Abstract
Abstract Objective: The goal of the project was to create recommendations and design specifications for a multimedia tool to enhance the informed consent process for clinical trials. The authors focused on the needs of patients with potential cognitive impairment.
Design: The authors first performed a needs assessment using focus groups and interviews with health care researchers, institutional review board members, and three groups of patients (who had depression, breast cancer, or schizophrenia). Their feedback was incorporated into the design of a prototype multimedia tool. The design included general modules with information about clinical trials and informed consent as well as trial-specific modules. The authors then used the resulting prototype multimedia tool for informed consent in follow-up focus groups and interviews to obtain feedback on the feasibility and potential effectiveness of using such a tool routinely for clinical trials.
Results: The authors showed that it was feasible to adapt a structured multimedia informed consent system to a specific clinical trial and to incorporate techniques to improve the understandability of informed consent content. Patients generally felt the prototype system was useful and could replace the paper document. They felt using the system would be less stressful, because they would have a greater sense of control and could proceed at their own pace. They liked the hierarchic and modular approach to providing information and felt that the use of video made information more understandable. Researchers and institutional review board members also found the system to be valuable in these ways but had concerns about how to review the system for potential biases in presentation and about the legal issues associated with replacing the paper document.
This paper explores the information needs of patients in the informed consent process for clinical research trials and how those needs might be met with the assistance of multimedia technology. Our objective was to use findings from the literature and feedback from patients and researchers to inform our recommendations for the design of a multimedia tool to enhance the informed consent process for clinical trials. We focused on the needs of patients with potential cognitive impairment. Our needs assessment included feedback from focus groups and interviews with patients who had depression, breast cancer, or schizophrenia, as well as interviews with health care researchers and institutional review board members.
Consumers are demanding a greater choice and involvement at all levels of health care. In addition, the advancing sophistication of medical research necessitates a more sophisticated understanding of the process of informed consent in both clinical and research settings. With the increasing number of informed consent protocols being developed for new therapeutic trials, there is a clear need for improvements in informed consent.1,2 Meaningful consent requires that a patient be given sufficient understandable information to make a valid choice. The consent form is not equivalent to informed consent.3 Lidz et al.4 observed the informed consent process and noted that patients had four major reasons for wanting information: to facilitate compliance in treatment decisions, as a sign of respect to them, to exercise veto power over a physician's decision, and for their own decision-making. Communicating highly technical and specialized knowledge to someone who is not educated in that subject is a challenging problem. The general anxiety of patients about their medical condition and the pressures of time also hamper effective communication.5,6 It is important that physicians understand that discussion is a necessary part of the physician-patient relationship and that patients are seeking something more important than the legally completed consent form.7
Background
The effectiveness of the informed consent process is poorly understood and not well researched. Many factors are involved: economic, legal, institutional, educational, cultural, religious, and interpersonal. Poor patient understanding can be due to poor communication techniques or to a lack of time on the part of health care professionals, to patient anxiety or denial or, as much of the existing literature reveals, to a lack of reading comprehension. Patients do not always realize the purpose of the information and the consent form.8,9,10 Cassileth et al.8 evaluated the recall of cancer patients who were given consent forms and verbal explanations. They found that the day after patients signed consent forms, only 60 percent understood the purpose of the forms and only 55 percent were able to identify a major risk. Expressing and understanding risk is a problem faced by physician and patient alike. Merz et al.11 in a study of informed consent litigation, noted that there was no consistency in the verbal expressions used by physicians to categorize risk. In general, physicians have difficulty in expressing subjective probabilities as odds ratios or decimal figures.
Studies of the comprehension of health education handouts show that, typically, only half the recipients are able to comprehend health education materials.12,13 Studies of readability suggest that the existing forms for informed consent are often too complex and difficult for the average person to understand.14,15,16,17,18,19,20,21,22 Morrow et al.17 noted that consent forms are less comprehensible than the popular press and that research consent forms may be as difficult to read as medical journals. When the Fry readability scale23 was used to analyze the grade level of consent forms for oncologic clinical trials, 73 percent of passages required a reading ability at college level or higher. A current challenge to educating patients is that only 28 percent of the American population have attended college, and that approximately 20 percent are functionally illiterate, reading at or below the fifth-grade level.25 These readability scores suggest that many patients lack real understanding, which leads to technically informed but uneducated consent.26,27
There is a long history of concern about research conducted on impaired human subjects.28 The competence of an impaired subject to provide consent is another issue that, with readability, is often neglected in the informed consent process. Although competence is a legal concept and can be formally determined only in court, it does play an important role in the informed consent process. At present, there is no universally accepted test of a patient's capacity to consent to treatment. With age, cognitive impairment becomes more common. Elderly patients are often not given full credit for decisional capacities.29,30,31 Competence may sometimes be questioned only because patients refuse medical treatment.32 Often, clinicians depend on mental status tests, such as the Folstein Mini-Mental Status Exam,33 to identify patients who may not be competent to give informed consent. Taub et al.34 studied the effect of vocabulary level and corrected feedback on informed consent in elderly patients. This study confirms the usefulness of a multi-step approach using a comprehension test as part of the informed consent process, as previously described by Miller and Willner.35
In addressing issues of competence, suggestions have been made that patients should be screened before making treatment decisions. One approach is a screening instrument, the Hopkins Competency Assessment Test,36 that requires the subject to answer a series of questions (true and false, and sentence completion) after reading a short essay presented at an appropriate reading comprehension level (sixth grade, eighth grade, or first-year college level). The authors concluded that screening for competency was possible and economically feasible.
The problem of assessing competence becomes more difficult in cognitively impaired persons and in those with mental disorders.37 Dresser38 reviewed the policy issues surrounding the use of mentally disabled persons as research subjects. Since no single accepted standard for determining decision-making capacity exists, issues of competence are linked with the level of comprehension of subjects. This can only be accomplished when the informed consent process provides understandable information and when the subject's comprehension is determined during that process. Dresser notes that assessment of competence should be task-specific and that conventional mental status tests may not be suitable for research settings.
It is also important to remember that the diagnosis of mental illness is not synonymous with incapacity or with loss of autonomy in decision-making. An investigation of the informed consent process in psychiatric research39 showed that psychiatric patients had problems understanding the purposes of research when investigators emphasized the therapeutic benefits rather than the research purposes. In a series of studies,40,41,42 three different standards for assessing decision-making capacity were tested on groups of patients with diagnoses of schizophrenia, major depression, and ischemic heart disease. Depending on which standard was used, patients were classified differently, pointing to the fact that no single standard is sufficiently broad to identify all patients. If all were used, the potential exists for compromising patients' autonomy in making decisions affecting their medical care. Physicians need to be aware of legal standards applicable in their community and how those standards can be applied correctly. Medical professionals often assume that refusal of therapy raises questions of a patient's competence. Lawyers do not necessarily take the same position,43 and the courts have helped solidify the legal boundaries of informed consent to the benefit of patient self-determination.
Clinical trials, especially in the area of cancer chemotherapy, offer another unique set of problems for informed consent. The distinction between informed consent for treatment and informed consent for research is becoming less clear and can present a conflict of interest for clinical investigators.44,45 The fundamental feature of clinical trials—namely, that participants are in a “research,” not a therapeutic, program—is not always clear to patients.46 Sutherland et al.47 suggest that “a more contemporary and comprehensive set of guidelines for planning and conducting trials is required to address the increasing influence of patients' preferences on the successful completion of clinical trials.” Differing cognitive style among patients characterize how information is gathered and how much information is desired in the informed consent process. The present informed consent procedure, which utilizes a single consent form, cannot address these differences.48 Other studies have shown that enhancing the process to provide more useful information for decision-making does not affect the clinical trial entry decision.49,50
Clinical trials of new therapies and procedures require informed consent of greater complexity. White et al.51 studied patient preferences for long, medium, or short consent forms for chemotherapy. Although the majority of patients expressed a preference for more detailed information, these patients, regardless of educational level, answered questions basic to the study design incorrectly. The authors stress the importance of the proper design and evaluation of consent forms. One aspect of this complexity relates to the terminology used in clinical trials. Few patients understand such terms as randomization and double-blind study.52 The results of one study53 suggest that patients who were better educated, encouraged by a partner, given adequate time to decide, or initially approached by a physician were more likely to consent.
Patients with cancer may be especially vulnerable as they deal with the distressing and difficult diagnosis of a terminal illness. Rodenhuis et al.54 studied the quality of the informed consent process in a phase I study of an anticancer drug. Patients were motivated by four factors: hope for improvement of their condition, pressure exerted by family and friends, the desire to help medical science, or simply the feeling that they really did not have a choice. When the authors interviewed patients five days after the informed consent process, 10 of 48 patients had unrealistic expectations and perceptions based on the information they had received during the consent process.
Although there has not been much change in standard practice for informed consent, there have been a few studies investigating alternative consent procedures and newer technologies, in an attempt to improve patient understanding and the meaningfulness of the informed consent process. Tymchuk et al.57 compared four methods of presenting informed consent information to elderly subjects. When those with severe cognitive limitations were excluded, findings showed that subjects benefited from simplified versions of forms more than from either storybook presentations or videotape. However, other studies have shown that oral presentations and videotaped presentations may help patients comprehend consent information.60,61,62,63 Yet video remains an under-utilized tool in the informed consent process.
Kieschnick et al.64 reviewed the available computer products for patient education. A number of specialized software programs are dedicated to handling informed consent both by providing the education (informing) and by automating the consent process. Llewellyn-Thomas et al.68 compared the effectiveness of using audiotape or an interactive computer program for teaching about a clinical trial in terms of patients' satisfaction, understanding of the information, and decision whether to enter the trial. They found no difference in understanding or satisfaction, but the computer users tended to report a more positive attitude.
Among various approaches to informed consent, computers represent potentially powerful and effective tools. Yet there are few existing informed consent programs, and those address only specific procedures.64 Because of the early stage of the field and the limited number of evaluations,65,66 it has been difficult to show compelling benefit from the use of computers in the informed consent process. No general programs currently exist for informed consent, and in those no attempt is made to assess either the competence of the patient or the appropriateness of the educational level of the material. In light of the ever-increasing number of new therapeutic interventions and complex research protocols, it remains important to continue to search for methods to improve the informed consent process. A multimedia computer tool has the potential to both enhance and standardize the quality of informed consent. However, because so little is understood about how to make it effective, it is important to first explore the needs of patients in the informed consent process and determine which multimedia approaches are likely to address those needs.
Study Design
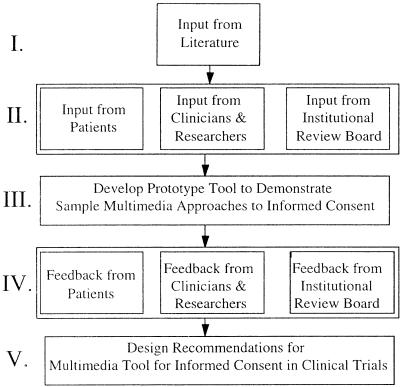
When creating technology for new applications where a variety of approaches are possible but untested, it is most useful to use qualitative techniques to determine how to focus the later system design efforts. Our approach to developing design recommendations for a multimedia tool to augment the informed consent process for clinical trial research is outlined in ▶. We first did a preliminary needs assessment to augment findings from the literature, using input from focus groups and interviews with patients who had been asked to participate in a clinical trial, with researchers responsible for obtaining informed consent, and with institutional review board members who oversee the informed consent process in research. Based on the findings from these focus groups and interviews, as well as on findings from the literature, we developed a prototype multimedia tool to test various suggested approaches to improving the informed consent process. The prototype tool was then demonstrated and used as stimulus material in follow-on focus groups and interviews with patients, researchers, and institutional review board members. We then used this feedback to generate design goals and specifications for a multimedia tool for informed consent.
Figure 1.
The five stages of needs assessment for a multimedia informed-consent tool for clinical trial research. The input and feedback in stages II and IV came from focus groups and interviews.
Preliminary Focus Groups and Interviews
Our review of the literature showed several unresolved deficiencies in the informed consent process that could be addressed in a well-designed multimedia tool. The major problems were the inability of some patients to read and comprehend many consent forms, the inadequacy and variability of the oral explanations of informed consent, and the lack of documentation of comprehension of the consent forms. To supplement these findings and to inform the design of our prototype multimedia tool for informed consent, we performed a preliminary needs assessment using focus groups and interviews with patients, health care professionals, and members of our institutional review board, as outlined below:
Focus group of patients with schizophrenia: 8 participants
Focus group of patients with breast cancer: 10 participants
Interviews with patients with depression: 11 participants
Focus group with institutional review board members: 8 participants
Interviews with researchers and experts in informed consent: 15 participants
We were interested in exploring the informational needs of patients with potential cognitive impairment who were making a decision about whether to participate in a clinical trial. We chose to look at three distinct groups of patients with different manifestations of possible cognitive impairment when making this decision. Patients with schizophrenia often have pronounced difficulty in concentrating on material long enough to take into account all aspects of informed consent documents when making a decision about trial participation. Patients with breast cancer are typically asked to participate in a trial very soon after diagnosis. They are often emotionally distraught and in a particularly vulnerable situation. Finally, patients with depression may have difficulty making a decision on whether to participate in a clinical trial since they often lack the motivation and emotional energy needed to absorb the large amounts of informed consent material.
Patients were recruited by clinical researchers at Oregon Health Sciences University and at the Portland, Oregon, Veterans Administration Hospital. The eligibility requirements were that patients be 18 years or older and that they had previously been asked to participate in a clinical trial. The subjects were compensated for time and travel ($20).
We also interviewed members of the Oregon Health Sciences Institutional Review Board as well as local researchers and informed consent experts to determine what would be viewed as acceptable and useful to them in using a multimedia tool to augment the informed consent process. These groups would continue to be closely involved with the design of a multimedia tool for the informed consent process.
Preliminary Focus Group/Interview Moderation
We used a “funnel” design of discussion organization for the focus groups and interviews, starting with open-ended broad topics regarding clinical trials and the process of informed consent to encourage unprompted discussion of issues. We also asked the patients and researchers to describe their actual experiences with informed consent, at some point asking them for feedback on the effects of possible cognitive impairment. In the second stage of the session, we showed subjects four representative sample informed consent documents from previous clinical trials at Oregon Health Sciences University. These documents ranged in length from 8 to 12 pages. Subjects were asked to imagine that they were being asked to participate in one of these trials and to read the document. We then asked for their comments on this process. In the third stage of the session, we asked subjects for specific recommendations for improving the informed consent process and suggestions on how to best design an interactive multimedia system to augment informed consent for clinical trials. Finally, to close each focus group and interview, we directed the discussion toward speculation about the future with regard to what might be available for patients involved in clinical trials.
Analysis of Preliminary Focus Groups and Interviews
We tape-recorded and transcribed each focus group and interview. The comments from these transcripts were then clustered and summarized for each group. The following lists detail the primary findings from our needs assessment.
Patients' experiences with informed consent:
Patients with schizophrenia often had difficulty in remembering the process. They depended on the doctor or researcher. Payment for participation was a large decision factor for this group.
The relationship with the doctor and trust was an important factor for patients with breast cancer. Many had high levels of anxiety and fear, leading to difficulty in remembering things. They did lots of reading and wanted as much literature as possible.
Patients with depression generally preferred an explanation from a doctor, instead of reading forms.
Patients' reactions to sample consent forms:
Patients generally felt the forms were much too long and the wording too complex.
The level of detail became confusing.
Patients' suggestions for improvement:
Make the consent forms simpler and shorter.
Provide a summary of highlights, with the details kept separate.
Provide a glossary; define terms.
Use lay language throughout.
Provide information on clinical trials in general.
Provide space to write questions.
Use larger font for text.
Emphasize what is important.
Use graphics and video.
The members of the institutional review board and the researchers had many similar comments. They felt it was important to manage information overload and address the reading level of documents. One suggestion was to require that consent forms be reviewed by an editor who specialized in writing for the lay public. They also suggested using graphics and visuals to explain risks and benefits, incorporating links to other data sources and support groups, customizing information to different patient groups (language, age, etc.), and incorporating mechanisms of providing feedback to the institutional review board.
Development of a Prototype Multimedia Tool for Informed Consent
We used the feedback from the preliminary focus groups and interviews and from the review of the literature to design a prototype multimedia tool for informed consent in clinical trials. The purpose of this prototype was to test various suggested methods of providing informed consent information in a single system that we would then test in follow-up focus groups and interviews. It was not a complete working system but rather an interactive tool that tested a variety of approaches. We developed this prototype using SuperCard and Macromedia authoring software an Apple PowerMac Computer with a Troll Touch monitor with touchscreen capability. Our design included general modules with information about clinical trials and informed consent as well as trial-specific modules. For the purposes of using this prototype as stimulus material in follow-up focus groups and interviews, we based the content of the specific modules on a drug study that had already taken place for patients with schizophrenia. The researcher-physician for this study provided both the informed consent content and video explanations that we used in the trial-specific modules.
Our prototype consisted of the following modules:
Welcome (general)
About Clinical Trials (general)
The Seroquel Study (trial-specific)
Questions for Your Doctor (general)
Available Resources (trial-specific)
Patient Experiences (trial-specific)
Self-test (trial-specific)
Ready to Decide (general)
Embedded in these modules were various approaches for testing how best to provide the following:
Easy, structured adaptation to a new research protocols, where general information appears in all applications and the researcher inputs trial-specific information (text and video) following a prescribed template.
Emphasis on a subject's understanding and consent (use of video, pictures, graphics, and audio)
Measurement and documentation of competency.
Measurement and documentation of comprehension.
Documentation of consent.
Figures ▶,▶,▶,▶,▶,▶ show representative screens from the prototype multimedia tool. Figures ▶, ▶, and ▶ show sample general screens that would remain the same from clinical trial to clinical trial (e.g., introductory and background material, competency testing, and written or voice signature). Figures ▶, ▶, and ▶, on the other hand, show screens representing material specific to a particular clinical trial, which would be replaced with new material each time the tool was used for a new research trial (e.g., description of study, patient experiences, patient resources, self-tests, and review material). We designed the multimedia prototype to be highly structured, with well-defined content definitions for the tailored materials to be inserted (text, video, and audio). Adapting the general tool by inserting trial-specific information would not require programming knowledge and could be performed by a member of the research team.
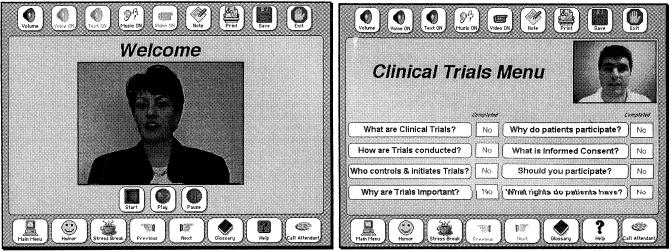
Figure 2.
Sample screens from the generic component of the system. Left, A welcome screen. Right, A menu of topics about clinical trials in general.
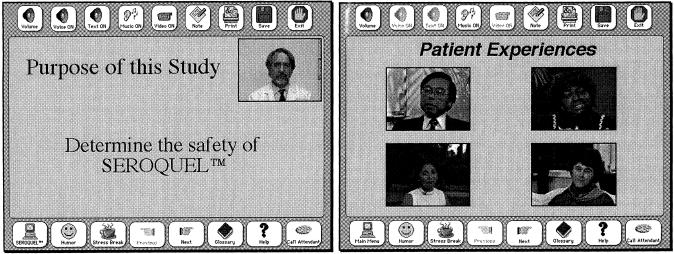
Figure 3.
Sample screens from the trial-specific component of the system. Left, One in a series of screens describing the Seroquel clinical trial, where the text on the screen is supplemented by a video of the physician describing the material. Right, Sample video clips of patient experiences that could be used to communicate possible patient outcomes during a clinical trial. (For the prototype we used kidney dialysis video clips from Lenert's research group at Stanford University.69)
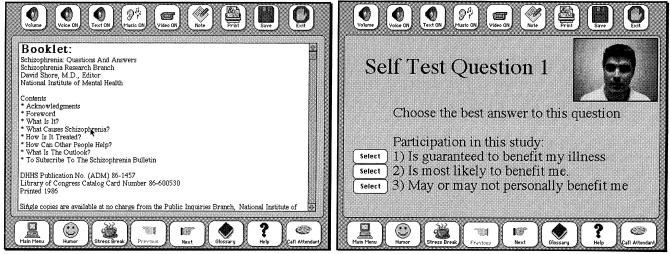
Figure 4.
Other trial-specific screens show a list of local resources, such as books and organizations (left) and a sample question from the self-test component (right).
Figure 5.
Sample screen from the cognitive test module. The patient is asked to put the pictured activities in order by touching the pictures in sequence. (Based on the Wang and Ennis Cognitive Competency Test.70)
Figure 6.
Summary screen describing the main points of the Seroquel study.
Figure 7.
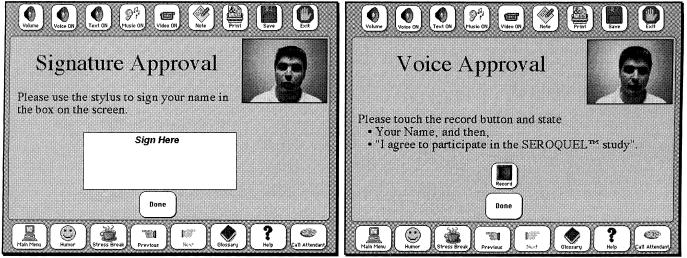
These screens show how a patient could use the touch screen to record an electronic signature (left) or to record an audio consent (right).
A sample welcome screen is shown on the left side of ▶, left. Video and audio were used throughout the system as often as possible to make the material more interesting to the patient and not dependent on the patient's ability to read. ▶, right, shows the topics covered in background material on clinical trials. This area was marked as optional, whereas material on the study itself (e.g., purpose, risks, benefits, alternatives, and costs) were required. Although the patient could browse through the topics as desired, the system recorded which areas were viewed and took the user back to any unseen but required topics before completing the session.
The screen shown in ▶, left, is representative of the material describing a particular clinical trial. For the purpose of the prototype tool for informed consent for clinical trials, we used the example of a study testing the efficacy of the drug Seroquel for patients with schizophrenia. This trial had already taken place at Oregon Health Sciences University under the direction of William Wilson, MD. Dr. Wilson adapted his consent form and typical discussion to a presentation format (bullet points and few concepts per page). We then videotaped his presentation of the informed consent material and included his pages with a video window of his comments as separate screens. This material was designed as required for the patient. Supplemental material, such as the descriptions of patient experiences with potential outcomes from the trial (shown in ▶, right) and resources for patients with schizophrenia (shown in Figure ▶, left) were designated as optional for the purposes of this prototype.
We used this modular, hierarchic approach to presenting informed consent information both to simplify the insertion of trial-specific information (prescribed bulleted slides and video) and to allow the patient to control the browsing of information. Other features that incorporated in this section were pop-up definitions of terms, which appeared when a word was touched; sample screens in Spanish; and a variety of graphics and icons.
In presenting informed consent, it is important to judge a patient's understanding of the material and competency to consent to treatment. This is especially important (and difficult to determine) with subjects who may have cognitive impairment. In the prototype, we included a self-test module in which multiple-choice questions were read to the patient (see ▶, right). Incorrect responses could lead the patient back to the original material or could simply be monitored to judge the patient's level of understanding. To demonstrate how competency could be tested in a multimedia tool, we adapted an existing validated paper-based method (the Wang and Ennis Cognitive Competency Test70). A sample question is shown in ▶. In this case, the pictures of activities associated with preparing a meal must be touched in the order they would be performed.
In response to some of the suggestions from patients and researchers regarding the need for a high-level summary of the important concepts, we tested the use of a summary screen that the patient could view before deciding on trial participation. This is shown in ▶. Finally, we tested a variety of methods for documenting the informed consent process. One option was to simply print out a form and have the patient sign it. ▶ shows the screens that accept an electronic signature and audio recording of consent.
Follow-up Focus Groups and Interviews
We used the prototype multimedia tool for informed consent in focus groups and interviews with patients and researchers to obtain feedback on the feasibility of using such a tool routinely for clinical trials. Five patients with schizophrenia, 7 with cancer, 9 with depression, 19 researchers, and 14 institutional review board members participated in these follow-up focus groups and interviews. Subjects were recruited from the pool of people who participated in the preliminary round of focus groups and interviews. Each subject was once again paid $20 for time and travel. The format of the focus groups and interviews included an initial overview of the project and a demonstration of the system, followed by a debriefing to elicit reactions, comments, and suggestions.
We found that subjects generally felt the system was useful and could replace the paper document. They felt that using the system would be less stressful, because they would have a greater sense of control and could proceed at their own pace. They liked the hierarchic and modular approach to providing information and felt that the use of video made information more understandable. When asked what we should keep in the system, most responded that they “liked it as it is” and that “everything was much friendlier than paper alone.” Specific features that were favored were the clinical trials background, the glossary (pop-up definitions on each page), the list of resources (which could be printed), the video and audio features with all text, and the hierarchic format. The subjects' suggestions for what should be changed about the prototype included reorganizing the information so that the important material is covered first (perhaps by an overview at the beginning). In addition, the self-test and competence test were not always viewed positively. Some patients felt the self-test “would create anxiety” if it were being recorded. The competence test took additional time (about three minutes), and the material was off the subject, making the interaction more confusing.
Researchers and institutional review board members found the system to be valuable in making the information more understandable with a consistent quality presentation by the physician/researcher. They realized that background information on clinical trials was often missing from informed consent and felt it was helpful to have this and other supplemental material available to the subject on demand, without interfering with the basic presentation. Several institutional review board members had concerns about being able to review the system for potential biases in presentation and about the legal issues associated with replacing the paper document. In particular, many felt that the section on subjects' experiences would be difficult to keep unbiased and were uncomfortable including that component in a system. Subjects, on the other hand, found the section on these experiences very valuable. Many researchers commented that such a tool could save significant physician time and could make the presentation of informed consent material more standard and less biased. The required-versus-optional and the overview-versus-detail modules were found to be both acceptable and useful.
When we discussed with the researchers the possible means of testing a patient's competence to decide on trial participation, we mentioned that it seemed difficult to find a way to measure competence without prolonging the time spent on seemingly irrelevant topics or without alienating the patient. However, some researchers mentioned that a computer-based competence tool, as tested in our prototype, would be a useful option, and more standard than a paper-based approach. The resulting suggestion was to develop a separate competence tool that could be used independently of the informed consent tool.
Finally, we probed the subjects on how such a multimedia tool should be worked into the informed consent process. Subjects still want to talk with a doctor, and institutional review board committees are not yet ready to relinquish the paper record. The current consensus seemed to be that subjects could use such a system to prepare for a conversation with the researcher/physician.
Conclusion
With our needs assessment and follow-up testing of a multimedia tool for informed consent, we showed that it was feasible to insert modules of structured trial-specific information into the larger system. Subjects generally felt the system was useful and could replace the paper document. They felt using the system would be less stressful, because they would have a greater sense of control and could proceed at their own pace. They liked the hierarchic and modular approach to providing information and felt that the use of video made information more understandable. Researchers and institutional review board members also found the system to be valuable but had concerns about being able to review the system for potential biases in presentation and about the legal issues associated with replacing the paper document. We developed both high-level and low-level design recommendations for a multimedia tool for informed consent based on the qualitative feedback from our prototype that demonstrated a variety of possible approaches. The basic design recommendations included:
A highly structured modular approach that reuses general informed consent and clinical trial information and makes use of scripted (fill-in-the-blank) trial-specific presentation screens and video from the physician/researcher.
An overview of the most important points near the beginning of the interaction and near the end, especially immediately before the subject decides whether to participate.
Glossary definitions included wherever possible.
A self-test module this is optional and mainly for patient use.
Graphic aids to explain risks.
Inclusion of the competence test as a separate tool or module.
A multimedia computer-based system has the potential to aid in the solution of some of the more common objections to the traditional informed consent process and paper document. These qualitative findings and recommendations are an important first step in creating a useful system. Future work should evaluate whether such a system can be integrated into a clinical setting and improve patient understanding and decision-making while conforming to the institutional requirements for informed consent.
This work was supported by grant R41-MH/LM57175-01 from the National Institute of Mental Health and the National Library of Medicine.
This paper was presented at the International Medical Informatics Association (IMIA) Nursing Informatics '97 Working Conference, “Informatics and Patient and Clinical Guidelines: The State of Our Knowledge and a Vision,” Stockholm, Sweden, October 2-4, 1997.
References
- 1.Kaufmann CL. Informed consent and patient decision making: two decades of research. Soc Sci Med. 1983;17(21): 1657-64. [DOI] [PubMed] [Google Scholar]
- 2.Varricchio CG, Jassak PF. Informed consent: an overview. Semin Oncol Nurs. 1985;5:(2): 95-8. [DOI] [PubMed] [Google Scholar]
- 3.Sloan J. The consent form revisited. Arch Intern Med. 1993; 153: 1170-2. [PubMed] [Google Scholar]
- 4.Lidz CW, Meisel A, Osterweis M, Holden JL, Marx JH, Munetz MR. Barriers to informed consent. Ann Intern Med. 1983;99(4): 539-43. [DOI] [PubMed] [Google Scholar]
- 5.Faden R, Beauchamp TL. A History and Theory of Informed Consent. New York: Oxford University Press, 1996.
- 6.Buchanan RGN. Enabling patients to make informed decisions. Nurs Times. 1995;91(18): 27-9. [PubMed] [Google Scholar]
- 7.Little JM, Leeder S. Logic, hermeneutics, and informed consent. Eur J Surg. 1996;162(1): 3-10. [PubMed] [Google Scholar]
- 8.Cassileth BR, Zupkis RV, Sutton-Smith K, March V. Informed consent: why are its goals imperfectly realized? N Engl J Med. 1980;302: 896-900. [DOI] [PubMed] [Google Scholar]
- 9.Robinson G, Merav A. Informed consent: recall by patients tested postoperatively. Ann Thorac Surg. 1976;22: 209-12. [DOI] [PubMed] [Google Scholar]
- 10.Olver IN, Turrell SJ, Olszewski NA, Willson KJ. Impact of an information and consent form on patients having chemotherapy. Med J Aust. 1995;162(2): 82-3. [DOI] [PubMed] [Google Scholar]
- 11.Merz JF, Druzdzel MJ, Mazur DJ. Verbal expressions of probability in informed consent litigation. Med Decis Making. 1991;11(4): 273-81. [DOI] [PubMed] [Google Scholar]
- 12.Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. Philadelphia, Pa: J.B. Lippincott, 1985.
- 13.Holt GA, Hallon JD, Hughes SE, Coyle R. OTC labels: can consumers read and understand them? Am Pharmacol NS. 1990;30: 51-4. [DOI] [PubMed] [Google Scholar]
- 14.Hopper KD, Ten Have TR, Hartzel J. Informed consent forms for clinical and research imaging procedures: how much do patients understand? Am J Roentgenol. 1995; 164(2): 493-6. [DOI] [PubMed] [Google Scholar]
- 15.Baker MT, Taub HA. Readability of informed consent forms for research in a Veterans Administration Medical Center. JAMA. 1983;250: 2646-8. [PubMed] [Google Scholar]
- 16.Morrow GR. How readable are subject informed consent forms? JAMA. 1980;244: 56-8. [PubMed] [Google Scholar]
- 17.Morrow GR, Bennett JM, Carpenter PJ. Informed consent to treatment in clinical trials. Biomedic Pharmacother. 1983; 37(1): 10-3. [PubMed] [Google Scholar]
- 18.Grundner TM. On the readability of surgical consent forms. N Engl J Med. 1980;302: 900-2. [DOI] [PubMed] [Google Scholar]
- 19.Tarnowski KJ, Allen DM, Mayhall C, Kelly PA. Readability of pediatric biomedical research informed consent forms. Pediatrics. 1990;85(1): 58-62. [PubMed] [Google Scholar]
- 20.Silva MC, Sorrell JM. Factors influencing comprehension of information for informed consent: ethical implications for nursing research. Int J Nurs Stud. 1984;21(4): 233-40. [DOI] [PubMed] [Google Scholar]
- 21.Jubelirer SJ, Linton JC, Magnetti SM. Reading versus comprehension: implications for patient education and consent in an outpatient oncology clinic. J Cancer Educ. 1994;9(1): 26-9. [DOI] [PubMed] [Google Scholar]
- 22.Meade CD, Howser DM. Consent forms: how to determine and improve readability. Oncol Nurs Forum. 1992;19: 1523-8. [PubMed] [Google Scholar]
- 23.Fry EA. A readability formula that saves time. J Reading. 1968;11: 513. [Google Scholar]
- 24.Hopper KD, Lambe HA, Shirk SJ. Readability of informed consent forms for use with iodinated contrast media. Radiology. 1993;187: 279-83. [DOI] [PubMed] [Google Scholar]
- 25.Doak LG, Doak CC. Lowering the silent barriers to compliance for patients with low literacy skills. Promot Health. 1987;8(4): 6-8. [PubMed] [Google Scholar]
- 26.Ingelfinger FJ. Informed (but uneducated) consent. N Engl J Med. 1972;287: 465-6. [DOI] [PubMed] [Google Scholar]
- 27.Hopper KD, Zajdel M, Hulse SF, et al. Interactive method of informing patients of the risks of intravenous contrast media. Radiology. 1994;192(1): 67-71. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher JC, Dommel FW, Cowell D. Consent to research with impaired human subjects. IRB Rev Hum Subj Res. 1985;7(6): 1-6. [PubMed] [Google Scholar]
- 29.High DM, Doole MM. Ethical and legal issues in conducting research involving elderly subjects. Behav Sci Law. 1995; 13(3): 319-35. [DOI] [PubMed] [Google Scholar]
- 30.Buehler DA. Informed consent and the elderly: an ethical challenge for critical care nursing. Crit Care Nurs Clin North Am. 1990;2(3): 461-71. [PubMed] [Google Scholar]
- 31.Bandman EL. Tough calls: making ethical decisions in the care of the elderly. Geriatrics. 1994;49(12): 46-51. [PubMed] [Google Scholar]
- 32.Weinstock R, Copelan R, Bagheri A. Competence to give informed consent for medical procedures. Bull Am Acad Psychiatry Law. 1984;12(2): 117-25. [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein S, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12: 189-98. [DOI] [PubMed] [Google Scholar]
- 34.Taub HA, Kline GE, Baker MT. The elderly and informed consent: effects of vocabulary level and corrected feedback. Exp Aging Res. 1981;7(2): 137-46. [DOI] [PubMed] [Google Scholar]
- 35.Miller R, Willner HS. The two-part consent form: a suggestion for promoting free and informed consent. N Engl J Med. 1974;290: 964-5. [DOI] [PubMed] [Google Scholar]
- 36.Janofsky JS, McCarty RJ, Folstein MF. The Hopkins Comptency Assessment Test: a brief method for evaluating patients' capacity to give informed consent. Hosp Commun Psychiatr. 1992;43(2): 132-6. [DOI] [PubMed] [Google Scholar]
- 37.Becker D, Kahana Z. Informed consent in demented patients: a question of hours. Med Law. 1993;12(3-5): 271-6. [PubMed] [Google Scholar]
- 38.Dresser R. Mentally disabled research subjects: the enduring policy issues. JAMA. 1996;276(1): 67-72. [PubMed] [Google Scholar]
- 39.Benson PR, Roth LH, Winslade WJ. Informed consent in psychiatric research: preliminary findings from an ongoing investigation. Soc Sci Med. 1985;20(12); 1331-41. [DOI] [PubMed] [Google Scholar]
- 40.Appelbaum PS, Grisso T. The MacArthur treatment competence study: mental illness and competence to consent to treatment. Law Hum Behav. 1995;19(2): 105-26. [DOI] [PubMed] [Google Scholar]
- 41.Grisso T, Appelbaum PS. Comparison of standards for accessing patients' capacities to make treatment decisions. Am J Psychiatry. 1995;152: 1033-7. [DOI] [PubMed] [Google Scholar]
- 42.Grisso T, Appelbaum PS, Mulvey EP, Fletcher K. The MacArthur treatment competence study, II: measures of abilities related to competence to consent to treatment. Law Human Behav. 1995;19(2): 127-48. [DOI] [PubMed] [Google Scholar]
- 43.Tancredi LR. The rights of mental patients: weighing the interests. J Health Polit Policy Law. 1980;5(2): 199-204. [DOI] [PubMed] [Google Scholar]
- 44.Lantos J. Informed consent: the whole truth for patients? Cancer. 1993;72(suppl): 2811-5. [DOI] [PubMed] [Google Scholar]
- 45.Prestifilippo J, Berkman BJ, Kaufman D, et al. The ethical treatment of cancer: what is right for the patient? Cancer. 1993;suppl 72(9): 2816-9. [DOI] [PubMed] [Google Scholar]
- 46.Howard JM, DeMets D. How informed is informed consent? the BHAT experience. Control Clin Trials. 1981;2(4): 287-303. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland HJ, Meslin EM, Till JE. What's missing from current clinical trial guidelines? A framework for integrating science, ethics, and the community context. J Clin Ethics. 1994;5(4): 297-303. [PubMed] [Google Scholar]
- 48.Schain WS. Barriers to clinical trials, II: knowledge and attitude of potential participants. Cancer. 1994;74(suppl 9): 2666-71. [DOI] [PubMed] [Google Scholar]
- 49.Simel DL, Feussner JR. A randomized controlled trial comparing quantitative informed consent formats. J Clin Epidemiol. 1991;44: 771-7. [DOI] [PubMed] [Google Scholar]
- 50.Llewellyn-Thomas HA, Thiel EC, McGreal MJ, Sem FW. Do interactive computer programs to present clinical trial information affect the trial entry decision? Med Decis Making. 1992;12(4): 342. [Google Scholar]
- 51.White DR, Muss HB, Michielutte R, et al. Informed consent: patient information forms in chemotherapy trials. Am J Clin Oncol. 1984;7(2): 183-90. [PubMed] [Google Scholar]
- 52.Wager E, Tooley PJ, Emanuel MB, Wood SF. How to do it: get patients' consent to enter clinical trials. Br Med J. 1995; 311(7007): 734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeLuca SA, Korcuska LA, Oberstar BH, Rosenthal ML, Welsh PA, Topol EJ. Are we promoting true informed consent in cardiovascular clinical trials? J Cardiovasc Nurs. 1995;9(3): 54-61. [DOI] [PubMed] [Google Scholar]
- 54.Rodenhuis S, van den Heuvel WJ, Annyas AA, Koops HS, Sleijfer DT, Mulder NH. Patient motivation and informed consent in a phase I study of an anti-cancer agent. Eur J Cancer Clin Oncol. 1984;20(4): 457-62. [DOI] [PubMed] [Google Scholar]
- 55.Goldman J, Katz MD. Inconsistency and institutional review boards. JAMA. 1982;248(2): 197-202. [PubMed] [Google Scholar]
- 56.Hammerschmidt DE. Institutional review board review lacks impact on readability of consent forms for research. Am J Med Sci. 1992;304(6): 348-51. [DOI] [PubMed] [Google Scholar]
- 57.Tymchuk AJ, Ouslander JG, Rader N. Informing the elderly: a comparison of four methods. J Am Geriatr Soc. 1986; 34(11): 818-22. [DOI] [PubMed] [Google Scholar]
- 58.Krynski MD, Tymchuk AJ, Ouslander JG. How informed can consent be? New light on comprehension among elderly people making decisions about enteral tube feeding. Gerontologist. 1994;34(1): 36-43. [DOI] [PubMed] [Google Scholar]
- 59.Dunn SM, Butow PN, Tattersall MN, et al. General information tapes inhibit recall of the cancer consultation. J Clin Oncol. 1993;11(11): 2279-85. [DOI] [PubMed] [Google Scholar]
- 60.Sorrell JM. Effects of writing/speaking on comprehension of information for informed consent. West J Nurs Res. 1991; 13: 110-22. [DOI] [PubMed] [Google Scholar]
- 61.Riecken HW, Ravich R. Informed consent to biomedical research in Veterans Administration hospitals. JAMA. 1982; 248: 344-8. [PubMed] [Google Scholar]
- 62.Barbour GL, Blumenkrantz MJ. Videotape aids informed consent decisions. JAMA. 1978;240: 2741-2. [PubMed] [Google Scholar]
- 63.Gagliano ME. A literature review on the efficacy of video in patient education. J Med Educ. 1988;63: 785-92. [DOI] [PubMed] [Google Scholar]
- 64.Kieschnick T, Adler LJ, Jimison HB. 1996 Health Informatics Directory. Baltimore: Williams & Wilkins, 1996.
- 65.Consoli SM, Said MB, Jean J, Menard J, Plouin P, Chatellier G. Benefits of a computer-assisted education program for hypertensive patients compared with standard education tools. Patient Educ Counsel. 1995;26: 343-7. [DOI] [PubMed] [Google Scholar]
- 66.Hanna P, Conley-Price D, McKiel EF, Soltes D, Hogen T, Wiens D. Computer applications for staff development patient education. Methods Inf Med. 1989;28: 261-6. [PubMed] [Google Scholar]
- 67.Kirby MD. Informed consent: what does it mean? J Med Ethics. 1983;9(2): 69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Llewellyn-Thomas HA, Thiel EC, Sem FWC, Woermke DEH. Presenting clinical trial information: a comparison of methods. Patient Educat Counsel. 1995;25: 97-107. [DOI] [PubMed] [Google Scholar]
- 69.Lenert LA, Michelson D, Flowers, Bergen MR. IMPACT: An object-oriented graphical environment for construction of multimedia preference assessment instruments. Proc 19th Annu Symp Comput Appl Med Care. 1995: 319-24. [PMC free article] [PubMed]
- 70.Wang PL, Ennis KE. Competency assessment in clinical populations: an introduction to the Cognitive Competency Test. In: Uzzell, Gross (eds). Clinical Neuropsychology of Intervention. Boston, Mass: Martinus Nijhoff, 1986.