Summary
Release of cytoplasmic proteins into the supernatant occurs both in bacteria and eukaryotes. Since the underlying mechanism remains unclear, the excretion of cytoplasmic proteins (ECP) has been referred to as ‘non-classical protein secretion’. We show that none of the known specific protein transport systems of Gram-positive bacteria are involved in ECP. However, the expression of the cationic and amphipathic α-type phenol-soluble modulins (PSMs), particularly of PSMα2, significantly increased ECP; while PSMβ peptides or δ-toxin have no effect on ECP. Since psm expression is strictly controlled by the accessory gene regulator (agr), ECP was also reduced in agr-negative mutants. PSMα peptides damage the cytoplasmic membrane as indicated by the release of not only CPs, but also lipids, nucleic acids and ATP. Thus, our results show that in Staphylococcus aureus, PSMα peptides non-specifically boost the translocation of CPs by their membrane damaging activity.
Keywords: cytoplasmic proteins, membrane damage, non-classical protein secretion, phenol-soluble-modulins, Staphylococcus aureus
Introduction
Normally, proteins that are translocated through the cytoplasmic membrane are distinguished by appropriate signal peptides and are translocated by defined transport systems. In eukaryotes such cytoplasmic proteins (CPs) are secreted distinct from the classical ER-Golgi route and the pathway was referred to as ‘nonclassical protein export’ (Muesch et al., 1990). Various distinct types of nonclassical export were described (Nickel, 2003). Some proteins are imbedded in endosomal sub-compartments (Rubartelli et al., 1990), others are N-terminal acylated and translocated via a flip-flop mechanism to the outer leaflet of the plasma membrane, and some proteins are translocated in exosomal vesicles that release their contents into the extracellular space. Sometimes the function of the protein in the intracellular milieu is completely different from that of the extracellular counterpart. For example the mammalian thymidine phosphorylase catalyzes the intracellular dephosphorylation of thymidine, but acts outside as a platelet-derived endothelial cell growth factor, which stimulates endothelial cell growth and chemotaxis (Jeffery, 1999). This is a paragon for a ‘moonlighting protein’, a term coined by Jeffery (Jeffery, 1999) and means that dependent on the localization one and the same protein can exert different functions.
But not only in eukaryotes, also in bacteria exists the ‘non-classical protein export’. In various streptococcal species and many other bacteria glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is not only found in the cytoplasm but was also present in large amounts on the cell surface and the supernatant (Pancholi and Chhatwal, 2003; Pancholi and Fischetti, 1992). The excreted streptococcal GAPDH functions as an ADP-ribosylating enzyme (Pancholi and Fischetti, 1993) and facilitates host colonization (D’Costa and Boyle, 2000; Madureira et al., 2007; Pancholi and Fischetti, 1993; Winram and Lottenberg, 1996). Thus GAPDH represents a bacterial example for a ‘moonlighting protein’. In Group B streptococci GAPDH is released by cell lysis and the exported enzyme induced apoptosis in murine macrophages (Oliveira et al., 2012).
With the rise of secretome analyses, it turned out that in bacteria the excretion of cytoplasmic proteins (ECP) is not restricted to individual species but is rather a general phenomenon in Gram-positive and Gram-negative bacteria (Götz et al., 2015). In particular, many glycolytic enzymes, chaperones, translation factors or enzymes involved in detoxification of reactive oxygen species were found in the supernatants (Boel et al., 2004; Sibbald et al., 2006; Tjalsma et al., 2004; Trost et al., 2005; Xia et al., 2008; Ziebandt et al., 2004). In the agr-positive S. epidermidis strain RP62A a surprisingly high number of CPs (80%) were found in the secretome (Siljamaki et al., 2014).
It is hotly debated whether the release of such proteins is due to cell lysis or whether they are exported by a so far unknown secretion mechanism. There are arguments for both possibilities. For example, cells in the stationary growth phase or mutants with increased autolysis activity or altered cell wall structure release more CPs to the supernatant (Ebner et al., 2015a; Nega et al., 2015). On the other hand there are also evidences for a defined mechanism in ECP. In E. coli for example excretion of enolase was significantly decreased by certain mutations in the active site region without affecting enzyme activity (Boel et al., 2004). In B. subtilis excretion of enolase was dependent on a hydrophobic alpha-helical domain (Yang et al., 2011; Yang et al., 2014). These examples are strong evidences that the protein structure plays a crucial role in ECP and speak therefore against an indiscriminate excretion by cell lysis.
In Staphylococcus aureus, our favorite model bacterium, we don’t know the underlying mechanism of ECP. But what we know is that pathogenic strains excrete more CPs than non-pathogenic strains and species (Ebner et al., 2016; Pasztor et al., 2010). For example in the agr-negative S. aureus SA113 strain only a defined set of CPs were found in the secretome, while other highly expressed CPs were absent (Pasztor et al., 2010), suggesting, that in this strain background only defined CPs were excreted. By analyzing some features of ECP in the agr-negative SA113 it has also been shown that ECP takes place mainly during the exponential growth phase, that the amount of excreted CPs is substantial and comparable to Sec-secreted proteins and that CPs are translocated particularly at the septal cleft of dividing cells (Ebner et al., 2015a; Ebner et al., 2015b). By investigating the potential role of excreted CPs in pathogenicity of S. aureus, it has been shown that the two model proteins, aldolase (FbaA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), increased adherence to host cells and exerted cytoxicity to various host cells (Ebner et al., 2016). The accessory gene regulator (agr) positively controls the expression of many toxins and has been assigned a central role in the pathogenesis of staphylococci, particularly in S. aureus (Novick, 2003). It is therefore not surprising that agr-mutants are severely attenuated in virulence (Abdelnour et al., 1993; Cheung et al., 2011), but why they excrete less CPs is unknown so far.
So far we don’t know the underlying mechanism of ECP in S. aureus; probably there is not just one but more mechanisms involved. We show, that in S. aureus ECP is not mediated by one of the known specific protein transport systems of Gram-positive bacteria. However, we found that ECP was significantly increased by alpha-type phenol soluble modulins (PSMα) expression, which was strictly controlled by the accessory gene regulator (agr). PSMα peptides disintegrated the cytoplasmic membrane thus triggering a rather unspecific release of CPs into the supernatant. This study explains why high ECP is correlated with pathogenicity.
Results
Known alternative protein transport systems in Gram-positive bacteria do not contribute to ECP - only the global regulator agr.
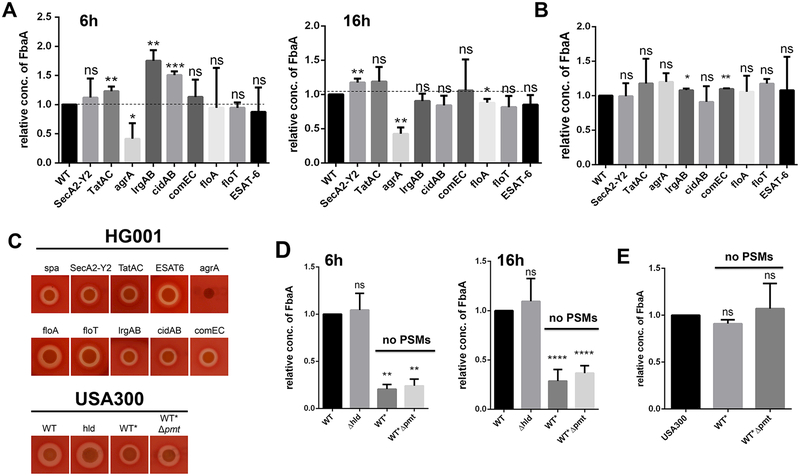
To facilitate analysis of ECP in S. aureus we have selected four CPs, namely aldolase (FbaA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), enolase (Eno) and the NADH-oxidorecuctase (Ndh2); the latter is membrane-associated (Ebner et al., 2015a; Pasztor et al., 2010). As CPs have no classical signal peptide, it is unlikely that they are “co-translocated” by the classical Sec-pathway. However, in Gram-positive bacteria, there are various alternative protein secretion systems by which certain CPs might be translocated or co-translocated with the target proteins. For this reason we created marker-less deletion mutants in all known alternative protein secretion systems in S. aureus HG001Δspa. In HG001, a derivative of NCTC8325, the global regulator agr has been repaired (Herbert et al., 2010). For better monitoring ECP in Western blots the spa gene (encoding protein A) was deleted. Deletions were generated in the accessory secretory system (secA2-Y2), the twin arginine translocation system (tatAC) (Biswas et al., 2009), the ESAT-6 secretion system (Burts et al., 2005), the holin (cidAB) (Rice et al., 2003) and anti-holin system (lrgAB) (Ranjit et al., 2011), and the competence system (comEC) (Draskovic and Dubnau, 2005). In addition we also created mutants in flotillin genes (floA and floT) that are potentially involved in lipid raft formation (Bach and Bramkamp, 2015). Finally, we also inactivated the global regulator agr, which controls a large number of secreted S. aureus toxins (Koenig et al., 2004; Peng et al., 1988). With all these mutants we investigated the amount of excreted FbaA (as a CP representative) in the culture supernatant of 6 and 16 h grown cells (Fig. 1A). None of the alternative protein transport systems or the flotillin deletions exhibited a convincing decrease of the FbaA content in the supernatant at both time points. In contrast, in the tatAC, lrgAB and cidAB mutants, the content of FbaA was even increased at the 6 h time point, but was equal to wild type (WT) levels after 16 h growth (Fig. 1A). Representative Western blots are shown in Fig. S1AB.
Figure 1: Excretion of FbaA is decreased in an agrA and PSM mutants.
(A) Relative amounts of FbaA in the supernatant of HG001 and its isogenic secA2-Y2, tatAC, agrA, lrgAB, cidAB, comEC, floA, floT and ESAT-6 deletion mutants after 6h and 16h. The dashed line represent an amount equal to that of the WT. (B) Relative amounts of FbaA in the cytoplasm of HG001 and its isogenic secA2-Y2, tatAC, agrA, lrgAB, cidAB, comEC, floA, floT and ESAT-6 deletion mutants after 16h of growth. (C) Hemolysis test of the HG001 and secA2-Y2, tatAC, agrA, lrgAB, cidAB, comEC, floA, floT, ESAT-6, USA300hld::hldMet→Ile, WT* and WT*Δpmt deletion mutants on sheep blood agar plates. (D) Relative amount of FbaA in the supernatant in USA300, USA300hld::hldMet→Ile, WT* and WT*Δpmt after 6h and 16h. (E) Relative amount of FbaA in the cytoplasm of USA300, hld::hldMet→Ile, WT* and WT*Δpmt after 6h. Representative data from three independent experiments are shown. For all graphs, each data point is the mean value ± SD (n = 3) *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001, by students t-test.
Only in the agr mutant the amount of FbaA in the supernatant was significantly decreased after both 6 and 16 h. As a control we quantified the amount of FbaA in the cytoplasm of the various mutants and the wild type; there was no marked difference indicating that FbaA expression was not affected (Fig. 1B). To verify whether the mutants were affected in the general Sec-dependent secretion pathway, we tested their hemolytic activity on sheep blood agar plates (Fig. 1C), because hemolysis is mostly dependent on α-toxin that is Sec-dependent secreted. With exception of the agrA mutant, all other mutants produced a clear hemolysis zone, indicating that the general Sec pathway was not affected in all these mutants. The absence of a hemolytic zone in the agrA mutant is expected because α-toxin expression is positively controlled by agr and there is usually no clear α-hemolysis observed in agr-negative backgrounds (Cheung et al., 1992; Herbert et al., 2010). This experiment clearly shows, that the decrease of FbaA excretion is not due to an abolished agr function itself, but needs to be affected by a downstream effect of the agr regulon.
FbaA excretion is decreased in PSM deficient mutants.
The question occurred why ECP is significantly decreased in the agr mutant. The most probable explanation is that agr positively controls other genes contributing to enhanced ECP. The phenol-soluble modulins (PSMs) seemed to be the most likely candidates, since they are under exceptionally strict regulation by agr (Queck et al., 2008) and because of their membrane-damaging and pore-forming properties (Wang et al., 2007). In order to investigate the impact of PSMs in ECP, we tested the PSM deletion mutants WT* (Δpsmα1–4Δpsmβ1–2hld::hldMet→Ile) and WT*Δpmt on their impact on ECP. In WT* the α- and β-psm genes were completely deleted, while hld (δ-hemolysin) was mutated only in the start codon to prevent toxin translation but not RNAIII transcription which is crucial for agr regulation. In WT*Δpmt the PSM transporter genes pmtABCD were additionally deleted. On blood agar plates WT* and WT*Δpmt showed a smaller hemolysis zone compared to the WT (Fig. 1D), which is likely due to a combined effect of PSMs and α-toxin (Cheung et al., 2012); furthermore, it has been shown that in psm mutants also α-toxin expression is decreased in S. aureus (Berube et al., 2014). In the hld mutant the hemolysis zone was not altered compared to wild type.
To verify whether PSMs affect FbaA excretion, we tested WT*, WT*Δpmt, and the δ-toxin deletion mutant USA300hld::hldMet→Ile. After 6 and 16 h growth, the amount of FbaA in the culture supernatants of WT* and WT*Δpmt, but not of USA300hld::hldMet→Ile, was significantly decreased compared to wild type (Fig. 1D). In order to rule out that the altered ECP was due to a decreased expression of cytoplasmic FbaA, we quantified the cytoplasmic FbaA, which showed no difference in the various mutants (Fig. 1C).
Endogenously expressed PSMα peptides are the major players in ECP.
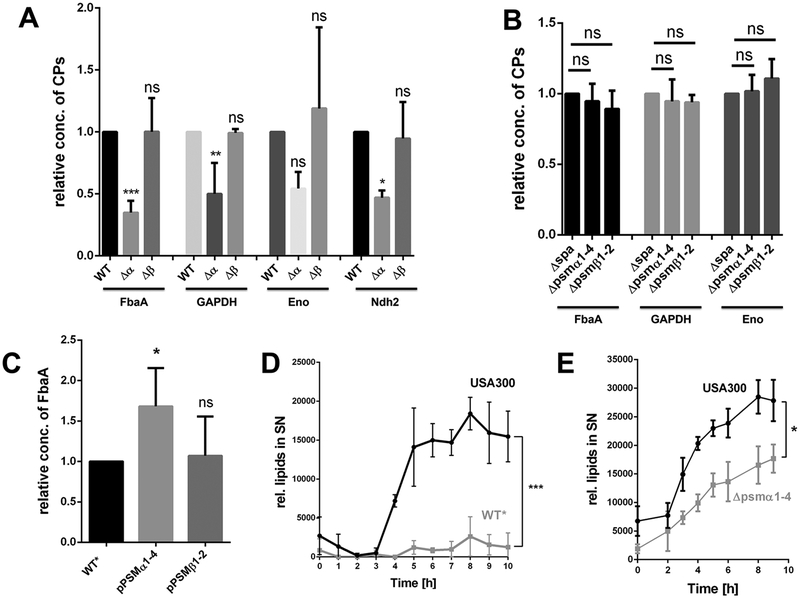
Next we verified whether the individual PSMα (PSMα1–4) or PSMβ peptides (PSMβ1–2) or a combination of both enhance ECP. We therefore deleted the psmα1–4 and psmβ1–2 operons, respectively in USA300. As shown, ECP was significantly decreased in the psmα1–4 deletion mutant but not in the psmβ1–2 mutant, suggesting that only PSMα peptides contributed to ECP (Fig. 2A). We also investigated the excretion other CPs, such as GAPDH, Eno and Ndh2, in the PSMα and PSMβ deletion mutants. All CPs showed decreased amounts in the supernatant of the PSMα deletion mutant, but not in the supernatant of the PSMβ mutant, indicating, that PSMα peptides were the major players in boosting ECP (Fig. 2A). As control, again the cytoplasmic amount of FbaA, GAPDH and Eno was investigated, which was unaltered; due to its membrane attachment the quantification of Ndh2 in the cytoplasm was hampered (Fig. 2B).
Figure 2: PSMα peptides are the driving force for ECP in USA300.
(A) Densitometric analysis of the relative amounts of FbaA, GAPDH, Enolase and Ndh2 in the supernatant of USA300Δspa and its corresponding PSMα1–4 and PSMβ1–2 mutants. (B) Relative amounts of FbaA, GAPDH and Enolase in the cytoplasm of USA300Δspa and its corresponding PSMα1–4 and PSMβ1–2 mutants. (C) Densitometric analysis for detecting the excreted FbaA in the supernatant of WT*, the complemented mutant pRAB11-psmα1–4 and WT* pRAB11-psmβ1–2 after 16h of PSM expression. (D) Release of lipids in USA300 and its and WT* mutant over a time period of 10 h, the cultures were diluted to OD578 = 1,85 for each time point. (E) Release of lipids in USA300 and its PSMα1–4 mutant over a time period of 9 h, the cultures were diluted to OD578 = 2 for each time point. Representative data from three independent experiments are shown. For all graphs, each data point is the mean value ± SD (n = 3 for ABCE, n = 2 for D) *p < 0.05; **p < 0.01; ***p < 0.001, by one-way ANOVA with Bonferroni posttest for ABC and unpaired t-test for DE.
The impaired excretion of FbaA in the WT* strain could be complemented by expressing plasmid-encoded PSMα1–4 (pPSMα1–4) but not by PSM1–2 β (pPSMβ1–2), supporting PSMα1–4 as major contributors (Fig. 2C). However, in the agr- and PSM mutants, there is still approximately 25% ECP observed, suggesting additional factors contributing to ECP. As we know that ECP is significantly decreased in an atlA deletion mutant of S. aureus SA113 (Pasztor et al., 2010), we compared the amount of FbaA in the supernatant of USA300 and its atlA mutant. Indeed, in the atlA mutant of USA300 the amount of FbaA in the supernatant was decreased by 50% (Fig. S5 B), indicating that AtlA also plays a crucial role in ECP.
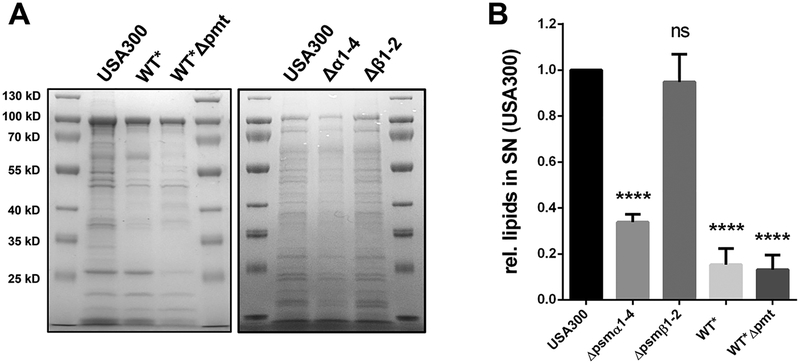
To further investigate the role of PSMα peptides in the life cycle of S. aureus; we tested USA300 and PSM deletion mutant WT* for their release of lipids to the supernatant, which was significantly lower in WT* than in USA300 (Fig. 2D). This decreased effect, was also seen in the PSMα1–4 deletion mutant (Fig. 2E). Comparative SDS-PAGE of extracellular proteins from 24h grown cultures revealed decreased protein amounts in WT*, WT*Δpmt the PSMα1–4 mutant was generally decreased compared to the USA300 or its PSMβ1–2 mutant (Fig. 3A). Additionally, the content of excreted lipids in stationary phase cells was significantly lower in the PSMα1–4 mutant, WT* or WT*Δpmt than in USA300 (Fig. 3B).
Figure 3: Deletion of of PSMα1–4 causes decreases proteins and lipids in the supernatant of USA300.
(A) SDS-PAGE of the extracellular proteins of USA300, WT*, WT*Δpmt, PSMα1–4 and PSMβ1–2 mutant after 24h of growth. (B) Relative amount of lipids in the supernatant of USA300, WT*, WT*Δpmt, PSMα1–4 and PSMβ1–2 mutant after 24h of growth. Representative data from three independent experiments are shown. For all graphs, each data point is the mean value ± SD (n = 3) *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001, by one-way ANOVA with Bonferroni posttest.
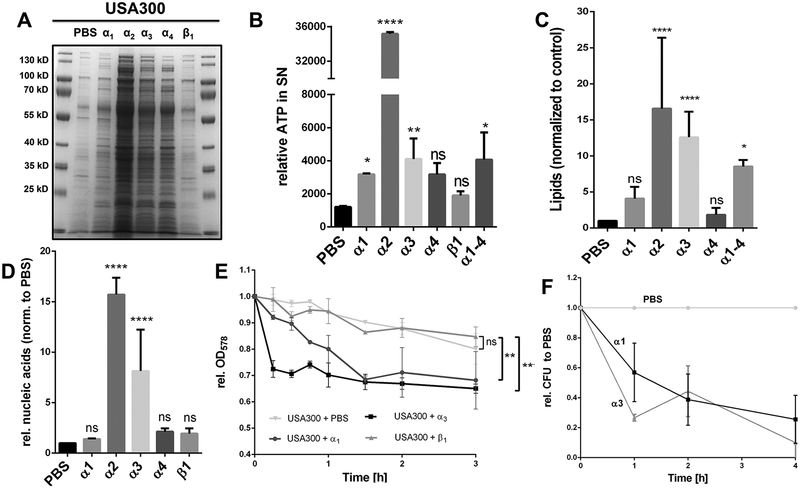
External supplied PSMα peptides increased release of proteins, ATP, lipids and nucleic acids to the supernatant due to cell leakage.
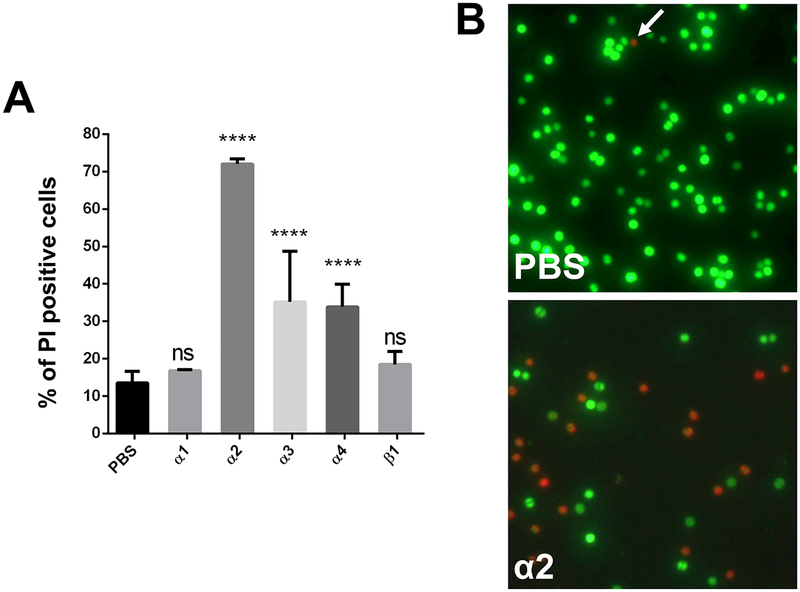
External supplied PSMα1, α2, α3 and α4, but not PSMβ1 increased the release of proteins into the supernatant of USA300 as shown in SDS-PAGE (Fig. 4A). PSMα2 showed by far the highest effect, followed by PSMα3. PSMα2 was also superior in triggering the release of ATP, lipids and nucleic acids (Fig. 4B–D). The impact of PSMβ1 on lipid release could not be determined since PSMβ1 itself interacts with the lipid dye FM5–95 (Fig. S5 A). When PSMα1 and PSMα3 (10 – 60 μg/ml) were added to a log-phase culture increased release of FbaA was only observed at high concentration (Fig. S2A), while PSMα2 triggered already at 25 μ/ml a clear increase in release of proteins (Fig. S2B). The release of CPs, ATP, nucleic acids (DNA and RNA) and lipids is indicative for particularly PSMα2 and PSMα3 triggered membrane damage. Membrane damage should affect growth, and that is what we observed in comparative growth curves with USA300, WT*, mutants lacking only PSMα1–4, or PSMβ1–2 (Fig. S3AB). Mutants where PSMα1–4 encoding genes were deleted grow significantly better than the wild type or the PSMβ1–2 mutant, indicating, that PSMα are the membrane damaging peptides. Interestingly, in accordance to the decreased growth, beginning in the mid-exponential phase, the release lipids also starts. Finally, external supplied PSMα1 and PSMα3, but not PSMβ1, enhanced cell lysis of a USA300 culture at mid exponential growth phase as indicated by both a decrease in absorbance (Fig. 4E), a decreased CFU over time (Fig. 4F), and by Live/Dead staining of PSM treated and untreated cells (Fig. 5AB); again, PSMα2 showed by far the strongest membrane damaging effect.
Figure 4: Exogenous supplied PSMα peptides enhance cell leakage and ECP.
(A) SDS-PAGE of the extracellular proteins of USA300, treated with PSMα1, α2, α3, α4 and β1. (B) Extracellular ATP levels of PBS washed USA300 cells incubated with PBS, α1, α2 α3, α4 and β1 after incubation for 4h. (C) Relative amount of membrane lipids in the supernatant of PSM treated USA300 cells after 4 h incubation of washed cells in the mid exponential growth phase. (D) Relative extracellular nucleic acids, normalized to PBS, after treatment of USA300 with synthetic PSM peptides. (E) Relative OD of mid exponential growth phase cells of USA300, WT* and WT*Δpmt resuspended in PBS and monitored for 3 h in PBS, PBS + α1, PBS + α3 and PBS + β1. (F) Relative CFU of PBS compared to PSMα1 and PSMα3 treated USA300 cells for a period of 4 h. Representative data from at least two independent experiments are shown. For all graphs, each data point is the mean value ± SD (n = 3 for BCD and n = 2 for EF) *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001, by one-way ANOVA with Bonferroni posttest for BCD and unpaired t-test for EF.
Figure 5: Monitoring of cytolytic effect of PSMs on S. aureus USA300 cells.
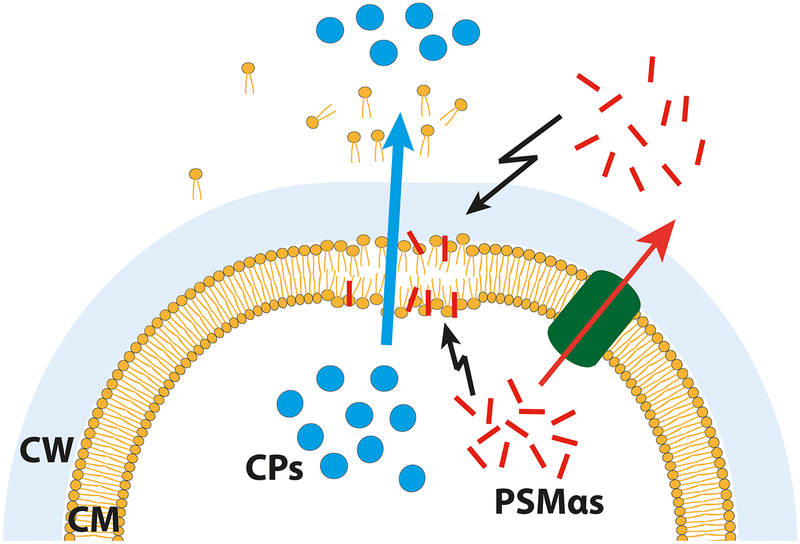
(A) Percentage of dead cells after treatment with PSM peptides. (B) Fluorescence microscopy of propidium iodide (red) and SYTO 9 (green) stained cells, either untreated or treated with PSMα2. Note: Propidium iodide positive cells (red) have damaged/leaky cell membranes. Scale bar represents 10 μm. (C) Proposed model for PSMα mediated excretion of cytoplasmic proteins. Either intracellular, as well as extracellular, PSMα peptides cause membrane perturbations and weaken the membrane integrity of S. aureus cells. This weakening causes cell leakage and therewith excretion of cytoplasmic proteins and other cytoplasmic compounds. Explanation: Cytoplasmic proteins, blue circles; PSMs, red rods; Pmt transporter, green tunnel; CM, cytoplasmic membrane; CW, cell wall. Representative data from three independent experiments are shown. For all graphs, each data point is the mean value ± SD (n = 3) *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001, by one-way ANOVA with Bonferroni posttest.
Recently it has been shown that FbaA and GAPDH interact with the surface exposed major autolysin (Atl) (Ebner et al., 2016). Therefore, we considered the question whether FbaA is not excreted but bound to the cell surface from where it might be detached by PSMα and released then into the supernatant. In an approach to answer this question, we treated washed USA300Δspa cells, cultivated to mid-exponential growth phase, for 15 min with PSMα1, PSMα3 and PBS as control. The protein pattern of the supernatant showed no difference in SDS-PAGE, and also the amount of FbaA was not altered as shown in the Western blot (Fig. S4AB), indicating that the presence of FbaA in the supernatant was not due to its detachment from the cell surface. All results indicate that both external supplied or endogenously expressed PSMα cause disintegration and leakage of the cytoplasmic membrane thus boosting ECP in agr-positive S. aureus strains.
Discussion
The excretion of cytoplasmic proteins (ECP) with no apparent secretion signal sequence has been described in pro- and eukaryotes. There are few examples that suggest that this type of ‘non-classical protein excretion’ underlies a specific mechanism. However, there many other examples, particularly in bacteria, where no defined mechanism could be identified, and where it therefore is generally believed that ECP is due to cell lysis or autolysis. From the energetic point of view it would be an enormous waste of energy and resources if bacteria excrete CPs without a good reason.
Here, we studied ECP in the community-acquired methicillin-resistant S. aureus (CA-MRSA) USA300 strain. This strain belongs to the clonal complex 8 (CC8) whose representatives are characterized by high transmissibility and virulence (Diep et al., 2006). As cytoplasmic model proteins we have chosen FbaA, GAPDH, Eno and Ndh2, which we can monitor in immune blots. One of the first questions we addressed was whether their abundance in the supernatant was dependent on one of the known alternative protein secretion systems such as Tat, accessory Sec-system (SacA2-Y2), ESAT-6 (Type VII), the holin-antiholin systems Cid and Lrg, or the competence system (com). We also investigated floA and floB mutants as these genes are involved in lipid raft-associated cellular processes, including membrane sorting, trafficking, cell polarization, and signal transduction (Bach and Bramkamp, 2015; Bramkamp and Lopez, 2015). However, none of the mutations showed a conspicuous decrease in FbaA excretion. The Sec-system could not be tested, as it is essential (Natale et al., 2008). However, a temperature sensitive mutant in secA in B. subtilis still had CPs in the supernatant (Hirose et al., 2000), suggesting that the classical Sec pathway is not directly involved in ECP.
The only mutant where excretion of FbaA was significantly decreased was the agr mutant. The accessory gene regulator (agr) system positively controls the expression of many toxins and plays a crucial role for the pathogenesis of S. aureus. We assumed that it was not agr itself but one of the agr-controlled genes causing the increase in FbaA excretion. The phenol soluble modulins (PSMs) belong to the strictly agr-regulated toxins, meaning that in agr-negative mutants no PSM expression can be observed (Cheung et al., 2011; Wang et al., 2007). PSMs can be divided into three types, the α- and β-type PSMs and δ-toxin; they all differ in size and net-charge (Bäsell et al., 2014; Peschel and Otto, 2013). All PSMs form an amphipathic α-helix. While in the longer β-type PSMs the α-helix is located at the carboxy-terminal region, the α-helix of the α-type PSMs extends over the whole length of the peptide (Peschel and Otto, 2013). PSMs are transported by the ATP transporter PmtABCD (Chatterjee et al., 2013); only when all psm genes are deleted the transporter genes can be deleted too. Consequently, expression of α-type PSMs or δ-toxin is lethal in the transporter mutant (Chatterjee et al., 2013). When we expressed the α1–4 and the β1–2 operons (pRAB vector) in WT*Δpmt, the α1–4 genes caused a massive release of FbaA, while the effect with β1–2 was only marginal (Fig. S2 C).
With their membrane disintegrative activity (Cheung et al., 2014b), PSMs appear to be the most likely candidates to boost ECP. Indeed, we could show that in the USA300 mutant where all all psm genes were deleted (WT*), as well as in a mutant where not only psm but also the pmt transporter genes were deleted (WT*Δpmt), excretion of FbaA was about four-fold decreased (Fig. 1D). Among the PSM peptides particularly the PSMα peptides exerted the strongest effect as shown in the psmα1–4 mutant and by complementation of WT* with pPSMα1–4 (Fig. 2A and C); while PSMβ1 expression showed no effect on ECP (Fig.S5). We assume that particularly the cationic amino acids present in PSMα peptides, which predominantly contribute to cytolytic activity (Cheung et al., 2014a; Wang et al., 2007), cause membrane damage and as a consequence the release CPs. Indeed, we could show that endogenously expressed and externally supplied PSMα peptides triggered the release of CPs, nucleic acids, ATP and membrane lipids (Fig. 2,3,4). PSMα2 was the most active peptide with respect to release of proteins, ATP, nucleic acids and lipids; PSMα3 and PSMα1 were less active (Fig. 4,5). This was a bit surprising, as it has been reported that PSMα3 has the highest cytolytic activity towards human leukocytes and erythrocytes (Wang et al., 2007). However, it is long known that mainly α-type PSMs but not β-types are cytotoxic. Both types are structurally different: α-types are shorter and over the entire length α-helical, while the β-types are longer and only the C-terminal part is α-helical. The PSMs of the β-type commonly have a negative net charge, while most, but not all, α-type PSMs have a neutral or positive net charge (Cheung et al., 2014a). In a biochemical approach the lytic activity of seven PSMs on phospholipid vesicles and T cells was compared with helical wheel projections and circular dichroism measurements. The authors concluded that the degree of alpha-helicity of the PSMs was the single most important property in predicting their lytic activity (Laabei et al., 2014). However, there is no evidence that PSM interaction with the membrane is dependent on a receptor (Cheung et al., 2014a; Kretschmer et al., 2010). However, the cytotoxic activity to human cells (HL-60) could be neutralized by serum lipoproteins (Surewaard et al., 2012).
Here we show that the global regulator, agr, plays a crucial role in ECP, but also caused some confusion. Originally, studies were carried out with S. aureus SA113, an agr negative strain (Pasztor et al., 2010). Proteome analysis suggested that only a subset of CPs were excreted and therefore it was assumed that there is a sophisticated selection procedure is at work. Furthermore, ECP takes place at the septum of dividing cells (Ebner et al., 2015a). However, in an agr-positive background the PSMα-induced ECP is about four-fold higher. Also in agr-positive cells excretion occurred in the septum region of dividing cells (Fig. S4 C); a site where the cells are most vulnerable because the cell wall biosynthesis is still in progress. We assume that the PSMα-induced ECP is largely unspecific. This assumption is supported in a compilation of annotated CPs in the secretome of agr-positive and -negative S. aureus strains (Supplementary Table 3). In the agr-negative strains only 88 CPs were found in the secretome while in the agr-positive it were 416, almost five-times more (Hanzelmann et al., 2016; Mekonnen et al., 2017; Sibbald et al., 2006). A model for the PSMα-induced release of CPs is shown in Fig. 5 C.
Conclusion
Our study raises a number of further questions. For example, what triggers ECP in the agr-negative S. aureus where ECP is restricted to a subset of CPs, is it also caused by membrane damage, is it tuned by autolysins such as the major autolysin Atl (Götz et al., 2014; Pasztor et al., 2010). Another question is related to the benefit of PSMα-induced ECP. The release of cytotoxic PSMs certainly contributes to virulence as shown in various publications. But does the membrane damage and accompanied release of CPs, ATP, nucleic acids, and lipids not kill the bacterium? Indeed, addition of PSMα1 or PSMα3 to washed cell culture decreased the CFU over time (Fig. 4E,F), and in Live/Dead staining PSMα2 showed a high percentage of propidium iodide positive cells, an indication of membrane damage (Fig. 5A,B). Despite affecting viability, ECP should have an advantage in infection, which we don’t understand yet. Some of the excreted CPs such as FbaA and GAPDH may contribute to pathogenicity due to their cytotoxic activity against host cells (Ebner et al., 2016). Another possibility is that the release of CPs and RNA might overload the immune system to the benefit of the bacterium. We shouldn’t assume that PSMα-induced ECP is a waste of resources and a mere collateral damage; it rather appears a deliberate process playing a crucial role in acute infections.
Supplementary Material
Experimental Procedures.
Detailed description of the experimental procedures for the following methods
Bacterial strains and growth conditions.
Construction of Staphylococcus aureus deletion mutants
Hemolysis assay
Preparation of protein samples for Western blot analysis
Live/Dead staining
Localization of the excreted aldolase via immunofluorescence
Relative quantification of cytoplasmic proteins
Membrane lipid, extracellular ATP and extracellular nucleic acid detection.
PSM synthesis
are described in Supplemental Information.
Statistical significance.
Multiple comparisons were analyzed using one-way ANOVA with Bonferroni posttest. Normal distributions were analyzed by Student’s t test. Statistical analyses were performed with GraphPad Prism software, with significance defined as p < 0.05. n represents independent biological replicates.
Acknowledgement
We thank Björn Watzer and Alexander Klotz for help and assistance with fluorescence microscopy. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) SFB766 and SFB/TRR24 to FG and graduate college GRK 1708/−2 to KF, and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (NIH).
Abbreviations:
- agr
accessory gene regulator
- CP
cytoplasmic protein(s)
- ECP
excretion of cytoplasmic proteins
- Eno
enolase
- FbaA
aldolase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- Ndh2
NADH:quinone oxidoreductase
- PSMs
phenol-soluble modulins
References
- Abdelnour A, Arvidson S, Bremell T, Ryden C, and Tarkowski A (1993). The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun 61, 3879–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JN, and Bramkamp M (2015). Dissecting the molecular properties of prokaryotic flotillins. PLoS One 10, e0116750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäsell K, Otto A, Junker S, Zuhlke D, Rappen GM, Schmidt S, Hentschker C, Macek B, Ohlsen K, Hecker M, et al. (2014). The phosphoproteome and its physiological dynamics in Staphylococcus aureus. International journal of medical microbiology : IJMM 304, 121–132. [DOI] [PubMed] [Google Scholar]
- Berube BJ, Sampedro GR, Otto M, and Bubeck Wardenburg J (2014). The psmalpha locus regulates production of Staphylococcus aureus alpha-toxin during infection. Infect Immun 82, 3350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas L, Biswas R, Nerz C, Ohlsen K, Schlag M, Schäfer T, Lamkemeyer T, Ziebandt AK, Hantke K, Rosenstein R, et al. (2009). Role of the twin-arginine translocation pathway in Staphylococcus. Journal of bacteriology 191, 5921–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boel G, Pichereau V, Mijakovic I, Maze A, Poncet S, Gillet S, Giard JC, Hartke A, Auffray Y, and Deutscher J (2004). Is 2-phosphoglycerate-dependent automodification of bacterial enolases implicated in their export? J Mol Biol 337, 485–496. [DOI] [PubMed] [Google Scholar]
- Bramkamp M, and Lopez D (2015). Exploring the existence of lipid rafts in bacteria. Microbiol Mol Biol Rev 79, 81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burts ML, Williams WA, DeBord K, and Missiakas DM (2005). EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A 102, 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, and Otto M (2013). Essential Staphylococcus aureus toxin export system. Nat Med 19, 364–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AL, Koomey JM, Butler CA, Projan SJ, and Fischetti VA (1992). Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci U S A 89, 6462–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Duong AC, and Otto M (2012). Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect 14, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Joo HS, Chatterjee SS, and Otto M (2014a). Phenol-soluble modulins--critical determinants of staphylococcal virulence. FEMS Microbiol Rev 38, 698–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Kretschmer D, Queck SY, Joo HS, Wang R, Duong AC, Nguyen TH, Bach TH, Porter AR, DeLeo FR, et al. (2014b). Insight into structure-function relationship in phenol-soluble modulins using an alanine screen of the phenol-soluble modulin (PSM) alpha3 peptide. FASEB J 28, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Wang R, Khan BA, Sturdevant DE, and Otto M (2011). Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79, 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Costa SS, and Boyle MD (2000). Interaction of group A streptococci with human plasmin(ogen) under physiological conditions. Methods 21, 165–177. [DOI] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, et al. (2006). Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739. [DOI] [PubMed] [Google Scholar]
- Draskovic I, and Dubnau D (2005). Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol Microbiol 55, 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner P, Prax M, Nega M, Koch I, Dube L, Yu W, Rinker J, Popella P, Flötenmeyer M, and Götz F (2015a). Excretion of cytoplasmic proteins (ECP) in Staphylococcus aureus. Mol Microbiol. [DOI] [PubMed] [Google Scholar]
- Ebner P, Rinker J, and Götz F (2015b). Excretion of cytoplasmic proteins in Staphylococcus is most likely not due to cell lysis. Curr Genet. [DOI] [PubMed] [Google Scholar]
- Ebner P, Rinker J, Nguyen MT, Popella P, Nega M, Luqman A, Schittek B, Di Marco M, Stevanovic S, and Götz F (2016). Excreted Cytoplasmic Proteins Contribute to Pathogenicity in Staphylococcus aureus. Infect Immun 84, 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F, Heilmann C, and Stehle T (2014). Functional and structural analysis of the major amidase (Atl) in Staphylococcus. International journal of medical microbiology : IJMM 304, 156–163. [DOI] [PubMed] [Google Scholar]
- Götz F, Yu W, Dube L, Prax M, and Ebner P (2015). Excretion of cytosolic proteins (ECP) in bacteria. International Journal of Medical Microbiology accepted. [DOI] [PubMed] [Google Scholar]
- Hanzelmann D, Joo HS, Franz-Wachtel M, Hertlein T, Stevanovic S, Macek B, Wolz C, Götz F, Otto M, Kretschmer D, et al. (2016). Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat Commun 7, 12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert S, Ziebandt AK, Ohlsen K, Schäfer T, Hecker M, Albrecht D, Novick R, and Götz F (2010). Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun 78, 2877–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose I, Sano K, Shioda I, Kumano M, Nakamura K, and Yamane K (2000). Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146, 65–75. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ (1999). Moonlighting proteins. Trends Biochem Sci 24, 8–11. [DOI] [PubMed] [Google Scholar]
- Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, and Hurlburt BK (2004). Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. Journal of bacteriology 186, 7549–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, et al. (2010). Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laabei M, Jamieson WD, Yang Y, van den Elsen J, and Jenkins AT (2014). Investigating the lytic activity and structural properties of Staphylococcus aureus phenol soluble modulin (PSM) peptide toxins. Biochim Biophys Acta 1838, 3153–3161. [DOI] [PubMed] [Google Scholar]
- Madureira P, Baptista M, Vieira M, Magalhaes V, Camelo A, Oliveira L, Ribeiro A, Tavares D, Trieu-Cuot P, Vilanova M, et al. (2007). Streptococcus agalactiae GAPDH is a virulence-associated immunomodulatory protein. J Immunol 178, 1379–1387. [DOI] [PubMed] [Google Scholar]
- Mekonnen SA, Palma Medina LM, Glasner C, Tsompanidou E, de Jong A, Grasso S, Schaffer M, Mader U, Larsen AR, Gumpert H, et al. (2017). Signatures of cytoplasmic proteins in the exoproteome distinguish community- and hospital-associated methicillin-resistant Staphylococcus aureus USA300 lineages. Virulence, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesch A, Hartmann E, Rohde K, Rubartelli A, Sitia R, and Rapoport TA (1990). A novel pathway for secretory proteins? Trends Biochem Sci 15, 86–88. [DOI] [PubMed] [Google Scholar]
- Natale P, Bruser T, and Driessen AJ (2008). Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane--distinct translocases and mechanisms. Biochim Biophys Acta 1778, 1735–1756. [DOI] [PubMed] [Google Scholar]
- Nega M, Dube L, Kull M, Ziebandt AK, Ebner P, Albrecht D, Krismer B, Rosenstein R, Hecker M, and Götz F (2015). Secretome analysis revealed adaptive and non-adaptive responses of the Staphylococcus carnosus femB mutant. Proteomics 15, 1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W (2003). The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 270, 2109–2119. [DOI] [PubMed] [Google Scholar]
- Novick RP (2003). Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48, 1429–1449. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Madureira P, Andrade EB, Bouaboud A, Morello E, Ferreira P, Poyart C, Trieu-Cuot P, and Dramsi S (2012). Group B streptococcus GAPDH is released upon cell lysis, associates with bacterial surface, and induces apoptosis in murine macrophages. PLoS One 7, e29963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V, and Chhatwal GS (2003). Housekeeping enzymes as virulence factors for pathogens. International journal of medical microbiology : IJMM 293, 391–401. [DOI] [PubMed] [Google Scholar]
- Pancholi V, and Fischetti VA (1992). A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med 176, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V, and Fischetti VA (1993). Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc Natl Acad Sci U S A 90, 8154–8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasztor L, Ziebandt AK, Nega M, Schlag M, Haase S, Franz-Wachtel M, Madlung J, Nordheim A, Heinrichs DE, and Götz F (2010). Staphylococcal major autolysin (atl) is involved in excretion of cytoplasmic proteins. J Biol Chem 285, 36794–36803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HL, Novick RP, Kreiswirth B, Kornblum J, and Schlievert P (1988). Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. Journal of bacteriology 170, 4365–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, and Otto M (2013). Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, and Otto M (2008). RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit DK, Endres JL, and Bayles KW (2011). Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. Journal of bacteriology 193, 2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KC, Firek BA, Nelson JB, Yang SJ, Patton TG, and Bayles KW (2003). The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. Journal of bacteriology 185, 2635–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, and Sitia R (1990). A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J 9, 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbald MJ, Ziebandt AK, Engelmann S, Hecker M, de Jong A, Harmsen HJ, Raangs GC, Stokroos I, Arends JP, Dubois JY, et al. (2006). Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev 70, 755–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siljamaki P, Varmanen P, Kankainen M, Sukura A, Savijoki K, and Nyman TA (2014). Comparative exoprotein profiling of different Staphylococcus epidermidis strains reveals potential link between non-classical protein export and virulence. J Proteome Res 13, 3249–3261. [DOI] [PubMed] [Google Scholar]
- Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, and van Strijp JA (2012). Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog 8, e1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalsma H, Antelmann H, Jongbloed JD, Braun PG, Darmon E, Dorenbos R, Dubois JY, Westers H, Zanen G, Quax WJ, et al. (2004). Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol Mol Biol Rev 68, 207–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, Wehmhoner D, Karst U, Dieterich G, Wehland J, and Jansch L (2005). Comparative proteome analysis of secretory proteins from pathogenic and nonpathogenic Listeria species. Proteomics 5, 1544–1557. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, et al. (2007). Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13, 1510–1514. [DOI] [PubMed] [Google Scholar]
- Winram SB, and Lottenberg R (1996). The plasmin-binding protein Plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology 142, 2311–2320. [DOI] [PubMed] [Google Scholar]
- Xia XX, Han MJ, Lee SY, and Yoo JS (2008). Comparison of the extracellular proteomes of Escherichia coli B and K-12 strains during high cell density cultivation. Proteomics 8, 2089–2103. [DOI] [PubMed] [Google Scholar]
- Yang CK, Ewis HE, Zhang X, Lu CD, Hu HJ, Pan Y, Abdelal AT, and Tai PC (2011). Nonclassical protein secretion by Bacillus subtilis in the stationary phase is not due to cell lysis. Journal of bacteriology 193, 5607–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CK, Zhang XZ, Lu CD, and Tai PC (2014). An internal hydrophobic helical domain of Bacillus subtilis enolase is essential but not sufficient as a non-cleavable signal for its secretion. Biochemical and biophysical research communications 446, 901–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebandt AK, Becher D, Ohlsen K, Hacker J, Hecker M, and Engelmann S (2004). The influence of agr and sigmaB in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 4, 3034–3047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.