Abstract
Background
There is no standardized definition of the asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO). Although the blood eosinophil count is regarded as a biomarker for identifying ACO, it has no distinct value. This study aimed to measure plasma levels of neutrophil gelatinase-associated lipocalin (NGAL), a potential biomarker for distinguishing between ACO and non-ACO COPD.
Methods
We used the Korean cohort in the COPD in dusty area (CODA) study which included 137 subjects with COPD confirmed by spirometry. We defined ACO by a positive bronchodilator response (forced expiratory volume in 1 s, FEV1 >12% and >200 mL from baseline) or based on a previous history of asthma. Plasma levels of NGAL were determined by enzyme immunoassay.
Results
Among the 137 subjects, 77 were ACO and 60 were non-ACO COPD. Overall, the plasma NGAL levels were 15.9±7.9 and 15.6±6.6 ng/mL for non-ACO and ACO subjects respectively, and not significantly different. However, NGAL levels were significantly higher in female subjects with ACO (17.0±6.4 vs. 11.1±4.5, P=0.01). In female subjects, NGAL levels showed a good predictive ability to discriminate between ACO and non-ACO COPD [area under the receiver operating characteristic curve (AUROC), 0.77]; the predictive ability was similar to that of the blood eosinophil count (AUROC, 0.79). There was a higher probability of discriminating ACO from non-ACO among subjects in the highest tertile of NGAL levels (odds ratio, 1.72; 95% confidence interval, 0.69–4.28; P for trend =0.01).
Conclusions
NGAL levels were significantly higher in ACO compared to non-ACO COPD in female subjects. After adjusting for gender as a confounding factor, the ability to distinguish ACO was better at higher levels of NGAL.
Keywords: Chronic obstructive pulmonary disease (COPD), asthma-COPD overlap, biomarker, neutrophil gelatinase-associated lipocalin (NGAL), eosinophil
Introduction
The asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) has recently received attention as a disease process that shares features of both asthma and COPD; it is generally recognized as one of the phenotypes of COPD rather than a distinct disease entity (1-3). In fact, since patients with features of the ACO are generally excluded from asthma and COPD studies, the precise pathogenesis, clinical features, treatment response, and prognosis of ACO are unclear. Furthermore, so far, there is no single, universally accepted diagnostic criterion for ACO. Hence, the prevalence of ACO varies widely according to the diagnostic criteria used. Even within the same cohort of COPD, ACO varied from 11.9–48.3% when different diagnostic criteria were applied (4).
Airway inflammation in asthma is characterized predominantly by eosinophilic, T-helper cell mediated airway inflammation, while in COPD, it is thought to be predominantly neutrophilic, cytotoxic, and T-cell mediated (5). Recently, eosinophilic inflammation has been reported in COPD also and it is considered as a biomarker of COPD with some features of asthma (6-8). However, as the diagnostic criteria of ACO are not uniform, the eosinophil count cut off that may exclude asthmatic features in COPD has not yet been established, and ranges from 3–5% or 300 cells/uL (8-10).
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25-kDa acute phase protein secreted by neutrophils (11), epithelial cells of the respiratory and intestinal mucosa (12,13), endothelial (14), and renal tubular cells (15). NGAL is considered to have possible roles in neutrophilic inflammation and epithelial injury by binding to matrix metalloproteinase (MMP)-9 released from neutrophils (16). Plasma levels of NGAL are reported to be higher in COPD patients compared to that of controls (17) and more recently, sputum NGAL levels were significantly elevated in ACO compared to COPD (18).
We hypothesized that patients with ACO and non-ACO COPD differ in their biomarker profiles, enabling clinician to have a clear diagnosis and better understanding of the pathophysiology. The aim of this study was to identify the ability of NGAL to discriminate ACO from non-ACO COPD and correlation of NGAL with the blood eosinophil count which has been widely accepted novel biomarker for ACO.
Methods
Subjects
We enrolled 60 patients with non-ACO COPD and 77 patients with ACO, who were matched for age and gender in the baseline survey of the COPD in dusty areas near cement plants (CODA) cohort (19). Fixed airflow limitation by spirometry, defined as a post-bronchodilator forced expiratory volume in 1 s over forced vital capacity value (FEV1/FVC) of less than 0.7, was confirmed in all patients. Patients were more than 40 years old; recruitment into the CODA cohort is ongoing, and it is planned for the subjects to be followed up for more than 10 years.
All CODA cohort studies comply with Good Clinical Practice guidelines and follow the Helsinki Declaration and written informed consent was obtained from all participants (2012-06-007). The present study has been approved by the ethics review boards of KNUH (IRB No. KNUH-KBB-2013-0005-009).
Data collection
Data from the CODA cohort includes medical interviews, comorbid conditions, smoking habits, exacerbation history in the year prior to enrollment and medication history. The CODA cohort provides volumetric CT scan measurements based on the protocol used in the Korean Obstructive Lung Disease (KOLD) (20) study, obtained from a 16-multidetector CT scanner (Somatom Sensation 16; Siemens Medical Systems, Bonn, Germany). Based on measurements obtained from the CT scan, the emphysema index, defined as the percentage of low attenuation area of ≤950 Hounsfield units (HU) and airway thickening by mean wall area (MWA, percentage of two segmental bronchi) are derived using an in-house software from the KOLD study group (20). The selection of subjects and data collection in the CODA cohort study has been published in greater detail (19).
Definitions of ACO
In the absence of a standard definition, we defined ACO if patients satisfied the criteria for COPD and asthma at the same time. COPD was diagnosed according to the ATS/ERS Task Force recommendations and defined by fixed airflow limitation (FEV1/FVC <0.7) on post-bronchodilator spirometry (1). The diagnosis of asthma was based on the Global Initiative for Asthma (GINA) guidelines (2), with a positive bronchodilator response of FEV1 >12% and >200 mL after inhalation of 2 puffs of salbutamol and/or a clinical history compatible with asthma. Spirometry was performed using standardized equipment, and lung volume measurements adhered to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines (21,22).
Measurement of biomarkers
In the CODA cohort, serum, plasma, and urine specimens are stored to enable research on biomarkers and genetic/proteomic analysis. Serum NGAL levels were measured by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D System, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. The detection sensitivity and upper limit of the test kit are 0.04 and 10 ng/mL, respectively. The ELISA test for NGAL was conducted three times for each sample and the mean value was used in the analysis. Blood eosinophil count and C-reactive protein (CRP) levels were measured. Interleukin-6 (IL-6) and interleukin-8 (IL-8) levels, measured by immunohistochemical analysis were also available from the CODA cohort dataset.
Statistical analysis
Propensity scores for age and gender-matched analysis were calculated; 60 subjects with non-ACO and 77 subjects with ACO COPD were included in the analysis. Categorical variables were compared with the unpaired t-test and presented as numbers and percentages. Statistical comparison of continuous variables was performed by the Chi-square or the Mann-Whitney U rank test and reported as mean and standard deviation. Correlations between serum NGAL levels and demographics, lung function, radiological features, and blood eosinophil counts were identified by Spearman’s rank correlation. NGAL levels were further analyzed by receiver operating characteristic (ROC) curves for the predictive ability to distinguish patients with ACO from non-ACO COPD based on gender; the predictive power of NGAL and blood eosinophil count were also compared by the ROC curve. Multivariable stepwise regression analysis was performed to identify independent factors affecting the differentiation of ACO from non-ACO COPD. A P value <0.05 was considered significant. All analyses were carried out using STATA version 14.2 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
Baseline characteristics including demographics, comorbidities, dyspnea score, disease-specific quality of life, previous exacerbation history and inhaler use are shown in Table 1. Among patients with ACO, 55 of 77 (71.4%) patients were previously diagnosed with asthma, whereas there was no prior diagnosis of asthma in patients with non-ACO COPD. Besides, 30 of 77 (39.0%) patients have positive bronchodilator response and 7 of 60 (11.7%) patients did in non-ACO COPD patients. Subjects with ACO were more obese and had relatively less airflow obstruction than non-ACO COPD. Among ACO patients, 66.2% were current or ex-smokers compared to 76.7% among non-ACO COPD patients.
Table 1. Baseline characteristics of patients with non-ACO and ACO.
| Characteristics | Non-ACO (n=60) | ACO (n=77) | P value |
|---|---|---|---|
| Age, years | 74.0±8.1 | 72.5±7.4 | 0.25 |
| Gender, male (%) | 50 (83.3) | 56 (72.7) | 0.14 |
| BMI, kg/m2 | 22.2±3.1 | 24.3±3.4 | <0.01 |
| Smoking status | |||
| Ever smoker (%) | 46 (76.7) | 51 (66.2) | 0.64 |
| Pack-years | 26.7±22.0 | 27.4±20.7 | 0.88 |
| Lung function, postbronchodilator | |||
| FEV1, L | 1.6±0.5 | 1.7±0.5 | 0.21 |
| FEV1, % | 71.1±15.8 | 77.6±16.6 | 0.02 |
| FVC, L | 2.9±0.8 | 2.9±0.7 | 0.85 |
| FVC, % | 92.5±17.5 | 96.5±16.1 | 0.17 |
| FEV1/FVC | 55.6±8.6 | 58.5±7.5 | 0.04 |
| ∆FEV1#, mL | 62.0±176.5 | 172.6±154.0 | <0.01 |
| ∆FEV1#, % | 2.9±8.5 | 8.4±6.8 | <0.01 |
| BDR positivity¶ (%) | 7 (11.7) | 30 (39.0) | <0.01 |
| Comorbid condition (%) | |||
| Asthma | 0 (0) | 55 (71.4) | <0.01 |
| DM | 13 (21.7) | 14 (18.2) | 0.48 |
| Ischemic heart disease | 3 (5.0) | 2 (2.6) | 0.42 |
| Congestive heart failure | 0 (0) | 2 (2.6) | 0.22 |
| Cerebrovascular accident | 1 (1.7) | 1 (1.3) | 0.85 |
| History of pulmonary tuberculosis | 10 (16.7) | 16 (20.8) | 0.62 |
| Chronic renal failure | 0 (0) | 2 (2.6) | 0.22 |
| Symptom score | |||
| mMRC | 1.6±1.1 | 1.9±1.2 | 0.21 |
| CAT | 18.7±9.1 | 20.9±8.8 | 0.17 |
| History of exacerbations in the past year (%) | |||
| Unplanned visit to an outpatient clinic (No./year) | 2 (3.3) | 9 (11.7) | 0.09 |
| Unplanned visit to an emergency department or hospital admission (No./year) | 3 (5.0) | 7 (9.1) | 0.40 |
| ≥2 events in the past year | 3 (5.0) | 7 (9.1) | 0.36 |
| Inhaler use (%) | |||
| LABA and/or LAMA | 10 (16.7) | 11 (14.3) | 0.70 |
| ICS/LABA | 10 (16.7) | 12 (15.6) | 0.86 |
| ICS/LABA/LAMA | 5 (8.3) | 7 (9.1) | 0.88 |
Data are presented as number (%) or mean ± standard deviation. ¶, positive bronchodilator response implies elevated FEV1 >12% and >200 mL from baseline FEV1 after the inhalation of 200 ìg salbutamol; ∆, change in FEV1; #, after the inhalation of 200 ìg salbutamol. ACO, asthma-COPD overlap; BMI, body mass index; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HTN, hypertension; ICS, inhaled corticosteroid; LABA, long acting beta2 receptor agonist; LAMA, long acting muscarinic receptor agonist; mMRC, modified Medical Research Council.
Moderate to severe exacerbation in the year prior to enrollment was higher in subjects with ACO, but this was not statistically significant. Fifteen patients (25%) with non-ACO were prescribed inhaled corticosteroids (ICS) alone or in combination with long-acting bronchodilators, compared to 19 (24.7%) with ACO, with no statistically significant difference between groups.
Inflammatory markers and radiographic features
Emphysema index on CT scan was higher in subjects with non-ACO COPD compared to ACO (9.7±8.3 vs. 7.2±7.1, P=0.06); the MWA was similar in both groups. Inflammatory markers including blood eosinophil count, IL-6, IL-8, CRP and NGAL levels were not significantly different between groups (Table 2). However, on analysis based on gender, NGAL levels were significantly higher in the ACO compared to the non-ACO group among female subjects (17.0±6.4 and 11.1±4.5 ng/mL, respectively) (Figure 1). There was no difference in plasma NGAL level between male ACO patients and non-ACO patients (15.0±6.7 and 16.8±8.2 ng/mL, respectively).
Table 2. Radiologic feature and inflammatory markers in patients with non-ACO and ACO.
| Variable | Non-ACO (n=60) | ACO (n=77) | P value |
|---|---|---|---|
| Radiologic finding | |||
| Emphysema index | 9.7±8.3 | 7.2±7.1 | 0.06 |
| Mean wall area (%) | 69.2±4.7 | 69.5±5.2 | 0.78 |
| Inflammatory markers | |||
| IL-6 (pg/mL) | 2.7±3.5 | 3.0±4.4 | 0.77 |
| IL-8 (pg/mL) | 16.6±20.8 | 21.2±23.5 | 0.20 |
| NGAL (ng/mL) | 15.9±7.9 | 15.6±6.6 | 0.92 |
| Blood eosinophil, % | 2.7±3.6 | 3.3±4.0 | 0.11 |
| Blood eosinophil, cell/ìL | 188.7±286.2 | 230.9±361.8 | 0.04 |
| hs-CRP, mg/dL | 0.22±0.28 | 0.42±0.88 | 0.91 |
Data are presented as number (%) or mean ± standard deviation. Emphysema index = volume fraction (%) of the lung below −950 HU; wall area (%) = wall area/(wall area + lumen area) ×100. ACO, asthma-chronic obstructive pulmonary disease overlap.
Figure 1.
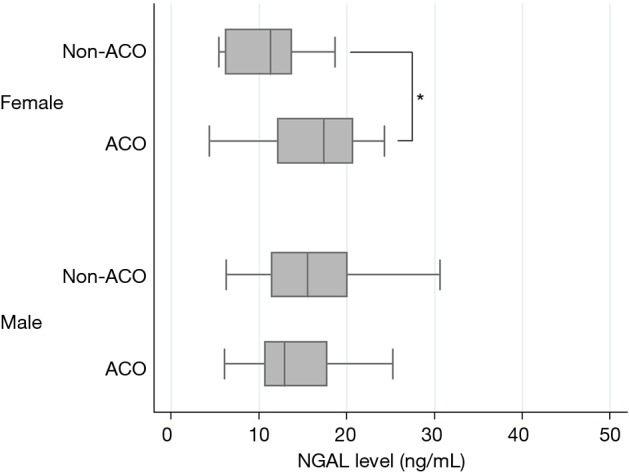
The level of plasma NGAL in patients with non-ACO and ACO. *, P<0.05. There was a significant difference in plasma NGAL levels between ACO and non-ACO only in female (17.0±6.4 and 11.1±4.5 ng/mL, respectively). NGAL, neutrophil gelatinase-associated lipocalin; ACO, asthma-chronic obstructive pulmonary disease overlap.
Gender differences were also analyzed for other inflammatory markers; however, there was no difference noted between subjects with ACO and non-ACO COPD.
Plasma concentration of NGAL to distinguish ACO from non-ACO COPD
Figure 2 shows the ROC curve of NGAL and blood eosinophil count to discriminate between subjects with ACO and non-ACO COPD based on gender. The ROC curve of NGAL and blood eosinophil count shows a fairly similar pattern. The area under ROC curve (AUROC) of NGAL and blood eosinophil count were 0.51 and 0.60 among all subjects; it was 0.77 and 0.79 in female subjects compared 0.44 and 0.57 in male subjects.
Figure 2.
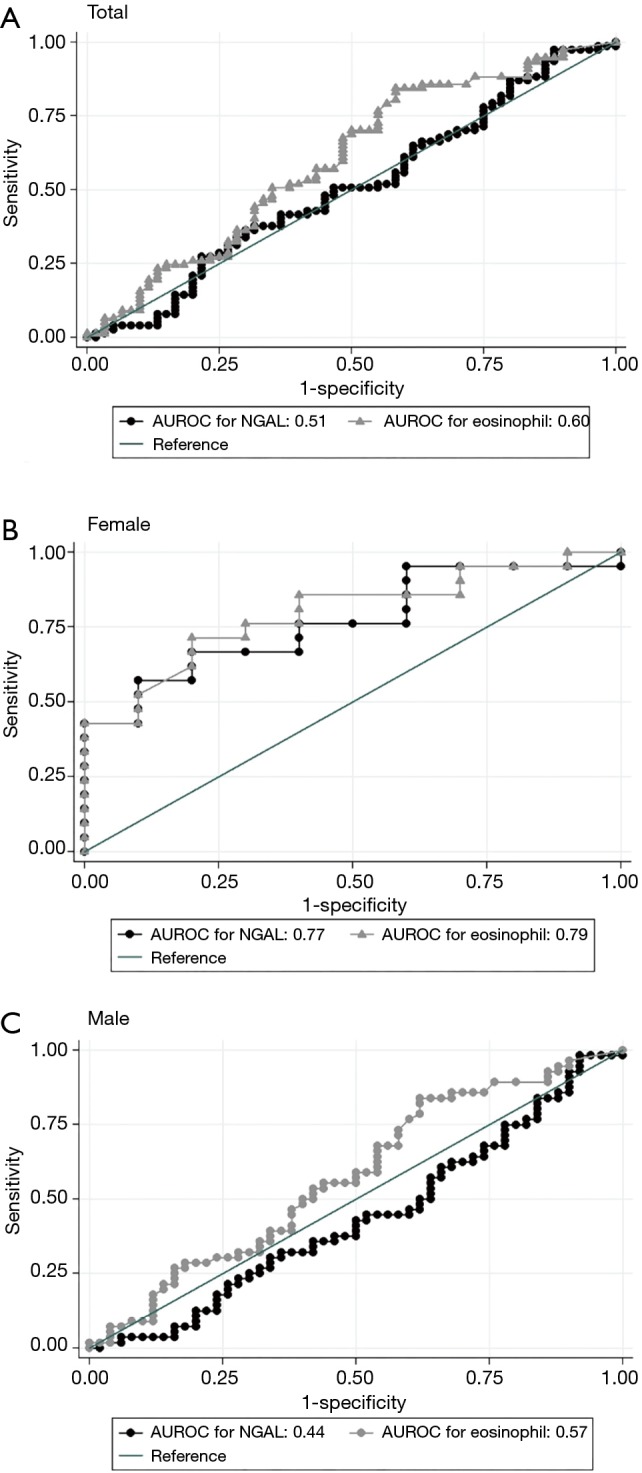
The ROC curve for predictive ability of NGAL and blood eosinophil to discriminate between patients with non-ACO and ACO: (A) total, (B) female and (C) male. ROC, receiver operating characteristic; NGAL, neutrophil gelatinase-associated lipocalin; ACO, asthma-chronic obstructive pulmonary disease overlap.
To determine the factors that affect plasma concentration of NGAL, Spearman rank correlation analysis was performed. Among variables including demographic, lung function and blood inflammatory markers, the emphysema index on the chest CT scan correlated significantly with the plasma level of NGAL (Spearman rank coefficient, 0.19; P=0.02).
The results of multivariate analyses adjusted for potential confounding factors are presented in Table 3. ACO was more likely in the third tertile of NGAL levels with odds ratio, 1.72; 95% CI, 0.69–4.28; P=0.24. There was a significantly higher likelihood of ACO with increasing NGAL levels (P for trend =0.01).
Table 3. Multivariable logistic regression model predicting the ACO.
| Variable | Univariate model | Multivariate model* | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | P for trend | aOR (95% CI) | P value | P for trend | ||
| Eosinophil | |||||||
| Tertile 1 | 1.00 (ref.) | N/A | 0.70 | 1.00 (ref.) | N/A | 0.01 | |
| Tertile 2 | 4.03 (0.76–21.29) | 0.10 | 4.24 (0.65–27.50) | 0.13 | |||
| Tertile 3 | 4.36 (0.81–23.65) | 0.09 | 5.18 (0.78–34.38) | 0.09 | |||
| NGAL | |||||||
| Tertile 1 | 1.00 (ref.) | N/A | 0.18 | 1.00 (ref.) | N/A | 0.01 | |
| Tertile 2 | 0.87 (0.38–1.99) | 0.75 | 1.03 (0.41–2.56) | 0.95 | |||
| Tertile 3 | 1.24 (0.54–2.87) | 0.61 | 1.72 (0.69–4.28) | 0.24 | |||
| Emphysema index | 0.96 (0.92–1.00) | 0.06 | 1.00 (0.95–1.06) | 0.95 | |||
*, adjusted by age, sex, BMI, initial FEV1 and use of ICS. ACO, asthma-chronic obstructive pulmonary disease overlap; aOR, adjusted odds ratio.
Discussion
The present study evaluated plasma NGAL level as a biomarker for differentiating ACO from non-ACO COPD. We found that in female patients, NGAL levels were significantly higher in the ACO compared to the non-ACO COPD group. Besides, significant higher likelihood of ACO was observed in the highest tertile of NGAL level which suggests the possibility that NGAL as a biomarker may distinguish subjects with ACO from those with non-ACO COPD. This result may be noteworthy considering that there was no biomarker other than blood eosinophil count and immunoglobulin E (IgE), that were conventionally used in the diagnosis of ACO.
Identification of patients with ACO may be important for several reasons. Patients with ACO are known to have more severe symptoms with impaired lung function (23,24). Besides, they tend to experience more frequent exacerbations than pure COPD (4,25) and have a lower self-perceived quality of life status (4,26). Consequently, patients with ACO use more healthcare resources at a higher cost of care compared to pure COPD (27). Overall, ACO is known to have more severe clinical features and a relatively unfavorable prognosis compared to pure COPD. Hence, it is important to distinguish this subgroup of patients early and provide appropriate treatment. Recently updated guidelines do not recommend the use of ICS as first-line treatment in COPD; the use of ICS is recommended in patients with features of asthma or those who need triple therapy because of persistent symptoms despite using long-acting bronchodilators (1). However, the lack of a clear, uniform definition of ACO is a fundamental issue. Several diagnostic criteria have been proposed to better differentiate between ACO and non-ACO COPD, and some helpful biomarkers have been proposed including blood eosinophil count and IgE. However, it is unclear whether a threshold value exists for the blood eosinophil count to diagnose ACO; cutoff values of IgE levels have also not been well defined.
There are several reports regarding the association of NGAL with COPD. Elevated NGAL levels have been reported in the bronchoalveolar lavage (BAL) fluid (28,29), sputum (30) and plasma (17,31) in patients with COPD compared to that of controls. Although it is not yet known exactly how NGAL affects small airway disease, it is known to have both positive and negative effects. It may inhibit bacterial growth by binding to siderophores secreted by bacteria (32) which might have a beneficial effect on airway disease by reducing infective complications. On the contrary, NGAL inhibits inactivation of MMP-9, secreted by activated neutrophils, leading to prolongation of collagen degradation (33,34). This may be of importance in patients with COPD as it may be related to the development of emphysema and small airway wall remodeling (28,31). Iwamoto et al. (18) reported that sputum NGAL levels in patients with ACO were significantly elevated compared to non-ACO COPD suggesting enhanced neutrophilic airway inflammation and/or airway epithelial cell injury in ACO. Considering the risk of contamination and difficulty in collecting adequate samples, sputum specimens are limited to homogeneous comparisons. In this context, we analyzed plasma levels of NGAL in the present study.
One of the strengths of this study was that quantitative CT findings were available in all subjects. Overall, the emphysema index was significantly higher in male compared to female subjects (9.6±8.1 vs. 4.0± 4.0, P<0.01); a similar result was observed in patients with ACO (8.8±7.6 vs. 3.1±2.6 for male and female respectively; P<0.01). In comparison, the MWA was higher in male subjects (71.1±4.4 vs. 68.9±5.1, P=0.03). However, among ACO patients, there was no statistically significant difference in the MWA according to gender ACO (68.8±5.3 vs. 71.2±4.7 for male and female subjects respectively, P=0.08).
Emphysema index was positively associated with NGAL levels, especially in female subjects. NGAL levels were significantly elevated in female subjects with ACO compared to those with non-ACO COPD. There have been several reports of gender differences in airway disease. There are some reports that suggest less severe emphysema in female subjects (35,36). In contrast, some reports suggest that female COPD patients may be more susceptible to lung parenchymal damage due to cigarette smoking and more likely to develop early-onset COPD with a more rapid decline in lung function (37-39). Although the phenotypic characteristics of COPD based on gender have not yet been accurately identified, several factors are thought to contribute. Women may be biologically more susceptible to toxic inhalation such as cigarette smoke; however, the precise role of sex hormones in pathways that regulate vulnerability to toxic inhalants with greater lung deposition of small particles has not been clarified (40). Also, the airway caliber in women is anatomically smaller (40); besides, 17β-estradiol, a female sex hormone, upregulates the mucin 5B gene which is related to mucus hypersecretion, and consequently may adversely affect COPD related respiratory symptoms (41).
Wang et al. (42) recently reported that plasma levels of NGAL was higher in patients with ACO than in asthma patients, and lower in ACO patients than in patients with COPD. The definition of asthmatic components was similar to our study, but our study did not show difference in NGAL level between ACO and non-ACO COPD. However, by showing the difference in the female patients, we suggested that gender difference may be involve in the complex pathophysiology of ACO which may be predominantly driven by both neutrophils and eosinophils.
The predictive value of NGAL and blood eosinophil count for ACO was similar. After adjustment for potential confounders, the likelihood of ACO was higher with increasing levels of NGAL. NGAL is known as a biomarker for acute kidney injury, and is associated with neutrophilic inflammation. Considering this, serum creatinine levels and neutrophil counts were used as potential confounding factors; however, the likelihood of ACO at higher tertiles of NGAL levels remained significant (data are not shown). In spite of this, it is difficult to conclude whether NGAL is a definitive biomarker for discriminating between ACO and non-ACO COPD. However, we hypothesize that this marker could offer supplementary information in defining ACO.
There are still few studies on biomarkers that help distinguish ACO patients from other chronic airway disease. NGAL is a biomarker that has recently received attention in the distinction between ACO and non-ACO COPD, and this study showed that there is a gender-related difference of NGAL level in distinguishing ACOs. However, there are several important weaknesses must be acknowledged. First, the small sample size of the study is likely to affect the statistical power. The small sample size also precluded analysis of NGAL levels according to the stage of COPD. Second, although we demonstrated a gender-related difference in NGAL levels, the number of female patients was small and hence we are unable to propose an appropriate cutoff level for distinguishing ACO from non-ACO COPD. Third, we did not evaluate other diagnostic biomarkers in ACO including total serum IgE and fractional exhaled nitric oxide levels. Fourth, although details of the physiological role of NGAL are unknown, considering its possible dichotomous effects in COPD, serial levels are required to clarify the short and long-term impact. Lastly, considering the number of current and ex-smokers, it is likely that there may have been some patients with asthma rather than ACO in our study.
Conclusions
This study showed elevated plasma levels of NGAL are related to a higher likelihood of ACO, especially in female. In addition, plasma NGAL levels corroborated with blood eosinophil count in distinguishing ACO patients. Regardless of whether ACO is a phenotype on the spectrum of obstructive airway diseases between asthma and COPD or not, it is clear that considering the clinical implications, more useful biomarkers are needed to clearly understand the pathophysiology and distinguish between pure asthma and COPD. Additional well-designed cohort studies with a larger sample size may be required to prove the clinical relevance of NGAL in defining ACO.
Acknowledgements
This study was supported by Ministry of Environment.
Ethical Statement: The present study has been approved by the ethics review boards of KNUH (IRB No. KNUH-KBB-2013-0005-009). Written informed consent was obtained from all participants (2012-06-007).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2017 update. Available online: http://www.goldcopd.org. Date last accessed: June 14, 2017.
- 2.Global Initiative for Asthma. Global strategy for asthma management and prevention: 2017 updated. Available online: http://www.ginasthma.org. Date last accessed: June 14, 2017.
- 3.Sin DD. Asthma-COPD Overlap Syndrome: What We Know and What We Don't. Tuberc Respir Dis (Seoul) 2017;80:11-20. 10.4046/trd.2017.80.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo YS, Lee J, Yoon HI, et al. Different prevalence and clinical characteristics of asthma–chronic obstructive pulmonary disease overlap syndrome according to accepted criteria. Ann Allergy Asthma Immunol 2017;118:696-703.e1. 10.1016/j.anai.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 2008;8:183. 10.1038/nri2254 [DOI] [PubMed] [Google Scholar]
- 6.Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015;3:435-42. 10.1016/S2213-2600(15)00106-X [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Therapeutic approaches to asthma–chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol 2015;136:531-45. 10.1016/j.jaci.2015.05.052 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, Hanagama M, Yamanda S, et al. Inflammatory biomarkers in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016;11:2117. 10.2147/COPD.S113647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ 2017;358:j3772. 10.1136/bmj.j3772 [DOI] [PubMed] [Google Scholar]
- 10.Plaza V, Álvarez F, Calle M, et al. Consensus on the Asthma-COPD Overlap Syndrome (ACOS) Between the Spanish COPD Guidelines (GesEPOC) and the Spanish Guidelines on the Management of Asthma (GEMA). Arch Bronconeumol 2017;53:443-9. [DOI] [PubMed] [Google Scholar]
- 11.Kjeldsen L, Bainton DF, Sengelov H, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994;83:799-807. [PubMed] [Google Scholar]
- 12.Cowland JB, Sørensen OE, Sehested M, et al. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol 2003;171:6630-9. 10.4049/jimmunol.171.12.6630 [DOI] [PubMed] [Google Scholar]
- 13.Nielsen BS, Borregaard N, Bundgaard JR, et al. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 1996;38:414-20. 10.1136/gut.38.3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bu DX, Hemdahl AL, Gabrielsen A, et al. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-κB. Am J Pathol 2006;169:2245-53. 10.2353/ajpath.2006.050706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14:2534-43. 10.1097/01.ASN.0000088027.54400.C6 [DOI] [PubMed] [Google Scholar]
- 16.Asimakopoulou A, Weiskirchen S, Weiskirchen R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front Physiol 2016;7:430. 10.3389/fphys.2016.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagan TM, Damås JK, Ueland T, et al. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest 2010;138:888-95. 10.1378/chest.09-2718 [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto H, Gao J, Koskela J, et al. Differences in plasma and sputum biomarkers between COPD and COPD-asthma overlap. Eur Respir J 2014;43:421-9. 10.1183/09031936.00024313 [DOI] [PubMed] [Google Scholar]
- 19.Hong Y, Kwon J, Lee S. Methodology of an observational cohort study for subjects with chronic obstructive pulmonary disease in dusty areas near cement plants. J Pulm Respir Med 2014;4:169. [Google Scholar]
- 20.Lee YK, Oh YM, Lee JH, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung 2008;186:157-65. 10.1007/s00408-008-9071-0 [DOI] [PubMed] [Google Scholar]
- 21.Crapo R, Hankinson J, Irvin C, et al. Single-breath carbon monoxide diffusing capacity (Transfer factor). Recommendations for a standard technique-1995 Update. Am J Respir Crit Care Med 1995;152:2185-98. 10.1164/ajrccm.152.6.8520796 [DOI] [PubMed] [Google Scholar]
- 22.Wanger J, Clausen J, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511. 10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 23.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64:728-35. 10.1136/thx.2008.108027 [DOI] [PubMed] [Google Scholar]
- 24.Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000;160:1683-9. 10.1001/archinte.160.11.1683 [DOI] [PubMed] [Google Scholar]
- 25.Menezes AMB, de Oca MM, Pérez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014;145:297-304. 10.1378/chest.13-0622 [DOI] [PubMed] [Google Scholar]
- 26.Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011;12:127. 10.1186/1465-9921-12-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee CK, Yoon HK, Yoo KH, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD 2014;11:163-70. 10.3109/15412555.2013.831061 [DOI] [PubMed] [Google Scholar]
- 28.Betsuyaku T, Nishimura M, Takeyabu K, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 1999;159:1985-91. 10.1164/ajrccm.159.6.9809043 [DOI] [PubMed] [Google Scholar]
- 29.Finlay GA, Russell KJ, McMahon KJ, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax 1997;52:502-6. 10.1136/thx.52.6.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med 1997;155:449-53. 10.1164/ajrccm.155.2.9032177 [DOI] [PubMed] [Google Scholar]
- 31.Ekberg-Jansson A, Andersson B, Bake B, et al. Neutrophil-associated activation markers in healthy smokers relates to a fall in DLCOand to emphysematous changes on high resolution CT. Respir Med 2001;95:363-73. 10.1053/rmed.2001.1050 [DOI] [PubMed] [Google Scholar]
- 32.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917. 10.1038/nature03104 [DOI] [PubMed] [Google Scholar]
- 33.Yan L, Borregaard N, Kjeldsen L, et al. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL) modulation of MMP-9 activity by NGAL. J Biol Chem 2001;276:37258-65. 10.1074/jbc.M106089200 [DOI] [PubMed] [Google Scholar]
- 34.Gupta K, Shukla M, Cowland JB, et al. Neutrophil gelatinase–associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum 2007;56:3326-35. 10.1002/art.22879 [DOI] [PubMed] [Google Scholar]
- 35.Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 2007;176:243-52. 10.1164/rccm.200606-828OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dransfield MT, Washko GR, Foreman MG, et al. Gender differences in the severity of CT emphysema in COPD. Chest 2007;132:464-70. 10.1378/chest.07-0863 [DOI] [PubMed] [Google Scholar]
- 37.Sørheim I-C, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65:480-5. 10.1136/thx.2009.122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langhammer A, Johnsen R, Gulsvik A, et al. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J 2003;21:1017-23. 10.1183/09031936.03.00053202 [DOI] [PubMed] [Google Scholar]
- 39.Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:2152-8. 10.1164/ajrccm.162.6.2003112 [DOI] [PubMed] [Google Scholar]
- 40.Jaques PA, Kim CS. Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal Toxicol 2000;12:715-31. 10.1080/08958370050085156 [DOI] [PubMed] [Google Scholar]
- 41.Choi HJ, Chung YS, Kim HJ, et al. Signal Pathway of 17β-Estradiol–Induced MUC5B Expression in Human Airway Epithelial Cells. Am J Respir Cell Mol Biol 2009;40:168-78. 10.1165/rcmb.2007-0377OC [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Lv H, Luo Z, et al. Plasma YKL-40 and NGAL are useful in distinguishing ACO from asthma and COPD. Respir Res 2018;19:47. 10.1186/s12931-018-0755-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


