Abstract
Background
To evaluate the effect of first-time and eventual reiterative surgery on overall survival (OS) and disease-free survival (DFS) in Caucasian patients affected by an invasive adenocarcinoma (ADC) with at least another ground-glass opacity (GGO).
Methods
We analysed 47 patients operated on for lung ADC, identified as main cancer (MC), with at least one synchronous GGO, from January 2003 to March 2017. Characteristics associated with the evolution of GGOs were investigated with logistic regression and overall and DFS were evaluated with Kaplan-Meier method.
Results
Forty-two (89%) patients received an anatomic resection of the MC, 5 patients were treated by a single or multiple wedge resections. In total, 9 (19.1%) patients had all the lesions resected undergoing simultaneous resection of ipsilateral GGOs at first surgery while the remaining 38 (80.9%) patients still had at least one GGO that was followed up by serial CT scan. At the median follow-up of 41 months, GGO evolved in 16 (42.1%) patients. The presence of solid component at the initial CT scan was the only risk factor for evolution of the GGO. Thirteen patients underwent surgical resection showing an invasive ADC in 9 patients, MIA in 3 and AIS in 1. New GGOs developed in 7 (14.9%) patients, in which three underwent surgery showing the presence of solid ADC, MIA and AAH. OS rate at 5 years was 97.4%. DFS at 3 years was 82% and was significantly influenced by the stage of MC.
Conclusions
Patients affected by an invasive ADC with at least another GGO nodule enjoy good OS and DFS with a surgical reiterative approach. Part-solid GGO is associated with GGO progression requiring treatment, but OS is not influenced by the new onset or evolution of GGOs. DFS is affected by the stage of the MC which dictates the treatment strategy.
Keywords: Lung adenocarcinoma, ground-glass opacity (GGO), multiple synchronous lung nodules, dominant lung tumour
Introduction
The recent advances in diagnostic imaging modalities and the widespread use of computed tomography (CT) scan resulted in increasing detection of ground glass opacities (GGOs) (1,2).
Many studies investigated the correlation between the radiologic findings of GGO lesions and their pathologic diagnosis (3-5) demonstrating that GGO is a nonspecific finding caused by various disorders, including benign conditions such as inflammatory disease and focal fibrosis, atypical adenomatous hyperplasia (AAH), and malignant lesions such as adenocarcinoma in situ (AIS) and invasive adenocarcinoma (ADC). However, GGO lesions are also detected as multiple lesions, and/or in addition to an invasive ADC, representing a diagnostic and therapeutic challenge. The possibility of multifocal ADC cannot be ruled out without a pathological diagnosis that is not always possible especially when the lesions are located deeply in the hilum or many lesions are scattered in different lungs. Since biopsy or excision of these lesions is relatively difficult, these slow growing GGOs are currently considered for follow-up instead of definitive diagnosis or treatment (6-8). However, according to previous studies, the patient’s history of lung cancer increases the risk of evolution of GGOs (5,6,8). Only few studies (9-13) reported the natural history of multiple GGOs associated with primary lung cancer with controversial results and no consensus regarding their appropriate follow-up period and treatment strategy has been defined (5,9-11). In particular no standard criteria have been established for the selection of which lesion should be resected simultaneously with the main cancer (MC) and which could be initially followed up with serial CT without surgical resection. Moreover, almost all published data are derived from Asian countries and may not be reliably transferrable to a Caucasian population.
The aim of this study was to evaluate the effect of first-time and eventual reiterative surgery on overall survival (OS) and disease-free survival (DFS) in a population of Caucasian patients affected by a lung ADC with at least another nodule showing GGO appearance. We also analysed the behaviour of un-resected GGOs at first-time surgery and the development of new lesions in order to determine if the presence of additional GGOs influenced the prognosis and render the surgical therapy inappropriate for this clinical scenario.
Methods
We analysed the prospective maintained database of two affiliated tertiary centres (Thoracic Surgery Unit, University Hospital Careggi, Florence and Thoracic Surgery Unit, Siena University Hospital, Siena) about all patients operated on for lung ADC identified as MC, associated with at least one synchronous pure or predominately GGO nodule located in a different lobe or lung from January 2003 to March 2017.
Pre-operative CT scans were reviewed to identify the subgroup of the patients who pre-operatively showed at least one separate, synchronous GGO lesion. Exclusion criteria were: presence of GGO in the same lobe of the MC (n=12); presence of uncountable GGOs (>10 nodules per patient) and patients with other synchronous completely solid nodules. The MC was defined as the lung lesion of larger diameter or that showing more radiologically invasiveness (margin of nodule, pleural indentation, presence of solid portion), while non-dominant nodules (NDNs) were any other non-calcified lung lesions radiologically evident. On the basis of its appearance on the thin-section CT scan, GGOs were classified as pure GGO (pGGO) lesions only if they had 100% GGO proportion and as part-solid (PSGGO) if a solid component was present.
The surgical procedure was determined according to the site of the MC and NDN, patient comorbidities and respiratory function. Initial surgical treatment included removal of the MC and any accessible ipsilateral NDN. However, if the homolateral NDN was smaller than 10 mm, impalpable and/or deep-located into the parenchyma requiring an extensive lung resection (for example a pneumonectomy), we decided to perform a close radiological follow-up with CT. Non dominant GGOs being followed or the newly developed NDNs were treated with surgical resection or stereotactic body radiation therapy (SBRT) in case of progression, defined as growth of the GGO of at least 2 mm in diameter (6), development of a new solid component in a pGGO or enlargement of the solid component in PSGGO.
Lung cancers were staged in accordance with the seventh edition of the TNM Classification for Lung and Pleural Tumours (14). ADC subtypes were defined according the 2011 Guidelines of the International Association for the Study of Lung, American Thoracic Society and European Respiratory Society (15). Consequently, 47 patients (1.7%) among 2,668 patients operated on for NSCLC in the same period, fulfilled these criteria and form the study population. Their medical records were reviewed to evaluate the clinical characteristics, histopathologic findings, long term outcome and recurrence. All patients were regularly evaluated by CT scan every 3 months for the first 2 years after surgery and every 6 months thereafter.
Patient and tumour characteristics were analysed to identify factors associated with OS and DFS and GGO progression requiring a therapeutic intervention.
Locoregional recurrence was defined as the development of ipsilateral recurrent tumour on bronchial or parenchymal resection margin or hilar/mediastinal lymph nodes. Distant recurrence was defined as metastasis to other organs.
Our institutional review boards granted approval and waived the requirement for specific informed consent for this retrospective study.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20.0 software (SPSS Inc., Chicago, IL). Continuous variables are expressed as median, mean values ± SD and compared with unpaired t-test. Categorical variables were analysed using χ-square test. The variables resulting significant at the 0.20 level fed the multivariate regression model to identify predictive factors of evolution of NDN. The Kaplan-Meier method was used to calculate overall and DFS. OS was calculated from the date of operation to death or date of the last follow-up (31/03/2017); DFS was calculated for those patients who received resection from the date of operation to the date of the first evidence of recurrence. Differences in OS and DFS were determined by log-rank analysis. The impact of variables on OS and DFS was then evaluated by Cox multivariable hazard regression model using a significance as P<0.05.
Results
Patient demographics
Patient characteristics are summarized in Table 1. Noteworthy, all patients were Caucasian and there was a slight predominance of men (53.2%).
Table 1. Patients demographics.
| Characteristics | Value |
|---|---|
| Age (years) | 70.02±6.66 [52–82] |
| Sex | |
| Female | 22 (46.8) |
| Male | 25 (53.2) |
| Ethnicity | |
| Caucasian | 47 (100.0) |
| Smoking history | |
| Never smokers | 17 (36.2) |
| Former smokers | 13 (27.6) |
| Current smokers | 17 (36.2) |
| Cancer history | 10 (21.3) |
| Other comorbidities | |
| Hypertension | 20 (42.5) |
| Heart disease | 9 (19.1) |
| COPD | 10 (21.2) |
| Diabetes mellitus | 2 (4.2) |
| Other | 3 (6.4) |
Data are shown as mean ± SD [range] or number (percentage). SD, standard deviation; COPD, chronic obstructive pulmonary disease.
Characteristics of the MC
The principal characteristics of the MC are depicted in Table 2. At pre-operative CT-scan, the MC had a mean maximal diameter of 25 mm (range, 6–100 mm) and in 40 patients (85%) the MC was completely solid or part-solid.
Table 2. Radiological findings, operative strategy and pathological results of MC.
| Variable | Value (n=47) |
|---|---|
| Pathological mean diameter in mm [range] | 28±20 [10–100] |
| Solid or part-solid GGO | 40 (85.1%) |
| First surgical procedure | |
| Lobectomy | 26 (55.3%) |
| Segmentectomy | 10 (21.3%) |
| Wedge resection | 2 (4.3%) |
| Combination | 9 (19.1%) |
| Lobectomy + wedge | 5 (10.6%) |
| Double segmentectomy | 1 (2.1%) |
| Wedge resections | 3 (6.4%) |
| Final pathology | |
| Acinar ADC | 25 (53.3%) |
| Papillary ADC | 5 (10.6%) |
| Solid ADC | 4 (8.5%) |
| Mucinous ADC | 1 (2.1%) |
| ADC NOS | 3 (6.4%) |
| Acinar + papillary ADC | 1 (2.1%) |
| Lepidic + acinar ADC | 1 (2.1%) |
| Lepidic ADC | 3 (6.4%) |
| MIA | 2 (4.2%) |
| AIS | 1 (2.1%) |
| ADC + neuroendocrine | 1 (2.1%) |
| Pathologic stage | |
| IA | 26 (55.3%) |
| IB | 11 (23.4%) |
| IIA | 2 (4.3%) |
| IIB | 4 (8.5%) |
| IIIA | 4 (8.5%) |
| Lymph node metastasis | |
| Hilar | 3 (6.3%) |
| Mediastinal | 2 (4.3%) |
| Patients with all lesions resected | 9 (19.1%) |
MC, main cancer; GGO, ground-glass opacity; ADC, adenocarcinoma; MIA, minimally invasive adenocarcinoma; AIS, adenocarcinoma in situ.
Lung resections were performed through traditional thoracotomies or, more recently, thoracoscopic approach (25%), but always associated with hilar and mediastinal systematic node dissection.
Forty-two (89%) patients received an anatomic resection of the MC, including 31 lobectomies and 11 segmentectomies, whereas only 5 patients were treated by wedge resections.
Regarding the pathology of the MC, 44 patients had an invasive ADC, while 3 patients had either MIA (n=2) or AIS (n=1).
Nine patients (19%) underwent to a combination of more than one resection, in particular: 5 patients underwent lobectomy for MC with an ipsilateral wedge resection of a NDN and one underwent two segmentectomies of different lobes; multiple wedge resections were performed in three patients.
Of 9 patients who had an additional GGO removed along with the MC at first surgery, 4 GGOs were determined on pathology to be acinar ADC, 1 predominant lepidic ADC, 2 MIAs, 1 AIS and AAH.
In total, 9 (19.1%) patients had all the lesions resected at first surgery while the remained 38 (80.9%) patients still had at least one GGO that was followed up by serial CT scan.
Characteristics of the NDNs (Table 3)
Table 3. Radiological findings, characteristics and post-surveillance treatment strategy of NDN.
| Variables | Value |
|---|---|
| Total number of GGOs | 102 |
| Number of GGOs per patient [range] | 2.17±1.90 [1–10] |
| CT dimension in mm [range] | 15.00±8.46 [6–35] |
| Pathological dimension in mm [range] | 18.63±14.31 [5–55] |
| Localization (n=47) | |
| Ipsilateral lung | 9 (19.1%) |
| Bilateral or contralateral | 38 (80.9%) |
| Type of GGOs (n=47) | |
| Pure | 36 (76.6%) |
| Part-solid | 11 (23.4%) |
| Multiple GGOs (n=47) | |
| More than one | 17 (36.2%) |
| One | 30 (63.8%) |
| GGOs behaviour (per patients) (n=38) | |
| Non-evolved | 18 (47.4%) |
| Disappeared | 4 (10.5%) |
| Evolved | 16 (42.1%) |
| Treatment of evolved GGOs (n=16) | |
| None | 1 (5.6%) |
| Surgery | 13 (81.3%) |
| SBRT | 2 (11.1%) |
| Surgical procedure of evolved GGO (n=13) | |
| Lobectomy + wedge | 1 (6.3%) |
| Lobectomy | 4 (25%) |
| Segmentectomy | 1 (6.3%) |
| Wedge resection | 7 (43.8%) |
| Pathology (n=13) | |
| ADC acinar | 3 (23.1%) |
| MIA | 3 (23.1%) |
| ADC lepidic | 1 (7.7%) |
| ADC NOS | 1 (7.7%) |
| ADC mucinous | 1 (7.7%) |
| ADC papillary | 1 (7.7%) |
| ADC solid | 1 (7.7%) |
| Adenosquamous | 1 (7.7%) |
| AIS | 1 (7.7%) |
| New onset GGOs (n=47) | 7 (14.9%) |
NDN, non-dominant nodules; GGO, ground-glass opacity; SBRT, stereotactic body radiation therapy; ADC, adenocarcinoma; MIA, minimally invasive adenocarcinoma; NOS, not otherwise specified; AIS, adenocarcinoma in situ.
The retrospective review of the CT scan showed a total of 102 GGOs in the study population of 47 patients. Thirty (63.8%) patients had a single GGO lesion, whereas 17 (36.2%) had multiple GGOs and the mean number of GGOs per patient was 2.17±1.90 (range, 1–10). The mean GGO lesion size was 15.00±8.46 mm (range, 6–35 mm) in the largest diameter at initial CT scan. Most of NDNs (80.9%) were located bilaterally or contralateral to the MC, while in only 19.1% of the cases they were ipsilaterally to the MC.
Regarding the radiological aspect of the NDN, 36 (76.6%) patients had a pGGO and 11 (23.4%) had a PSGGO.
At the median follow-up of 41 months (range, 3–161 months), 16 (18%) out of 86 GGOs increased in size or developed a solid component, 4 (4.5%) lesions disappeared and 66 (77.5%) showed no change in size. On a per-person basis, the frequency of progression of unresected GGOs was 42% (16 of 38). In 13 patients (34%), there was growth of GGO with an average increase of 5 mm (range, 2–25 mm). Nine patients (23.6%) had at least one GGO that developed a solid component. All 7 PSGGO in observation evolved and required resection, while in 9 out of 31 (29%) patients the NDN with pGGO appearance evolved (HR =3.444; 95% CI, 1.987–5.972; P=0.001). Among the 16 patients who showed GGO progression, 13 underwent surgical resection, two patients were treated by radiotherapy, with a median time to treatment of 19 months (range, 5–110 months), while one patient is still on follow-up, because there has been no further change in size. The final pathological analysis showed the presence of invasive ADC in 9 patients, MIA in 3 and AIS in 1; the two patients treated by SBRT did not undergo to histological confirmation.
The only risk factor for evolution of the GGO, identified at univariate and confirmed at multivariable analysis, was the presence of a solid component at the initial CT scan (Table 4). In addition, during the follow-up new GGOs developed in 7 (14.9%) patients and five of them received treatment, including surgery in 3 cases and radiotherapy in 2. The pathological examination of new nodules resected showed the presence of solid ADC, MIA and AAH.
Table 4. Univariate and multivariable analysis of risk factors influencing the evolution of GGOs.
| Variable | No GGO progression (n=22) | Any GGO progression (n=16) | P | Multivariable analysis | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | ||||
| Sex | 1 | |||||
| Male | 12 (54.5%) | 9 (56.3%) | ||||
| Female | 10 (45.5%) | 7 (43.8%) | ||||
| Age (years) | 70.68±6.542 | 71.19±6.337 | 0.813 | |||
| Smoking history | 0.238 | |||||
| Current smokers | 8 (36.4%) | 7 (43.8%) | ||||
| Former smokers | 8 (36.4%) | 2 (12.4%) | ||||
| Never smokers | 6 (27.2%) | 7 (43.8%) | ||||
| Maximum diameter of MC | 2.65±1.818 | 3.07±1.875 | 0.510 | |||
| Final pathological histology of MC | 0.601 | |||||
| ADC NOS | 2 (9.1%) | 1 (6.3%) | ||||
| ADC papillary/acinar | 0 | 1 (6.3%) | ||||
| ADC acinar | 13 (59.1%) | 6 (37.5%) | ||||
| ADC lepidic | 1 (4.5%) | 1 (6.3%) | ||||
| ADC lepidic/acinar | 0 | 1 (6.3%) | ||||
| ADC mucinous | 0 | 1 (6.3%) | ||||
| ADC papillary | 3 (13.6%) | 2 (12.5%) | ||||
| ADC solid | 2 (9.1%) | 2 (12.5%) | ||||
| AIS | 0 | 1 (6.3%) | ||||
| MIA | 1 (4.5%) | 0 | ||||
| Pathological stage of MC | 0.928 | |||||
| IA | 11 (50.0%) | 9 (56.3%) | ||||
| IB | 7 (31.8%) | 3 (18.8%) | ||||
| IIA | 1 (4.5%) | 1 (6.3%) | ||||
| IIB | 1 (4.5%) | 1 (6.3%) | ||||
| IIIA | 2 (9.1%) | 2 (12.5%) | ||||
| Lymph node metastasis | 3 (13.6%) | 2 (12.5%) | 1 | |||
| Side of GGO respect to MC | 1 | |||||
| Ipsilateral | 2 (9.1%) | 1 (6.3%) | ||||
| Contralateral | 20 (90.9%) | 15 (93.8%) | ||||
| Mean number of GGOs | 2.14±1.754 | 2.00±2.251 | 0.835 | |||
| GGO | 0.729 | |||||
| Presence of one GGO | 15 (68.2%) | 12 (75.0%) | ||||
| More than one GGO | 7 (31.8%) | 4 (25.0%) | ||||
| Maximum diameter on CT scan of NDN | 14.95±8.95 | 15.07±8.040 | 0.969 | |||
| Pathological maximum diameter of NDN | 1.47±0.935 | 2.46±16.100 | 0.428 | |||
| GGO | 0.001 | 3.444 | 1.987–5.972 | 0.001 | ||
| Pure | 22 (100.0%) | 9 (56.3%) | ||||
| Part-solid | 0 | 7 (43.8%) | ||||
GGO, ground-glass opacity; MC, main cancer; ADC, adenocarcinoma; NOS, not otherwise specified; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; NDN, non-dominant nodule.
Prognostic analysis and survival
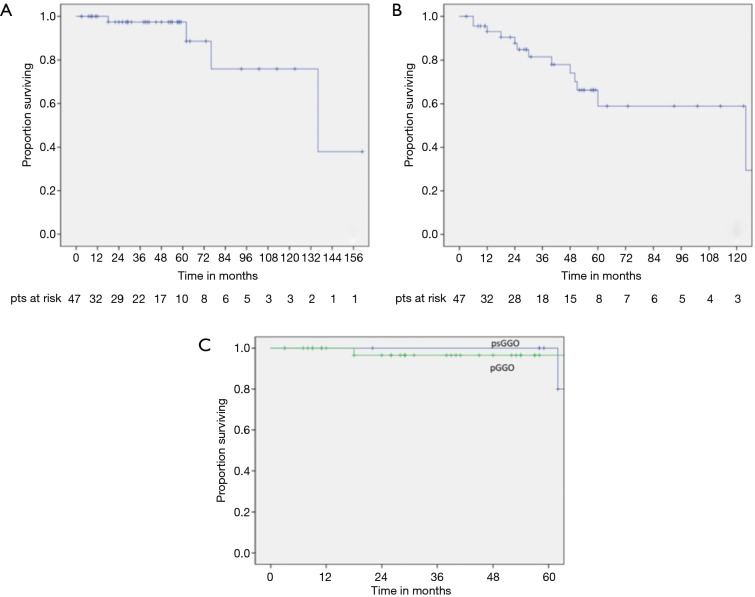
OS rate at 3 and 5 years were 97.4% (Figure 1A). No factor had influence on OS at univariate and multivariable analysis (Table 5).
Figure 1.
(A) Overall survival curve; (B) disease-free survival curve; (C) survival curve in patients affected by pGGO and PSGGO. pGGO, pure ground glass opacity; PSGGO, part-solid ground glass opacity.
Table 5. Univariate and multivariable analysis on OS and on disease-free survival.
| Variable | 5-year OS | P | Multivariable analysis | 5-year DFS | P | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||||
| Age (years) | 0.774 | 0.872 | ||||||||
| <70 | 100% | 71% | ||||||||
| >70 | 95% | 59% | ||||||||
| Sex | 0.464 | 0.212 | ||||||||
| Male | 95% | 47% | ||||||||
| Female | 100% | 74.5% | ||||||||
| NDN | 0.501 | 0.735 | ||||||||
| Bilateral | 96% | 59.2% | ||||||||
| Ipsilateral | 100% | 62.8% | ||||||||
| NDN | 0.531 | 0.098 | 0.3 | 0.066–1.363 | 0.119 | |||||
| Multiple | 100% | 90% | ||||||||
| Single | 100% | 53.5% | ||||||||
| Pathological stage of MC | 0.246 | 0.040 | 0.3 | 0.089–1.015 | 0.049 | |||||
| I | 100% | 73% | ||||||||
| II–IIIA | 85.5% | 30% | ||||||||
| Lymph node metastasis | 0.133 | 4.703 | 0.562–39.34 | 0.153 | 0.178 | 2.513 | 0.635–9.944 | 0.189 | ||
| Presence | 80% | 60% | ||||||||
| Absence | 100% | 68% | ||||||||
| Completeness of resection | 0.592 | |||||||||
| Resection of all lesions at first-time surgery | 100% | |||||||||
| Un-resected lesions on follow-up | 97% | |||||||||
| Recurrence | 0.008 | 144.1 | 0.23–92204 | 0.266 | ||||||
| With | 92% | |||||||||
| Without | 100% | |||||||||
| Development of new GGOs | 0.503 | 0.381 | ||||||||
| With | 100% | 44.2% | ||||||||
| Without | 96% | 70.2% | ||||||||
| Type of GGO | 0.105 | 0.178 | 0.018–1.799 | 0.144 | 0.486 | |||||
| pGGO | 96.3% | 68.4% | ||||||||
| PSGGO | 100% | 62.9% | ||||||||
| Behaviour of GGO | 0.662 | 0.804 | ||||||||
| Evolved NDN | 100% | 61% | ||||||||
| Not evolved NDN | 95.2% | 70% | ||||||||
NDN, non-dominant nodule; MC, main cancer; GGO, ground-glass opacity.
Thirteen (27.6%) patients developed cancer recurrence, including 5 (38%) local recurrences, 7 (54%) distant recurrence and 1 (8%) both local and distant recurrence. DFS at 3 and 5 years were 82% and 66% respectively (Figure 1B).
Stage I of the MC, both on univariate and multivariable analysis, was the only prognostic factor of favourable DFS (HR =0.3; 95% CI, 0.089–1.015; P=0.049) (Table 5).
The type (Figure 1C), the CT-scan initial diameter, the evolution of GGOs, the development of new GGOs, the multiplicity of GGOs, and if all the lesions were treated or not at the initial surgery, did not have any influence on OS and DFS (Table 5).
Discussion
In this study we evaluated the clinic-pathological features and long term outcomes of patients with a dominant invasive ADC in the setting of multifocal GGOs, in order to determine if there is a role for surgery and what can be the optimal management of residual and new developed lesions. We demonstrated that a totally Caucasian group of patients with an invasive lung ADC and additional GGOs (apparently multifocal lesions in the spectrum of ADC from AAH to invasive ADC) had favorable long term survival with surgery. The DFS of the patients was significantly associated with the stage of MC, whereas no association was observed between survival and the number or characteristics of additional GGOs, the emergence of new GGO or whether or not the GGOs required treatment because of their evolution during follow-up. Several studies, all from Asia and only one from USA have shown similar long term results. In a series of 23 Japanese patients with predominantly invasive ADC and other multifocal pure GGOs undergoing surgical resection, a 90% OS and 80% DFS was reported in a 5-year follow-up period (11). Similar long-term results (93% of 3-year OS rate and 78% of 3-year DFS rate) were described by Nakata (12) and also by Gu and co-workers, who, in 39 patients affected by a dominant ADC with multifocal GGOs, showed a mid-term DFS of 95% (9). In the study from Shimada et al. (13) the survival of patients with multifocal GGOs was strongly affected by the radiological findings of the MC, with 5-year OS of 95,8% and 68% when the MC had a CTR <0.5 or >0.5, respectively. These results are clearly better that those reported in patients with multifocal solid tumors (16,17), highlighting a possible difference in biology between a clinical situation of an invasive ADC with additional GGOs and multifocal solid invasive tumours. In the last case the possibility that some lesions could represent a lung metastasis cannot be excluded, if not in case of different histology, while GGOs associated to a MC behave as a different kind of lung cancer that has a propensity for multifocal involvement but also less propensity to metastatize (18), making them good candidates for parenchymal sparing surgery with curative intent. All these data demonstrate that in case of an ADC with additional GGOs, prognosis is largely dependent on the characteristics and stage of MC and that there is a clear indication to surgery at least of the MC. For this reason, the resection of the MC should be adequate in terms of radicality, but at the same time permitting to spare parenchyma in view of eventual future treatments. So, lobectomy and when possible segmentectomy (19), with simultaneous resection of ipsilateral accessible GGOs, if amenable to wedge, is the most appropriate surgical procedure as suggested by our data, because all GGOs simultaneously resected with the MC were pre-invasive or invasive form of ADC. This recommendation is based on the fact that some of these GGOs will evolve requiring future local treatment, that, in case of surgery, will be more complex (redo-surgery) and sometimes more extensive compared to the initial procedure. On the other hand, in case of a central parenchymal pure GGO lesion that would require removal of a second lobe ipsilateral to the MC, and then possibly a pneumonectomy, we prefer to leave it, and like in case of contralateral GGOs, follow them for growth or invasiveness with serial CT scans.
The appropriate surveillance strategy and eventual treatment for the residual or newly developed GGOs is still unclear. The chance of progression of a GGO over 2 to 5 years is quite variable depending on different background, but generally around 20–30%, although few lesions will require additional local treatment, because of their indolent nature (most are AIS with very few ADC) (9-11,13). In comparison to all these previous studies, we observed a higher percentage of evolution of GGO (42%) and consequently a major incidence of GGOs requiring resection. In total, 15 patients had their GGOs treated during the follow-up period because of the GGO progression. This result could be considered an overtreatment, since many of these lesions have been reported to correspond histologically to pre-invasive lesions or lepidic growing tumors with an indolent clinical course. However, in our study, 70% of resected specimens resulted to be an invasive ADC. This difference from previous studies is probably related to the fact that the initial mean size of the GGOs was larger in our study. The initial size of GGO lesion has been reported to be a predictive factor of future growth. Hiramatsu et al. (6) showed initial GGO size to be the strongest risk factor for future evolution with GGO growth incidence at 5 years of 66% when the GGOs are larger than 10 mm. In the study of Kim, although the overall malignancy rate was only 20%, the chance of malignancy was 67% for lesions larger than 10 mm (10). These observations suggest that larger GGOs are more likely to have an invasive character, and a high growth incidence can reasonably be expected. Two other studies (1,6) found that a previous history of lung cancer is a strong predictive factor of future growth of GGO, strengthening the concept that the context is very important, and the significance of GGOs in patients with a primary lung cancer is different from those detected in otherwise healthy individuals in a screening program. The reason for the higher growth incidence of GGOs in patients with a history of lung cancer is unclear. We can only speculate that it could be related to an alteration of the host, resulting in a favorable local microenvironment or in altered immunocompetence due to the presence of an invasive cancer. In our study, the only risk factor for evolution of the GGO was the presence of a solid component at the initial CT scan. Among 11 patients harboring PSGGOs, all 7 patients that we put on follow-up evolved. The other 4 cases were operated, at first the surgery, concomitantly with the MC, demonstrating the presence at pathological examination of invasive ADC. In the long-term study of Sawada et al. dealing with 226 patients with GGO <3 cm in maximal diameter, invasive cancer was observed in only 8% of the patients whose tumors increased in size during the follow-up and in up to 66,7% of patients with a CTR between 0.25 and 0.50 (20). Ohta et al. (5) showed that PSGGOs have a statistically significant higher Nm 23 and MIB1 expression compared to pGGOs, which support the hypothesis that PSGGOs represent relatively high-grade malignancy requiring prompt treatment. Considering the results of these reports and the present study, resection without a long follow-up period should be considered for PSGGOs. Thus, the radiographic features of GGOs are highly predictive of biologic behavior and are the most relevant criteria for intervention of a GGO, although, also other patients factors as age, estimated postoperative function and major comorbidities should be taken into consideration at the time of initial evaluation, for planning the more appropriate treatment.
All studies dealing with this topic showed that some new nodules have developed during the follow-up period. In Gu et al. one or more new GGOs developed in 41% of the patients, but only 1 required treatment during the study period (9). Instead, in our series only 7 (14.9%) patients developed new GGOs and 5 of 7 patients had their new lesions resected. This could be related to our longer follow-up because it is likely that a higher percentage of the un-resected or new GGOs would become clinically relevant over a long follow-up period.
The limitations of this study include all of the inherent biases proper to retrospective analyses with our cohort composed by selected patients. The presence of synchronous lung ADC associated with GGOs is still an infrequent situation in Western countries and we were still limited by this sample size and the resulting limited statistical power. In particular, we did not find any difference in OS between the groups due to the few events (deaths) observed.
Nowadays, the molecular assessment of ADC (EGFR, ALK, ROS-1) is basilar for the treatment of advanced stage with TKI, but in our study, due principally to the long study period, the evaluation of biomarkers was not routinely determined in all patients.
In conclusion, patients affected by multiple synchronous lung ADC with GGO appearance enjoy good long-term survival with anatomic resection of the MC, wedge resection of accessible ipsilateral GGOs and subsequent observation and resection of remaining evolving GGOs. This clinical scenario should not be considered as an advanced disease because both OS and DFS are favourable and not influenced by the new onset or evolution of GGOs. DFS is affected by the stage of the MC and then even those patients affected by characteristics of GGO requiring future treatment, such as part-solid GGOs, should not be denied potentially curative surgery for the MC.
Acknowledgements
None.
Ethical Statement: Our institutional review boards granted approval and waived the requirement for specific informed consent for this retrospective study.
Footnotes
Conflicts of Interest: Presented at 31st EACTS Annual Meeting, 7-11 October 2017, Wien. Session: Lung cancer-controversies (09/10/2017 10:15-11:45).
References
- 1.Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT. Chest 2002;121:1464-7. 10.1378/chest.121.5.1464 [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Lee HJ, Jeon JH, et al. Natural history of ground-glass nodules detected on the chest computed tomography scan after major lung resection. Ann Thorac Surg 2013;96:1952-7. 10.1016/j.athoracsur.2013.07.071 [DOI] [PubMed] [Google Scholar]
- 3.Chang B, Hwang JH, Choi YH, et al. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest 2013;143:172-8. 10.1378/chest.11-2501 [DOI] [PubMed] [Google Scholar]
- 4.Ohta Y, Shimizu Y, Kobayashi T, et al. Pathologic and biological assessment of lung tumors showing ground-glass opacity. Ann Thorac Surg 2006;81:1194-7. 10.1016/j.athoracsur.2005.10.037 [DOI] [PubMed] [Google Scholar]
- 5.Kodama K, Higashiyama M, Yokouchi H, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years. Ann Thorac Surg 2002;73:386-92; discussion 392-3. 10.1016/S0003-4975(01)03410-5 [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu M, Inagaki T, Inagaki T, et al. Pulmonary ground-glass opacity (GGO) lesions-large size and a history of lung cancer are risk factors for growth. J Thorac Oncol 2008;3:1245-50. 10.1097/JTO.0b013e318189f526 [DOI] [PubMed] [Google Scholar]
- 7.Libby DM, Smith JP, Altorki NK, et al. Managing the small pulmonary nodule discovered by CT. Chest 2004;125:1522-9. 10.1378/chest.125.4.1522 [DOI] [PubMed] [Google Scholar]
- 8.Haro A, Yano T, Kohno M, et al. Ground-glass opacity lesions on computed tomography during postoperative surveillance for primary non-small cell lung cancer. Lung Cancer 2012;76:56-60. 10.1016/j.lungcan.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Gu B, Burt BM, Merritt RE, et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. Ann Thorac Surg 2013;96:411-8. 10.1016/j.athoracsur.2013.04.048 [DOI] [PubMed] [Google Scholar]
- 10.Kim HK, Choi YS, Kim K, et al. Management of ground-glass opacity lesions detected in patients with otherwise operable non-small cell lung cancer. J Thorac Oncol 2009;4:1242-6. 10.1097/JTO.0b013e3181b3fee3 [DOI] [PubMed] [Google Scholar]
- 11.Tsutsui S, Ashizawa K, Minami K, et al. Multiple focal pure ground-glass opacities on high-resolution CT images: Clinical significance in patients with lung cancer. AJR Am J Roentgenol 2010;195:W131-8. 10.2214/AJR.09.3828 [DOI] [PubMed] [Google Scholar]
- 12.Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. 10.1016/j.athoracsur.2004.03.102 [DOI] [PubMed] [Google Scholar]
- 13.Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer 2015;88:174-80. 10.1016/j.lungcan.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C. editors. TNM classification of malignant tumours (UICC International Union Against Cancer). 7th ed. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 15.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AA, Barfield ME, Kelsey CR, et al. Outcomes after surgical management of synchronous bilateral primary lung cancers. Ann Thorac Surg 2012;93:1055-60; discussion 1060. 10.1016/j.athoracsur.2011.12.070 [DOI] [PubMed] [Google Scholar]
- 17.Voltolini L, Rapicetta C, Luzzi L, et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg 2010;37:1198-204. 10.1016/j.ejcts.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 18.Garfield DH, Cadranel JL, Wislez M, et al. The bronchioloalveolar carcinoma and peripheral adenocarcinoma spectrum of diseases. J Thorac Oncol 2006;1:344-59. 10.1016/S1556-0864(15)31593-8 [DOI] [PubMed] [Google Scholar]
- 19.Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. 10.1016/j.lungcan.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 20.Sawada S, Yamashita N, Sugimoto R., et al. Long-term outcomes of patients with ground-glass opacities detected using CT scanning. Chest 2017;151:308-15. 10.1016/j.chest.2016.07.007 [DOI] [PubMed] [Google Scholar]