Diterpene synthases are characterized that may contribute to abiotic stress responses in the bioenergy crop switchgrass (Panicum virgatum).
Abstract
Diterpenoids constitute a diverse class of metabolites with critical functions in plant development, defense, and ecological adaptation. Major monocot crops, such as maize (Zea mays) and rice (Oryza sativa), deploy diverse blends of specialized diterpenoids as core components of biotic and abiotic stress resilience. Here, we describe the genome-wide identification and functional characterization of stress-related diterpene synthases (diTPSs) in the dedicated bioenergy crop switchgrass (Panicum virgatum). Mining of the allotetraploid switchgrass genome identified an expansive diTPS family of 31 members, and biochemical analysis of 11 diTPSs revealed a modular metabolic network producing a diverse array of diterpenoid metabolites. In addition to ent-copalyl diphosphate (CPP) and ent-kaurene synthases predictably involved in gibberellin biosynthesis, we identified syn-CPP and ent-labda-13-en-8-ol diphosphate (LPP) synthases as well as two diTPSs forming (+)-labda-8,13E-dienyl diphosphate (8,13-CPP) and ent-neo-cis-trans-clerodienyl diphosphate (CT-CLPP) scaffolds not observed previously in plants. Structure-guided mutagenesis of the (+)-8,13-CPP and ent-neo-CT-CLPP synthases revealed residue substitutions in the active sites that altered product outcome, representing potential neofunctionalization events that occurred during diversification of the switchgrass diTPS family. The conversion of ent-CPP, ent-LPP, syn-CPP, and ent-neo-CT-CLPP by promiscuous diTPSs further yielded distinct labdane-type diterpene olefins and alcohols. Of these metabolites, the formation of 9β-hydroxy-syn-pimar-15-ene and the expression of the corresponding genes were induced in roots and leaves in response to oxidative stress and ultraviolet irradiation, indicating their possible roles in abiotic stress adaptation. Together, these findings expand the known chemical space of diterpenoid metabolism in monocot crops toward systematically investigating and ultimately improving stress resilience traits in crop species.
Switchgrass (Panicum virgatum; Poaceae) is a perennial C4 grass native to North America valued primarily as a next-generation feedstock for lignocellulosic biofuel production (Schmer et al., 2008; Keshwani and Cheng, 2009; Allwright and Taylor, 2016). Its high net energy yield and wide habitat range make switchgrass an attractive crop for cultivation on marginal lands with minimal agronomic inputs, thus promising environmentally sustainable bioenergy production (Sarath et al., 2008; Schmer et al., 2008; Liu et al., 2015). However, increasing drought stress and the accompanying disease and pest damage are projected to become major impediments for scalable switchgrass cultivation (Barney et al., 2009; Keshwani and Cheng, 2009; Liu et al., 2015; Sykes et al., 2016). Two major switchgrass ecotypes, namely lowland and upland, are distinguished based on their distinct environmental adaptability, morphological characteristics, and ploidy levels (Uppalapati et al., 2013; Liu et al., 2015). Lowland ecotypes are predominantly tetraploid (2n = 4x = 36), whereas upland ecotypes mostly contain octoploid (2n = 8x = 72) genomes (Casler et al., 2011). This genetic diversity and the common reproductive isolation of different switchgrass ecotypes have hampered crop optimization. However, the availability of genome sequences for the diploid genome of Panicum hallii var filipes (∼600 Mb; DOE-JGI, 2018b and the allotetraploid lowland ecotype P. virgatum var Alamo (∼1.23 Gb; DOE-JGI, 2018a, as well as advances in switchgrass transformation (Nelson et al., 2017; Biswal et al., 2018), now provide the resources necessary to investigate and optimize switchgrass performance and stress resilience. For example, silencing of the switchgrass 4-coumarate:CoA ligase resulted in plants with reduced lignin formation (Xu et al., 2011), and genetic alteration of pectin biosynthesis in cell walls profoundly improved biomass production (Biswal et al., 2018). Similarly, characterization and overexpression of a vacuolar ion transporter enhanced switchgrass abiotic stress tolerance (Huang et al., 2017). By contrast, the biosynthetic and functional diversity of chemical defense mechanisms in switchgrass have remained largely unknown, although drought-induced alterations in carbohydrate, lipid, phenylpropanoid, and terpenoid metabolism have been reported (Meyer et al., 2014).
In related monocot crops, including the global staples maize (Zea mays) and rice (Oryza sativa), unique arrays of diterpenoid natural products serve as major defenses against pests, pathogens, and abiotic stressors (Xu et al., 2004; Peters, 2006; Schmelz et al., 2011, 2014; Vaughan et al., 2015). For example, extensive rice studies illustrated the stress-elicited biosynthesis of a diverse suite of oryzalexin, phytocassane, and momilactone diterpenoid phytoalexins with antimicrobial and allelopathic properties (Peters, 2006; Bagnaresi et al., 2012; Kato-Noguchi and Peters, 2013; Toyomasu et al., 2014; Lu et al., 2018). Similarly, unique groups of diterpenoids identified in maize have been demonstrated to exhibit potent antimicrobial activity against key Fusarium and Aspergillus spp. pathogens (Dafoe et al., 2011; Schmelz et al., 2011). Beyond their relevance as antibiotics and feeding deterrents, possible roles in conferring resistance against abiotic perturbations have been suggested for diterpenoids of both rice and maize. For instance, rice diterpenoids were demonstrated to accumulate in response to UV irradiation (Kodama et al., 1988; Horie et al., 2015), and maize diterpenoids showed inducible patterns of gene and metabolite accumulation after exposure to drought and belowground oxidative stress (Vaughan et al., 2015; Mafu et al., 2018).
The enzyme family of diterpene synthases (diTPSs) is the gatekeeper to specialized diterpenoid metabolism, catalyzing the committed cyclization and rearrangement of the central precursor geranylgeranyl diphosphate (GGPP) into an array of different scaffolds (Peters, 2010; Chen et al., 2011; Zerbe and Bohlmann, 2015). In monocots, diterpenoid metabolism, to current knowledge, invariably recruits consecutive pairs of class II and class I diTPS reactions, where class II diTPSs convert GGPP into a handful of prenyl diphosphate intermediates that then undergo secondary cyclization and/or rearrangements facilitated by class I diTPSs (Peters, 2010; Zerbe and Bohlmann, 2015). The resulting diTPS products most commonly undergo various regiospecific and stereospecific oxygenation and other tailoring reactions facilitated by cytochrome P450 monooxygenases (P450s) and a few other enzyme classes to form more than 10,000 known plant diterpenoids (Hamberger and Bak, 2013). This expansive chemical and functional diversity of diterpenoid metabolism is rooted in repeated events of gene duplication and neofunctionalization of diTPS and P450 genes (Pichersky and Lewinsohn, 2011). Ancestral genes of general metabolism, especially GA biosynthesis, will have been recruited repeatedly as blueprints for this evolutionary diversification and the emergence of species-specific diterpenoid metabolic networks (Chen et al., 2011; Zi et al., 2014). This evolutionary path is supported by the high active-site plasticity of diTPSs, where as little as a single residue substitution may alter product outcomes (Xu et al., 2007b; Morrone et al., 2008; Criswell et al., 2012; Potter et al., 2014, 2016a, 2016b; Mafu et al., 2016; Pelot et al., 2017b; Jia et al., 2018). Additionally, plants can employ functionally distinct multigene diTPS and P450 families as modular biosynthetic grids, where different enzyme combinations expand the chemical space of possible products beyond the sum of distinct enzyme functions (Peters, 2010; Morrone et al., 2011; Zerbe and Bohlmann, 2015). Such matrix-type biosynthetic networks have been identified in rice (Wilderman et al., 2004; Xu et al., 2004, 2007a; Swaminathan et al., 2009; Wang et al., 2011, 2012a, 2012b; Wu et al., 2011), maize (Harris et al., 2005; Schmelz et al., 2014; Fu et al., 2016; Mafu et al., 2018), and wheat (Triticum aestivum; Wu et al., 2012; Zhou et al., 2012). These insights exemplify the diverse protective biological roles of diterpenoid metabolism and support the distribution of species-specific diterpenoid defense networks more broadly throughout the family of monocot crops.
This study describes the identification of the 31-membered switchgrass diTPS family using a functional genomics approach. Biochemical characterization and structure-function analysis of select diTPSs demonstrate common and species-specific diterpenoid biosynthetic pathways in switchgrass. Combined with patterns of stress-elicited diterpenoid accumulation and gene expression in leaves and roots, these findings support a complex repertoire of diterpenoids in switchgrass with potential roles in environmental adaptation.
RESULTS
The Switchgrass diTPS Family
To elucidate the biosynthetic machinery underlying diterpenoid metabolism in switchgrass, we mined the allotetraploid P. virgatum [v4.1; DOE-JGI(a)] and diploid P. hallii [v3.1; DOE-JGI(b)] genomes for diTPS gene candidates using a previously described gene discovery strategy that combines custom database searches and phylogenetic analyses (Zerbe et al., 2013). This approach identified 31 diTPS candidates in the allotetraploid P. virgatum genome (Fig. 1; Supplemental Table S1), thus representing a diTPS family substantially larger than those identified in the related monocot species maize, rice, and wheat, which are composed of 10 to 12 members (Schmelz et al., 2014). By comparison, the diploid genome of P. hallii contained a smaller diTPS family with nine diTPS gene candidates. Of the 31 identified diTPS gene candidates, 15 were classified as class II diTPSs based on their characteristic γβα-domain architecture and the presence of a catalytic DxDD motif in the N-terminal domain (Peters et al., 2001; Peters and Croteau, 2002). The remaining 16 genes were classified as γβα- or βα-domain class I diTPSs featuring the signature DDxxD divalent metal-binding motif in the C-terminal active site.
Figure 1.
Discovery and functional prediction of the P. virgatum and P. hallii diTPS families. A, Relative locations of class II (black) and class I (blue) diTPS candidates on the pseudochromosomes of the P. virgatum and P. hallii genomes as based on the chromosome karyotype of switchgrass (Young et al., 2012). B, Amino acid alignment of characterized active-site residues with impact on class II diTPS product specificity (residue positions are numbered based on ZmAn2; Harris et al., 2005). C and D, Maximum-likelihood phylogenetic trees of P. virgatum and P. hallii class II (C) and class I (D) diTPSs and known monocot diTPSs. Trees were rooted with the gymnosperm Picea sitchensis ent-CPP synthase (PsCPS) and ent-kaurene synthase (PsEKS; Keeling et al., 2010), respectively. Black dots represent branches with bootstrap support of greater than 80% (500 repetitions). The definitions of protein abbreviations and accession numbers are given in Supplemental Table S1.
The identified diTPS candidates were dispersed across chromosomes 1, 2, 3, 7, and 9 in the homologous subgenomes K and N of P. virgatum as well as the P. hallii genome (Fig. 1). Several groups of genes showed close phylogenetic relationships and similar genome loci across the P. virgatum and P. hallii genomes (Fig. 1; Supplemental Table S2). For instance, phylogenetic analysis placed the class II diTPSs PhH01630 (P. hallii genome), PvCPS14 (P. virgatum subgenome K), and PvCPS15 (P. virgatum subgenome N) in the same phylogenetic cluster, and all three genes shared similar positions on chromosome 9 of the individual genomes. Likewise, among the class I diTPS genes, P. hallii PhG02322 shared a similar chromosomal location and close phylogenetic relationship with PvKSL13 and PvKSL15 on P. virgatum chromosomes 7K and 7N, respectively. Notably, chromosome 3 of P. virgatum subgenomes K and N contained an array of six and seven distinct diTPSs, respectively, whereas only two diTPS genes were present on chromosome 3 of P. hallii, consistent with the possible occurrence of multiple events of gene duplication at this locus during the polyploidization and diversification of the allotetraploid switchgrass genome. The expanded gene arrays of chromosome 3K and 3N contained colocalized class II diTPSs (PvCPS11 and PvCPS12) and class I diTPSs (PvKSL2-12) that could be indicative of biosynthetic gene clusters, as described previously for the formation of momilactone diterpenoids in rice (Sakamoto et al., 2004).
Next, we combined phylogenetic analysis with sequence comparison of key active-site residues to deduce possible functions and guide the biochemical characterization of identified P. virgatum (herein called switchgrass) diTPSs (Fig. 1). Among the class II diTPS candidates, phylogenetic analysis placed PvCPS11, PvCPS12, PvCPS14, and PvCPS15 adjacent to known ent-copalyl diphosphate synthases (CPSs) from other monocot species. Specifically, PvCPS14 and PvCPS15 were closely related to the maize ent-CPS ZmAn1 involved in GA metabolism (Bensen et al., 1995). An ent-CPS activity of these diTPSs was supported further by the presence of a conserved catalytic His-Asn dyad shown previously to determine ent-CPS activity (Fig. 1; Potter et al., 2014, 2016b; Cui et al., 2015). PvCPS11 and PvCPS12 formed a separate branch together with another maize ent-CPS, namely ZmAn2, which functions in the biosynthesis of defense-related specialized diterpenoids (Harris et al., 2005; Schmelz et al., 2011; Mafu et al., 2018). However, both PvCPS11 and PvCPS12 featured a Gly residue rather than the Asn conserved in known monocot ent-CPSs, thus indicating a related yet possibly distinct activity. PvCPS8, PvCPS9, and PvCPS10 formed a phylogenetic branch together with rice and wheat diTPSs of specialized metabolism and were most closely related to the rice syn-CPS OsCPS4 (Otomo et al., 2004b; Xu et al., 2004; Schmelz et al., 2014). Consistently, these diTPSs contained a signature His residue shown to contribute to defining syn-CPS activity in OsCPS4 (His-501; Potter et al., 2016a). The remaining eight class II diTPS candidates (PvCPS1–PvCPS7 and PvCPS13) were placed in a separate cluster that also contained uncharacterized class II diTPSs of maize. Among the investigated active-site determinants, these class II enzymes differed largely from known class II diTPSs (Fig. 1), suggesting distinct functions.
Unlike class II diTPSs that are typically product specific, class I diTPSs of specialized metabolism often show latent substrate and product promiscuity (Morrone et al., 2011; Wu et al., 2012; Zhou et al., 2012), which limits reliable functional annotation on the basis of active-site determinants. However, phylogenetic comparison with characterized enzymes positioned PvKSL13 and PvKSL15 most closely with maize ZmKSL3 that was identified recently as an ent-kaurene synthase with possible roles in both GA biosynthesis and specialized diterpenoid metabolism (Fu et al., 2016; Fig. 1). PvKSL14 and PvKSL16 were placed within a group of diTPSs from maize and wheat that lack the N-terminal γ-domain and adopt a βα-domain structure more commonly found in monoterpene and sesquiterpene synthases. Known enzymes in this phylogenetic group are multifunctional, forming ent-kaurene as well as specialized sesquiterpenoids and diterpenoids (Hillwig et al., 2011; Zhou et al., 2012; Fu et al., 2016). Similar to the switchgrass class II diTPS family, a large group of 12 class I diTPSs formed a separate group also including rice diTPSs of specialized diterpenoid metabolism and a recently identified dolabradiene synthase from maize (Mafu et al., 2018). Notably, PvKSL3/4 (representing enzymes with identical protein sequences), PvKSL6, and PvKSL12 within this group also lacked the γ-domain, suggesting functions in specialized diterpenoid metabolism.
Switchgrass Class II diTPSs Catalyze Diverse Functions
To biochemically verify the hypothesized class II diTPS activities and examine the functional landscape of switchgrass diterpenoid metabolism, we selected six class II diTPS candidates (PvCPS1, PvCPS3, PvCPS8, PvCPS11, PvCPS14, and PvCPS15) representing members of all observed phylogenetic branches (Fig. 1) for functional characterization. Enzyme activities were assayed in vivo by coexpressing gene candidates with a GGPP synthase from Abies grandis using an Escherichia coli system engineered for increased diterpenoid precursor supply (Morrone et al., 2010) and in vitro via activity assays of recombinant and affinity-purified enzymes with commercial GGPP as a substrate (Zerbe et al., 2012). Enzymatic products were identified by gas chromatography-mass spectrometry (GC-MS) based on comparison with authentic enzyme products or de novo via NMR analysis. Notably, all class II diTPS products were analyzed in the form of their respective dephosphorylated diterpene alcohols, but for simplicity they are described below as the naturally occurring prenyl diphosphate products.
In vivo activity assays of the predicted ent-CPSs PvCPS14 and PvCPS15 identified copalyl diphosphate (CPP; compound 1) as compared with the authentic enzyme product of the maize ent-CPS ZmAn2 (Harris et al., 2005; Fig. 2). Along with CPP, an unidentified diterpene olefin (compound 2) was detected that likely occurred through dephosphorylation and/or rearrangement of CPP by E. coli endogenous phosphatases, as concluded previously for other class II diTPSs (Sallaud et al., 2012; Zerbe et al., 2015; Pelot et al., 2017b). Therefore, additional in vitro assays of affinity-purified enzymes were performed with GGPP as a substrate and subsequent dephosphorylation of the diTPS products using calf intestinal alkaline phosphatase (Zerbe et al., 2012). For both PvCPS14 and PvCPS15, in vitro assays verified CPP 1 as the enzyme product (Fig. 2).
Figure 2.
GC-MS analysis of switchgrass class II diTPS reaction products. Shown are individually scaled extracted or total ion chromatograms (EIC/TIC) and mass spectra of reaction products from in vivo and in vitro enzyme assays of the switchgrass class II diTPSs PvCPS15 and PvCPS14 (A), PvCPS11 (B), PvCPS8 (C), PvCPS3 (D and E), and PvCPS1 (F and G). Products of PvCPS15, PvCPS14, PvCPS11, and PvCPS8 were identified by comparison with theproducts of the known diTPSs ent-CPS (ZmAn2; Harris et al., 2005), ent-LPS (SdCPS2:W360A; Pelot et al., 2017b), and syn-CPS (OsCPS4; Xu et al., 2004). The PvCPS3 and PvCPS1 reaction products were identified by GC-MS and NMR analysis as (+)-labda-8,13E-dienyl diphosphate (E) and ent-neo-cis-trans-clerodienyl diphosphate (G), respectively. Details on NMR structural validation and the proposed absolute stereochemistries are given in Supplemental Figures S2 to S4. Numbered peaks are as follows: 1, ent-copalol (i.e. dephosphorylated ent-copalyl diphosphate; CPP); 2, unidentified diterpene; 5, ent-8β-labda-13E-en-8,15-diol (i.e. dephosphorylated ent-labda-13-en-8-ol diphosphate; LPP); 6, ent-8α-labda-13E-en-8,15-diol; 9, syn-copalol (i.e. dephosphorylated syn-CPP); 10, unidentified diterpene; 11, (+)-labda-8,13E-dien-15-ol (i.e. dephosphorylated [+]-labda-8,13E-dienyl diphosphate; 8,13-CPP); 12, ent-neo-cis-trans-kolavenol (i.e. dephosphorylated ent-neo-cis-trans-clerodienyl diphosphate; CT-CLPP); 13, unidentified diterpene.
To determine the stereochemistry of CPP 1 formed by PvCPS14 and PvCPS15, we took advantage of the natural sequential activity of class II diTPSs with class I diTPSs that are specific to select prenyl diphosphate stereoisomers (Zerbe et al., 2013; Kitaoka et al., 2015; Andersen-Ranberg et al., 2016). Applying this combinatorial concept, PvCPS14 and PvCPS15 were coexpressed with characterized class I diTPSs that are specific to converting either the ent-stereoisomer (Grindelia robusta GrEKS; Zerbe et al., 2015) or the (+)-stereoisomer (Marrubium vulgare MvELS; Zerbe et al., 2014) of CPP. As controls, we coexpressed GrEKS with the ent-CPS ZmAn2 forming ent-kaurene (compound 3) and MvELS with the (+)-CPS A. grandis abietadiene synthase (variant D621A; Peters et al., 2001) producing miltiradiene (compound 4). Coexpression of PvCPS14 or PvCPS15 with GrEKS afforded ent-kaurene 3 (Supplemental Fig. S1), whereas no class I diTPS product was detected in combined assays with MvELS. These results identified PvCPS14 and PvCPS15 as ent-CPSs, consistent with their predicted activities.
Phylogenetic and sequence analyses indicated a function of PvCPS11 that was related to yet distinct from known ent-CPSs (Fig. 1). In vivo coexpression assays of PvCPS11 yielded the β- (compound 5) and α- (compound 6) 8-hydroxy epimers of labda-13-en-8-ol diphosphate (LPP), as verified by comparison with the relative retention times and mass spectra of the authentic products formed by a characterized ent-LPP synthase (LPS) from Salvia divinorum (Pelot et al., 2017b; Fig. 2). Additional in vitro assays of PvCPS11 showed the formation of 8β-LPP 5 as the sole enzyme product. As observed frequently during in vivo activity analyses of class II diTPSs (Sallaud et al., 2012; Zerbe et al., 2015; Pelot et al., 2017a, 2017b), the discrepancy in the product profiles observed during in vitro and in vivo activity assays is presumably due to the presence of endogenous phosphatases in the in vivo E. coli system that are capable of dephosphorylating the diTPS product, resulting in spontaneous rearrangements yielding an epimeric mixture of the labda-13-en-8-ol product. To assess the stereospecificity of PvCPS11, we conducted coexpression assays with GrEKS and MvELS. As controls, we used 13R-ent-manoyl oxide (compound 7), formed through the pairwise activity of GrEKS with the ent-LPS SdCPS2:W360A (Pelot et al., 2017b), and 13R-(+)-manoyl oxide (compound 8), formed by combining MvELS with the (+)-LPS GrTPS1 (Zerbe et al., 2013; Supplemental Fig. S1). The sequential activity of PvCPS11 and GrEKS produced 13R-ent-manoyl oxide 7, whereas no new product was observed when coexpressing PvCPS11 with MvELS. These results are consistent with an ent-stereochemistry of the PvCPS11 product.
Confirming our functional prediction, in vivo and in vitro activity assays of PvCPS8 afforded syn-CPP (compound 9) as based on characteristic mass ions of mass-to-charge ratio (m/z) 290, 275, 257, 192, 177, and 137, matching those of syn-CPP produced by the rice syn-CPS OsCPS4 (Xu et al., 2004; Fig. 2). An additional unidentified diterpene olefin (compound 10) was observed only during in vivo assays and likely represents a by-product of the E. coli coexpression system. Although a syn-ent-stereochemistry could not be excluded entirely, mass spectral comparison of the PvCPS8 and OsCPS4 products and the presence of signature active-site determinants (His-493 in the position of PvCPS8) strongly support syn-CPP as the PvCPS8 product.
Phylogenetic analysis placed PvCPS1 and PvCPS3 distant from known class II diTPSs (Fig. 1). Coexpression and in vitro assays of PvCPS3 resulted in a single major product (compound 11; Fig. 2) with a fragmentation pattern containing mass ions of m/z 290, 275, and 257 characteristic for labdane-type scaffolds and mass ions of m/z 149, 135, 204, and 205 similar to, but distinct from, labda-7,13E-dienyl diphosphate (7,13-CPP; Mafu et al., 2011; Zerbe et al., 2015; Pelot et al., 2017b). To define the activity of PvCPS3, enzyme product in excess of 1 mg was produced using large-scale E. coli coexpression assays and purified by silica gel column chromatography and semipreparative HPLC for high-resolution 1D and 2D NMR analyses. The obtained 1D NMR 13C and 1H chemical shifts matched those reported previously for the diterpenoid (+)-labda-8,13E-dien-15-ol isolated from Nicotiana setchellii (Suzuki et al., 1983; Supplemental Fig. S2). Additional 2D NMR correlation spectroscopy, heteronuclear single quantum coherence, heteronuclear multiple bond correlation, and heteronuclear two bond correlation spectra identified the PvCPS3 product as (+)-labda-8,13E-dien-15-ol 11 [i.e. dephosphorylated (+)-labda-8,13E-dienyl diphosphate (8,13-CPP); Fig. 2], with the (+)-absolute stereochemistry tentatively assigned on the basis of active-site mutations as described below (Supplemental Fig. S3).
E. coli coexpression assays of PvCPS1 resulted in two reaction products (compounds 12 and 13), whereas PvCPS1 in vitro assays yielded only compound 12, with a retention time of 11.62 min (Fig. 2). With characteristic mass ions of m/z 290, 275, 257, 189, and 107, the fragmentation pattern of compound 12 was similar to the clerodane-type diterpenoids trans-cis-kolavenol and trans-trans-kolavenol (Hamano et al., 2002; Nakano et al., 2015; Andersen-Ranberg et al., 2016; Pelot et al., 2017b). However, the additional prominent mass fragments of m/z 121 and 191 indicated a distinct structure. Hence, NMR analyses were conducted and identified the PvCPS1 product as cis-trans-kolavenol 12 (i.e. dephosphorylated cis-trans-clerodienyl diphosphate [CT-CLPP]; Fig. 2), based on the nomenclature of clerodane-related diastereomer configurations derived from GGPP (Tokoroyama, 2000; Li et al., 2016). Specifically, the cis-trans-relative stereochemistry (cis-configuration for the C-5 and C-10 substituents and trans-configuration for the C-8 and C-9 substituents) was determined on the basis of comparison with available NMR spectra from the four different kolavenol diastereomers (Ohsaki et al., 1994; Freiburghaus et al., 1998; Nogueira et al., 2001; Pelot et al., 2017b; Supplemental Fig. S3). In addition, an ent-neo-absolute stereochemistry of CT-CLPP is proposed on the basis of active-site mutations as outlined below (Supplemental Fig. S4).
Active-Site Determinants Controlling PvCPS3 and PvCPS1 Catalytic Specificity
Having identified the (+)-8,13-CPS PvCPS3 and the ent-neo-CT-CLPS PvCPS1 as two previously unrecognized class II diTPS enzymes in switchgrass, we employed homology modeling based on the crystal structure of the Arabidopsis (Arabidopsis thaliana) ent-CPS (Köksal et al., 2014) to gain insight into the active-site determinants that govern these distinct functions. Molecular docking of GGPP in the active-site cavity of both protein structures identified several amino acids located in sufficient proximity to the substrate to infer a role in controlling catalytic specificity (Fig. 3). Of these residues, several were shown previously to impact product outcome in other plant class II diTPSs (Potter et al., 2014, 2016a, 2016b; Mafu et al., 2016; Hansen et al., 2017b; Pelot et al., 2017b). Residues F284 and H476 of PvCPS3 were located at the top of the active-site cavity near to the terminal double bond of GGPP, whereas F331 was seated between GGPP and D341 of the DxDD motif (Fig. 3). Gly substitution of PvCPS3:F331 through site-directed mutagenesis resulted in multiple products (Fig. 3; Supplemental Fig. S4), including the wild-type product 8,13-CPP 11 and the double bond isomers 7,13-CPP (compound 14) and (+)-CPP (compound 15). The production of 7,13-CPP had been reported similarly for a protein variant of the S. divinorum class II diTPS SdCPS2, carrying a mutation of the corresponding Phe residue (Pelot et al., 2017b). Notably, product formation in coupled assays of PvCPS3:F331G with MvELS, but not GrEKS, supported a (+)-stereochemistry of the PvCPS3 products (Supplemental Fig. S4). Ala substitution of active-site residues corresponding to position F284 in PvCPS3 has been shown to alter the product specificity of functionally distinct class II diTPSs toward producing isomers of LPP (Potter et al., 2014; Hansen et al., 2017b; Pelot et al., 2017b). Consistently, the protein variant PvCPS3:F284A partially redirected product outcome toward (+)-7,13-CPP 14 and the 8α/β-stereoisomers of (+)-LPP 16/17 (Fig. 3; Supplemental Fig. S4). The adjacent H476 in PvCPS3 is occupied typically by a Tyr residue in other class II diTPSs, whereas a His residue is found in only select enzymes such as the rice syn-CPS OsCPS4 (Potter et al., 2016a) and the C-9-hydroxylated peregrinol diphosphate synthase MvCPS1 of M. vulgare (Mafu et al., 2016), where it was demonstrated to impact catalytic specificity. The mutagenesis of PvCPS3:H476 to Ser altered product outcome toward the formation of the structurally related (+)-7,13-CPP 14 as the predominant product (Fig. 3; Supplemental Fig. S4).
Figure 3.
Structure-function analysis of PvCPS3 and PvCPS1. Homology models are shown for PvCPS3 (top) and PvCPS1 (bottom) with GGPP docked into the class II active site situated at the γ-domain (orange) and β-domain (teal) interface. Models were generated using the crystal structure of Arabidopsis ent-CPS (Protein Data Bank code 4LIX) as a template (Köksal et al., 2014). Residues targeted for site-directed mutagenesis in this study are indicated. Reaction products resulting from E. coli coexpression assays of the wild type and mutant variants of PvCPS3 and PvCPS1 are highlighted as labdane (orange), hydroxylated labdane (pink), and clerodane (blue) scaffolds and numbered as follows: 11, 8,13-CPP; 12, ent-neo-CT-CLPP; 14, 7,13-CPP; 15, (+)-CPP; 16, (+)-8β-LPP; 17, (+)-8α-LPP; 18, syn-halima-5,13E-dienyl diphosphate (5,13-HPP). Corresponding GC-MS chromatograms and mass spectra are given in Supplemental Figure S4.
Similar to the PvCPS3:F331G variant, Gly substitution of the corresponding F357 site in PvCPS1 resulted in several products (Fig. 3; Supplemental Fig. S4). Major products included the native product CT-CLPP 12, halima-5,13E-dienyl diphosphate (5,13-HPP; compound 18) as compared with the authentic product of the diTPS variant OsCPS4:H501F (Potter et al., 2016a), and 8,13-CPP 11 formed by PvCPS3. In addition, several minor products were observed that featured mass ions of m/z 290, 275, 272, and 257 characteristic for labdane diterpene olefins or alcohols. However, the low abundance of these products prevented the structural validation of these compounds. Since the PvCPS1:F357G product of 5,13-HPP 18 was likely derived from the characteristic syn-labda-13E-en-8-yl+ diphosphate intermediate rather than the syn-ent-enantiomer that has yet to be observed enzymatically (Peters, 2010), we propose an ent-neo-absolute stereochemistry for the PvCPS1 wild-type CT-CLPP product 12. However, since the absolute configuration of the PvCPS1 product CT-CLPP 12 could not be verified empirically, either an ent-neo- or neo-stereochemistry is theoretically possible.
Specific and Promiscuous Class I diTPSs Expand Switchgrass Terpenoid Diversity
Following the identification of several known and uncharacterized class II diTPS functions, we tested the conversion of these enzyme products by pairwise reactions with select class I diTPSs with predicted functions in GA and specialized diterpenoid metabolism. To validate a hypothesized ent-kaurene synthase function of PvKSL13 and PvKSL15 (Fig. 1), both enzymes were applied to E. coli coexpression assays, with class II diTPSs producing CPP isomers of ent-ZmAn2 + AgAS:D621A (Peters and Croteau, 2002) and syn-(PvCPS8) stereochemistry (Fig. 4). Both PvKSL13 and PvKSL15 converted ent-CPP into ent-kaurene 3, as verified by comparison with an authentic standard. Conversely, no class I diTPS products were observed when PvKSL13 or PvKSL15 was coexpressed with the class II diTPSs AgAS:D621A and PvCPS8.
Figure 4.
Functional characterization of PvKSL13 and PvKSL15 as ent-kaurene synthases. GC-MS extracted ion chromatograms (EIC; m/z 257) are shown for reaction products obtained from E. coli coexpression assays of the class I diTPSs PvKSL13 and PvKSL15 with select class II diTPSs: ent-CPS ZmAn2 (Harris et al., 2005), (+)-CPS AgAS:D621A (Peters and Croteau, 2002), and syn-CPS PvCPS8. Numbered peaks are as follows: 1, ent-copalol (i.e. dephosphorylated ent-CPP); 2, unidentified diterpene; 3, ent-kaurene; 9, syn-copalol; 15, (+)-copalol; 23, unidentified diterpene.
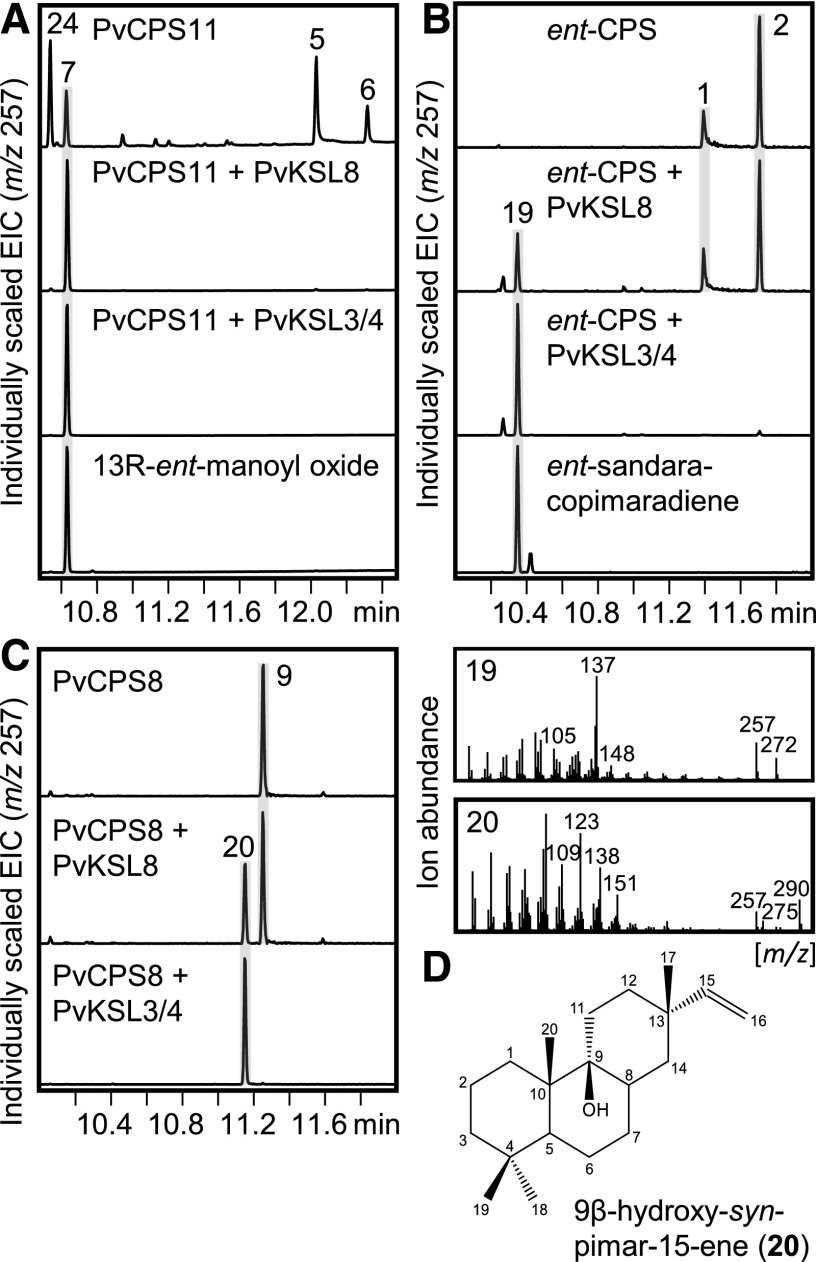
Among the large group of class I diTPSs with predicted functions in specialized metabolism, PvKSL3/4 (representing two diTPS genes of identical protein sequence) and PvKSL8 represented a pair of closely related enzymes, featuring βα- and a γβα-domain architecture, respectively (Fig. 1). In addition, PvKSL3/4 and PvKSL8 colocalized with the ent-LPS PvCPS11 and the related class II diTPS PvCPS12, respectively, on chromosomes 3 of the switchgrass subgenomes. Coexpression assays of PvKSL3/4 or PvKSL8 with PvCPS11 afforded 13R-ent-manoyl oxide 7, as verified by comparison with an authentic enzyme product (Andersen-Ranberg et al., 2016; Fig. 5). To further probe the catalytic range of PvKSL3/4 or PvKSL8, both enzymes were coexpressed with the maize ent-CPS ZmAn2, the syn-CPS PvCPS8, the (+)-8,13-CPS PvCPS3, and the ent-neo-CT-CLPS PvCPS1. No activity of PvKSL3/4 or PvKSL8 was detected in pairwise reactions with PvCPS3 or PvCPS1 (Supplemental Fig. S5). By contrast, PvKSL3/4 and PvKSL8 showed nearly identical product profiles resulting from sequential reactions with ZmAn2 or PvCPS8, albeit with relatively lower product quantities formed by PvKSL8 (Fig. 5). Both enzymes converted ent-CPP into ent-sandaracopimaradiene (compound 19), as verified by comparison with an authentic enzyme product (Fig. 5), and syn-CPP into a diterpenoid product (compound 20) with a retention time of 11.15 min and mass ions of m/z 290 and 275, indicating an unknown hydroxylated labdane structure (Fig. 5). NMR analysis identified this product as 9β-hydroxy-syn-pimar-15-ene (Fig. 5; Supplemental Fig. S6). The β-configuration of the C-9 hydroxyl group was tentatively assigned on the basis of the absolute stereochemistry of the syn-CPP substrate. In addition, selective 1D nuclear Overhauser effect analysis determined the relative stereochemistry of the methyl groups at C-17, C-20, and C-19 to be in cis-configuration, further supporting a syn-stereochemistry of the methyl group at C-17 (Supplemental Fig. S6). Thus, PvKSL3/4 and PvKSL8 belong to the rare group of class I diTPSs capable of incorporating hydroxyl groups into their prenyl diphosphate substrate, which, to current knowledge, also include Salvia sclarea sclareol synthase and Isodon rubescens nezukol synthase (Caniard et al., 2012; Schalk et al., 2012; Pelot et al., 2017a). Given the two-domain structure of PvKSL3/4, a possible additional sesquiterpene synthase activity similar to those shown for multisubstrate TPS from wheat (TaKSL5; Hillwig et al., 2011) and maize (ZmTPS1; Schnee et al., 2002) would be plausible. To test this hypothesis, we performed E. coli coexpression assays of PvKSL3/4 and the characterized maize FPP synthase ZmFPPS3 (Richter et al., 2015). However, no conversion of FPP was observed that would indicate a sesquiterpene synthase activity of PvKSL3/4.
Figure 5.
Functional characterization of the promiscuous class I diTPSs PvKSL3/4 and PvKSL8. A to C, GC-MS traces of products resulting from E. coli coexpression assays of the class I diTPSs PvKSL3/4 and PvKSL8 with the ent-LPS PvCPS11 (A), the ent-CPS ZmAn2 (Harris et al., 2005; B), and the syn-CPS PvCPS8 (C). Individually scaled extracted ion chromatograms (EIC; m/z 257) are shown with the corresponding mass spectra of select diterpenoids. D, Structure of 9β-hydroxy-syn-pimar-15-ene as verified by NMR analysis. Numbered peaks are as follows: 1, ent-copalol (i.e. dephosphorylated ent-CPP); 2, unidentified diterpene; 5, ent-8β-labda-13E-en-8,15-diol (i.e. dephosphorylated ent-LPP); 6, ent-8α-labda-13E-en-8,15-diol; 7, 13R-ent-manoyl oxide; 9, syn-copalol; 19, ent-sandaracopimaradiene; 20, 9β-hydroxy-syn-pimar-15-ene; 24, 13S-ent-manoyl oxide.
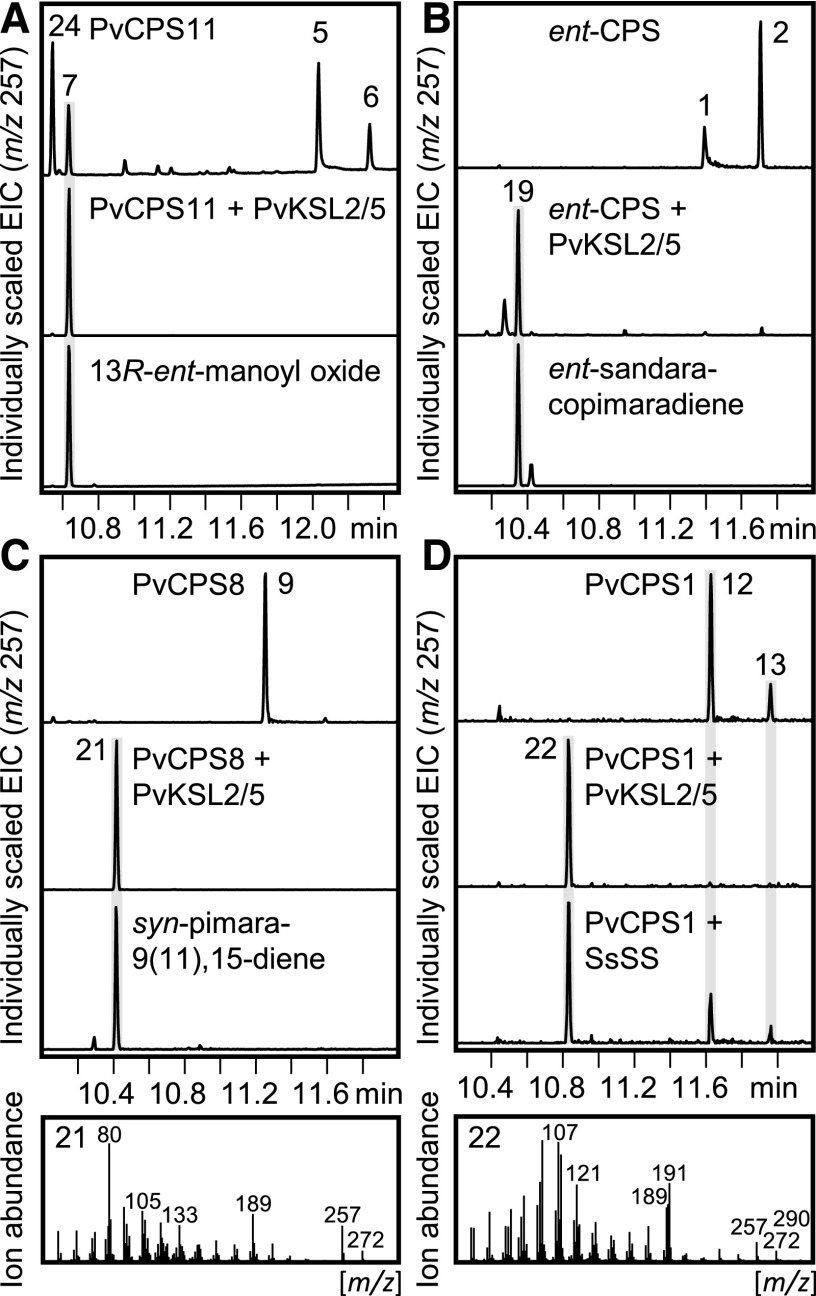
Next, we probed the function of PvKSL2/5 (a gene pair of identical protein sequence), which showed a close genomic and phylogenetic interrelatedness with PvKSL3/4 (Fig. 1). Similar to PvKSL3/4, PvKSL2/5 converted ent-LPP and ent-CPP into 13R-ent-manoyl oxide 7 and ent-sandaracopimaradiene 19, respectively (Fig. 6). However, coupled activity with the syn-CPS PvCPS8 did not yield the PvKSL3/4 product 9β-hydroxy-syn-pimar-15-ene 20 but syn-pimara-9(11),15-diene (compound 21), as verified by an authentic standard (Zhou et al., 2012; Fig. 6). Similar to PvKSL3/4, no activity was observed when PvKSL2/5 was paired with PvCPS3 (Supplemental Fig. S5). In contrast, when coexpressed with PvCPS1, PvKSL2/5 showed activity with ent-neo-CT-CLPP as a substrate, resulting in ent-neo-cis-trans-kolavelool (compound 22; Fig. 6), as compared with a previously reported enzymatic reaction of S. sclarea sclareol synthase (Caniard et al., 2012; Jia et al., 2016).
Figure 6.
Functional characterization of the promiscuous class I diTPS PvKSL2/5. Shown are GC-MS extracted ion chromatograms (EIC; m/z 257) and corresponding mass spectra of select products resulting from E. coli coexpression assays of the class I diTPS PvKSL2/5 with the ent-LPS PvCPS11 (A), the ent-CPS ZmAn2 (Harris et al., 2005; B), the syn-CPS PvCPS8 (C), and the ent-neo-CT-CLPS PvCPS1 (D). Numbered peaks are as follows: 1, ent-copalol (i.e. dephosphorylated ent-CPP); 2, unidentified diterpene; 5, ent-8β-labda-13E-en-8,15-diol (i.e. dephosphorylated ent-LPP); 6, ent-8α-labda-13E-en-8,15-diol; 7, 13R-ent-manoyl oxide; 9, syn-copalol; 12, ent-neo-cis-trans-kolavenol (i.e. dephosphorylated ent-neo-CT-CLPP); 13, unidentified diterpene; 19, ent-sandaracopimaradiene; 21, syn-pimara-9(11),15-diene; 22, ent-neo-cis-trans-kolavelool; 24, 13S-ent-manoyl oxide.
Abundance of diTPS Transcripts in Switchgrass Tissues
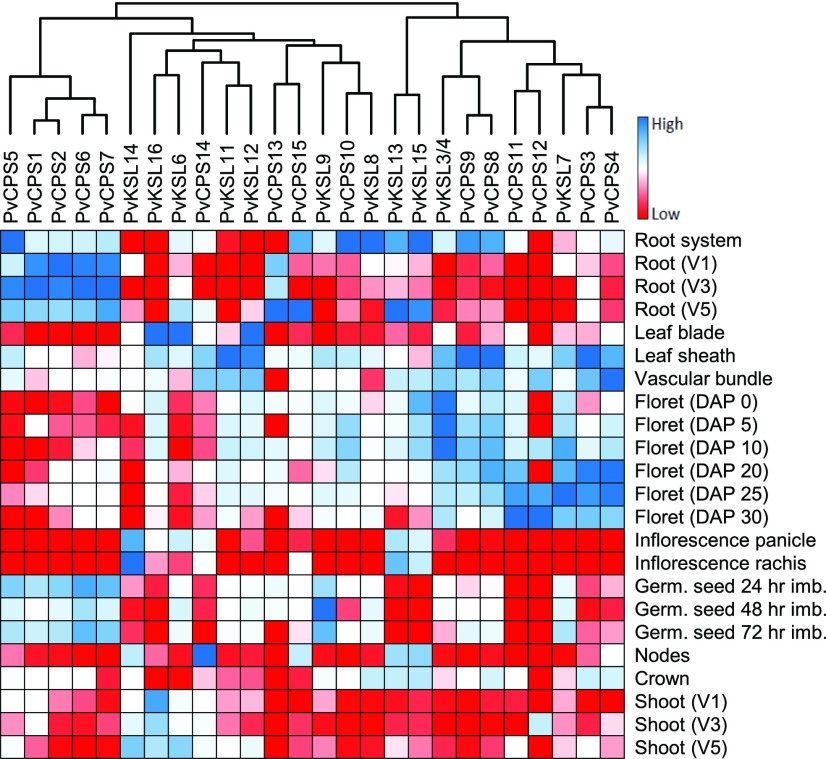
To assess the abundance of switchgrass diTPS genes in planta, we investigated publicly available gene expression profiles for different switchgrass tissues [DOE-JGI(a)]. A heat map displaying relative transcript levels for each diTPS gene candidate across a set of 23 different untreated switchgrass tissues was generated by grouping genes on the basis of similar gene expression profiles using an agglomerative hierarchical cluster analysis. Transcripts of PvKSL1, PvKSL2/5, and PvKSL10 were not present in this data set, probably due to the low abundance of these genes. For the majority of the identified diTPSs, transcript abundance was highest in either roots or leaf and floret tissues (Fig. 7). Of the functionally characterized diTPSs, the pairs of ent-CPSs (PvCPS14 and PvCPS15) and ent-kaurene synthases (PvKSL13 and PvKSL15) showed highest abundance in roots, with the exception of PvCPS14, which was most abundant in leaves and nodes. The syn-CPS PvCPS8 clustered closely with the sequentially acting 9β-hydroxy-syn-pimar-15-ene synthase PvKSL3/4 and the predicted syn-CPS homolog PvCPS9, showing transcript levels widely distributed in roots, leaves, and florets. By contrast, the functionally redundant 9β-hydroxy-syn-pimar-15-ene synthase PvKSL8 was expressed predominantly in roots and clustered with the predicted syn-CPS homolog PvCPS10 showing a similar expression profile. Gene expression of the (+)-8,13-CPS PvCPS3 and the ent-LPS PvCPS11 clustered with their respective homologs (+)-8,13-CPS PvCPS4 and ent-LPS PvCPS12 and showed predominant gene expression in leaf sheath, vascular bundle, and floret tissues. The transcript abundance of the ent-neo-CT-CLPS PvCPS1 was highest in roots and germinating seeds, along with several other clustered class II diTPSs (PvCPS2, PvCPS5, PvCPS6, and PvCPS7) that also were observed to cluster phylogenetically (Fig. 1).
Figure 7.
Hierarchical cluster analysis of diTPS expression profiles across switchgrass tissues. The heat map shows the relative transcript abundance for each diTPS gene candidate in FPKM (fragments per kilobase of transcript per million mapped reads) expression profiles of 23 different untreated switchgrass plant tissues (https://phytozome.jgi.doe.gov/). Transcript levels for PvKSL1, PvKSL2/5, and PvKSL10 were not detected. DAP, Days after pollination; V, vegetative stage.
Stress-Elicited Biosynthesis of Switchgrass Diterpenoids
To identify diTPS products in planta and investigate the possible role of switchgrass diterpenoids in abiotic stress responses, we performed metabolite and transcript analyses in stress-elicited leaves and roots. Aboveground stress-induced diterpenoid biosynthesis was examined by treating detached leaf blades of 15-week-old switchgrass (var Alamo) plants with UV irradiation (20 min), which was shown previously to induce diterpenoid accumulation in rice and wheat (Nemoto et al., 2004; Otomo et al., 2004b; Prisic et al., 2004; Sakamoto et al., 2004; Wilderman et al., 2004; Xu et al., 2004; Zhou et al., 2012). Reverse transcription quantitative PCR (RT-qPCR) analysis revealed overall low abundance of diTPS transcripts in leaves. However, several genes showed significantly increased expression levels, which peaked at 6 to 12 h post treatment (Fig. 8). This included PvCPS3 (∼50-fold increase), PvCPS1 (∼100-fold increase), PvCPS8 (∼15-fold increase), PvKSL3/4 (∼8-fold increase), and PvKSL8 (∼2-fold increase) of specialized metabolism. In contrast, no stress-elicited increase in gene expression was detected for the bona fide GA biosynthetic diTPSs PvCPS15, PvCPS14, PvKSL13, and PvKSL15, all of which were detected at only trace levels. Likewise, the promiscuous class I diTPS PvKSL2/5 were detected at only trace amounts. Notably, the identical nucleotide sequences of PvKSL2/5 and PvKSL3/4 prevented a separate analysis of their respective gene expression. Diterpenoid metabolite analysis of the same tissue samples identified small quantities of 9β-hydroxy-syn-pimar-15-ene in leaves, which accumulated over 24 h post UV treatment to 79 ± 9 ng g−1 dry weight (Fig. 8; Supplemental Fig. S7), consistent with the gene expression patterns of PvCPS8 and PvKSL3/4. In addition, manoyl oxide accumulated up to 181 ± 32 ng g−1 dry weight at 48 h after treatment.
Figure 8.
Relative transcript and metabolite abundance in stress-induced switchgrass leaf blade and root tissues. A and B, Analysis of relative transcript (A) and metabolite (B) abundance in stress-treated (blue) and control (gray) tissues. In the left column, detached leaf blades were treated with 20 min of UV irradiation (254 nm). In the right column, roots were treated by adding 10 mm CuSO4 solution daily to the soil of switchgrass plants. RT-qPCR was used to measure relative transcript abundance and normalized to the internal reference gene elongation factor 1α (EF-1α). Relative metabolite abundance was determined by GC-MS analysis. Error bars represent propagated se of the mean (n = 3). Asterisks depict statistically significant differences between stress-treated and control samples based on Student’s t test (P < 0.05). DW, Dry weight.
In conjunction, alterations of diterpenoid metabolism in response to belowground perturbations were examined by soil treatment with 10 mm CuSO4 as a proxy for oxidative stress, which was demonstrated recently to induce diterpenoid accumulation in maize (Mafu et al., 2018). Gene expression analysis in stress-elicited roots as compared with controls treated with only nutrient water showed transcript abundance of PvCPS8 (∼1,700-fold increase), PvKSL3/4 (∼1,400-fold increase), PvKSL8 (∼1,100-fold increase), and PvKSL2/5 (∼800-fold increase) to be increased significantly after CuSO4 treatment, with highest abundance at 6 to 12 h post treatment (Fig. 8). Increased transcript abundance of PvCPS8 and PvKSL3/4 was accompanied by the accumulation of 9β-hydroxy-syn-pimar-15-ene up to a concentration of 907 ± 56 ng g−1 dry weight 48 h post treatment (Fig. 8B; Supplemental Fig. S7). None of the remaining tested diTPS genes showed altered gene expression levels in response to CuSO4 treatment. In both treated and control samples, the transcript of PvCPS14 was not detected.
DISCUSSION
Shifting environmental pressures and associated biotic and abiotic stressors can overwhelm the natural defense systems of plants and are foreseeable impediments to crop production (Chakraborty and Newton, 2011; de Sassi and Tylianakis, 2012). To mitigate crop loss, a better understanding of the often species-specific biosynthetic machinery and diversity of chemical defense mechanisms that contribute to plant resilience is essential. In this context, elucidating the metabolic network driving switchgrass diterpenoid metabolism and its contribution to stress tolerance can provide useful resources for improving crop traits, as demonstrated in other major crops such as maize and rice (Degenhardt, 2009; Schmelz et al., 2011; Bagnaresi et al., 2012; Mafu et al., 2018). This study highlights the utility of functional genomics approaches to efficiently decode the biosynthetic networks underlying plant terpenoid metabolism. Despite the predominantly taxonomic relatedness of plant diTPSs, the biochemical verification of the predicted functions of PvCPS15 and PvCPS14 as ent-CPSs and PvCPS8 as a syn-CPS exemplifies the increasing applicability of sequence- and phylogeny-based functional annotations as more diTPS activities are being discovered. However, the still incomplete knowledge of the diTPS functional space and the profound catalytic plasticity, especially among class I diTPSs, continue to present notable hurdles to diTPS functional annotation and underscore the necessity of biochemical characterization to unambiguously validate enzyme activities.
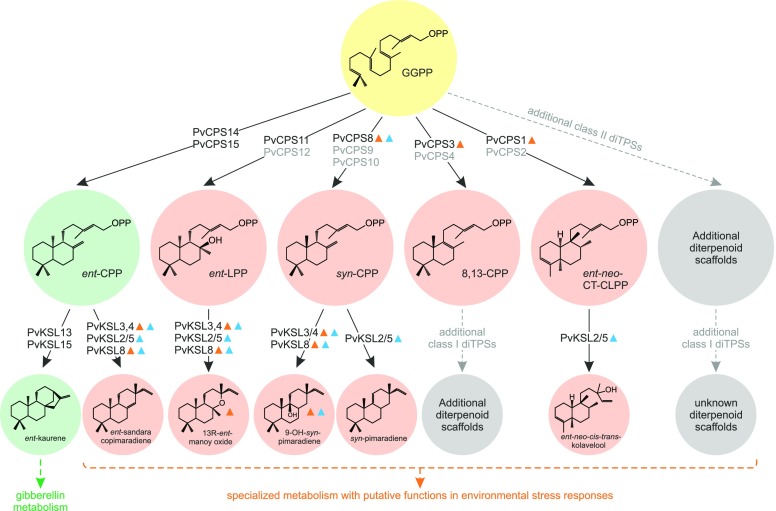
The presence of at least five distinct class II diTPS functions demonstrates a large class II diTPS family in switchgrass, providing the committed precursors to a diverse arsenal of diterpenoids that fall into the broad family of labdane-related natural products (Fig. 9). This comprised common intermediates such as ent-CPP (PvCPS15 and PvCPS14), ubiquitously required for GA biosynthesis and shown to be involved in the biosynthesis of maize and rice diterpenoid phytoalexins (Cho et al., 2004; Otomo et al., 2004a, 2004b; Sakamoto et al., 2004; Xu et al., 2007a; Schmelz et al., 2011; Mafu et al., 2018), and syn-CPP (PvCPS8), as the core precursor for antimicrobial and allelopathic oryzalexin and momilactone diterpenoids in rice (Peters, 2006; Xu et al., 2007a; Bagnaresi et al., 2012; Kato-Noguchi and Peters, 2013; Toyomasu et al., 2014). In addition, with PvCPS11, we also identified an ent-LPS in switchgrass, which thus far were known only from dicot species such as Salvia miltiorrhiza (Cui et al., 2015) and Tripterygium wilfordii (Hansen et al., 2017a), whereas the respective (+)-stereoisomer is distributed broadly among Lamiaceae and Asteraceae species (Caniard et al., 2012; Zerbe et al., 2013; Pateraki et al., 2014). Furthermore, PvCPS3 and PvCPS1 add, to the best of our knowledge, previously unrecognized functions to the catalytic landscape of the plant diTPS family, facilitating the biosynthesis of (+)-8,13-CPP and ent-neo-CT-CLPP, respectively. The PvCPS3-catalyzed formation of (+)-8,13-CPP will proceed through terminal deprotonation of the common (+)-labda-13E-en-8-yl+ diphosphate intermediate at C-9 (Supplemental Fig. S8). Conversely, direct deprotonation of the intermediate carbocation at the exocyclic C-17 methyl would lead to the related doubled bond CPP isomer (Peters, 2010), whereas deprotonation at the endocyclic C-7 methylene would afford 7,13-CPP (Mafu et al., 2011; Zerbe et al., 2015). With the ent-neo-CT-CLPS PvCPS1, switchgrass further has the capacity to produce clerodane-type diterpenoids. Notably, PvCPS1 produces a rare cis-clerodane scaffold known in members of the Euphorbiaceae, Salicaceae, and Menispermaceae, whereas the more common transclerodanes are abundant in select Lamiaceae and Asteraceae species (Li et al., 2016). The proposed PvCPS1-catalyzed reaction toward ent-neo-CT-CLPP likely involves rearrangement of the intermediary syn-labda-13E-en-8-yl+ diphosphate carbocation via a series of 1,2-hydride and methyl shifts, with the final 1,2-methyl migration occurring at the C-18 methyl to yield a cis-clerodane, whereas migration of the C-19 methyl would result in trans-clerodane scaffolds (Hamano et al., 2002; Peters, 2010; Supplemental Fig. S8). The formation of a characteristic syn-5,13-HPP product by the protein variant PvCPS1:F357G supports this general reaction mechanism and suggests ent-neo-absolute stereochemistry for the PvCPS1 product (Fig. 3).
Figure 9.
Diterpene biosynthesis in switchgrass. Shown is an overview of identified and proposed (gray) biosynthetic pathways, involving monofunctional class II and class I diTPSs that convert the central precursor GGPP into a range of diterpenoid metabolites with putative roles in general (GA; green) and specialized (orange) diterpenoid metabolism. Triangles indicate genes and metabolites showing patterns of stress-inducible formation in roots (blue) and leaves (orange).
Consistent with the modular architecture of diterpenoid metabolic networks described in maize, wheat, and rice (Wu et al., 2012; Zhou et al., 2012; Schmelz et al., 2014; Fu et al., 2016; Mafu et al., 2018), we identified functionally diverse class I diTPSs that act sequentially with the array of distinct class II enzymes to form a complex branched grid of diterpenoid pathways in switchgrass (Fig. 9). Considering the nine class II and nine class I diTPSs with yet unidentified activities, a more expansive and diverse diterpenoid network is expected to operate in this species. With PvCPS14, PvCPS15, PvKSL13, and PvKSL15, we identified pairs of ent-CPP and ent-kaurene synthases in switchgrass (Fig. 4). Similar functionally redundant copies of ent-CPSs and ent-kaurene synthases also were described in maize, where the ent-CPS ZmAn1 is required for GA biosynthesis, whereas the second ent-CPS ZmAn2 was shown to be stress inducible and functions in specialized diterpenoid metabolism (Schmelz et al., 2011; Vaughan et al., 2015; Mafu et al., 2018). The close phylogenetic relatedness of PvCPS14 and PvCPS15 to ZmAn1 and the lack of stress inducibility of the corresponding genes in leaves or roots supported a role in GA metabolism. However, the predominant abundance of PvKSL13, PvKSL15, and PvCPS15 transcripts in roots may point to alternative or additional functions in specialized metabolism. The conversion of ent-CPP into ent-sandaracopimaradiene by PvKSL3/4, PvKSL2/5, and PvKSL8 also is supportive of the allocation of ent-CPP toward specialized diterpenoid pathways in switchgrass. However, ent-sandaracopimaradiene or related pimarane diterpenoids were not detected in leaf and root extracts. By contrast, albeit at low abundance, the presence of manoyl oxide and 9β-hydroxy-syn-pimar-15-ene in switchgrass leaves and roots verified their formation in planta. Furthermore, the in vitro formation of these metabolites by PvKSL3/4, PvKSL2/5, and PvKSL8, combined with the presence of additional gene copies and distinct patterns of tissue-specific and stress-inducible gene expression, suggest that multiple metabolic routes toward these and related diterpenoids may exist in switchgrass. Strong coexpression of PvCPS8 and PvKSL3/4 may point to one such tissue-specific enzyme pairing. Furthermore, given the low abundance of 9β-hydroxy-syn-pimar-15-ene and manoyl oxide in extracts from 0.5 to 2.5 g of switchgrass leaf and root tissue, it appears likely that the observed diTPS products will be elaborated further toward yet unknown and more complex diterpenoid metabolites that may contribute to stress defenses in switchgrass. Likewise, it must be considered that so far unknown downstream pathway products of higher polarity may not be readily abundant in hexane extracts and/or may accumulate only under stimuli not yet investigated. However, patterns of diterpenoid accumulation and the increased expression of relevant switchgrass diTPS genes in response to UV irradiation and oxidative stress also have been observed for specialized diterpenoid metabolism in maize and rice (Kodama et al., 1988; Horie et al., 2015; Vaughan et al., 2015) and support diterpenoid functions in aboveground and belowground protective mechanisms in switchgrass. This hypothesis also is supported by recent maize studies that demonstrated increased drought susceptibility of the maize an2 mutant that is deficient in select kauralexin and dolabralexin diterpenoids (Vaughan et al., 2015; Mafu et al., 2018). Although a role of diterpenoids in conferring abiotic stress resistance has yet to be empirically proven, these results point toward a broader spectrum of diterpenoid bioactivities beyond their more common antifeedant and antimicrobial functions (Schmelz et al., 2014; Vaughan et al., 2015; Christensen et al., 2018; Mafu et al., 2018).
Gene and whole-genome duplications have been suggested as factors in generating new genes and expanding gene families of plant specialized metabolism (Jiao and Paterson, 2014), as shown, for example, in the diversification of glucosinolate metabolism in the Brassicaceae family (Hofberger et al., 2013). The large size of the 31-membered switchgrass diTPS family (as compared with the nine diTPSs identified in its diploid relative P. hallii) suggests that the polyploidization of allotetraploid switchgrass has had a substantial impact on the expansion of the switchgrass diTPS family since the split from the P. hallii genome approximately 5 million years ago (Huang et al., 2003; Zhang et al., 2011). This hypothesis is consistent with a recent phylogenomics study of multiple plant genomes, including those of maize and sorghum (Sorghum bicolor), that demonstrated the impact of both gene and genome duplication on the expansion of isoprenoid metabolic gene families (Hofberger et al., 2015). Notably, whereas a relatively smaller size of diTPS gene families in diploid genomes is consistent with the nine and 14 diTPSs identified in maize and rice, respectively (Schmelz et al., 2014), the hexaploid wheat genome also contains a diTPS family of, to current knowledge, nine members (Schmelz et al., 2014). Thus, polyploidization is not implicitly linked to the expansion of the terpenoid metabolic gene families, underscoring the need for further studies on the evolution of terpenoid metabolism in this context. In the case of switchgrass, it is plausible that the tetraploidy and heterozygosity of the switchgrass genome (due, at least partly, to the largely obligate outcrossing nature of this species) have provided robustness for genome rearrangements, gene duplications, and subsequent domain loss and/or neofunctionalization events fueling the evolutionary diversification of the diTPS family (Zi et al., 2014). As compared with P. hallii, in both switchgrass subgenomes, a larger number of class II diTPSs were identified on chromosome 1 and expansive blooms of class I diTPSs were observed on chromosome 3 (Fig. 1), suggesting repeated and independent events of gene duplication and functional divergence in both switchgrass subgenomes. This included PvCPS1, PvCPS3, PvCPS11, PvKSL3/4, PvKSL2/5, and PvKSL8, demonstrated here to form a range of specialized diterpenoids. Altered product profiles resulting from single-residue modifications in the PvCPS1 and PvCPS3 active sites (Fig. 3) underscore the ease of functional diversification among the switchgrass diTPS family, as also demonstrated for diterpenoid metabolism in maize, rice, and wheat (Xu et al., 2007b; Morrone et al., 2008; Schmelz et al., 2014; Potter et al., 2016a). Notably, the formation of (+)-8,13-CPP by the protein variant PvCPS1:F357G may resemble evolutionary events leading to the emergence of the (+)-8,13-CPS and ent-neo-CT-CLPS activities of PvCPS3 and PvCPS1 on subgenome K. Additionally, loss of the N-terminal γ-domain appears to have occurred multiple times in the evolution of the switchgrass diTPS family, with PvKSL3/4, PvKSL6, PvKSL12, PvKSL14, and PvKSL16 representing such two-domain enzymes. Similar TPSs from wheat (TaKSL5) and maize (ZmTPS1) were shown to possess sesquiterpene synthase activity while retaining the ancestral ent-kaurene synthase function (Schnee et al., 2002; Hillwig et al., 2011; Fu et al., 2016). By contrast, no such dual activity was observed for PvKSL3/4 in coexpression assays, which is consistent with the phylogenetic positions of PvKSL3/4 and TaKSL5 on distant branches among the class I diTPS family. Conversely, TaKSL5 and ZmTPS1 are closely related to the thus far uncharacterized PvKSL14 and PvKSL16, which may suggest more closely related dual functionalities.
Whereas the chemical space of switchgrass diterpenoid metabolism and its role in plant defense and environmental adaptation require further study, the genome-wide identification of the switchgrass diTPS family expands our insight into the distribution and biosynthesis of common and species-specific diterpenoid networks operating in monocot crops. Patterns of inducible diterpenoid formation in response to abiotic stress are consistent with a growing body of knowledge suggesting that diterpenoid bioactivities span beyond established antibiotic activities and include roles in countering abiotic stress, as proposed, for example, for diterpenoid phytoalexins in maize (Vaughan et al., 2015; Mafu et al., 2018) and the diterpenoid isorosmanol in rosemary (Rosmarinus officinalis; Munné-Bosch and Alegre, 2000). These findings underscore the need for a closer examination of the precise ecological functions of monocot diterpenoids, which ultimately necessitates the generation and analysis of genetic switchgrass mutants in future work. With increasing capacities for computational and biochemical annotation of gene functions, the defensive potential of modular diterpenoid networks in monocot crop species can be of substantial value to elucidate and ultimately utilize the genetic basis of crop stress resilience (Schmelz et al., 2014).
MATERIALS AND METHODS
Plant Material
Switchgrass (Panicum virgatum var Alamo) was obtained as seed from Roundstone Native Seed and as whole plants from the U.S. Department of Agriculture Germplasm Resources Information Network (accession no. PI 422006). Plants were grown in a greenhouse using nutrient water solution at an output of ∼300 mL d−1 for up to 15 weeks under ambient photoperiod and ∼22°C/17°C day/night temperatures.
Elicitation of Switchgrass Leaves and Roots
Leaf blades and roots were elicited with UV irradiation and oxidative stress (CuSO4 treatment), respectively. For UV irradiation, leaf blades were detached from 15-week-old plants, placed on wet paper towels, irradiated for 20 min using a 254-nm UV lamp situated 20 cm from the leaf surface, and then kept in the dark in a high-humidity incubator at 30°C until sample collection. As a negative control, leaf blades were handled in the same manner, but without UV treatment. Root treatments with CuSO4 were used as a proxy for oxidative stress compared with controls treated with nutrient water only. Here, 300 mL of a 10 mm CuSO4 solution (in nutrient water) was added daily to the soil of 15-week-old plants. Tissues were collected at 0, 6, 12, 24, and 48 h and immediately flash frozen in liquid N2 until used for metabolite and gene expression analysis as described below.
RT-qPCR Analysis
Total RNA from P. virgatum leaf and root tissue was extracted using an optimized SDS method (Das et al., 2013), and residual genomic DNA was removed using the TURBO DNA-free kit (Thermo Fisher Scientific). The resulting total RNA was reverse transcribed into first-strand cDNA using the iScript Reverse Transcription Supermix for RT-qPCR kit (Bio-Rad). qPCR was performed on a Bio-Rad CFX96 real-time system using iTaq SYBR Green Supermix (Bio-Rad) with gene-specific oligonucleotides (Supplemental Table S3). Oligonucleotide specificity was verified by melt curve analysis and sequencing of representative amplicons. Relative transcript abundance was determined using efficiency-corrected ΔCT values based on eukaryotic EF1α as a previously validated switchgrass reference gene (Gimeno et al., 2014) and duplicate measurements of three biological replicates.
Metabolite Analysis in Plant Tissues
Metabolites were extracted from flash-frozen switchgrass leaf blades (∼0.5 g) and roots (∼2.5 g) by grinding the tissue to a fine powder in liquid N2. Metabolites were hexane extracted under vigorous shaking overnight at 16°C, air dried, and resuspended in hexane for fractionation by silica gel (70-230 mesh; Sigma-Aldrich) with an ethyl acetate/hexane mobile phase at a stepwise gradient of 10%, 20%, 30%, and 40% (5 mL each) for leaf blade tissue and a gradient of 10%, 25%, 50%, and 75% (1 mL each) for root tissue. The resulting fractions were concentrated to a volume of 0.5 mL of hexane for GC-MS analysis. Metabolite quantification was based on three biological replicates using 1-Eicosene (Sigma-Aldrich) as an external standard (linear calibration curve R2 = 0.99). GC-MS analysis of extracted metabolites was conducted on an Agilent 7890B GC device interfaced with a 5977 Extractor XL MS Detector at 70 eV and 1.2 mL min−1 He flow, using an Agilent HP5-ms column (30 m, 250 µm i.d., 0.25 µm film) and the following GC parameters: 50°C for 2 min, 25°C min−1 to 300°C, hold 3 min with pulsed splitless injection at 250°C. MS data from 40 to 400 m/z were collected after an 8.5-min solvent delay.
Gene Synthesis
Full-length or N-terminally truncated (removal of the predicted plastidial transit peptides) genes used in this study were synthesized with the support of a Department of Energy Joint Genome Institute Community Science Program grant. For coexpression in Escherichia coli, synthesized class II diTPSs were cloned into the pET-28b(+) vector using the NdeI restriction site and class I diTPS genes were inserted into the pETDuet-1 vector (Novagen) using the SacI restriction site. Details of the constructs used in this study are given in Supplemental Table S3.
Escherichia coli in Vivo Coexpression Assays of diTPSs
For microbial coexpression assays of diTPSs, E. coli BL21DE3-C41 was cotransformed with individual target diTPS constructs in the pET-28b(+) and pETDuet-1 vectors for compatible plasmid replication, the Abies grandis GGPP synthase (plasmid pGG), and key enzymes of the methyl-erythritol phosphate pathway (plasmid pIRS), as described previously (Morrone et al., 2010). Cultures were grown at 37°C and 180 rpm in 50 mL of Terrific Broth medium to an OD600 of ∼0.6 before cooling the cultures to 16°C and induction with 1 mm isopropyl-β-d-1-thiogalactopyranoside for 72 h with the addition of 25 mm sodium pyruvate. Products were extracted with 50 mL of hexane, air dried, and resuspended in 1 mL of n-hexane for GC-MS analysis.
NMR Analysis
For NMR analysis, 1 mg or more of diterpene products was produced through microbial coexpression assays as described above using 500-mL cultures. Hexane extracts were air dried and redissolved in 10 mL of hexane for purification on silica gel (Sigma-Aldrich) using ethyl acetate/hexane as a mobile phase at a stepwise gradient of 10%, 20%, 30%, and 40% (5 mL each). Fractions containing the desired product were further HPLC purified using an Agilent 1100 HPLC system with an RRHD Eclipse Plus C18 column (2.1 × 50 mm) and an acetonitrile/deionized water mobile phase at a flow rate of 1 mL min−1. For NMR analysis, products were dissolved in deuterated chloroform (CDCl3; Sigma-Aldrich) containing 0.03% (v/v) tetramethylsilane. NMR 1D (1H, 13C, nuclear Overhauser effect) and 2D (heteronuclear single quantum coherence), 1H-1H correlation spectroscopy, heteronuclear multiple bond correlation, and heteronuclear two bond correlation spectra were acquired on a Bruker Avance III 800-MHz spectrometer equipped with a 5-mm CPTCI cryoprobe. All NMR spectra were acquired with TopSpin 3.2 software (Bruker) and analyzed with MestReNova 11.0.2 software (Mestrelab Research). Chemical shifts were calibrated against known chloroform (1H 7.26 ppm and 13C 77 ppm) signals.
In Vitro Enzyme Assays
Class II diTPS constructs in the pET-28b(+) vector were expressed in E. coli BL21DE3-C41 cells and Ni2+-NTA affinity purified as described elsewhere (Zerbe et al., 2013). Single-vial diTPS enzyme assays were performed using 100 µg of recombinant protein and 15 µm (E,E,E)-GGPP (Sigma-Aldrich) as a substrate with gentle shaking for 1 h at 30°C (Zerbe et al., 2012). Enzyme products were dephosphorylated by treatment with 10 units of calf intestinal alkaline phosphatase (Thermo Fisher Scientific) overnight with gentle shaking at 37°C to remove the diphosphate moiety and enable hexane extraction for subsequent GC-MS analysis.
Homology Modeling and Site-Directed Mutagenesis
The crystal structure of the Arabidopsis (Arabidopsis thaliana) ent-copalyl diphosphate synthase (Protein Data Bank code 4LIX; Köksal et al., 2014) served as a template to create homology models for PvCPS3 and PvCPS1 using the SWISS-MODEL server. The stereochemical correctness of the generated models was assessed on the basis of Ramachandran plots using ProCheck (Laskowski et al., 1993). Ligand docking of GGPP within the active site of each homology model was performed using Molegro Virtual Docker with a manually positioned 12 Å search space around the active site (Thomsen and Christensen, 2006). Select point mutants were generated using whole-plasmid PCR amplification with site-specific sense and antisense oligonucleotides (Supplemental Table S3). Treatment with DpnI was used to remove template plasmids. All protein variants were sequence verified and analyzed using E. coli coexpression assays as described above.
Heat Map and Hierarchical Cluster Analysis
Publicly available transcriptome-based gene expression data on the Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html) were analyzed for each diTPS gene candidate among 23 different tissue types to create a heat map. FPKM values were normalized by feature scaling and used to generate individual heat maps for each investigated diTPS gene candidate. Normalized FPKM values were first grouped manually by tissue type to simplify the comparison of similar tissue types and then grouped by genes for hierarchical clustering analysis using the statistical software package XLSTAT 2018 with the agglomeration method of complete linkage, enabling the generation of a binary clustering tree (dendrogram) based on Euclidian distance to measure the dissimilarity between two data points.
Phylogenetic Analysis
A maximum-likelihood phylogenetic tree was generated using the PhyML server (Guindon et al., 2010) with four rate substitution categories, LG substitution model, BIONJ starting tree, and 500 bootstrap repetitions.
Accession Numbers
Nucleotide sequences of the enzymes characterized in this study are available on Phytozome (https://phytozome.jgi.doe.gov) and have been submitted to the GenBank/EMBI Data Bank with the following gene identifiers and accession numbers: PvCPS1 (Pavir.1KG382200, MH124141), PvCPS3 (Pavir.1KG339400, MH124142), PvCPS8 (Pavir.2KG192900, MH124143), PvCPS11 (Pavir.3KG444700, MH124144), PvCPS14 (Pavir.9KG170400, MH124145), PvCPS15 (Pavir.9NG385700, MH124146), PvKSL2 (Pavir.3KG458200, MH124147), PvKSL3 (Pavir.3KG458100, MH124148), PvKSL4 (Pavir.3KG443800, MH124149), PvKSL5 (Pavir.3KG443700, MH124150), PvKSL8 (Pavir.3NG231500, MH124151), PvKSL13 (Pavir.7KG337500, MH124152), and PvKSL15 (Pavir.7NG404900, MH124153).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Biochemical characterization of PvCPS15 and PvCPS14 as an ent-CPS and PvCPS11 as an ent-LPS.
Supplemental Figure S2. NMR analysis of (+)-labda-8,13E-dien-15-ol (compound 11).
Supplemental Figure S3. NMR analysis of ent-neo-cis-trans-kolavenol (compound 12).
Supplemental Figure S4. E. coli coexpression assays of PvCPS3 and PvCPS1 protein variants.
Supplemental Figure S5. Nonreactive E. coli in vivo coexpression assays of class I diTPSs.
Supplemental Figure S6. NMR analysis of 9β-hydroxy-syn-pimar-15-ene (compound 20).
Supplemental Figure S7. Metabolite analysis of switchgrass leaf blades and roots.
Supplemental Figure S8. Putative reaction mechanisms of uncharacterized switchgrass diTPSs.
Supplemental Table S1. Abbreviations and accession numbers of proteins used for phylogenetic analysis.
Supplemental Table S2. Comparative genomic locations of P. virgatum and P. hallii diTPS genes.
Supplemental Table S3. Oligonucleotides and gene constructs used in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Dr. Reuben Peters (Iowa State University) for providing the pIRS and pGGxC constructs and select diterpenoid standards and Dr. Jörg Bohlmann for providing select diterpenoid authentic standards.
Footnotes
This work was supported by a University of California-Davis Academic Senate Faculty Research Grant (to P.Z.), a DOE Joint Genome Institute Community Science Program Grant (CSP2568 to P.Z. and D.T.), and the NSF Graduate Research Fellowship Program (to K.A.P.).
Articles can be viewed without a subscription.
References
- Allwright MR, Taylor G (2016) Molecular breeding for improved second generation bioenergy crops. Trends Plant Sci 21: 43–54 [DOI] [PubMed] [Google Scholar]
- Andersen-Ranberg J, Kongstad KT, Nielsen MT, Jensen NB, Pateraki I, Bach SS, Hamberger B, Zerbe P, Staerk D, Bohlmann J, et al. (2016) Expanding the landscape of diterpene structural diversity through stereochemically controlled combinatorial biosynthesis. Angew Chem Int Ed Engl 55: 2142–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnaresi P, Biselli C, Orrù L, Urso S, Crispino L, Abbruscato P, Piffanelli P, Lupotto E, Cattivelli L, Valè G (2012) Comparative transcriptome profiling of the early response to Magnaporthe oryzae in durable resistant vs susceptible rice (Oryza sativa L.) genotypes. PLoS ONE 7: e51609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barney JN, Mann JJ, Kyser GB, Blumwald E, Van Deynze A, DiTomaso JM (2009) Tolerance of switchgrass to extreme soil moisture stress: ecological implications. Plant Sci 177: 724–732 [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP (1995) Cloning and characterization of the maize An1 gene. Plant Cell 7: 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal AK, Atmodjo MA, Li M, Baxter HL, Yoo CG, Pu Y, Lee YC, Mazarei M, Black IM, Zhang JY, et al. (2018) Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat Biotechnol 36: 249–257 [DOI] [PubMed] [Google Scholar]
- Caniard A, Zerbe P, Legrand S, Cohade A, Valot N, Magnard JL, Bohlmann J, Legendre L (2012) Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casler MD, Tobias CM, Kaeppler SM, Buell CR, Wang Z, Cao P, Schmutz J, Ronald P (2011) The switchgrass genome: tools and strategies. Plant Genome J 4: 273–282 [Google Scholar]
- Chakraborty S, Newton AC (2011) Climate change, plant diseases and food security: an overview. Plant Pathol 60: 2–14 [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J 66: 212–229 [DOI] [PubMed] [Google Scholar]
- Cho EM, Okada A, Kenmoku H, Otomo K, Toyomasu T, Mitsuhashi W, Sassa T, Yajima A, Yabuta G, Mori K, et al. (2004) Molecular cloning and characterization of a cDNA encoding ent-cassa-12,15-diene synthase, a putative diterpenoid phytoalexin biosynthetic enzyme, from suspension-cultured rice cells treated with a chitin elicitor. Plant J 37: 1–8 [DOI] [PubMed] [Google Scholar]
- Christensen SA, Sims J, Vaughan MM, Hunter C, Block A, Willett D, Alborn HT, Huffaker A, Schmelz EA (2018) Commercial hybrids and mutant genotypes reveal complex protective roles for inducible terpenoid defenses in maize. J Exp Bot 69: 1693–1705 [DOI] [PubMed] [Google Scholar]
- Criswell J, Potter K, Shephard F, Beale MH, Peters RJ (2012) A single residue change leads to a hydroxylated product from the class II diterpene cyclization catalyzed by abietadiene synthase. Org Lett 14: 5828–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Duan L, Jin B, Qian J, Xue Z, Shen G, Snyder JH, Song J, Chen S, Huang L, et al. (2015) Functional divergence of diterpene syntheses in the medicinal plant Salvia miltiorrhiza. Plant Physiol 169: 1607–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafoe NJ, Huffaker A, Vaughan MM, Duehl AJ, Teal PE, Schmelz EA (2011) Rapidly induced chemical defenses in maize stems and their effects on short-term growth of Ostrinia nubilalis. J Chem Ecol 37: 984–991 [DOI] [PubMed] [Google Scholar]
- Das A, Saha D, Mondal KT (2013) An optimized method for extraction of RNA from tea roots for functional genomics analysis. Int J Biotechnol 12: 129–132 [Google Scholar]
- Degenhardt J. (2009) Indirect defense responses to herbivory in grasses. Plant Physiol 149: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sassi C, Tylianakis JM (2012) Climate change disproportionately increases herbivore over plant or parasitoid biomass. PLoS ONE 7: e40557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOE-JGI (2018a) Panicum virgatum v4.1 (Switchgrass) AP13 var. Alamo. http://phytozome.jgi.doe.gov/
- DOE-JGI (2018b) Panicum hallii v3.1 (Hall’s panicgrass) FIL2 var. filipes. http://phytozome.jgi.doe.gov/
- Freiburghaus F, Steck A, Pfander H, Brun R (1998) Bioassay-guided isolation of a diastereoisomer of kolavenol from Entada abyssinica active on Trypanosoma brucei rhodesiense. J Ethnopharmacol 61: 179–183 [DOI] [PubMed] [Google Scholar]
- Fu J, Ren F, Lu X, Mao H, Xu M, Degenhardt J, Peters RJ, Wang Q (2016) A tandem array of ent-kaurene synthases in maize with roles in gibberellin and more specialized metabolism. Plant Physiol 170: 742–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno J, Eattock N, Van Deynze A, Blumwald E (2014) Selection and validation of reference genes for gene expression analysis in switchgrass (Panicum virgatum) using quantitative real-time RT-PCR. PLoS ONE 9: e91474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321 [DOI] [PubMed] [Google Scholar]
- Hamano Y, Kuzuyama T, Itoh N, Furihata K, Seto H, Dairi T (2002) Functional analysis of eubacterial diterpene cyclases responsible for biosynthesis of a diterpene antibiotic, terpentecin. J Biol Chem 277: 37098–37104 [DOI] [PubMed] [Google Scholar]
- Hamberger B, Bak S (2013) Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Philos Trans R Soc Lond B Biol Sci 368: 20120426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen NL, Heskes AM, Hamberger B, Olsen CE, Hallström BM, Andersen-Ranberg J, Hamberger B (2017a) The terpene synthase gene family in Tripterygium wilfordii harbors a labdane-type diterpene synthase among the monoterpene synthase TPS-b subfamily. Plant J 89: 429–441 [DOI] [PubMed] [Google Scholar]
- Hansen NL, Nissen JN, Hamberger B (2017b) Two residues determine the product profile of the class II diterpene synthases TPS14 and TPS21 of Tripterygium wilfordii. Phytochemistry 138: 52–56 [DOI] [PubMed] [Google Scholar]
- Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, Allard S, Kathiresan A, Ouellet T, Peters RJ (2005) The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol 59: 881–894 [DOI] [PubMed] [Google Scholar]
- Hillwig ML, Xu M, Toyomasu T, Tiernan MS, Wei G, Cui G, Huang L, Peters RJ (2011) Domain loss has independently occurred multiple times in plant terpene synthase evolution. Plant J 68: 1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofberger JA, Lyons E, Edger PP, Pires JC, Schranz ME (2013) Whole genome and tandem duplicate retention facilitated glucosinolate pathway diversification in the mustard family. Genome Biol Evol 5: 2155–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofberger JA, Ramirez AM, van den Bergh E, Zhu X, Bouwmeester HJ, Schuurink RC, Schranz ME (2015) Large-scale evolutionary analysis of genes and supergene clusters from terpenoid modular pathways provides insights into metabolic diversification in flowering plants. PLoS ONE 10: e0128808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue Y, Sakai M, Yao Q, Tanimoto Y, Koga J, Toshima H, Hasegawa M (2015) Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J Agric Food Chem 63: 4050–4059 [DOI] [PubMed] [Google Scholar]
- Huang S, Su X, Haselkorn R, Gornicki P (2003) Evolution of switchgrass (Panicum virgatum L.) based on sequences of the nuclear gene encoding plastid acetyl-CoA carboxylase. Plant Sci 164: 43–49 [Google Scholar]
- Huang Y, Guan C, Liu Y, Chen B, Yuan S, Cui X, Zhang Y, Yang F (2017) Enhanced growth performance and salinity tolerance in transgenic switchgrass via overexpressing vacuolar Na+ (K+)/H+ antiporter gene (PvNHX1). Front Plant Sci 8: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Potter KC, Peters RJ (2016) Extreme promiscuity of a bacterial and a plant diterpene synthase enables combinatorial biosynthesis. Metab Eng 37: 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, O’Brien TE, Zhang Y, Siegel JB, Tantillo DJ, Peters RJ (2018) Changing face: a key residue for the addition of water by sclareol synthase. ACS Catal 8: 3133–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Paterson AH (2014) Polyploidy-associated genome modifications during land plant evolution. Philos Trans R Soc Lond B Biol Sci 369: 20130355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Noguchi H, Peters RJ (2013) The role of momilactones in rice allelopathy. J Chem Ecol 39: 175–185 [DOI] [PubMed] [Google Scholar]
- Keeling CI, Dullat HK, Yuen M, Ralph SG, Jancsik S, Bohlmann J (2010) Identification and functional characterization of monofunctional ent-copalyl diphosphate and ent-kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms. Plant Physiol 152: 1197–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100: 1515–1523 [DOI] [PubMed] [Google Scholar]
- Kitaoka N, Lu X, Yang B, Peters RJ (2015) The application of synthetic biology to elucidation of plant mono-, sesqui-, and diterpenoid metabolism. Mol Plant 8: 6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama O, Suzuki T, Miyakawa J, Akatsuka T (1988) Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric Biol Chem 52: 2469–2473 [Google Scholar]
- Köksal M, Potter K, Peters RJ, Christianson DW (2014) 1.55Å-resolution structure of ent-copalyl diphosphate synthase and exploration of general acid function by site-directed mutagenesis. Biochim Biophys Acta 1840: 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26: 283–291 [Google Scholar]
- Li R, Morris-Natschke SL, Lee KH (2016) Clerodane diterpenes: sources, structures, and biological activities. Nat Prod Rep 33: 1166–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Tran H, Shan L, Kim J, Childs K, Ervin EH, Frazier T, Zhao B (2015) Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol Biofuels 8: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zhang J, Brown B, Li R, Rodríguez-Romero J, Berasategui A, Liu B, Xu M, Luo D, Pan Z, et al. (2018) Inferring roles in defense from metabolic allocation of rice diterpenoids. Plant Cell 30: 1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafu S, Hillwig ML, Peters RJ (2011) A novel labda-7,13e-dien-15-ol-producing bifunctional diterpene synthase from Selaginella moellendorffii. ChemBioChem 12: 1984–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafu S, Fischer E, Addison JB, Riberio Barbosana I, Zerbe P (2016) Substitution of two active-site residues alters C9-hydroxylation in a class II diterpene synthase. ChemBioChem 17: 2304–2307 [DOI] [PubMed] [Google Scholar]
- Mafu S, Ding Y, Murphy KM, Yaacoobi O, Addison JB, Wang Q, Shen Z, Briggs SP, Bohlmann J, Castro-Falcón G, et al. (2018) Discovery, biosynthesis and stress-related accumulation of dolabradiene-derived defenses in maize. Plant Physiol 176: 2677–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Aspinwall MJ, Lowry DB, Palacio-Mejía JD, Logan TL, Fay PA, Juenger TE (2014) Integrating transcriptional, metabolomic, and physiological responses to drought stress and recovery in switchgrass (Panicum virgatum L.). BMC Genomics 15: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone D, Xu M, Fulton DB, Determan MK, Peters RJ (2008) Increasing complexity of a diterpene synthase reaction with a single residue switch. J Am Chem Soc 130: 5400–5401 [DOI] [PubMed] [Google Scholar]
- Morrone D, Lowry L, Determan MK, Hershey DM, Xu M, Peters RJ (2010) Increasing diterpene yield with a modular metabolic engineering system in E. coli: comparison of MEV and MEP isoprenoid precursor pathway engineering. Appl Microbiol Biotechnol 85: 1893–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone D, Hillwig ML, Mead ME, Lowry L, Fulton DB, Peters RJ (2011) Evident and latent plasticity across the rice diterpene synthase family with potential implications for the evolution of diterpenoid metabolism in the cereals. Biochem J 435: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2000) Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 210: 925–931 [DOI] [PubMed] [Google Scholar]
- Nakano C, Oshima M, Kurashima N, Hoshino T (2015) Identification of a new diterpene biosynthetic gene cluster that produces O-methylkolavelool in Herpetosiphon aurantiacus. ChemBioChem 16: 772–781 [DOI] [PubMed] [Google Scholar]
- Nelson RS, Stewart CN Jr, Gou J, Holladay S, Gallego-Giraldo L, Flanagan A, Mann DGJ, Hisano H, Wuddineh WA, Poovaiah CR, et al. (2017) Development and use of a switchgrass (Panicum virgatum L.) transformation pipeline by the BioEnergy Science Center to evaluate plants for reduced cell wall recalcitrance. Biotechnol Biofuels 10: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto T, Cho EM, Okada A, Okada K, Otomo K, Kanno Y, Toyomasu T, Mitsuhashi W, Sassa T, Minami E, et al. (2004) Stemar-13-ene synthase, a diterpene cyclase involved in the biosynthesis of the phytoalexin oryzalexin S in rice. FEBS Lett 571: 182–186 [DOI] [PubMed] [Google Scholar]
- Nogueira RT, Shepherd GJ, Laverde A Jr, Marsaioli AJ, Imamura PM (2001) Clerodane-type diterpenes from the seed pods of Hymenaea courbaril var. stilbocarpa. Phytochemistry 58: 1153–1157 [DOI] [PubMed] [Google Scholar]
- Ohsaki A, Yan LT, Ito S, Edatsugi H, Iwata D, Komoda Y (1994) The isolation and in vivo potent antitumor activity of clerodane diterpenoid from the oleoresin of the Brazilian medicinal plant, Copaiferu langsdorfii Desfon. Bioorg Med Chem Lett 4: 2889–2892 [Google Scholar]
- Otomo K, Kanno Y, Motegi A, Kenmoku H, Yamane H, Mitsuhashi W, Oikawa H, Toshima H, Itoh H, Matsuoka M, et al. (2004a) Diterpene cyclases responsible for the biosynthesis of phytoalexins, momilactones A, B, and oryzalexins A-F in rice. Biosci Biotechnol Biochem 68: 2001–2006 [DOI] [PubMed] [Google Scholar]
- Otomo K, Kenmoku H, Oikawa H, König WA, Toshima H, Mitsuhashi W, Yamane H, Sassa T, Toyomasu T (2004b) Biological functions of ent- and syn-copalyl diphosphate synthases in rice: key enzymes for the branch point of gibberellin and phytoalexin biosynthesis. Plant J 39: 886–893 [DOI] [PubMed] [Google Scholar]
- Pateraki I, Andersen-Ranberg J, Hamberger B, Heskes AM, Martens HJ, Zerbe P, Bach SS, Møller BL, Bohlmann J, Hamberger B (2014) Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii. Plant Physiol 164: 1222–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelot KA, Hagelthorn DM, Addison JB, Zerbe P (2017a) Biosynthesis of the oxygenated diterpene nezukol in the medicinal plant Isodon rubescens is catalyzed by a pair of diterpene synthases. PLoS ONE 12: e0176507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelot KA, Mitchell R, Kwon M, Hagelthorn DM, Wardman JF, Chiang A, Bohlmann J, Ro DK, Zerbe P (2017b) Biosynthesis of the psychotropic plant diterpene salvinorin A: discovery and characterization of the Salvia divinorum clerodienyl diphosphate synthase. Plant J 89: 885–897 [DOI] [PubMed] [Google Scholar]
- Peters RJ. (2006) Uncovering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry 67: 2307–2317 [DOI] [PubMed] [Google Scholar]
- Peters RJ. (2010) Two rings in them all: the labdane-related diterpenoids. Nat Prod Rep 27: 1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RJ, Croteau RB (2002) Abietadiene synthase catalysis: conserved residues involved in protonation-initiated cyclization of geranylgeranyl diphosphate to (+)-copalyl diphosphate. Biochemistry 41: 1836–1842 [DOI] [PubMed] [Google Scholar]
- Peters RJ, Ravn MM, Coates RM, Croteau RB (2001) Bifunctional abietadiene synthase: free diffusive transfer of the (+)-copalyl diphosphate intermediate between two distinct active sites. J Am Chem Soc 123: 8974–8978 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Lewinsohn E (2011) Convergent evolution in plant specialized metabolism. Annu Rev Plant Biol 62: 549–566 [DOI] [PubMed] [Google Scholar]
- Potter K, Criswell J, Zi J, Stubbs A, Peters RJ (2014) Novel product chemistry from mechanistic analysis of ent-copalyl diphosphate synthases from plant hormone biosynthesis. Angew Chem Int Ed Engl 53: 7198–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter KC, Jia M, Hong YJ, Tantillo D, Peters RJ (2016a) Product rearrangement from altering a single residue in the rice syn-copalyl diphosphate synthase. Org Lett 18: 1060–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter KC, Zi J, Hong YJ, Schulte S, Malchow B, Tantillo DJ, Peters RJ (2016b) Blocking deprotonation with retention of aromaticity in a plant ent-copalyl diphosphate synthase leads to product rearrangement. Angew Chem Int Ed Engl 55: 634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisic S, Xu M, Wilderman PR, Peters RJ (2004) Rice contains two disparate ent-copalyl diphosphate synthases with distinct metabolic functions. Plant Physiol 136: 4228–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Seidl-Adams I, Köllner TG, Schaff C, Tumlinson JH, Degenhardt J (2015) A small, differentially regulated family of farnesyl diphosphate synthases in maize (Zea mays) provides farnesyl diphosphate for the biosynthesis of herbivore-induced sesquiterpenes. Planta 241: 1351–1361 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, et al. (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134: 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallaud C, Giacalone C, Töpfer R, Goepfert S, Bakaher N, Rösti S, Tissier A (2012) Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J 72: 1–17 [DOI] [PubMed] [Google Scholar]
- Sarath G, Mitchell RB, Sattler SE, Funnell D, Pedersen JF, Graybosch RA, Vogel KP (2008) Opportunities and roadblocks in utilizing forages and small grains for liquid fuels. J Ind Microbiol Biotechnol 35: 343–354 [DOI] [PubMed] [Google Scholar]
- Schalk M, Pastore L, Mirata MA, Khim S, Schouwey M, Deguerry F, Pineda V, Rocci L, Daviet L (2012) Toward a biosynthetic route to sclareol and amber odorants. J Am Chem Soc 134: 18900–18903 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PE (2011) Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci USA 108: 5455–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Huffaker A, Sims JW, Christensen SA, Lu X, Okada K, Peters RJ (2014) Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J 79: 659–678 [DOI] [PubMed] [Google Scholar]
- Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci USA 105: 464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol 130: 2049–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Noma M, Kawashima N (1983) Two labdane diterpenoids from Nicotiana setchellii. Phytochemistry 22: 1294–1295 [Google Scholar]
- Swaminathan S, Morrone D, Wang Q, Fulton DB, Peters RJ (2009) CYP76M7 is an ent-cassadiene C11α-hydroxylase defining a second multifunctional diterpenoid biosynthetic gene cluster in rice. Plant Cell 21: 3315–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes VR, Allen FL, Mielenz JR, Stewart CN Jr, Windham MT, Hamilton CY, Rodriguez M Jr, Yee KL (2016) Reduction of ethanol yield from switchgrass infected with rust caused by Puccinia emaculata. BioEnergy Res 9: 239–247 [Google Scholar]
- Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49: 3315–3321 [DOI] [PubMed] [Google Scholar]
- Tokoroyama T. (2000) Synthesis of clerodane diterpenoids and related compounds: stereoselective construction of the decalin skeleton with multiple contiguous stereogenic centers. Synthesis 5: 611–633 [Google Scholar]
- Toyomasu T, Usui M, Sugawara C, Otomo K, Hirose Y, Miyao A, Hirochika H, Okada K, Shimizu T, Koga J, et al. (2014) Reverse-genetic approach to verify physiological roles of rice phytoalexins: characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiol Plant 150: 55–62 [DOI] [PubMed] [Google Scholar]
- Uppalapati SR, Serba DD, Ishiga Y, Szabo LJ, Mittal S, Bhandari HS, Bouton JH, Mysore KS, Saha MC (2013) Characterization of the rust fungus, Puccinia emaculata, and evaluation of genetic variability for rust resistance in switchgrass populations. BioEnergy Res 6: 458–468 [Google Scholar]
- Vaughan MM, Christensen S, Schmelz EA, Huffaker A, McAuslane HJ, Alborn HT, Romero M, Allen LH, Teal PE (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ 38: 2195–2207 [DOI] [PubMed] [Google Scholar]
- Wang Q, Hillwig ML, Peters RJ (2011) CYP99A3: functional identification of a diterpene oxidase from the momilactone biosynthetic gene cluster in rice. Plant J 65: 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hillwig ML, Okada K, Yamazaki K, Wu Y, Swaminathan S, Yamane H, Peters RJ (2012a) Characterization of CYP76M5-8 indicates metabolic plasticity within a plant biosynthetic gene cluster. J Biol Chem 287: 6159–6168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hillwig ML, Wu Y, Peters RJ (2012b) CYP701A8: a rice ent-kaurene oxidase paralog diverted to more specialized diterpenoid metabolism. Plant Physiol 158: 1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilderman PR, Xu M, Jin Y, Coates RM, Peters RJ (2004) Identification of syn-pimara-7,15-diene synthase reveals functional clustering of terpene synthases involved in rice phytoalexin/allelochemical biosynthesis. Plant Physiol 135: 2098–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Hillwig ML, Wang Q, Peters RJ (2011) Parsing a multifunctional biosynthetic gene cluster from rice: biochemical characterization of CYP71Z6 & 7. FEBS Lett 585: 3446–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou K, Toyomasu T, Sugawara C, Oku M, Abe S, Usui M, Mitsuhashi W, Chono M, Chandler PM, et al. (2012) Functional characterization of wheat copalyl diphosphate synthases sheds light on the early evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry 84: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Escamilla-Treviño LL, Sathitsuksanoh N, Shen Z, Shen H, Zhang YH, Dixon RA, Zhao B (2011) Silencing of 4-coumarate:coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol 192: 611–625 [DOI] [PubMed] [Google Scholar]
- Xu M, Hillwig ML, Prisic S, Coates RM, Peters RJ (2004) Functional identification of rice syn-copalyl diphosphate synthase and its role in initiating biosynthesis of diterpenoid phytoalexin/allelopathic natural products. Plant J 39: 309–318 [DOI] [PubMed] [Google Scholar]
- Xu M, Wilderman PR, Morrone D, Xu J, Roy A, Margis-Pinheiro M, Upadhyaya NM, Coates RM, Peters RJ (2007a) Functional characterization of the rice kaurene synthase-like gene family. Phytochemistry 68: 312–326 [DOI] [PubMed] [Google Scholar]
- Xu M, Wilderman PR, Peters RJ (2007b) Following evolution’s lead to a single residue switch for diterpene synthase product outcome. Proc Natl Acad Sci USA 104: 7397–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HA, Sarath G, Tobias CM (2012) Karyotype variation is indicative of subgenomic and ecotypic differentiation in switchgrass. BMC Plant Biol 12: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbe P, Bohlmann J (2015) Plant diterpene synthases: exploring modularity and metabolic diversity for bioengineering. Trends Biotechnol 33: 419–428 [DOI] [PubMed] [Google Scholar]
- Zerbe P, Chiang A, Bohlmann J (2012) Mutational analysis of white spruce (Picea glauca) ent-kaurene synthase (PgKS) reveals common and distinct mechanisms of conifer diterpene synthases of general and specialized metabolism. Phytochemistry 74: 30–39 [DOI] [PubMed] [Google Scholar]
- Zerbe P, Hamberger B, Yuen MM, Chiang A, Sandhu HK, Madilao LL, Nguyen A, Hamberger B, Bach SS, Bohlmann J (2013) Gene discovery of modular diterpene metabolism in nonmodel systems. Plant Physiol 162: 1073–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbe P, Chiang A, Dullat H, O’Neil-Johnson M, Starks C, Hamberger B, Bohlmann J (2014) Diterpene synthases of the biosynthetic system of medicinally active diterpenoids in Marrubium vulgare. Plant J 79: 914–927 [DOI] [PubMed] [Google Scholar]
- Zerbe P, Rodriguez SM, Mafu S, Chiang A, Sandhu HK, O’Neil-Johnson M, Starks CM, Bohlmann J (2015) Exploring diterpene metabolism in non-model species: transcriptome-enabled discovery and functional characterization of labda-7,13E-dienyl diphosphate synthase from Grindelia robusta. Plant J 83: 783–793 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zalapa JE, Jakubowski AR, Price DL, Acharya A, Wei Y, Brummer EC, Kaeppler SM, Casler MD (2011) Post-glacial evolution of Panicum virgatum: centers of diversity and gene pools revealed by SSR markers and cpDNA sequences. Genetica 139: 933–948 [DOI] [PubMed] [Google Scholar]
- Zhou K, Xu M, Tiernan M, Xie Q, Toyomasu T, Sugawara C, Oku M, Usui M, Mitsuhashi W, Chono M, et al. (2012) Functional characterization of wheat ent-kaurene(-like) synthases indicates continuing evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry 84: 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi J, Mafu S, Peters RJ (2014) To gibberellins and beyond! Surveying the evolution of (di)terpenoid metabolism. Annu Rev Plant Biol 65: 259–286 [DOI] [PMC free article] [PubMed] [Google Scholar]