Introduction
Surgical treatment with complete resection is the standard of care in the management of thymic tumors. Although minimally invasive surgery is not routinely recommended due to the lack of long-term oncologic data according to NCCN guideline, video-assisted thoracic surgery (VATS) technique has been proved to be promising alternative to open approach in the surgical treatment of early-stage thymoma by previous studies (1). Postoperative chylothorax can occur in every cardiothoracic surgical procedure, especially esophagectomy, but it is a rare complication accompanied by thoracoscopic extended thymectomy.
Case presentation
A 57-year-old Chinese male patient presented with detection of hilar bulging on chest X-ray for 2 weeks prior to his admission to our hospital. He was 165 cm in height and 72 kilograms in weight and the body mass index (BMI) was 26.4. He had no symptoms such as myasthenia gravis (MG), cough, hemoptysis, dyspnea, and chest pain and did not have previous medical history. A chest computed tomography (CT) scan revealed a solid mass approximately 6 cm in diameter with non-homogeneous density located in anterior mediastinum (Figure 1).
Figure 1.
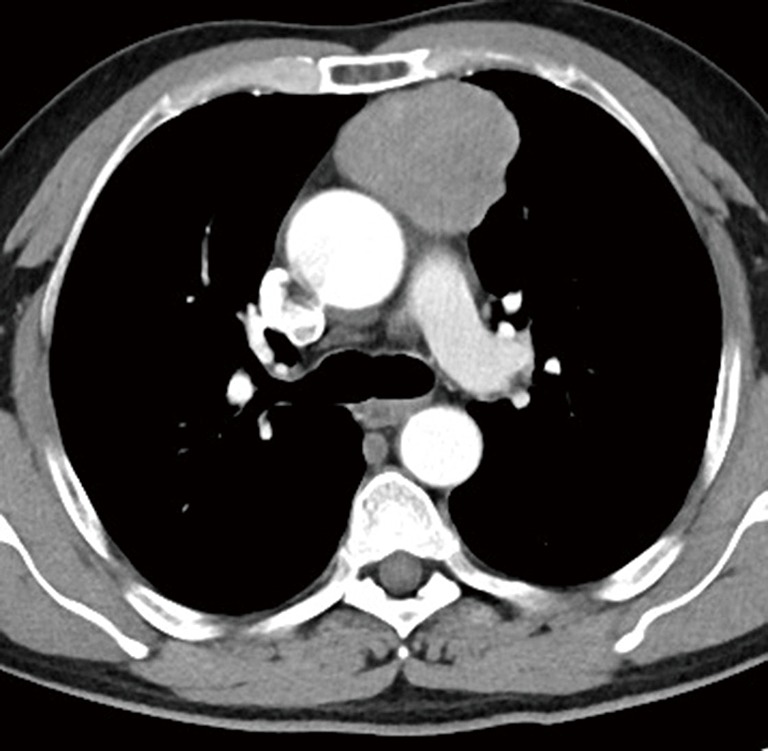
A solid mass with non-homogeneous density located in anterior mediastinum is revealed on chest CT. CT, computed tomography.
The patient was advised to have an operation and underwent video-assisted thoracoscopic thymectomy with left-sided approach on August 7, 2017. An extended thymectomy with en bloc resection of the anterior mediastinal fat tissue was performed in this patient. The adipose tissue around the upper poles of the tumor, both innominate veins, the retrosternal area, left phrenic nerve, and the pericardium were resected meticulously using only the Harmonic Scalpel (Ethicon Endo-Surgery). The surgical procedure was uneventful with 40ml bleeding amount and 110 minutes of operation time and a 28-Fr chest tube was placed and there was no lymphorrhea observed during the operation. The excised mass was 6.1×5.5×4 cm3 and the pathological report demonstrated type AB thymoma encapsulated completely in surrounding fatty tissue. According to the Masaoka staging system it was a stage I thymoma.
On the 3rd postoperative day, chylous effusion with milky and turbid fluid with the amount of 400 mL for 24 h was noted. Chylothorax was confirmed because the biochemical examination of pleural fluid revealed that triglyceride was 1,030 mg/dL. Conservative treatment including absolute fast and total parenteral nutrition was initiated immediately at the same day and the pleural fluid turned out to be clear on the next day, but the amount of chest tube drainage did not decrease obviously with maintaining at a level of 300 to 350 mL per day during the following period. On the 9th day, we allowed the drinking of water instead of fasting and the pleural effusion drainage increased to 600 to 700 mL/day for 6 days. Surgical intervention with thoracic duct ligation was suggested for this patient but it failed due to his refusal. A low fat diet was prescribed to this patient on the 16th postoperative day and 4 days later the outflow began to drop from approximately 500 to 100 mL/day, and we observed the chest tube drainage for 2 days. The chest tube was removed on the 26th day and he was discharged uneventful with normal diet. No remarkable signs on chest radiograph or symptoms were shown after 3 months postoperative routine follow-up. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
Radical thymectomy with removing all the soft tissue is the most important factor in influencing survival for patients with thymoma. Some studies have proved that minimally invasive thymectomy is safe and as effective as open thymectomy in achieving comparable survival data and oncologic outcomes, and even better because of less postoperative complications (2). It is rare that chylothorax is complicated after minimally invasive thymectomy and the incidence of postoperative chylothorax following extended thymectomy thus far has not been known. From January 2010 to December 2017, there were 205 patients who underwent extended thymectomy in our center, and actually two cases (0.98%) developed chylothorax after surgery including one case we mentioned in our study but another one was lost follow-up.
Chylothorax remains an uncommon but challenging clinical problem and the management varies depending on the etiology, the duration or the degree of chylothorax (3,4). In this case, conservative treatment including total parenteral nutrition, fasting and low fat diet and chest tube drainage was performed and chylothorax was controlled successfully, although the duration of chylothorax was relatively long (26 days). To avoid the metabolic problems, we once proposed surgical intervention with thoracic duct ligation to this patient and he refused it when conservative policy was not effective for 2 weeks. Therefore, as to postoperative chylothorax following thymectomy, conservative therapy could be the possible and effective approach instead of early surgical intervention if there were no metabolic and immunologic consequences.
According to what has been reported in literature, including this case, 11 cases in total with chylothorax after thymectomy via transthorax and sternotomy approach have been reported from 1994 to 2017 (Table 1) (5-10). In all cases, chylothorax occurred mostly in patients with MG symptoms and who had sternotomy approach. Three patients who underwent minimally invasive thymectomy including 1 robotic-assisted surgery and 2 video-assisted surgeries had postoperative chylothorax. The majority of patients were diagnosed with chylothorax on the 2nd or 3rd postoperative day and can be treated successfully by conservative means for approximately 2 weeks, which indicates that postoperative chylothorax after thymectomy could be caused by dissection of small mediastinal lymphatic channels during removal of perithymic fatty tissue instead of thoracic duct injury.
Table 1. Chylothorax following extended thymectomy via transthorax and sternotomy approach.
| Author [time] | No. | Incidence | Age (years) | Gender | MG | Approach | Diagnosis | Day of chylothorax | Pattern | Diet | Treatment | Day of tube removal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flaherty [1994] (5) | 1 | – | 24 | Female | Y | Sternotomy | – | 7th day | Bilateral | – | Unilateral chest tube | 8th day |
| Mafé [2003] (6) | 2 | – | 49 | Female | Y | Sternotomy | – | – | Bilateral | Fasting 7 days, oral diet 8th day | Unilateral chest tube, TPN, octreotide | 11th day |
| Liu [2004] (7) | 3 | – | 59 | Male | Y | Sternotomy | Invasive thymoma | 3rd day | Unilateral | Low fat diet | Unilateral chest tube, Somatostatin | 9th day |
| Huang [2007] (8) | 4 | 1.38% | 62 | Male | Y | Sternotomy | Invasive thymoma | 3rd day | – | Fasting 3rd day, low fat diet 9th day | Chest tube, TPN | 11th day |
| 5 | 21 | Female | Y | VATS | Lymphoid hyperplasia | 3rd day | – | Fasting 3rd day, MCT diet 5th day | Unilateral chest tube | 12th day | ||
| 6 | 25 | Female | Y | Sternotomy | Follicular hyperplasia | 3rd day | – | Fasting 4th day, MCT diet 14 days | 1st: TPN; 2nd: TDL | 1st: 44th day; 2nd: 43rd day | ||
| Wang [2007]* | 7 | – | 49 | Female | Y | Sternotomy | Invasive thymoma | 3rd day | Fasting 3rd day | Chest tube with aspiration, TPN | 7th day | |
| Paul [2009] (3) | 8 | – | 39 | Male | – | Sternotomy | Thymoma | – | – | – | TDL | – |
| Marulli [2013] (9) | 9 | – | – | – | – | RATS | – | 2nd day | – | – | Conservative means | 14th day |
| Kim [2016] (10) | 10 | – | 50 | Male | Y | Sternotomy | – | 2nd day | Bilateral | Fasting 2nd day, low fat diet 3 days, normal diet | Bilateral chest tube, TPN | 12–16th day |
| Zhang [2018] | 11 | 0.98% | 57 | Male | N | VATS | Thymoma | 3rd day | Unilateral | Fasting 3rd day, water diet 9th day, low fat diet 16th day | Unilateral chest tube, TPN | 26th day |
*, only Chinese version found in WanFang Database. MG, myasthenia gravis; VATS, video-assisted thoracic surgery; RATS, robotic-assisted thoracic surgery; MCT, medium-chain triglyceride diet; TPN, total parenteral nutrition; TDL, thoracic duct ligation.
As to the diet on conservative treatment, absolute fast may not be essential but low fat diet could be beneficial. In this case, the persistent output of chylous fluid increased but the color of effusion was not changeable obviously after offering water or low fat diet. The reason why we did not continue fasting was because we considered this complication was caused by the injury of minor lymphatic channels flowing into thoracic duct in the upper horn of the thymus. Somatostatin or octreotide might advance the treatment of chylothorax by reducing the pleural effusion drainage and promoting absorption of thoracic duct in some previous report (6,11,12). We did not know whether the duration of chest tube would be shorter if octreotide was administered in this patient.
Although postoperative chylothorax after extended thymectomy is rare, thoracic surgeons should be attentive to this complication no matter what kind of surgical approach is, especially in patients with MG symptoms. During the procedure, careful dissection of thymectomy including fatty tissue must be performed and enough attention on minor lymphatic channels should be paid. Conservative management would be the first choice and ideal intervention for postoperative chylothorax patients, absolute fast may not be essential and low fat diet could be introduced. Chylothorax in most patients would be controlled within 2 to 4 weeks, but thoracic duct ligation is still needed to be considered if the high output of chylous effusion is persistent.
Acknowledgements
We thank the patient for his approval to publication.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ruffini E, Filosso PL, Guerrera F, et al. Optimal surgical approach to thymic malignancies: New trends challenging old dogmas. Lung Cancer 2018;118:161-70. 10.1016/j.lungcan.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 2.Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. 10.1016/j.jtho.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul S, Altorki NK, Port JL, et al. Surgical management of chylothorax. Thorac Cardiovasc Surg 2009;57:226-8. 10.1055/s-0029-1185457 [DOI] [PubMed] [Google Scholar]
- 4.Chiarelli M, Achilli P, Guttadauro A, et al. Chylothorax after mediastinal ganglioneuroma resection treated with fibrin sealant patch: a case report. J Thorac Dis 2017;9:E748-51. 10.21037/jtd.2017.08.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty S, Ellison R, Grishkin BA. Bilateral chylothorax following thymectomy: resolution following unilateral drainage. Mil Med 1994;159:627-8. 10.1093/milmed/159.9.627 [DOI] [PubMed] [Google Scholar]
- 6.Mafé JJ, Caravajal JM, Baschwitz B, et al. Bilateral chylothorax after thymectomy via median sternotomy and resolution through conservative treatment. Eur J Cardiothorac Surg 2003;24:466-8. 10.1016/S1010-7940(03)00335-X [DOI] [PubMed] [Google Scholar]
- 7.Liu CP, Zhao YP, Xue ZQ, et al. A case of chylothorax after tymectomy via median sternotomy in a patient with thymoma. Acta Acad Med Mil Tert 2004;26:1896. [Google Scholar]
- 8.Huang CS, Hsu HS, Kao KP, et al. Chylothorax following extended thymectomy for myasthenia gravis. Thorac Cardiovasc Surg 2007;55:274-6. Erratum in: Thorac Cardiovasc Surg 2007;55:405. 10.1055/s-2006-955882 [DOI] [PubMed] [Google Scholar]
- 9.Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. 10.1016/j.jtcvs.2012.12.031 [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Chang JW, Kim SW, et al. Bilateral chylothorax after transsternal total thymectomy: resolution with short period of fasting and total parenteral nutrition. J Thorac Dis 2016;8:E255-7. 10.21037/jtd.2016.01.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bac DJ, Van Hagen PM, Postema PT, et al. Octreotide for protein-losing enteropathy with intestinal lymphangiectasia. Lancet 1995;345:1639. 10.1016/S0140-6736(95)90145-0 [DOI] [PubMed] [Google Scholar]
- 12.Caverly L, Rausch CM, da Cruz E, et al. Octreotide treatment of chylothorax in pediatric patients following cardiothoracic surgery. Congenit Heart Dis 2010;5:573-8. 10.1111/j.1747-0803.2010.00464.x [DOI] [PubMed] [Google Scholar]


