Fig. 4.
Predicted protein-protein interaction map of proteins identified in the An. stephensi ovary for which, their An. gambiae orthologs were found to have a role in vector-pathogen interactions.
Fig. 5.
Predicted protein-protein interaction map of proteins and having a potential role in immunity (predicted by mapping to ImmunoDB database). Proteins associated with regulation of autophagy, spliceosome machinery, gamete generation and peroxisomes were predicted to be interacting among each other.
The data presented here, thus facilitates further analysis and studies on An. stephensi by providing a baseline data.
Acknowledgements
We thank ICMR- National Institute of Malaria Research, Institute of Bioinformatics for institutional support. We thank Science and Engineering Research Board (SERB), Government of India, for funding (EMR/2014/000444) to NIMR and IOB to characterize proteome and transcriptome of mosquito vectors. This manuscript has been approved by the NIMR publication committee (approval number 062/2017). We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). Gourav Dey and Sreelakshmi K. Sreenivasamurthy are recipients of Senior Research Fellowship from the University Grants Commission (UGC), Government of India. Manish Kumar is a recipient of Senior Research Fellowship from Council of Scientific & Industrial Research (CSIR), Government of India.
Footnotes
Transparency data associated with this article can be found in the online version at 10.1016/j.dib.2018.08.066.
Supplementary data associated with this article can be found in the online version at 10.1016/j.dib.2018.08.066.
Contributor Information
T.S. Keshava Prasad, Email: keshav@yenepoya.edu.in.
Ashwani Kumar, Email: ashwani07@gmail.com.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material Supplementary Table S1: List of proteins identified in the ovaries of An. stephensi using high-resolution mass spectrometry-based proteomics approach.
.
Supplementary material Supplementary Table S2: List of peptides and their corresponding proteins that were identified in the ovaries of An. stephensi through high-resolution LC-MS/MS analysis.
.
Supplementary material Supplementary Table S3: List of proteins identified in An. stephensi (Indian strain) along with their orthologs in An. stephensi (SDA500 strain) and An. gambiae. Ortholog information was fetched from VectorBase using the provided Biomart tool.
.
Supplementary material Supplementary Table S4: List of proteins identified in ovaries of An. stephensi and predicted to be interacting among each other. Interaction map was constructed using STRING online tool with default search parameters.
.
Supplementary material Supplementary Table S5: Predicted protein-protein interactions of ovary proteins in An. stephensi (Indian strain) that may have a potential role in vector-parasite interactions.
.
Supplementary material Supplementary Table S6: List of proteins identified to be expressed in the ovary of An. stephensi and orthologs of which, were experimentally validated in An. gambiae for their role in Plasmodium development inside the vector mosquito.
.
Supplementary material Supplementary Table S7: List of proteins identified in An. stephensi ovaries that may have potential immune related functions according to the data obtained from Immunodb.
.
Supplementary material Supplementary Table S8: List of proteins expressed in An. stephensi ovary predicted to be associated with immune-related functions and predicted to be interacting with each other.
.
References
- 1.Prasad T.S.K. Integrating transcriptomic and proteomic data for accurate assembly and annotation of genomes. Genome Res. 2017;27(1):133–144. doi: 10.1101/gr.201368.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwivedi S.B. Brain proteomics of Anopheles gambiae. OMICS. 2014;18(7):421–437. doi: 10.1089/omi.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelkar D.S. Annotation of the zebrafish genome through an integrated transcriptomic and proteomic analysis. Mol. Cell. Proteom. 2014;13(11):3184–3198. doi: 10.1074/mcp.M114.038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dey G. Proteome data of Anopheles stephensi hemolymph using high resolution mass spectrometry. Data Brief. 2018;18:1441–1447. doi: 10.1016/j.dib.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mi H. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013;8(8):1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smedley D. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res. 2015;43(W1):W589–W598. doi: 10.1093/nar/gkv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giraldo-Calderon G.I. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015;43:D707–D713. doi: 10.1093/nar/gku1117. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szklarczyk D. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sreenivasamurthy S.K. A compendium of molecules involved in vector-pathogen interactions pertaining to malaria. Malar. J. 2013;12:216. doi: 10.1186/1475-2875-12-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sreenivasamurthy S.K. Mosquito-borne diseases and Omics: tissue-restricted expression and alternative splicing revealed by transcriptome profiling of Anopheles stephensi. OMICS. 2017;21:488–497. doi: 10.1089/omi.2017.0073. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M. Response to blood meal in the fat body of Anopheles stephensi using quantitative proteomics: towards new vector control strategies against malaria. OMICS. 2017;21:520–530. doi: 10.1089/omi.2017.0092. [DOI] [PubMed] [Google Scholar]
- 12.Mohanty A.K. Mapping Anopheles stephensi midgut proteome using high-resolution mass spectrometry. Data Brief. 2018;17:1295–1303. doi: 10.1016/j.dib.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material Supplementary Table S1: List of proteins identified in the ovaries of An. stephensi using high-resolution mass spectrometry-based proteomics approach.
Supplementary material Supplementary Table S2: List of peptides and their corresponding proteins that were identified in the ovaries of An. stephensi through high-resolution LC-MS/MS analysis.
Supplementary material Supplementary Table S3: List of proteins identified in An. stephensi (Indian strain) along with their orthologs in An. stephensi (SDA500 strain) and An. gambiae. Ortholog information was fetched from VectorBase using the provided Biomart tool.
Supplementary material Supplementary Table S4: List of proteins identified in ovaries of An. stephensi and predicted to be interacting among each other. Interaction map was constructed using STRING online tool with default search parameters.
Supplementary material Supplementary Table S5: Predicted protein-protein interactions of ovary proteins in An. stephensi (Indian strain) that may have a potential role in vector-parasite interactions.
Supplementary material Supplementary Table S6: List of proteins identified to be expressed in the ovary of An. stephensi and orthologs of which, were experimentally validated in An. gambiae for their role in Plasmodium development inside the vector mosquito.
Supplementary material Supplementary Table S7: List of proteins identified in An. stephensi ovaries that may have potential immune related functions according to the data obtained from Immunodb.
Supplementary material Supplementary Table S8: List of proteins expressed in An. stephensi ovary predicted to be associated with immune-related functions and predicted to be interacting with each other.