Enhancing amino acid allocation to leaves represents an effective strategy for improving photosynthetic nitrogen use efficiency and oilseed yields in Arabidopsis thaliana under reduced nitrogen input.
Abstract
The coordinated distribution of nitrogen to source leaves and sinks is essential for supporting leaf metabolism while also supplying sufficient nitrogen to seeds for development. This study aimed to understand how regulated amino acid allocation to leaves affects photosynthesis and overall plant nitrogen use efficiency in Arabidopsis (Arabidopsis thaliana) and how soil nitrogen availability influences these processes. Arabidopsis plants with a knockout of AAP2, encoding an amino acid permease involved in xylem-to-phloem transfer of root-derived amino acids, were grown in low-, moderate-, and high-nitrogen environments. We analyzed nitrogen allocation to shoot tissues, photosynthesis, and photosynthetic and plant nitrogen use efficiency in these knockout plants. Our results demonstrate that, independent of nitrogen conditions, aap2 plants allocate more nitrogen to leaves than wild-type plants. Increased leaf nitrogen supply positively affected chlorophyll and Rubisco levels, photosynthetic nitrogen use efficiency, and carbon assimilation and transport to sinks. The aap2 plants outperformed wild-type plants with respect to growth, seed yield and carbon storage pools, and nitrogen use efficiency in both high and deficient nitrogen environments. Overall, this study demonstrates that increasing nitrogen allocation to leaves represents an effective strategy for improving carbon fixation and photosynthetic nitrogen use efficiency. The results indicate that an optimized coordination of nitrogen and carbon partitioning processes is critical for high oilseed production in Arabidopsis, including in plants exposed to limiting nitrogen conditions.
Nitrogen (N) is an essential nutrient that plants require for the synthesis of amino acids, proteins, and many other important metabolites. Consequently, the amount of N that is assimilated and distributed from roots to source leaves and finally to developing sinks, like fruits and seeds, has significant consequences for plant metabolism and growth. Importantly, N and carbon (C) metabolism are highly interrelated (Palenchar et al., 2004; Krapp et al., 2005; Nunes-Nesi et al., 2010). Photosynthesis and subsequent respiration provide the C skeletons and energy required for the synthesis of amino acids (Lewis et al., 2000; Nunes-Nesi et al., 2010), whereas the majority of leaf N is present in proteins essential for C assimilation and metabolism (Evans, 1989a; Hikosaka and Terashima, 1996). In fact, Rubisco, the primary enzyme in C fixation, alone can account for up to 30% of the total N and 60% of the soluble proteins in mature source leaves (Sage et al., 1987; Makino and Osmond, 1991; Osaki et al., 1993; Warren et al., 2000). Accordingly, photosynthetic nitrogen use efficiency (PNUE) is related to the proportion of leaf N used for C fixation per unit of leaf area (Poorter and Evans, 1998; Onoda et al., 2004; Warren and Adams, 2006).
It has been demonstrated that PNUE can be improved in Brassicaceae when N assimilation in leaves is stimulated through exogenous ethylene supply (Iqbal et al., 2012). In addition, repression of Rubisco synthesis in transgenic rice (Oryza sativa) plants resulted in higher Rubisco activity and increased PNUE, although overall C fixation was decreased (Makino et al., 1997). In general, PNUE is influenced by the amount of photosynthetic machinery that is produced and is negatively correlated with N supply (Nakano et al., 1997; Onoda et al., 2004; Takashima et al., 2004). Furthermore, PNUE is impacted by C metabolism and the C demand of the plant (Hikosaka et al., 1998) and, subsequently, is an important factor for the production of carbohydrates, energy, and structural compounds as well as for plant growth and seed development. Plants must balance N allocation in support of leaf metabolism with supplying sufficient N to developing sinks. Plants that display improved PNUE, therefore, also tend to exhibit higher overall plant nitrogen use efficiency (NUE) for seed production (Brown, 1978). However, strategies for crop improvement generally have not integrated PNUE with plant NUE (Hirel et al., 2007; Kant et al., 2012).
NUE is determined by the seed yield relative to N supply and generally is divided into two major components, nitrogen uptake efficiency (NUpE) and nitrogen utilization efficiency (NUtE). Plant NUE is a complex trait that is governed by many physiological processes, including N uptake, assimilation, metabolism, allocation, and remobilization, as well as environmental factors, such as the availability of soil N (Habash et al., 2001; Tsay et al., 2011; Girondé et al., 2015). Many plants display a relatively low NUE because they are ineffective at accessing soil N and may take up as little as 41% of supplied N fertilizer (Hodge et al., 2000; Kumar and Goh, 2002; Thornton and Robinson, 2005; Yang et al., 2015; Zhu et al., 2016). Research on improving NUE has focused on the manipulation of nitrate and ammonium transporters (Fraisier et al., 2000; Yuan et al., 2007; Tsay et al., 2011; Fang et al., 2013; Ranathunge et al., 2014; Bao et al., 2015; Chen et al., 2016, 2017; Fan et al., 2016; Wang et al., 2018a, 2018b), associated transcription factors (Qu et al., 2015; Araus et al., 2016), or the ectopic expression of genes involved in amino acid synthesis and N metabolism (Ameziane et al., 2000; Chichkova et al., 2001; Habash et al., 2001; Yamaya et al., 2002; Seiffert et al., 2004; Good et al., 2007; Shrawat et al., 2008; McAllister et al., 2012; Peña et al., 2017). Although promising results have been achieved with respect to seed yields and NUE, these studies rarely analyzed both NUE-contributing factors, NUpE and NUtE, or the effects of different N environments (Kiba and Krapp, 2016; Tegeder and Masclaux-Daubresse, 2018).
In most plant species, N partitioning within the plant and to sinks occurs in the form of amino acids, and recent studies have demonstrated that altering amino acid transport processes from leaves to seeds impacts N and C assimilation, seed development (Rolletschek et al., 2005; Schmidt et al., 2007; Weigelt et al., 2008; Zhang et al., 2010, 2015; Carter and Tegeder, 2016; Santiago and Tegeder, 2016, 2017), and overall plant NUE (Perchlik and Tegeder, 2017). Here, it was hypothesized that regulated amino acid allocation from the root to leaves influences PNUE and also affects plant NUE. Root-synthesized amino acids are transported in the xylem to mature source leaves, where they are used for metabolism, stored, or loaded into the phloem to supply sinks with N (Atkins et al., 1983). In addition, along the path from root to leaf, up to 21% of the organic N may be transferred from the xylem to the phloem to directly supply developing sinks with N (Pate et al., 1975; van Bel, 1984). In Arabidopsis (Arabidopsis thaliana), AMINO ACID PERMEASE2 (AAP2) is involved in this xylem-to-phloem transfer step (Zhang et al., 2010), and knockout of AAP2 results in decreased N delivery to seeds and reduced seed protein levels (Zhang et al., 2010). However, in these aap2 mutants, amino acid allocation to leaves was increased, positively affecting photosynthesis and Suc transport to sinks. This resulted in higher seed yields and seed oil levels (Zhang et al., 2010). This study aimed to resolve how regulated amino acid allocation to leaves impacts photosynthesis and PNUE, how plant N and C status and overall NUE are affected, and how soil N availability influences these processes.
It is demonstrated that, independent of the amount of N nutrition, aap2 plants move significantly more N to leaves, promoting leaf growth and photosynthetic surface area. In addition, shifts in leaf N pools and rates of electron transport occurred in favor of photosynthesis, resulting in enhanced C fixation and PNUE. Furthermore, the transgenic plants displayed improved NUpE and significant increases in total shoot N and C. Together, the increased amino acid delivery to aap2 leaves and a subsequent enhanced sink C supply resulted in higher NUE, including under N stress conditions. Overall, our work demonstrates that, independent of the soil N conditions, increasing N transport to leaves provides a promising approach for optimizing N use in support of C fixation as well as for sink development and oilseed yield.
RESULTS
C Fixation Is Elevated in aap2 Plants under High-N and N-Stress Conditions
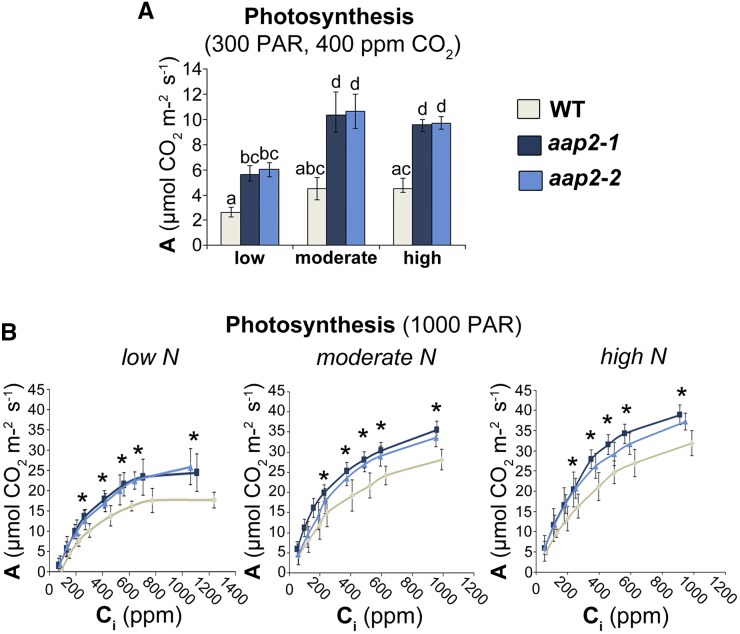
The aap2 and wild-type plants were grown in N-deficient soil containing low, moderate, or high N, and photosynthetic rates were analyzed under ambient light and CO2 concentrations (Fig. 1A). Our results demonstrate that photosynthesis generally increased with higher fertilization, similar to prior reports (Fredeen et al., 1991; Cechin and Fumis, 2004; Dordas and Sioulas, 2008). Within each N fertilization regime, photosynthetic rates were always higher in aap2 plants compared with the wild type, with increases ranging between 113% and 136% (Fig. 1A). Similarly, analysis of photosynthesis under high light showed an increase in C assimilation rates in aap2 plants when atmospheric CO2 levels were 400 µL L−1 or higher (Fig. 1B). This supports that the knockout of AAP2 leads to improved photosynthesis, even in N-limited environments and when measured at higher CO2 concentrations.
Figure 1.
Photosynthesis in Arabidopsis aap2 and wild-type (WT) plants grown for 5 weeks in 12-h days under low-, moderate-, or high-N conditions. A, Photosynthetic rate (A) under ambient light (300 µmol photons m−2 s−1 photosynthetically active radiation [PAR]) and CO2 (400 µL L−1; n ≥ 4). B, Response of A to internal CO2 concentration (Ci) under light-saturating conditions (1,000 PAR; n ≥ 4). Data are means ± sd. Significant differences are indicated by letters (A) or asterisks (B; ANOVA; P ≤ 0.05).
Rubisco Content and Rates of Electron Transport Are Elevated in aap2 Plants under High and Deficient N Supply
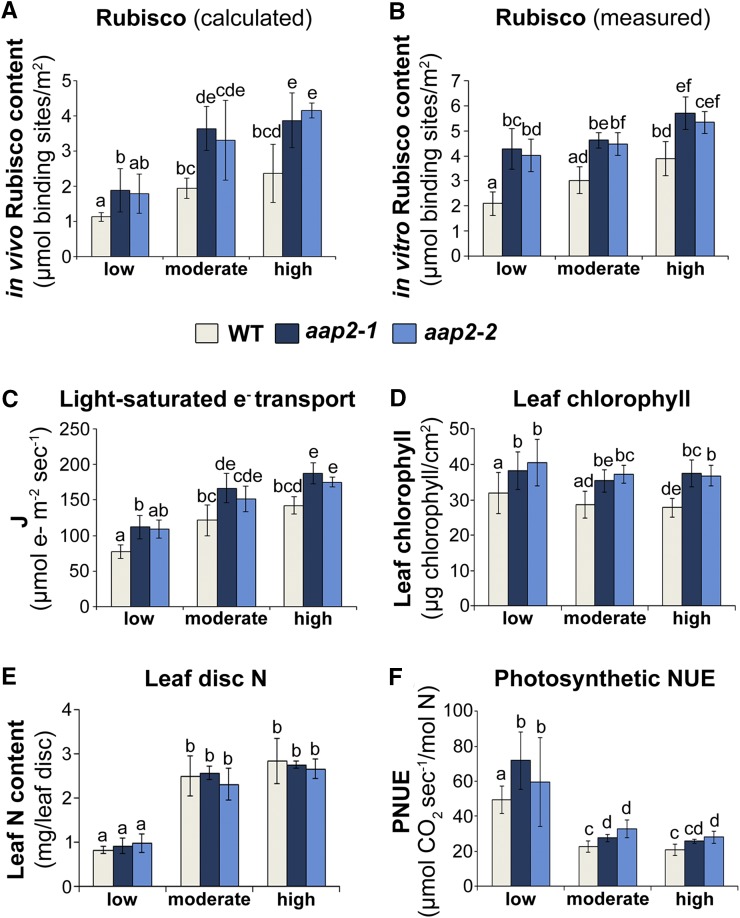
To understand which components of the photosynthetic machinery contribute to the increase in C fixation, the Rubisco content as well as the electron transport rate under light-saturated conditions were determined. Leaf Rubisco content was both calculated based on in vivo photosynthetic CO2 response curves (Fig. 2A) and measured using an in vitro assay (Fig. 2B). Both methods showed that the aap2 plants contain significantly higher Rubisco levels in leaves than wild-type plants under all N conditions. Depending on the method and N treatment, observed increases in Rubisco contents ranged from 37% to 88% (Fig. 2, A and B).
Figure 2.
Analysis of components of the photosynthetic apparatus and PNUE. Arabidopsis aap2 and wild-type (WT) plants were grown in 12-h days for 5 weeks under low-, moderate-, or high-N conditions. A, Calculated apparent Rubisco content (n ≥ 4). B, Measured Rubisco content per rosette leaf disc (n = 5). C, Calculated electron transport rates (J) based on the CO2-response curves measured under light-saturating conditions (n ≥ 4). D, Total chlorophyll content (n ≥ 15). E, N content in rosette leaf discs (n = 5). F, PNUE, calculated based on photosynthesis at 400 µL L−1 CO2 relative to the amount of N in a leaf disc (n = 5). Data are means ± sd. Significant differences are indicated by letters (ANOVA; P ≤ 0.05).
Maximum CO2 assimilation is limited by the regeneration of ribulose bisphosphate (RuBP), and successively, RuBP regeneration is dependent on the ATP and NADH supply from the light-dependent electron transport in the chloroplast (Farquhar, 1979). Therefore, the light-saturated electron transport rate provides an appropriate measure for the effects of N fertilization on the CO2 assimilation capacity of aap2 leaves. Electron transport rate was calculated using the A-Ci response curves and standard biochemical models of C3 photosynthesis (see “Materials and Methods”; von Caemmerer, 2000). The results show that the electron transport rate was significantly higher in the aap2 plants relative to wild-type plants under all N treatments (Fig. 2C). Since electron transport rates are generally impacted by chlorophyll abundance (von Caemmerer and Farquhar, 1981; Friend, 1995), leaf chlorophyll content was assayed. The data demonstrate that aap2 source leaves contained 16% to 46% more chlorophyll than wild-type leaves within each N condition (Fig. 2D). Together, photosynthesis, electron transport, Rubisco, and chlorophyll analyses (Figs. 1 and 2, A–D) support that the aap2 lines have a higher CO2 assimilation capacity than the control plants.
PNUE of aap2 Plants Is Elevated under All N Conditions
To determine the PNUE of aap2 plants, first, the total elemental N content of source leaf discs was determined. Within each N treatment, no differences in total N per leaf area were observed between mutant and wild-type plants (Fig. 2E). PNUE was then calculated by relating photosynthesis rates measured under ambient growth conditions to leaf N content (Fig. 1A; see “Materials and Methods”). As expected, PNUE was higher when plants were exposed to severe N deficiency compared with moderate- and high-N conditions (Fig. 2F; Kochsiek et al., 2006; Ibrahim et al., 2010). More importantly, the aap2 plants exhibited higher PNUE than wild-type plants, regardless of the N condition (Fig. 2F). Furthermore, increases in Rubisco and chlorophyll levels in mutants versus wild-type plants (Fig. 2, A, B, and D) but no changes in elemental N per leaf area (Fig. 2E) suggest that a higher proportion of leaf N is channeled into the synthesis of the photosynthetic machinery in aap2 plants, resulting in enhanced C fixation.
Total Leaf Area and Total C and N Content of Rosette Leaves Are Elevated during Vegetative Growth under All N Conditions
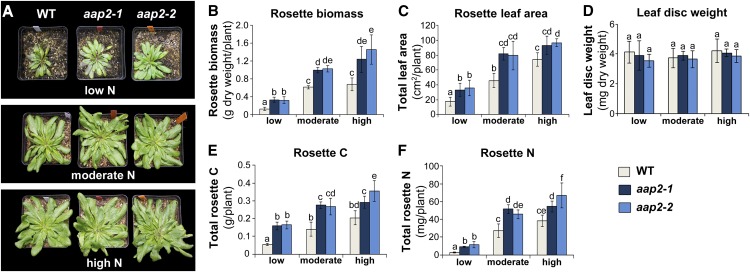
The effect of N supply on rosette leaf growth was examined at the vegetative stage (Fig. 3, A–D). Our results showed that the rosette leaf (i.e. shoot) biomass was increased significantly in aap2 plants compared with wild-type plants under all N treatments (Fig. 3, A and B). The transgenic plants produced 109%, 106%, and 42% more leaf biomass than wild-type plants in low-, moderate-, and high-N environments, respectively (Fig. 3B). Additionally, aap2 plants displayed a 26% to 106% increase in total leaf area per rosette in all N treatments (Fig. 3, A and C). No change in leaf disc weight was detected between aap2 and wild-type plants or among the different N conditions (Fig. 3B), suggesting no differences in leaf density or thickness.
Figure 3.
Analysis of leaf development and total elemental N and C content in rosette leaves of Arabidopsis aap2 and wild-type (WT) plants. Plants were grown in 12-h days for 5 weeks under low-, moderate-, or high-N conditions. A, Composite images of two aap2 mutant lines and wild-type plants exposed to the different N regimes. Photographs of single plants were put together on black backgrounds, assembled in a panel, and labeled using Adobe Photoshop CS5. B, Total dry weight of source rosette leaves per plant (n ≥ 4). C, Total surface area of rosette leaves (n ≥ 3). D, Weight of rosette leaf discs with 2-cm2 surface area (n ≥ 5). E, Total C content of rosette leaves per plant (n = 5). F, Total N content of rosette leaves per plant (n = 5). Data are means ± sd. Significant differences are indicated by letters (ANOVA; P ≤ 0.05).
To determine the effects of the loss of AAP2 function on total shoot C and N gain, elemental C and N levels of rosettes were analyzed (Fig. 3, E and F). Within each N treatment and dependent on the amount of N supplied, the leaves of aap2 lines showed a 43% to 202% higher C content than wild-type leaves (Fig. 3E). Similarly, the transgenic plants contained significantly more total shoot N than wild-type plants in all N fertilization regimes (Fig. 3F). Dependent on the N condition, the increases ranged from 42% to 323%. The differences in C and N content seem to be related to differences in biomasses, since leaf disc weights and N (and C) content per leaf disc were unchanged. Together, these data support that the transgenic plants assimilate and allocate more C and N to the shoot/rosette leaves, resulting in increased leaf size and biomass.
Shoot Biomass and Silique Number Are Elevated in aap2 Plants at the Reproductive Stage
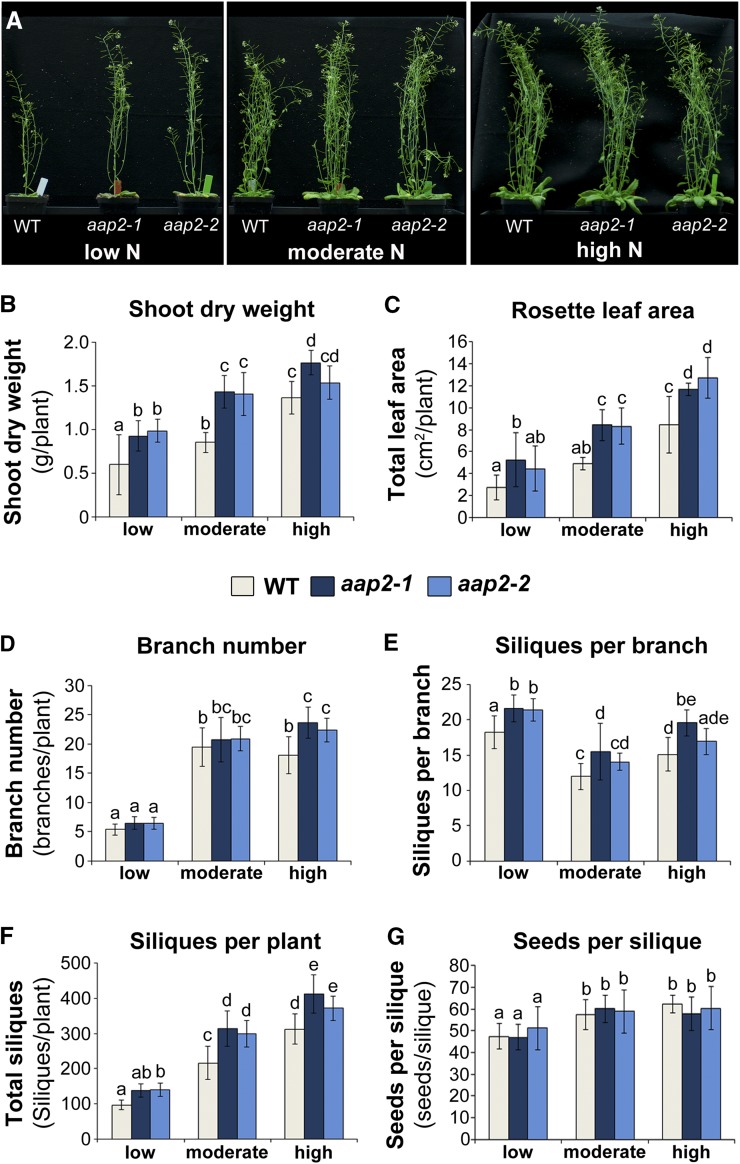
The effects of N fertilization were analyzed at the reproductive stage (i.e. leaves, stem, branches, siliques, and seeds) in aap2 plants grown under a 16-h photoperiod (Fig. 4). Within each N treatment, the total shoot dry weight was increased in aap2 plants relative to wild-type plants, and depending on the N fertilization, the increases ranged between 13% and 68% (Fig. 4, A and B). As seen at the vegetative stage and when plants were grown in 12-h (short) days (Fig. 3C), total leaf surface area of the rosettes also was increased in 16-h (long) days and at the reproductive stage in aap2 mutants versus wild-type plants under all N conditions (Fig. 4C). Furthermore, aap2 and wild-type plants produced fewer branches with high numbers of siliques per branch under low N versus more branches with fewer siliques per branch at moderate and high N (Fig. 4, D and E). When comparing the mutants with wild-type plants, the branch number was only increased significantly under high-N conditions (Fig. 4D). However, aap2 mutants produced more siliques per branch under all N treatments (Fig. 4E), resulting in an increased number of total siliques per plant (Fig. 4F). Analysis of the seed number per silique showed no differences between aap2 mutants and wild-type plants (Fig. 4G).
Figure 4.
Analysis of shoot, fruit, and seed development. Arabidopsis aap2 and wild-type (WT) plants were grown in 16-h days for 6 weeks under low-, moderate-, or high-N conditions. A, Composite images of two aap2 mutant lines and wild-type plants. Mutant and wild-type plants from each N treatment were photographed. The three images were put on black backgrounds, assembled in a panel, and labeled using Adobe Photoshop CS5. B, Total shoot dry weight per plant (n ≥ 10). C, Total surface area of rosette leaves (n ≥ 3). D, Total branch number per plant (n ≥ 8). E, Siliques per branch (n ≥ 8). F, Total siliques per plant (n ≥ 7). G, Seeds per silique on the main branch (n ≥ 17). Data are means ± sd. Significant differences are indicated by letters (ANOVA; P ≤ 0.05).
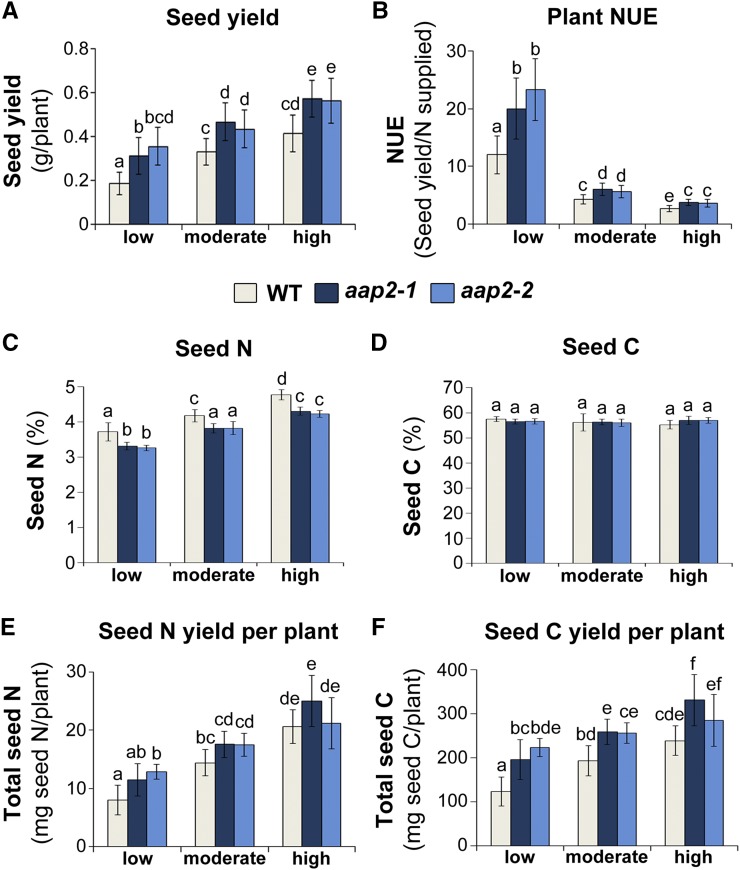
Seed Yield, NUE, and Seed C Gain Are Elevated in aap2 Plants under All N Conditions
The effects of N fertilization on seed yield and NUE of aap2 plants were analyzed. Generally, with increasing N supply, transgenic and wild-type plants displayed higher seed yields but lower NUE (Fig. 5, A and B). More importantly, under each N condition tested, aap2 plants achieved significantly higher seed yields and NUE than wild-type plants (Fig. 5, A and B). Seed yield increases of 32% to 41% were obtained in aap2 plants under moderate and high N and up to 91% in low N (Fig. 5A). Notably, aap2 mutants grown with moderate N produced similar yields to wild-type plants that were grown with twice the amount of N (i.e. high N) and displayed up to 125% higher NUE (Fig. 5, A and B). Together, these results demonstrate that aap2 mutants outperform wild-type plants with respect to both seed yield and NUE under all N conditions.
Figure 5.
Analysis of plant NUE and seed N and C content of Arabidopsis aap2 and wild-type (WT) plants at harvest. Plants were grown in 16-h days for 8 weeks under low-, moderate-, or high-N conditions. A, Seed yield per plant (n ≥ 12). B, Plant NUE, calculated based on seed yield relative to N supply (n ≥ 12). C, N content of seeds in percentage (n = 6). D, C content of seeds in percentage (n = 6). E, Total seed N content per plant (n = 6). F, Total seed C content per plant (n = 6). Data are means ± sd. Significant differences are indicated by letters (ANOVA; P ≤ 0.05).
In order to evaluate the effects of N nutrition on N and C allocation to aap2 seeds, seed N and C content was analyzed. Overall, with increasing N fertilization, seed N content was increased in all Arabidopsis plants, while seed C content was unaffected (Fig. 5, C and D). Within each N treatment, seed N levels were decreased in aap2 relative to wild-type plants (Fig. 5C) and seed C content was unchanged (Fig. 5D). When calculating the total seed N and C yield per plant by using seed yield and seed N/C content data (Fig. 5, A and D), no differences were observed for seed N per plant within each N treatment (Fig. 5E), whereas seed C yields were increased from 19% to 80% for aap2 plants (Fig. 5F). These data support that, in the aap2 mutants, more C is allocated to seed sinks, while N source-sink partitioning is unchanged under all N fertilization regimes.
Total Shoot C and N Levels of aap2 Plants Are Elevated under All N Conditions
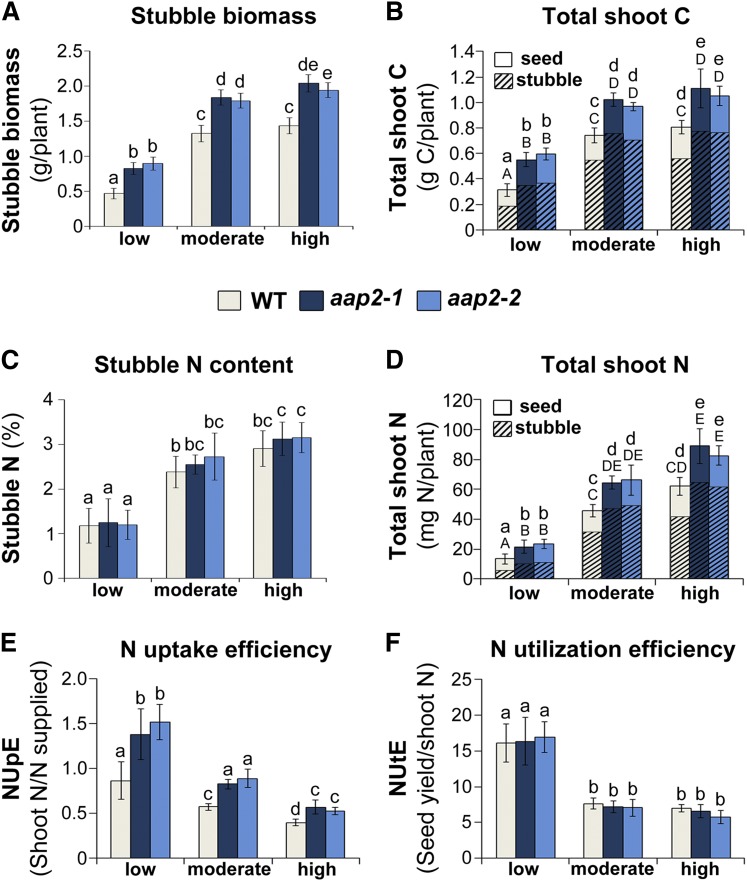
At the harvest time point, total shoot biomass is composed of seed (Fig. 5A) and stubble tissue (Fig. 6A), which included rosette leaves, cauline leaves, stems, and silique walls (i.e. all aboveground tissue except seeds). Analysis of stubble materials showed that, independent of the N fertilization, aap2 plants produced significantly more stubble biomass (Fig. 6A), which agrees with the observed increases in rosette leaf size, silique (wall) amounts, and, in some cases, branch numbers (Fig. 4, C, D, and F).
Figure 6.
Analysis of stubble biomass, shoot C and N distribution, NUpE, and NUtE of Arabidopsis aap2 and wild-type (WT) plants at harvest. Plants were grown in 16-h days for 8 weeks under low-, moderate-, or high-N conditions. Stubble includes rosette and cauline leaves, stems, and silique walls and excludes seeds. For total shoot analyses, all aboveground tissue was used (i.e. stubble and seeds). A, Stubble dry weight per plant (n = 6). B, Total shoot C as a sum of stubble and seed C per plant (n = 6). C, N content of stubble in percentage (n = 6). D, Total shoot N as a sum of stubble and seed N per plant (n = 6). E, Plant NUpE, calculated based on total shoot N relative to N supply (n = 6). F, Plant NUtE, calculated based on seed yield relative to total shoot N (n = 6). Data are means ± sd. Significant differences are indicated by letters (ANOVA; P ≤ 0.05).
To resolve the contribution of stubble to total shoot C and N content, elemental C and N levels in stubble were analyzed. Stubble C represented the major C pool in the shoot of all Arabidopsis plants. It contributed between 60% and 64% of the total shoot C when N supply was low and between 70% and 75% in moderate- and high-N environments, respectively (Fig. 6B). When comparing aap2 mutants with wild-type plants, total stubble C was increased significantly in the mutants under all N regimes. Together with the increases in total C seed yield (Fig. 5F), this led to an overall C gain in aap2 versus wild-type shoots from 30% to 90%, depending on the N fertilization treatment (Fig. 6B).
Analysis of the stubble N content showed that, while the percentage of N content of stubble tissues was similar between mutants and wild-type plants at low, moderate, or high N (Fig. 6C), total stubble N content per plant was increased significantly in aap2 plants within each N treatment (Fig. 6D) due to more stubble biomass (Fig. 6A). Adding up the stubble and seed N to determine total shoot N per plant (Figs. 5E and 6D) resolved that the majority of shoot N was present in stubble tissue for both aap2 and wild-type plants grown under moderate and high N, with stubble contributing between 68% and 74% of the total shoot N. In low-N environments, however, only 40% to 47% of the total shoot N was present in stubble, while seed N represented the major N pool (Fig. 6D). This is in line with previous studies showing that Arabidopsis plants remobilize more leaf N and allocate a higher proportion of stubble N to seeds when the N supply is low (Schulze et al., 1994; Masclaux-Daubresse and Chardon, 2011). Furthermore, within each N treatment, aap2 mutants contained 75%, 45%, and 43% more total shoot N than wild-type plants when grown in low-, moderate-, and high-N environments, respectively (Fig. 6D). For all N fertilization regimes, this increase in total shoot N was due solely to significant increases in total stubble N and not to changes in seed N (Figs. 5E and 6D). Overall, these results support that aap2 plants take up and allocate more N to the shoot than wild-type plants.
The NUpE of aap2 Mutants Is Elevated under All N Conditions
To analyze if aap2 mutants are more effective than wild-type plants in N uptake and/or in the utilization of N for seed production, both NUpE and NUtE were calculated. NUpE describes the amount of N supplied relative to the N that was taken up and allocated to the shoot, while NUtE conveys the relationship between seed yield and total shoot N. In general, NUpE and NUtE increased in both aap2 and wild-type plants with lower N supply, similar to previous reports (Fig. 6, E and F; Kaul et al., 2005; Beatty et al., 2010). When comparing aap2 and wild-type plants within each N treatment, the transgenic plants displayed significantly higher NUpE (Fig. 6E), while no differences in NUtE were observed (Fig. 6F). Together, these data support that the increases in seed yield and NUE of aap2 plants (Fig. 5, A and B) are due to higher NUpE and N allocation to leaves as well as to the subsequent improvement in C capture and transport to seeds (Fig. 6, B, D, and E).
DISCUSSION
Amino Acid Allocation to Source Leaves Influences C Fixation and PNUE under High-N and N-Stress Conditions
Photosynthetic capacity is highly related to the leaf N content, as the majority of leaf N is present in chlorophyll and in proteins of the thylakoids and the Calvin-Benson cycle (e.g. Rubisco; Evans, 1989a; Evans and Poorter, 2001; Ripullone et al., 2003). This study demonstrates that knocking out the Arabidopsis amino acid transporter AAP2 leads to increased N allocation to photosynthetically active rosette/source leaves, independent of how much soil N is available to the plant (Fig. 3F). The additional N then stimulates shoot biomass production (Figs. 3, A and B, and 4, A and B), including the development of larger leaves (Figs. 3C and 4C). Leaf N content per unit of area was not changed (Fig. 2E), but the levels of chlorophyll and Rubisco per leaf area were increased in the mutants versus wild-type plants (Fig. 2, A, B, and D), suggesting that less N is invested in the transient storage of amino acids (Zhang et al., 2010) or in insoluble N pools (Evans and Poorter, 2001; Ripullone et al., 2003) and that a higher proportion of leaf N is channeled into synthesis of the photosynthetic machinery. In fact, the optimized N allocation to, and within, aap2 leaves resulted in increased photosynthesis in all N environments (Fig. 1).
During high as well as limited N nutrition, photosynthetic activity can be influenced by affecting components of (1) the light reaction, and thereby in the production of ATP and NADPH through light absorption and electron transport in the chloroplast, and/or (2) the dark reaction, in which ATP and NADPH from the light reaction are consumed to reduce inorganic C and to regenerate RuBP, which is then carboxylated by Rubisco (von Caemmerer and Farquhar, 1981; Sage et al., 1988; Genty et al., 1989). Furthermore, the physiological state and the C demand of the plant impact ATP and NADPH consumption (Schwender et al., 2006; Vanhercke et al., 2014), which, in turn, controls electron movement through the light-dependent electron transport chain (Khamis et al., 1990; Fernie et al., 2004). This study supports that enhanced N partitioning to leaves can improve the performance of both the light and dark reactions, as chlorophyll amounts, electron transport rate (and RuBP regeneration), and Rubisco content were increased (Fig. 2, A–D). This is in line with previous reports showing a positive correlation between leaf Rubisco content, electron transport, and N nutrition (Ripullone et al., 2003; Bown et al., 2007; Liberloo et al., 2007; Eichelmann et al., 2009) and between leaf N content and photosynthesis (Evans, 1989a, 1989b; Onoda et al., 2004; Takashima et al., 2004). This work also suggests that pushing increased amounts of N into leaves induces adjustments in the leaf N balance and modifications in N allocation to different components of the photosynthetic apparatus (Fig. 2; Fuentes et al., 2001; Iqbal et al., 2012). While the signal(s) for cellular N distribution remains unclear, the shift in N usage in aap2 leaves may be triggered by alterations in local N metabolite pools or by changes in sink C demand (Fig. 3, A, B, and E; Paul and Foyer, 2001; Stitt et al., 2010; Zhang et al., 2010). On the other hand, pulling N into specific photosynthetic elements, such as Rubisco, by using, for example, an overexpression approach, seems less successful (Suzuki et al., 2007), as it might affect photosynthetic plasticity.
PNUE depends on the leaf N content as well as the proportion of leaf N used for the different components of the photosynthetic machinery (Westbeek et al., 1999; Onoda et al., 2004; Pons and Westbeek, 2004; Takashima et al., 2004). Typically, higher N fertilization raises the leaf N concentration more than corresponding increases in photosynthetic rates, thereby lowering PNUE (Kochsiek et al., 2006; Dordas and Sioulas, 2008; Ibrahim et al., 2010). However, as pointed out above, the N content was not changed in aap2 leaves; rather, a higher proportion of leaf N was used for the photosynthetic apparatus, supporting higher C assimilation rates per unit of N, or PNUE (Figs. 1 and 2). This is in line with studies comparing different plant species and/or developmental stages of leaves, where high PNUE was associated with a favorable distribution of N to photosynthetic elements (e.g. chlorophyll, Rubisco, and RuBP carboxylase) versus other cellular N pools (e.g. cell wall proteins and structural compounds; Evans, 1989b; Warren et al., 2000; Onoda et al., 2004; Takashima et al., 2004). Most importantly, our results demonstrate that optimizing N allocation to leaves results in an up-regulation of photosynthesis and PNUE under high and deficient N as well as in a substantial increase in plant C gain (up to 90%; Figs. 1, 2F, and 6B).
Optimized N Allocation to and within Leaves Leads to Improved NUE
The efficiency of N use and plant adaptation to less favorable N environments are highly complex processes (Moll et al., 1982; Ortiz-Monasterio et al., 1997; Xu et al., 2012) but generally are associated with both N uptake capacity and the efficiency of N utilization for seed production (Kaul et al., 2005; Beatty et al., 2010). Strategies to increase N uptake have focused on the overexpression of root transporters involved in nitrate and ammonium uptake or related transcription factors. But often, studies only demonstrated improvements in a particular N environment (Yuan et al., 2007; Ranathunge et al., 2014; Qu et al., 2015; Chen et al., 2016, 2017; Fan et al., 2016), showed no or negative effects on N uptake and/or growth (Fraisier et al., 2000; Kaiser et al., 2002; Kumar et al., 2006; Bao et al., 2015; Araus et al., 2016), or did not determine NUpE (Katayama et al., 2009; Fang et al., 2013; Wang et al., 2018a). However, some studies support that amino acid transport processes in the shoot and/or associated changes in the shoot N status exert regulatory control over N uptake in roots (Tan et al., 2010; Zhang et al., 2015; Santiago and Tegeder, 2016; Perchlik and Tegeder, 2017). For example, overexpression of AAP1 in pea (Pisum sativum) plants led to increased amino acid phloem loading, which positively affected N acquisition and, subsequently, N assimilation and usage in source and sink tissues (Zhang et al., 2015). Furthermore, NUpE was improved in these plants when grown with sufficient N but unchanged under N deficiency (Perchlik and Tegeder, 2017). In this study, the Arabidopsis aap2 plants showed significantly higher shoot N levels and increased NUpE in all N conditions (Figs. 3F and 6, D and E), supporting that the mutant lines take up and allocate more N to the shoot than wild-type plants, even under severe N deficiency. Why Arabidopsis aap2 mutants and pea AAP1-overexpressing plants perform differently with respect to NUpE at low N is unclear, but the effects may be species specific due to the respective genetic manipulation or caused by differences in shoot N utilization (see below; Perchlik and Tegeder, 2017). Nevertheless, changes in leaf N concentrations or pools in aap2 plants probably affect shoot-to-root signaling and trigger positive feedback regulation of N uptake (Ruffel et al., 2011; Zhang et al., 2015; Santiago and Tegeder, 2016; Ohkubo et al., 2017; Perchlik and Tegeder, 2017). Such phloem-mobile signals, for example, may involve nitrate or particular amino acids (Miller et al., 2008; Forde, 2014) or require the production of specific molecules, including the C-terminally encoded peptide downstream1 (CEPD1) and CEPD2 (Tabata et al., 2014; Ohkubo et al., 2017; Tegeder and Masclaux-Daubresse, 2018).
A number of studies have demonstrated a positive correlation between N uptake and seed development (López-Bellido and López-Bellido, 2001; Fageria and Baligar, 2005; Coque and Gallais, 2007). However, in this study, the increased amount of N taken up and allocated to aap2 shoots was not used directly for seed production, as individual seeds contain less N and the total seed N yield per plant is not changed (Fig. 5, C and E). Compared with other plant species, Arabidopsis plants generally display low N remobilization and utilization efficiency, and a large portion of the shoot N remains in the leaf or stubble tissue at harvest (Fig. 6D; Masclaux-Daubresse et al., 2010; Masclaux-Daubresse and Chardon, 2011; Guiboileau et al., 2012). Obviously, changes in amino acid xylem-to-phloem transfer did not affect N usage for seed production in aap2 plants; instead, the additional N was invested in growth and the physiological function of aap2 stubble tissue, especially of rosette leaves (Figs. 3F, 5E, and 6D). During the reproductive phase, the extra N in aap2 leaves/shoot seems not to be remobilized for seed production, since aap2 stubble tissue still contained more N than wild-type stubble tissue at the harvest time point and seed N levels were lower in the mutants compared with the control (Figs. 5E and 6, C and D). Limited N remobilization also is supported by previous work showing that aging aap2 leaves maintain higher chlorophyll levels relative to wild-type leaves (Zhang et al., 2010). As discussed for stay-green mutants, the preservation of the photosynthetic apparatus in older leaves may be critical for seed nutrient supply during the seed-filling phase (Borrel et al., 2001; Spano et al., 2003; Christopher et al., 2008; Derkx et al., 2012).
Coordinated N and C Partitioning Processes Are Essential for Efficient Plant N Use
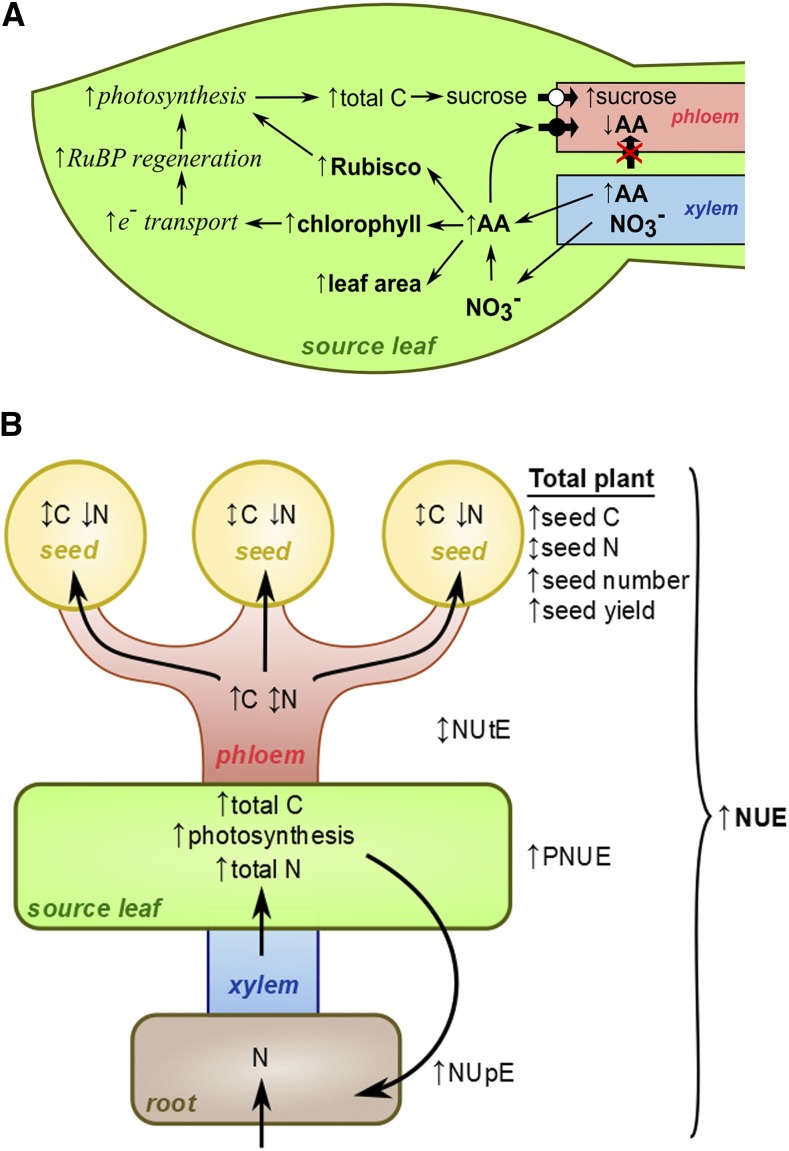
Despite displaying similar shoot NUEs for seed development to the wild-type plants, aap2 plants produced significantly more fruits and higher seed yields and displayed increased NUE under high as well as suboptimal N conditions (Figs. 4F, 5, A and B, and 6F). A number of factors may contribute to this success, as follows. (1) Increased N uptake and allocation to aap2 leaves support an increase in total leaf area for photosynthesis as well as enhanced C fixation per unit of leaf area, together leading to strongly improved C capture as well as C transport from leaves to growing sinks (see above; Fig. 7A). (2) Overall source-to-sink N supply is unchanged (Fig. 5E), thereby avoiding N-limitation conditions for seed development (Guiboileau et al., 2012; Santiago and Tegeder, 2016). (3) Increases in C partitioning to sinks (Fig. 5F) and/or in the C-N ratio in the phloem under all N regimes (Fig. 7B; Zhang et al., 2010) may trigger increased fruit and seed development (Munier-Jolain and Salon, 2003; Munier-Jolain et al., 2008; Braun et al., 2014).
Figure 7.
Effects of optimized amino acid allocation to and within Arabidopsis aap2 leaves at the leaf (A) and whole-plant (B) level. In general, improvements in aap2 versus wild-type plants were achieved independently of how much N was supplied. A, Effects on the photosynthetically active leaf area, components of the photosynthetic machinery, C fixation, and leaf export of C (Suc) are shown. Overall, increased amino acid allocation to leaves positively affects both the light and dark reactions of photosynthesis as well as the leaf area available for C fixation, leading to a significant increase in C capture and export in the leaf phloem. B, Effects on root-to-shoot N supply, N and C source-to-sink partitioning, N and C tissue status, plant productivity, and NUE are demonstrated. Increased amino acid allocation to leaves positively affects NUpE and root-to-shoot N transport, probably by a feedback regulatory mechanism. In leaves, the N is used effectively for photosynthesis (see A), resulting in increased PNUE and enhanced total C gain. Increased C transport to sinks and/or changes in the C-to-N ratio lead to increases in seed number and yield as well as overall NUE. Within seeds, N amounts are reduced while C levels are unchanged, which, in Arabidopsis, relate to a decrease in protein and an increase in oil levels, respectively (Zhang et al., 2010). NUtE or total harvestable seed N is unchanged, while total seed C yield per plant is improved. Overall, this work supports the notion that increasing N allocation to leaves provides an effective strategy for improving photosynthetic and plant NUE and harvestable seed C yield in a range of N conditions. Arrows with circles represent transporters for amino acids (black) or Suc (white). Arrows located to the left of features analyzed indicate significant changes in aap2 mutants compared with wild-type plants (up, increase; down, decrease; up and down, no change).
Clearly, in aap2 plants, changes in N uptake and allocation to leaves, as well as increases in leaf C metabolism and partitioning to seeds, have positive consequences for seed yield, independent of how much N is supplied. However, in all N environments, N levels in individual aap2 seeds were decreased and their C content was unchanged, likely due to the decreased supply of amino acids to seeds and increased Suc transport to individual seeds (Zhang et al., 2010; Figs. 5, A, C, and D, and 6, B and D). As shown previously, these changes in total N and C correspond to decreases in seed protein and increases in seed oil levels, respectively (Kirkman et al., 1982; Mossé, 1990; Zhang et al., 2010). Nevertheless, since seed yields generally were higher in aap2 plants compared with wild-type plants, the results support that the seed N/protein yields are unchanged in aap2 plants, while the total seed C/oil yields were improved (Zhang et al., 2010; Fig. 5, E and F). Overall, this demonstrates that reduced amino acid xylem-to-phloem transfer and a successive increase in N allocation to leaves trigger increases in both total seed yield and seed C/oil yield under high-N and N-stress conditions.
CONCLUSION
This study established that regulated N partitioning to and within Arabidopsis aap2 leaves positively affects both NUpE and PNUE, irrespective of how much N is fed to the transgenic plants (Fig. 7). In aap2 plants, more N is channeled into leaf growth to provide more surface area for photosynthesis and the photosynthetic machinery, together resulting in higher C assimilation and transport to sinks (Fig. 7A). The transgenic plants outperformed wild-type plants with respect to shoot biomass and seed production and displayed significantly improved NUE in high-N as well as N-deficient environments. In fact, aap2 plants achieved similar biomass and seed production to wild-type plants with half the N supply, clearly demonstrating more efficient N use. Even on marginal N soils or under severe N limitation, the aap2 plants attained a biomass increase of up to 65% and a seed yield increase of up to 91% (Figs. 4B and 5A). This suggests that altering xylem-to-phloem transfer and N allocation to leaves presents a highly promising approach for increasing shoot biomass production in plant species used for biofuels or forage. In addition, this strategy might be employed for seed yield improvements of grain crops if the goal is to simultaneously increase seed C storage pools, specifically starch or oil levels, while keeping the seed storage protein levels relatively low. This would be relevant, for example, is the seed meal remaining after oil extraction is economically useless or undesirable (Russo and Reggiani, 2012, 2013). However, for soybean (Glycine max) and other crop species, high seed protein levels are of nutritional and economical value, as the seed meal is used in poultry and livestock feed (Knudsen, 1997; Patil et al., 2017;). In those cases, it would be essential to combine the approach described here with strategies that result in increased NUtE, remobilization of stubble N, and its redistribution to seed sinks, especially during senescence (Masclaux-Daubresse and Chardon, 2011; Avila-Ospina et al., 2015; Girondé et al., 2015; Yang and Udvardi, 2018). Overall, this work demonstrates that alterations in the leaf N balance result in increased biomass production and seed yield even under severe N stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia wild-type plants and AAP2 T-DNA insertion lines (Zhang et al., 2010) were grown in N-deficient medium (Sun Gro growing mix LB2) consisting of 70% to 80% peat, perlite, and gypsum and 20% to 30% domestic limestone. Plants were grown in environmentally controlled growth chambers with photosynthetically active radiation between 200 and 300 µmol photons m−2 s−1 and at day and night temperatures of 20°C and 16°C, respectively. Plants were grown either for 5 weeks (i.e. vegetative stage) in 12-h days to promote leaf growth and a relatively large leaf area, allowing photosynthesis measurements with the 2-cm2 LI-COR exchange chamber system (see below), or in 16-h days for 6 weeks (i.e. midreproductive stage) or 8 weeks (i.e. harvest time point). Fertilization was performed weekly using a modified Hoagland solution containing 1 mm MgSO4, 1 mm CaCl2, 0.25 mm KH2PO4, 0.25 mm K2SO4, 50 μm CoCl2, 50 μm H3BO4, 25 μm MnCl2, 2 μm Fe-EDTA, 0.5 μm ZnSO4, 0.5 μm CuSO4, and 0.5 μm Na2MoO4 (Hoagland and Arnon, 1950). In addition, N was supplied once per week using a 1 mm (low), 5 mm (moderate), and 10 mm (high) NH4NO3 solution at total amounts of 20, 100, and 200 mg NH4NO3, respectively, for the plants kept in 12-h days and 4.4, 22, and 44 mg NH4NO3 for plants grown at a 16-h daylength. For both 12- and 16-h day growth conditions, high N fertilization resulted in maximum shoot growth, moderate N nutrition caused a significant decrease in shoot biomass, and plants grown under low N supply showed N deficiency symptoms, including leaf anthocyanin accumulation and severely reduced shoot growth (Figs. 3, A and B, and 4, A and B).
Plants grown in 12-h days were used to determine the leaf phenotype and biomass and to collect leaf discs for protein, chlorophyll, and elemental N and C analyses. Leaf discs were collected from the first four fully expanded, mature source leaves (Zhang et al., 2010) 6 h after the onset of light (i.e. middle of the photoperiod), flash frozen in liquid N, and stored at −80°C until analysis. Plants kept for 6 weeks in 16-h days were used for the analysis of shoot, fruit, and seed development. The bottom 10 siliques were collected from the main inflorescence, cleared with 100% ethanol, and seeds per silique were counted using a compound light microscope (Leitz). Finally, stubble tissue (i.e. leaves, stems, and silique walls) and seeds were harvested from plants grown in 16-h days for 8 weeks until desiccation.
Photosynthesis Measurements and Calculation of Photosynthetic NUE
Gas-exchange measurements were performed using the LI-6400XT photosynthesis system (LI-COR) with a 2-cm2 fluorescence leaf chamber. The leaf temperature was maintained at 25°C during measurements. Photosynthesis was determined under ambient growth conditions using a light intensity of 300 µmol quanta m−2 s−1 and 400 µL L−1 CO2. In addition, based on photosynthetic light-response curves (50–2,000 μmol quanta m−2 s−1) established for each N treatment, measurements for photosynthetic CO2-response curves were performed at a light intensity of 1,000 μmol quanta m−2 s−1 in order to avoid light limitation of photosynthesis. Photosynthetic rates (A) and internal leaf CO2 concentrations (Ci) were determined at a range of reference CO2 concentrations (100, 200, 300, 400, 600, 800, 1,000, and 1,500 µL L−1 CO2). PNUE was calculated by relating the photosynthesis rates measured under ambient growth conditions to the N content of a specific leaf area:
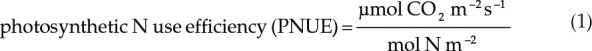 |
Calculations of Apparent Rubisco Content and Electron Transport Rates
In order to calculate the apparent Rubisco content, Rubisco-limited photosynthesis (Ac) responses to CO2 chloroplast concentrations (Cc) were modeled. To calculate Cc, first, the ratio of measured A values to the CO2 transfer resistance between the intercellular airspace and chloroplasts (gm) was determined. The gm value used for the calculations (0.22 mol m−2 s−1 bar−1) has been reported previously for Arabidopsis at 25°C (von Caemmerer and Evans, 2015). The resulting value was subtracted from the measured Ci values (von Caemmerer, 2000):
Next, Ac versus Cc curves were modeled using standard biochemical equations (Eq. 3; von Caemmerer, 2000). Subsequently, the maximum Rubisco carboxylation (Vcmax) was estimated so that the measured A and Ci values of the Rubisco-limited, initial slope of the A-Ci curve would best fit the modeled Ac-Cc curve (Eq. 3). Modeling was performed using measured values of the internal leaf oxygen concentration (O) and the Michaelis-Menten constants for carboxylase (Kc; 26.5 Pa) and oxygenase (Ko; 20.1 kPa), as well as the CO2 compensation point of Rubisco without respiration (Γ*; 3.64 Pa), as reported for Arabidopsis at 25°C by Walker et al. (2013):
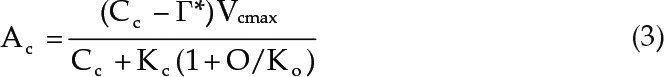 |
Finally, the apparent Rubisco content of leaves was calculated by dividing Vcmax by the rate of Rubisco carboxylation (kcat; Eq. 4). The kcat value of 3.3 used for calculations has been reported previously for Arabidopsis at 25°C by Walker et al. (2013):
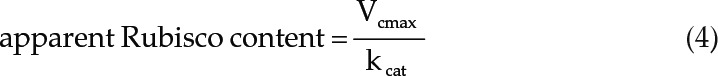 |
In order to calculate the electron transport rate (J) under light-saturated conditions, electron transport-limited photosynthesis (Aj) versus Cc curves were modeled using standard biochemical equations (Eq. 5; von Caemmerer, 2000; Buckley and Diaz-Espejo, 2015). Values of J then were estimated so that the measured A and Ci values of the electron transport-limited plateau of the A-Ci curve would best fit the modeled Aj-Cc curve (Eq. 5). The value for Γ* was the same as that used for the Ac calculations (Eq. 3):
 |
Analysis of Leaf Rubisco and Chlorophyll Content
Soluble proteins were extracted from leaf discs (2 cm2), and Rubisco content was determined by the enzymatically coupled conversion of NADH to NAD+ as described by Walker et al. (2013). The consumption of the supplied NADH was measured in vitro at a 340-nm wavelength and by monitoring the decreasing light absorption using an Evolution 300 UV-Vis spectrophotometer (Thermo Fisher Scientific). Total chlorophyll (a and b) was extracted from leaf discs (2 cm2) with 100% ethanol, measured by extracting the absorbance of light at 645 and 663 nm with a BioMate 3 Series spectrophotometer (Thermo Spectronic), and the amount was calculated using standard equations (Arnon, 1949; Ritchie, 2006).
Imaging and Leaf Area Analysis
Photographs of plants were taken with a Canon EOS Rebel T2i. Rosette leaves were collected and imaged with an Epson Perfection 3200 Photo flatbed scanner. Leaf surface area was determined using ImageJ 1.47v.
Analysis of Elemental N and C as well as NUpE, NutE, and NUE
Total elemental N and C content of lyophilized rosette leaves, dry stubble tissue, and seeds was quantified using gas chromatography followed by mass spectrometry as described previously (Sanders et al., 2009). NUpE, NutE, and NUE were calculated according to Moll et al. (1982). NUpE is the ratio of total N in the aboveground shoot mass to total N supplied. NUtE is the ratio of seed yield to total N in aboveground shoot tissue. NUE is described as the combination of these two parameters, or the ratio of seed yield to the total N supplied (Eq. 6; Moll et al., 1982). The amount of total elemental N supplied to plants grown in 16-h days and used for NUE analyses was 16, 78, or 156 mg N per plant and life cycle in low-, moderate-, and high-N conditions, respectively:
 |
Statistical Analysis
Results presented are from one growth set of plants but are representative of at least two independently grown sets of plants. Data generally are shown as means ± sd of at least three biological repetitions. Statistical analyses were performed using SigmaPlot 11.0 Systat software. Data were tested for normality. Data sets that failed the normality test were transformed so that statistical significance could be analyzed. Specifically, data sets for PNUE, NUE, NUpE, rosette biomass, stubble N, and total shoot N were transformed using log10 (Bartlett and Kendall, 1946), and total shoot C content data were transformed using square root (Bartlett, 1936; Freeman and Tukey, 1950). Statistical significance was determined by performing a one-way ANOVA followed by a pairwise Holm-Sidak mean separation test. Data were considered significantly different at P ≤ 0.05.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AT5G09220 (AAP2), AY956395 (AAP1), AT1G06830 (CEPD1), and AT2G47880 (CEPD2).
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Dr. Asaph Cousins at Washington State University for valuable discussions and technical help. The support from our greenhouse manager, Chuck Cody, and Dr. Raymond Lee with elemental N and C analyses is highly appreciated.
Footnotes
This work was funded by grants from the U.S. National Science Foundation (IOS 1021286 and IOS 1457183).
Articles can be viewed without a subscription.
References
- Ameziane R, Bernhard K, Lightfoot D (2000) Expression of the bacterial gdhA gene encoding a NADPH glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil 22: 147–157 [Google Scholar]
- Araus V, Vidal EA, Puelma T, Alamos S, Mieulet D, Guiderdoni E, Gutiérrez RA (2016) Members of BTB gene family of scaffold proteins suppress nitrate uptake and nitrogen use efficiency. Plant Physiol 171: 1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CA, Pate JS, Peoples MB, Joy KW (1983) Amino acid transport and metabolism in relation to the nitrogen economy of a legume leaf. Plant Physiol 71: 841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Ospina L, Marmagne A, Talbotec J, Krupinska K, Masclaux-Daubresse C (2015) The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (Hordeum vulgare L.), and their expression during leaf senescence. J Exp Bot 66: 2013–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A, Liang Z, Zhao Z, Cai H (2015) Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int J Mol Sci 16: 9037–9063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. (1936) The square root transformation in analysis of variance. J R Stat Soc 3: 68–78 [Google Scholar]
- Bartlett MS, Kendall DG (1946) The statistical analysis of variance-heterogeneity and the logarithmic transformation. J R Stat Soc 8: 128–138 [Google Scholar]
- Beatty PH, Anbessa Y, Juskiw P, Carroll RT, Wang J, Good AG (2010) Nitrogen use efficiencies of spring barley grown under varying nitrogen conditions in the field and growth chamber. Ann Bot 105: 1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrel A, Hammer G, Van Oosterom E (2001) Stay-green: a consequence of the balance between supply and demand for nitrogen during grain filling. Ann Appl Biol 138: 91–95 [Google Scholar]
- Bown HE, Watt MS, Clinton PW, Mason EG, Richardson B (2007) Partititioning concurrent influences of nitrogen and phosphorus supply on photosynthetic model parameters of Pinus radiata. Tree Physiol 27: 335–344 [DOI] [PubMed] [Google Scholar]
- Braun DM, Wang L, Ruan YL (2014) Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot 65: 1713–1735 [DOI] [PubMed] [Google Scholar]
- Brown RH. (1978) A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci 18: 93–98 [Google Scholar]
- Buckley TN, Diaz-Espejo A (2015) Reporting estimates of maximum potential electron transport rate. New Phytol 205: 14–17 [DOI] [PubMed] [Google Scholar]
- Carter AM, Tegeder M (2016) Increasing nitrogen fixation and seed development in soybean requires complex adjustments of nodule nitrogen metabolism and partitioning processes. Curr Biol 26: 2044–2051 [DOI] [PubMed] [Google Scholar]
- Cechin I, Fumis TF (2004) Effect of nitrogen supply on growth and photosynthesis of sunflower plants grown in the greenhouse. Plant Sci 166: 1379–1385 [Google Scholar]
- Chen J, Zhang Y, Tan Y, Zhang M, Zhu L, Xu G, Fan X (2016) Agronomic nitrogen-use efficiency of rice can be increased by driving OsNRT2.1 expression with the OsNAR2.1 promoter. Plant Biotechnol J 14: 1705–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fan X, Qian K, Zhang Y, Song M, Liu Y, Xu G, Fan X (2017) pOsNAR2.1:OsNAR2.1 expression enhances nitrogen uptake efficiency and grain yield in transgenic rice plants. Plant Biotechnol J 15: 1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichkova S, Arellano J, Vance CP, Hernández G (2001) Transgenic tobacco plants that overexpress alfalfa NADH-glutamate synthase have higher carbon and nitrogen content. J Exp Bot 52: 2079–2087 [DOI] [PubMed] [Google Scholar]
- Christopher JT, Manschadi AM, Hammer GL, Borrell AK (2008) Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Aust J Agric Res 59: 354–364 [Google Scholar]
- Coque M, Gallais A (2007) Genetic variation for nitrogen remobilization and postsilking nitrogen uptake in maize recombinant inbred lines: heritabilities and correlations among traits. Crop Sci 47: 1787–1796 [Google Scholar]
- Derkx AP, Orford S, Griffiths S, Foulkes MJ, Hawkesford MJ (2012) Identification of differentially senescing mutants of wheat and impacts on yield, biomass and nitrogen partitioning. J Integr Plant Biol 54: 555–566 [DOI] [PubMed] [Google Scholar]
- Dordas CA, Sioulas C (2008) Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind Crops Prod 27: 75–85 [Google Scholar]
- Eichelmann H, Talts E, Oja V, Padu E, Laisk A (2009) Rubisco in planta kcat is regulated in balance with photosynthetic electron transport. J Exp Bot 60: 4077–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. (1989a) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19 [DOI] [PubMed] [Google Scholar]
- Evans JR. (1989b) Partitioning of nitrogen between and within leaves grown under different irradiances. Aust J Plant Physiol 16: 533–548 [Google Scholar]
- Evans J, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24: 755–767 [Google Scholar]
- Fageria NK, Baligar VC (2005) Enhancing nitrogen use efficiency in crop plants. Adv Agron 88: 97–185 [Google Scholar]
- Fan X, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, Lian X, Shen Q, Miller AJ, Xu G (2016) Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc Natl Acad Sci USA 113: 7118–7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Xia K, Yang X, Grotemeyer MS, Meier S, Rentsch D, Xu X, Zhang M (2013) Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol J 11: 446–458 [DOI] [PubMed] [Google Scholar]
- Farquhar GD. (1979) Models describing the kinetics of ribulose biphosphate carboxylase-oxygenase. Arch Biochem Biophys 193: 456–468 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, Sweetlove LJ (2004) Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 7: 254–261 [DOI] [PubMed] [Google Scholar]
- Forde BG. (2014) Glutamate signalling in roots. J Exp Bot 65: 779–787 [DOI] [PubMed] [Google Scholar]
- Fraisier V, Gojon A, Tillard P, Daniel-Vedele F (2000) Constitutive expression of a putative high-affinity nitrate transporter in Nicotiana plumbaginifolia: evidence for post-transcriptional regulation by a reduced nitrogen source. Plant J 23: 489–496 [DOI] [PubMed] [Google Scholar]
- Fredeen AL, Gamon JA, Field CB (1991) Responses of photosynthesis and carbohydrate-partitioning to limitations in nitrogen and water availability in field-grown sunflower. Plant Cell Environ 14: 963–970 [Google Scholar]
- Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Ann Math Stat 21: 607–611 [Google Scholar]
- Friend AD. (1995) PGEN: an integrated model of leaf photosynthesis, transpiration, conductance. Ecol Modell 77: 233–255 [Google Scholar]
- Fuentes SI, Allen DJ, Ortiz-Lopez A, Hernández G (2001) Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J Exp Bot 52: 1071–1081 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Girondé A, Etienne P, Trouverie J, Bouchereau A, Le Cahérec F, Leport L, Orsel M, Niogret MF, Nesi N, Carole D, et al. (2015) The contrasting N management of two oilseed rape genotypes reveals the mechanisms of proteolysis associated with leaf N remobilization and the respective contributions of leaves and stems to N storage and remobilization during seed filling. BMC Plant Biol 15: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good A, Johnson S, De Pauw M, Carroll R, Savidov N, Vidmar J, Lu Z, Taylor G, Stroeher V (2007) Engineering nitrogen use efficiency with alanine aminotransferase. Can J Bot 85: 252–262 [Google Scholar]
- Guiboileau A, Yoshimoto K, Soulay F, Bataillé MP, Avice JC, Masclaux-Daubresse C (2012) Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytol 194: 732–740 [DOI] [PubMed] [Google Scholar]
- Habash DZ, Massiah AJ, Rong HL, Wallsgrove RM, Leigh RA (2001) The role of cytosolic glutamine synthetase in wheat. Ann Appl Biol 138: 83–89 [Google Scholar]
- Hikosaka K, Terashima I (1996) Nitrogen partitioning among photosynthetic components and its consequence in sun and shade plants. Funct Ecol 10: 335–343 [Google Scholar]
- Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Funct Ecol 12: 896–905 [Google Scholar]
- Hirel B, Le Gouis J, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58: 2369–2387 [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The Water-Culture Method for Growing Plants without Soil. California Agricultural Experiment Station Circular 347 [Google Scholar]
- Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5: 304–308 [DOI] [PubMed] [Google Scholar]
- Ibrahim MH, Jaafar HZ, Rahmat A, Rahman ZA (2010) The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 16: 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Khan NA, Nazar R, da Silva JAT (2012) Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ Exp Bot 78: 84–90 [Google Scholar]
- Kaiser BN, Rawat SR, Siddiqi MY, Masle J, Glass AD (2002) Functional analysis of an Arabidopsis T-DNA “knockout” of the high-affinity NH4+ transporter AtAMT1;1. Plant Physiol 130: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Seneweera S, Rodin J, Materne M, Burch D, Rothstein SJ, Spangenberg G (2012) Improving yield potential in crops under elevated CO2: integrating the photosynthetic and nitrogen utilization efficiencies. Front Plant Sci 3: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Mori M, Kawamura Y, Tanaka T, Mori M, Hasegawa H (2009) Production and characterization of transgenic rice plants carrying a high-affinity nitrate transporter gene (OsNRT2.1). Breed Sci 59: 237–243 [Google Scholar]
- Kaul HP, Kruse M, Aufhammer W (2005) Yield and nitrogen utilization efficiency of the pseudocereals amaranth, quinoa, and buckwheat under differing nitrogen fertilization. Eur J Agron 22: 95–100 [Google Scholar]
- Khamis S, Lamaze T, Lemoine Y, Foyer C (1990) Adaptation of the photosynthetic apparatus in maize leaves as a result of nitrogen limitation: relationships between electron transport and carbon assimilation. Plant Physiol 94: 1436–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Krapp A (2016) Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol 57: 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman MA, Shewry PR, Miflin BJ (1982) The effect of nitrogen nutrition on the lysine content and protein composition of barley seeds. J Sci Food Agric 33: 115–127 [Google Scholar]
- Knudsen KE. (1997) Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol 67: 319–338 [Google Scholar]
- Kochsiek A, Ciganda V, Bryan N, Hite L, Awada T (2006) Ecophysiological responses of Schizachyrium scoparium to water and nitrogen manipulations. Great Plains Res 16: 29–36 [Google Scholar]
- Krapp A, Saliba-Colombani V, Daniel-Vedele F (2005) Analysis of C and N metabolisms and of C/N interactions using quantitative genetics. Photosynth Res 83: 251–263 [DOI] [PubMed] [Google Scholar]
- Kumar K, Goh K (2002) Recovery of 15N-labelled fertilizer applied to winter wheat and perennial ryegrass crops and residual 15N recovery by succeeding wheat crops under different crop residue management practices. Nutr Cycl Agroecosyst 62: 123–130 [Google Scholar]
- Kumar A, Kaiser BN, Siddiqi MY, Glass AD (2006) Functional characterisation of OsAMT1.1 overexpression lines of rice, Oryza sativa. Funct Plant Biol 24: 339–346 [DOI] [PubMed] [Google Scholar]
- Lewis CE, Noctor G, Causton D, Foyer CH (2000) Regulation of assimilate partitioning in leaves. Funct Plant Biol 27: 507–519 [Google Scholar]
- Liberloo M, Tulva I, Raïm O, Kull O, Ceulemans R (2007) Photosynthetic stimulation under long-term CO2 enrichment and fertilization is sustained across a closed Populus canopy profile (EUROFACE). New Phytol 173: 537–549 [DOI] [PubMed] [Google Scholar]
- López-Bellido RJ, López-Bellido L (2001) Efficiency of nitrogen in wheat under Mediterranean conditions: effect of tillage, crop rotation and N fertilization. Field Crops Res 71: 31–46 [Google Scholar]
- Makino A, Osmond B (1991) Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol 96: 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Shimada T, Takumi S, Kaneko K, Matsuoka M, Shimamoto K, Nakano H, Miyao-Tokutomi M, Mae T, Yamamoto N (1997) Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense RbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol 114: 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Chardon F (2011) Exploring nitrogen remobilization for seed filling using natural variation in Arabidopsis thaliana. J Exp Bot 62: 2131–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105: 1141–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CH, Beatty PH, Good AG (2012) Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol J 10: 1011–1025 [DOI] [PubMed] [Google Scholar]
- Miller AJ, Fan X, Shen Q, Smith SJ (2008) Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J Exp Bot 59: 111–119 [DOI] [PubMed] [Google Scholar]
- Moll R, Kamprath E, Jackson W (1982) Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron J 74: 562–564 [Google Scholar]
- Mossé J. (1990) Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds: a reappraisal of its definition and determination. Variation according to species and to seed protein content. J Agric Food Chem 38: 18–24 [Google Scholar]
- Munier-Jolain N, Salon C (2003) Can sucrose content in the phloem sap reaching field pea seeds (Pisum sativum L.) be an accurate indicator of seed growth potential? J Exp Bot 54: 2457–2465 [DOI] [PubMed] [Google Scholar]
- Munier-Jolain N, Larmure A, Salon C (2008) Determinism of carbon and nitrogen reserve accumulation in legume seeds. C R Biol 331: 780–787 [DOI] [PubMed] [Google Scholar]
- Nakano H, Makino A, Mae T (1997) The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol 115: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3: 973–996 [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y (2017) Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat Plants 3: 17029. [DOI] [PubMed] [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18: 419–425 [Google Scholar]
- Ortiz-Monasterio R, Sayre KD, Rajaram S, McMahon M (1997) Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci 37: 898–904 [Google Scholar]
- Osaki M, Shinano T, Tadano T (1993) Effect of nitrogen application on the accumulation of ribulose-1,5-bisphosphate carboxylase/oxygenase and chlorophyll in several field crops. Soil Sci Plant Nutr 39: 427–436 [Google Scholar]
- Palenchar PM, Kouranov A, Lejay LV, Coruzzi GM (2004) Genome-wide patterns of carbon and nitrogen regulation of gene expression validate the combined carbon and nitrogen (CN)-signaling hypothesis in plants. Genome Biol 5: R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JS, Sharkey PJ, Lewis OAM (1975) Xylem to phloem transfer of solutes in fruiting shoots of legumes, studied by a phloem bleeding technique. Planta 122: 11–26 [DOI] [PubMed] [Google Scholar]
- Patil G, Chauldry J, Vuong TD, Jenkins B, Qui D, Kadan S, Shannon GJ, Nguyen HT (2017) Development of SNP genotyping assays for seed compositiontraits in soybean. Int J Plant Genomics 2017: 1–12 10.1155/2017/6572969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Peña PA, Quach T, Sato S, Ge Z, Nersesian N, Changa T, Dweikat I, Soundararajan M, Clemente TE (2017) Expression of the maize Dof1 transcription factor in wheat and sorghum. Front Plant Sci 8: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchlik M, Tegeder M (2017) Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol 175: 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TL, Westbeek MH (2004) Analysis of differences in photosynthetic nitrogen-use efficiency between four contrasting species. Physiol Plant 122: 68–78 [Google Scholar]
- Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116: 26–37 [DOI] [PubMed] [Google Scholar]
- Qu B, He X, Wang J, Zhao Y, Teng W, Shao A, Zhao X, Ma W, Wang J, Li B, et al. (2015) A wheat CCAAT box-binding transcription factor increases the grain yield of wheat with less fertilizer input. Plant Physiol 167: 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, El-Kereamy A, Gidda S, Bi YM, Rothstein SJ (2014) AMT1;1 transgenic rice plants with enhanced NH4+ permeability show superior growth and higher yield under optimal and suboptimal NH4+ conditions. J Exp Bot 65: 965–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripullone F, Grassi G, Lauteri M, Borghetti M (2003) Photosynthesis-nitrogen relationships: interpretation of different patterns between Pseudotsuga menziesii and Populus × euroamericana in a mini-stand experiment. Tree Physiol 23: 137–144 [DOI] [PubMed] [Google Scholar]
- Ritchie RJ. (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89: 27–41 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Hosein F, Miranda M, Heim U, Götz KP, Schlereth A, Borisjuk L, Saalbach I, Wobus U, Weber H (2005) Ectopic expression of an amino acid transporter (VfAAP1) in seeds of Vicia narbonensis and pea increases storage proteins. Plant Physiol 137: 1236–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci USA 108: 18524–18529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Reggiani R (2012) Antinutritive compounds in twelve Camelina sativa genotypes. Am J Plant Sci 3: 1408 [Google Scholar]
- Russo R, Reggiani R (2013) Variability of antinutritive compounds in flaxseed flours. Int J Plant Biol 4: 11–13 [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR (1987) The nitrogen use efficiency of C3 and C4 plants. III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol 85: 355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR (1988) The in-vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgaris L. Planta 174: 407–416 [DOI] [PubMed] [Google Scholar]
- Sanders A, Collier R, Trethewy A, Gould G, Sieker R, Tegeder M (2009) AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J 59: 540–552 [DOI] [PubMed] [Google Scholar]
- Santiago JP, Tegeder M (2016) Connecting source with sink: the role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol 171: 508–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JP, Tegeder M (2017) Implications of nitrogen phloem loading for carbon metabolism and transport during Arabidopsis development. J Integr Plant Biol 59: 409–421 [DOI] [PubMed] [Google Scholar]
- Schmidt R, Stransky H, Koch W (2007) The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 226: 805–813 [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Stadler J, Heilmeier H, Stitt M, Mooney HA (1994) Growth and reproduction of Arabidopsis thaliana in relation to storage of starch and nitrate in the wild-type and in starch-deficient and nitrate-uptake-deficient mutants. Plant Cell Environ 17: 795–809 [Google Scholar]
- Schwender J, Shachar-Hill Y, Ohlrogge JB (2006) Mitochondrial metabolism in developing embryos of Brassica napus. J Biol Chem 281: 34040–34047 [DOI] [PubMed] [Google Scholar]
- Seiffert B, Zhou Z, Wallbraun M, Lohaus G, Möllers C (2004) Expression of a bacterial asparagine synthetase gene in oilseed rape (Brassica napus) and its effect on traits related to nitrogen efficiency. Physiol Plant 121: 656–665 [Google Scholar]
- Shrawat AK, Carroll RT, DePauw M, Taylor GJ, Good AG (2008) Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol J 6: 722–732 [DOI] [PubMed] [Google Scholar]
- Spano G, Di Fonzo N, Perrotta C, Platani C, Ronga G, Lawlor DW, Napier JA, Shewry PR (2003) Physiological characterization of ‘stay green’ mutants in durum wheat. J Exp Bot 54: 1415–1420 [DOI] [PubMed] [Google Scholar]
- Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism: more than the icing on the cake. Plant J 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ohkubo M, Hatakeyama H, Ohashi K, Yoshizawa R, Kojima S, Hayakawa T, Yamaya T, Mae T, Makino A (2007) Increased Rubisco content in transgenic rice transformed with the ‘sense’ rbcS gene. Plant Cell Physiol 48: 626–637 [DOI] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346: 343–346 [DOI] [PubMed] [Google Scholar]
- Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27: 1047–1054 [Google Scholar]
- Tan Q, Zhang L, Grant J, Cooper P, Tegeder M (2010) Increased phloem transport of S-methylmethionine positively affects sulfur and nitrogen metabolism and seed development in pea plants. Plant Physiol 154: 1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder M, Masclaux-Daubresse C (2018) Source and sink mechanisms of nitrogen transport and use. New Phytol 217: 35–53 [DOI] [PubMed] [Google Scholar]
- Thornton B, Robinson D (2005) Uptake and assimilation of nitrogen from solutions containing multiple N sources. Plant Cell Environ 28: 813–821 [Google Scholar]
- Tsay YF, Fan SC, Chen HY (2011) Method for changing nitrogen utilization efficiency in plants. US Patent Application No. 12/832: 234. January 13, 2011.
- van Bel AJ. (1984) Quantification of the xylem-to-phloem transfer of amino acids by use of inulin [14C] carboxylic acid as xylem transport marker. Plant Sci Lett 35: 81–85 [Google Scholar]
- Vanhercke T, El Tahchy A, Liu Q, Zhou XR, Shrestha P, Divi UK, Ral JP, Mansour MP, Nichols PD, James CN, et al. (2014) Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol J 12: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S. (2000) Biochemical Models of Leaf Photosynthesis, Ed 2 CSIRO Publishing, Collingwood, Australia [Google Scholar]
- von Caemmerer S, Evans JR (2015) Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ 38: 629–637 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Walker B, Ariza LS, Kaines S, Badger MR, Cousins AB (2013) Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ 36: 2108–2119 [DOI] [PubMed] [Google Scholar]
- Wang J, Lu K, Nie H, Zeng Q, Wu B, Qian J, Fang Z (2018a) Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield. Rice (N Y) 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hu B, Yuan D, Liu Y, Che R, Hu Y, Ou S, Liu Y, Zhang Z, Wang H, et al. (2018b) Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 30: 638–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR, Adams MA (2006) Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ 29: 192–201 [DOI] [PubMed] [Google Scholar]
- Warren CR, Adams MA, Chen Z (2000) Is photosynthesis related to concentrations of nitrogen and Rubisco in leaves of Australian native plants? Funct Plant Biol 27: 407–416 [Google Scholar]
- Weigelt K, Küster H, Radchuk R, Müller M, Weichert H, Fait A, Fernie AR, Saalbach I, Weber H (2008) Increasing amino acid supply in pea embryos reveals specific interactions of N and C metabolism, and highlights the importance of mitochondrial metabolism. Plant J 55: 909–926 [DOI] [PubMed] [Google Scholar]
- Westbeek MH, Pons TL, Cambridge ML, Atkin OK (1999) Analysis of differences in photosynthetic nitrogen use efficiency of alpine and lowland Poa species. Oecologia 120: 19–26 [DOI] [PubMed] [Google Scholar]
- Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63: 153–182 [DOI] [PubMed] [Google Scholar]
- Yamaya T, Obara M, Nakajima H, Sasaki S, Hayakawa T, Sato T (2002) Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. J Exp Bot 53: 917–925 [DOI] [PubMed] [Google Scholar]
- Yang J, Udvardi M (2018) Senescence and nitrogen use efficiency in perennial grasses for forage and biofuel production. J Exp Bot 69: 855–865 [DOI] [PubMed] [Google Scholar]
- Yang L, Cao W, Thorup-Kristensen K, Bai J, Gao S, Chang D (2015) Effect of Orychophragmus violaceus incorporation on nitrogen uptake in succeeding maize. Plant Soil Environ 61: 260–265 [Google Scholar]
- Yuan L, Loqué D, Ye F, Frommer WB, von Wirén N (2007) Nitrogen-dependent posttranscriptional regulation of the ammonium transporter AtAMT1;1. Plant Physiol 143: 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tan Q, Lee R, Trethewy A, Lee YH, Tegeder M (2010) Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. Plant Cell 22: 3603–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Garneau MG, Majumdar R, Grant J, Tegeder M (2015) Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J 81: 134–146 [DOI] [PubMed] [Google Scholar]
- Zhu S, Vivanco JM, Manter DK (2016) Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl Soil Ecol 107: 324–333 [Google Scholar]