Mitochondrial alternative oxidase respiration supports source leaf carbon and energy balance during growth at elevated CO2.
Abstract
Plants will experience an elevated atmospheric concentration of CO2 (ECO2) in the future. Growth of tobacco (Nicotiana tabacum) at ECO2 more than doubled the leaf protein amount of alternative oxidase (AOX), a non-energy-conserving component of mitochondrial respiration. To test the functional significance of this AOX increase, wild-type tobacco was compared with AOX knockdown and overexpression lines, following growth at ambient CO2 or ECO2. The ECO2-grown AOX knockdowns had a reduced capacity for triose phosphate use (TPU) during photosynthesis compared with the other plant lines. This TPU limitation of CO2 assimilation was associated with an increased accumulation of glucose-6-phosphate, sucrose, and starch in the leaves of the knockdowns. Under TPU-limiting conditions, the size of the proton gradient and proton motive force across the thylakoid membrane was enhanced in the knockdowns relative to the other plant lines, suggesting a restriction of chloroplast ATP synthase activity. This restriction was not due to a decline in ATP synthase (AtpB) protein amount. The knockdowns also displayed a photosystem stoichiometry adjustment at ECO2, which was absent in the other plant lines. Additional experiments showed that the way in which AOX supports photosynthesis at ECO2 is distinct from its previously described role in supporting photosynthesis during water deficit. The results are discussed in terms of how AOX contributes to TPU capacity and the maintenance of chloroplast ATP synthase activity at ECO2. Overall, the evidence suggests that AOX respiration is needed to maintain both the carbon and energy balance in photosynthetic tissues during growth at ECO2.
The plant respiratory electron transport chain (ETC) is branched, such that electrons in the ubiquinone pool can be passed to oxygen through the usual cytochrome (cyt) pathway (involving complex III, cyt c, and cyt oxidase) or via alternative oxidase (AOX; Plaxton and Podestá, 2006; Noctor et al., 2007; Millar et al., 2011; Del-Saz et al., 2018; Selinski et al., 2018). Unlike the cyt pathway, electron flow through AOX is not coupled to proton translocation across the inner mitochondrial membrane and, hence, does not support ATP synthesis. This distinct property of AOX respiration could be useful in contending with cellular energy imbalances that result in too high an ATP/ADP ratio, too high an NAD(P)H/NAD(P)+ ratio, or both (Vanlerberghe, 2013). For example, if increases in AOX activity are accompanied by decreases in cyt oxidase activity, then it is possible to simultaneously increase respiratory NAD(P)H turnover and decrease respiratory ATP yield.
In recent years, AOX knockdown/knockout plants have been used to evaluate the impact of AOX respiration on photosynthetic metabolism, which can be prone to energy imbalances, for example due to changes in irradiance or CO2 availability. In many of these studies, short-term increases in irradiance were used to rapidly challenge chloroplast energy balance. Yoshida et al. (2011a) showed that such an irradiance shift rapidly increased the reduction state of both the chloroplast plastoquinone and mitochondrial ubiquinone pools and that the change in both pools was exaggerated in an Arabidopsis (Arabidopsis thaliana) aox1a knockout compared with the wild type. That study and others have shown that a lack of AOX during such short-term irradiance shifts can perturb photosynthesis and perhaps increase the risk of photodamage (Zhang et al., 2010; Florez-Sarasa et al., 2011, 2016; Yoshida et al., 2011a, 2011b; Vishwakarma et al., 2014; Watanabe et al., 2016). On the other hand, current evidence from studies of AOX knockout/knockdown Arabidopsis and tobacco (Nicotiana tabacum) plants suggests that AOX is not essential in maintaining photosynthetic performance following a long-term increase in irradiance (Yoshida et al., 2011a, 2011b; Dahal et al., 2017).
AOX becomes a more prominent component of respiration when plants experience a water deficit, as seen in soybean (Glycine max; Ribas-Carbo et al., 2005) and Nicotiana sylvestris (Galle et al., 2010). Water deficit challenges chloroplast energy balance, since the closure of stomata, meant to reduce water loss, also restricts CO2 supply to the Calvin cycle. Under water deficit, the fraction of closed (reduced) PSII reaction centers was higher in tobacco AOX knockdowns and lower in AOX overexpressors when compared with the wild type, indicating that AOX respiration had a role in maintaining chloroplast energy balance (Dahal et al., 2014, 2015). Subsequently (i.e. as water deficit became more severe), the knockdowns displayed a greater loss of chloroplast ATP synthase protein compared with the wild type, whereas the overexpressors lost less. When severe enough, this loss reduced photosynthesis to rates below those caused by the reduced CO2 availability under water deficit (Dahal et al., 2014). A water deficit-induced biochemical limitation of photosynthesis, due to the loss of ATP synthase, has been seen in several plant species, suggesting that it is a common phenomenon (Tezara et al., 1999; Kohzuma et al., 2009; Hoshiyasu et al., 2013; Dahal et al., 2014). While the specific signal(s) that triggers the decline of ATP synthase remains elusive, it clearly relates to AOX respiration and its ability to maintain chloroplast energy balance (Dahal and Vanlerberghe, 2018). Furthermore, the importance of AOX is evident during both short-term (Dahal et al., 2014) and long-term (Dahal and Vanlerberghe, 2018) water deficit. During long-term water deficit, improved photosynthetic performance due to AOX respiration positively impacts overall growth (Dahal and Vanlerberghe, 2018).
Growth at elevated concentrations of atmospheric CO2 (ECO2) has a major impact on C3 photosynthesis (Harley et al., 1992; Socias et al., 1993; Sage, 1994; Drake et al., 1997; Long et al., 2004; Ainsworth and Rogers, 2007; Leakey et al., 2009a, 2012). For example, ECO2 increases the rate of CO2 fixation relative to that of photorespiration due to a promotion of the carboxylase activity and suppression of the oxygenase activity of Rubisco (Ehlers et al., 2015). This shift has a number of consequences for carbon and energy metabolism. First, the Calvin cycle demand for ATP relative to NADPH is lower under nonphotorespiratory than photorespiratory conditions (Kramer and Evans, 2011), meaning that growth at ECO2 reduces this demand, all else being equal. This reduced demand for ATP might, in turn, reduce reliance on cyclic electron transport (CET) as a means to supplement the chloroplast supply of ATP relative to NADPH (Foyer et al., 2012). Second, increased net CO2 assimilation (A) at ECO2 could increase the carbohydrate status of the plant, which could have numerous potential downstream effects on metabolism, growth, and development (Paul and Foyer, 2001; MacNeill et al., 2017). Third, a reduction in photorespiratory Gly oxidation in mitochondria could reduce the need for NADH turnover by the mitochondrial ETC (Oliver and Gardeström, 2004). Some studies suggest that this turnover is preferentially linked to AOX respiration (Igamberdiev et al., 1997). Fourth, there is evidence that growth at ECO2 increases leaf amounts of hydrogen peroxide (H2O2; Cheeseman, 2006) and leaf protein carbonylation (Qiu et al., 2008). These results may seem surprising, since ECO2 suppresses photorespiration, a dominant source of H2O2. On the other hand, disruptions in metabolism by ECO2, such as those described above, might cause energy imbalances that then promote H2O2 generation from other sources, such as the photosynthetic and respiratory ETCs (Møller, 2001; Asada, 2006). Given the impacts of ECO2 on carbon and energy metabolism described above, as well as others, it is possible that growth at ECO2 alters the interactions that occur between photosynthesis and respiration. These interactions act in part to optimize photosynthetic metabolism under different growth conditions (Hoefnagel et al., 1998; Raghavendra and Padmasree, 2003; Sweetlove et al., 2006; Tcherkez et al., 2012; Gandin et al., 2014; Gardestrӧm and Igamberdiev, 2016; Dahal and Vanlerberghe, 2018).
Surprisingly, only a few studies have examined the response of AOX to growth at ECO2. Gandin et al. (2009) found that growth at ECO2 increased AOX protein and capacity, as well as overall respiration rate, in the sink (bulb) tissue of Erythronium americanum. They suggested that this acted to prevent a buildup of carbohydrate in this sink-limited species under conditions where carbohydrate supply by photosynthesis was being stimulated by the ECO2. In another study, using the Crassulacean acid metabolism plant Opuntia ficus-indica, growth at ECO2 was associated with both a decrease in cyt oxidase activity and an increase in AOX activity, as measured in the dark using the noninvasive oxygen isotope discrimination technique (Gomez-Casanovas et al., 2007). In Arabidopsis, a high irradiance-induced rapid increase in AOX1A transcript amount could be enhanced further if accompanied by transfer to ECO2 (Yoshida and Noguchi, 2009). A short-term transfer of tobacco to ECO2 also increased AOX protein amount, relative to that of cyt oxidase subunit II (Wang et al., 2014), and AOX transcripts increased in tomato (Solanum lycopersicum) plants following transfer to ECO2 (Li et al., 2013). Another study used an Arabidopsis aox1a knockout to investigate how the absence of AOX impacted the response of photosynthesis to instantaneous changes in CO2 amount (i.e. measurement concentrations of CO2). In this case, A at saturating CO2 and irradiance was slightly lower in the knockout compared with the wild type (Gandin et al., 2012). Another interesting study compared Arabidopsis hybrids selected for high seed yield at 700 μmol mol−1 CO2 with those selected for high seed yield at 200 μmol mol−1 CO2. When the respiration of both sets of plants was compared under equivalent conditions (i.e. at a growth and measurement CO2 concentration of 360 μmol mol−1), those that had been selected for high seed yield at 700 μmol mol−1 showed higher AOX activity than those that had been selected for high seed yield at 200 μmol mol−1 CO2 (Gonzàlez-Meler et al., 2009).
The above studies that investigated AOX respiration variously at the transcript, protein, and activity levels collectively suggest that this respiratory pathway can respond dynamically to short-term and long-term changes in atmospheric CO2. However, we are unaware of any studies utilizing AOX knockdown/knockout or overexpression plants to investigate the significance of such respiratory changes during long-term growth at ECO2. This represents a major gap in our understanding of carbon and energy metabolism in a future ECO2 world (Gifford, 2003; Gonzalez-Meler et al., 2004; Atkin et al., 2010; Wang et al., 2014; Tcherkez et al., 2017). Hence, we compared wild-type tobacco with transgenic AOX knockdown and overexpression lines, following their growth and development from germination, in either ambient concentrations of atmospheric CO2 (ACO2) or ECO2. Since AOX is known to interact with photosynthesis (see above), this study focused on the metabolism of a mature source leaf. The results show that AOX amount has little influence on photosynthesis in well-watered plants grown at ACO2 but becomes essential in maintaining carbon and energy balance in plants grown at ECO2. The results also indicate that the role of tobacco AOX in supporting photosynthetic metabolism at ECO2 is clearly distinct from its previously described role in supporting photosynthesis during water deficit.
RESULTS
Tobacco (wild type, AOX knockdown [RI9 and RI29], and AOX overexpression [B7 and B8] lines) were germinated and grown at either ACO2 or ECO2. As expected, ECO2-grown plants had higher concentrations of CO2 in the leaf intercellular air space (Ci) and at the site of Rubisco (Cc) than ACO2-grown plants when measured at their respective growth CO2 concentrations (Supplemental Fig. S1). The ECO2-grown plants also displayed a lower stomatal conductance and mesophyll conductance than the ACO2-grown plants, again when measured at their respective growth CO2 concentrations. Similar trends were seen whether the plants were measured at their growth irradiance (photosynthetic photon flux density of 700 µmol m−2 s−1 [700 PPFD]) or a saturating irradiance (1,600 PPFD). However, regardless of measurement or growth conditions, none of the above-mentioned parameters differed across the five plant lines differing in AOX amount (Supplemental Fig. S1).
Under well-watered conditions, the relative water content (RWC) of leaf 5 was 88% in ACO2-grown and 91% in ECO2-grown wild-type plants (Supplemental Fig. S2A). When water was withheld from ACO2-grown wild-type plants for 5 d, RWC dropped to 73%, which was described previously as a moderate water deficit (Dahal and Vanlerberghe, 2017; Supplemental Fig. S2B). A similar RWC (75%) could be achieved in the ECO2-grown wild-type plants by withholding water for an additional day (i.e. 6 d). Hence, comparing ACO2-grown plants following 5 d of withholding water with ECO2-grown plants following 6 d of withholding water provided a comparison of the two sets of plants under equivalent moderate water deficit conditions. Furthermore, the AOX knockdowns and overexpressors displayed a similar RWC to the wild type, regardless of growth condition (Supplemental Fig. S2).
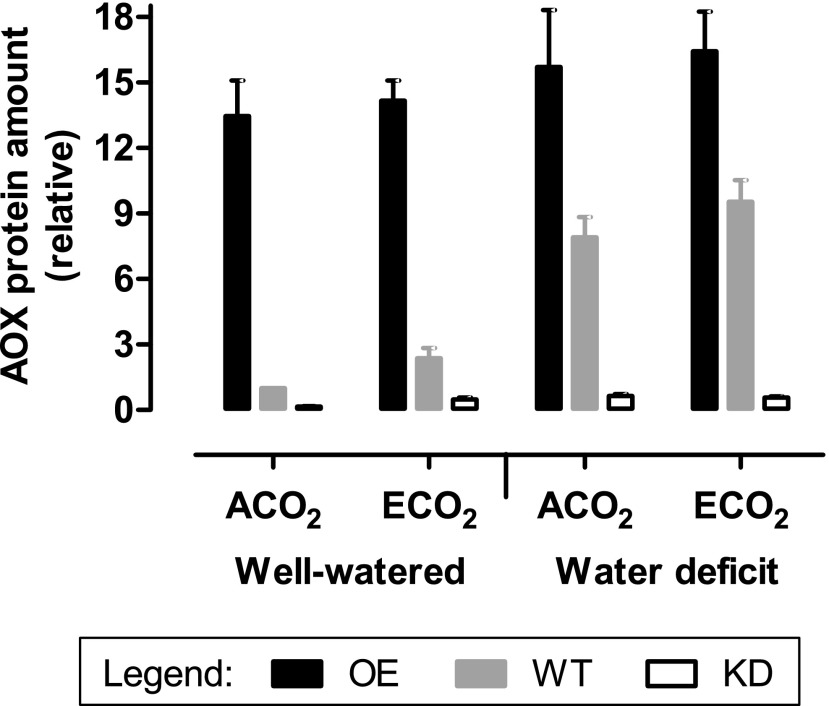
AOX protein amount was 2.4-fold higher in leaf 5 of well-watered wild-type plants grown at ECO2 compared with ACO2 (Fig. 1). In response to moderate water deficit, AOX amount in wild-type plants increased severalfold, regardless of growth CO2 concentration. As expected, AOX knockdown lines (RI9 and RI29) had much lower AOX amounts and AOX overexpression lines (B7 and B8) had much higher AOX amounts, compared with the wild type, regardless of growth condition (Fig. 1). Representative western blots are shown in Supplemental Figure S3. As described below, the different plant lines were compared with one another under each of the four growth conditions (ACO2, well watered; ECO2, well watered; ACO2, water deficit; and ECO2, water deficit).
Figure 1.
Amounts of AOX protein in leaf 5 of wild-type and transgenic tobacco. Plants were grown at ACO2 or ECO2 and either well watered or experiencing a moderate water deficit. Data are presented for the wild type (WT; gray bars), combined AOX overexpressors (OE) B7 and B8 (black bars), and combined AOX knockdowns (KD) RI9 and RI29 (white bars). The AOX protein amounts are all relative to the amount in well-watered ACO2-grown wild-type plants, which was set to 1. Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3).
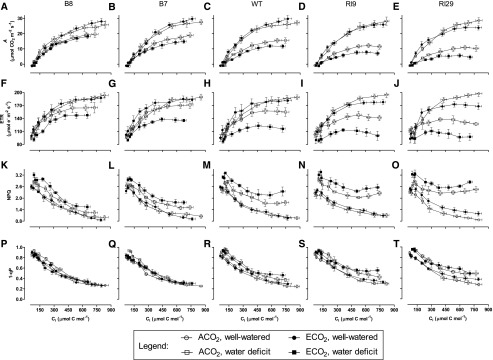
Simultaneous gas exchange and chlorophyll a fluorescence were used to characterize photosynthesis. Figure 2, A to E, shows CO2 response curves for A (i.e. A/Ci curves) for each of the five plant lines under each of the four growth conditions. In well-watered plants, the wild type and AOX overexpressors displayed slightly higher maximal A rates (the rates at high Ci) when grown at ECO2 compared with ACO2 (Fig. 2, A–C). Furthermore, A in these lines was not truly maximal but rather continued to increase incrementally with increasing Ci, regardless of growth CO2. However, in the AOX knockdowns, A at high Ci was lower in the ECO2-grown plants than in the ACO2-grown plants (Fig. 2, D and E). Furthermore, A in the ECO2-grown knockdowns appeared to level off to a true maximum at high Ci. Similar results were evident when A was plotted as a function of Cc rather than Ci (Supplemental Fig. S4).
Figure 2.
Photosynthetic parameters in leaf 5 of tobacco. CO2 response curves are shown for A (A–E), ETR (F–J), NPQ (K–O), and 1 − qP (P–T) in B8 (A, F, K, and P), B7 (B, G, L, and Q), the wild type (C, H, M, and R), RI9 (D, I, N, and S), and RI29 (E, J, O, and T). Data are shown for well-watered ACO2-grown plants (white circles), well-watered ECO2-grown plants (black circles), ACO2-grown plants experiencing a moderate water deficit (white squares), and ECO2-grown plants experiencing a moderate water deficit (black squares). Measurements were done at saturating irradiance (1,600 PPFD). Each experiment included single individuals for each plant line and growth condition. Data are means ± se of five independent experiments (n = 5).
In well-watered wild-type and overexpression lines, the electron transport rate (ETR) through PSII at high Ci was similar for plants grown at ACO2 or ECO2 (Fig. 2, F–H). However, in the knockdowns, ETR at high Ci was noticeably lower in the plants raised at ECO2 (Fig. 2, I and J). In all plant lines, nonphotochemical energy quenching (NPQ) declined with increasing Ci (Fig. 2, K–O). In well-watered wild-type plants, the minimum NPQ (i.e. NPQ at high Ci) was similar, regardless of growth CO2, while in the overexpressors, ECO2-grown plants exhibited a slightly lower minimum NPQ than ACO2-grown plants (Fig. 2, K–M). The opposite was seen in knockdowns, where it was the ACO2-grown rather than the ECO2-grown plants that exhibited the lowest minimum NPQ (Fig. 2, N and O). This was particularly so in RI29, which displays the strongest reduction of AOX protein amount of these two knockdowns (Wang et al., 2011). As with NPQ, PSII excitation pressure (1 − qP; the fraction of closed [reduced] PSII reaction centers) declined with increasing Ci (Fig. 2, P–T). In the well-watered overexpression plants, the minimum 1 − qP was similar between plants grown at ACO2 or ECO2, while wild-type plants had a slightly lower minimum 1 − qP when grown at ACO2 than ECO2 (Fig. 2, P–R). This trend was exaggerated further in the knockdowns, particularly RI29 (Fig. 2, S and T).
In all plant lines, A at high Ci was suppressed by the water deficit (Fig. 2, A–E). In the wild type and knockdowns, A at high Ci was lower in the ECO2- than in the ACO2-grown plants (Fig. 2, C–E), while in the overexpressors (particularly B8), this difference was less evident (Fig. 2, A and B). In both ACO2- and ECO2-grown plants, A at high Ci depended upon AOX amount. Comparing lines, the AOX overexpressors had the highest A, and A in these lines continued to creep upward, even at the highest Ci. The knockdowns had the lowest A, and A in these lines came to a maximum at intermediate Ci and even began to decline at the highest Ci levels. Wild-type plants showed an intermediate response. The differences in A across lines under water deficit were mirrored by differences in ETR (Fig. 2, F–J). In the knockdowns, ETR also came to a maximum at intermediate Ci and then began to decline at higher Ci (Fig. 2, I and J).
In all plant lines, NPQ under water deficit was higher in ECO2- than in ACO2-grown plants and higher than in the well-watered condition (Fig. 2, K–O). Under water deficit, NPQ depended upon AOX amount in both ACO2- and ECO2-grown plants. At high Ci, the overexpressors displayed a lower NPQ than the wild type. This was particularly the case in ECO2-grown plants, where NPQ in the wild type began to increase again at the highest Ci. The knockdowns displayed the highest NPQ values across plant lines, in part because NPQ in these lines began to increase again at intermediate Ci in both ACO2- and ECO2-grown plants (Fig. 2, K–O). In the wild type, 1 − qP at high Ci was higher under water deficit than under the well-watered condition and slightly higher following growth at ECO2 than ACO2 (Fig. 2R). These effects were exaggerated further in the knockdowns (Fig. 2, S and T). Remarkably, the overexpressors always displayed a similar low 1 − qP at high Ci, regardless of growth condition (Fig. 2, P and Q).
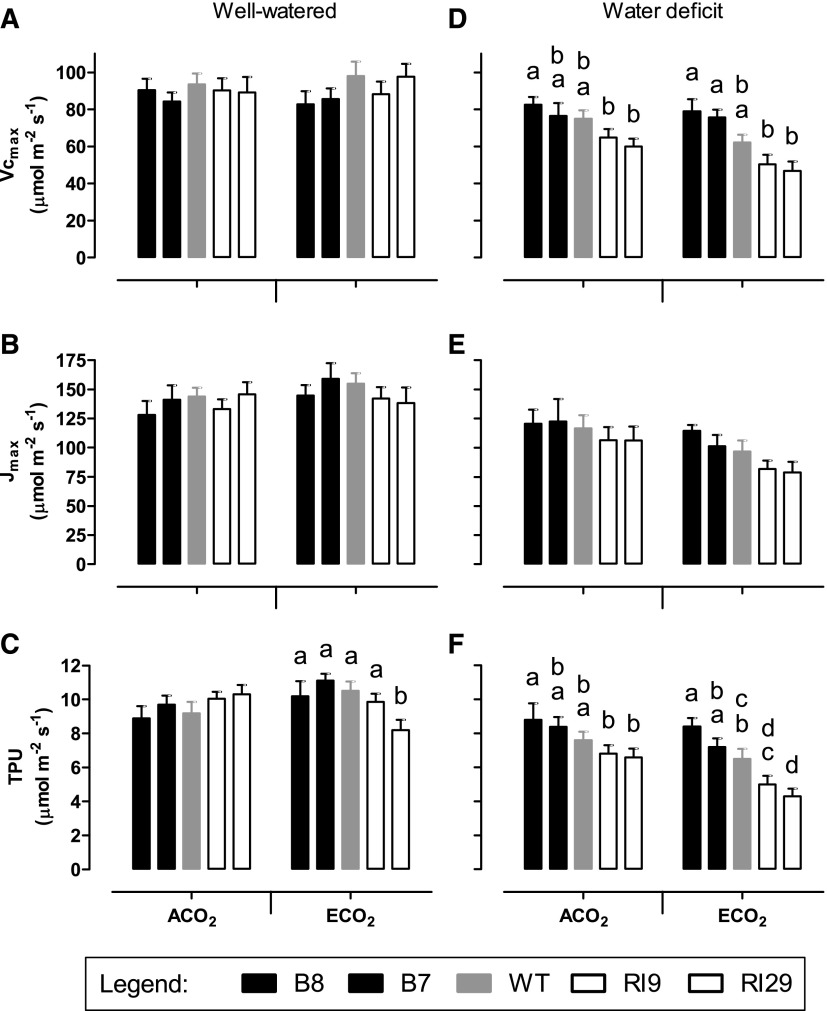
The CO2 response curves were used to calculate the maximum carboxylation capacity (Vcmax), the maximum capacity for ribulose 1,5-bisphosphate regeneration (Jmax), and the maximum capacity for triose phosphate use (TPU; Sharkey et al., 2007). There were no differences in Vcmax or Jmax across plant lines when well watered, regardless of growth CO2 (Fig. 3). In ACO2-grown plants, there also was no difference in TPU across lines. However, in ECO2-grown plants, the knockdowns (particularly RI29) displayed a lower TPU than the wild type and overexpressors. This was because, while the TPU of wild-type and overexpression plants increased slightly in response to growth at ECO2, the TPU of both knockdowns responded in the opposite manner (Fig. 3).
Figure 3.
Photosynthetic parameters in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered (A–C) or experiencing a moderate water deficit (D–F). A and D, Vcmax. B and E, Jmax. C and F, TPU. Data are presented for the wild type (WT; gray bars), AOX overexpressors (B8, left black bars; B7, right black bars), and AOX knockdowns (RI9, left white bars; RI29, right white bars). These data are calculated from the A/Ci curves shown in Figure 2. Each experiment included single individuals for each plant line and growth condition. Data are means ± se of five independent experiments (n = 5). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines.
In plants experiencing water deficit, both Vcmax and TPU were higher in the overexpressors and lower in the knockdowns compared with the wild type (Fig. 3). These differences were most pronounced in ECO2-grown plants but also were evident in ACO2-grown plants. Vcmax and TPU were 24% and 20% higher, respectively, in the ECO2-grown overexpressors (average of the two lines) than in the wild type. Conversely, Vcmax and TPU were 22% and 28% lower, respectively, in the ECO2-grown knockdowns (average of the two lines) than in the wild type. There were no significant differences in Jmax across plant lines, although the trend was similar to that of Vcmax and TPU, particularly in ECO2-grown plants (Fig. 3).
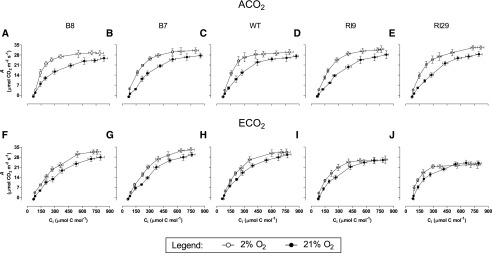
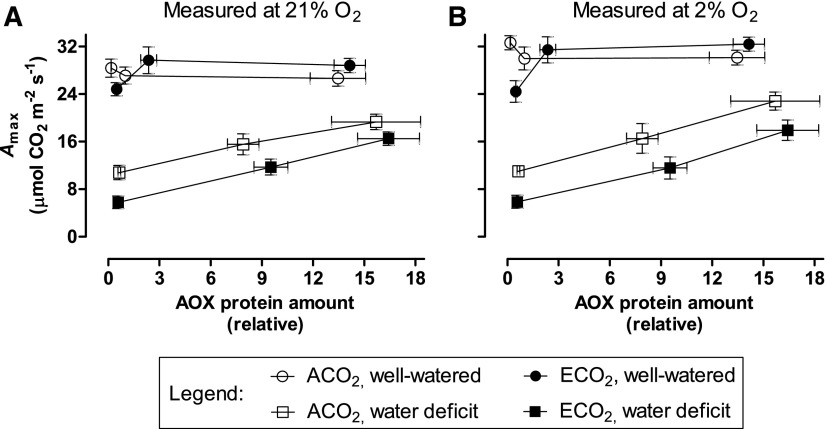
Two additional approaches were taken to examine whether photosynthesis in the well-watered ECO2-grown knockdowns was more prone to be TPU limited than in the other plant lines. First, A measured in 21% oxygen was compared with that measured in 2% oxygen. For ACO2-grown plants, A measured at 2% oxygen and high Ci was greater than that measured at 21% oxygen and high Ci, regardless of plant line (Fig. 4, A–E). The enhanced A was due to the suppression of photorespiration at 2% oxygen. However, in ECO2-grown plants, only the overexpressors and the wild type showed such stimulation at high Ci (Fig. 4, F–H). In the knockdowns, 2% oxygen did not increase A at high Ci (Fig. 4, I and J), consistent with A in these plants being TPU limited at high Ci and, hence, unable to increase A, despite the suppression of photorespiration (Sharkey, 1985; Yang et al., 2016).
Figure 4.
CO2 response curves for A in leaf 5 of well-watered tobacco. Plants were grown at ACO2 (A–E) or ECO2 (F–J). Data are presented for B8 (A and F), B7 (B and G), the wild type (WT; C and H), RI9 (D and I), and RI29 (E and J). A was measured in an atmosphere of either 21% oxygen (black circles) or 2% oxygen (white circles). Measurements were done at saturating irradiance (1,600 PPFD). Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3).
The A measured at 21% oxygen was again compared with that measured at 2% oxygen but for plants experiencing water deficit. At high Ci, the overexpressors continued to display higher A at 2% oxygen than 21% oxygen, particularly following growth at ACO2 but also ECO2 (Supplemental Fig. S5, A, B, F, and G). However, the other plant lines showed no stimulation of A at high Ci by 2% oxygen, regardless of growth at ACO2 or ECO2 (Supplemental Fig. S5).
Supplemental Figure S6 summarizes, from CO2 response curves, the A rates measured at the highest atmospheric concentration of CO2(Ca) tested (1,200 µmol mol−1) and when measured at either 21% or 2% oxygen. In well-watered ACO2-grown plants, there were no differences in A across plant lines, regardless of the measurement oxygen concentration (Supplemental Fig. S6, A and B). In well-watered ECO2-grown plants, A was lower in the knockdowns than in other plant lines, and the magnitude of this difference was greater at 2% than 21% oxygen (Supplemental Fig. S6, A and B). Under water deficit, the overexpressors and knockdowns maintained higher and lower A rates, respectively, than the wild type, regardless of the growth CO2 concentration or measurement oxygen concentration (Supplemental Fig. S6, C and D). Figure 5 plots these A data as a function of AOX protein amount in the plant lines.
Figure 5.
Effect of AOX protein amount on maximal net CO2 assimilation rate (Amax) of tobacco. Plants were grown at ACO2 or ECO2 and either well watered or experiencing a moderate water deficit. Measurements were done in an atmosphere of either 21% oxygen (A) or 2% oxygen (B). Data are shown for well-watered ACO2-grown plants (white circles), well-watered ECO2-grown plants (black circles), ACO2-grown plants experiencing a moderate water deficit (white squares), and ECO2-grown plants experiencing a moderate water deficit (black squares). Within each growth condition, the left-most data point is the average of RI9 and RI29 (AOX knockdown) plants, the middle data point is wild-type plants, and the right-most data point is the average of B7 and B8 (AOX overexpression) plants. This figure is based on the AOX protein amount data shown in Figure 1 and the CO2 assimilation values shown in Supplemental Figure S6. Data are means ± se.
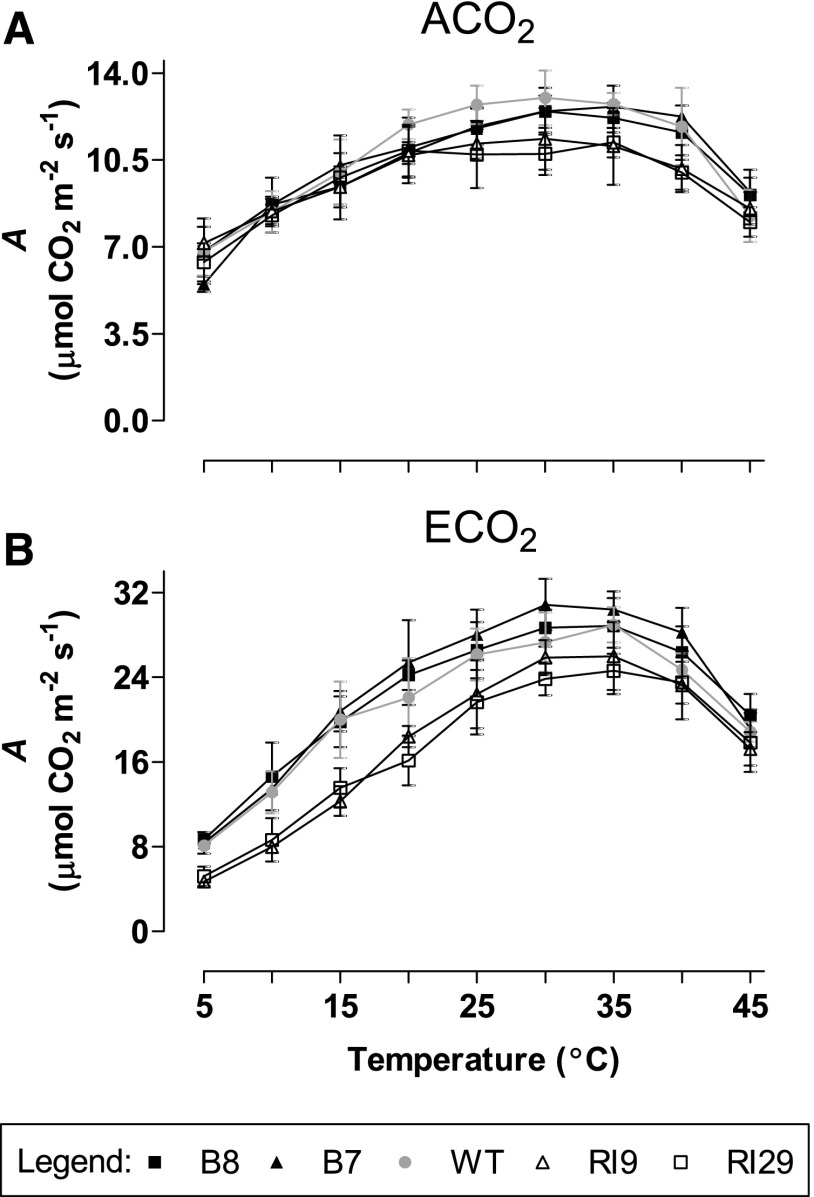
Another approach to examine whether photosynthesis in the well-watered ECO2-grown knockdowns was more prone to TPU limitation than the other plant lines was to measure A at growth irradiance and CO2 concentration but over a wide temperature range. TPU is known to be more sensitive to low temperature than either Vcmax or Jmax (Sharkey and Bernacchi, 2012). At higher measurement temperatures (25°C–45°C), the knockdowns maintained a slightly lower A than the wild type and overexpressors, regardless of the growth CO2 (Fig. 6). At lower temperatures (5°C–20°C), all lines displayed similar A when grown at ACO2 (Fig. 6A). However, in ECO2-grown plants, the knockdowns had much lower A at lower temperatures than the other lines. For example, at 15°C, A was 20 μmol CO2 m−2 s−1 in the wild type, 20.3 μmol CO2 m−2 s−1 in the overexpressors (average of the two lines), and 12.9 μmol CO2 m−2 s−1 in the knockdowns (average of the two lines; Fig. 6B). This result is consistent with a lower TPU capacity in the ECO2-grown knockdowns than in the other plant lines when measured at growth irradiance and CO2 concentration.
Figure 6.
Temperature response curves for A in leaf 5 of well-watered tobacco. Plants were grown at ACO2 (A) or ECO2 (B). Data are presented for the wild type (WT; gray circles), B7 (black triangles), B8 (black squares), RI9 (white triangles), and RI29 (white squares). Measurements were done at growth irradiance (700 PPFD), growth Ca (i.e. 400 or 1,000 µmol mol−1), and the temperature indicated. Each experiment included single individuals for each plant line and growth condition. Data are means ± se of two independent experiments (n = 2).
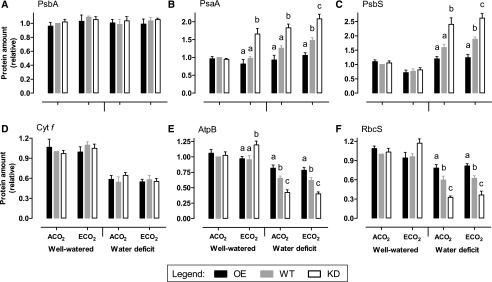
In well-watered plants, PsbA (D1 reaction center protein of PSII) protein amount was similar between ACO2- and ECO2-grown plants and did not differ across plant lines (Fig. 7A). PsaA (a reaction center protein of PSI) amount also was similar between well-watered ACO2- and ECO2-grown plants and across plant lines, but with one notable exception. PsaA amount in the ECO2-grown knockdowns was 1.7-fold that of the wild type and 2-fold that of the overexpressors grown under the same condition (Fig. 7B). PsbS (a PSII-associated sensor of lumen pH necessary for NPQ induction) amount was slightly lower in well-watered plants grown at ECO2 compared with ACO2 but did not differ in either condition across plant lines (Fig. 7C). Cyt f (the c-type cyt subunit of the cyt b6f complex) amount was similar between well-watered ACO2- and ECO2-grown plants and did not differ across plant lines (Fig. 7D). Interestingly, the amounts of AtpB (the β-subunit of the F1 catalytic subcomplex of ATP synthase) and RbcS (small subunit of Rubisco) displayed similar patterns in well-watered plants. While both proteins declined slightly in the wild type and overexpressors at ECO2 compared with ACO2, both increased slightly in the knockdowns. As a result, AtpB and RbcS amounts were 1.3- and 1.2-fold, respectively, in the ECO2-grown knockdowns compared with the wild type (Fig. 7, E and F). Under water deficit, there were again no differences in PsbA or cyt f protein amount across the plant lines, regardless of growth CO2 (Fig. 7, A and D). However, large differences were seen for the other proteins. Regardless of growth CO2, PsaA and PsbS were higher in the knockdowns and lower in the overexpressors compared with the wild type (Fig. 7, B and C). On the other hand, AtpB and RbcS were lower in the knockdowns and higher in the overexpressors compared with the wild type (Fig. 7, E and F). For all of the above-described proteins, representative western blots are shown in Supplemental Figure S3.
Figure 7.
Photosynthetic protein amounts in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered or experiencing a moderate water deficit. Shown are the protein amounts of PsbA (A), PsaA (B), PsbS (C), cyt f (D), AtpB (E), and RbcS (F). Data are presented for the wild type (WT; gray bars), combined AOX overexpressors (OE) B7 and B8 (black bars), and combined AOX knockdowns (KD) RI9 and RI29 (white bars). The protein amounts are all relative to the amount in well-watered ACO2-grown wild-type plants, which was set to 1. Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines.
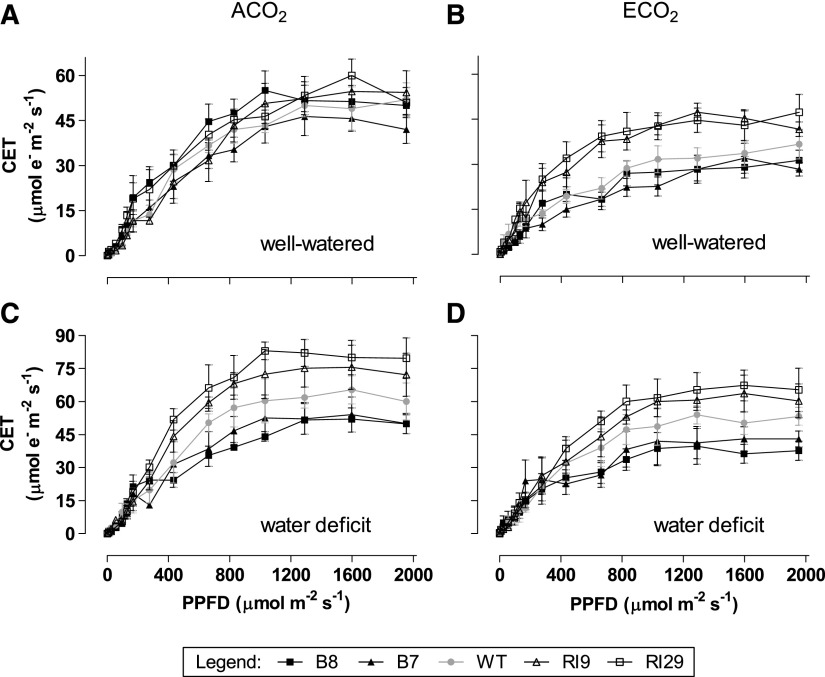
In well-watered plants grown at ACO2, the rate of CET was similar across plant lines (Fig. 8A). However, at ECO2, the knockdowns maintained higher CET rates than the wild type across a wide range of irradiance (Fig. 8B). Under water deficit, the knockdowns maintained higher rates of CET and the overexpressors maintained lower rates of CET than the corresponding wild-type plants, regardless of growth CO2 (Fig. 8, C and D).
Figure 8.
Rates of CET in leaf 5 of tobacco. Plants were grown at ACO2 (A and C) or ECO2 (B and D) and either well watered (A and B) or experiencing a moderate water deficit (C and D). Data are presented for the wild type (WT; gray circles), B7 (black triangles), B8 (black squares), RI9 (white triangles), and RI29 (white squares). Measurements were done at growth Ca (i.e. 400 or 1,000 µmol mol−1). Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3).
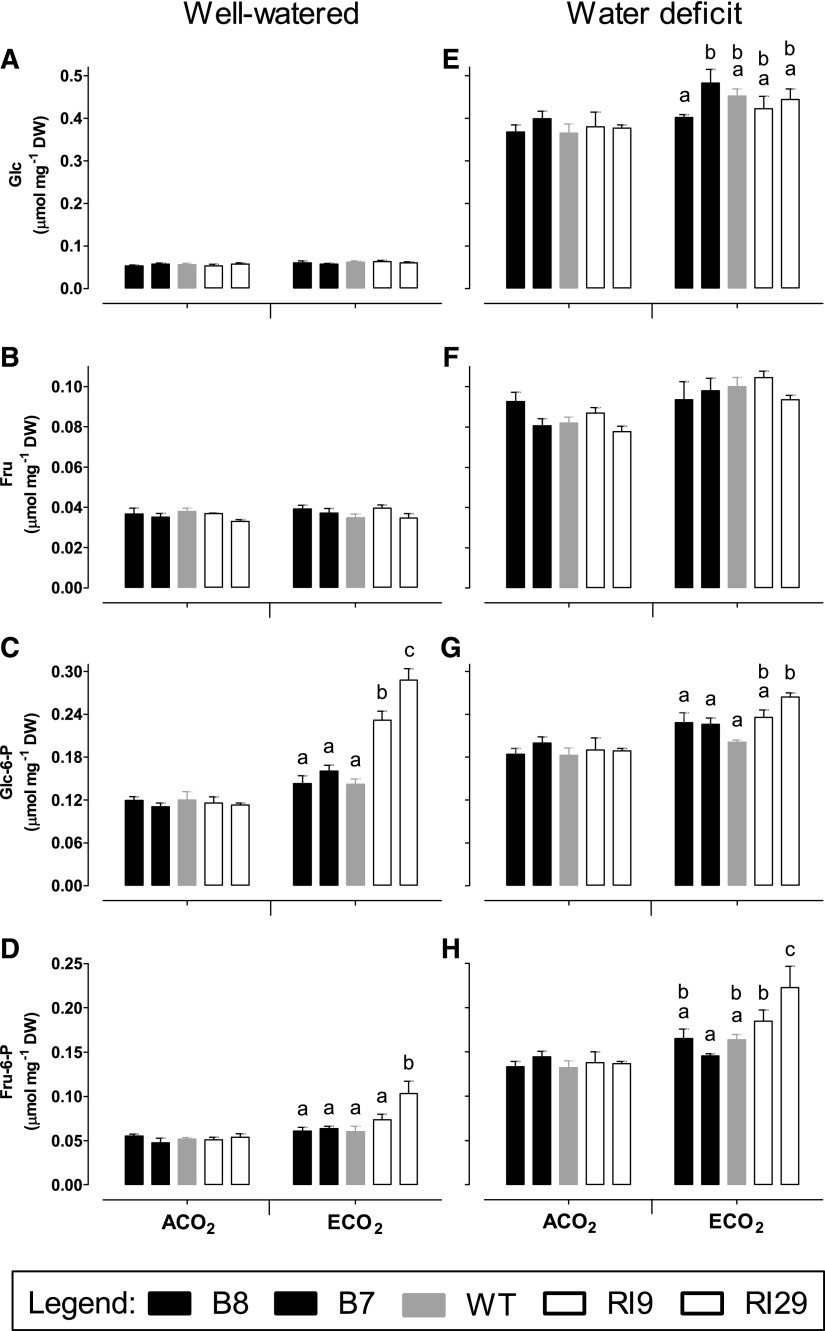
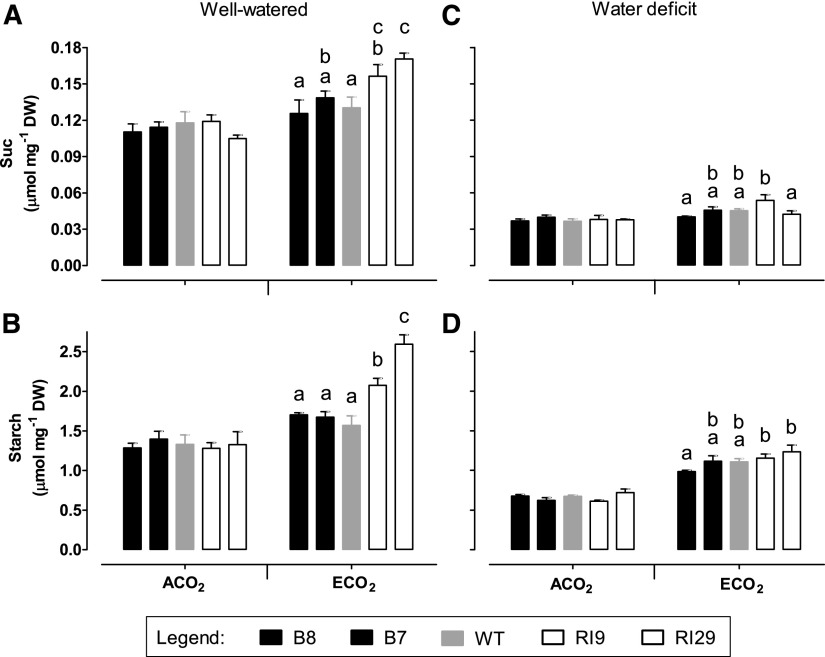
In well-watered plants, hexose (Glc and Fru) amounts were similar between ACO2- and ECO2-grown plants and across plant lines (Fig. 9, A and B). Hexose phosphates (Glc-6-P and Fru-6-P) also were similar across lines, but only for plants grown at ACO2 (Fig. 9, C and D). At ECO2, the knockdowns displayed higher Glc-6-P and Fru-6-P amounts than wild-type or overexpression plants. Glc-6-P was 1.6- and 2-fold in RI9 and RI29, respectively, compared with the wild type (Fig. 9C). Fru-6-P was 1.2- and 1.7-fold in RI9 and RI29, respectively, compared with the wild type (Fig. 9D). In the wild type, Suc and starch amounts were similar between well-watered plants grown at ACO2 and ECO2 (Fig. 10, A and B). Suc and starch also were similar across lines, but only at ACO2. At ECO2, Suc was 1.2- and 1.3-fold in RI9 and RI29, respectively, compared with the wild type (Fig. 10A) At ECO2, starch was 1.3- and 1.7-fold in RI9 and RI29, respectively, compared with wild type (Fig. 10B).
Figure 9.
Carbohydrate amounts in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered (A–D) or experiencing a moderate water deficit (E–H). Shown are the amounts of Glc (A and E), Fru (B and F), Glc-6-P (C and G), and Fru-6-P (D and H). Data are presented for the wild type (WT; gray bars), AOX overexpressors (B8, left black bars; B7, right black bars), and AOX knockdowns (RI9, left white bars; RI29, right white bars). Sampling was done at the midpoint of the light period. Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines. DW, Dry weight.
Figure 10.
Carbohydrate amounts in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered (A and B) or experiencing a moderate water deficit (C and D). Shown are the amount of Suc (A and C) and starch (B and D). Data are presented for the wild type (WT; gray bars), AOX overexpressors (B8, left black bars; B7, right black bars), and AOX knockdowns (RI9, left white bars; RI29, right white bars). Sampling was done at the midpoint of the light period. Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines. DW, Dry weight.
In ACO2-grown plants experiencing water deficit, none of the hexoses, hexose phosphates, Suc, or starch differed across plant lines (Figs. 9, E–H, and 10, C and D). Under ECO2, there was a slightly higher amount of hexose phosphates in one knockdown (RI29) than in the other plant lines. However, the large differences that had been seen in hexose phosphates, Suc, and starch between the knockdowns and other plant lines when grown at ECO2 and well watered were largely absent under water deficit (Figs. 9 and 10).
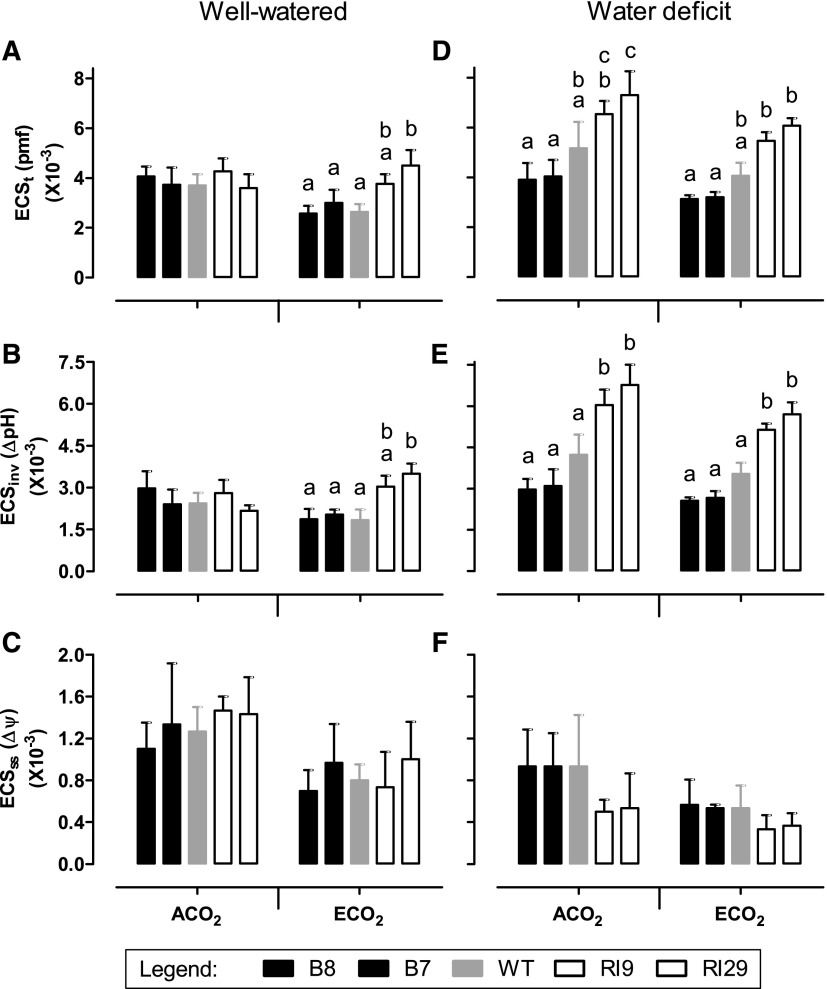
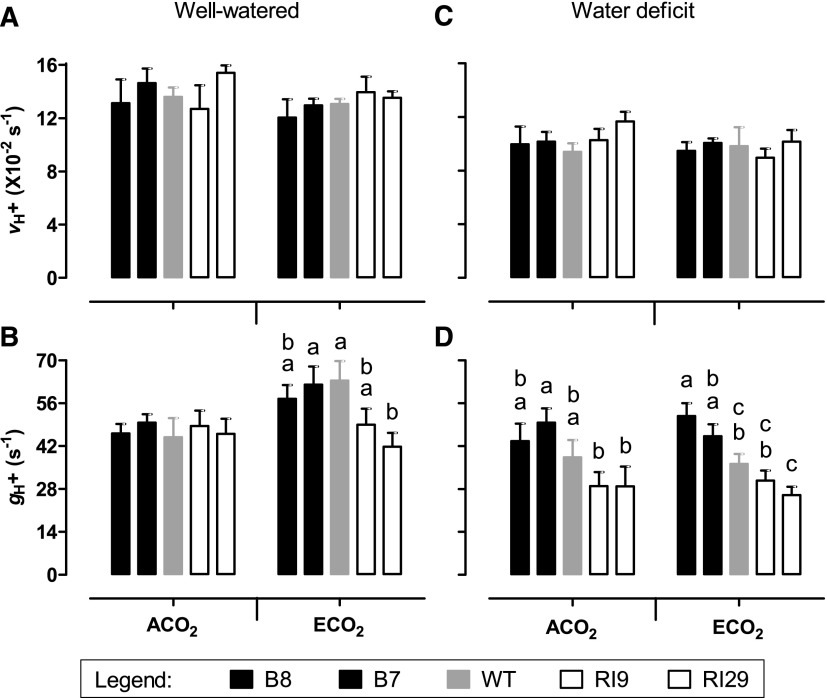
In well-watered ECO2-grown plants, hexose phosphates, particularly Glc-6-P, were higher in knockdowns than in the wild type (see above). We hypothesized that this might limit inorganic phosphate (Pi) availability in the knockdowns, thereby restricting chloroplast ATP synthase activity. To examine this possibility, a dark interval relaxation kinetics (DIRK) analysis (Cruz et al., 2005; Baker et al., 2007) was performed at growth irradiance and CO2 concentration. In well-watered ACO2-grown plants, the proton motive force (pmf) across the thylakoid membrane (measured as ECSt), the chemical potential (∆pH) component of pmf (measured as ECSinv), and the electrical potential (∆ψ) component of pmf (measured as ECSss) did not differ across plant lines (Fig. 11, A–C). However, in ECO2-grown plants, ∆pH was higher in the knockdowns than in the wild type (1.7- and 1.9-fold in RI9 and RI29, respectively). This resulted in a higher pmf in the knockdowns than in the wild type, since there was no difference in ∆ψ across plant lines (Fig. 1, A–C). The DIRK analysis also estimated the rate of proton flux from stroma to lumen (vH+) and the conductance of the chloroplast ATP synthase to proton movement from lumen to stroma (gH+). In well-watered ACO2-grown plants, there were no differences in vH+ or gH+ across plant lines (Fig. 12, A and B). In ECO2-grown plants, vH+ was similar across plant lines, but gH+ was 23% lower in RI9 and 34% lower in RI29 compared with the wild type (Fig. 12, A and B). This reduced proton conductivity was evident despite the knockdowns having higher amounts of ATP synthase protein (measured as AtpB) than the wild type (Fig. 7E).
Figure 11.
Thylakoid membrane pmf and the partitioning of pmf into its ∆ψ and ∆pH components in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered (A–C) or experiencing a moderate water deficit (D–F). A and D, ECSt, a measure of pmf. B and E, ECSinv, a measure of ∆pH. C and F, ECSss, a measure of ∆ψ. Data are presented for the wild type (WT; gray bars), AOX overexpressors (B8, left black bars; B7, right black bars), and AOX knockdowns (RI9, left white bars; RI29, right white bars). Measurements were done at growth irradiance (700 PPFD) and growth Ca (i.e. 400 or 1,000 µmol mol−1). Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines.
Figure 12.
Thylakoid membrane proton flux parameters in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered (A and B) or experiencing a moderate water deficit (C and D). A and C, vH+. B and D, gH+. Data are presented for the wild type (WT; gray bars), AOX overexpressors (B8, left black bars; B7, right black bars), and AOX knockdowns (RI9, left white bars; RI29, right white bars). Measurements were done at growth irradiance (700 PPFD) and growth Ca (i.e. 400 or 1,000 µmol mol−1). Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines.
The DIRK analyses were repeated with plants experiencing water deficit. When measured at growth irradiance and CO2 concentration, ECO2-grown knockdowns maintained a larger ∆pH than the wild type, similar to the results seen with well-watered plants (Fig. 11E). However, under water deficit, also the ACO2-grown knockdowns maintained a larger ∆pH than the wild type, as described before (Dahal and Vanlerberghe, 2018). Furthermore, the overexpressors now tended to maintain a smaller ∆pH than the wild type under both growth CO2 conditions, although this effect was not significant (Fig. 11E). The differences in ∆pH across plant lines translated into similar differences in pmf (Fig. 11D), while ∆ψ did not differ across lines in either ACO2- or ECO2-grown plants (Fig. 11F). In ECO2-grown plants experiencing water deficit, the knockdowns had a lower gH+ and the overexpressors had a higher gH+ than the wild type (Fig. 12D). A similar trend was evident in the ACO2-grown plants, although the results were more variable. There were no differences in vH+ across plant lines, regardless of growth CO2 (Fig. 12C).
The DIRK analyses were repeated at saturating irradiance (1,600 PPFD) and at both the growth CO2 concentrations (i.e. 400 and 1,000 μmol mol−1) and a saturating CO2 concentration (1,200 μmol mol−1). For both well-watered plants (Supplemental Figs. S7 and S8) and plants experiencing water deficit (Supplemental Figs. S9 and S10), these analyses yielded similar results to all those described above, except that the previously observed differences in ∆pH, pmf, and gH+ across plant lines were, in many cases, exaggerated even further. Interestingly, under the most saturating measurement conditions (1,600 PPFD and 1,200 µmol mol−1 CO2), ECO2-grown plants under water deficit also displayed some difference in vH+ across lines (Supplemental Fig. S10C).
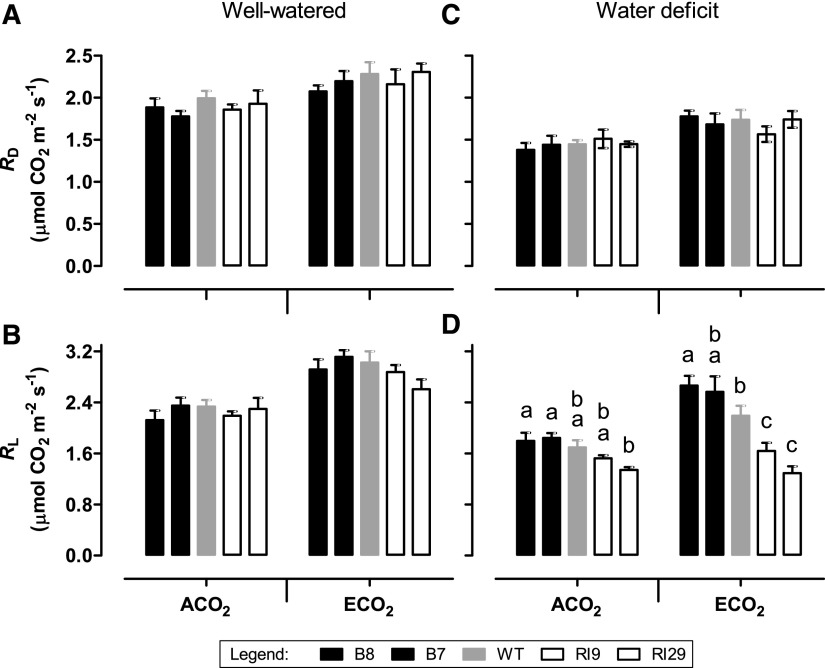
For well-watered plants, there were no differences in respiration rate in the dark (RD) or light (RL) across plant lines, regardless of the growth CO2 (Fig. 13, A and B). There was a slightly lower RL in the ECO2-grown knockdowns (particularly RI29) compared with the other plant lines, but this was not statistically significant (Fig. 13B). During water deficit, there were no differences in RD across plant lines, regardless of growth CO2 (Fig. 13C). However, RL tended to be higher in overexpressors and lower in knockdowns compared with the wild type. This was most evident in ECO2-grown plants but also was seen following growth at ACO2 (Fig. 13D). The RL/RD ratio also differed across plant lines during water deficit, while such a trend was not evident in well-watered plants (Supplemental Fig. S11).
Figure 13.
Respiration rate in leaf 5 of tobacco. Plants were grown at ACO2 or ECO2 and either well watered (A and B) or experiencing a moderate water deficit (C and D). A and C, RD. B and D, RL. Data are presented for the wild type (WT; gray bars), AOX overexpressors (B8, left black bars; B7, right black bars), and AOX knockdowns (RI9, left white bars; RI29, right white bars). Each experiment included single individuals for each plant line and growth condition. Data are means ± se of three independent experiments (n = 3). Within a growth condition, plant lines not sharing a common letter are significantly different from one another (P < 0.05). In data sets without letters, there are no significant differences across plant lines.
DISCUSSION
There is a paucity of data on how AOX respiration will respond in a future ECO2 world. Wild-type tobacco plants that were germinated and grown long term at ECO2 displayed more than double the AOX protein amount in source leaves of comparable plants grown at ACO2 (Fig. 1). A comparison of wild-type plants with multiple AOX knockdown and overexpression lines revealed that AOX substantially influences the carbon and energy metabolism of ECO2-grown plants.
During Growth at ECO2, AOX Respiration Contributes to TPU Capacity and Supports Chloroplast ATP Synthase Activity
The growth of plants at ECO2 can result in carbohydrate accumulation, which then can act as a signal to down-regulate the capacity of the photosynthetic apparatus (Paul and Foyer, 2001; Long et al., 2004). However, whether such carbohydrate accumulation and subsequent photosynthetic acclimation occurs is dependent upon numerous variables such as nutrient availability, sink strength, and even pot size (Sage, 1994; Geiger et al., 1999; Paul and Foyer, 2001; Ainsworth and Rogers, 2007; Ruiz-Vera et al., 2017). In this work, carbohydrate (hexoses, hexose phosphates, Suc, and starch) amounts were only marginally higher in well-watered wild-type tobacco plants grown at ECO2 compared with ACO2 (Figs. 8 and 9). There also was no indication that photosynthetic capacity had been reduced at ECO2 compared with ACO2. For example, the amount of most photosynthetic proteins examined was similar between the two growth conditions (Fig. 7), as were Vcmax, Jmax, and TPU values (Fig. 3). These values were actually marginally higher in the ECO2-grown plants. Hence, we conclude that, under the study conditions, there was no particular propensity for photosynthetic acclimation in the wild-type plants due to growth at ECO2.
Following growth at ACO2, there were no differences in photosynthetic parameters (e.g. Vcmax, Jmax, TPU, and amounts of photosynthetic proteins) between well-watered wild-type, AOX overexpression, and AOX knockdown plants, indicating that AOX amount had little influence on photosynthesis under these conditions (Figs. 3 and 7). This is consistent with a previous study, also done with well-watered ACO2-grown plants, which showed that photosynthesis across these plant lines was similar over a wide range of growth and measurement irradiances (Dahal et al., 2017).
At high measurement Ci, the A of the wild type or AOX overexpressors was slightly higher in ECO2- compared with ACO2-grown well-watered plants, but the opposite was seen in the AOX knockdowns (Fig. 2). Analysis of A/Ci curves indicated a lower TPU (the capacity at which triose phosphates being generated by the Calvin cycle could be utilized) in the knockdowns than in other plant lines following growth at ECO2 (Fig. 3). The relative insensitivity of A to low oxygen seen in the knockdowns (Fig. 4), as well as the increased sensitivity of A to low temperature seen in the knockdowns (Fig. 6), provided additional independent confirmations that, during growth at ECO2, there is an increased propensity, in the absence of AOX, for photosynthesis to become TPU limited. These results suggest that AOX respiration contributes to TPU capacity during growth at ECO2 and, hence, in its absence, TPU capacity is reduced. On the other hand, AOX overexpression did not increase TPU capacity or affect other photosynthetic parameters compared with the wild type. This indicates that the increased capacity for AOX respiration in these lines was inconsequential, at least for photosynthesis, and suggests that the increase in AOX protein amount seen in wild-type plants at ECO2 compared with ACO2 (greater than 2-fold) was sufficient to optimize photosynthesis under the new conditions.
Following growth at ECO2, the well-watered AOX knockdowns clearly had elevated amounts of hexose phosphates, Suc, and starch in leaf 5 (the source leaf being used for all of the analyses) compared with the other plant lines (Figs. 9 and 10). The simplest interpretation of this finding is that AOX respiration acts to consume carbohydrates during growth at ECO2 and, hence, in its absence, carbohydrate accumulates. One possibility is that the source leaf itself is the most critical site of AOX respiration acting to prevent source leaf carbohydrate accumulation. However, it also is possible that AOX respiration at a sink tissue is the most critical site for consuming carbohydrates, which then acts to prevent source leaf carbohydrate accumulation by promoting Suc translocation to the sink. Regardless, the large accumulation of source leaf carbohydrate in the knockdowns is likely to be an important factor contributing to the perturbation of photosynthesis in these plants.
The accumulation of hexose phosphates in the well-watered ECO2-grown knockdowns is particularly noteworthy, as this may relate directly to the reduction of TPU evident in these plants. A consequence of excessive sugar phosphate accumulation in the cytosol is that it sequesters Pi that would otherwise be returned to the stroma in exchange for triose phosphates, following consumption of the cytosolic sugar phosphates by Suc synthesis or by respiration (Stitt et al., 2010). In turn, too low Pi in the stroma could restrict ATP synthase activity (Sharkey and Vanderveer, 1989; Kanazawa and Kramer, 2002; Takizawa et al., 2008; Kiirats et al., 2009; Morales et al., 2018). Consistent with this possibility, the well-watered ECO2-grown knockdowns maintained higher pmf (ECSt) and ∆pH (ECSinv) across the thylakoid membrane than the other plant lines (Fig. 11). They also displayed lower gH+, an estimate of ATP synthase activity (Fig. 12). Since NPQ engages in response to declines in lumen pH, TPU limitation in the knockdown plants was associated with an increase in NPQ and a resultant decline in ETR, which then limited A (Fig. 2). Interestingly, the knockdowns displayed a significant increase in AtpB protein amount relative to the other plant lines (Fig. 7). This may represent an attempt by the knockdowns to compensate for low ATP synthase activity by up-regulating ATP synthase protein amount. (The ECO2-grown knockdowns displayed a similar but nonsignificant increase in RbcS protein amount relative to the other plant lines.) Low cytosolic and stromal Pi during TPU also should favor Suc and starch synthesis, respectively (Sharkey, 1985). Indeed, both Suc and starch (especially starch) amounts were higher in the ECO2-grown knockdowns compared with the other plant lines (Fig. 10).
The above discussion explicitly implicates low stromal Pi as a key driver of the photosynthetic perturbations in the well-watered ECO2-grown AOX knockdowns. However, an alternative view also should be considered, one in which ATP synthase activity in the absence of AOX is not restricted by low Pi per se but, rather, by a high ATP/ADP ratio in the stroma. An important consequence of growth at ECO2 is that it promotes the carboxylase activity and suppresses the oxygenase activity of Rubisco. As outlined in the introduction, this will lower demand in the chloroplast for ATP relative to NADPH. This reduced demand has been shown to reduce the expression of CET chain components at ECO2 (Foyer et al., 2012), consistent with the idea that CET acts to supplement the supply of ATP relative to NADPH in the chloroplast (Kramer and Evans, 2011). In wild-type tobacco and the AOX overexpressors, CET rates were indeed lower in ECO2- compared with ACO2-grown plants (Fig. 8). However, this was not the case in the knockdowns, which maintained similar rates of CET under the two growth conditions. It is not clear why the AOX knockdowns maintain higher rates of CET at ECO2 than the other plant lines, which could result in an overabundance of ATP relative to NADPH. Elucidating the metabolic conditions that activate CET in C3 plants remains an area of active research. Overreduction of the stromal pyridine nucleotide pool (perhaps a good indicator that NADPH is being supplied in excess of ATP), changes in the redox state of intersystem ETC components, and/or increased stromal H2O2 all are potential contributing factors, and collectively, they suggest that CET activation occurs in response to energy imbalances in the chloroplast (Joët et al., 2002; Okegawa et al., 2008; Livingston et al., 2010; Joliot and Johnson, 2011; Strand et al., 2015, 2017; Yamori and Shikanai, 2016; Johnson, 2018). At ECO2, the AOX knockdowns displayed a large increase in PsaA (PSI) relative to PsbA (PSII) compared with the other plants lines (Fig. 7). Such a photosystem stoichiometry adjustment is a common response to the overreduction of intersystem electron transport (Pfannschmidt and Yang, 2012; Puthiyaveetil et al., 2012; Rochaix et al., 2012). Despite this adjustment, the knockdowns still displayed higher 1 − qP (the fraction of closed [reduced] PSII reaction centers) at ECO2 than did the other plant lines. These are indications that chloroplast energy balance is perturbed in the ECO2-grown knockdowns and may provide an explanation for their higher rates of CET than in the other plant lines. However, this exaggerated CET rate may be higher than necessary to meet the ATP demands of the chloroplast at ECO2. The resulting high chloroplast ATP/ADP ratio would then act to slow ATP synthase activity (Morales et al., 2018), resulting in the high ECSt and low gH+ mentioned above. The higher CET rate in the knockdowns also may explain why vH+ did not differ across plant lines at ECO2, even though the knockdowns tended to maintain a lower ETR (rate of linear electron transport) than the other plant lines. In knockdowns, the lesser proton influx due to the lower ETR could be compensated by the additional proton influx associated with a higher CET rate.
The above discussion has outlined two different hypotheses for why photosynthesis in AOX knockdowns grown at ECO2 becomes restricted. The low-Pi hypothesis is one in which AOX aids in preventing a carbon imbalance in the cell that is being promoted by the high rates of A typical of growth at ECO2. The high-ATP/ADP ratio hypothesis is one in which AOX aids in preventing an energy imbalance in the chloroplast, which may relate in part to the different energy requirements of the chloroplast at ECO2, when photorespiration is being suppressed. In reality, the photosynthetic perturbations could be due to a combination of both these factors, which are not mutually exclusive but rather complementary. This emphasizes the fact that AOX respiration is a well-suited mechanism for both the oxidation of excess carbohydrate (Lambers, 1980) and/or the consumption of excess reductant (Noguchi and Yoshida, 2008), in both cases minimizing the resultant ATP yield. Our results also show that restriction of ATP synthase activity can be an important component of TPU-limited photosynthesis, as emphasized before (Kiirats et al., 2009; Yang et al., 2016).
Respiration rates tended to be slightly higher in ECO2- compared with ACO2-grown well-watered wild-type tobacco plants (Fig. 13). This is consistent with most physiological and molecular evidence in the recent literature, which shows that, across a wide range of species, long-term growth at ECO2 results in a mild to moderate increase in leaf respiration (Lewis et al., 1999; Davey et al., 2004; Leakey et al., 2009b; Fukayama et al., 2011; Gillespie et al., 2012; Markelz et al., 2014a, 2014b). However, while the knockdown of AOX clearly affected photosynthesis in well-watered ECO2-grown plants, such plants did not show any obvious differences in respiration rate (either RD or RL) in the source leaf relative to comparable wild-type and overexpression plants (RL was slightly lower in the ECO2-grown knockdowns than other plant lines, but that result was not statistically significant). This result clearly differs from how respiration in these plant lines responds to water deficit. Our previous work (with just ACO2-grown plants) showed that, during water deficit, RL is lower in AOX knockdowns and higher in AOX overexpressors compared with the wild type (Dahal and Vanlerberghe, 2017). This study confirms these results and shows that a similar respiratory pattern across plant lines is evident in ECO2-grown plants experiencing water deficit. One possibility is that, in the well-watered ECO2-grown knockdowns, there is no change in overall respiration rate compared with the other plant lines because (most) electrons are able to divert toward cyt oxidase under these growth conditions (while this apparently cannot occur during water deficit). Such a phenomenon, if occurring in the well-watered knockdowns, presumably would increase the ATP yield of respiration in the knockdowns relative to the other plant lines and, hence, further exacerbate the high-ATP/ADP conditions that may be perturbing photosynthesis in these plants. Another possibility is that a lack of AOX reduced respiration elsewhere in the plant, rather than in the source leaf being measured (see above).
Growth at ECO2 Does Not Protect against the Reduced Chloroplast ATP Synthase Amount Seen during Water Deficit
We showed previously that moderate water deficit resulted in a loss of tobacco chloroplast ATP synthase protein, which then limited photosynthetic rate. Furthermore, the severity of this biochemical limitation was enhanced in AOX knockdowns and delayed in AOX overexpressors relative to the wild-type response (Dahal and Vanlerberghe, 2018). This effect related to the ability of AOX to dampen energy imbalances in the chloroplast, which can arise when stomatal closure restricts the availability of CO2.
Given the above previous results, we tested whether an increased availability of CO2 during water deficit (i.e. water deficit during growth at ECO2) could stave off the ATP synthase decline. The results were clear: growth at ECO2 provided no protection against the onset of this limitation, which, again, was most severe in the AOX knockdowns and least severe in the AOX overexpressors, with the wild type showing an intermediate response. In fact, growth at ECO2 exaggerated the differences between plant lines under water deficit relative to the differences seen in a parallel set of ACO2-grown plants subjected to a similar severity of water deficit. These results suggest that energy imbalances persist at ECO2, despite the potential for increased Calvin cycle activity, and that AOX continues to provide an important means to minimize this imbalance. The results also suggest that low Ci or Cc is not, in itself, the signal that initiates the loss of ATP synthase; otherwise, the loss of ATP synthase would be expected to be less severe in ECO2- than in ACO2-grown wild-type plants experiencing comparable water deficit.
CONCLUSION
TPU capacity has been linked to the activity of key enzymes in starch and Suc synthesis, such as ADP-Glc pyrophosphorylase, cytosolic Fru-1,6-bisphosphatase, and Suc phosphate synthase (Sharkey et al., 1988; Vassey and Sharkey, 1989; Vassey et al., 1991; Socias et al., 1993; Sun et al., 1999, 2011; Tamoi et al., 2011; Yang et al., 2016). During growth at ECO2, TPU capacity was less in tobacco AOX knockdowns compared with the wild type, indicating that AOX respiration also was an important component of TPU under such growth conditions. An underlying characteristic of this TPU limitation is the restriction of chloroplast ATP synthase activity, likely due to low Pi and/or high ATP/ADP ratio in the stroma. This restriction enhances the ∆pH across the thylakoid membrane, which then limits photosynthesis by promoting pH-dependent feedback controls on the ETR, such as the engagement of NPQ. As a non-energy-conserving electron sink, AOX respiration acts against these changes in the chloroplast by preventing both carbohydrate and energy imbalances in the photosynthetic leaf during growth at ECO2. During water deficit in either ACO2- or ECO2-grown plants, AOX respiration also is critical for maintaining photosynthesis, but in this case by preventing a down-regulation of ATP synthase protein amount, which otherwise also restricts ATP synthase activity. During water deficit, AOX respiration appears not critical to prevent carbohydrate imbalances, even at ECO2, but nonetheless remains critical in preventing energy imbalances in the photosynthetic leaf that promote the loss of ATP synthase protein.
Our results suggest a need to better understand how future ECO2 growth conditions will affect the partitioning of respiratory electrons between the energy-conserving cyt pathway and the energy-dissipating AOX. If future growth conditions are accompanied by a significant change in this partitioning, then this will have important consequences for overall plant carbon and energy balance (Gonzalez-Meler et al., 2004; Becklin et al., 2017). Our results also may be relevant to the efforts being made to boost photosynthetic rates, such as through the engineering of C4 metabolism into C3 plants (Sage and Zhu, 2011). The success of these efforts may depend, in part, on how the respiratory pathways respond and how those changes affect the photosynthesis-respiration interaction.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Experiments used wild-type and transgenic tobacco (Nicotiana tabacum ‘Petit Havana’). The transgenic lines have decreased (lines RI9 and RI29) or elevated (lines B7 and B8) amounts of mitochondrial AOX protein compared with the wild type, as described previously (Dahal and Vanlerberghe, 2017). Seeds were germinated in vermiculite in controlled-environment growth chambers (model PGC-20; Conviron) with a 16-h photoperiod, temperature of 28°C/22°C (light/dark), relative humidity of 60%, PPFD of 700 µmol m−2 s−1, and in an atmosphere containing either ACO2 (400 μmol mol−1 Ca) or ECO2 (1,000 μmol mol−1 Ca). Following germination, seedlings were transplanted to 6-inch pots containing a growth medium that consisted of four parts (v/v) soil (Promix BX; Premier Horticulture) and one part vermiculite. Plants were then watered daily with a one-fifth-strength Hoagland solution (Hoagland and Arnon, 1950). Physiological and biochemical analyses (see below) were performed on a fully expanded leaf (leaf 5). The ACO2-grown plants were analyzed at 18 d after transplanting, while the ECO2-grown plants were analyzed at 16 d after transplanting. At these time points, the two sets of plants were of similar size. In some cases, plants at this stage were subjected to a moderate water deficit by withholding water for 5 d (ACO2-grown plants) or 6 d (ECO2-grown plants) prior to analysis of leaf 5.
Leaf Gas Exchange and Chlorophyll a Fluorescence
Measurements of leaf CO2 exchange and chlorophyll a fluorescence from PSII were performed in the growth chamber using a portable system (GFS-3000; Heinz Walz). In general, all measurements and calculations were done as described previously (Dahal et al., 2014; Dahal and Vanlerberghe, 2017). Specific measurement conditions were as indicated in the figure legends.
CO2 response curves of A were generated at saturating irradiance (1,600 PPFD) by supplying nine different CO2 concentrations over the range of 50 to 1,200 µmol mol−1. Vcmax, Jmax, and maximum capacity of TPU were then estimated by fitting the CO2 response curves to a model of photosynthesis, as discussed previously (Long and Bernacchi, 2003), and using the spreadsheet provided by Sharkey et al. (2007). RD and RL were estimated as described previously (Dahal et al., 2014). Temperature response curves of A were measured at the growth irradiance and respective growth CO2 concentration and over the range of 5°C to 45°C.
Leaf Absorption Spectroscopy
A DUAL-PAM-100 measuring system (Heinz Walz) equipped with DUAL-E and DUAL-DB modules was used to simultaneously measure PSI absorbance and PSII fluorescence at intervals over the range of 0 to 2,000 PPFD (from low to high PPFD, with 6 min at each irradiance). These were used to estimate the rate of CET around PSI as described previously (Johnson, 2011) and as outlined in a previous study (Dahal et al., 2014). A DUAL-PAM-100 measuring system equipped with the emitter module DUAL-EP515 and the detector module DUAL-DP515 was used to measure the 550- to 515-nm absorbance difference signal, which provides a linear measure of thylakoid membrane potential (∆ψ) due to a ∆ψ-induced shift in the absorption spectrum of thylakoid pigments, known as the electrochromic shift (ECS; Schreiber and Klughammer, 2008). A DIRK analysis of the ECS signal during a light-to-dark transition can be used to estimate parameters related to thylakoid proton flux (Cruz et al., 2005; Baker et al., 2007). These include gH+, vH+, ECSt, and the partitioning of the pmf into its ∆pH and ∆ψ components, known as ECSinv and ECSss, respectively. To perform the DIRK analysis, plants were dark adapted for 1 h, followed by illumination with actinic light (700 or 1,600 PPFD) for 10 min prior to the light-dark transition. Analyses were done as described previously (Cruz et al., 2005; Baker et al., 2007) and as outlined in a previous study (Dahal and Vanlerberghe, 2018). It is also noted that the ECS signal and its meaning remain controversial due to the possibility of confounding overlapping absorbance signals (Johnson and Ruban, 2014).
Biochemical and Other Analyses
Hexoses, hexose phosphates, Suc, and starch were quantified by enzyme-coupled assays, as described previously (Jones, 1981; Stitt et al., 1989) and as outlined in a previous study (Wang et al., 2011). Immunoblot analyses were performed as described previously (Dahal et al., 2014; Dahal and Vanlerberghe, 2018), as was leaf RWC (Wang and Vanlerberghe, 2013). Statistical analyses were performed using Prism 5.0 (GraphPad Software). Two-way ANOVAs were performed, with plant line and growth CO2 as the two independent variables. Such analyses were performed separately on well-watered plants or plants experiencing a water deficit. Bonferroni post tests were then used to compare the five plant lines within each of the four growth conditions.
Accession Number
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number X79768 (AOX1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Leaf 5 parameters of well-watered tobacco.
Supplemental Figure S2. RWC of leaf 5 of tobacco.
Supplemental Figure S3. Representative immunoblots for AOX and several chloroplast-localized photosynthesis-related proteins in tobacco.
Supplemental Figure S4. CO2 response curves (A/Cc) for leaf 5 of tobacco.
Supplemental Figure S5. CO2 response curves for A in leaf 5 of tobacco.
Supplemental Figure S6. A of leaf 5 of tobacco.
Supplemental Figure S7. Thylakoid membrane pmf and the partitioning of pmf into its ∆ψ and ∆pH components in leaf 5 of well-watered tobacco.
Supplemental Figure S8. Thylakoid membrane proton flux parameters in leaf 5 of well-watered tobacco.
Supplemental Figure S9. Thylakoid membrane pmf and the partitioning of pmf into its ∆ψ and ∆pH components in leaf 5 of tobacco.
Supplemental Figure S10. Thylakoid membrane proton flux parameters in leaf 5 of tobacco.
Supplemental Figure S11. RL/RD ratio in leaf 5 of tobacco.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Jia Wang, Ahmed El-Banbawy, Farkhunda Rahimy, and Harindra Rajasekeran for their contributions to this work. G.C.V. thanks the Natural Sciences and Engineering Research Council of Canada for the Discovery Grant and Green Crop Network strategic funding that has supported this work.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant no. RGPIN-2014-06553 to G.C.V.)
Articles can be viewed without a subscription.
References
- Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Asada K. (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141: 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin O, Millar H, Turnbull M (2010) Plant respiration in a changing world. New Phytol 187: 268–272 [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125 [DOI] [PubMed] [Google Scholar]
- Becklin KM, Walker SM II, Way DA, Ward JK (2017) CO2 studies remain key to understanding a future world. New Phytol 214: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM. (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57: 2435–2444 [DOI] [PubMed] [Google Scholar]
- Cruz JA, Avenson TJ, Kanazawa A, Takizawa K, Edwards GE, Kramer DM (2005) Plasticity in light reactions of photosynthesis for energy production and photoprotection. J Exp Bot 56: 395–406 [DOI] [PubMed] [Google Scholar]
- Dahal K, Vanlerberghe GC (2017) Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytol 213: 560–571 [DOI] [PubMed] [Google Scholar]
- Dahal K, Vanlerberghe GC (2018) Improved chloroplast energy balance during water deficit enhances plant growth: more crop per drop. J Exp Bot 69: 1183–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal K, Wang J, Martyn GD, Rahimy F, Vanlerberghe GC (2014) Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiol 166: 1560–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal K, Martyn GD, Vanlerberghe GC (2015) Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytol 208: 382–395 [DOI] [PubMed] [Google Scholar]
- Dahal K, Martyn GD, Alber NA, Vanlerberghe GC (2017) Coordinated regulation of photosynthetic and respiratory components is necessary to maintain chloroplast energy balance in varied growth conditions. J Exp Bot 68: 657–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey PA, Hunt S, Hymus GJ, DeLucia EH, Drake BG, Karnosky DF, Long SP (2004) Respiratory oxygen uptake is not decreased by an instantaneous elevation of [CO2], but is increased with long-term growth in the field at elevated [CO2]. Plant Physiol 134: 520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Saz NF, Ribas-Carbo M, McDonald AE, Lambers H, Fernie AR, Florez-Sarasa I (2018) An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci 23: 206–219 [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzàlez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48: 609–639 [DOI] [PubMed] [Google Scholar]
- Ehlers I, Augusti A, Betson TR, Nilsson MB, Marshall JD, Schleucher J (2015) Detecting long-term metabolic shifts using isotopomers: CO2-driven suppression of photorespiration in C3 plants over the 20th century. Proc Natl Acad Sci USA 112: 15585–15590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-Sarasa I, Flexas J, Rasmusson AG, Umbach AL, Siedow JN, Ribas-Carbo M (2011) In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions. Plant Cell Environ 34: 1373–1383 [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Ribas-Carbo M, Del-Saz NF, Schwahn K, Nikoloski Z, Fernie AR, Flexas J (2016) Unravelling the in vivo regulation and metabolic role of the alternative oxidase pathway in C3 species under photoinhibitory conditions. New Phytol 212: 66–79 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63: 1637–1661 [DOI] [PubMed] [Google Scholar]
- Fukayama H, Sugino M, Fukuda T, Masumoto C, Taniguchi Y, Okada M, Sameshima R, Hatanaka T, Misoo S, Hasegawa T, et al. (2011) Gene expression profiling of rice grown in free air CO2 enrichment (FACE) and elevated soil temperature. Field Crops Res 121: 195–199 [Google Scholar]
- Galle A, Florez-Sarasa I, Thameur A, de Paepe R, Flexas J, Ribas-Carbo M (2010) Effects of drought stress and subsequent rewatering on photosynthetic and respiratory pathways in Nicotiana sylvestris wild type and the mitochondrial complex I-deficient CMSII mutant. J Exp Bot 61: 765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin A, Lapointe L, Dizengremel P (2009) The alternative respiratory pathway allows sink to cope with changes in carbon availability in the sink-limited plant Erythronium americanum. J Exp Bot 60: 4235–4248 [DOI] [PubMed] [Google Scholar]
- Gandin A, Duffes C, Day DA, Cousins AB (2012) The absence of alternative oxidase AOX1A results in altered response of photosynthetic carbon assimilation to increasing CO2 in Arabidopsis thaliana. Plant Cell Physiol 53: 1627–1637 [DOI] [PubMed] [Google Scholar]
- Gandin A, Denysyuk M, Cousins AB (2014) Disruption of the mitochondrial alternative oxidase (AOX) and uncoupling protein (UCP) alters rates of foliar nitrate and carbon assimilation in Arabidopsis thaliana. J Exp Bot 65: 3133–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeström P, Igamberdiev AU (2016) The origin of cytosolic ATP in photosynthetic cells. Physiol Plant 157: 367–379 [DOI] [PubMed] [Google Scholar]
- Geiger M, Haake V, Ludewig F, Stitt M (1999) The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ 22: 1177–1199 [Google Scholar]
- Gifford RM. (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30: 171–186 [DOI] [PubMed] [Google Scholar]
- Gillespie KM, Xu F, Richter KT, McGrath JM, Markelz RJC, Ort DR, Leakey ADB, Ainsworth EA (2012) Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell Environ 35: 169–184 [DOI] [PubMed] [Google Scholar]
- Gomez-Casanovas N, Blanc-Betes E, Gonzalez-Meler MA, Azcon-Bieto J (2007) Changes in respiratory mitochondrial machinery and cytochrome and alternative pathway activities in response to energy demand underlie the acclimation of respiration to elevated CO2 in the invasive Opuntia ficus-indica. Plant Physiol 145: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Taneva L, Trueman RJ (2004) Plant respiration and elevated atmospheric CO2 concentration: cellular responses and global significance. Ann Bot 94: 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Blanc-Betes E, Flower CE, Ward JK, Gomez-Casanovas N (2009) Plastic and adaptive responses of plant respiration to changes in atmospheric CO2 concentration. Physiol Plant 137: 473–484 [DOI] [PubMed] [Google Scholar]
- Harley PC, Thomas RB, Reynolds JF, Strain BR (1992) Modelling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15: 271–282 [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method of growing plants without soil. Calif Agric Exp Sta Circ 347: X. [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT (1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta 1366: 235–255 [Google Scholar]
- Hoshiyasu S, Kohzuma K, Yoshida K, Fujiwara M, Fukao Y, Yokota A, Akashi K (2013) Potential involvement of N-terminal acetylation in the quantitative regulation of the ε subunit of chloroplast ATP synthase under drought stress. Biosci Biotechnol Biochem 77: 998–1007 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova NV, Gardeström P (1997) Involvement of cyanide-resistant and rotenone-insensitive pathways of mitochondrial electron transport during oxidation of glycine in higher plants. FEBS Lett 412: 265–269 [DOI] [PubMed] [Google Scholar]
- Joët T, Cournac L, Peltier G, Havaux M (2002) Cyclic electron flow around photosystem I in C3 plants: in vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128: 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GN. (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807: 384–389 [DOI] [PubMed] [Google Scholar]
- Johnson MP. (2018) Metabolic regulation of photosynthetic membrane structure tunes electron transfer function. Biochem J 475: 1225–1233 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Ruban AV (2014) Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth Res 119: 233–242 [DOI] [PubMed] [Google Scholar]
- Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MG. (1981) Enzymic assay for starch and glycogen. Tech Carbohyd Metab 303: 1–13 [Google Scholar]
- Kanazawa A, Kramer DM (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99: 12789–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiirats O, Cruz JA, Edwards GE, Kramer DM (2009) Feedback limitation of photosynthesis at high CO2 acts by modulating the activity of the chloroplast ATP synthase. Funct Plant Biol 36: 893–901 [DOI] [PubMed] [Google Scholar]
- Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage YN, Yokota A, Kramer DM (2009) The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ 32: 209–219 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Evans JR (2011) The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155: 70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H. (1980) The physiological significance of cyanide-resistant respiration in higher plants. Plant Cell Environ 3: 293–302 [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009a) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR (2009b) Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc Natl Acad Sci USA 106: 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Zhu X, Long SP, Ort DR (2012) Photosynthesis in a CO2-rich atmosphere. In Eaton-Rye JJ, Tripathy BC, Sharkey TD, eds, Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation., Advances in Photosynthesis and RespirationVol Vol 34 Springer Science+Business Media, The Netherlands, pp 733–768 [Google Scholar]
- Lewis CE, Peratoner G, Cairns AJ, Causton DR, Foyer CH (1999) Acclimation of the summer annual species, Lolium temulentum, to CO2 enrichment. Planta 210: 104–114 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang G, Sun B, Zhang S, Zhang Y, Liao Y, Zhou Y, Xia X, Shi K, Yu J (2013) Stimulated leaf dark respiration in tomato in an elevated carbon dioxide atmosphere. Sci Rep 3: 3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston AK, Kanazawa A, Cruz JA, Kramer DM (2010) Regulation of cyclic electron flow in C3 plants: differential effects of limiting photosynthesis at ribulose-1,5-bisphosphate carboxylase/oxygenase and glyceraldehyde-3-phosphate dehydrogenase. Plant Cell Environ 33: 1779–1788 [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54: 2393–2401 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ (2017) Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot 68: 4433–4453 [DOI] [PubMed] [Google Scholar]
- Markelz RJC, Lai LX, Vosseler LN, Leakey ADB (2014a) Transcriptional reprogramming and stimulation of leaf respiration by elevated CO2 concentration is diminished, but not eliminated, under limiting nitrogen supply. Plant Cell Environ 37: 886–898 [DOI] [PubMed] [Google Scholar]
- Markelz RJC, Vosseller LN, Leakey ADB (2014b) Developmental stage specificity of transcriptional, biochemical and CO2 efflux responses of leaf dark respiration to growth of Arabidopsis thaliana at elevated [CO2]. Plant Cell Environ 37: 2542–2552 [DOI] [PubMed] [Google Scholar]
- Millar AH, Whelan J, Soole KL, Day DA (2011) Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol 62: 79–104 [DOI] [PubMed] [Google Scholar]
- Møller IM. (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591 [DOI] [PubMed] [Google Scholar]
- Morales A, Yin X, Harbinson J, Driever SM, Molenaar J, Kramer DM, Struik PC (2018) In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants. Plant Physiol 176: 1247–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12: 125–134 [DOI] [PubMed] [Google Scholar]
- Noguchi K, Yoshida K (2008) Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8: 87–99 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Kagawa Y, Kobayashi Y, Shikanai T (2008) Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol 49: 825–834 [DOI] [PubMed] [Google Scholar]
- Oliver DJ, Gardeström P (2004) Photorespiration: photosynthesis in the mitochondria. In Day DA, Millar AH, Whelan J, eds, Plant Mitochondria: From Genome to Function. Advances in Photosynthesis and Respiration, Vol 17 Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 293–306 [Google Scholar]
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Yang C (2012) The hidden function of photosynthesis: a sensing system for environmental conditions that regulates plant acclimation responses. Protoplasma (Suppl 2) 249: S125–S136 [DOI] [PubMed] [Google Scholar]
- Plaxton WC, Podestá FE (2006) The functional organization and control of plant respiration. Crit Rev Plant Sci 25: 159–198 [Google Scholar]
- Puthiyaveetil S, Ibrahim IM, Allen JF (2012) Oxidation-reduction signalling components in regulatory pathways of state transitions and photosystem stoichiometry adjustment in chloroplasts. Plant Cell Environ 35: 347–359 [DOI] [PubMed] [Google Scholar]
- Qiu QS, Huber JL, Booker FL, Jain V, Leakey ADB, Fiscus EL, Yau PM, Ort DR, Huber SC (2008) Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated [CO2]. Photosynth Res 97: 155–166 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8: 546–553 [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Taylor NL, Giles L, Busquets S, Finnegan PM, Day DA, Lambers H, Medrano H, Berry JA, Flexas J (2005) Effects of water stress on respiration in soybean leaves. Plant Physiol 139: 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix JD, Lemeille S, Shapiguzov A, Samol I, Fucile G, Willig A, Goldschmidt-Clermont M (2012) Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment. Philos Trans R Soc Lond B Biol Sci 367: 3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Vera UM, De Souza AP, Long SP, Ort DR (2017) The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front Plant Sci 8: 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. (1994) Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res 39: 351–368 [DOI] [PubMed] [Google Scholar]
- Sage RF, Zhu XG (2011) Exploiting the engine of C4 photosynthesis. J Exp Bot 62: 2989–3000 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C (2008) New accessory for the DUAL-PAM-100: the P515/535 module and examples of its application. PAM Appl Notes 1: 1–10 [Google Scholar]
- Selinski J, Hartmann A, Deckers-Hebestreit G, Day DA, Whelan J, Scheibe R (2018) Alternative oxidase isoforms are differentially activated by tricarboxylic acid cycle intermediates. Plant Physiol 176: 1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. (1985) O2-insensitive photosynthesis in C3 plants: its occurrence and a possible explanation. Plant Physiol 78: 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ (2012) Photosynthetic responses to high temperature. In Flexas J, Loreto F, Medrano H, eds, Terrestrial Photosynthesis in a Changing Environment: A Molecular, Physiological, and Ecological Approach. Cambridge University Press, Cambridge, UK, pp 294–302 [Google Scholar]
- Sharkey TD, Vanderveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91: 679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Kobza J, Seemann JR, Brown RH (1988) Reduced cytosolic fructose-1,6-bisphosphatase activity leads to loss of O2 sensitivity in a Flaveria linearis mutant. Plant Physiol 86: 667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Socias FX, Medrano H, Sharkey TD (1993) Feedback limitation of photosynthesis of Phaseolus vulgaris L. grown in elevated CO2. Plant Cell Environ 16: 81–86 [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW (1989) Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174: 518–552 [Google Scholar]
- Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism: more than the icing on the cake. Plant J 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Strand DD, Livingston AK, Satoh-Cruz M, Froehlich JE, Maurino VG, Kramer DM (2015) Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci USA 112: 5539–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand DD, Livingston AK, Satoh-Cruz M, Koepke T, Enlow HM, Fisher N, Froehlich JE, Cruz JA, Minhas D, Hixson KK, et al. (2017) Defects in the expression of chloroplast proteins leads to H2O2 accumulation and activation of cyclic electron flow around photosystem I. Front Plant Sci 7: 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Okita TW, Edwards GE (1999) Modification of carbon partitioning, photosynthetic capacity, and O2 sensitivity in Arabidopsis plants with low ADP-glucose pyrophosphorylase activity. Plant Physiol 119: 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhang J, Larue CT, Huber SC (2011) Decrease in leaf sucrose synthesis leads to increased leaf starch turnover and decreased RuBP regeneration-limited photosynthesis but not Rubisco-limited photosynthesis in Arabidopsis null mutants of SPSA1. Plant Cell Environ 34: 592–604 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR (2006) Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 103: 19587–19592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Kanazawa A, Kramer DM (2008) Depletion of stromal Pi induces high ‘energy-dependent’ antenna exciton quenching (qE) by decreasing proton conductivity at CFO-CF1 ATP synthase. Plant Cell Environ 31: 235–243 [DOI] [PubMed] [Google Scholar]
- Tamoi M, Hiramatsu Y, Nedachi S, Otori K, Tanabe N, Maruta T, Shigeoka S (2011) Increase in the activity of fructose-1,6-bisphosphatase in cytosol affects sugar partitioning and increases the lateral shoots in tobacco plants at elevated CO2 levels. Photosynth Res 108: 15–23 [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Boex-Fontvieille E, Mahé A, Hodges M (2012) Respiratory carbon fluxes in leaves. Curr Opin Plant Biol 15: 308–314 [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Gauthier P, Buckley TN, Busch FA, Barbour MM, Bruhn D, Heskel MA, Gong XY, Crous KY, Griffin K, et al. (2017) Leaf day respiration: low CO2 flux but high significance for metabolism and carbon balance. New Phytol 216: 986–1001 [DOI] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917 [Google Scholar]
- Vanlerberghe GC. (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci 14: 6805–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassey TL, Sharkey TD (1989) Mild water stress of Phaseolus vulgaris plants leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant Physiol 89: 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassey TL, Quick P, Sharkey TD, Stitt M (1991) Water stress, carbon dioxide, and light effects on sucrose phosphate synthase in Phaseolus vulgaris. Physiol Plant 81: 37–44 [Google Scholar]
- Vishwakarma A, Bashyam L, Senthilkumaran B, Scheibe R, Padmasree K (2014) Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana. Plant Physiol Biochem 81: 44–53 [DOI] [PubMed] [Google Scholar]
- Wang J, Vanlerberghe GC (2013) A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol Plant 149: 461–473 [DOI] [PubMed] [Google Scholar]
- Wang J, Rajakulendran N, Amirsadeghi S, Vanlerberghe GC (2011) Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol Plant 142: 339–351 [DOI] [PubMed] [Google Scholar]
- Wang J, Cheung M, Rasooli L, Amirsadeghi S, Vanlerberghe GC (2014) Plant respiration in a high CO2 world: how will alternative oxidase respond to future atmospheric and climatic conditions? Can J Plant Sci 94: 1091–1101 [Google Scholar]
- Watanabe CKA, Yamori W, Takahashi S, Terashima I, Noguchi K (2016) Mitochondrial alternative pathway-associated photoprotection of photosystem II is related to the photorespiratory pathway. Plant Cell Physiol 57: 1426–1431 [DOI] [PubMed] [Google Scholar]
- Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67: 81–106 [DOI] [PubMed] [Google Scholar]
- Yang JT, Preiser AL, Li Z, Weise SE, Sharkey TD (2016) Triose phosphate use limitation of photosynthesis: short-term and long-term effects. Planta 243: 687–698 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Noguchi K (2009) Differential gene expression profiles of the mitochondrial respiratory components in illuminated Arabidopsis leaves. Plant Cell Physiol 50: 1449–1462 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Hachiya T, Tholen D, Shibata M, Terashima I, Noguchi K (2011a) Distinct responses of the mitochondrial respiratory chain to long- and short-term high-light environments in Arabidopsis thaliana. Plant Cell Environ 34: 618–628 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Terashima I, Noguchi K (2011b) Physiological impact of mitochondrial alternative oxidase on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Environ 34: 1890–1899 [DOI] [PubMed] [Google Scholar]
- Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH (2010) Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ 33: 2121–2131 [DOI] [PubMed] [Google Scholar]