Abstract
Trenbolone acetate is widely-used in some parts of the world for its desirable anabolic effects on livestock. Several metabolites of the acetate, including 17β-trenbolone (βTRB), have been detected in at low ng/L concentrations in surface waters associated with animal feedlots. The βTRB isomer can affect androgen receptor (AR) signaling pathways in various vertebrate species at comparatively low concentrations/doses. This paper provides a comprehensive review and synthesis of the existing literature concerning exposure to and biological effects of βTRB, with an emphasis on potential risks to aquatic animals. In vitro studies indicate that, although βTRB can activate several nuclear hormone receptors, its highest affinity is for the AR in all vertebrate taxa examined, including fish. Short-term exposures of fish to ng/l water concentrations of βTRB can cause changes in endocrine function, and adverse apical effects in longer exposures during development and reproduction. Impacts on endocrine function typically are indicative of inappropriate AR signaling, such as changes in sex steroid metabolism, impacts on gonadal stage, and masculinization of females. Exposure of fish to βTRB during sexual differentiation in early development can greatly skew sex ratios, while adult exposures can adversely impact fertility and fecundity. To fully assess ecosystem-level risks, additional research is warranted to address uncertainties as to the degree/breadth of environmental exposures and potential population-level effects of βTRB in sensitive species.
Keywords: 17β-Trenbolone, Exposure, Effects, Review
Background
In early 2016, the Society of Environmental Toxicology and Chemistry (SETAC) sponsored an international Pellston workshop focused on hazard- versus risk-based approaches for the ecological assessment of endocrine active chemicals (EACs; Matthiessen et al. 2017). For this comparative evaluation, workshop participants focused on several facets of EAC assessments, including linkage of mechanistic data indicative of altered endocrine function to adverse outcomes (Mihaich et al. 2017), limitations and proposed improvements in current screening and testing approaches (Coady et al. 2017), attributes of EACs that might complicate test interpretation and prediction of hazard/risk (Parrott et al. 2017), and population-level effects of EACs (Marty et al. 2017). To support and illustrate these analyses, relatively extensive literature reviews were conducted for six well-established EACs that affect different pathways, endpoints an d/or species, including an estrogen receptor agonist (17α-ethinylestradiol), a sex steroid synthesis inhibitor (propiconazole), a compound associated with the induction of intersex in in vertebrates (tributyltin), a thyroid-active substance (perchlorate), and chemicals that interact with the vertebrate androgen receptor (AR) as an antagonist (vinclozolin) or an agonist (17β-trenbolone, βTRB) (Matthiessen et al. 2017). Several of these chemicals have been the subject of detailed and, in some cases, relatively recent reviews in the open literature concerning their environmental fate and biological effects. One chemical that has not been recently and/or comprehensively assessed based on existing literature is βTRB. The purpose of this paper is to build upon the analysis started in conjunction with the Pellston workshop to develop a current, thorough review of the occurrence and potential effects of βTRB, with an emphasis on aquatic environments and species.
Trenbolone (TRB) acetate has been used for decades as anabolic steroid to enhance feed conversion and increase muscle mass in livestock (Neumann 1976a; 1976b; Roche and Quirke 1986; Meyer 2001). Although banned as a livestock supplement in parts of the world (e.g., European Union), the use of anabolic steroids for meat production in other areas of the world is extensive (Stephany 2010). For example, in the United States (US), the majority of beef cattle receive steroid implants as part of the finishing process (US Department of Agriculture 2000). TRB acetate also is a popular drug used illicitly by some body builders and athletes (Thevis et al. 2009; Quan et al. 2011). While TRB acetate typically is the administered form of the steroid, in the body, the molecule is rapidly hydrolyzed to form βTRB, a very high affinity ligand for the vertebrate AR (Neumann 1976b; Meyer and Rapp 1985; Wilson et al. 2002); it is the activation of the AR by βTRB that causes its anabolic properties. Several metabolites of TRB acetate, including βTRB, 17α-trenbolone (αTRB), and trendione have been detected in water samples, primarily associated with livestock operations, occasionally at biologically-relevant concentrations (e.g., Schiffer et al. 2001; Durhan et al. 2006; Kolodziej et al. 2007; Gall et al. 2011; Bartelt-Hunt et al. 2012; Khan and Lee 2012).
In addition to being a high potency AR agonist that can occur in aquatic systems, βTRB has been used extensively as a model androgen in studies focused in the development of EAC test methods, and research assessing toxicity pathways associated with AR activation. A major reason for this is that, unlike many other natural (e.g., androstenedione, testosterone) or synthetic (e.g., methyltestostrone) steroidal androgens, βTRB is not aromatized to estrogenic metabolites (Neumann 1976b). Consequently, βTRB produces a relatively unambiguous AR-mediated response suitable both for test method validation and mechanistic toxicology studies. One outcome of the use of βTRB as a model androgen is the existence of a comparatively large amount of information concerning its biological effects in a variety of non-mammalian species, especially fish. In this review, we focus on that knowledgebase, as well as relevant data concerning the fate and occurrence of βTRB and related metabolites in the environment. We also identify areas of uncertainty and associated research needs relative to the occurrence and potential environmental effects of βTRB. While the summarized information provided herein could support in-depth hazard or risk assessments for βTRB, the present paper is not intended to provide that level of analysis.
Literature Search and Evaluation
An intensive TRB literature search was conducted during summer of 2015 using a wide variety of search terms (Matthiessen et al. 2017), which yielded about 800 papers. Several additional papers from 2016 and 2017 were identified using a more targeted approach in PubMed. The abstracts of all papers were reviewed in order to identify those most relevant for further evaluation and summarization. Route of exposure, species, endpoints examined, and experimental design aspects were all considered relative to utility of a paper for assessing potential ecological effects. For example, several hundred of the identified papers dealt either with the efficacy of various forms of TRB in terms of biological responses in livestock (e.g., comparative weight gain) or residues detected in the flesh of treated livestock (from a human consumption perspective), and these were not considered relevant for assessing ecological effects. Similarly, we did not further consider studies that involved the illicit use of βTRB in humans. Following review of the abstracts for general relevance, approximately 160 papers were selected for more in depth evaluation. These references included those concerning environmental fate of, and potential exposure to metabolites of TRB acetate, as well as analyses of in vitro activity, and in vivo effects in ecologically-relevant animals.
Although our focus on effects in this review was primarily on non-mammalian species, some mammalian in vitro data also are included in the analysis which, based on conserved structural homology of nuclear hormone receptors such as the AR (LaLone et al. 2013), could be useful in terms of lending mechanistic insights into βTRB effects in a variety of vertebrate species.
Herein we summarize information from the papers identified as relevant to assessing potential environmental exposure and/or effects in aquatic species. Supplemental Information files associated with Matthiessen et al. (2017) describe content of most of the papers in detail, with respect to experimental design (e.g., full range of test concentrations, study conditions, etc.), and also present Klimisch scores for many of the studies, which are designed to evaluate possible utility of a given dataset for contributing to an assessment of risk (Klimisch et al. 1997). In the current paper, however, we focus on a briefer description of salient results from, and synthesis of the reviewed papers, rather than any sort of formal hazard or risk assessment.
Following an overview of knowledge concerning fate and exposure, information concerning biological effects of βTRB is presented in four broad categories: (a) in vitro studies focused on potentially affected pathways, most prominently AR signaling and potency, (b) in vivo work evaluating “mechanistic” endpoints reflective of direct impacts on the hypothalamic-pituitary-gonadal (HPG) axis, (c) in vivo studies assessing apical responses such as survival, growth, development and reproduction, most commonly used in population-level risk assessments, and (d) efforts to integrate mechanistic and apical responses to βTRB. We chose to present data in this manner because screening and testing programs for EACs focus not only upon perturbation of specific endocrine pathways of concern, but linkage of these perturbations to adverse apical effects (Matthiessen et al. 2017; Coady et al. 2017; Mihaich et al. 2017).
Summary of Available Literature
Environmental fate and exposure
The principle route of βTRB entering the environment is through runoff of wastes (urine and feces) from livestock dosed with TRB acetate (Lange et al. 2002; Kolok and Sellin 2008; Snow et al. 2013; Jones et al. 2014a, b). Hence, aquatic species would be most prone to unintended exposures. However, there is at least some potential for exposure in terrestrial environments, either through land-applied manure from treated animals (Lange et al. 2002, Jones et al. 2014a), or via aerial transport of TRB acetate metabolites associated with dust (Blackwell et al. 2011; 2015; Wooten et al. 2015).
Detection of αTRB, βTRB and/or trendione in run-off associated with cattle feedlots has been reported in several studies, typically at low ng/L concentrations, with the stereoisomer αTRB the most commonly detected and at the highest concentrations (Table 1). TRB acetate is predominantly excreted from cattle as αTRB (about 90% of dosed TRB acetate) with lesser quantities of βTRB (about 10%) and trendione (about 1%) (Blackwell et al. 2014). This is reflected in measured environmental concentrations from a number of studies where αTRB was greater than βTRB in liquid manure (0.4–1.8 versus 0.06–0.16 μg/kg), dung (4.7–75 versus 0.5–4.2 μg/kg), soil (11.8 versus 6.1 μg/kg), final effluent water (0.3–1.5 versus 0.02–0.1 μg/L) and run-off (<0.034 μg/L versus non-detectable) from cattle feedlots where TRB acetate had been used (Schiffer et al. 2001; Bartelt-Hunt et al. 2012, Khan and Lee 2012, Webster et al. 2012). Somewhat in contrast to these studies, Gall et al. (2011) measured maximum concentrations of βTRB similar to or even greater than those of αTRB (0.001–0.023 versus 0.003–0.162 μg/L) in run-off from fields fertilized with manure from TRB acetate-implanted cattle. Another run-off study, which featured a 2 y monitoring program, recorded a maximum measured concentration of βTRB of 270 ng/L, while not detecting αTRB (Bartelt-Hunt et al. 2012). Both the Gall et al. (2011) and Bartlet-Hunt (2012) studies are in contrast with other simulated cattle-dosing studies, where concentrations of αTRB were consistently higher than βTRB in wastes originating from dosed animals (Webster et al. 2012; Jones et al. 2014a–c).
Table 1.
Summary of concentrations of trenbolone acetate metabolites, 17β-trenbolone (βTRB), 17α-trenbolone (αTRB) and trendione, in field-collected samples
| TRB acetate metabolite | Matrix | Concentration range | Reference | |
|---|---|---|---|---|
| αTRB | Water | Feedlot run-off/lagoon | 0.005–1720 ng/L | (1) (2) (3) (4) (5) |
| water | 1.4–23 ng/L | (6) | ||
| Tile drain run-off Stream water | 0.002–50 ng/L | (1) (5) | ||
| Solids | Manure | 5–31 pg/kg | (2) (4) (7) | |
| Soil | 0.6–11.8 pg/kg | (4) (8) | ||
| Airborne Particulate Matter | NDa−26.5 pg/kg | (9) | ||
| βTRB | Water | Feedlot run-off/lagoon | 0.0015 ng/L- | (1) (2) (3) (4) (5) |
| water | 270 ng/L | (7) (8) | ||
| Tile drain run-off | 3.3–162 ng/L | (6) | ||
| Stream water | 0.0013 ng/L- <8 ng/L | (1) (5) | ||
| Solids | Manure | 0.5–4.3 pg/kg | (2) (4) | |
| Soil | 0.5–6.1 pg/kg | (4) | ||
| Airborne Particulate Matter | NDa−63 pg/kg | (9) | ||
| Trendione | Water | Feedlot run-off/lagoon | 5–180 ng/L | (2) (3) (4) (8) |
| water | 6.5–35.3 ng/L | (6) | ||
| Tile drain run-off | 0.008 ng/L | (5) | ||
| Lagoon water Stream water | 0.002–0.016 ng/L | (5) | ||
| Solids | Manure | 0.4–4.7 pg/kg | (2) | |
| Soil | 0.7–1.2 pg/kg | (4) | ||
| Airborne Particulate Matter | NDa−38.8 pg/kg | (9) | ||
not detectable, (1) Durhan et al. (2006), (2) Schiffer et al. (2001), (3) Khan and Lee (2012), (4) Webster et al. (2012), (5) Soto et al. (2004), (6) Gall et al. (2011), (7) Bartelt-Hunt et al. (2012), (8) Parker et al. (2012), (9) Blackwell et al. (2011)
Based on other fate assessments of TRB acetate metabolites, the βTRB isomer is expected to be less mobile and persistent compared with the other TRB acetate metabolites. For example, the affinity of βTRB for solids is expected to be greater than that of αTRB, based on a number of sorption studies (Khan et al. 2009; Card et al. 2012; Qu et al. 2014). Also, Qu et al. (2014) found that sorption of βTRB was less reversible than αTRB, suggesting a lesser degree of mobility of the former isomer in soils. In that same study, the degradation rate of βTRB was also greater than αTRB in a soil solution. Preferential degradation of βTRB has also been observed in other soil and water or sediment and water studies (Khan et al. 2008; Khan and Lee 2010; Khan and Lee 2012; Cole et al. 2015; Robinson et al. 2017b). Photolysis of TRB also appears to be isomer specific, with degradation of βTRB more rapid than αTRB and reversion of hydroxylated photoproducts (reported to be endocrine active) to the parent compounds occurring to a larger degree for αTRB (Kolodziej et al. 2013; Qu et al. 2013). Overall, based on the preponderance of evidence, we expect that the concentrations of βTRB sourced from TRB acetate would be less than those of αTRB in solution, based on the extent of excretion from cattle, relative mobility and affinity to solids in the environment, and relative environmental degradation rates of the α and β isomers.
Stereochemistry clearly is important for considering the overall environmental risk of TRB acetate, as in many instances αTRB appears likely to be the most prevalent metabolite in the environment. There is evidence, however, that αTRB may have a potency similar to βTRB for reproductive effects in the fathead minnow, possibly due in part to in vivo conversion of αTRB to βTRB (Jensen et al. 2006). This may not occur in all fish species though, as Robinson et al. (2017c) reported that αTRB was much less potent in medaka than in fathead minnows. Another complicating factor relative to assessing the overall risk of TRB acetate is the reported interconversion of trendione to βTRB under some conditions (Khan et al. 2008; Cole et al. 2015). Irrespective, however, of the exact nature of the chemistry leading to the occurrence of βTRB in aquatic environments or species, the isomer clearly can and does occur at low ng/L concentrations that, as described below, would be sufficient to cause biological effects in fish (e.g., Durhan et al. 2006; Khan and Lee 2012).
There has been some work concerning the bioconcentration, toxicokinetics and toxicodynamics of βTRB in non-mammalian species. For example, Ankley et al. (2003) noted a relatively low bioconcentration factor, on the order of 13, for fathead minnows exposed to βTRB for 21-d. Schultz et al. (2013) used single concentrations (generally 0.5 μg/L) to investigate toxicokinetics and toxicodynamics of βTRB in adult female fathead minnows and rainbow trout. In both species, βTRB was shown to have rapid uptake, reaching a steady state at approximately 8 h. Plasma concentrations suggested different toxicokinetics between the two species, with levels approximately two to six times higher in the trout than in the minnows. Depuration was also measured in rainbow trout and was quite rapid with a half-life of 14.5 h. Thus, changes in environmental concentrations would be predicted to be rapidly reflected in changes in fish plasma and other tissues.
Biological effects: In vitro studies
There is a substantial amount of information for βTRB in different in vitro systems with a variety of vertebrate species. In competitive receptor binding assays with a recombinant rat AR, βTRB displaced the strong synthetic androgen R1881, as well as the endogenous AR agonist 5α-dihydro-testosterone (DHT), with IC50 (chemical concentration producing a 50% inhibition in binding of the competing ligand) values in the range of 1.3 to 5.7 × 10−8 M (Kim et al. 2010). Studies with a recombinant human AR showed a similar binding affinity for βTRB relative to DHT (Bauer et al. 2000). In that same study, relative binding affinities for αTRB and trendione were approximately 5 and 0.4%, respectively, of that determined for βTRB (Bauer et al. 2000). The significantly lower affinity of αTRB compared to βTRB for the human AR is consistent with results from AR transcriptional activation assays using cell lines derived from human tissue (Blake et al. 2010). Further, as a whole, results of the βTRB binding assays with mammalian ARs are consistent with data obtained for the steroid in functionally equivalent AR transcriptional activation assays with mammalian cell lines (Blankvoort et al. 2001; Blake et al. 2010; Wilson et al. 2002). In support of these AR binding and transcriptional activation assays, Wilson et al. (2002) confirmed nuclear translocation of the AR after binding βTRB at a concentration as low as 10−12 M.
Competitive receptor binding and transcriptional activation assays performed with βTRB and AR receptors from fish species (fathead minnow, mosquitofish) have shown comparable values to mammalian assays (Wilson et al. 2004; Katsu et al. 2007). This result is not surprising given conservation of AR sequence homology across vertebrate species (LaLone et al. 2013). In studies with another fish species, βTRB was shown to induce AR-dependent spiggin production in stickleback kidney cell culture assay at a concentration as low as 10−10 M (Jolly et al. 2006). Work also has been published characterizing binding of βTRB to other nuclear receptors and binding globulins associated with endocrine function. For example, βTRB was shown to have a higher affinity for the bovine progesterone receptor than progesterone (Bauer et al. 2000). Conversely, βTRB has been shown to only poorly compete with DHT in binding to the human sex-hormone binding globulin, and failed to activate human glucocorticoid receptor-mediated gene transcription (Bauer et al. 2000; Wilson et al. 2002). Forsgren et al. (2014) assessed the relative estrogenicity of βTRB, αTRB and trendione using a rainbow trout cell line. Both βTRB and trendione showed some limited estrogenic activity, while αTRB did not (Kurauchi et al. 2008). Interestingly, at relatively high water concentrations, βTRB has been reported to exhibit estrogenic activity in fish in vivo, inducing vitellogenin (VTG; egg yolk protein precursor) in adult male fathead minnows (Ankley et al. 2003), and VTG mRNA in estrogen-sensitive, transgenic medaka larvae (Kurauchi et al. 2008).
A broader analysis of pathway-specific biological activities of βTRB was achieved via the USEPA ToxCast™ program, which employs a collection of high-throughput (HTP) cell-based and cell-free assays to screen chemicals for biological effects (www.epa.gov/ncct/toxcast/index.html; see Supplemental Information for detailed methods and results and the Data Availability statement at the end of this paper). The majority of the HTP assays are based on mammalian systems. Results from ToxCast™ assays typically are summarized as AC50 values, the chemical concentration required to cause 50% activity response in a given test system. In all, βTRB (accessed July 20, 2017) was evaluated for a total of 876 assay endpoints. βTRB showed activity (defined as calculable AC50 values) in 14.5% of the assays (127 positive hits), nine of which are designated as cytotoxicity assays in the ToxCast™ battery. Based on the results from these nine cytotoxicity assays, the lower cytotoxicity limit for βTRB was determined to be 2.29 μM. Those biological activities below the lower cytotoxicity limit are of most interest for determining specific activities of possible concern; βTRB had AC50 values below the lower cytotoxicity limit for assays related to the: AR, progesterone receptor, estrogen receptor, mineralocorticoid receptor, glucocorticoid receptor, and serine proteinase inhibitor, (see Supplemental Information Table S1). In further HTP testing focused specifically on estrogen, androgen and thyroid pathways of current regulatory concern (actor.epa.gov/edsp21/), βTRB was active in 11 of 12 AR-related assays, 14 of 17 estrogen-related assays, and one of three assays associated with thyroid function (see Supplemental Information Table S2). The one non-responsive AR assay measures receptor antagonism, so a lack of βTRB activity is not surprising. Notably, 10 of the AR assay AC50 values and nine of the estrogen receptor assay AC50 values were less than the lower cytotoxicity limit for βTRB, indicating specific activity in both pathways. The positive response in the thyroid battery had an AC50 value much higher than the lower cytotoxicity limit, suggesting that this result was likely non-specific.
Biological effects: In vivo studies with mechanistic endpoints
A wide variety of pathway-based mechanistic endpoints has been examined in aquatic species in response to βTRB exposure, including: changes in expression of genes, proteins and metabolites potentially sensitive to the actions of EACs, alterations in sex steroid concentrations, gonad and kidney histopathology, and variations in secondary sex characteristics. The majority of this information has been collected from different fish species. Endpoints such as changes in female plasma VTG, male gonad histopathology, and presence of male secondary sex characteristics in female fish all appear to be especially robust diagnostic endpoints that directly reflect the ability of βTRB to act as an AR agonist in vivo (Ankley and Jensen 2014).
A number of studies have evaluated changes in gene expression in βTRB-exposed fish at different life-stages, using both targeted (polymerase chain reaction [PCR]) and non-targeted (e.g., microarray) techniques (Hook et al. 2006; Garcia-Reyero et al. 2009; Dorts et al. 2009; Ekman et al. 2011; Brockmeier et al. 2013b; Leet et al. 2015; Mizukami-Murata et al. 2016). For example, Leet et al. (2015) observed down-regulation of several genes involved in steroid synthesis in larval fathead minnows exposed to βTRB, consistent with a compensatory response to over-stimulation of the HPG axis by exogenous steroids. Ekman et al (2011) reported analogous results for several HPG-responsive genes in adult fathead minnows exposed to βTRB during a time-course study. This over-stimulation and resultant compensation could also explain the observation by Sone et al. (2005) that mRNA expression of AR genes was up-regulated by βTRB in the anal fin of adult female Western mosquitofish after 3 d of exposure but not after 28 d. Dorts et al. (2009) demonstrated downregulation of liver VTG and brain aromatase transcripts in fathead minnows exposed to βTRB. Collectively, the preponderance of transcriptomic responses in the Dorts et al. (2009) study indicated that genes in the fathead minnow ovary were generally down-regulated, supporting the inhibitory effect of androgens on estrogen-related functions in the ovary. Garcia-Reyero et al. (2009) used a microarray approach to evaluate ovarian gene expression in βTRB-exposed fathead minnows and noted several up- and down- regulated genes, the majority of which were not seemingly directly related to AR interactions. Similarly, Brockmeier et al. (2013b) used a custom microarray to investigate transcriptomic responses in the liver of female mosquitofish exposed to βTRB for 14 d. Many metabolic processes not necessarily directly linked to the AR were increased in response to βTRB exposure, including lipid metabolic processes, regulation of protein metabolic processes, lipoprotein metabolic processes, cholesterol biosynthesis processes, and cholesterol transport and metabolism. Finally, Mizukami-Murata et al. (2016) used both microarray and PCR measurements to evaluate the effects of βTRB on 10 genes related to cholesterol synthesis and observed an induction of these genes which could be a secondary response to depletion of cellular cholesterol stores.
There have been several non-targeted proteomic and metabolomic studies with βTRB-exposed fathead minnows (e.g., Collette et al. 2010; Ekman et al. 2011; Martyniuk et al. 2009) that have yielded results qualitatively similar to those of Garcia-Reyero et al. (2009), in that the majority of the observed protein or metabolite changes could not be directly related to known AR-signaling pathways. Martyniuk and Denslow (2012) conducted a review of the ‘omic’ responses of several known androgenic chemicals in teleost fish and noted the difficulty in separating molecular responses that are a result of general toxicity from those reflecting activation of the AR. In the case of βTRB, significant cell processes that were affected at the level of the proteome included lymphocyte differentiation, xenobiotic clearance, low-density lipid oxidation, proliferation of smooth muscle cells, permeability of blood vessels, and DNA degradation.
A number of studies have documented decreases in sex steroid synthesis and/or plasma steroid concentrations in fish of both sexes exposed to βTRB (e.g., Ankley et al. 2003; Garcia-Reyero et al. 2009; Boettcher 2012; Ekman et al. 2011; Zhang et al. 2008; Shultz et al. 2013; Massart et al. 2015). Steroids reported as affected include testosterone (T), 11-ketotestosterone (KT) and 17β- estradiol (E2). Down-regulation of steroid synthesis is consistent with feedback inhibition in the HPG axis associated with over-stimulation by an exogenous androgen. Concurrent with decreased steroid synthesis in adult females are observations of decreased plasma VTG levels (or hepatic expression of VTG mRNA) associated with βTRB exposure, consistent with decreased activation of the estrogen receptor by E2 (Ankley et al. 2003; Ankley et al. 2004; Ankley et al. 2010; Miracle et al. 2006; Martinovic et al. 2007; Ekman et al. 2011; Zhang et al. 2008b; Schultz et al. 2013).
Based on the mode of action of βTRB, it is expected that, in general, gonads would be a primary target organ for effects observed at the histopathological level. But, the type and magnitude of effects on the gonads is highly dependent upon the timing and duration of exposure. For example, organisms undergoing active sexual differentiation and gonadal development are oftentimes particularly sensitive to EACs (Ankley and Johnson 2004). The presence of ovotestes (i.e., intersex tissue) has also been observed in fish (Sone et al. 2005) and amphibians (Olmstead et al. 2012) exposed to βTRB during critical windows of development. Exposure to βTRB also has been shown to affect facets of testicular development (Wolffian and Müllerian ducts) in amphibians (Xenopus tropicalis, X. laevis) exposed during early life-stages (Olmsted et al. 2012; Haselman et al. 2016). Various effects of βTRB on ovarian tissue have been observed in female fish exposed as adults, including oocyte atresia (Ankley et al. 2003; Cripe et al. 2010; Hemmer et al. 2008), mild to severe fibrosis (Boettcher 2012), and abnormal oocyte development and ovarian degeneration (Cripe et al. 2010). In adult female medaka exposed to βTRB, αTRB, or trenedione for 14 d, there was a decrease in the percentage of primary ovarian follicles in the treated fish relative to controls (Forsgren et al. 2014). Histopathological effects of βTRB on testicular tissue have also been observed in adult exposures of several species of fish, in which signs of increased maturity in the testes are a common pattern. For example, male zebrafish exposed to βTRB during had testes that were larger relative to controls and contained more spermatozoa (Baumann et al. 2014; Orn et al. 2006). Increased spermatozoa (and fewer spermatogonia) were also observed among adult male medaka exposed for 7 d to βTRB (Park et al. 2009). Additionally, adult male fathead minnows exposed to βTRB over 21 d had testes with a thinned germinal epithelial and greatly expanded, sperm-filled lumens relative to controls (Ankley et al. 2003).
In addition to gonads, TRB can affect the histology of other tissues in aquatic vertebrates. For example, at a relatively large water concentration (1.26 μg/L), increased kidney epithelial height was noted among bullhead fish exposed to trenbolone acetate (Villeret et al. 2013). As a general recommendation for future studies with TRB, it would be desirable to assess histopathological effects on the liver and kidneys as an indication of potential systemic effects, although changes in these tissues likely would occur only at concentrations higher than those associated with effects in the gonads.
Several studies have demonstrated morphological masculinization of adult female fish exposed to water-borne βTRB (e.g., nuptial tubercle formation in fathead minnows, Ankley et al. 2003; 2004; Ankley et al. 2010; Martinovic et al. 2007; Seki et al. 2006; anal fin elongation in Eastern and Western mosquitofish; Sone et al. 2005; Brockmeier et al. 2013a). This is, of course, highly diagnostic of activation of the AR (Ankley and Jensen 2014). However, the actual physiological consequences of masculinization of adult females on reproductive function is uncertain.
Finally, it is worth noting that there have been a number of mixture studies with fish that have used βTRB as a model AR agonist to investigate interactions with known/possible AR antagonists like flutamide, vinclozolin and bisphenol A (e.g., Ankley et al. 2004; Ankley et al. 2010; Martinovic et al. 2007; Garcia-Reyero et al. 2009; Hogan et al. 2008; Hogan et al. 2012; Collette et al. 2010; Ekman et al. 2012; Sun et al. 2016; Villeneuve et al. 2017). These studies typically examine the ability of a putative AR antagonist to “block” a known AR-mediated effect of βTRB, such as masculinization of females. While these types of mechanistic studies can be very useful in demonstrating how a chemical interacts with the AR in vivo, their results typically are of little direct utility in assessing the potential environmental hazard/risk of βTRB, as the work usually employs just one, fairly large test concentration of the androgen.
Biological effects: In vivo studies with apical endpoints
The effects of βTRB on different facets of survival, reproduction and development have been evaluated in several fish species. These studies are particularly important for providing endpoints that could serve as the basis for population-level risk assessments.
Sex steroids, in general, have relatively minimal effects on survival of fish, with acute (lethality)/chronic toxicity ratios of several thousand (Ankley et al. 2005). Consistent with this, Ankley et al. (2003) noted no significant effects of up to 50 μg βTRB/L on survival of adult male or female fathead minnows in a 21-d exposure. Similarly, Holbech et al. (2006) reported no significant effects on survival of embryonic/larval zebrafish exposed to 3 μg βTRB/L for about 60 d, and Seki et al. (2006) reported no effects on survival up to 5 μg βTRB /L following exposure of adult medaka and fathead minnows for 14–21 d. Amphibian toxicity studies with βTRB have reported effects on survival at concentrations lower than those not affecting fish (Olmstead et al. 2012; Li et al. 2015). In these studies, significantly increased mortality of tadpoles/metamorphs was noted at 0.1 – 0.3 μg/L βTRB via hypothesized effects on the developing larynx. Laryngeal development in frogs is a sexually dimorphic process, and the formation of a larynx capable of male calling behavior is androgen-dependent (Kelley et al. 1989). When tadpoles were exposed to βTRB this seemingly led to laryngeal hypertrophy and restricted air passage to the lungs. This was hypothesized to facilitate an accumulation of air in the digestive tract of the developing frogs and eventual suffocation (Olmstead et al. 2012; Li et al. 2015).
There have been several reports of βTRB affecting growth of fish. Most of these have involved increases in the size (especially weight) of exposed organisms, likely due to the anabolic nature of βTRB. For example, Ankley et al. (2003) found a concentration-dependent increase in the weight of adult female fathead minnows exposed to βTRB for 21 d. Similarly, Hemmer et al. (2008) found that a 21-d exposure of sheepshead minnows to 5 μg βTRB/L significantly increased the weight of adult females. The anabolic effects of βTRB appear not to be limited to adult fish. For example, Baumann et al. (2014) reported that the growth of zebrafish of both sexes was increased by exposure to low levels of βTRB (0.003 μg/L) in a 60 d embryo-larval design.
Endocrine-active chemicals, such as βTRB, typically cause their most pronounced apical effects in fish during two windows of susceptibility--early sexual development/differentiation and active reproduction (Ankley and Johnson 2004). Research has shown that sexual differentiation is indeed a very sensitive window in fish exposed to βTRB (Iguchi et al. 2007). For example, six studies with zebrafish covering the period of sexual differentiation consistently report skewed sex ratios, with occurrence of all male populations at about 0.01 μg βTRB /L (Holbech et al. 2006, Larsen et al. 2010; Morthorst et al. 2010; Boettcher 2012; Baumann et al. 2013; 2014). Three of these studies investigated reversibility of the masculinization during long-term recovery periods in clean water, and all found that the phenotypic sex change was irreversible.
Permanently skewed sex ratios would, of course, have substantial implications in terms of maintaining sustainable fish populations (Marty et al. 2017). In a comparative study, Orn et al. (2006) investigated VTG, sex ratio, and gonad morphology in zebrafish and Japanese medaka exposed to βTRB for 60 d post hatch. Production of phenotypic males was more sensitive in zebrafish (100% males at 0.05 μg/L) compared to medaka (no effect at 0.05 μg/L). Mizukami-Murata et al. (2016) confirmed in a developmental study (exposure from 0 to 60 d post-fertilization) the relative insensitivity of Japanese medaka, with phenotypic masculinization occurring only at concentrations greater than 0.1 μg βTRB/L. The enhanced sensitivity to βTRB during sex differentiation in zebrafish could be a consequence of the extended period of juvenile hermaphroditism that occurs in this species compared to medaka. Early-life stage exposures with amphibians also produced male-skewed cohorts in X. tropicalis and Pelophylax nigromaculatus at βTRB test concentrations of 0.078 and 0.1 μg/L, respectively (Olmstead et al. 2012; Li et al. 2015), although similar concentrations did not affect phenotypic sex ratios in X. laevis (Haselman et al. 2016).
Aquaculture studies using comparatively high concentrations of TRB acetate either in the diet or during brief aqueous immersions have also demonstrated effects on sexual differentiation/sex ratios in a wide range of fish species, including bluegill, black crappie, Nile tilapia and channel catfish (Galvez et al. 1996; Al-Ablani and Phelps 2002; Arslan and Phelps 2004, Bart et al. 2003, Davis et al. 2000). These types of treatments typically are conducted in juvenile fish to purposefully produce populations with skewed sex ratios. Depending on the exposure regime and doses, up to 100% phenotypic male populations can be achieved. Of interest is a study by Davis et al. (2000) which showed that, although exposure to TRB acetate seemingly resulted in all male populations, the treatment did not totally masculinize the catfish from a behavioral perspective. Resultant phenotypic males paired with untreated females mated and produced spawns; however, the eggs were not fertile and did not develop.
A second window of biological susceptibility to EACs is active reproduction. Ankley et al. (2003) conducted a 21-d fathead minnow reproduction assay and observed decreases in egg production at a βTRB water concentration as low as 0.027 μg/L. In a subsequent analysis, Miller and Ankley (2004) developed a fathead minnow population model and utilized data from Ankley et al. (2003) to project that fish continually exposed to this concentration would have an average equilibrium population size that approached zero in a long-term exposure. Notably, mechanistic endpoints (morphological masculinization and decreased plasma VTG in females) measured in that same study exhibited significant effects at the same test concentration affecting egg production. In 7-d reproduction studies with another fish species, Japanese medaka exhibited decreased egg production at βTRB water concentrations of 0.5 (Park et al. 2009) and 5 μg/L (Zhang et al. 2008a).
There have been several longer-term studies with βTRB in fish that have encompassed both stages of susceptibility—early sexual development and active reproduction—in some instances in multiple generations. Cripe et al. (2010) conducted a three-generation βTRB study with sheepshead minnows, an euryhaline species. Reproduction was consistently the most sensitive parameter measured. Reproductive rate (egg production per day per female) and cumulative egg production both were significantly reduced at water concentrations of 0.87, 0.13 and 0.027 μg βTRB/L in the F0, F1 and F2 generations, respectively. These data suggest the possibility of adverse effects occurring at lower concentrations in the F1 and F2 generations compared to F0. The effects of βTRB also were evaluated in a multigenerational toxicity study with the Japanese medaka (USEPA 2013; Flynn et al., 2017). In that study, fecundity was significantly reduced in the F0 and F1 generations at βTRB concentrations of 0.084 and 0.032 μg/L, respectively. No eggs were produced in F2 generation fish exposed to 0.032 μg βTRB/L (USEPA 2013; Flynn et al. 2017). Finally, Boettcher (2012) conducted a test with zebrafish starting with adults exposed to βTRB for 21 d (F0), continuing through an F1 generation, and culminating when the F2 generation reached 35 d post-hatch. There were a variety of effects, most notably a skewed sex ratio toward males in the F1 generation at a βTRB concentration of 0.004 μg/L.
Biological effects: Integrating responses across biological levels of organization
Screening and testing programs throughout the world have been implemented to address possible human health and ecological effects of EACs, including chemicals that activate the AR. Although these programs differ with regard to scope, data requirements, and assessment endpoints, all require that biologically-plausible linkages be made between impacts on endocrine function and actual adverse outcomes. Historically, chemical hazard or risk assessments have focused only on adverse apical outcomes (e.g., reduced survival, growth/development, reproduction) associated with exposure to chemicals of concern, rather than the mechanistic basis of the undesired effects. Consistently establishing this linkage for potential EACs has proven challenging (Mihaich et al. 2017). One approach to addressing this challenge has been to employ assays that directly measure both mechanistic and apical responses; however, the types of tests needed for the latter (long-term assays with sublethal endpoints) can be resource-intensive, particularly if there is a need/desire to assess large numbers (hundreds, thousands) of chemicals. An alternative to direct evaluation of adverse effects in long-term tests is to employ adverse outcome pathways (AOPs), in conjunction with mechanistic data, to make the critical linkage between molecular or biochemical changes associated with perturbation of the HPG axis, and possible apical effects (Ankley et al. 2010; Mihaich et al. 2017). This requires, of course, robust AOPs for endocrine activities of scientific and regulatory concern.
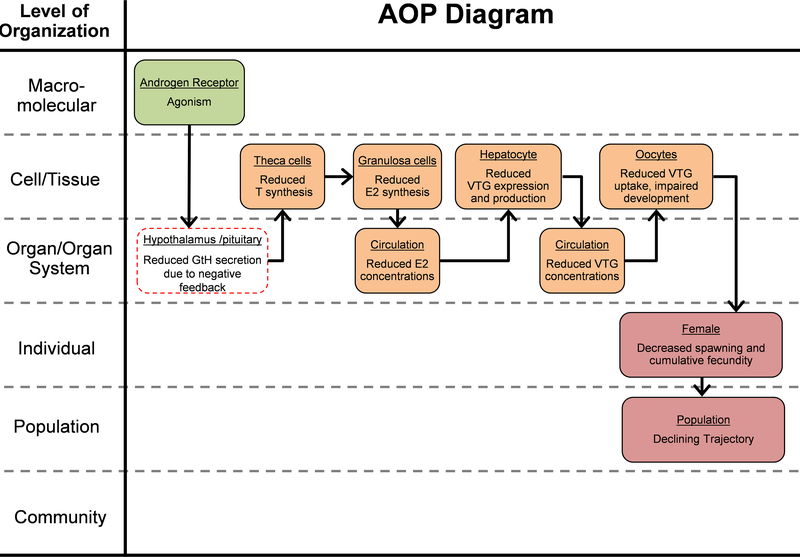
Based on the substantial amount of work that has been conducted with βTRB in spawning female fish it has been possible to derive a robust AOP that establishes clear, causal linkages across biological levels of organization, starting with activation of the AR, resulting in reduced sex steroid and, consequently, VTG synthesis, culminating in decreased egg production (Figure 1). In considering cross-species conservation of the AR (LaLone et al. 2013) as well as structure/function of the HPG axis (Norris and Carr 2014), it is likely that aspects of this basic AOP are applicable not only to many fishes, but other oviparous vertebrates as well. Further, based on recent work defining predictive relationships for key events in this AOP from steroid synthesis to egg production, it is possible to contemplate development of a quantitative AOP, whereby in vitro measures of AR activation by an otherwise untested chemical could be directly translated into potential effects on egg production (Li et al. 2011a; Li et al. 2011b; Conolly et al. 2017). Of course, the consequences of activation of the AR in terms of adverse effects of regulatory significance will be both species- and life-stage dependent. For example, based on the studies described above, exposure of many fish species to a strong AR agonist such as βTRB during sexual development/differentiation can result in phenotypic sex ratios skewed toward males, a response that could have population-level consequences (Hazlerigg et al. 2014; Marty et al. 2017). While there is not yet an AOP available for this developmental outcome, there are sufficient physiological and toxicological data whereby one could be developed. In particular, the many high-quality, early life-stage studies with zebrafish exposed to βTRB would provide a logical basis for derivation of an AOP focused on sexual differentiation (Holbech et al. 2006, Larsen et al. 2010; Morthorst et al. 2010; Boettcher et al. 2011; Baumann et al. 2013; 2014).
Figure 1.
Adverse outcome pathway (AOP) linking activation of the androgen receptor to decreased reproductive output in fish (https://aopwiki.org/aops/23). GtH=gonadotrophic stimulating hormone; T=testosterone; E2=17β-estradiol; VTG=vitellogenin
Synthesis of Current Knowledge, Identification of Uncertainties and Data Needs
Fate and exposure
Data from different studies confirm that a number of TRB acetate metabolites, including βTRB, can occur in surface water at concentrations of potential biological significance. Given known use patterns, the majority of these monitoring studies have been conducted in conjunction with livestock operations known or likely to be using TRB acetate. However, even for sites impacted by livestock wastes, the overall knowledgebase concerning the possible occurrence of βTRB is inadequate in several regards. For example, only a relatively limited number of sites actually have been assessed; to this end, a survey of βTRB incidence in environmental samples (water, soil, manure) from a more diverse representation of a larger number of livestock operations using TRB acetate would be very useful. This ideally would include livestock operations in countries other than the US, where the majority of monitoring work to date has been done. Additional empirical data concerning the persistence of βTRB under different use/degradation scenarios and climatological conditions also would be valuable. For example, while recent studies suggest that aerial transport of βTRB can occur (Blackwell et al. 2015), data concerning this phenomenon are relatively limited. Basically, at present, there are insufficient empirical data to conduct a robust probabilistic assessment of exposure for aquatic organisms to βTRB.
Another uncertainty concerning exposure assessment involves the possible conversion of other TRB acetate metabolites to βTRB. For example, based on in vitro data, both trendione and αTRB appear to be less potent AR agonists than βTRB. However, there is evidence that trendione can be converted to βTRB under certain environmental conditions (Khan et al. 2008; Cole et al. 2015), and that αTRB can be transformed to βTRB by fish in vivo (Jensen et al. 2006). If either of these scenarios is widespread, approaches to considering the integrated ecological risk of TRB acetate would be required. For example, some recent ecological assessments of possible risks of TRB acetate have focused more (or solely) on αTRB, as the prevalent environmental metabolite of the drug (Zoetis, 2014 Robinson et al. 2017a; 2017b 2017c). This type of approach could quite possibly underestimate hazards and risks of TRB acetate.
Effects: Mechanism of action, susceptible species and sensitive endpoints
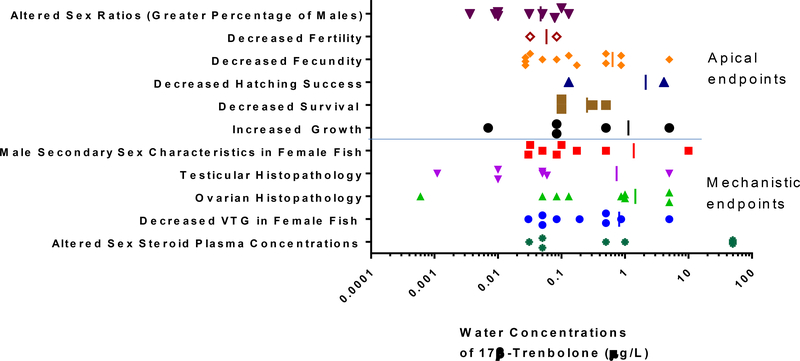
Both in vitro (receptor binding, transcriptional activation) and mechanistic in vivo studies strongly support that βTRB is a potent AR agonist. High-throughput in vitro studies evaluating a wide variety of biological pathways suggest that additional βTRB interactions are largely limited to nuclear hormone receptors, including the estrogen, progesterone, mineralocorticoid and glucocorticoid receptors. It is not uncommon for steroidal compounds to exhibit some degree of in vitro cross-reactivity with a wide variety of nuclear hormone receptors. Based on mechanistic in vivo data, however, the most sensitive endpoints affected are seemingly related to AR signaling, as opposed to pathways associated with the other receptors. Figure 2 shows the lowest observable effect concentrations for βTRB for a range of mechanistic and apical endpoints from in vivo fish and amphibian toxicity studies. Notably, effects on gonadal histopathology, increased growth, and altered sex ratios (toward greater percentages of males) are all sensitive endpoints in response to βTRB exposure. Overall, based on available in vitro and in vivo data, βTRB is expected to elicit its most sensitive biological effects through AR activation.
Figure 2:
Lowest observable effect concentrations for a variety of mechanistic and apical endpoints from in vivo fish and amphibian toxicity studies with 17β-trenbolone.
Based on evidence of interactions of βTRB with the AR, it is possible to identify those species most likely to be affected by low-level exposures to the chemical. Specifically, comparative evaluation of protein sequence information across roughly 40,000 species represented in the NCBI database indicates that vertebrates would be the most sensitive to androgens like βTRB (LaLone et al. 2013). There is little evidence for a functional AR in invertebrates, plants, or bacteria, suggesting that these taxonomic groups would be comparatively insensitive to βTRB (LaLone et al. 2013). Limited empirical data support this hypothesis. For example, as noted above, fish and amphibians can be affected by aqueous concentrations of the androgen in the range of 0.001 to 0.01 μg/L, but reproduction in the New Zealand mud snail was unaffected by a 28-d exposure to βTRB at test concentrations as high as 1 μg/L (Geiss et al. 2017). Similarly, full life-cycle (7 d) studies with Ceriodaphnia dubia found reproductive effects in the cladoceran only at a βTRB concentration of 50 μg/L (C. LaLone, USEPA, unpublished data). We were unable to identify data concerning possible sensitivity of plants to βTRB, but Radl et al. (2005) evaluated effects of a one-time dose of 15 μg βTRB/L on a microbial community, noting a slight change in function (N-acetyl glucosamine activity) but not in genetic composition after 19 d.
While more data confirming the possible effects (or lack thereof) of βTRB in non-vertebrate taxa might be useful, additional information concerning the sensitivity of different classes of potentially-sensitive vertebrates to the androgen likely would be more important to conducting robust assessments of ecological risk. A comparatively rich toxicology dataset exists for a variety of fish species, including multigenerational tests, but there is far less information concerning possible effects of βTRB in different amphibian species. And, even less is known about the toxicity of βTRB in non-mammalian vertebrates who might be exposed via contact with soil or ingestion of organisms (e.g., invertebrates) contaminated with βTRB. There have been some studies in which Japanese quail were exposed to βTRB either via the diet or egg injection (e.g., Henry et al. 2012; Quinn et al. 2007a; Quinn et al. 2007b; Karouna-Renier et al. 2017). The most thorough of these was by Karouna-Renier et al. (2017) who conducted a relatively-intensive three-generation assay at doses ranging from 1 to 40 mg βTRB/kg feed. They reported significant decreases in egg production in the F0 and F1 generations at βTRB doses of 1 to 5 mg/kg and higher (egg production was not evaluated in the F2), as well as changes in l evels of circulating steroids and gene expression consistent with compensatory responses in the HPG axis in response to βTRB exposure. Work with additional avian species that might be exposed to βTRB in a realistic environmental setting (e.g., insectivores associated with aquatic systems) may be warranted. Finally, we were unable to identify any reptile studies with βTRB, which is consistent with the relative paucity of toxicity data for any chemicals in this vertebrate class.
A variety of endpoints at multiple biological levels of organization have been evaluated in non-mammalian vertebrates (particularly fish) exposed to βTRB. As described above, studies examining changes in global gene expression have found that βTRB affects an assortment of pathways, many of which may not be related to HPG function; however, it can be difficult to ascertain which changes in gene expression are related to direct versus indirect (secondary) effects. Studies focused on βTRB-mediated responses of specific genes, proteins and/or metabolites likely related to the HPG axis typically have found patterns consistent with exposure to a strong androgen, in the context of both direct and compensatory responses (Ankley et al. 2003; Ekman et al. 2011; Ankley and Villeneuve 2015). For example, exposure to βTRB decreases sex steroid synthesis, likely through feedback inhibition which, in turn decreases production of VTG in adult females (Figure 1). These alterations subsequently appear to result in a compensatory upregulation of gene products associated with steroid synthesis (Ekman et al. 2011; Karouna-Renier et al. 2017). So, while responses of mechanistic endpoints associated with HPG function clearly can be highly dynamic, they nonetheless often are consistent with expectations from an HPG systems perspective (Villeneuve et al. 2007).
Apical responses to βTRB in aquatic vertebrates also are largely consistent with expectations relative to exposure to a potent androgenic steroid (Figure 1). For example, sensitive apical effects such as altered sex ratio and decreased fecundity are associated with exposures occurring during sexual differentiation/development and active reproduction, respectively. Effects on somatic growth (including increases) can be also be observed in βTRB-exposed animals, but these typically occur at higher doses that those impacting sexual development or reproduction (Figure 2). Effects on survival of βTRB-exposed fish would appear to be the least sensitive of any of the routinely-monitored apical endpoints in fish studies, however the limited βTRB toxicity information available for developing amphibians indicate that survival may be a sensitive apical endpoint for these organisms, likely due to the specific responsiveness of the androgen-sensitive laryngeal tissue during development. Although most βTRB toxicity information for non-mammalian species is from fish, apical effects of the androgen in both amphibians and birds are generally consistent with the larger fish dataset. However, additional data concerning βTRB effects on apical endpoints in under-represented vertebrate classes (amphibians, birds, reptiles) could reduce uncertainties in assessing ecological risk of the androgen.
An additional uncertainty involves the interpretation of behavioral and immunological endpoints and their relationship to potential adverse effects of EACs such as βTRB. For example, Bertram et al. (2015) reported an alteration in mating behavior in guppies exposed to βTRB which they felt could affect male reproductive success. Saaristo et al. (2013) and Tomkins et al. (2016) observed changes in female mating behavior in Eastern mosquitofish and guppies at comparatively low βTRB concentration of 0.006 and 0.004 μg/L, respectively. Also, Heintz et al. (2015) noted that exposure to βTRB affected the risk-taking behavior in both male and female guppies. Tompkins et al. (2017) observed more frequent aggressive behaviors, as well as less courting and more “sneak” mating attempts among male guppies exposed to 0.008 μg/L βTRB. In terms of immune function, several authors have noted that TRB acetate might cause relevant adverse effects in fish and birds (Quinn et al. 2007b; Bado-Milles et al. 2014; Massart et al. 2015). Neither behavioral nor immunological endpoints have been commonly used in ecological assessments of contaminant effects, but given the role of the endocrine system in both processes (Milla et al. 2011; Norris and Carr 2014), this could be an important oversight relative to assessing potential impacts of EACs.
Effects: Mixture considerations and population-level responses
There are other data gaps that result in uncertainty relative to a robust effects assessment of the potential ecological risks of βTRB. One involves the fact that the primary metabolites of TRB acetate in the environment will consist not only of βTRB, but αTRB and trendione, both of which may be able to interact—at least to some extent—with the AR in exposed animals. Complicating this, as described above, is the fact that the forms may be interconvertible to some degree. There have been a couple short-term fish reproduction studies with αTRB (Jensen et al. 2006; Robinson et al. 2017c), but little is known concerning possible biological effects of trendione. Additional toxicity data in non-target species for both of these TRB acetate metabolites would better enable consideration of their effects as a component of a mixture with βTRB.
Finally, available data concerning the occurrence of βTRB in aquatic systems adjacent to livestock operation, although limited, indicate that it can occur at concentrations that overlap those causing adverse effects on developmental and reproductive endpoints in fish in a laboratory setting. Further, simple population modeling based on laboratory data indicate that a sustained exposure to βTRB would cause long-term population-level effects in fish (Miller and Ankley 2004; Hazlerigg et al. 2014). However, whether fish population responses to βTRB might actually be occurring in a field setting is entirely unknown. Determination of this is, of course, a difficult challenge and one hardly unique to βTRB. Ascertaining effects of EACs in general on wildlife populations is an ongoing uncertainty in the field (Marty et al. 2017).
Supplementary Material
Acknowledgment
We thank B. Blackwell for helpful review comments on an earlier version of the manuscript, and D. Villeneuve for providing a graphical version of the AR AOP. We also thank the organizers and sponsors of the SETAC Pellston conference that resulted in this analysis/paper (see Matthiessen et al. 2017 for details). This paper has been reviewed in accordance with USEPA policy.
Footnotes
Supplemental Information
Supplemental information for the paper is available on the Wiley Online Library at DOI: 10.1002/etc.xxxx
Disclaimer
The views and statements expressed in this paper are those of the authors alone, and do not necessarily represent the views of the organisations to which the authors are affiliated, so those organisations cannot accept any responsibility for such views or statements.
Data Availability Statement
ToxCast™ is the United States Environmental Protection Agency’s (EPA’s) publicly available high-throughput toxicity data on thousands of chemicals. ToxCast™ is part of the Toxicology in the 21st Century (Tox21) federal collaboration. All the computational toxicology data are publicly available for anyone to access and use. EPA’s computational toxicology data are considered “open data”, and thus are free of all copyright restrictions, and fully and freely available for both non-commercial and commercial use.
References
- Al-Ablani SA, Phelps RP. 2002. Paradoxes in exogenous androgen treatments of bluegill. J Appl Ichthyol 18:61–64. [Google Scholar]
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, Henry TR, Denny JS, Leino RL, Wilson VS, Cardon MC, Hartig PC, Gray LE. 2003. Effects of the androgenic growth promoter 17β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem 22:1350–60. [PubMed] [Google Scholar]
- Ankley GT, Johnson RD. 2004. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. Inst Lab Animal Res J 45:469–483. [DOI] [PubMed] [Google Scholar]
- Ankley GT, DeFoe DL, Kahl MD, Jensen KM, Miracle A, Hartig P, Gray LE, Cardon M, Wilson V. 2004. Evaluation of the model anti-androgen flutamide for assessing the mechanistic basis of responses to an androgen in the fathead minnow (Pimephales promelas). Environ Sci Technol 38:6322–6327. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Black MC, Garric J, Hutchinson TH, Iguchi T. 2005. A framework for assessing the hazard of pharmaceutical materials to aquatic species In Williams R, ed, Science for Assessing the Impacts of Human Pharmaceutical Materials on Aquatic Ecosystems, Williams R, Ed. SETAC Press, Pensacola, FL, USA: pp 183–238. [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL. 2010a. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Kahl MD, Durhan EJ, Makynen EA, Cavallin JE, Martinovic D, Wehmas LC, Mueller ND, Villeneuve DL. 2010b. Use of chemical mixtures to differentiate mechanisms of endocrine action in small fish model. Aquat Toxicol 99:389–396. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM. 2014. A novel framework for interpretation of data from the fish short term reproduction assay (FSTRA) for the detection of endocrine-disrupting chemicals. Environ Toxicol Chem 33:2529–2540. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Villeneuve DL. 2015. Temporal changes in biological responses and uncertainty in assessing risk of endocrine-disrupting chemicals: Insights from intensive time-course studies with fish. Toxicol Sci 144:259–275. [DOI] [PubMed] [Google Scholar]
- Arslan T, Phelps RP. 2004. Production of monosex male black crappie, Promoxis nigromaculatus, populations by multiple androgen immersion. Aquaculture 234:561–573. [Google Scholar]
- Bado-Nilles A, Techer R, Porcher JM, Geffard A, Gagnaire B, Betoulle S, Sanchez W. 2014. Detection of immunotoxic effects of estrogenic and androgenic endocrine disrupting compounds using splenic immune cells of the female three-spined stickleback, Gasterosteus aculeatus (L.). Environ Toxicol Pharmacol 38:672–83. [DOI] [PubMed] [Google Scholar]
- Bart AN, Athauda ARSB, Fitzpatrick MS, Contreras-Sanchez WM. 2003. Ultrasound enhanced immersion protocols for masculinization of Nile tilapia, Oreochromis niloticus. J World Aquaculture Soc 34:210–216. [Google Scholar]
- Bartelt-Hunt SL, Snow DD, Kranz WL, Mader TL, Shapiro CA, van Donk SJ, Shelton DP, Tarkalson DD, Zhang TC. 2012. Effect of growth promotants on the occurrence of endogenous and synthetic steroid hormones on feedlot soils and in runoff from beef cattle feeding operations. Environ Sci Technol 46:1352–1360. [DOI] [PubMed] [Google Scholar]
- Bauer ERS, Daxenberger A, Petri T, Sauerwein H, Meyer HHD. 2000. Characterization of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS 109 (Suppl 103): S452–S460. [DOI] [PubMed] [Google Scholar]
- Baumann L, Holbech H, Keiter S, Kinnberg KL, Knorr S, Nagel T, Braunbeck T. 2013. The maturity index as a tool to facilitate the interpretation of changes in vitellogenin production and sex ratio in the fish sexual development test. Aquat Toxicol 128:34–42. [DOI] [PubMed] [Google Scholar]
- Baumann L, Knórr S, Keiter S, Nagel T, Rehberger K, Volz S, Oberrauch S, Schiller V, Fenske M, Holbech H, Segner H, Braunbeck T. 2014. Persistence of endocrine disruption in zebrafish (Danio rerio) after discontinued exposure to the androgen 17β-trenbolone. Environ Toxicol Chem 33:2488–96. [DOI] [PubMed] [Google Scholar]
- Bertram MG, Saaristo M, Baumgartner JB, Johnstone CP, Allinson M, Allinson G, Wong BB. 2015. Sex in troubled waters: Widespread agricultural contaminant disrupts reproductive behavior in fish. Horm Behav 70:85–91. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Cai Q, Smith PN, Cobb GP. 2011. Liquid chromatography-tandem mass spectrometry analysis of 17α-trenbolone and 17β-trendione in airborne particulate matter. Talanta 85:1317–23. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Brown TR, Broadway PR, Buser MD, Brooks JC, Johnson BJ, Cobb GP, Smith PN. 2014. Characterization of trenbolone acetate and estradiol metabolite excretion profiles in implanted steers. Environ Toxicol Chem 33:2850–2858. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Wooten KJ, Buser MD, Johnson BJ, Cobb GP, Smith PN. 2015. Occurrence and characterization of steroid growth promoters associated with particulate matter originating from beef cattle feedyards. Environ Sci Technol 49:8796–8803. [DOI] [PubMed] [Google Scholar]
- Blake LS, Martinović D, Gray LE, Wilson VS, Regal RR, Villeneuve DL, Ankley GT. 2010. Characterization of the androgen-sensitive MDA-kb2 cell line for assessing complex environmental mixtures. Environ Toxicol Chem 29:1367–76. [DOI] [PubMed] [Google Scholar]
- Blankvoort BMG, de Groene EM, van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJT, Aarts JMMJG. 2001. Development of an androgen reporter gene assay (AR-LUX) utilizing a human cell line with an endogenously regulated androgen receptor. Anal Biochem 298:93–102. [DOI] [PubMed] [Google Scholar]
- Boettcher M 2012, Genotoxicity and endocrine effects - population relevant impacts of steroids, PhD Thesis, University of Heidelberg, Germany, 332 pp. http://nbn-resolving.de/urn:nbn:de:bsz:16-heidok-139778. [Google Scholar]
- Brockmeier EK, Ogino Y, Iguchi T, Barber DS, Denslow ND. 2013a. Effects of 17β-trenbolone on eastern and western mosquitofish (Gambusia holbrooki and G. affinis) anal fin growth and gene expression patterns. Aquat Toxicol 123:163–170. [DOI] [PubMed] [Google Scholar]
- Brockmeier EK, Yu FH, Amador DM, Bargar TA, Denslow ND. 2013b. Custom microarray construction and analysis for determining potential biomarkers of subchronic androgen exposure in the Eastern mosquitofish (Gambusia holbrooki). BMC Genomics 14:10.1186/1471-2164-14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card ML, Chin YP, Lee LS, Khan B. 2012. Prediction of experimental evaluation of soil sorption by natural hormones and hormone mimics. J Agric Food Chem 60:1480–1487. [DOI] [PubMed] [Google Scholar]
- Coady KK, Biever RC, Denslow ND, Gross M, Guiney PD, Holbech H, Karouna-Renier NK, Katsiadaki I, Krueger H, Levine SL, Maack G, Williams M, Wolf JC, Ankley GT. 2017. Current limitations and recommendations to improve testing for the environmental assessment of endocrine active substances. Integr Environ Assess Manag 13:302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EA, McBride SA, Kimbrough KC, Lee J, Marchand EA, Cwiertny DM, Kolodziej EP. 2015. Rates and product identification for trenbolone acetate metabolite biotransformation under aerobic conditions. Environ Toxicol Chem 34: 1472–1482. [DOI] [PubMed] [Google Scholar]
- Collette TW, Teng Q, Jensen KM, Kahl MD, Makynen EA, Durhan EJ, Villeneuve DL, Martinovic-Weigelt D, Ankley GT, Ekman DR. 2010. Impacts of an anti-androgen and an androgen/anti-androgen mixture of the metabolite profile of male fathead minnow urine. Environ Sci Technol 44:6881–6886. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Ankley GT, Cheng WY, Mayo ML, Miller DH, Perkins EJ, Villeneuve DL, Watanabe KH. 2017. Quantitative adverse outcome pathways and their application to predictive toxicology. Environ Sci Technol 51:4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe GM, Hemmer BL, Raimondo S, Goodman LR, Kulaw DH. 2010. Exposure of three generations of the estuarine sheepshead minnow (Cyprinodon variegatus) to the androgen 17β-trenbolone: Effects on survival, development, and reproduction. Environ Toxicol Chem 29:207987. [DOI] [PubMed] [Google Scholar]
- Davis KB, Morrison J, Galvez JL. 2000. Reproduction characteristics of adult channel catfish treated with trenbolone acetate during the phenocritical period of sex differentiation. Aquaculture 189:351–360. [Google Scholar]
- Dorts J, Richter CA, Wright-Osment MK, Ellersieck MR, Carter BJ, Tillitt DE. 2009. The genomic transcriptional response of female fathead minnows (Pimephales promelas) to an acute exposure to the androgen, 17β-trenbolone. Aquat Toxicol 91:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, Gray LE, Ankley GT. 2006. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ Health Perspect 114:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman DR, Hartig PC, Cardon M, Skelton DM, Teng Q, Durhan EJ, Jensen KM, Kahl MD, Villeneuve DL, Gray LE, Collette TW, Ankley GT. 2012. Metabolite profiling and a transcriptional activation assay provide direct evidence of androgen receptor antagonism by bisphenol A in fish. Environ Sci Technol 46:9673–80. [DOI] [PubMed] [Google Scholar]
- Ekman DR, Villeneuve DL, Teng Q, Ralston-Hooper KJ, Martinovic-Weigelt D, Kahl MD, Jensen KM, Durhan EJ, Makynen EA, Ankley GT, Collette TW. 2011. Use of gene expression, biochemical and metabolite profiles to enhance exposure and effects assessment of the model androgen 17β-trenbolone in fish. Environ Toxicol Chem 30:319–329. [DOI] [PubMed] [Google Scholar]
- Flynn K, Lothenbach D, Whiteman F, Hammermeister D, Touart LW, Swintek J, Tatarazako N, Onishi Y, Iguchi T, Johnson R. 2017. Summary of the development the US Environmental Protection Agency’s Medaka Extended One Generation Reproduction Test (MEOGRT) using data from 9 multigenerational medaka tests. Environ Toxicol Chem 36:3387–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren KL, Qu S, Lavado R, Cwiertny D, Schlenk D. 2014. Trenbolone acetate metabolites promote ovarian growth and development in adult Japanese medaka (Oryzias latipes). Gen Comp Endocrinol 202:1–7. [DOI] [PubMed] [Google Scholar]
- Gall HE, Sassman SA, Lee LS, Jafvert CT. 2011. Hormone discharges from a Midwest tile-drained agroecosystem receiving animal wastes. Environ Sci Technol 45:8755–8764. [DOI] [PubMed] [Google Scholar]
- Galvez JI, Morrison JR, Phelps RP. 1996. Efficacy of trenbolone acetate in sex inversion of the blue tilapia Oreochromis aureus. J World Aquaculture Soc 27:483–486. [Google Scholar]
- Garcia-Reyero N, Villeneuve DL, Kroll KJ, Liu L, Orlando EF, Watanabe KH, Sepulveda MS, Ankley GT, Denslow ND. 2009. Expression signatures for a model androgen and antiandrogen in the fathead minnow (Pimephales promelas) ovary. Environ Sci Technol 43:2614–2619. [DOI] [PubMed] [Google Scholar]
- Geiss C, Ruppert K, Askem C, Barroso C, Faber D, Ducrot V, Holbech H, Hutchinson TH, Kajankari P, Kinnberg KL, Lagadic L, Matthiessen P, Morris S, Neiman M, Penttinen OP, Sanchez-Marin P, Teigeler M, Weltje L, Oehlmann J. 2017. Validation of the OECD reproduction test guideline with the New Zealand mudsnail Potamopyrgus antipodarum using trenbolone and prochloraz. Ecotoxicology 26:370–382. [DOI] [PubMed] [Google Scholar]
- Haselman JT, Kosian PA, Korte JJ, Olmstead AW, Iguchi T, Johnson RD, Degitz SJ. 2016. Development of the larval amphibian growth and development assay: Effects of chronic 4-tert-octylphenol or 17β-trenbolone exposure in Xenopus laevis from embryo to juvenile. J Appl Toxicol 36:1639–1650. [DOI] [PubMed] [Google Scholar]
- Hazlerigg CRE, Tyler CR, Lorenzen K, Wheeler JR, Thorbek P. 2014. Population relevance of toxicant mediated changes in sex ratio in fish: An assessment using an individual-based zebrafish (Danio rerio) model. Ecological Modelling 280:76–88. [Google Scholar]
- Heintz MM, Brander SM, White JW. 2015. Endocrine disrupting compounds alter risk-taking behavior in guppies (Poecilia reticulate). Ethol 121:480–491. [Google Scholar]
- Hemmer MJ, Cripe GM, Hemmer BL, Goodman LR, Salinas KA, Fournie JW, Walker CC. 2008. Comparison of estrogen-responsive plasma protein biomarkers and reproductive endpoints in sheepshead minnows exposed to 17β-trenbolone. Aquat Toxicol 88:128–136. [DOI] [PubMed] [Google Scholar]
- Henry PFP, Akuffo VG, Chen Y, Karouna-Renier NK, Sprague DT, Bakst MR. 2012. Effect of 17β-trenbolone on male and female reproduction in Japanese quail (Coturnix japonica). Avian Biol Res 5:61–68. [Google Scholar]
- Hogan NS, Gallant MJ, van den Heuvel MR. 2012. Exposure to the pesticide linuron affects androgen-dependent gene expression in the three-spined stickleback (Gasterosteus aculeatus). Environ Toxicol Chem 31:1391–1395. [DOI] [PubMed] [Google Scholar]
- Hogan NS, Wartman CA, Finley MA, van der Lee JG, van den Heuvel MR. 2008. Simultaneous determination of androgenic and estrogenic endpoints in the threespine stickleback (Gasterosteus aculeatus) using quantitative RT-PCR. Aquat Toxicol 90:269–276. [DOI] [PubMed] [Google Scholar]
- Holbech H, Kinnberg K, Petersen GI, Jackson P, Hylland K, Norrgren L, Bjerregaard P. 2006. Detection of endocrine disrupters: Evaluation of a fish sexual development test (FSDT). Comp Biochem Physiol 144C:57–66. [DOI] [PubMed] [Google Scholar]
- Hook SE, Skillman AD, Small JA, Schultz IR. 2006. Gene expression patterns in rainbow trout, Oncorhynchus mykiss, exposed to a suite of model toxicants. Aquat Toxicol 77:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Katsu Y, Urushitani H, Lange A, Tyler CR. 2007. Developmental reproductive effects of exposure to pharmaceutical steroids in the aquatic environment: Studies on mosquitofish (Gambusia affinis affinis), roach (Rutilus rutilus) and medaka (Oryzias latipes). J Mar Sci Tech 15:29–36. [Google Scholar]
- Jensen KM, Makynen EA, Kahl MD, Ankley GT. 2006. Effects of the feedlot contaminant 17α-trenbolone on reproductive endocrinology of the fathead minnow. Environ Sci Technol 40:3112–3117. [DOI] [PubMed] [Google Scholar]
- Jolly C, Katsiadaki I, Le BN, Mayer I, Dufour S. 2006. Development of a stickleback kidney cell culture assay for the screening of androgenic and anti-androgenic endocrine disrupters. Aquat Toxicol 79:158–166. [DOI] [PubMed] [Google Scholar]
- Jones GD, Benchetler PV, Tate KW, Kolodziej EP. 2014a. Surface and subsurface attenuation of trenbolone acetate metabolites and manure-derived constituents in irrigation runoff on agro-ecosystems. Environ Sci Process Impacts 16:2507–2516. [DOI] [PubMed] [Google Scholar]
- Jones GD, Benchetler PV, Tate KW, Kolodziej EP. 2014b. Trenbolone acetate metabolite transport in rangelands and irrigated pasture: Observations and conceptual approaches for agro-ecosystems. Environ Sci Technol 48:12569–12576. [DOI] [PubMed] [Google Scholar]
- Jones GD, Benchetler PV, Tate KW, Kolodziej EP. 2014c. Mass balance approaches to characterizing the leaching potential of trenbolone acetate metabolites in agro-ecosystems. Environ Sci Technol 48:3715–3723. [DOI] [PubMed] [Google Scholar]
- Karouna-Renier NK, Chen Y, Henry PFP, Maddox CM, Sprague DT. 2017. Effects of circulating steroid hormones and gene expression along the hypothalamus-pituitary-gonadal axis in adult Japanese quail exposed to 17β-trenbolone across multiple generations. Toxicol Sci 157:62–73. [DOI] [PubMed] [Google Scholar]
- Katsu Y, Hinago M, Sone K, Urushitani H, Guillette LI, Iguchi T. 2007. In vitro assessment of transcriptional activation of the estrogen and androgen receptors of mosquitofish, Gambusia affinis affinis. Mol Cell Endocrinol 276:10–17. [DOI] [PubMed] [Google Scholar]
- Kelley D, Sassoon D, Segil N, Scudder M. 1989. Development and hormone regulation of androgen receptor levels in the sexually dimorphic larynx of Xenopus laevis. Developmental Biol 131:111–118. [DOI] [PubMed] [Google Scholar]
- Khan B, Lee LS. 2010. Soil temperature and moisture effects on the persistence of synthetic androgen 17α-trenbolone, 17β-trenbolone and trendione. Chemosphere 79:873–879. [DOI] [PubMed] [Google Scholar]
- Khan B, Lee LS. 2012. Estrogens and synthetic androgens in manure slurry from trenbolone acetate/estradiol implanted cattle and in waste-receiving lagoons used for irrigation. Chemosphere 89:1443–1449. [DOI] [PubMed] [Google Scholar]
- Khan B, Lee LS, Sassman SA. 2008. Degradation of synthetic androgens 17α- and 17β-trenbolone and trendione in agricultural soils. Environ Sci Technol 42:3570–3574. [DOI] [PubMed] [Google Scholar]
- Khan B, Qiao X, Lee LS. 2009. Stereoselective sorption by agricultural soils and liquid-liquid partitioning of trenbolone (17α and 17β) and trendione. Environ Sci Technol 43:8827–8833. [DOI] [PubMed] [Google Scholar]
- Kim TS, Yoon CY, Jung KK, Kim SS, Kang IH, Baek JH, Jo MS, Kim HS, Kang TS. 2010. In vitro study of organization for Economic Co-operation and Development (OECD) endocrine disruptor screening and testing methods- establishment of a recombinant rat androgen receptor (rrAR) binding assay. J Toxicol Sci 35:239–243. [DOI] [PubMed] [Google Scholar]
- Klimisch HJ, Andrae M, Tillmann U 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Reg Toxicol Pharmacol 25:1–5. [DOI] [PubMed] [Google Scholar]
- Kolodziej EP, Qu S, Forsgren KL, Long SA, Gloer JB, Jones GD, Schlenk D, Baltrusaitis J, Cwiertny DM. 2013. Identification and environmental implications of photo-transformation products of trenbolone acetate metabolites. Environ Sci Technol 47:5031–5041. [DOI] [PubMed] [Google Scholar]
- Kolodziej EP, Sedlak DL. 2007. Rangeland grazing as a source of steroid hormones to surface waters. Environ Sci Technol 41:3514–3520. [DOI] [PubMed] [Google Scholar]
- Kolok AS, Sellin MK. 2008. The environmental impact of growth-promoting compounds employed by the United States beef cattle industry: History, current knowledge, and future directions. Rev Environ Contam Toxicol 195:1–30. [DOI] [PubMed] [Google Scholar]
- Kurauchi K, Hirata T, Kinoshita M. 2008. Characteristics of ChgH-GFP transgenic medaka lines, an in vivo estrogenic compound detection system. Mar Poll Bull 57:442–448. [DOI] [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Burgoon LD, Russom CL, Helgen HW, Beringer JP, Tietge JE, Severson MN, Cavallin JE, Ankley GT. 2013. Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action. Aquat Toxicol 144–145:141–54. [DOI] [PubMed] [Google Scholar]
- Lange IG, Daxenberger A, Schiffer B, Witters H, Ibarreta D, Meyer HHD. 2002. Sex hormones originating from different livestock production systems: Fate and potential disrupting activity in the environment. Anal Chim Acta 473:27–37. [Google Scholar]
- Larsen MG, Baatrup E. 2010. Functional behavior and reproduction in androgenic sex reversed zebrafish (Danio rerio). Environ Toxicol Chem 29:1828–1833. [DOI] [PubMed] [Google Scholar]
- Leet JK, Sassman S, Amberg JJ, Olmstead AW, Lee LS, Ankley GT, Sepulveda MS. 2015. Environmental hormones and their impacts on sex differentiation in fathead minnows. Aquat Toxicol 158:98–107. [DOI] [PubMed] [Google Scholar]
- Li YY, Xu W, Chen XR, Lou QQ, Wei WJ, Qin ZF. 2015. Low concentrations of 17β-trenbolone induce female-to-male reversal and mortality in the frog Pelophylax nigromaculatus. Aquat Toxicol 158:230–7. [DOI] [PubMed] [Google Scholar]
- Li Z, Kroll KJ, Jensen KM, Villeneuve DL, Ankley GT, Brian JV, Sepúlveda MS, Orlando EF, Lazorchak JM, Kostich M, Armstrong B, Denslow ND, Watanabe KH. 2011a. A computational model of the hypothalamic: pituitary: gonadal axis in female fathead minnows (Pimephales promelas) exposed to 17α-ethynylestradiol and 17β-trenbolone. 2011. BMC Syst Biol 5:63 doi:10.1186/1752-0509-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Villeneuve DL, Jensen KM, Ankley GT, Watanabe KH. 2011b. A computational model for asynchronous oocyte growth dynamics in a batch-spawning fish. Can J Fish Aquat Sci 68:1528–1538. [Google Scholar]
- Martinović D, Blake LS, Durhan EJ, Greene KJ, Kahl MD, Jensen KM, Makynen EA, Villeneuve DL, Ankley GT. 2008. Reproductive toxicity of vinclozolin in the fathead minnow: Confirming an anti-androgenic mode of action. Environ Toxicol Chem 27:478–88. [DOI] [PubMed] [Google Scholar]
- Marty MS, Blankinship A, Chambers J, Constantine L, Kloas W, Kumar A, Lagadic L, Meador J, Pickford D, Schwarz T, Verslycke T. 2017. Population-relevant endpoints in the evaluation of endocrine-active substances (EAS) for ecotoxicological hazard and risk assessment. Integr Environ Assess Manag 13:317–330. [DOI] [PubMed] [Google Scholar]
- Martyniuk CL, Alvarez S, McClung S, Villeneuve DL, Ankley GT, Denslow ND. 2009. Quantitative proteomic profiles of androgen receptor signaling in the liver of fathead minnows (Pimephales promelas). J Proteome Res 8:2186–2200. [DOI] [PubMed] [Google Scholar]
- Martyniuk CL, Denslow ND. 2012. Exploring androgen-regulated pathways in teleost fish using transcriptomics and proteomics. Integr Comp Biol 52:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart S, Redivo B, Flarnion E, Mandiki SNM, Falisse E, Milla S, Kestemont P. 2015. The trenbolone acetate affects the immune system in rainbow trout, Oncorhynchus mykiss. Aquat Toxicol 163:109–120. [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Ankley GT, Biever RC, Bjerregaard P, Borgert C, Brugger K, Blankinship A, Chambers J, Coady KK, Constantine L, Dang Z, Denslow ND, Dreier DA, Dungey S, Gray LE, Gross M, Guiney PD, Hecker M, Holbech H, Iguchi T, Kadlec S, Karouna-Renier NK, Katsiadaki I, Kawashima Y, Kolas W, Krueger H, Kumar A, Lagadic L, Leopold A, Levine SL, Maack G, Marty S, Meador J, Mihaich E, Odum J, Ortego L, Parrott J, Pickford D, Roberts M, Schaefers C, Schwarz T, Solomon K, Verslycke T, Weltje L, Sheeler JR, Williams M, Wolf JC, Yamazaki K. 2017. Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances. Integr Environ Assess Manag 13:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HHD. 2001. Biochemistry and physiology of anabolic hormones used for improvement of meat production. Acta Pathol Microbiol Immunol Scand 109:1–8. [DOI] [PubMed] [Google Scholar]
- Meyer HHD, Rapp M. 1985. Reversible binding of the anabolic steroid trenbolone to steroid receptors. Acta Endocrinol 108 (Suppl 267):129. [Google Scholar]
- Mihaich EM, Schafers C, Dreier DA, Hecker M, Kawashima Y, Ortego L, Kawashima Y, Dang Z-C, Solomon K 2017. Challenges in assessing endocrine-specific modes of action: Recommendations for researchers and regulators. Integr Environ Assess Manag 13:280–292. [DOI] [PubMed] [Google Scholar]
- Milla S, Depiereux S, Kestemont P. 2011. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review. Ecotoxicology 20:305–319. [DOI] [PubMed] [Google Scholar]
- Miller DH, Ankley GT. 2004. Modeling impacts on populations: fathead minnow (Pimephales promelas) exposure to the endocrine disruptor 17β-trenbolone as a case study. Ecotoxicology Environ Safety 59:1–9. [DOI] [PubMed] [Google Scholar]
- Miracle A, Ankley G, Lattier D. 2006. Expression of two vitellogenin genes (vg1 and vg3) in fathead minnow (Pimephales promelas) liver in response to exposure to steroidal estrogens and androgens. Ecotoxicology Environ Safety 63:337–342. [DOI] [PubMed] [Google Scholar]
- Mizukami-Murata S, Kishi-Kadota K, Nishida T. 2016. 17β-Trenbolone exposure programs metabolic dysfunction in larval medaka. Environ Toxicol 31:1539–1551. [DOI] [PubMed] [Google Scholar]
- Morthorst JE, Holbech H, Bjerregaard P. 2010. Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations. Aquat Toxicol 98:336–343. [DOI] [PubMed] [Google Scholar]
- Neumann F 1976a. Pharmacological and endocrinological studies on anabolic agents In Lu FC, Rendal J, eds. Anabolic Agents in Animal Production. Verlag Georg Thieme, Stuttgart, Germany, pp 253–264. [PubMed] [Google Scholar]
- Neumann F 1976b. Pharmacological and endocrinological studies on anabolic agents. Environ Qual Safety Suppl 7:253–264. [PubMed] [Google Scholar]
- Norris DO, Carr JA. 2014. Vertebrate Endocrinology, 5th Edition Elsevier, 580 pg. [Google Scholar]
- Olmstead AW, Kosian PA, Johnson R, Blackshear PE, Haselman J, Blanksma C, Korte JJ, Holcombe GW, Burgess E, Lindberg-Livingston A, Bennett BA, Woodis KK, Degitz SJ. 2012. Trenbolone causes mortality and altered sexual differentiation in Xenopus tropicalis during larval development. Environ Toxicol Chem 31:2391–2398. [DOI] [PubMed] [Google Scholar]
- Orn S, Yamani S, Norrgren L. 2006. Comparison of vitellogenin induction, sex ratio, and gonad morphology between zebrafish and Japanese medaka after exposure to 17α-ethinylestradiol and 17β-trenbolone. Arch Environ Contam Toxicol 51:347–43. [DOI] [PubMed] [Google Scholar]
- Park JW, Tompsett AR, Zhang X, Newsted JL, Jones PD, Au DW, Kong R, Wu RS, Giesy JP, Hecker M. 2009. Advanced fluorescence in situ hybridization to localize and quantify gene expression in Japanese medaka (Oryzias latipes) exposed to endocrine-disrupting compounds. Environ Toxicol Chem 28:1951–62. [DOI] [PubMed] [Google Scholar]
- Parrott JL, Bjerregaard P, Brugger KE, Gray LE, Kguchi T, Kadlec SM, Weltje L, Wheeler JR. 2017. Uncertainties in biological responses that influence hazard and risk approaches to the regulation of endocrine active substances. Integr Environ Assess Manag 13:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Kolodziej EP, Long SA, Gloer JB, Patterson EV, Baltrusaitis J, Jones GD, Benchetler PV, Cole EA, Kimbrough KC, Tarnoff MD, Cwiertny DM. 2013. Product-to-parent reversion of trenbolone: Unrecognized risks for endocrine disruption. Science 342:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Kolodziej EP, Cwiertny DM. 2014. Sorption and mineral- promoted transformation of synthetic hormone growth promoters in soil systems. J Agric Food Chem 62:12277–12286 [DOI] [PubMed] [Google Scholar]
- Quan C, Su F, Wang H, Li H. 2011. Development of anabolic-androgenic steroids purity certified reference materials for anti-doping. Steroids 76:1527–1534. [DOI] [PubMed] [Google Scholar]
- Quinn MJ, Lavoie ET, Ottinger MA. 2007a. Reproductive toxicity of trenbolone acetate in embryonically exposed Japanese quail. Chemosphere 66:1191–1196. [DOI] [PubMed] [Google Scholar]
- Quinn MJ, McKernan M, Lavoie ET, Ottinger MA. 2007b. Immunotoxicity of trenbolone acetate in Japanese quail. J Toxicol Environ Health – Part A 70:88–93. [DOI] [PubMed] [Google Scholar]
- Radl V, Pritsch K, Munch JC, Schloter M. 2005. Structural and functional diversity of microbial communities from lake sediment contaminated with trenbolone, an endocrine-disrupting chemical. Environ Poll 137:345–353. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Qingli M, Staveley JP, Smolenski WJ. 2017a. Sorption and desorption of 17α-trenbolone and trendione on five soils. Environ Toxicol Chem 36:613–620. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Qingli M, Staveley JP, Smolenski WJ, Erickson J. 2017b. Degradation and transformation of 17α-trenbolone in aerobic water-sediment systems. Environ Toxicol Chem 36:630–635. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Staveley JP, Constantine L. 2017c. Reproductive effects on freshwater fish exposed to 17α-trenbolone and 17α-estradiol. Environ Toxicol Chem 36:636–644. [DOI] [PubMed] [Google Scholar]
- Roche JF, Quirke JF. 1986. The effects of steroid hormones and xenobiotics on growth of farm animals Buttery PJ, Hayes NB, Lindsay DB, eds. Control and Manipulation of Animal Growth. Butterworth, London, UK, pp 39–51. [Google Scholar]
- Saaristo M, Tomkins P, Allinson M, Allinson G, Wong BB. 2013. An androgenic agricultural contaminant impairs female reproductive behavior in a freshwater fish. PLoS One 8:e62782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer B, Daxenberger A, Meyer K, Meyer HH. 2001. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: Environmental studies. Environ Health Perspec 109:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz IR, Nagler JJ, Swanson P, Wunsche D, Sunschel D, Skillman AD, Burnett V, Smith D, Barry R. 2013. Toxicokinetic, toxicodynamic, and toxicoproteomic aspects of short-term exposure to trenbolone in female fish. Toxicol Sci 136:413–29. [DOI] [PubMed] [Google Scholar]
- Seki M, Fujishima S, Nozaka T, Maeda M, Kobayashi K. 2006. Comparison of response to 17β-estradiol and 17β-trenbolone among three small fish species. Environ Toxicol Chem 25:2742–2752. [DOI] [PubMed] [Google Scholar]
- Snow DD, Cassada DA, Papastavros E, Bartelt-Hunt SL, Li X, Zhang Y, Zhang YP, Yuan Q, Sallach JB. 2013. Detection, occurrence and fate of emerging contaminants in agricultural environments. Water Environ Res 85:869–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone K, Hinago M, Itamoto M, Katsu Y, Watanabe H, Urushitani H, Tooi O, Guillette LJ, Iguchi T. 2005. Effects of an androgenic growth promoter 17β-trenbolone on masculinization of mosquitofish (Gambusia affinis affinis). Gen Comp Endocrinol 143:151–60. [DOI] [PubMed] [Google Scholar]
- Soto AM, Calabro JM, Prechtl NV, Yau AY, Orlando EF, Daxenberger A, Kolok AS, Guilette LJ Jr., le Bizec B, Lange IG and Sonnenschein C 2004. Androgenic and estrogenic activity inwater bodies receiving cattle feedlot effluent in eastern Nebraska, USA. Environ Health Perspect 112:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephany RW. 2010. Hormonal growth promoting agents in food producing animals. Handbook Exp Pharmacol 355–367. [DOI] [PubMed] [Google Scholar]
- Sun L, Peng T, Liu F, Ren L, Peng Z, Ji G, Zhou Y, Fu Z. 2016. Transcriptional responses in male Japanese medaka exposed to antiandrogens and antiandrogen/androgen mixtures. Environ Toxicol 31:1591–1599. [DOI] [PubMed] [Google Scholar]
- Thevis M, Guddat S, Schänzer W 2009. Doping control analysis of trenbolone and related compounds using liquid chromatography-tandem mass spectrometry. Steroids 74:315–321. [DOI] [PubMed] [Google Scholar]
- Tomkins P, Saaristo M, Allinson M, Wong BB. 2016. Exposure to an agricultural contaminant, 17β-trenbolone, impairs female mate choice in a freshwater fish. Aquat Toxicol 170:365–370. [DOI] [PubMed] [Google Scholar]
- Tompkins P, Saaristo M, Bertram MG, Tomkins RB, Allinson M, Wong BB. 2017. The agricultural contaminant 17β-trenbolone disrupts male-male competition in the guppy (Poecilla reticulata). Chemosphere 187:286–293. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture. 2000. Part I: Baseline reference of feedlot management practices, 1999 USDA:APHIS:VS, CEAH, #N327.0500. National Animal Health Monitoring System, Fort Collins, CO: [cited 2015 October 27] Available from: http://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot99/Feedlot99_dr_PartI.pdf. [Google Scholar]
- US Environmental Protection Agency (EPA). 2013. Validation of the Medaka Multigeneration Test: Integrated Summary Report. U.S Environmental Protection Agency Endocrine Disruptor Screening Program; Washington, DC: http://www.oecd.org/env/ehs/testing/MMT%20ISR%20final.pdf [Google Scholar]
- Villeneuve DL, Jensen KM, Cavallin JE, Durhan EJ, Garcia-Reyero N, Kahl MD, Leino RL, Makynen EA, Wehmas LC, Perkins EJ, Ankley GT. 2017. Effects of the anti-microbial contaminant triclocarban, and co-exposure with the androgen 17β-trenbolone, on reproductive function and ovarian transcriptome of the fathead minnow (Pimephales promelas). Environ Toxicol Chem 36:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Larkin P, Knoebl I, Miracle AL, Kahl MD, Jensen KM, Makynen EA, Durhan EJ, Carter BJ, Denslow ND, Ankley GT. 2007. A graphical systems model to facilitate hypothesis-driven ecotoxicogenomics research on the brain-pituitary-gonadal axis. Environ Sci Technol 40:321–330. [DOI] [PubMed] [Google Scholar]
- Villeret M, Jolly S, Wiest L, Vulliet E, Bado-Nilles A, Porcher JM, Betoulle S, Minier C, Sanchez W. 2013. A potential biomarker of androgen exposure in European bullhead (Cottus sp.) kidney. Fish Physiol Biochem 39:573–580. [DOI] [PubMed] [Google Scholar]
- Webster JP, Kover SC, Bryson RJ, Harter T, Mansell DS, Sedlak DL, Kolodzielj EP. 2012. Occurrence of trenbolone acetate metabolites in simulated confined animal feeding operation (CAFO) runoff. Environ Sci Technol 46:3803–3810. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Cardon MC, Thornton J, Korte JJ, Ankley GT, Welch J, Gray LE, Hartig PC. 2004. Cloning and in vitro expression and characterization of the androgen receptor and isolation of estrogen receptor alpha from the fathead minnow (Pimephales promelas). 2004. Environ Sci Technol 38:6314–6321. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Ostby J, Gray LE. 2002. In vitro and in vivo effects of 17β-trenbolone: A feedlot effluent contaminant. Toxicol Sci 70:202–211. [DOI] [PubMed] [Google Scholar]
- Wooten KJ, Blackwell BR, McEachran AD, Mayer GD, Smith PN. 2015. Airborne particulate matter collected near beef cattle feedyards induces androgenic and estrogenic activity in vitro. Agric Ecosys Environ 203:29–35. [Google Scholar]
- Zhang X, Hecker M, Park JW, Tompsett AR, Newsted J, Nakayama K, Jones PD, Au D, Kong R, Wu RS, Giesy JP. 2008a. Real-time PCR array to study effects of chemicals on the hypothalamic-pituitary-gonadal axis of the Japanese medaka. Aquat Toxicol 88:173–82. [DOI] [PubMed] [Google Scholar]
- Zhang XW, Hecker M, Park JW, Tompsett AR, Jones PD, Newsted J, Au DWT, Kong R, Wu RSS, Giesy JP. 2008b. Time-dependent transcriptional profiles of genes of the hypothalamic-pituitary-gonadal axis in medaka (Oryzias latipes) exposed to fadrozole and 17β-trenbolone. Environ Toxicol Chem 27:2504–2511. [DOI] [PubMed] [Google Scholar]
- Zoetis. 2014. Environmental Assessment for Synovex ONE (Estradiol Benzoate and Trenbolone Acetate Extended Release Implant) Feedlot and Grass for Beef Steers and Heifers. Environmental Assessment PNU-0090851, PNU-0023173
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.