Abstract
Objectives
Despite evidence for many potential risks, use of benzodiazepines (BZD) among older adults is common. The authors evaluated the available evidence for BZD effectiveness and tolerability for use in older adults in three psychiatric conditions for which BZD are commonly prescribed: insomnia, anxiety disorders, and behavioral and psychological symptoms of dementia.
Design
Electronic databases including PubMed/MEDLINE were searched to identify articles that included: 1) patients ≥50 years of age; 2) focused on patients diagnosed with insomnia, anxiety disorders, or behavioral and psychological symptoms of dementia; and 3) were either a randomized, placebo-controlled trial or a randomized trial comparing a BZD with either another psychotropic medication or psychotherapy.
Results
A total of 31 studies met the inclusion criteria. Of the three clinical indications evaluated, treatment of insomnia had the greatest available evidence for use of BZD among older adults, with 21 of 25 trials demonstrating improvement in sleep outcomes with use of BZD. Only one trial was found to meet eligibility criteria for BZD use in anxiety disorders, demonstrating benefit over placebo. A total of 5 studies for use in behavioral disturbances in dementia were included; of which only one trial demonstrated improvement over placebo.
Conclusion
The results of this systematic review suggest that BZD prescribing to older adults is significantly in excess of what the available evidence would suggest is appropriate. Future trials should focus on efforts to reduce both acute and chronic BZD use among older adults while improving access to effective non-pharmacologic treatment alternatives.
Keywords: Benzodiazepine, older adults, anxiety, insomnia, behavioral and psychological symptoms of dementia
INTRODUCTION
Despite evidence for many potential risks, use of benzodiazepines (BZD) among older adults is common. Recent national estimates suggest that 8.7% of older Americans greater than age 65 were prescribed BZD within the past year.1 Because a proportion of all new users convert to long-term use,2,3 long-term use accumulates among patients as they age, so the prevalence of BZD prescribing is greatest among older adults.4 Roughly one third of all BZD use is long term (≥120 days in a recent analysis), with older adults accounting for the greatest proportion of patients with long term use.1
Understanding the prescribing of BZD among older adults is of particular importance given the greater risk for polypharmacy and increased sensitivity to medication side effects within this population. Use of BZD among older adults has been associated with many adverse effects including falls,5 fractures,6,7 and motor vehicle accidents.8,9 While several studies have suggested a potential association between BZD use and increased risk of dementia,10–12 a large prospective study found no increase in risk with greater BZD exposure.13 However, a review of randomized, placebo-controlled trials by Tannenbaum and colleagues demonstrated that BZD clearly cause amnestic and non-amnestic cognitive impairment.14 Following opioids, BZD are the second most-common medication class associated with prescription drug overdose mortality, and BZD-related overdoses have been increasing.4,15 While opioids are the most common prescription medication associated with overdose mortality, BZD are the medication class mostly commonly combined with opioids in such deaths.15,16 In light of these concerns, the U.S. Food and Drug Administration (FDA) recently issued a black box warning advising potential respiratory suppression and death caused by co-prescribing opioids with other CNS depressants, including BZD.17
Given these concerns, multiple professional societies have identified reducing prescribing of BZD among older adults as a national priority. Since its inception, the American Geriatrics Society (AGS) Beers Criteria for potentially inappropriate medication use in the elderly have included BZD, with the most recent update in 2015 now listing all BZD as potentially inappropriate for older adults.18 As part of the Choosing Wisely Campaign, the AGS identified use of BZD in older adults as one of ten things physicians and patients should question.19 Additionally, the American Psychiatric Association (APA) has recommended special caution when prescribing BZD to older adults given high potential for side effects.20
In light of high prevalence of use of BZD among older adults and known associated harms with medication use, we sought to evaluate the available evidence for their effectiveness and tolerability for use specifically in older adults. For the purpose of this study, we chose to focus on the three psychiatric conditions for which BZD are commonly prescribed: insomnia, anxiety disorders, and behavioral and psychological symptoms of dementia.
METHODS
Inclusion and Exclusion Criteria
Articles were included if they: 1) included patients ≥50 years of age; 2) focused on patients diagnosed with insomnia, anxiety disorders, or behavioral and psychological symptoms of dementia; and 3) were either a randomized, placebo-controlled trial or a randomized trial comparing a BZD with either another psychotropic medication (e.g., antidepressant) or psychotherapy. We did not include studies in languages other than English or where an English language translation was not available. Additionally, studies were excluded if the primary outcome was not symptoms of insomnia, anxiety disorders, or behavioral and psychological symptoms of dementia (e.g., sleep disturbances, agitation, psychosis).
Search Strategy
Electronic databases including PubMed/MEDLINE were searched from inception through August 2017 to identify studies that met the inclusion criteria. Databases were searched for peer-reviewed, English language articles using the following combinations of keywords and medical subject headings (MeSH terms) in title, abstract, or index terms:
Older adults: aged [MeSH terms] or aged or elderly or “older adults”
Benzodiazepines: benzodiazepine [MeSH terms] or alprazolam or benzodiazepinones or bromazepam or clonazepam or devazepide or diazepam or flumazenil or flunitrazepam or flurazepam or lorazepam or nitrazepam or oxazepam or chlordiazepoxide or clorazepate dipotassium or estazolam or medazepam or midazolam or halazepam or quazepam or triazolam
Insomnia: sleep disorder [MeSH terms] or insomnia or sleep initiation and maintenance disorders or sleep or sleep wake disorder or sleep initiation or sleep onset or sleep latency
Anxiety disorders: anxiety disorder [MeSH terms] or agoraphobia or obsessive-compulsive disorder or panic disorder or stress disorders, traumatic or combat disorders or stress disorders, post-traumatic, acute or phobias or claustrophobia or social phobia or specific phobia
Dementia: dementia [MeSH terms] or BPSD or neuropsychiatric symptoms of dementia or disruptive behaviors or behavioral and psychological symptoms of dementia
Search terms including a combination of older adults, BZD, and the clinical diagnosis of interest (i.e. insomnia, anxiety, or dementia). The final search was completed September 1, 2017. Reference lists of the relevant literature were also searched manually to identify additional studies to potentially include.
Study Selection
One author (LG) screened initial titles and abstracts; selected abstracts were then screened by two independent authors (LG and DM). Studies were selected for inclusion if two authors agreed that they met eligibility criteria. The extracted data from each study was collected into a data-collection spreadsheet. Abstracted data included total sample size, age range, placebo controlled versus comparison of other treatment, summary of findings, and whether study favored use of a BZD or comparison treatment.
RESULTS
The initial search yielded 2073 results, which was reduced to 661 after excluding studies that were not randomized clinical trials. Forty-five studies were identified as potentially relevant; the full text was then reviewed by two of the study authors (LG and DM). After review of all potentially relevant studies, the authors initially agreed whether or not to include 39 of 45 articles (87%). The reviewers were able to reach discussion-based consensus on the remaining 6 articles; further review by a third party was unnecessary. Ultimately, 31 studies met inclusion criteria and were included in this review (Figure 1). Findings from these studies are reviewed below, organized by diagnosis type and placebo controlled or BZD versus another treatment (e.g., psychotropic medication or psychotherapy).
Figure 1.

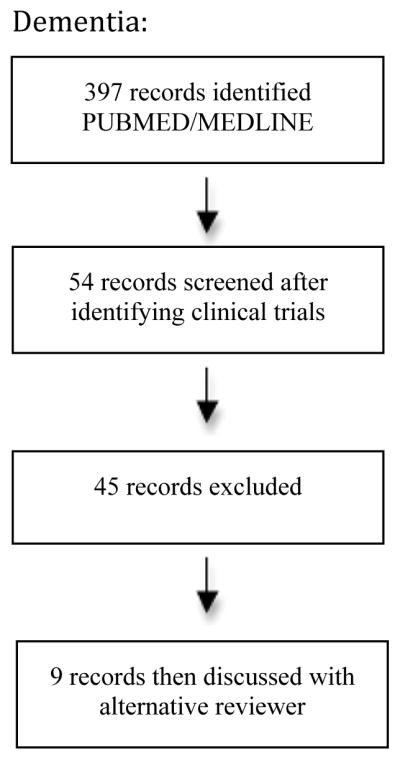
Flow chart for the selection of studies
Insomnia
A total number of 25 trials were identified evaluating of BZD for treatment of insomnia in older adults. Study sample sizes ranged from 6 to 335 participants (Table 1).
Table 1.
Benzodiazepine (BZD) use for treatment of insomnia
| Insomnia | |||||
|---|---|---|---|---|---|
| Study | N | Age | BZD/Other Treatment | Summary of Findings | Favors BZD |
| Placebo Controlled | |||||
| Glass et al. 200821 | 20 | Mean 73.9 Range 70–89 |
Temazepam 15 mg Diphenhydramine 50 mg Placebo |
Sleep improvements greater with temazepam versus placebo and diphenhydramine | Yes, temazepam was more effective but advantage is mitigated by risk of falls |
| Morin et al. 199930 | 78 | Mean 65 | Temazepam 7.5 to 30 mg CBT Temazepam + CBT Placebo |
Three active treatments were more effective than placebo. Improvements in sleep favored combination treatment, then CBT, followed by pharmacotherapy. | Yes, pharmacologic approaches are effective but sleep improvements are better sustained with CBT |
| Leppik et al. 199722 | 335 | Range 60–85 | Triazolam 0.125 mg Temazepam 15 mg Zolpidem 5 mg Placebo |
Compared to placebo sleep latency improved with zolpidem and temazepam (not triazolam). Sleep latency shorter than placebo in temazepam group. Temazepam and triazolam had significantly more side effects. | Yes, but potentially offset but side effects (drowsiness, fatigue, nervousness) |
| Roth et al. 199761 | 30 | Mean 66 | Quazepam 7.5 to 15 mg Placebo |
Improved total sleep time and sleep latency versus placebo | Yes |
| Roger et al. 199362 | 205 | Mean 80 Range 60–90 |
Triazolam 0.25 mg Zolpidem 5 to 10 mg Placebo |
Both groups favored treatment, no difference between triazolam and zolpidem | Yes |
| Elie et al. 199063 | 44 | Mean 76 Range 60–90 |
Triazolam 0.125 or 0.25 mg Zopiclone 5 or 7.5 mg Placebo |
Both drugs more effective than placebo | Yes |
| Overstall & Oldman 198764 | 62 | Mean 82 Mean 80 Mean 80 |
Lormetazepam Chlormethiazole Placebo |
Both improved sleep duration, sleep latency, improved quality of sleep | Yes |
| Klimm et al. 198723 | 74 | Mean 73 | Nitrazepam 5 mg Zopiclone 7.5 mg Placebo |
Improvements in sleep quality and latency over placebo for both nitrazepam and zopiclone | Yes, but improvements with nitrazepam were offset by more side effects |
| Bayer & Pathy 198665 | 89 | Range 67–89 | Loprazolam 0.5 or 1 mg Placebo |
Improved sleep latency, satisfaction with sleep, decreased number of nocturnal awakenings with loprazolam | Yes |
| Viukari et al. 198466 | 29 | Mean 81 Range 62–98 |
Brotizolam 0.125 mg Nitrazepam 2.5 mg Placebo |
Both better than placebo | Yes |
| Vogel 198467 | 10 | Mean 60 Range 55–71 |
Lormetazepam 0.5 mg Placbo |
Improved total sleep time and sleep latency over placebo | Yes |
| Viukari et al. 198368 | 37 | Mean 74 | Flunitrazepam 1 mg Nitrazepam 5 mg Placebo |
Both BZDs were more effective in inducing and maintaining sleep | Yes |
| Elie et al. 198369 | 30 | Mean 75 Range 60–93 |
Flurazepam 15 mg Zopiclone 5, 7.5, and 10 mg Placebo |
Both active groups better than placebo, flurazepam and zopiclone equally effective | Yes |
| Martinez & Serna 198270 | 60 | Range 54–90 | Quazepam 15 mg Placebo |
Improved quantity and quality of sleep with benzo | Yes |
| Caldwell 198271 | 57 | Range 60–81 | Quazepam 15 mg Placebo |
Improved sleep measures for BZD | Yes |
| Fillingim 198272 | 75 | Mean 81 | Temazepam 30 mg Flurazepam 30 mg Placebo |
Both better than placebo in improving quality of sleep | Yes |
| Piccione et al. 198073 | 27 | Mean 70 Range 60–94 |
Triazolam 0.25 or 0.5 mg Chloral hydrate 250 or 500 mg Placebo |
Triazolam improved sleep over placebo and chloral hydrate | Yes |
| Frost & DeLucchi 197974 | 6 | Range 67–82 | Flurazepam 15 mg Placebo |
Improved sleep latency and total sleep time with BZD | Yes |
| Goldstein et al. 197875 | 17 | Mean 82 | Oxazepam 15 mg Flurazepam 15 mg Chloral hydrate 500 mg Placebo |
Only oxazepam was better than placebo in reducing nighttime awakenings and improved quality of sleep. Flurazepam was better than placebo in decreasing sleep latency although had residual daytime sedative effect. | Yes |
| Reeves 197776 | 41 | 69 70 70 Range 61–83 |
Triazolam 0.25 mg Flurazepam 15 mg Placebo |
Triazolam better than placebo in sleep onset, duration of sleep, nighttime awakenings, and feeling restless. Flurzepam was better than placebo in onset of sleep and quality of sleep. Triazolam out performed flurazepam in all measures | Yes |
| Mamelak et al. 198977 | 36 | Range 60–72 | Brotizolam 0.25 mg Flurazepam 15 mg Placebo |
Sleep improved with all treatments, at end of the study only placebo treated group was sleeping significantly longer | No |
| Meuleman et al. 198778 | 17 | Mean 78 Range 56–97 |
Temazepam 15 Diphenhydramien 50 mg Placebo |
Diphenhydramine and temazepam resulted in decreased sleep latency than placebo, longer duration of sleep with diphenhydramine versus temazepam, more deficits on neurologic functioning with temazepam | No |
| Vs. Other Treatment | |||||
| Lachnit et al. 198325 | 46 | Mean 77 Range 54–90 |
Midazolam 15 mg Vesparax (hydroxyzine 50 mg, brallobarbital 50 mg, and secobarbital 150 mg) |
Identical in terms of efficacy, greater side effects with Vesparax | Yes |
| Dehlin et al. 199579 | 102 | Mean 79 | Flunitrazepam 1 mg Zopiclone 5 mg |
No difference in effectiveness between groups | No |
| Bayer et al. 198680 | 53 | Mean 82 Range 70–91 |
Triazolam 0.125 mg Chlormethiazole 384 |
Similar hypnotic effect during early phase, results not sustained by triazolam. Worse daytime withdrawal effects with triazolam | No |
BZD vs placebo
We were able to identify 22 trials comparing use of BZD with placebo for treatment of insomnia. Among these trials, 20 studies demonstrated greater improvement in sleep measures with use of BZD versus placebo. Improvements were generally seen in decreased sleep latency, increased duration of sleep, and reduced nighttime awakenings, provided mostly by patient self-report. However, 3 of the positive trials favoring use of BZD concluded that potential for benefit was likely offset by risk of adverse effects including falls, confusion, and daytime sedation.21–23 Only one study by Morin et al. specifically compared use of cognitive behavioral therapy (CBT) for insomnia to use of BZD in older adults.24 In this study the authors compared use of temazepam, CBT, combined treatment with temazepam and CBT, or placebo and find that improvements in sleep continuity and efficiency favored combination treatment, then CBT, followed by pharmacotherapy. However, sleep improvements were better sustained with the CBT-alone condition compared to CBT + BZD or BZD alone.
BZD vs other treatment
Three trials compared BZD to another treatment for insomnia. Only one of the three studies favored use of a BZD over another medication for treatment of insomnia. Lachnit el al. found that use of oral midazolam was similar in efficacy but led to less side effects as compared to the combination medication Vesparax (combination of hydroxyzine, brallobarbital, and secobarbital), which includes strong anticholinergic and barbiturate components.25
Anxiety Disorders
Only one trial was found to meet eligibility criteria, with a sample size of 220 patients (Table 2).
Table 2.
Benzodiazepine (BZD) use for treatment of anxiety disorders
| Anxiety Disorders | |||||
|---|---|---|---|---|---|
| Study | N | Age | BZD/Other Treatment | Summary of Findings | Favors BZD |
| Placebo Controlled | |||||
| Koepke et al. 198226 | 220 | Mean 68 | Oxazepam 30 to 60 mg Placebo |
Oxazepam demonstrated reductions in the Hamilton Anxiety Scale and Physician Target Symptom Scale over placebo | Yes |
| Vs. Other Treatment | |||||
| None | |||||
BZD vs placebo
We only identified one study comparing a BZD to placebo for treatment of anxiety disorders among older adults. This 1982 study by Koepke et al. found that use of oxazepam was associated with reductions in the Hamilton Anxiety rating scale as compared to placebo.26 Patients on oxazepam had roughly a 7-point reduction in their Hamilton Anxiety rating scale total score, as compared to a 3-point reduction in the placebo group.
BZD vs other treatment
No eligible studies were found.
Dementia
A total of 5 studies met eligibility criteria, with sample sizes ranging from 7 to 610 (Table 3).
Table 3.
Benzodiazepine (BZD) use for treatment of behavioral and psychological symptoms of dementia
| Behavioral and Psychological Symptoms of Dementia | |||||
|---|---|---|---|---|---|
| Study | N | Age | BZD/Other Treatment | Summary of Findings | Favors BZD |
| Placebo Controlled | |||||
| Meehan et al. 200227 | 272 | Mean 78 Range 54–97 |
Lorazepam 1 mg (up to 3) intramuscular (IM) injections Olanzapine 2.5 and 5 mg IM injections Placebo |
Significant improvement with lorazepam 1 mg on the Agitation-Calmness Scale, Cohen-Mansfield Agitation Inventory, and PANSS Excited Component versus placebo | Yes, however effect of olanzapine was longer lasting compared with lorazepam |
| McCarten et al. 199581 | 7 | Mean 73 Range 62–81 |
Triazolam 0.125 mg Placebo |
Triazolam had no significant improvement across sleep measures. | No |
| Vs. Other Treatment | |||||
| Christensen & Benfield 199882 | 48 | Mean 83 Range 65–98 |
Alprazolam 1 mg Haloperidol 0.1 to 1.0 mg (mean 0.64 mg) |
No significant difference between medications | No |
| Coccaro et al. 199028 | 59 | Mean 76 Range 58–99 |
Oxazepam 60 mg Haloperidol 5 mg Diphenhydramine 200 mg |
Modest but significant improvement with all medications in the Alzheimer’s Disease Assessment Scale. Magnitude of improvement was greater for haloperidol and diphenhydramine than for oxazepam (not significant) | No |
| Stotsky 198429 | 610 | Mean 81 (nursing home) 72 (inpatient) |
Diazepam 2 to 40 mg Thioridazine 10 to 200 mg |
Thioridazine performed better than diazepam in the Hamilton Anxiety Scale, modified Nurses’ Observation Scale for Inpatient Evaluation (NOISE), and global ratings | No |
BZD vs placebo
Two trials compared BZD use with placebo for treatment of patients with dementia; one demonstrated benefit over placebo. Meehan et al. evaluated use of lorazepam and olanzapine intramuscular injections in managing behavioral disturbances in dementia.27 They found a significant reduction on the Agitation-Calmness Scale, Cohen-Mansfield Agitation Inventory, and Positive and Negative Symptom Scale (PANNS) with use of intramuscular lorazepam. However, the effect of treatment with olanzapine was longer lasting and outperformed that of the BZD.
BZD vs other treatment
Three trials were included comparing BZD use to another treatment. Of these trials, none favored use of BZDs for treatment of behavioral and psychological symptoms of dementia. In two of the three studies, antipsychotic agents (haloperidol and thioridazine) led to greater reductions in the Alzheimer’s Disease Assessment Scale and Nurses’ Observation Scale for Inpatient Evaluation (NOISE) as compared a BZD (oxazepam and diazepam, respectively).28,29
DISCUSSION
This systematic review highlights several key features regarding use of BZD among older adults. First, the high rate of BZD prescribing among older adults is strikingly out of proportion to the body of evidence supporting such use. Our search identified a total of only 31 trials evaluating use of BZD across combined indications of insomnia, anxiety disorders, and behavioral disturbances in dementia, with only two studies published after 2000. Given the high risk of potential harms associated with use of these medications in older adults, it is concerning that there is such limited evidence to support their efficacy and safety in treatment of conditions for which they are commonly prescribed.
Of the three clinical indications that we evaluated, treatment of insomnia had the greatest available evidence for use of BZD among older adults, accounting for three fourths of the total studies evaluated. For insomnia, 21 of 25 trials demonstrated improvement in sleep outcomes with use of BZD over placebo or another treatment. However, among these studies, 3 of the positive trials concluded that high side effect burden limited recommended use of BZD in older adults21–23 with an additional study finding no improvement in use of BZD over CBT for insomnia.30 Additionally, while the positive trials reported here demonstrated improvement with use of BZD over placebo or another treatment, the overall magnitude of the treatment effect was relatively small. Many published trials included here followed patients over a short follow up period, with limited evaluation of longer term sleep outcomes. Of the 21 trials demonstrating improvement in sleep measures, only 6 of the studies followed patients for greater than 14 days.
Additionally, for insomnia, many of the trials included here used patient self-report for sleep measures. Studies suggest that patients may overestimate the potential subjective improvement in sleep with use of pharmacotherapy, with one study finding that use of sedative hypnotics only increase total sleep time objectively by 25 minutes.31,32 While there may be some evidence to support use of BZD for treatment of insomnia in the short-term, there is little evidence supporting chronic use of BZD for insomnia.33 Several recent studies have shown that long term BZD use for insomnia is associated with worsening sleep quality, including difficult falling asleep and more nighttime awakenings, as compared to non-users.34–37 Clinicians may consider use of non-benzodiazepine receptor agonist (“Z-drugs”) as a safer alternative, however, studies demonstrate a similar concerning side effect profile for use in older adults including increased risk of falls,38 fracture,39 impaired cognition,31 and motor vehicle accidents40 due to morning after impairment. In addition, a variety of newer studies have established that CBT is an effective treatment for insomnia, if not more effective than BZD and sedative hypnotics in a general adult population,41,42 yet without any of the associated potential risks.
Despite BZD routinely being prescribed to older adults for treatment of anxiety disorders, we were only able to find one randomized controlled trial to support their use, which was completed 35 years ago.26 Additionally, for treatment of anxiety disorders there is also growing concern that BZD may in fact reduce the benefit of other more effective therapies. Analyses of treatment of panic disorder demonstrate that concurrent use of BZD can reduce the effectiveness of exposure-based psychotherapies.43,44 For treatment of post-traumatic stress disorder (PTSD), a recent systematic review and meta-analysis recommends against use of BZD treatment, finding that concurrent use of BZD and psychotherapy can lead to worse patient outcomes.45 Despite the lack of efficacy for BZD in treating symptoms of PTSD, 30% of veterans with PTSD received a BZD prescription in 2012.46
Lastly, there is limited evidence to support use of BZD in management of behavioral and psychological symptoms of dementia with only one study demonstrating potential benefit with use of intramuscular lorazepam.27 However, across all included trials, BZD performed worse than antipsychotics in reducing agitation. Since BZD are not subject to the black box warnings for increased mortality that apply to antipsychotics,47 some prescribers may consider using a BZD to be safer than an antipsychotic for treating the behavioral and psychological symptoms of dementia. However, their use of BZD can still lead to significant harms among these older adults including paradoxical disinhibition, increased confusion, and worsening agitation,48 while they have less evidence for benefit than antipsychotics.
The results of this systematic review suggest that BZD prescribing to older adults is significantly in excess of what the available evidence would suggest is appropriate. While there is some amount of evidence, albeit limited, to guide risk-benefit decisions regarding use of BZD for the above indications, a significant amount of BZD prescribing also appears to occur in the absence of any clear mental health diagnosis or for chronic pain, for which BZD are not indicated.2,3,49,50 Where there is not evidence to support the prescribing of BZD, a provider then only has the ample evidence of potential harms associated with use in older adults. Recent analyses of general use of BZD in older adults suggest nearly half of patients receiving a BZD do not have a diagnosis of a mental health disorder or significant psychiatric symptoms to support their use.3,49,51
This study has several limitations. Our inclusion of only randomized controlled trials limits our sample size of included studies and the results may have been different with inclusion of observational studies. Additionally, we did not assess for potential publication bias, or perform a systematic search to account for unpublished data. However, it is our assumption that trials without significant findings would be more likely to be represented in unpublished work, so our findings would likely be reinforced by inclusion of unpublished studies.
Unfortunately, for patients accustomated to using BZD, the prospect of discontinuing the medication can be intimidating for both patients and their prescribers. A 2007 study by Cook et al. explored both physician and older adult perspectives on chronic BZD use and reducing use. Among patients,52 BZD were viewed as being very important in managing their anxiety, sleep, and stress. Patients felt that these medications provided them a “lifeline” and without them, feared that they would not fare well. Patients generally underestimated or were unaware of the potential side effects of their BZD use and resisted the idea of a taper. In the companion paper focusing on physician attitudes,53 physicians did not view BZD use among older adults as a health problem and perceived that BZD were more effective than other available treatment alternatives. Providers assumed that patients would be resistant to changing their BZD dose and perceived that a taper would cause distress for patients. This reluctance from both patients and physicians to discuss tapering may help account for the persistently high use of BZD among older adults.
While patients may be reluctant to participant in studies of benzodiazepine tapers, there is evidence supporting several successful strategies. Structured algorithms to reduce polypharmacy use among older adults—including use of BZD—demonstrate successful discontinuation of medications without adverse effects for the patient.54 In the EMPOWER trial, Tannenbaum et al. used direct-to-patient education that successfully encouraged older adults to initiate discussions with their providers regarding safe and appropriate prescribing, which ultimately lead to reduced BZD use.55,56 Studies evaluating older adults on chronic BZD for treatment of insomnia have shown success from utilizing a combination of CBT with supervised tapering, with the majority of participants that successfully tapered were also BZD-free at one-year follow up.57,58 Finally, structured interventions providing a written stepped-dose reduction tapering schedule for patients have been effective in reducing chronic BZD use among primary care patients.59 A recent meta-analysis evaluating interventions for reducing BZD use among older adults found that the combination of psychotherapy and supervised BZD tapering resulted in the highest rate of BZD discontinuation—with patients having a five times greater odds of discontinuing treatment as compared to control interventions.60
Use of prescription BZD among older adults remains a significant concern and ongoing challenge. BZD are associated with multiple adverse outcomes and, following opioids, BZD are the medication class most associated with mortality in overdose4. Despite these known harms and relative lack of evidence to support use in conditions such as anxiety disorders and behavioral and psychological symptoms of dementia, BZD remain widely prescribed to many older adults. While BZD use may be appropriate in select populations, use should be considered only after assessment of the potential benefits, risks and effective alternatives and following efforts to use evidence-based treatments that pose less risk of harm. While both patients and providers may consider the possibility of stopping a BZD with dread, recent intervention trials have shown promise in reducing BZD use. Future trials should focus on efforts to reduce both acute and chronic BZD use among older adults while improving access to effective non-pharmacologic treatment alternatives
Acknowledgments
Funding Source: DTM was supported by the Beeson Career Development Award Program (NIA K08AG048321, AFAR, The John A. Hartford Foundation, and The Atlantic Philanthropies).
Footnotes
Conflict of Interest: No disclosures to report
Author Contributions: All authors have made substantive contributions to the study, and all authors endorse the data and conclusions.
Sponsor’s Role: The sponsors did not play any role in the design, methods, data collection, analysis, or preparation of this manuscript.
References
- 1.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA psychiatry. 2015;72:136–42. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 2.Simon GE, Ludman EJ. Outcome of new benzodiazepine prescriptions to older adults in primary care. Gen Hosp Psychiatry. 2006;28:374–8. doi: 10.1016/j.genhosppsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maust DT, Kales HC, Wiechers IR, Blow FC, Olfson M. No End in Sight: Benzodiazepine Use in Older Adults in the United States. J Am Geriatr Soc. 2016 doi: 10.1111/jgs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996–2013. Am J Public Health. 2016;106:686–8. doi: 10.2105/AJPH.2016.303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–60. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 6.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158:892–8. doi: 10.1176/appi.ajp.158.6.892. [DOI] [PubMed] [Google Scholar]
- 7.Schneeweiss S, Wang PS. Claims data studies of sedative-hypnotics and hip fractures in older people: exploring residual confounding using survey information. J Am Geriatr Soc. 2005;53:948–54. doi: 10.1111/j.1532-5415.2005.53303.x. [DOI] [PubMed] [Google Scholar]
- 8.Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving. Drug safety. 2011;34:125–56. doi: 10.2165/11539050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Barbone F, McMahon AD, Davey PG, et al. Association of road-traffic accidents with benzodiazepine use. The Lancet. 1998;352:1331–6. doi: 10.1016/s0140-6736(98)04087-2. [DOI] [PubMed] [Google Scholar]
- 10.Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterniti S, Dufouil C, Alperovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22:285–93. doi: 10.1097/00004714-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Islam MM, Iqbal U, Walther B, et al. Benzodiazepine Use and Risk of Dementia in the Elderly Population: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2016;47:181–91. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 13.Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ (Clinical research ed) 2016;352:i90. doi: 10.1136/bmj.i90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29:639–58. doi: 10.1007/BF03262280. [DOI] [PubMed] [Google Scholar]
- 15.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 16.Chen LH, Hedegaard H, Warner M. Drug-poisoning Deaths Involving Opioid Analgesics: United States, 1999–2011. NCHS Data Brief. 2014:1–8. [PubMed] [Google Scholar]
- 17.FDA Drug Safety Communication. FDA Warns About Serious Risks and Death When Combining Opioid Pain and Cough Medicines with Benzodiazepines; Requires Its Strongest Warning. U.S. Food & Drug Administration; [Accessed August 1, 2017]. [on-line]. Available at http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. [Google Scholar]
- 18.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227–46. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 19.American Geriatrics Society. [Accessed August 1, 2017];Ten Things Physicians and Patients Should Question. Available at http://www.choosingwisely.org/societies/american-geriatrics-society/
- 20.Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148:151–2. doi: 10.1176/ajp.148.2.151. [DOI] [PubMed] [Google Scholar]
- 21.Glass JR, Sproule BA, Herrmann N, Busto UE. Effects of 2-week treatment with temazepam and diphenhydramine in elderly insomniacs: a randomized, placebo-controlled trial. J Clin Psychopharmacol. 2008;28:182–8. doi: 10.1097/JCP.0b013e31816a9e4f. [DOI] [PubMed] [Google Scholar]
- 22.Leppik IE, Roth-Schechter GB, Gray GW, Cohn MA, Owens D. Double-blind, placebo-controlled comparison of zolpidem, triazolam, and temazepam in elderly patients with insomnia. Drug Development Research. 1997;40:230–8. [Google Scholar]
- 23.Klimm HD, Dreyfus JF, Delmotte M. Zopiclone versus nitrazepam: a double-blind comparative study of efficacy and tolerance in elderly patients with chronic insomnia. Sleep. 1987;10(Suppl 1):73–8. doi: 10.1093/sleep/10.suppl_1.73. [DOI] [PubMed] [Google Scholar]
- 24.Morin CM, Vallières A, Guay B, et al. Cognitive Behavioral Therapy, Singly and Combined With Medication, for Persistent Insomnia: A Randomized Controlled Trial. JAMA : the journal of the American Medical Association. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lachnit KS, Proszowski E, Rieder L. Midazolam in the treatment of sleep disorders in geriatric patients. Br J Clin Pharmacol. 1983;16(Suppl 1):173s–7s. doi: 10.1111/j.1365-2125.1983.tb02291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koepke HH, Gold RL, Linden ME, Lion JR, Rickels K. Multicenter controlled study of oxazepam in anxious elderly outpatients. Psychosomatics. 1982;23:641–5. doi: 10.1016/S0033-3182(82)73363-8. [DOI] [PubMed] [Google Scholar]
- 27.Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology. 2002;26:494–504. doi: 10.1016/S0893-133X(01)00365-7. [DOI] [PubMed] [Google Scholar]
- 28.Coccaro EF, Kramer E, Zemishlany Z, et al. Pharmacologic treatment of noncognitive behavioral disturbances in elderly demented patients. Am J Psychiatry. 1990;147:1640–5. doi: 10.1176/ajp.147.12.1640. [DOI] [PubMed] [Google Scholar]
- 29.Stotsky B. Multicenter study comparing thioridazine with diazepam and placebo in elderly, nonpsychotic patients with emotional and behavioral disorders. Clin Ther. 1984;6:546–59. [PubMed] [Google Scholar]
- 30.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Jama. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 31.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeck JL, Ford J, Conway EL, et al. Review of Safety and Efficacy of Sleep Medicines in Older Adults. Clinical therapeutics. 2016;38:2340–72. doi: 10.1016/j.clinthera.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Lader MH. Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified? European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1999;9(Suppl 6):S399–405. doi: 10.1016/s0924-977x(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Bell JS, Visvanathan R, et al. The association between benzodiazepine use and sleep quality in residential aged care facilities: a cross-sectional study. BMC geriatrics. 2016;16:196. doi: 10.1186/s12877-016-0363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beland SG, Preville M, Dubois MF, et al. Benzodiazepine use and quality of sleep in the community-dwelling elderly population. Aging & mental health. 2010;14:843–50. doi: 10.1080/13607861003781833. [DOI] [PubMed] [Google Scholar]
- 36.Beland SG, Preville M, Dubois MF, et al. The association between length of benzodiazepine use and sleep quality in older population. International journal of geriatric psychiatry. 2011;26:908–15. doi: 10.1002/gps.2623. [DOI] [PubMed] [Google Scholar]
- 37.Bourgeois J, Elseviers MM, Van Bortel L, Petrovic M, Vander Stichele RH. Sleep quality of benzodiazepine users in nursing homes: a comparative study with nonusers. Sleep medicine. 2013;14:614–21. doi: 10.1016/j.sleep.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Tom SE, Wickwire EM, Park Y, Albrecht JS. Nonbenzodiazepine Sedative Hypnotics and Risk of Fall-Related Injury. Sleep. 2016;39:1009–14. doi: 10.5665/sleep.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin FY, Chen PC, Liao CH, Hsieh YW, Sung FC. Retrospective population cohort study on hip fracture risk associated with zolpidem medication. Sleep. 2014;37:673–9. doi: 10.5665/sleep.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen RN, Boudreau DM, Ebel BE, Grossman DC, Sullivan SD. Sedative Hypnotic Medication Use and the Risk of Motor Vehicle Crash. Am J Public Health. 2015;105:e64–9. doi: 10.2105/AJPH.2015.302723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morin CM, Vallières A, Guay B, et al. Cognitive Behavioral Therapy, Singly and Combined With Medication, for Persistent Insomnia: A Randomized Controlled Trial. JAMA : the journal of the American Medical Association. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClusky HY, Milby JB, Switzer PK, Williams V, Wooten V. Efficacy of behavioral versus triazolam treatment in persistent sleep-onset insomnia. The American journal of psychiatry. 1991;148:121–6. doi: 10.1176/ajp.148.1.121. [DOI] [PubMed] [Google Scholar]
- 43.Marks IM, Swinson RP, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto The British Journal of Psychiatry. 1993;162:776–87. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- 44.Otto MW, Pollack MH, Sabatino SA. Maintenance of remission following cognitive behavior therapy for panic disorder: Possible deleterious effects of concurrent medication treatment. Behav Ther. 1996;27:473–82. [Google Scholar]
- 45.Rothbaum BO, Price M, Jovanovic T, et al. A Randomized, Double-Blind Evaluation of d-Cycloserine or Alprazolam Combined With Virtual Reality Exposure Therapy for Posttraumatic Stress Disorder in Iraq and Afghanistan War Veterans. Am J Psychiatry. 2014;171:640–8. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund BC, Bernardy NC, Alexander B, Friedman MJ. Declining Benzodiazepine Use in Veterans With Posttraumatic Stress Disorder. J Clin Psychiatry. 2011;73:292–6. doi: 10.4088/JCP.10m06775. [DOI] [PubMed] [Google Scholar]
- 47.Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:957–70. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern TA, Celano CM, Gross AF, et al. The assessment and management of agitation and delirium in the general hospital. Prim Care Companion J Clin Psychiatry. 2010:12. doi: 10.4088/PCC.09r00938yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiechers IR, Leslie DL, Rosenheck RA. Prescribing of Psychotropic Medications to Patients Without a Psychiatric Diagnosis. Psychiatric services. 2013;64:1243–8. doi: 10.1176/appi.ps.201200557. [DOI] [PubMed] [Google Scholar]
- 50.Chou R, Deyo R, Friedly J, et al. Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:480–92. doi: 10.7326/M16-2458. [DOI] [PubMed] [Google Scholar]
- 51.Maust DT, Mavandadi S, Eakin A, et al. Telephone-based behavioral health assessment for older adults starting a new psychiatric medication. Am J Geriatr Psychiatry. 2011;19:851–8. doi: 10.1097/JGP.0b013e318202c1dc. [DOI] [PubMed] [Google Scholar]
- 52.Cook JM, Biyanova T, Masci C, Coyne JC. Older patient perspectives on long-term anxiolytic benzodiazepine use and discontinuation: a qualitative study. J Gen Intern Med. 2007;22:1094–100. doi: 10.1007/s11606-007-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303–7. doi: 10.1007/s11606-006-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Archives of internal medicine. 2010;170:1648–54. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 55.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of Inappropriate Benzodiazepine Prescriptions Among Older Adults Through Direct Patient Education. JAMA internal medicine. 2014;174:890–8. doi: 10.1001/jamainternmed.2014.949. [DOI] [PubMed] [Google Scholar]
- 56.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174:890–8. doi: 10.1001/jamainternmed.2014.949. [DOI] [PubMed] [Google Scholar]
- 57.Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161:332–42. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 58.Baillargeon L, Landreville P, Verreault R, Beauchemin JP, Gregoire JP, Morin CM. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomized trial. Cmaj. 2003;169:1015–20. [PMC free article] [PubMed] [Google Scholar]
- 59.Vicens C, Bejarano F, Sempere E, et al. Comparative efficacy of two interventions to discontinue long-term benzodiazepine use: cluster randomised controlled trial in primary care. The British journal of psychiatry : the journal of mental science. 2014;204:471–9. doi: 10.1192/bjp.bp.113.134650. [DOI] [PubMed] [Google Scholar]
- 60.Gould RL, Coulson MC, Patel N, Highton-Williamson E, Howard RJ. Interventions for reducing benzodiazepine use in older people: meta-analysis of randomised controlled trials. The British journal of psychiatry : the journal of mental science. 2014;204:98–107. doi: 10.1192/bjp.bp.113.126003. [DOI] [PubMed] [Google Scholar]
- 61.Roth TG, Roehrs TA, Koshorek GL, Greenblatt DJ, Rosenthal LD. Hypnotic effects of low doses of quazepam in older insomniacs. J Clin Psychopharmacol. 1997;17:401–6. doi: 10.1097/00004714-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Roger M, Attali P, Coquelin JP. Multicenter, double-blind, controlled comparison of zolpidem and triazolam in elderly patients with insomnia. Clin Ther. 1993;15:127–36. [PubMed] [Google Scholar]
- 63.Elie R, Frenay M, Le Morvan P, Bourgouin J. Efficacy and safety of zopiclone and triazolam in the treatment of geriatric insomniacs. Int Clin Psychopharmacol. 1990;5(Suppl 2):39–46. [PubMed] [Google Scholar]
- 64.Overstall PW, Oldman PN. A comparative study of lormetazepam and chlormethiazole in elderly in-patients. Age Ageing. 1987;16:45–51. doi: 10.1093/ageing/16.1.45. [DOI] [PubMed] [Google Scholar]
- 65.Bayer AJ, Pathy MS. Clinical and psychometric evaluation of two doses of loprazolam and placebo in geriatric patients. Curr Med Res Opin. 1986;10:17–24. doi: 10.1185/03007998609111088. [DOI] [PubMed] [Google Scholar]
- 66.Viukari M, Vartio T, Verho E. Low doses of brotizolam and nitrazepam as hypnotics in the elderly. Neuropsychobiology. 1984;12:130–3. doi: 10.1159/000118125. [DOI] [PubMed] [Google Scholar]
- 67.Vogel GW. Sleep laboratory study of lormetazepam in older insomniacs. Psychopharmacology Suppl. 1984;1:69–78. doi: 10.1007/978-3-642-69659-6_5. [DOI] [PubMed] [Google Scholar]
- 68.Viukari M, Jaatinen P, Kylmamaa T. Flunitrazepam and nitrazepam as hypnotics in psychogeriatric inpatients. Clin Ther. 1983;5:662–70. [PubMed] [Google Scholar]
- 69.Elie R, Deschenes JP. Efficacy and tolerance of zopiclone in insomniac geriatric patients. Pharmacology. 1983;27(Suppl 2):179–87. doi: 10.1159/000137925. [DOI] [PubMed] [Google Scholar]
- 70.Martinez HT, Serna CT. Short-term treatment with quazepam of insomnia in geriatric patients. Clin Ther. 1982;5:174–8. [PubMed] [Google Scholar]
- 71.Caldwell JR. Short-term quazepam treatment of insomnia in geriatric patients. Pharmatherapeutica. 1982;3:278–82. [PubMed] [Google Scholar]
- 72.Fillingim JM. Double-blind evaluation of temazepam, flurazepam, and placebo in geriatric insomniacs. Clin Ther. 1982;4:369–80. [PubMed] [Google Scholar]
- 73.Piccione P, Zorick F, Lutz T, Grissom T, Kramer M, Roth T. The efficacy of triazolam and chloral hydrate in geriatric insomniacs. J Int Med Res. 1980;8:361–7. doi: 10.1177/030006058000800513. [DOI] [PubMed] [Google Scholar]
- 74.Frost JD, Jr, DeLucchi MR. Insomnia in the elderly: treatment with flurazepam hydrochloride. J Am Geriatr Soc. 1979;27:541–6. doi: 10.1111/j.1532-5415.1979.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 75.Goldstein SE, Birnbom F, Lancee WJ, Darke AC. Comparison of oxazepam, flurazepam and chloral hydrate as hypnotic sedatives in geriatric patients. J Am Geriatr Soc. 1978;26:366–71. doi: 10.1111/j.1532-5415.1978.tb03686.x. [DOI] [PubMed] [Google Scholar]
- 76.Reeves RL. Comparison of triazolam, flurazepam, and placebo as hypnotics in geriatric patients with insomnia. J Clin Pharmacol. 1977;17:319–23. doi: 10.1002/j.1552-4604.1977.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 77.Mamelak M, Csima A, Buck L, Price V. A comparative study on the effects of brotizolam and flurazepam on sleep and performance in the elderly. J Clin Psychopharmacol. 1989;9:260–7. [PubMed] [Google Scholar]
- 78.Meuleman JR, Nelson RC, Clark RL., Jr Evaluation of temazepam and diphenhydramine as hypnotics in a nursing-home population. Drug Intell Clin Pharm. 1987;21:716–20. doi: 10.1177/106002808702100908. [DOI] [PubMed] [Google Scholar]
- 79.Dehlin O, Rubin B, Rundgren A. Double-blind comparison of zopiclone and flunitrazepam in elderly insomniacs with special focus on residual effects. Curr Med Res Opin. 1995;13:317–24. doi: 10.1185/03007999509110492. [DOI] [PubMed] [Google Scholar]
- 80.Bayer AJ, Bayer EM, Pathy MS, Stoker MJ. A double-blind controlled study of chlormethiazole and triazolam as hypnotics in the elderly. Acta Psychiatr Scand Suppl. 1986;329:104–11. doi: 10.1111/j.1600-0447.1986.tb10544.x. [DOI] [PubMed] [Google Scholar]
- 81.McCarten JR, Kovera C, Maddox MK, Cleary JP. Triazolam in Alzheimer’s disease: pilot study on sleep and memory effects. Pharmacol Biochem Behav. 1995;52:447–52. doi: 10.1016/0091-3057(95)00116-e. [DOI] [PubMed] [Google Scholar]
- 82.Christensen DB, Benfield WR. Alprazolam as an alternative to low-dose haloperidol in older, cognitively impaired nursing facility patients. J Am Geriatr Soc. 1998;46:620–5. doi: 10.1111/j.1532-5415.1998.tb01081.x. [DOI] [PubMed] [Google Scholar]


