Abstract
Objective
Late Life Depression (LLD) is characterized by poor antidepressant response and cognitive dysfunction. We examined whether transdermal nicotine benefits mood symptoms and cognitive performance in LLD.
Methods
In a 12-week open-label outpatient study between November 2016 and August 2017, transdermal nicotine was given to 15 nonsmoking older adults. Eligible participants met DSM-IV-TR criteria for Major Depressive Disorder with ≥15 on the Montgomery-Asberg Depression Rating scale (MADRS) and endorsed subjective cognitive impairment. Transdermal nicotine patches were applied daily and titrated in a rigid dose escalation strategy to a maximum dose of 21.0 mg/day, allowing dose reductions for tolerability. Our primary mood outcome was MADRS change measured every 3 weeks. Our primary cognitive outcome was the Conners Continuous Performance Test (CPT), a test of attention.
Results
We observed robust response (86.7%) and remission rates (53.3%). There was a significant decrease in MADRS over the study (β = −1.51, p < 0.001), with improvement seen as early as three weeks (Bonferroni-adjusted p-value = 0.004). We also observed improvement in apathy and rumination. We did not observe improvement on the CPT, but did observe improvement in subjective cognitive performance and signals of potential drug effects on secondary cognitive measures of working memory, episodic memory, and self-referential emotional processing. Overall transdermal nicotine was well tolerated, although 6 participants could not reach the maximum targeted dose.
Conclusions
Nicotine may be a promising therapy for depressed mood and cognitive performance in LLD. A definitive placebo-controlled trial is necessary and longer-term safety established before clinical usage.
Trial Registration
ClinicalTrials.gov identifier: NCT02816138
INTRODUCTION
Late Life Depression (LLD), or major depressive disorder occurring in adults 60 years or older is associated with a poor response to current antidepressants,1,2 with greater than 50% failing to respond to initial treatments.3 LLD is further characterized by impaired cognitive performance that, even with successful antidepressant treatment, often does not return to the level of age-matched peers.4,5 Such cognitive deficits in LLD are themselves associated with greater disability6,7 and predict poor antidepressant response.6,8–12 There is currently no accepted therapy for LLD that effectively treats both mood and cognitive symptoms. We propose that, given past work, nicotine is an intriguing molecule to test for both symptom domains in LLD.13
Preliminary evidence and preclinical models support that nicotine may benefit mood.13–16 In population studies, those with current or lifetime history of Major Depressive Disorder (MDD) are twice as likely to become smokers, suggesting a self-medication effect.17 Although there are no published studies in geriatric populations, several small trials in non-smokers with midlife MDD report that nicotine reduces depressive symptom severity.18–20 These studies suggest that transdermal nicotine benefits depression severity and attentional performance as early as after 8 days of administration19 and may exhibit longer-term benefits comparable to fluoxetine for up to six months.20
Preclinical and clinical investigations similarly demonstrate cognitive benefits of nicotine.13 The most consistent results in healthy, cognitively intact adults are that nicotine improves attentional performance.21–28 A recent meta-analysis identifies specific improvements in five cognitive domains: fine motor, alerting attention, orienting attention, short-term episodic memory, and working memory.29 Comparable findings are reported in older, cognitively impaired populations, including Alzheimer’s disease,30 Age Associated Memory Impairment (AAMI),31 and Mild Cognitive Impairment (MCI).32 In our previously published placebo controlled blinded trial examining amnestic MCI, over six months transdermal nicotine administration resulted in improvement in attentional performance, psychomotor speed, and long-term verbal recall. The drug was well tolerated and its effect on cognition did not diminish over the course of the study.32
Given this background and as its mechanism of action is distinct from currently approved antidepressant medications, nicotine may be a promising pharmacotherapy for mood and cognitive symptoms of LLD. To our knowledge, there has not yet been a clinical trial assessing nicotine’s effects in LLD. We hypothesized that nicotine would benefit both mood and cognitive symptoms of LLD. In this proof-of-concept, open label clinical trial in LLD, we describe the clinical effects, safety, and tolerability of transdermal nicotine.
METHODS
Participants
Participants were recruited at Vanderbilt University Medical Center from clinical referrals and community advertisements from November 2016 through April 2017, with the study ending in August 2017. Core entry criteria focused on adults aged 60 years or older meeting DSM-IV-TR criteria for Major Depressive Disorder, recurrent or single episode, with a baseline depression severity measured by the Montgomery-Asberg Depression Rating Scale (MADRS)33 of ≥15. Criteria related to cognitive function specified a Montreal Cognitive Assessment (MoCA)34 score of ≥ 24 and subjective cognitive decline, defined as endorsing ≥ 20% of items on the Cognitive Complaint Index (CCI).35 Eligible participants could either be antidepressant-free or currently taking antidepressant monotherapy, however those taking antidepressants needed to be on a stable dose for at least eight weeks.
Additional exclusion criteria included: 1) Current tobacco or nicotine use in last year; 2) Other psychiatric disorders, except for anxiety symptoms occurring during a depressive episode; 3) History of alcohol or drug abuse over last three years; 4) Primary neurological disorders including dementia; 5) Regular use of drugs with centrally acting cholinergic or anticholinergic properties in the last 4 weeks; 6) Current psychotherapy.
All participants provided written informed consent. The study was approved by the Vanderbilt University Medical Center Institutional Review Board. The study was registered with ClinicalTrials.gov (NCT02816138).
Assessments
Diagnostic and Medical
The Mini-International Neuropsychiatric Interview (MINI, version 5.0)36 assessed current and lifetime depression and other psychiatric disorders. Diagnoses and duration of current episode were confirmed by clinical interview with a geriatric psychiatrist (WDT). Medical burden was quantified using the clinician-rated Cumulative Illness Rating Scale (CIRS).37 The Antidepressant Treatment History Form (ATHF) administered by the psychiatrist quantified the intensity of antidepressant treatment received for the current episode.38
Mood and Neuropsychiatric Assessments
As our primary depression outcome, MADRS was assessed every three weeks by the study psychiatrist. Secondary neuropsychiatric measures included specific symptom questionnaires administered at baseline and week 12. These symptoms included: Anhedonia (Snaith-Hamilton Pleasure Scale);39 Anxiety (Penn State Worry Questionnaire);40 Apathy (Apathy Evaluation Scale);41 Fatigue (Fatigue severity scale);42 and Rumination (Ruminative Response Scale that includes a total score and subscales for depressive rumination, reflective rumination, and brooding rumination).43
Cognitive Assessments
Participants were screened with subjective and objective instruments. Subjective cognitive impairment was assessed using the CCI, an index integrating questions from multiple assessments assessing perceived cognitive function. The MoCA screened for objective impairment.
Subjective cognitive performance was monitored using the primary outcome of change on the Memory Functioning Questionnaire (MFQ),44 administered at baseline and week 12. A secondary measure was the 8-item PROMIS Applied Cognition - Abilities questionnaire,45,46 administered every three weeks. The MFQ focuses on subjective memory performance, whereas the PROMIS assesses broader subjective performance.
Objective cognitive performance was assessed at baseline and week 12 using a combination of computerized and non-computerized tasks with repeatable conditions. Computerized tests were primarily conducted using elements of the Cogstate battery (Cogstate Inc., New Haven, CT, USA), supplemented by computerized tests of attention and specific paper-and-pencil neuropsychological tests that have demonstrated specific deficits in LLD in past work.5,47 Testing focused on cognitive domains affected by aging, impaired in LLD, or previously reported to be influenced by nicotine administration. Specific tests in each domain included:
Attention was assessed using the Conners Continuous Performance Test (CPT),48 Choice Reaction Time (CRT) task,49 and the card Identification Test (Cogstate). The primary objective cognitive outcome measure was the CPT hit reaction time standard error over inter-stimulus interval, given its known sensitivity to nicotinic receptor stimulation in Alzheimer’s disease50 and MCI.32
Executive Function was assessed using the Trail-Making Test Part B,51 the color-word interference condition of the Stroop, and the Groton Maze Learning Task (Cogstate).
Episodic Memory, both immediate and delayed memory was assessed using the Shopping List Task (Cogstate), the one-card learning task (Cogstate), and the NYU Paragraph Recall test.52
Working Memory was assessed using the one-back test (Cogstate).
Processing Speed was assessed using the color naming condition of the Stroop, the Trail-Making Test Part A,51 and the Detection Test (Cogstate).
We also examined self-referential negativity bias using the Trait Adjectives Task.53 This task rapidly presents a series of positively and negatively valenced adjectives (for example, talented, selfish, hostile, etc). Participants must indicate whether each word applies (endorsed) or does not apply (rejected) to them. The task probes self-referential negativity bias and has been proposed as an early predictor of antidepressant response.54
Study Visits
Participants were seen every 3 weeks plus a telephone call assessing tolerability at week 1. At each study clinic visit: 1) Depression severity was assessed by the study physician using the MADRS, 2) Subjective cognitive performance was assessed using the PROMIS, 3) Vital signs were assessed including sitting blood pressure, heart rate and weight, 4) Medication adherence was assessed using the Medication Adherence Questionnaire (MAQ)55 and a patch count.
Study Drug Administration and Dosing
Transdermal nicotine was administered in a rigid dose escalation strategy with the ability to reduce to previous or intermediate doses for tolerability. The dose escalation strategy was: 3.5mg (half of 7mg patch) in week 1, 7mg in weeks 2 and 3, 14mg in weeks 4 through 6, and 21mg in weeks 7 through 12. We selected 21mg as the maximum dose as it was well tolerated and demonstrated a signal for benefit to cognition in older adults with MCI.32 Dose reductions were based on reports of adverse events. For example, if a participant could not tolerate the 14mg patch, the dose was initially reduced to an intermediate dose of 10.5mg (half of 21mg patch) and could be further reduced to 7mg if needed. Participants were instructed to wear the study patch during the day and remove it at bedtime (approximately 16 hours daily); they were also instructed to move the patch location daily.
Discontinuation and Follow-up
Following trial completion, doses were tapered and discontinued over 3 weeks. Participants were seen at week 15 for a final visit.
Statistical Analysis
Statistical analyses were conducted in R Statistical Software version 3.4.0 (http://CRAN.R-project.org) and SAS Studio 3.6 (Cary, NC, USA). Baseline characteristics were summarized using mean (standard deviation) for continuous variables and N (%) for categorical variables. For MADRS and PROMIS (measured every 3 weeks), values were trended over time using a linear mixed effects model with autoregressive of order 1 (AR (1)) temporal process. To avoid potential bias, Last Observation Carried Forward (LOCF) approach was not used to impute missing data for the one participant who withdrew early. Missing values were instead imputed using the mean value of the sample at that time point56. We then calculated the N (%) for Remitters (MADRS ≤8) and Responders: (≥50% improvement in MADRS from baseline). For all measures obtained only at baseline and week 12, we only examined results from individuals who completed the study and values were compared using Wilcoxon signed-rank test.
In secondary analyses, we examined the effects of several variables on outcome measures. Smoking history (defined as having smoked cigarettes daily for 6 months in lifetime), final patch dose, and concurrent antidepressant use were analyzed for their potential effects on all analyses. Outcome measures were also examined for correlations with MADRS change to determine the relationship with change in depression severity.
RESULTS
24 subjects provided informed consent and were screened for eligibility. 9 subjects were excluded because they did not meet MADRS criteria, had comorbid psychiatric illness, or exhibited MRI contraindications for a linked MRI pilot project. 15 subjects started study patches, of whom 14 completed all 12 weeks. 1 subject stopped study medication at week 4 due to reported “cloudiness and fogginess” and “feeling tense and anxious” after increasing to the 14 mg dose; these effects did not resolve after decreasing the dose. The mean final patch dose was 15.4mg (SD = 6.3mg, range 7 – 21mg) with N=8 able achieving the maximum 21mg dose.
Baseline sample characteristics are detailed in Table 1. Our sample primarily consisted of early-onset LLD, with a mean age of initial onset at 26.0 years. Individuals receiving the patch as monotherapy did not achieve as high an average final patch dose as those who received it as augmentation to an antidepressant (t = 7.03, p < 0.001). No other demographic measures, including smoking history, were significantly associated with maximum tolerated dose (data not shown). Participants exhibited > 90% medication adherence with study patches.
Table 1.
Baseline characteristics
| Total Group N=15 |
Augmentation N=9 |
Monotherapy N=6 |
|
|---|---|---|---|
| Age, yrs | 64.9 (4.6) | 65.3 (4.9) | 64.3 (4.6) |
| Sex, women, N (%) | 10 (66.7%) | 7 (77.8%) | 3 (50%) |
| Education, yrs | 18.2 (1.8) | 17.8 (2.1) | 18.8 (1.2) |
| Past smoker, N (%) | 5 (33.3%) | 2 (22.2%) | 3 (50%) |
| CIRS | 7.1 (4.6) | 6.8 (2.7) | 7.7 (3.8) |
| MADRS | 27.7 (4.0) range 21–34 |
27.3 (4.8) | 28.2 (2.6) |
| ATHF | 3.3 (3.0) | 4.3 (3.0) | 1.7 (2.3) |
| Duration of current episode, days | 666.0 (648.6) | 852.7 (762.3) | 386.0 (302.3) |
| Age at first episode | 26.0 (16.4) | 26.9 (19.9) | 24.7 (10.9) |
| MoCA | 27.9 (1.4) | 28.0 (1.3) | 27.7 (1.6) |
| MFQ | 234.8 (40.3) | 225.3 (49.3) | 249.0 (15.8) |
| PROMIS Cognition SF | 20.7 (6.3) | 19.4 (7.3) | 22.5 (4.7) |
| Maximum dose, mg | 15.4 (6.3) | 19.8 (3.5) | 8.8 (1.9) |
Data presented as mean (SD), unless specified. Past smoker is defined as having smoked a cigarette daily for at least 6 months over the participant’s lifetime. CIRS = Cumulative Illness Rating Scale, MADRS = Montgomery-Asberg Depression Rating Scale, ATHF = Antidepressant Treatment History Form, MoCA = Montreal Cognitive Assessment, MFQ = Memory Functioning Questionnaire
Clinical Effect
Depression
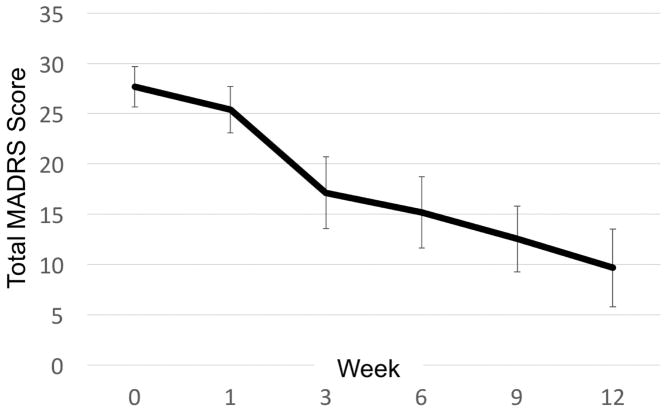
Transdermal nicotine treatment resulted in a significant change over time in depression severity measured by total MADRS score (β = −1.51, p < 0.001; Figure 1). Over the trial, we observed a mean MADRS reduction of 18.45 (SD=7.98) that significantly differed from baseline beginning at week 3 (Bonferroni-adjusted p-value = 0.004). 13 of 15 participants responded (86.7%) while 8 of 15 remitted (53.3%). This was true for both mean imputation and LOCF approaches. Change in depression severity was not significantly related to patch dose, smoking history, or concurrent antidepressant use.
Figure 1.
Effects of transdermal nicotine patches on depression severity
Data Points show mean MADRS and 95% CI over time. MADRS = Montgomery-Asberg Depression Rating Scale
Secondary Neuropsychiatric Measures
We observed improvement in some secondary symptoms (Table 2). These included increases in Apathy Evaluation Scale scores, indicating a reduction in apathy, and significant decreases in the Ruminative Response Scale scores, as well as for all subscales. Change in neither scale was significantly correlated with change in MADRS.
Table 2.
Change in Secondary Neuropsychiatric Measures
| Measure | Baseline | Week 12 | Mean Change | p value |
|---|---|---|---|---|
| Anhedonia | ||||
| Snaith-Hamilton Pleasure Scale | 18.4 (4.9) | 14.9 (3.9) | −3.4 (6.6) | P = 0.084 |
| Anxiety | ||||
| Penn State Worry Questionnaire | 55.1 (11.1) | 50.0 (14.5) | −5.1 (13.4) | P = 0.073 |
| Apathy | ||||
| Apathy Evaluation Scale | 25.3 (6.4) | 33.0 (6.6) | 7.7 (5.4) | P < 0.001 |
| Fatigue | ||||
| Fatigue Severity Scale | 41.7 (13.8) | 36.1 (14.1) | −5.6 (17.4) | P = 0.197 |
|
Rumination Ruminative Response Scale |
||||
| Total Score | 33.8 (11.8) | 24.8 (12.1) | −9.0 (10.0) | P = 0.002 |
| Depressive Subscore | 21.1 (6.3) | 15.5 (6.7) | −5.6 (6.08) | P = 0.003 |
| Reflective Subscore | 5.8 (3.3) | 4.4 (3.4) | −1.4 (2.3) | P = 0.047 |
| Brooding Subscore | 6.9 (3.4) | 4.9 (3.7) | −1.9 (2.9) | P = 0.028 |
Data presented as mean (SD). For all scales, higher scores indicate greater levels of symptoms, aside from the Apathy Evaluation Scale where higher scores indicate less apathy. Statistical testing utilized Wilcoxon signed-rank test.
Cognitive Function
Subjective Cognitive Performance
Subjective cognitive performance significantly improved over the trial. The primary subjective outcome of MFQ score increased (representing improved subjective function) by 23.64 points (SD = 40.96, p = 0.049). Using a mean imputation approach, 6 missing data points for PROMIS were imputed. Mean PROMIS score increased (representing improved subjective functioning) from baseline to week 12 by 6.21 (SD = 7.35) with participants exhibiting significant increases over time (β = 0.46, p = 0.001). Changes were not affected by dose, smoking history, or concurrent antidepressant use. Changes in MFQ and PROMIS were significantly correlated (PCC = 0.73, p = 0.005). Similarly, MADRS change was significantly correlated with changes in MFQ (PCC = −0.75, p = 0.002) and PROMIS (PCC = −0.63, p = 0.020).
Objective Cognitive Performance
We did not observe a statistically significant change in our primary cognitive outcome variable of the CPT. However, at an alpha=0.05, we did observe significant improvement in performance on specific secondary cognitive measures (Table 3). For working memory, there was improvement in one-back test speed. For episodic memory, there was significant improvement in the Cogstate shopping list task immediate recall.
Table 3.
Change in Cognitive Test Performance
| Measure | Baseline | Week 12 | Mean Change | p value |
|---|---|---|---|---|
| Attention | ||||
| Conners CPT - Standard Error Reaction Time | 0.021 (0.094) | 0.019 (0.103) | −0.003 (0.135) | P = 0.761 |
| Conners CPT - Reaction Time | 0.064 (0.028) | 0.054 (0.030) | − 0.009 (0.019) | P = 0.099 |
| CRT -Total Response Time | 862.9 (141.3) | 847.1 (124.8) | −16.0 (85.9) | P = 0.502 |
| Identification Test – Speed | 2.767 (0.070) | 2.771 (0.069) | 0.004 (0.063) | P = 0.626 |
| Identification Test – Accuracy | 1.474 (0.120) | 1.476 (0.121) | 0.002 (0.187) | P = 0.959 |
| Executive Function | ||||
| Trail-Making Test Part B | 78.8 (17.4) | 71.4 (13.5) | −7.4 (16.1) | P = 0.177 |
| Stroop Color-word interference | 37.2 (8.1) | 34.7 (8.5) | −2.5 (7.2) | P = 0.420 |
| Groton Maze Learning Task-Errors | 53.4 (16.7) | 62.6 (26.4) | 9.3 (16.9) | P = 0.064 |
| Groton Maze Learning Task-Time (seconds) | 466.5 (258.7) | 471.2 (244.4) | 4665.5 (113.6) | P = 0.715 |
| Episodic Memory | ||||
| Shopping-list task- Immediate Recall | 25.5 (2.7) | 27.7 (3.1) | 2.2 (3.4) | P = 0.049 |
| Shopping-list task – Delayed Recall | 9.0 (2.3) | 9.6 (1.7) | 0.6 (2.2) | P = 0.444 |
| One-Card learning - Correct | 59.7 (8.7) | 61.8 (6.4) | 2.1 (6.4) | P = 0.289 |
| One-Card Learning – Accuracy | 0.977 (0.092) | 0.994 (0.077) | 0.017 (0.064) | P = 0.367 |
| NYU Paragraph Recall | 30.5 (13.1) | 33.9 (13.3) | 3.4 (5.8) | P = 0.068 |
| Working Memory | ||||
| One-back test - Speed | 2.974 (0.102) | 2.934 (0.076) | −0.040 (0.065) | P = 0.049 |
| One-back test - Accuracy | 1.355 (0.108) | 1.415 (0.118) | 0.060 (0.162) | P = 0.160 |
| Processing Speed | ||||
| Stroop Color Naming | 67.4 (11.0) | 68.8 (10.0) | 1.4 (4.3) | P = 0.195 |
| Trail-Making Test Part A | 32.8 (8.6) | 30.0 (10.2) | −2.8 (7.0) | P = 0.277 |
| Detection Test – Speed | 2.622 (0.089) | 2.634 (0.130) | 0.012 (0.123) | P =0.426 |
| Detection Test – Accuracy | 1.524 (0.135) | 1.559 (0.045) | 0.035 (0.147) | P = 0.586 |
Data presented as mean (SD). Statistical comparisons utilized the Wilcoxon signed-rank test. CPT = Continuous Performance Test, CRT = Choice Reaction Time
Negativity Bias
On the Trait Adjectives Task, there was an increase in both good adjectives endorsed (mean increase = 2.0, SD = 3.4, p = 0.046) and bad adjectives rejected (mean increase = 2.6, SD = 2.3, p = 0.004). We also observed decreases in reaction time (RT) when endorsing good items (−101.2 msec, p = 0.035) and rejecting bad items (−133.3 msec, p = 0.017).
Safety and Tolerability
Vital Signs and weight
We observed no significant changes in blood pressure or heart rate, but did observe a decrease in weight (mean change = −6.7lb, p < 0.001) and BMI (mean change = −1.0 kg/m2, p < 0.001).
Side Effects
There were no serious adverse events. Nausea was most commonly reported (N=7), along with dizziness/lightheadedness (N=4), headache (N=4), increased tension/anxiety (N=3), vivid dreams (N=3), and patch site reactions (N=3). These events required dose decreases in 7 participants, specifically for nausea (N=4), dizziness/lightheadedness (N=2), tension/anxiety (N=2), and headache (N=1).
Follow-up
After discontinuing patches, at the 15-week visit, no side effects or nicotine cravings were reported.
DISCUSSION
Open-label administration of transdermal nicotine in LLD resulted in a robust response (86.7%) and remission rates (53.3%), with improvement occurring as early as 3 weeks. Change in depression severity was not related to smoking history, final patch dose, or whether used as monotherapy or augmentation. We also observed improvements in subjective cognitive performance, self-referential negativity bias, and in specific cognitive tests. Overall transdermal nicotine was well tolerated, although some participants could not reach the maximum dose.
The observed improvement in depression severity is concordant with studies in younger depressed adults,18–20 as is our observation that depression severity improved by 3 weeks.19 Our response and remission rates are comparable to open-label trials of approved antidepressants in LLD.57,58 We further observed improvement in self-report of apathy and rumination. As change in these measures was not correlated with MADRS change, these measures may not be simple surrogate markers of overall depression severity. We further observed improvement in subjective cognitive performance, but these changes were correlated with MADRS change. Although clinically important, it is unclear whether improvement in subjective cognitive performance is independent of the improvement in mood. Final patch dose was not associated with change in depression severity, suggesting that doses lower than our maximum dose may provide benefit.
Although limited by multiple comparisons and no significant change in our primary cognitive outcome, we observed improvement in measures of episodic memory and working memory speed. Per a meta-analysis, these domains are improved by nicotine in younger smoking and non-smoking populations.29 Although reporting subjective cognitive deficits, subjects were objectively non-impaired per MOCA scores. As nicotine may have its greatest cognitive benefit in those with baseline cognitive deficits or during conditions of high difficulty,13,59 either the population or test selection may have contributed to this overall negative finding. Our findings for episodic and working memory are thus encouraging but not confirmatory.
We similarly observed that nicotine reduces self-referential negativity bias in LLD. Reductions in negativity bias are early predictors of antidepressant response54 and may be fundamental to the mechanism of conventional antidepressants.60
Through activity at nicotinic acetylcholine receptors (nAChRs) nicotine modulates serotonin, norepinephrine, and dopamine.61 In our recently proposed network model,13 at the neural system’s level, nicotine may exert effects through modulation of the Cognitive Control Network (CCN; or Central Executive Network). The CCN is an intrinsic functional network involved in emotional regulation, inhibiting irrelevant information, and working memory that exhibits altered connectivity and function in depression and LLD.62 These effects and any resultant clinical benefit may depend upon broad agonist activity across nAChR subtypes 63 as previous trials utilizing specificα7 nAChR agonists and nAChR antagonists have demonstrated limited efficacy for mood and cognitive improvement.64,65
Concordant with past geriatric studies,31,32 transdermal nicotine was tolerated reasonably well with no withdrawal symptoms or cravings. Vital signs were stable, and the modest decrease in weight and BMI may be a benefit as many antidepressants contribute to weight gain.66 Higher doses of transdermal nicotine were not widely tolerated, with only 8 of 15 subjects able to tolerate the maximum dose. This may have been secondary to an overly aggressive dosing strategy. Interestingly, those taking concurrent antidepressants were able to tolerate higher patch doses. Because nicotine is metabolized through a different cytochrome system than utilized by study participants’ antidepressants,67 it is unlikely that pharmacokinetic interactions explain this finding. Instead, this may represent a selection bias as individuals who were not taking antidepressants at study entry were likely not doing so for specific reasons, including tolerability.
The primary study limitations are its open-label design, small sample size, and multiple comparisons. Open-label studies in LLD report higher response rate than blinded placebo controlled trials and the response rates we observed are comparable to other open-label trials of approved antidepressants.57 The small sample size limits the power of the study and requires replication, particularly as our sample primarily included individuals with early-life onset of depression. It is unclear whether study results would generalize to individuals with a later life onset. Due to individual differences in metabolism, variable plasma nicotine levels may be achieved from the same dose.68 Because we did not measure plasma levels, it is not possible to determine whether the bioavailable nicotine dose for a given patch strength had an effect on efficacy or tolerability. Lastly, because of the exploratory nature of this study, multiple comparisons were made in our analyses so statistically significant values, particularly in analyses of cognitive performance, should be viewed with caution.
In summary, our study is the first trial utilizing transdermal nicotine in the context of LLD and supports its further investigation as a potential novel treatment for LLD. More definitive studies are necessary before nicotine’s clinical usage. Future trials examining transdermal nicotine as a therapeutic for LLD should have a slower dose titration and measure of plasma metabolites to assess bioavailability. The optimal dose of transdermal nicotine benefitting both mood and cognitive performance also requires further investigation. We propose that as transdermal nicotine is unlikely to be used as monotherapy to treat depression, given its different mechanism of action, it should be examined as an augmentation agent in a more definitive blinded placebo-controlled clinical trial to examine clinical response, safety, and tolerability.
CLINICAL POINTS.
Late life depression has no currently approved treatment that improves both its mood and cognitive symptoms.
This study provides preliminary evidence that transdermal nicotine may safely benefit mood, and potentially cognitive symptoms, in late-life depression.
Transdermal Nicotine may be a promising novel therapy for Late Life Depression. However, definitive studies examining efficacy and long-term safety are necessary before its clinical usage.
Acknowledgments
This research was supported by NIH grant K24 MH110598 and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. The sponsor provided funding for the study but did not influence the design or conduct of the study.
Footnotes
The authors deny any conflicts of interest.
References
- 1.Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008;16(7):558–567. doi: 10.1097/JGP.0b013e3181693288. [DOI] [PubMed] [Google Scholar]
- 2.Taylor WD, Doraiswamy PM. A systematic review of antidepressant placebo-controlled trials for geriatric depression: limitations of current data and directions for the future. Neuropsychopharmacology. 2004;29(12):2285–2299. doi: 10.1038/sj.npp.1300550. [DOI] [PubMed] [Google Scholar]
- 3.Dew MA, Whyte EM, Lenze EJ, et al. Recovery from major depression in older adults receiving augmentation of antidepressant pharmacotherapy. Am J Psychiatry. 2007;164(6):892–899. doi: 10.1176/ajp.2007.164.6.892. [DOI] [PubMed] [Google Scholar]
- 4.Sexton CE, McDermott L, Kalu UG, et al. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychol Med. 2012;42(6):1195–1202. doi: 10.1017/S0033291711002352. [DOI] [PubMed] [Google Scholar]
- 5.Sheline YI, Barch DM, Garcia K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60(1):58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- 7.Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2004;12(1):50–56. [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57(3):285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29(12):2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 10.Kalayam B, Alexopoulos GS. Prefrontal Dysfunction and Treatment Response in Geriatric Depression. Arch Gen Psychiatry. 1999;56(8):713. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 11.Pimontel MA, Culang-Reinlieb ME, Morimoto SS, Sneed JR. Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry. 2012;27(9):893–899. doi: 10.1002/gps.2808. [DOI] [PubMed] [Google Scholar]
- 12.McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25(10):933–944. doi: 10.1002/gps.2431. [DOI] [PubMed] [Google Scholar]
- 13.Gandelman JA, Newhouse P, Taylor WD. Nicotine and networks: Potential for enhancement of mood and cognition in late-life depression. Neuroscience and Biobehavioral Reviews. 2017 doi: 10.1016/j.neubiorev.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tizabi Y, Overstreet DH, Rezvani AH, et al. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142(2):193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- 15.Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43(5):389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- 16.Vázquez-Palacios G, Bonilla-Jaime H, Velázquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine] Pharmacol Biochem Behav. 2004;78(1):165–169. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Jama. 2000;284(20):2606. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 18.Salín-Pascual RJ, Drucker-Colín R. A novel effect of nicotine on mood and sleep in major depression. Neuroreport. 1998;9(1):57–60. doi: 10.1097/00001756-199801050-00012. [DOI] [PubMed] [Google Scholar]
- 19.McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2006;189(1):125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 20.Haro R, Drucker-Colín R. Effects of long-term administration of nicotine and fluoxetine on sleep in depressed patients. Arch Med Res. 2004;35(6):499–506. doi: 10.1016/j.arcmed.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav. 2006;87(3):614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-Related Enhancement of Mood and Cognition in Smokers Administered Nicotine Nasal Spray. Neuropsychopharmacology. 2008;33(3):588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36(3):539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 24.Kelemen WL, Fulton EK. Cigarette abstinence impairs memory and metacognition despite administration of 2 mg nicotine gum. Exp Clin Psychopharmacol. 2008;16(6):521–531. doi: 10.1037/a0014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harte CB, Kanarek RB. The effects of nicotine and sucrose on spatial memory and attention. Nutr Neurosci. 2004;7(2):121–125. doi: 10.1080/10284150410001704543. [DOI] [PubMed] [Google Scholar]
- 26.Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127(1–2):31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- 27.File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. Int J Neuropsychopharmacol. 2001;4(4):371–376. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- 28.Levin ED, Conners CK, Silva D, et al. Transdermal nicotine effects on attention. Psychopharmacol. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- 29.Heishman SJJ, Kleykamp BAa, Singleton EGG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newhouse PA, Potter A, Kelton M, Corwin J. Nicotinic treatment of Alzheimer’s disease. Biol Psychiatry. 2001;49(3):269–278. doi: 10.1016/s0006-3223(00)01069-6. [DOI] [PubMed] [Google Scholar]
- 31.White HK, Levin ED. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berl) 2004;171(4):465–471. doi: 10.1007/s00213-003-1614-8. [DOI] [PubMed] [Google Scholar]
- 32.Newhouse P, Kellar K, Aisen P, et al. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology. 2012;78(2):91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 35.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 37.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 38.Keller MB. The Longitudinal Interval Follow-up Evaluation. Arch Gen Psychiatry. 1987;44(6):540. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 39.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995 Jul;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 40.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav Res Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 41.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 42.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 43.Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. J Abnorm Psychol. 1993;102(1):20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- 44.Gilewski MJ, Zelinsky EM, Schaie KW. The Memory Functioning Questionnaire for Assessement of Memory Complaints in Adulthood and Old Age. Psychol Aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 45.Saffer BY, Lanting SC, Koehle MS, Klonsky ED, Iverson GL. Assessing cognitive impairment using PROMIS® applied cognition-abilities scales in a medical outpatient sample. Psychiatry Res. 2015;226(1):169–172. doi: 10.1016/j.psychres.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 46.Howland M, Tatsuoka C, Smyth KA, Sajatovic M. Evaluating PROMIS((R)) applied cognition items in a sample of older adults at risk for cognitive decline. Psychiatry Res. 2017;247:39–42. doi: 10.1016/j.psychres.2016.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nebes RD, Butters MA, Mulsant BH, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30:679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- 48.Conners C. The Conners Continuous Performance Test. Toronto, Canada: Multi-Health Systems; 1994. [Google Scholar]
- 49.Bates T, Mangan G, Stough C, Corballis P. Smoking, processing speed and attention in a choice reaction time task. Psychopharmacol. 1995;120(2):209–212. doi: 10.1007/BF02246195. [DOI] [PubMed] [Google Scholar]
- 50.White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 1999;143(2):158–165. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- 51.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 52.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12(4):168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 53.Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161(7):1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- 54.Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J Affect Disord. 2009;118(1–3):87–93. doi: 10.1016/j.jad.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 55.Morisky DE, Green LW, Levine DM. Concurrent and Predictive Validity of a Self-reported Measure of Medication Adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Streiner DL. The case of the missing data: Methods of dealing with dropouts and other research vagaries. Can J Psychiatry. 2002;47(1):68–75. [PubMed] [Google Scholar]
- 57.Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design Makes a Difference: A Meta-Analysis of Antidepressant Response Rates in Placebo-Controlled Versus Comparator Trials in Late-Life Depression. Am J Geriatr Psychiatry. 2008;16(1):65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 58.Roose SP, Schatzberg AF. The efficacy of antidepressants in the treatment of late-life depression. J Clin Psychopharmacol. 2005;25(4 Suppl 1):S1–S7. doi: 10.1097/01.jcp.0000162807.84570.6b. [DOI] [PubMed] [Google Scholar]
- 59.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4(1):36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195(2):102–108. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- 61.Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48(15):4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 62.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Picciotto MR, Lewis AS, Van Schalkwyk GI, Mineur YS, Schalkwyk GI, Van Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96(PB):235–243. doi: 10.1016/j.neuropharm.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Möller HJ, Demyttenaere K, Olausson B, et al. Two Phase III randomised double-blind studies of fixed-dose TC-5214 (dexmecamylamine) adjunct to ongoing antidepressant therapy in patients with major depressive disorder and an inadequate response to prior antidepressant therapy. World J Biol Psychiatry. 2015;16(7):483–501. doi: 10.3109/15622975.2014.989261. [DOI] [PubMed] [Google Scholar]
- 65.Lewis AS, van Schalkwyk GI, Bloch MH. Alpha-7 nicotinic agonists for cognitive deficits in neuropsychiatric disorders: A translational meta-analysis of rodent and human studies. Prog Neuro-Psychopharmacology Biol Psychiatry. 2017;75:45–53. doi: 10.1016/j.pnpbp.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kivimäki M, Hamer M, Batty GD, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: A population-based study. Diabetes Care. 2010;33(12):2611–2616. doi: 10.2337/dc10-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 68.Hurt RD, Dale LC, Offord KP, et al. Serum nicotine and cotinine levels during nicotine-patch therapy. Clin Pharmacol Ther. 1993;54(1):98–106. doi: 10.1038/clpt.1993.117. [DOI] [PubMed] [Google Scholar]